
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 05 October 2021
Sec. Veterinary Epidemiology and Economics
Volume 8 - 2021 | https://doi.org/10.3389/fvets.2021.735076
 Lin Wang
Lin Wang Na Liu
Na Liu Yubin Gao
Yubin Gao Junhui Liu
Junhui Liu Xiumei Huang
Xiumei Huang Qingqing Zhang
Qingqing Zhang Yuehua Li
Yuehua Li Jianmei Zhao
Jianmei Zhao Junwei Wang*
Junwei Wang* Ge Zhao*
Ge Zhao*The microbial contamination of pork during the slaughter process, especially that of the hygiene indicator bacteria, Escherichia coli, is closely related to the safety and quality of the meat. Some diarrheagenic E. coli can cause serious foodborne diseases, and pose a significant threat to human life and health. In order to ascertain the current status of E. coli and diarrheagenic E. coli contamination during the pig slaughter process in China, we conducted thorough monitoring of large-sized slaughterhouses, as well as small- or medium-sized slaughterhouses, in different provinces of China from 2019 to 2020. The overall positive rate of E. coli on the pork surface after slaughter was very high (97.07%). Both the amount of E. coli contamination and the positive ratio of diarrheagenic E. coli in large-sized slaughterhouses (7.50–13.33 CFU/cm2, 3.44%) were lower than those in small- or medium-sized slaughterhouses (74.99–133.35 CFU/cm2, 5.71%). Combined with the current status of sanitary control in slaughterhouses, we determined that pre-cooling treatment significantly reduced E. coli and diarrheagenic E. coli in pork after slaughter, while microbiological testing reduced E. coli. Based on our monitoring data, China urgently needs to establish relevant standards to better control microbial contamination during pig slaughtering progress. This study provided a theoretical basis for the hygiene quality management of the pig slaughter industry in China.
In recent years, foodborne pathogenic infections have occurred frequently, which has a significant impact on human health and economy, and has become a major public health problem worldwide (1). Foodborne pathogens are the primary cause of foodborne diseases. Foodborne diseases caused by foodborne pathogens have been a major threat to food safety (2–4). With the increase in demand for fresh livestock and poultry products, an increasing amount of attention has been paid to food safety. Previous studies have shown that the number of outbreaks associated with fresh products increased from 0.7% in the 1970s to 6% in the 1990s in the United States (5, 6). According to the annual report statistics of the Rapid Alert System for Food and Feed (RASFF), the incidence of food notifications caused by pathogenic microorganisms has been fluctuating at a high level. Among the hazard category were concerned, pathogenic microorganisms were the most common hazard in meat and meat products in the years 2011–2015 (7). As a large country with high levels of pork production, pork accounts for 75% of the meat consumption in China. In recent years, domestic and foreign foodborne diseases caused by pathogenic microorganisms in pork have become very common (8–11). Quantitative microbiological risk assessment shows that slaughtering plays a significant role in the contamination of pig carcasses; therefore, it is very important to assess the microbial index in pork during the slaughter process in China (12–14).
According to reports, common pathogenic bacteria include: Salmonella, Shigella, diarrheagenic E. coli, Campylobacter, Yersinia enterocolitica, Clostridium perfringens, Vibrio and Listeria monocytogenes, etc (1). Studies have shown that in China, pork from pig slaughtering process and retail market was contaminated by various pathogenic bacteria in varying degrees, including Campylobacter (15), Shiga toxin–producing E. coli (11), Listeria monocytogenes (16), Salmonella (17), etc., which posed a great threat to people's life and health. Therefore, it is essential for the prevention and control of foodborne diseases by strengthening the public health surveillance of food-borne pathogens during animal production and formulating related hygiene specification and control standards in the process of “from farm to fork” (1).
Escherichia coli is a hygiene indicator bacteria for pig carcasses during slaughter; the level of E. coli contamination in the slaughtering process of live pigs has been found to be key to the health of the consumers. As a Gram-negative bacteria, most E. coli are generally non-pathogenic (18, 19). They are normally found living in human and animal intestines. Usually, E. coli is utilized as a hygiene indicator bacteria, reflecting the sanitary and safety status of food (20). A low amount of detected E. coli indicates that the food processing process is well-controlled and the product safety is good. In contrast, a high amount of detected E. coli indicates a poor hygiene level.
Presently, the risk of cross-contamination of various pathogenic microorganisms is also high during the slaughter of pigs. In this process, factors such as intestinal damage, fecal contamination, environment, equipment, or workers' hands may cause cross-contamination of pathogenic microorganisms. Therefore, the safety of the product cannot be guaranteed. Some serotypes of E. coli, known as diarrheagenic E. coli, including enteropathogenic E.coli (EPEC), Shiga toxin-producing E. coli/enterohemorrhagic E.coli (STEC/EHEC), Shigella/enteroinvasive E.coli (EIEC), enteroaggregative E.coli (EAEC), and enterotoxigenic E.coli (ETEC), can cause diarrhea, with food transmission as the main route of infection (21, 22). Diarrheagenic E. coli is the focus of pathogenic microorganism monitoring in livestock and poultry products in various countries. Monitoring data from the United States in 2015 showed that 5.0% of fresh pork was contaminated with diarrheagenic E. coli (23). Meanwhile, monitoring data from the European Union in 2017 showed that the contamination rate of diarrheagenic E. coli in fresh pork was 4.4% (24). Although the rate of diarrheagenic E. coli in pork is not high overall, once food safety issues are triggered, they can easily escalate and are more likely to be fatal to people with low immunity. Therefore, risk monitoring and assessment of diarrheagenic E. coli in pork products at the slaughter stage is very important.
In this study, we comprehensively analyzed microbial contamination of 2,013 samples from 71 pig slaughterhouses in 14 provinces of China from 2019 to 2020 to determine the current contamination status of E. coli and diarrheagenic E. coli in slaughtered pork in the country. This study provided technical support for the sanitary quality supervision and management of the pig slaughter industry in China.
Based on daily slaughter scales, in which the data calculation was in accordance with GB503175-2007 “Code for design of pig slaughtering and cutting rooms,” the slaughterhouses were divided into large-sized slaughterhouses, and small- or medium-sized slaughterhouses. The large-sized pig slaughter companies operate on daily slaughter scales exceeding 1,000 pigs, with slaughter and segmentation processing. The small- or medium-sized slaughterhouses have daily slaughter scales under 1,000 pigs. We conducted investigations into hygiene inspection and microbiological control of large-sized, and small- or medium-sized pig slaughter companies, in different regions, to determine the current hygiene control status of the industry.
We selected 71 pig slaughterhouses in 14 provinces of China from 2019 to 2020 [including ten provinces (Anhui, Shanxi, Shandong, Yunnan, Guangxi, Heilongjiang, Guangdong, Shanghai, Hubei, Xinjiang) in 2019 and eight provinces (Anhui, Shanxi, Shandong, Yunnan, Jiangsu, Sichuan, Henan, Jilin) in 2020; four of the provinces was sampled in both years]. There were 33 large slaughterhouses, and 38 small- or medium-sized slaughterhouses. The configuration of the slaughter cold chain and the development of microbiological testing in the slaughterhouses are shown in Table 1. In each province, at least two large-sized pig slaughter companies, and three small- or medium-sized slaughter companies, were selected for sampling.
The period of May to October was selected for centralized sampling. According to the current situation of large slaughterhouses in China, the slaughtered pork is placed at 4°C for more than 6 h for precooling. In our study, at least 20 carcass surfaces swabbed before precooling, and 25 carcass surfaces swabbed after precooling, were collected from large slaughterhouses. At least 20 carcass surface swabbed samples were collected from small- or medium-sized slaughterhouses without precooling. A total of 1,074 carcass surfaces swabs were collected from large slaughterhouses, and 939 carcass surfaces swabs were collected from small- or medium-sized slaughterhouses. The total number of samples was 2013.
Cotton swab (SWAB-10, Elabscience, USA) samples (involving pre-cooling) were collected from the surface of carcasses after slaughtering. Three sampling sites (belly, ham, and jowl) were selected on each sample, and 100 cm2 of cotton swab samples were applied to each sampling site. Environmental samples comprised the surface swabs of facilities and equipment, workers' hands, utensils, and the ground that directly or indirectly came into contact with the pig carcass during the slaughter process. The collected cotton swab samples were loaded into transport medium containing 10 mL phosphate-buffered saline, stored at low temperature, and transported to the laboratory for testing within 24–48 h.
Escherichia coli counts were detected using commercial kits according to the industry standard SN/T 4547-2017, specifically using the Petrifilm E. coli test sheet (3M PetrifilmTM, USA), with reference to the instructions and the methods specified in the standard for detection. In short, 1 mL sample with appropriate dilution was added to the center of the Petrifilm plate, then slowly covering the upper film of the Petrifilm plate. After the sample was evenly covered the Petrifilm plate, place it horizontally at 36 ± 1°C and incubate for 24 ± 2 h. Record the number of blue colonies with gas on the Petrifilm plate and the corresponding dilution factor, expressed in colony forming units (CFU).
Samples from four randomly selected sampled provinces were tested for diarrheagenic E. coli. Diarrheagenic E. coli was identified using the multiplex polymerase chain reaction detection kit developed by our laboratory referring the study of Toma et al. (25).
The microbial contamination rate was analyzed by Excel software, and the amount of E. coli contamination was analyzed by MiniTab v.17 software (Minitab, Inc, State College, PA, USA). The contamination rate of pathogenic microorganisms was analyzed by the Pert distribution fitting in @RISK7 software (Palisade Corporation, New York, NY, USA), and the amount of E. coli contamination was analyzed by random distribution fitting. The function is obtained by random fitting. Monte Carlo simulation was performed using the Latin hypercube sampling method. Probability distributions were obtained from each time point in simulations of 10,000 iterations.
As a hygiene indicator, E. coli were detected and counted in pork after slaughter. Overall, there was a very high positive detection rate of E. coli in pork after slaughter in China, with an overall rate of 97.07% (1,954/2,013). Due to the fact that 55.26% (21/38, Table 1) small- or medium-sized slaughterhouses lack cold chain facilities, and considering the comparability of data between large-sized, and small- or medium-sized slaughterhouses, we detected and counted E. coli in pork before pre-cooling after slaughter in different types of slaughterhouses, and found that the isolation rate of E. coli was comparative between large-sized (97.30%) and small- or medium-sized slaughterhouses (96.81%).
We separately compared the data distribution of E. coli detected in large-sized, and small- or medium-sized slaughterhouses. As shown in Figure 1A, the E. coli contamination of pork in large-scale slaughterhouses was generally lower than that in small- or medium-sized slaughterhouses. The highest frequency of contamination detected in small- or medium-sized slaughterhouses was 74.99 colony-forming units (CFU)/cm2-133.35 CFU/cm2, while the most frequently detected contamination in large-sized slaughterhouses was 7.50 CFU/cm2-13.33 CFU/cm2, which is exactly an order of magnitude difference. These results suggested that although the detection rate of E. coli in small- or medium-sized slaughterhouses was significantly different from that of large-sized slaughterhouses, the amount of E. coli contamination was still higher than that of large-sized slaughterhouses, indicating that overall hygiene control was poorer than that of large-sized slaughterhouses.
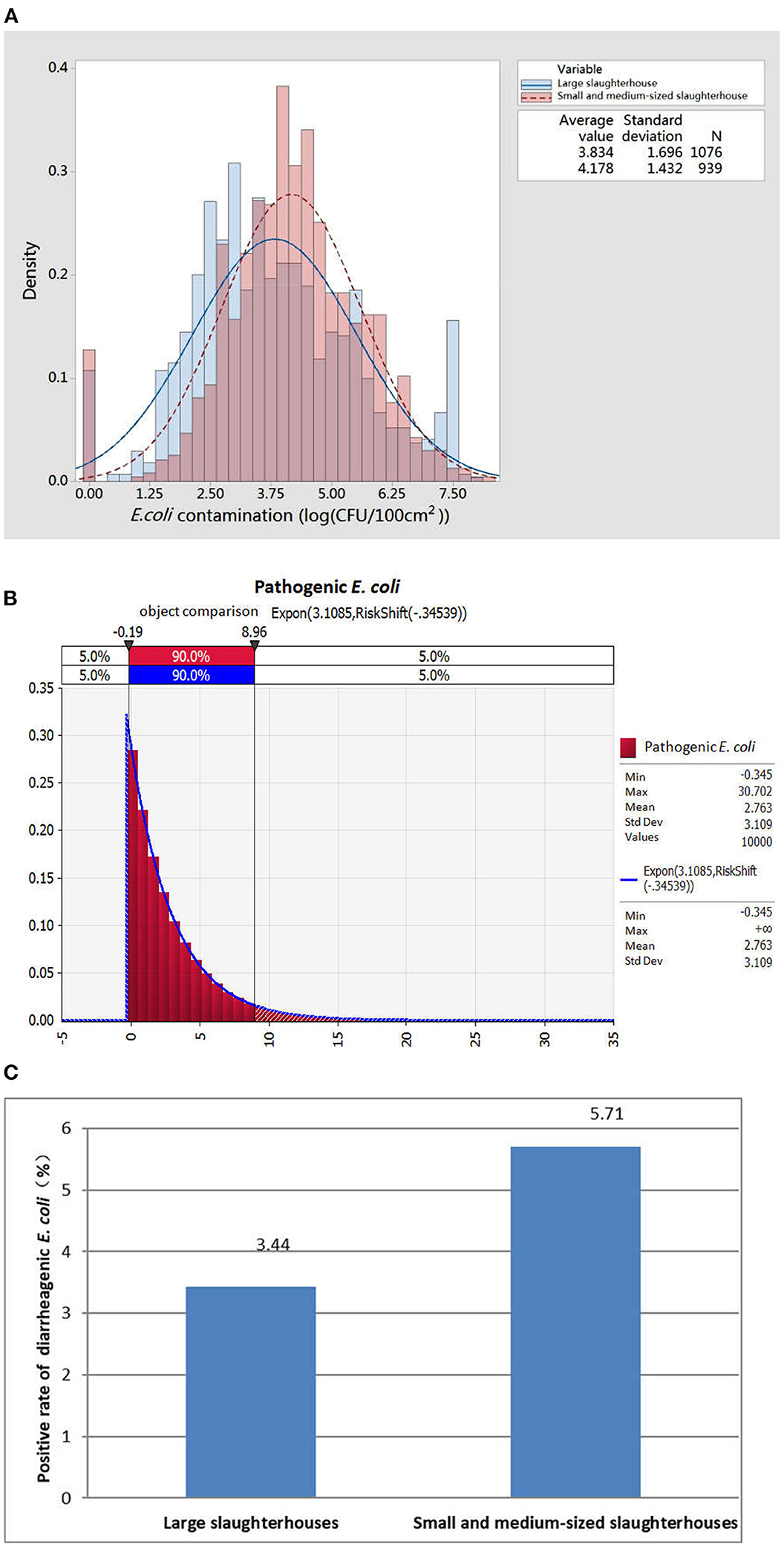
Figure 1. Monitoring of Escherichia coli and diarrheagenic E. coli contamination in pork produced in different types of slaughterhouses. (A) Distribution of E. coli contamination in pork from different types of slaughterhouses. (B) Exposure assessment results of diarrheagenic E. coli in pork during the slaughter process. (C) Exposure of diarrheagenic E. coli in pork produced in different types of slaughterhouses.
Next, the contamination rate of diarrheagenic E. coli in slaughtered pork was randomly distributed and fitted by @Risk RISK assessment software to determine the optimal function, and an exposure assessment was then carried out by Monte Carlo sampling (Figure 1B). The results showed that the contamination rate of diarrheagenic E. coli was mainly distributed in the range of 0–8.69% (mean value 2.76%). Meanwhile, the overall contamination rate of diarrheagenic E. coli in pork samples produced by large-sized slaughterhouses was 3.44% (32/931), and that of small- or medium-sized slaughterhouses was 5.71% (25/438) (Figure 1C). These results showed that the contamination of diarrheagenic E. coli in pork produced by small- or medium-sized slaughterhouses was greater than that of large slaughterhouses.
From a hygiene perspective, after pre-cooling cold fresh meat, the temperature and humidity changes of the carcass surface can deactivate the pathogenic microorganisms contaminated on the surface of the pork during the slaughter process, which better guarantees the quality and safety of the meat. Therefore, in this study, cotton swabbed samples from pork surfaces before and after pre-cooling were collected in some slaughterhouses with pre-cooling treatment for E. coli count analysis.
By fitting the random probability distribution of E. coli contamination on the pork surface before and after pre-cooling (Figure 2A), the probability of 90% of the E. coli contamination before pre-cooling was 0.95–6.19 log (CFU/100 cm2) or 0.09 CFU/cm2-15,488.17 CFU/cm2; while the average was 3.76 log (CFU/100 cm2) or 57.54 CFU/cm2. After pre-cooling, the 90% probability of E. coli contamination was −0.25–5.19 log (CFU/100 cm2) or 0.005 CFU/cm2-1,548.82 CFU/cm2, and the average was 2.40 log (CFU/100 cm2) or 2.45 CFU/cm2 (Figure 2B). The results in Figure 2C show that pre-cooling treatment can greatly reduce the amount of E. coli in pork after slaughter. Considering that E. coli is a hygiene indicator, pre-cooling treatment may greatly reduce the contamination of pathogenic microorganisms in pork, thereby avoiding the chance of cross-contamination or infection in the subsequent circulation and consumption links.
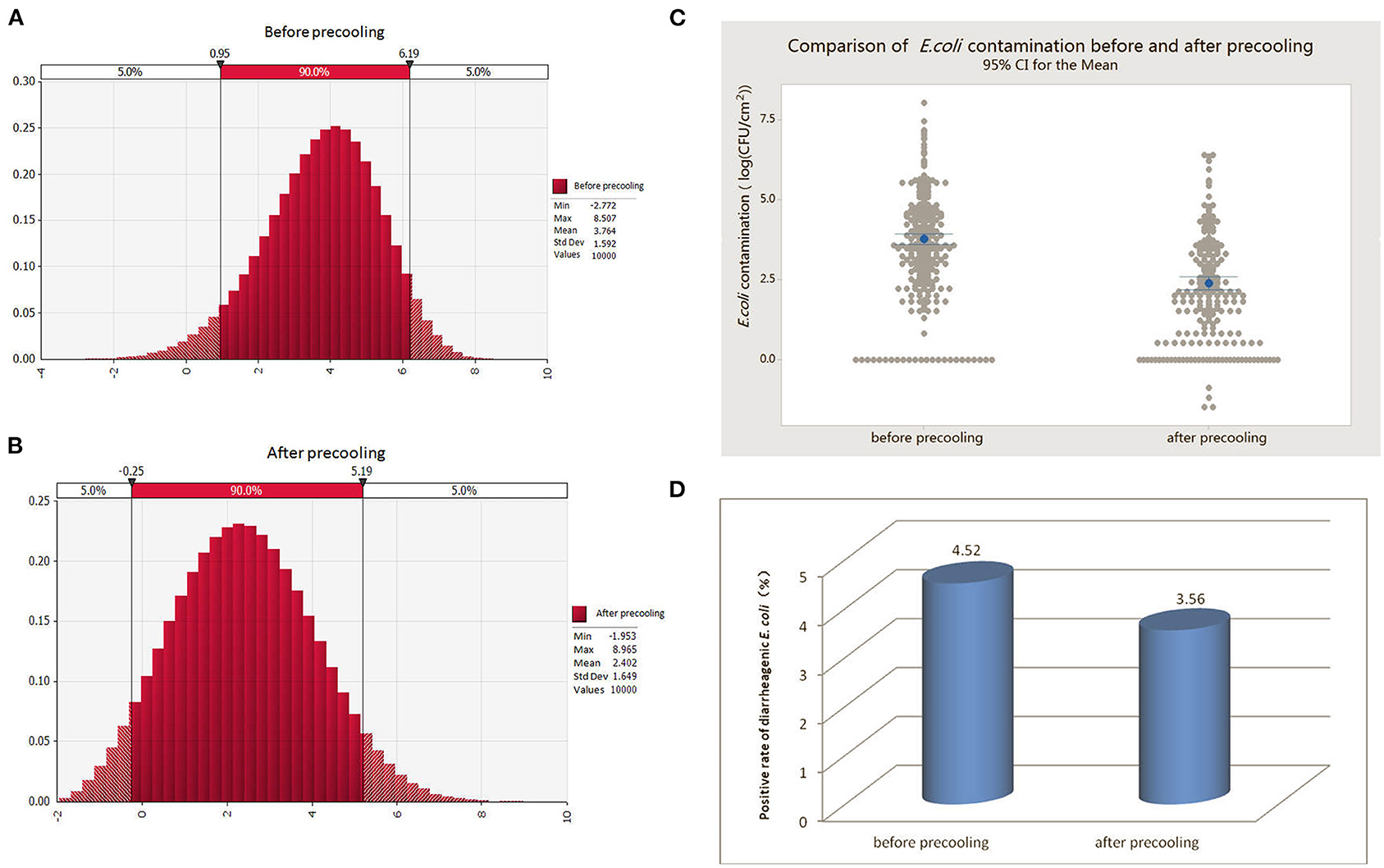
Figure 2. Comparison of the amount of E. coli and diarrheagenic E. coli contamination in pre-cooled and non-pre-cooled pork. (A) Probability distribution of E. coli contamination in pork before pre-cooling. (B) Probability distribution of E. coli contamination in pork after pre-cooling. (C) Comparison of E. coli contamination in pork before and after pre-cooling. (D) Exposure status of diarrheagenic E. coli contamination in pork before and after pre-cooling treatment.
In addition, we conducted exposure assessments of diarrheagenic E. coli in pork after slaughter before pre-cooling and after pre-cooling. The results showed that the overall contamination rate of diarrheagenic E. coli in pork samples was 4.52% (39/863) before pre-cooling and 3.56% (18/506) after pre-cooling (Figure 2D). In conclusion, pre-cooling significantly reduced the contamination of diarrheagenic E. coli in pork. Of note, we found that trend was similar to that of slaughterhouses, probably because large slaughterhouses generally performed pre-cooling treatment.
Based on the preliminary investigation, we found that nearly half of the pig slaughterhouses carried out microbiological inspections. We compared and analyzed the contamination status of E. coli under the conditions of microbial inspection, not microbial detection in pork samples, before pre-cooling. By fitting the random probability distribution of E. coli contamination on the surface of pork after slaughter in two types of slaughterhouses, with and without microbial detection (Figure 3A), the probability of 90% of E. coli contamination without microbial detection was 2.19–7.19 log (CFU/100 cm2) or 1.55 CFU/cm2-15, 4881.66 CFU/cm2; while the average was 4.92 log (CFU/100 cm2) or 831.76 CFU/cm2. In contrast, the 90% probability of the contamination of E. coli with microbiological detection was 0.61–6.23 log (CFU/100cm2) or 0.04 CFU/cm2-16982.43 CFU/cm2; while the average was 3.60 log (CFU/100 cm2) or 39.82 CFU/cm2 (Figure 3B). The results in Figure 3C showed that microbial inspection played a significant role in promoting hygiene control in slaughterhouses, which could significantly reduce the amount of E. coli in pork after slaughter, thereby reducing the risk of potential pathogenic microorganisms in the slaughter process.
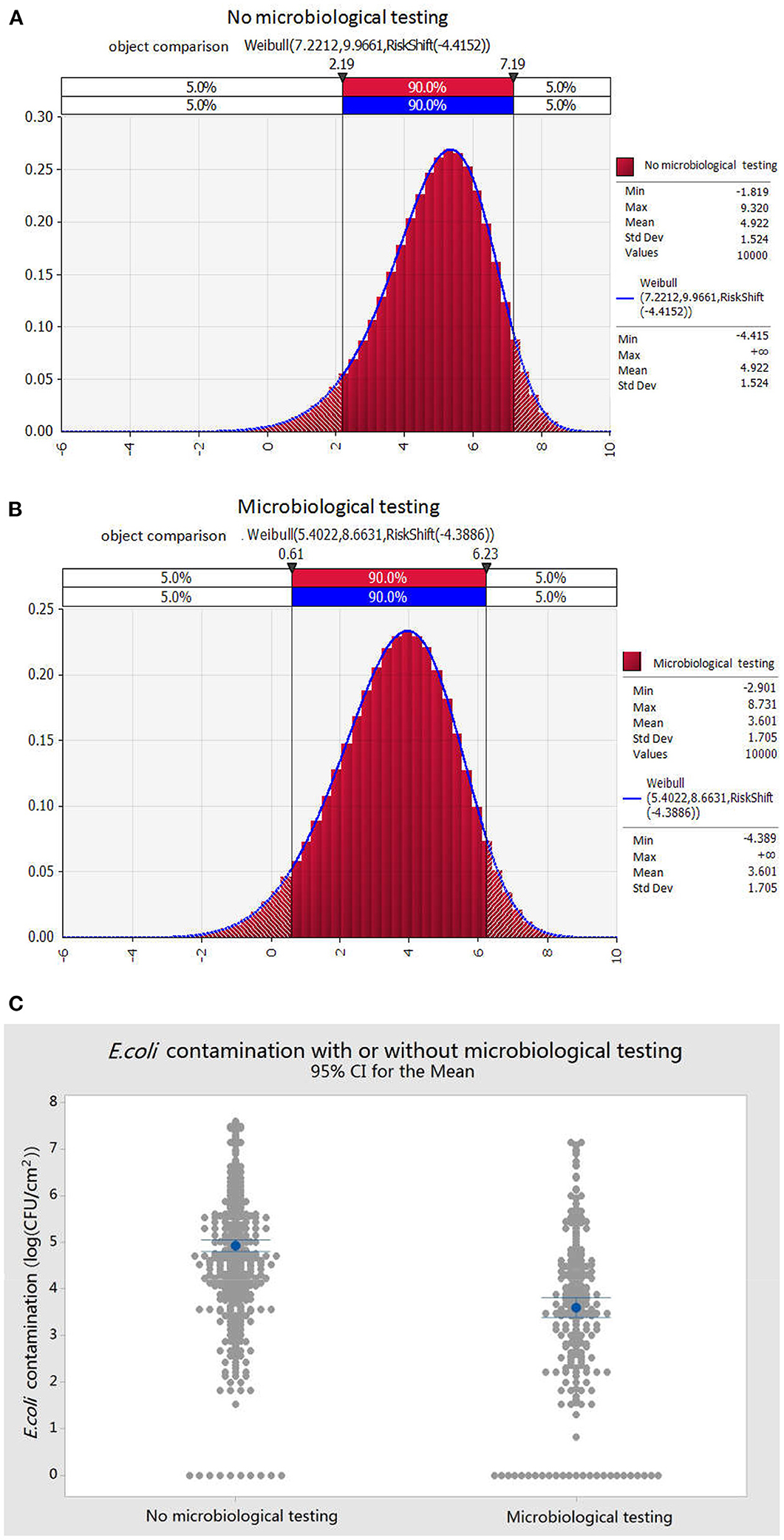
Figure 3. Effects of microbiological testing on E. coli contamination of pork after slaughter. (A) Contamination distribution of E. coli in pork during slaughter with microbiological detection. (B) Contamination distribution of E. coli in pork during slaughter without microbiological detection. (C) Comparison of E. coli contamination in pork during slaughter with or without microbiological detection.
In this study, we selected pig slaughterhouses in major pig producing provinces (respectively located in East China, South China, southwest China, central China, northwest China, and northeast China) for the monitoring of E. coli, which reflects the overall hygienic situation of pig slaughtering in China at the present stage. We found that the positive detection rate of E. coli in pork after slaughtering without precooling in China is very high, reaching 97.07% overall (1,954/2,013), which is similar to the 95.81% positive detection rate before evisceration in the United States (26). However, the United States pig slaughterhouses are equipped with cold chain facilities, after pre-cooling, the positive rate of E. coli decreased to 11.78% (26). In China, large-sized slaughterhouses with cold chain facilities account for ~80% of the total number of slaughtered pigs (Table 1). However, most small- or medium-sized slaughterhouses lack cold chain facilities (55.26%, 21/38, Table 1); therefore, the pork carries a large amount of E. coli, which enters the circulation. In the sales link, this undoubtedly increases the risk of food safety incidents. In addition, the total amount of E. coli contamination in pork after precooling in China was 2.45 CFU/cm2, which was even better than the monitoring data of 4.67 CFU/cm2 in the United States in 2011 (26). In addition, the exposure assessment results showed that overall, the contamination rate of diarrheagenic E. coli generally ranged between 0 and 8.69%, with an average of 2.76%, which is lower than the surveillance results for pathogenic diarrheagenic E. coli in the United States in 2015 (5%) and the European Union in 2017 (4.4%) (23, 24).
Research by H. Holly Wang et al. has shown that chilled meat and hot meat are the main pork types among Chinese consumers in the cities surveyed, accounting for 45 and 46%, respectively (27). The pre-cooling process can decrease the central temperature of the pork to ~4°C after slaughter. This process not only makes the pork taste better after the acid removal, but more importantly deactivates the microorganisms on the surface of the pork to ensure the safety of the meat. The average amount of E. coli contamination was 57.54 CFU/cm2 before precooling and 2.45 CFU/cm2 after precooling. Similarly, the contamination rate of diarrheagenic E. coli in pork before pre-cooling was 4.52%, which was reduced to 3.56% after pre-cooling. Thus, chilled pork will become a meat consumption trend in the future. GB/T 20551-2006 “Evaluating specification on the HACCP certification in the slaughter of livestock and poultry” (28) clearly states that the procedures for the inspection of Coliforms, Salmonella spp., and other harmful microorganisms should be established during the pig slaughter process and must meet the qualified requirements. We also found that the contamination of E. coli in pork produced in the slaughterhouses where microbiological inspection was carried out was greatly reduced. It is indicated that routine microbiological inspections in slaughterhouses had a positive effect on hygiene control and better guarantee product safety. However, in our investigations, we found that more than half of the pig slaughter companies did not carry out microbiological related testing and control, and even the companies that did this did not use a unified reference for monitoring technical specifications. Therefore, we recommend a standard operation of hygiene control in the slaughter process, effective implementation of microbial reduction measures (especially the development of microbiological inspections, etc.), establishment of a hygiene evaluation method for pig slaughter, and formulation of an evaluation index system, in order to improve slaughter hygiene, and the quality and safety of pork products.
Developed countries such as Europe and the United States place great importance on the monitoring, assessment, and prevention and control of pathogenic microbial contamination in livestock and poultry products during the slaughter process, and have successively established a strict scientific regulatory and standards system (29, 30). However, in China, gaps remain in the standards for microbial control during the slaughter process. Because the monitoring results of E. coli in large-sized slaughterhouses are comparable to those in developed countries, and the microorganisms in the slaughter process can also be effectively controlled by measures such as pre-cooling, the control standards of E. coli in the pig slaughtering process must be urgently addressed. In fact, China is also taking a series of measures to improve the hygienic control of pig slaughtering process in recent years. For example, firstly, we promotes the standardization of pig slaughterhouses, which requires microbiological monitoring; secondly, we promotes the quality and safety risk monitoring of the slaughter process, which also stipulates the monitoring of common pathogens such as E. coli and Salmonella; thirdly, the overall bio-safety prevention and control measures of the slaughterhouse under the influence of African Swine fever (ASF) have been strengthened. It is believed that the development of standards for the detection of E. coli at the slaughtering stage can directly ensure the health and safety of the pork entering the market, and is also conducive to international standards.
In conclusion, the control of pork E. coli in large-sized slaughterhouses is better than that in small or medium-sized slaughterhouses in China, and E. coli contamination in the pig slaughtering chain in large-sized slaughterhouses after pre-cooling is comparable to that in developed countries. Therefore, corresponding standards are urgently needed to better control microbial pollution in pork during the slaughtering process.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
This work was funded by the National Key Research and Development Program of China (2018YFD0500505).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Christine ER, Aldsworth DT, Stein RA, Cliver DO, Riemann HP. Foodborne Diseases, 3rd Edn. London: Academic Press (2017).
2. Jones TF, Yackley J. Foodborne disease outbreaks in the United States: a historical overview. Foodborne Pathog Dis. (2018) 15:11–5. doi: 10.1089/fpd.2017.2388
3. Dalton CB, Gregory J, Kirk MD, Stafford RJ, Givney R, Kraa E, et al. Foodborne disease outbreaks in Australia, 1995 to 2000. Commun Dis Intell Q Rep. (2004) 24:211–24.
4. Chen Y, Guo YC, Wang ZT, Liu XM, Liu H, Dai Y, et al. Foodborne disease outbreaks in 2006 report of the National Foodborne Disease Surveillance Network, China. Wei Sheng Yan Jiu. (2010) 39:331–4.
5. Sivapalasingam S, Friedman CR, Cohen L, Tauxe RV. Fresh produce: a growing cause of outbreaks of foodborne illness in the United States, 1973 through 1997. J Food Prot. (2004) 67:2342–53. doi: 10.4315/0362-028X-67.10.2342
6. Miller C, Heringa S, Kim J, Jiang X. Analyzing indicator microorganisms, antibiotic resistant Escherichia coli, and regrowth potential of foodborne pathogens in various organic fertilizers. Foodborne Pathog Dis. (2013) 10:520–7. doi: 10.1089/fpd.2012.1403
7. Anna D. K, Małgorzata K. Meat and meat products-analysis of the most common threats in the years 2011-2015 in Rapid Alert System for Food and Feed (FASFF). Rocz Panstw Zakl Hig. (2017) 68:289–96.
8. Dewey-Mattia D, Manikonda K, Hall AJ, Wise ME, Crowe SJ. Surveillance for foodborne disease outbreaks–United States, 2009–2015. MMWR Surveill Summ. (2018) 67:1–11. doi: 10.15585/mmwr.ss6710a1
9. Nastasijevic I, Schmidt JW, Boskovic M, Glisic M, Kalchayanand N, Shackelford SD, et al. Seasonal prevalence of Shiga toxin-producing Escherichia coli on pork carcasses for three steps of the harvest process at two commercial processing plants in the United States. Appl Environ Microbiol. (2020) 87:e01711–20. doi: 10.1128/AEM.01711-20
10. Erickson MC, Doyle MP. Food as a vehicle for transmission of Shiga toxin-producing Escherichia coli. J Food Prot. (2007) 70:2426–49. doi: 10.4315/0362-028X-70.10.2426
11. Bai X, Wang H, Xin Y, Wei R, Tang X, Zhao A, et al. Prevalence and characteristics of Shiga toxin-producing Escherichia coli isolated from retail raw meats in China. Int J Food Microbiol. (2015) 200:31–8. doi: 10.1016/j.ijfoodmicro.2015.01.018
12. EFSA. Scientific opinion on a quantitative microbiological risk assessment of Salmonella in 382 slaughter and breeder pigs. EFSA J. (2010) 8:1547. doi: 10.2903/j.efsa.2010.1547
13. Miller GY, Liu X, McNamara PE, Barber DA. Influence of Salmonella in pigs Pre-harvest 407 and during pork processing on human health costs and risks from pork. J Food Prot. (2005) 68:1788–98. doi: 10.4315/0362-028X-68.9.1788
14. Biasino W, De Zutter L, Mattheus W, Bertrand S, Uyttendaele M, Van Damme I. Correlation between slaughter practices and the distribution of Salmonella and hygiene indicator bacteria on pig carcasses during slaughter. Food Microbiol. (2018) 70:192–9. doi: 10.1016/j.fm.2017.10.003
15. Huang JL, Zang XQ, Lei TY, Ren FZ, Jiao XA. Prevalence of Campylobacter spp. in pig slaughtering line in Eastern China: analysis of contamination sources. Foodborne Pathog Dis. (2020) 17:712–9. doi: 10.1089/fpd.2020.2800
16. Li H, Wang PF, Lan RT, Luo LJ, Cao XL, Wang Y, et al. Risk factors and level of Listeria monocytogenes contamination of raw pork in retail markets in China. Front Microbiol. (2018) 9:1–10. doi: 10.3389/fmicb.2018.01090
17. Zhu Z, Huang X, Chen X, Lu Y, Chen Z, Wang C, et al. Isolation and characterization of Salmonella in pork samples collected from retail and wholesale markets in each season from 2016 to 2018 in Wuhan, China. J Appl Microbiol. (2020) 128:875–83. doi: 10.1111/jam.14515
18. Jang J, Hur HG, Sadowsky MJ, Byappanahalli MN, Yan T, Ishii S. Environmental Escherichia coli: ecology and public health implications-a review. J Appl Microbiol. (2017) 123:570–81. doi: 10.1111/jam.13468
19. Croxen MA, Finlay BB. Molecular mechanisms of Escherichia coli pathogenicity. Nat Rev Microbiol. (2010) 8:26–38. doi: 10.1038/nrmicro2265
20. Ghafir Y, China B, Dierick K, De Zutter L, Daube G. Hygiene indicator microorganisms for selected pathogens on beef, pork, and poultry meats in Belgium. J Food Prot. (2008) 71:35–45. doi: 10.4315/0362-028X-71.1.35
21. Yang SC, Lin CH, Aljuffali IA, Fang JY. Current pathogenic Escherichia coli foodborne outbreaks cases and therapy development. Arch Microbiol. (2017) 199:811–25. doi: 10.1007/s00203-017-1393-y
22. Aijuka M, Buys EM. Persistence of foodborne diarrheagenic Escherichia coli in the agricultural and food production environment: implications for food safety and public health. Food Microbiol. (2019) 82:363–70. doi: 10.1016/j.fm.2019.03.018
23. United States Department of Agriculture. Food Safety and Inspection Service. (2020). Available online at: https://www.fsis.usda.gov/sites/default/files/media_file/2020-09/RPPESP-Phase-I-Transitional-Results.pdf
24. European Food Safety Authority and European Centre for Disease Prevention and Control (EFSA and ECDC). The European Union summary report on trends and sources of Zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. (2018) 16:e05500. doi: 10.2903/j.efsa.2018.5500
25. Toma C, Lu Y, Higa N, Nakasone N, Chinen I, Baschkier A, et al. Multiplex PCR assay for identification of human diarrheagenic Escherichia coli. J Clin Microbiol. (2003) 41:2669–71. doi: 10.1128/JCM.41.6.2669-2671.2003
26. United States Department of Agriculture, Food Safety and Inspection Service Office of Public Health Science, Microbiology Division. The Nationwide Microbiological Baseline Data Collection Program: Market Hogs Survey August 2010–August 2011. Available online at: https://www.fsis.usda.gov/sites/default/files/media_file/2020-07/Baseline_Data_Market_Hogs_2010-2011.pdf.
27. Wang HH, Chen J, Bai J, Lai J. Meat packaging, preservation and marketing implications: consumer perferences in an emerging economy. Meat Sci. (2018) 145:300–7. doi: 10.1016/j.meatsci.2018.06.022
28. Standardization Administration, General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China. GB/T 20551-2006 Evaluating Specification on the HACCP Certification in the Slaughter of Livestock and Poultry (2006).
29. Federal Register. Pathogen Reduction; Hazard Analysis and Critical Control Point (HACCP) Systems; Final Rule. Available online at: https://www.fsis.usda.gov/sites/default/files/media_file/2020-08/93-016F_0.pdf
30. Legislation.gov.uk. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. Available online at: https://www.legislation.gov.uk/eur/2005/2073/contents
Keywords: Escherichia coli, diarrheagenic E. coli, pig, slaughtering progress, pre-cooling treatment, microbiological testing
Citation: Wang L, Liu N, Gao YB, Liu JH, Huang XM, Zhang QQ, Li YH, Zhao JM, Wang JW and Zhao G (2021) Surveillance and Reduction Control of Escherichia coli and Diarrheagenic E. coli During the Pig Slaughtering Process in China. Front. Vet. Sci. 8:735076. doi: 10.3389/fvets.2021.735076
Received: 02 July 2021; Accepted: 01 September 2021;
Published: 05 October 2021.
Edited by:
Miguel Angel Prieto Lage, University of Vigo, SpainReviewed by:
Paula García Oliveira, Polytechnic Institute of Bragança (IPB), PortugalCopyright © 2021 Wang, Liu, Gao, Liu, Huang, Zhang, Li, Zhao, Wang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junwei Wang, eWZmczIwMDBAc2luYS5jb20=; Ge Zhao, Y2F0aHlnZUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.