- 1Laboratory of Theriogenology and Reproductive Health, College of Animal Science and Veterinary Medicine, Heilongjiang Bayi Agricultural University, Daqing, China
- 2Heilongjiang Provincial Key Laboratory of Prevention and Control of Bovine Diseases, College of Animal Science and Veterinary Medicine, Heilongjiang Bayi Agricultural University, Daqing, China
- 3Laboratory of Stem Cell Therapy and Regenration Biology, College of Life Science and Biotechnology, Heilongjiang Bayi Agricultural University, Daqing, China
- 4Cofeed Feedmill (Changchun) Co., Ltd., Changchun, China
Endometritis is a disease that affects reproductive health in dairy cows and causes serious economic damage to the dairy industry world-wide. Although in recent years, the application of mesenchymal stem cell (MSC) therapy for the treatment of inflammatory diseases has attracted much attention, there are few reports of the use of MSCs in dairy cows. In the present study, our objective was to explore the inhibitory effects of bovine adipose-derived mesenchymal stem cells (bAD-MSCs) on lipopolysaccharide (LPS) induced inflammation in bovine endometrial epithelial cells (bEECs) along with the potential underlying molecular mechanisms. We characterized isolated bAD-MSCs using cell surface marker staining and adipogenic/osteogenic differentiation, and analyzed them using immunofluorescence, flow cytometry (surface marker staining), and adipogenic and osteogenic differentiation. Furthermore, to understand the anti-inflammatory effects of bAD-MSCs on LPS induced bEEC inflammation, we used a bAD-MSC/bEEC co-culture system. The results showed that bAD-MSC treatments could significantly decrease LPS induced bEEC apoptosis and pro-inflammatory cytokine expression levels, such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α). Furthermore, our results showed that bAD-MSC treatments could also significantly downregulate LPS induced p38, IkB-a, and JAK1 phosphorylation and Bax protein expression levels, which are closely related to inflammatory progress and cellular apoptosis in bEECs. Our findings demonstrate that bAD-MSCs play an inhibitory role in LPS induced bEEC inflammation and provide new insights for the clinical therapy of endometritis in dairy cows.
Introduction
In recent years, the incidence of uterine disease in dairy cows has shown an increasing trend, and has become one of the most important factors affecting their reproductive efficiency (1). The endometrium plays an essential role in embryo implantation, embryo development, and sperm motility (2). Endometritis is a type of refractory uterine disease, which is mainly caused by pathogenic microorganisms (such as Escherichia coli and Streptococcus) infecting the endometrium during or after parturition (3). Endometritis is one of the most common reasons for infertility in dairy cows and causes huge annual losses to the dairy industry. A survey of the literature shows that in the United States uterine inflammation can cause losses of around US$171.9 ± 47.88 in first lactation primiparous heifers, while this figure is around US$262.65 ± 56.15 per lactation in multiparous cows (4). Currently, the treatment for bovine endometritis includes injection with antibiotics and prostaglandin F2 alpha (PGF2α), and flushing of the uterus with iodine solution, but the cure rate is not satisfactory. Therefore, there is an urgent need to find new and effective treatments for endometritis in dairy cows.
Mesenchymal stem cells (MSCs) are a type of early undifferentiated adult stem cells that have the potential for self-renewal and multilineage differentiation. Compared with other stem cells (such as embryonic stem cell, germinal stem cell and neural stem cell), MSCs have a wide range of sources and can be obtained from bone marrow, adipose tissue, umbilical cords, and the placenta (5–7). MSCs can differentiate into adipocytes, chondrocytes, cardiomyocytes, nerve cells, hepatocytes, osteoblasts, and other cell types (8–13); MSCs also show characteristics of immune regulation, anti-inflammation, and inhibit malignant cell proliferation (14). With in-depth research, scholars are starting to realize that the paracrine effects of MSCs play an important role in disease treatment. MSCs secrete soluble bioactive factors to that inhibit apoptosis, promote angiogenesis, stimulate the differentiation of progenitor cells, and regulate immune response to promote the regeneration of injured tissues (15). Based on these characteristics, MSCs have been widely used in the treatment of bone, heart, kidney, lung, brain, and liver injuries (16–20). It has been reported that MSCs can regulate natural killer cells (NK cells), dendritic cells, T lymphocytes, and B lymphocytes, either through cell contact or cytokine secretion to modulate immune responses (21–23). In addition, paracrine factors [such as transforming growth factor-β (TGF-β), tumor necrosis factor-inducible gene 6 protein (TSG-6), indoleamine 2,3-dioxygenase (IDO), and prostaglandin-E2 (PGE2)] released by MSCs also have strong anti-inflammatory effects (24). Consequently, MSCs and/or MSC-based biological preparations are becoming novel therapeutic tools for solving issues of antibiotic residues and intractable diseases in livestock (25). Bone marrow is the most common source of MSCs, but sample collection is not easy; whereas MSCs from adipose tissue, namely adipose-derived MSCs (AD-MSCs) have become a research hotspot due to their abundance and convenience of sample collection.
Based on the above information, this study focused on the inhibitory effects of bAD-MSCs on LPS induced inflammation in bovine endometrial epithelial cells (bEECs) along with the underlying molecular mechanisms in vitro. It provides theoretical support for the application of bovine adipose-derived mesenchymal stem cells (bAD-MSCs) in the prevention and treatment of endometritis in dairy cows.
Materials and Methods
Isolation and Culture of bAD-MSCs
Bovine adipose tissue cells were isolated using 0.25% enzymatic digestion. bADs were collected under sterile conditions from the neck of a Holstein cow (age, 3–4 years; female; weight, 640 ± 30 kg) at a slaughterhouse (Daqing Longda Food Co., Ltd., China). Samples were washed three times with phosphate buffer saline (PBS) containing 1% (vol/vol) penicillin and streptomycin (penicillin 100 U/mL, streptomycin 100 μg/mL; Beijing Solarbio Science & Technology Co., Ltd., Beijing, China). The vascular, fascia, and connective tissues were discarded, and the adipose tissue was quickly homogenized and subsequently digested using 0.25% trypsinase (Beijing Solarbio Science & Technology Co., Ltd.) for 6 h at 37°C, and cells were filtered using a 70 μm cell strainer (Wuxi NEST Biotechnology Co., Ltd., China). Cells were cultured in Dulbecco's modified Eagle medium, nutrient mixture F-12 (DMEM/F-12, Thermo Fisher Scientific, Grand Island, NY, USA) containing 10% (vol/vol) fetal bovine serum (Zhejiang Tianhang Biotechnology Co., Ltd., China), and 1% (vol/vol) penicillin and streptomycin (penicillin 100 U/mL, streptomycin 100 μg/mL; Beijing Solarbio Science & Technology Co., Ltd.) at 37 °C in a humidified atmosphere of 5% CO2. Each medium was changed every 24 h. The cells were passaged when the confluence was 70–80%.
Detection of the Surface Antigens and Multilineage Differentiation Potential of bAD-MSCs
It has been previously demonstrated that the most common lineage for MSCs is to differentiate down the osteogenic and adipogenic lines (26). The fourth passage of bAD-MSCs was obtained, and the cell density was adjusted to 1 × 105 cells/3.5 cm culture dishes. The cells were cultured in DMEM/F-12 (Thermo Fisher Scientific, Grand Island, NY, USA) containing 10% (vol/vol) FBS, and 1% (vol/vol) penicillin and streptomycin (penicillin 100 U/mL, streptomycin 100 μg/mL; Beijing Solarbio Science & Technology Co., Ltd.) at 37°C in a humidified atmosphere of 5% CO2 in 3.5 cm culture dishes. For osteogenic differentiation and adipogenic differentiation, the cells were divided into two groups: a differentiation culture group and a control group. When the cells achieved a confluency of 80%, the differentiation culture groups were treated with osteogenic induction medium (Biowit BW12007) and adipogenic induction medium (Biowit BW12006), respectively. Each medium was changed every 48 h. After 21 d of culture, the media were discarded, the cells were washed with PBS (calcium and magnesium free) three times and fixed with 4% formaldehyde solution for 20 min. The fixation fluid was then discarded followed by three washes with PBS. In the osteogenic and adipogenic induction groups, Alizarin Red S (Beijing Solarbio Science & Technology Co., Ltd.) and Oil Red O (Beijing Solarbio Science & Technology Co., Ltd.) were added for staining for 5 and 15 min, respectively, the staining solution was discarded, and the cells were washed three times with PBS. Finally, after mounting with neutral resin, the samples were observed under a microscope.
The bAD-MSCs from passage 4 were used for all assays, phenotypic analysis took place by immunofluorescence, flow cytometry, and differentiation culture. For immunofluorescence assays, cells were placed in 24-well plates at an initial density of 4 × 104 with DMEM/F-12 supplemented with 10% fetal bovine serum (FBS; Zhejiang Tianhang Biotechnology Co., Ltd.) culture media, in a 37°C incubator with 5% CO2. After 24 h, each culture medium was removed and cells were washed three times with PBS; the cells were fixed with 4% formaldehyde in the dark for 10 min at room temperature (RT), and washed with PBS a further three times, each time for 5 min. Cells were then permeabilized with a PBS dilution of 0.1% Triton X-100 (T9284; Sigma-Aldrich, St. Louis, MO, USA) for 10 min, followed by a further three washes with PBS. The cells were then blocked with bovine serum albumin (BSA, Sigma-Aldrich) for 30 min. Subsequently, cells were incubated with primary antibodies CD73 (Proteintech; 1:200), CD44 (Biolegend; 1:200), CD45 (Biolegend; 1:200), and CD34 (Biolegend; 1:200) at 4°C overnight, followed by three washes with PBS. Different from the treatment methods of CD44, CD45 and CD34, the detection of the CD73 surface marker needed a secondary antibody [goat anti-mouse IgG conjugated with Cy3 (Beyotime Institute of Biotechnology, Shanghai, China)] incubation for 2 h. Cells were then stained with 4', 6-diamidino-2-phenylindole (DAPI, 1 μg/mL; Sigma-Aldrich) for 10 min. Subsequently, cells were visualized with a laser scanning confocal microscope (TCS SP5, Leica Microsystems, Wetzlar, Germany), and images were taken under an Olympus (Tokyo, Japan) FLUOVIEW FV1000 microscope.
Single-cell suspensions were prepared using enzymatic dissociation with trypsin-EDTA solution, fixed with 70% ethanol, and permeabilized with 0.05% Triton X-100 (Sigma- Aldrich) for 1.5 min. Subsequently, cells were centrifuged at 4°C for 3 min and blocked with BSA for 30 min. Cells were then incubated with the primary antibodies CD73 (Proteintech; 1:200), CD44 (Biolegend; 1:200), CD45 (Biolegend; 1:200), and CD34 (Biolegend; 1:200) at 4°C overnight, followed by three washes with PBS. CD73 was incubated with Cy3 conjugated goat anti-rabbit IgG (Beyotime Institute of Biotechnology, Shanghai, China; 1:200) at RT for 1 h. The samples were centrifuged at 4°C for 3 min and suspended in PBS. Lastly, cells were detected using flow cytometry (FACSCalibur, BD Biosciences, Franklin Lakes, NJ, USA) and results were analyzed using WinMDI (v.2.9, BD Biosciences) software.
Establishment of a Bovine Endometritis Model Induced by LPS in vitro
A bovine endometrial epithelial cell (bEEC) line was established in our laboratory. Briefly, the caruncle tissue was cut into small sections (3–5 mm2), which were washed three times with phosphate-buffered saline (PBS; pH 7.3). Roswell Park Memorial Institute-1640 medium (RPMI-1640; HyClone, Beijing, China) containing 10% fetal calf serum (FCS; Sijiqing Biological Engineering Materials Co., Ltd., Hangzhou, Zhejiang, China) and 1% penicillin/streptomycin (10,000 IU/10,000 μg/ml; Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) were added to the tissue sections attached to the cell culture dish (60 × 15 mm, NEST, China) and then incubated in an atmosphere of 5% CO2/95% air at 37°C. After 6 h, 1.5 ml of RPMI-640 was added to the bottom of the cell culture dish. Epithelioid cells were separated into two populations based on enzyme sensitivity. In order to identify the purity of endo epithelial cells, immunofluorescence was performed with cultures passaged three times using a primary antibody against cytokeratin 18 (CK 18; Abcam, Cambridge, UK) as described previously (27). The bEECs were removed from the liquid nitrogen tank for resuscitation culture and passage. They were then plated in 24-well plates at a density of 4 × 104 cells/well in RPMI-1640 standard complete medium (Hyclone, Logan, UT, USA) containing 10% (vol/vol) FBS, and 1% (vol/vol) penicillin and streptomycin (penicillin 100 U/mL, streptomycin 100 μg/mL) at 37°C in a humidified atmosphere of 5% CO2. After bEEC adhesion (about 4 h), LPS (Sigma-Aldrich) at concentrations of free, 0.1, 1, and 10 μg/mL were added into different wells for 6 h to screen the best stimulation concentration. Then, after determining the optimal concentration, the optimal concentration (0.1ug/mL) is used to stimulate different times (0, 3, 6, 12, and 24 h) to screen the optimal stimulation time. Finally, the expression of inflammatory related genes (e.g., IL-1 β, IL-6, and TNF- α) were detected by real-time quantitative PCR. The same genes expressed at each concentration and each time point were analyzed to determine the optimal treatments.
Co-culture Assays
The bAD-MSCs and bEECs were co-cultured in transwell plates with a pore size of 0.4 μm (3413, Corning, NY, USA) at ratios of 0.5:1 and 1:1, in the upper and lower compartments, respectively. Briefly, bAD-MSCs were resuscitated from frozen storage. The density of bAD-MSCs was adjusted to 3 × 104 cells/well and 6 × 104 cells/well. The cells were seeded into transwell chambers and placed in an incubator at 37°C and 5% CO2 for use. The bEECs (6 × 104 cells/well) were then plated in 24-well plates and cultured for 4 h, and LPS at a concentration of 0.1 μg/mL (deemed the ideal concentration following experiment 2.3 above) was added after most of the cells had adhered to the plate wall. After stimulation with LPS for 6 h, all the original culture medium was discarded and 500 μL of DMEM/F-12 medium was supplemented. The transwell chambers were transferred into 24-well plates for co-culture for 48 h. After co-culture, each culture medium was discarded and the cells were washed with PBS, and then stained using an Annexin V-PE kit (Beijing Solarbio Science & Technology Co., Ltd.) to detect apoptosis. The stained cells were observed under a fluorescence microscope after incubation in accordance with the manufacturer's instructions. At the same time, the bEECs were collected and total RNA was extracted to detect the expression of IL-1 β, IL-6, and TNF-α. The experimental group was co-cultured after LPS stimulation. The LPS stimulated, but not the co-cultured, bEECs were set aside as the positive control group and bEECs (no LPS stimulation) were set as the negative control group.
Detection of RNA Expression of Cytokines
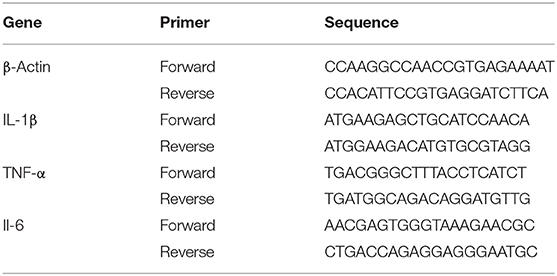
Genes were detected using Quantitative Reverse-Transcription PCR (qRT-PCR) as described by Sun et al. (28). Trizol was used to extract total RNA from bEECs. Then, an ultraviolet visible spectrophotometer (UV-Vis; Thermo Fisher Scientific) was used to measure RNA purity. The optical density (OD)260/(OD)280 ratio of the total RNA was determined to be 1.9, and the intensity of the 28S ribosomal RNA band was about twice the intensity of the 18S ribosomal RNA band in total RNA samples; this indicated that the total RNA was of high quality. The cDNA was synthesized from total RNA using a PrimeScript RT Reagent Kit (Takara Biotechnology Co., Ltd., Dalian, China). The gene expression specific sequences of cows are shown in Table 1 (the primer sequences were generated as described previously) (29, 30). Subsequently, cDNA was synthesized using a reverse transcription kit (Takara Biotechnology Co., Ltd.). Total cDNA was used as the starting material for real-time PCR with FastStart Universal SYBR Green Master Mix (Roche Applied Science, Germany). Finally, the 2−ΔΔCt method was used to analyze expression levels. All reactions were run in triplicate.
Protein Extraction and Western Blotting Analysis
To verify bAD-MSCs mediated processes, we extracted the total protein from endometrial cells and performed SDS-PAGE; the immunoreactive proteins visualized were IκB-α, P-JAK1, P-p38, p-38, and Bax. bAD-MSCs were seeded into transwell chambers at a density of 6 × 104 cells per chambers and placed in an incubator at 37°C and 5% CO2 for use. bEECs were seeded onto 24-well plates at a density of 6 × 104 cells per well for 4 h to adhere and then treated with LPS for 6 h. Then, bAD-MSCs and bEECs were co-cultured in transwell plates in the upper and lower compartments, respectively. Cells were cultured in DMEM/F-12 medium supplemented with 10% FBS in an incubator at 37°C and 5% CO2 for 12, 24, and 48 h, and then cells were harvested and added to the lysate. Lysates were then centrifuged at 13,201 × g for 30 min at 4°C and collected for further analysis. The protein concentration was determined using the Bradford protein assay. The absorbance of the mixture at 595 nm was determined using a UV-Visible spectrophotometer (Thermo Fisher Scientific). Total proteins were extracted from endometrial cells and SDS-PAGE was performed. Briefly, equal amounts of protein (25 μg) were separated on an 8–15% Tris-glycine gel and electro transferred onto polyvinylidene difluoride (PVDF) membranes. The PVDF membranes were blocked with 5% non-fat dried milk, and membranes were incubated overnight at 4°C with primary antibodies against β-actin (1:1,000, Sigma-Aldrich), IκB-α (1:1,000, Bioss biology, Beijing, China), P-JAK1 (1:2,000, Santa Cruz, CA, USA), P-p38 (1:1,000, Sigma-Aldrich), p-38 (1:1,000, Bioss Biology), and Bax (1:1,000, Bioss Biology). These were followed by horse-radish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse IgG (Thermo Fisher Scientific) at RT for 1 h. Target protein bands were revealed with an enhanced chemiluminescence (ECL) detection kit (Thermo Fisher Scientific), and the images were analyzed using Image-Pro Plus 6.0 software.
Statistical Analysis
All experiments and determinations were repeated three times. Data were analyzed using IBM SPSS Statistics v.20.0 and were tested for normality. The first, data of mRNA expression of IL-1β, IL-6, TNF-α and the protein expression of IκB-α, pJAK-1, Bax, pP38 were analyzed through Excel. And then differences of IL-1β, IL-6, TNF-α between control group and LPS group and differences of IκB-α, pJAK-1, Bax, pP38 between LPS group and co-culture group were compared using one-way ANOVA followed by Bonferroni correction. All values for variables are expressed as the mean and standard error of the mean (mean ± SEM). P < 0.05 were considered to be significant; when <0.01, they were considered to be highly significant.
Results
Isolation and Characterization of bAD-MSCs
We isolated and cultured bAD-MSCs as described in the Methods section. In vitro observation showed that cells were spindle-shaped and exhibited fibroblast-like adherent growth (Figures 1A,B). Furthermore, we cultured bAD-MSCs in differentiation medium and found that the isolated bAD-MSCs were able to differentiate into osteoblasts (Figures 1C,D) and adipocytes (Figures 1E,F), as demonstrated by Alizarin Red and Oil Red O staining; thus indicating the successful extraction of bAD-MSCs. The results of immunofluorescence microscopy and flow cytometry assays showed that isolated cells were strongly stained with CD73 and CD44 antibodies, the positive surface markers for bAD-MSCs, but showed a low binding affinity to CD45 and CD34 (negative surface markers for bAD-MSCs; Figures 2A,B).
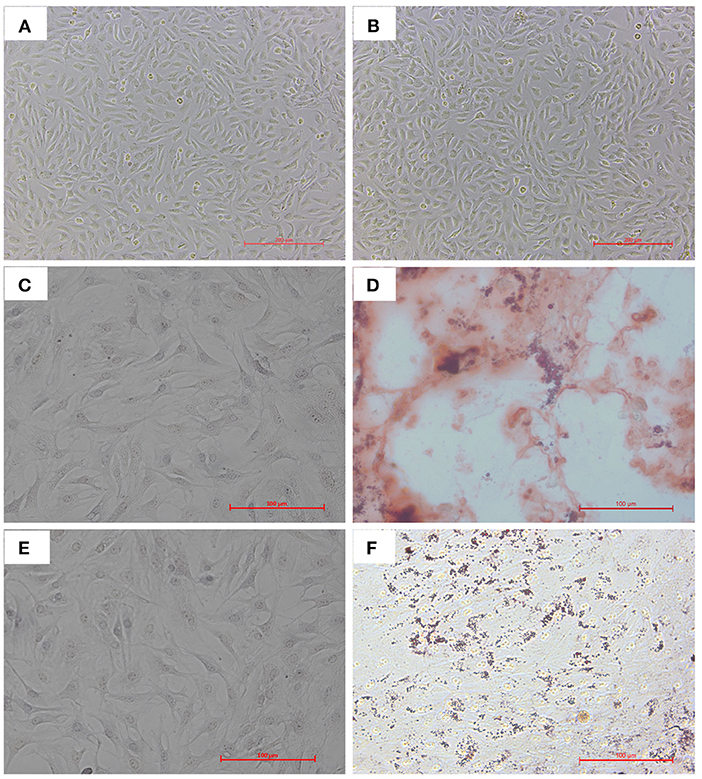
Figure 1. Cultivation and differentiation of bAD-MSCs. (A,B) Cell morphology of different parts at P0 and P4. (C,D) Osteogenic differentiation after 21 d showing calcium deposits stained with Alizarin Red S (C is control). (E,F) Adipogenic differentiation after 21 d showing lipid droplets stained with Oil Red O (E is control) (Scale bar = 200 μm).
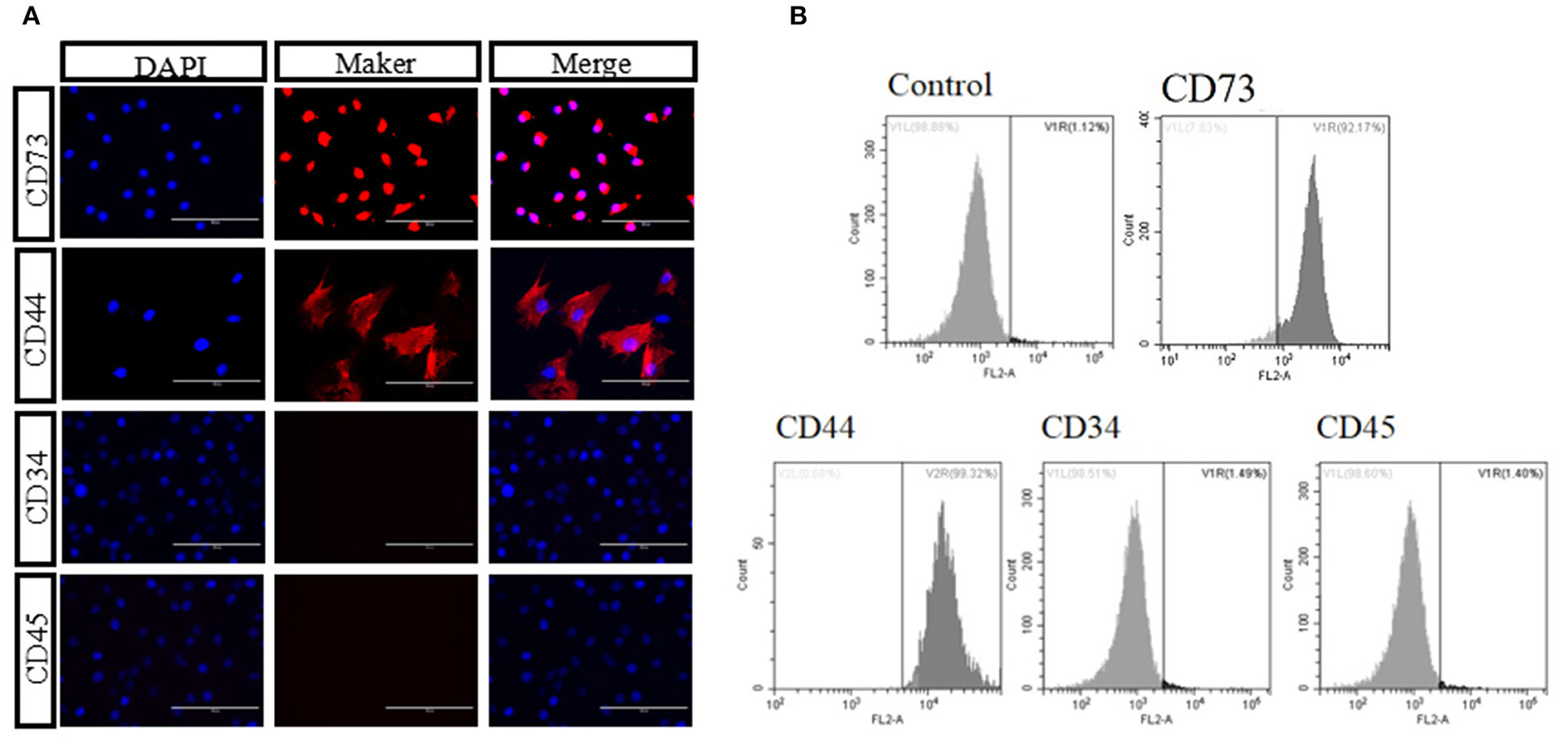
Figure 2. Identification of bAD-MSCs. (A,B) Identification marker CD44, CD73, CD45, and CD34 using immunofluorescence and flow cytometry (Scale bar = 100 μm).
Effect of Different Concentrations of LPS on Pro-inflammatory Cytokine Relate Gene Expression in bEECs
We treated bEECs with various concentrations of LPS (free, 0.1, 1, and10 μg/mL) and analyzed pro-inflammatory gene expression levels using qRT- PCR. In the screening of LPS stimulation concentration we found that, compared with the control group (LPS free), the relative expression levels of IL-1β, IL-6, and TNF-α were 6.19, 5.61, and 5.37, respectively, and were significantly increased in the 0.1μg/mL treatment of the LPS group (P < 0.01; Figure 3A). In the screening of LPS (0.1 μg/mL) stimulation time, when compared with the control group (0 h = none), the relative expression levels of IL-1β, IL-6, and TNF-α were 34.65, 7.81, and 4.33, respectively, and were increased highly significantly in the 6 h group (P < 0.01; Figure 3B). The data were processed and analyzed using Graphpad.
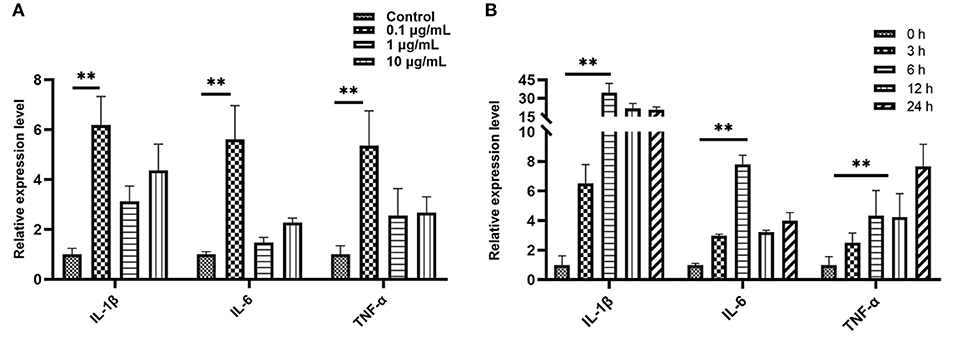
Figure 3. Quantitative PCR analysis for the expression of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α. (A) Screening stimulation optimum concentration of LPS (stimulation for 6 h). (B) Screening stimulation optimum timing with 0.1 μg/mL. Values are mean ± SD (n = 4). Significance is indicated by **P < 0.01.
bAD-MSC Treatment Downregulates Pro-inflammatory Cytokine Expression in LPS Induced bEECs
To investigate the anti-inflammatory effect of bAD-MSCs in the in vitro endometritis model, we seeded bAD-MSCs and bEECs into the transwell co-culture system with the indicated number of cells, followed by LPS stimulation. As shown in Figure 4, the relative expression levels of IL-1β, IL-6, and TNF-α were 5.45, 2.82, and 12.28 in LPS stimulated group, respectively, while the relative expression levels of IL-1β, IL-6, and TNF-α were 3.14, 1.53, and 1.52 in co-culture 60,000 group, respectively. The bAD-MSCs treatments highly significantly decreased the LPS induced pro-inflammatory cytokine expression levels, including IL-1β, IL-6, and TNF-α, in the bEECs (P < 0.01). This result demonstrated that the paracrine factors of bAD-MSCs had an anti-inflammatory effect in the endometrial epithelial cell inflammatory model.
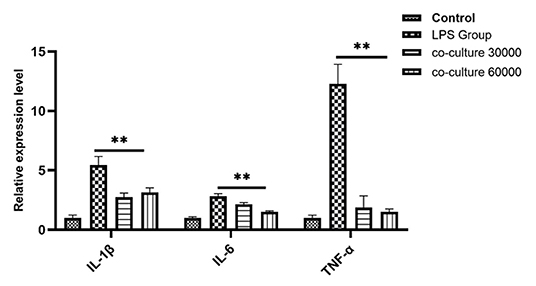
Figure 4. Quantitative PCR analysis for the expression of pro-inflammatory markers such as IL-1β, IL-6, and TNF-α when co-cultured with bAD-MSC 30,000 cells and 60,000 cells in the presence of 0.1 μg/mL of LPSs. Expression levels normalized to the reference gene β-actin. Values are mean ± SD (n = 4). Significance is indicated by **P < 0.01.
bAD-MSCs Downregulate Iκb-α and p-JAK1 Protein Expression in LPS Induced bEECs
To understand the possible molecular mechanisms for the inhibitory effects of bAD-MSCs on LPS induced pro-inflammatory cytokine expression in bEECs, we examined the activation level of the Iκb-α and p-JAK1 signaling pathways in bAD-MSC/bEEC co-culture systems after LPS stimulation. As shown in Figure 5, treatment with bAD-MSCs significantly downregulated LPS induced Iκb-α protein expression (relative expression of Iκb-α protein were 0.92 and 0.58 in LPS group and in co-culture 48 h, respectively) and p-JAK1 phosphorylation in bEECs (relative expression of p-JAK1 phosphorylation protein were 0.62 and 0.29 in LPS group and in co-culture 48 h, respectively)(P < 0.01).
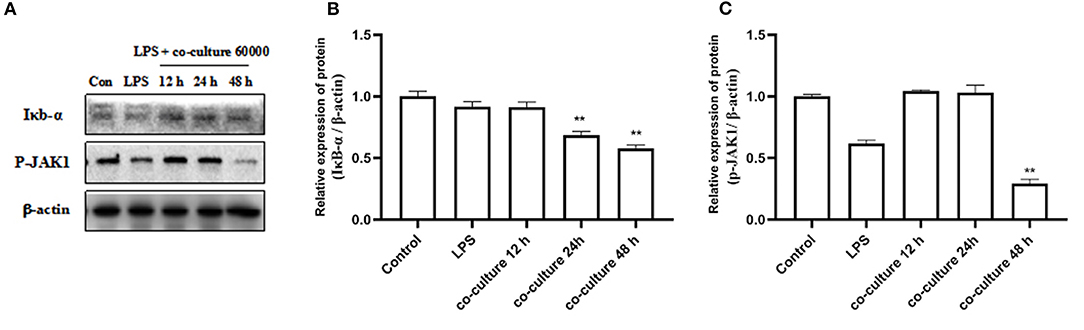
Figure 5. Expression of inflammation related proteins. bEECs were treated with 0.1 μg/mL LPSs for 6 h, co-cultured with bAD-MSCs 60 000 cells for 12, 24, and 48 h. (A) Western blot analysis of IκB-α and P-JAK1 proteins. (B) Relative expression levels of IκB-α protein. (C) Relative expression levels of P-JAK1 protein. Significance is indicated by **P < 0.01.
bAD-MSCs Inhibit LPS Induced bEEC Apoptosis
To verify the effect of bAD-MSCs on LPS induced apoptosis in bEECs, we observed the cellular apoptosis levels of bEECs after LPS stimulation in a bAD-MSC/bEEC co-culture system. According to microscopic examination, we found that the bAD-MSC treatment significantly decreased LPS induced apoptosis in bEECs stained with an Annexin-V-FITC/PI detection kit (Figure 6A). Furthermore, to understand the possible mechanisms of the anti-apoptotic effects of bAD-MSCs on LPS induced bEECs, we also examined the p38 signaling pathway and Bax (pro-apoptotic protein) protein expression levels. As shown in Figures 6B–D, the bAD-MSC treatment significantly downregulated LPS induced p38 phosphorylation (P-p38) level (relative expression levels were 2.56 and 0.22 in LPS group and in co-culture 48 h group, respectively, P < 0.01) as well as Bax protein expression (relative expression levels were 1.38 and 1.06 in LPS group and in co-culture 48 h group, respectively, P < 0.05) in bAD-MSC/bEEC co-culture systems. These results suggest that bAD-MSCs can inhibit LPS induced bEEC apoptosis.
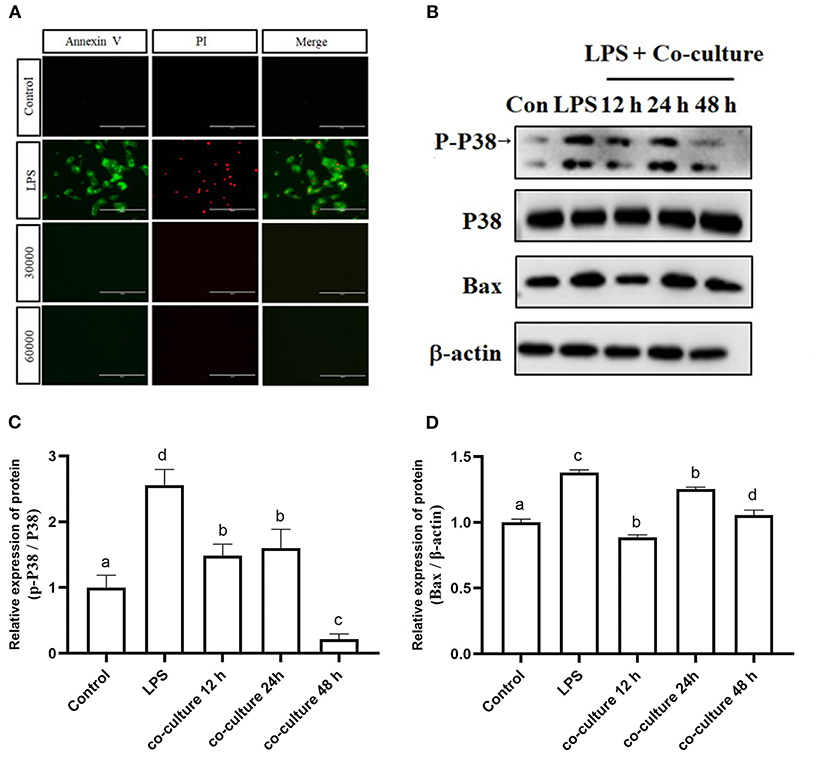
Figure 6. Effects of bAD-MSCs on LPS induced apoptosis of bEECs. (A) Immunofluorescence reactions for bEEC apoptosis by Annexin V-FITC and PI. bEECs were treated with 0.1 μg/mL LPSs for 6 h, co-cultured with bAD-MSCs 30,000 cells, and 60,000 cells for 48 h. The first column shows Annexin V by FITC, while the second column shows cell nuclei labeled with PI. The last column shows merged images with FITC and PI. (Scale bar = 100 μm). (B) Western blot analysis of p38, P-p38, and Bax proteins. bEECs were treated with 0.1 μg/mL LPSs for 6 h, and co-cultured with bAD-MSCs for 12, 24, and 48 h. (C) Relative protein expression levels of P-p38/p38. (D) Relative expression levels of Bax protein. Different lowercase letters in bar charts indicate significant differences (P < 0.05).
Discussion
MSCs are adult stem cells that originate from the mesoderm. MSCs have been successfully isolated and cultured from the skin, tooth tissue, peripheral blood, the dermis, and adipose tissue (31–33). They were initially found in bone marrow, but studies have shown that MSCs constitute between 0.001 and 0.01% of the total number of bone marrow cells, while in adipose tissue this figure can be as high as 2% of nucleated cells (34, 35). Therefore, adipose tissue can be an excellent source of MSCs. Recently, researchers have paid close attention to the therapeutic performance of MSCs. MSCs used in cell therapy need to satisfy the following three conditions: in the process of standard culture: they must have the characteristics of adherent growth; express positive markers, such as CD105, CD90, CD44, CD73, instead of negative markers, such as CD34, CD45, CD19; and can exhibit osteogenic and adipogenic differentiation in vitro (36). In the present study, we found that the isolated bAD-MSCs from bovine adipose tissues were not only strongly positive to the CD73 and CD44 antibodies, while negative to the antibodies for CD45 and CD34, but also exhibited osteogenic and adipogenic differentiation, which indicated that the cells we isolated and cultured had the characteristics of MSCs and could be used in the further study.
Through their ability to secrete endogenous regulatory factors of inflammation, MSCs have an anti-inflammatory effect, which can affect the progression of various inflammatory diseases through immunomodulation (37). For example, IL-10 (Interleukin-10), the activated IL-10 receptor complex can activate Janus kinase (JAK) and cause the phosphorylation of transcription (STAT), thus affecting the transcription of target genes and playing the anti-inflammatory regulatory role (38, 39). Likewise, IDO (Indoleamine 2,3-dioxygenase), a study shows that IFN-γ production by activated T-cells is correlated with the induction of IDO expression in MSCs via the IFN-γ-JAK-STAT1 pathway, thereby inhibiting T-cell proliferation (40). MSCs have a high degree of plasticity and the inherent ability to perceive changes in the microenvironment, so the different immunoregulatory effects of MSCs depend on their environment (41). In the process of inflammation, MSCs can inhibit archetypical inflammatory behaviors of target cells and promote anti-inflammatory, pro-apoptosis, and/or the generation of regulatory cells (42, 43). Studies have shown that MSCs interact with vascular endothelial cells to regulate inflammatory infiltration (44). On the other hand, bone marrow mesenchymal stem cells act through the release of soluble factors, so there are still many unknown areas to explore in cell therapy using mesenchymal stem cells (45).
Endometritis is an obstetric disease, which seriously reduces the reproductive performance of cattle. The etiology of endometritis is very complex; it is mostly caused by fungal, bacterial, and parasitic infections (46, 47). LPSs are one of the important causes of endometritis in dairy cows; they can induce an inflammatory response by stimulating bEECs to secrete inflammatory cytokines (48). Therefore, we chose LPSs to establish an in vitro bovine endometritis model to study the anti-inflammatory effect of MSCs. The expression of IL-1β, IL-6, TNF-α, and other inflammatory genes was increased after LPS stimulation. The results showed that LPSs successfully induced a cellular immune response by activating IL-1β and TNF-α secretion pathways. At the same time overexpression of IL-1β and TNF-α often leads to the recruitment of macrophages and activation of inflammatory cytokine interleukin-6 (IL-6) (49). In this experiment, bAD-MSCs were co-cultured within a bovine endometrial epithelial cell inflammation model; we found that the levels of IL-1β, TNF-α, and IL-6 were significantly lower than those in the endometritis model group. These results suggest that bAD-MSCs have a certain immunosuppressive capacity and can play an anti-inflammatory role. Therefore, we went on to detect the expression of inflammatory related proteins. Inflammation is a complex process when tissue cells are infected or damaged, such as during LPS stimulation. When stimulated by LPSs, the TLR4 pathway was activated, NF-κB was transferred to the nucleus, IκB-α was induced, and other proteins were expressed. Janus kinases (JAKs) are important signaling mediators downstream of many pro-inflammatory cytokines. Small molecule inhibitors of JAKs have been used as therapeutic agents for the treatment of inflammation, in which the Jak1/STAT3 signal is involved in the pathogenesis of inflammatory diseases (50, 51). Cytokine receptors use the Janus kinase signal transduction and transcription activation (JAK-STAT) pathway for signal transduction (52). When the cytokines bind to their cognate receptors, this activates the receptor-associated JAK (53). In our study, the expression of IκB-α and P-JAK1 proteins in endometrial epithelial cells of dairy cows was significantly increased when stimulated by LPSs. However, the expression of IκB-α and p-JAK1 were decreased when co-cultured with bAD-MSCs. This result may be related to some immunoregulatory characteristics of MSCs (such as TGF-β, IL-10, PGE2, TSG-6), and we will continue to investigate this issue. These outcomes suggest that bAD-MSCs play an anti-inflammatory role via the regulation of the IκB-α and JAK1 signaling pathways activated by LPSs. This is the first study of the role of the JAK1 signaling pathway in the treatment of endometritis by bAD-MSCs, and the results of this study offer a new therapeutic target for the treatment of bovine endometritis.
LPS induced inflammatory damage usually leads to changes in cell membrane permeability and the expression of apoptosis related proteins (54). We found that co-culture with bAD-MSCs can also reduce bEEC apoptosis. In the current experiment, the apoptosis of bEECs was induced by LPSs. Under an inverted fluorescence microscope, phosphatidylserine, and nuclei were stained by Annexin V-FITC and PI. However, the staining of Annexin V-FITC and PI in bEECs decreased significantly after co-culture with bAD-MSCs. To this point in the study, we had made a preliminarily elucidation of the anti-apoptotic ability of bAD-MSCs. We then detected changes in apoptosis related proteins when LPSs stimulated bAD-MSCs. The P38 MAPK signaling pathway is a recognized stress response pathway. After being stimulated and activated, p38 MAPK regulates cell apoptosis and proliferation through various means. Studies have shown that p38 MAPK can regulate the synthesis of Bax protein and then mediate cell apoptosis (55). When bEECs treated with LPSs were co-cultured with bAD-MSCs, the expression of P-p38, p38, and Bax protein decreased significantly. From this we can infer that bAD-MSCs can inhibit the p38 signaling pathway activated by LPSs, thus reducing the pro-apoptotic protein Bax and playing an anti-apoptotic role. However, it is still not clear how bAD-MSCs regulate the changes of these signaling pathways. According to the existing literature, we speculate that it may be regulated by the release of paracrine factors and/or exosomes; this theory requires further study in the future.
Conclusions
In conclusion, in this paper, we studied bAD-MSC therapy of endometritis using a bovine in vitro model. The results shed further light on the molecular mechanisms underlying the suggestion that bAD-MSCs can inhibit LPS induced bEEC inflammatory response and apoptosis by downregulating p38, IkB-a, and JAK1 phosphorylation and Bax protein expression levels. This study provides a theoretical basis for the future application of MSC therapy in the prevention and treatment of endometritis in dairy cows.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author Contributions
WL, Z-MX, and Y-HH conceived the study and wrote the manuscript. WL, Z-MX, QL, N-NY, J-BY, W-LL, Y-YM, ZD, LS, SY, JJ, SF, and DS carried out experiments and data analysis. WL, DS, and Y-HH interpreted the data. All authors approved the final version.
Funding
This work was supported by Heilongjiang Provincial Natural Science Foundation of China (Grant No. LH2020C084), the Key Scientific Research Program of the General Bureau of State Farms of Heilongjiang Proviance (Grant No: HKKY190302), and Three longitudinal and three transverse scientific research talent support programs-Basic cultivation plan projects of Heilongjiang Bayi Agricultural University (Grant No. ZRCPY201808).
Conflict of Interest
JJ is employed by Cofeed Feedmill (Changchun) Co., Ltd., Changchun, Jilin Province, China.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pascottini O B., Opsomer G. Postpartum uterine diseases in dairy cows: a review with emphasis on subclinical endometritis. Vlaams Diergeneeskundig Tijdschrift. (2016) 85.
2. He B, Ni Z, Kong S, Lu J, Wang, H. Homeobox genes for embryo implantation:From mouse to human. Animal Models Exp Med. (2018) 1:14–22. doi: 10.1002/ame2.12002
3. Li T, Hai L, Liu B, Mao W, Liu K, Yuan S, et al. TLR2/4 promotes PGE production to increase tissue damage in Escherichia coli-infected bovine endometrial explants via MyD88/p38 MAPK pathway. Theriogenology. (2020) 152:129–38. doi: 10.1016/j.theriogenology.2020.04.004
4. Liang D, Arnold LM, Stowe CJ, Harmon RJ, Bewley JM. Estimating US dairy clinical disease costs with a stochastic simulation model. J Dairy Sci. (2017) 100:1472–86. doi: 10.3168/jds.2016-11565
5. Song L, Tuan, R. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J. (2004) 18:980–2. doi: 10.1096/fj.03-1100fje
6. Aust L, Devlin B, Foster S, Halvorsen Y, Hicok K, du Laney T, et al. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. (2004) 6:7–14. doi: 10.1080/14653240310004539
7. Corrao S, La Rocca G, Lo Iacono M, Corsello T, Farina F, Anzalone R. Umbilical cord revisited: from Wharton's jelly myofibroblasts to mesenchymal stem cells. Histol Histopathol. (2013) 28:1235–44. doi: 10.14670/HH-28.1235
8. Hemmingsen M, Vedel S, Skafte-Pedersen P, Sabourin D, Collas P, Bruus H, et al. The role of paracrine and autocrine signaling in the early phase of adipogenic differentiation of adipose-derived stem cells. PLoS ONE. (2013) 8:e63638. doi: 10.1371/journal.pone.0063638
9. Wang FS, Zhou HH, Liu ZQ, Zhang SR. Conditions for inducing the differentiation of human bone marrow mesenchymal stem cells into chondrocytes. J Clin Rehabil Tissue Eng Res. (2007) 11:3920–3. doi: 10.1089/ten.tea.2009.0625
10. Tropel P, Platet N, Platel J, Noël D, Albrieux M, Benabid A, et al. Functional neuronal differentiation of bone marrow-derived mesenchymal stem cells. Stem Cells. (2006) 24:2868–76. doi: 10.1634/stemcells.2005-0636
11. Feng GH, Wang XX, Liu MY, Zhang XL, Wen QS, et al. Transformation of rat fatty mesenchymal stem cells into hepatocytes. (in Chinese) Progress Modern Biomed. (2008) 8:249–51. doi: 10.13241/j.cnki.pmb.2008.02.036
12. Zhou S, Greenberger J, Epperly M, Goff J, Adler C, Leboff M, et al. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. (2008) 7:335–43. doi: 10.1111/j.1474-9726.2008.00377.x
13. Liao JM. Transplantation of umbilical cord blood-derived mesenchymal stem cells for treatment of liver cirrhosis: research progress. World Chinese J Digestol. (2013) 21:508. doi: 10.11569/wcjd.v21.i6.508
14. Gao F, Chiu SM, Motan DA, Zhang Z, Chen L, Ji HL, et al. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. (2016) 7:e2062. doi: 10.1038/cddis.2015.327
15. Savukinas UB, Enes SR, Sjöland AA, Westergren-Thorsson G. Concise review: the bystander effect: mesenchymal stem cell-mediated lung repair. Stem Cells. (2016) 34:1437–44. doi: 10.1002/stem.2357
16. Horwitz E, Prockop D, Fitzpatrick L, Koo W, Gordon P, Neel M, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. (1999) 5:309–313. doi: 10.1038/6529
17. Ohnishi S, Nagaya N. Prepare cells to repair the heart: mesenchymal stem cells for the treatment of heart failure. Am J Nephrol. (2007) 27:301–7. doi: 10.1159/000102000
18. Yang J, Zhang N, Wang H, Gao P, Yang Q, Wen Q. CXCR4 receptor overexpression in mesenchymal stem cells facilitates treatment of acute lung injury in rats. J Biol Chem. (2015) 290:1994–2006. doi: 10.1074/jbc.M114.605063
19. Guan J, Zhu Z, Zhao R, Xiao Z, Wu C, Han Q, et al. Transplantation of human mesenchymal stem cells loaded on collagen scaffolds for the treatment of traumatic brain injury in rats. Biomaterials. (2013) 34:5937–46. doi: 10.1016/j.biomaterials.2013.04.047
20. Tgel FE, Westenfelder C. Treatment of Acute Kidney Injury with Allogeneic Mesenchymal Stem Cells: Preclinical and Initial Clinical Data. In: Regenerative Nephrology. (2011). p. 315–39.
21. Meisel R, Zibert A, Laryea M, Göbel U, Däubener W., Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. (2004) 103:4619–21. doi: 10.1182/blood-2003-11-3909
22. Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. (2006) 24:386–98. doi: 10.1634/stemcells.2005-0008
23. Ryan J, Barry F, Murphy J, Mahon B. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol. (2007) 149:353–63. doi: 10.1111/j.1365-2249.2007.03422.x
24. Lo Sicco C, Reverberi D, Balbi C, Ulivi V, Principi E, Pascucci L, et al. Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: endorsement of macrophage polarization. Stem Cells Transl Med. (2017) 6:1018–28. doi: 10.1002/sctm.16-0363
25. Hill A, Bressan F, Murphy B, Garcia J. Applications of mesenchymal stem cell technology in bovine species. Stem Cell Res Ther. (2019) 10:44. doi: 10.1186/s13287-019-1145-9
26. Chen Q, Shou P, Zheng C, Jiang M, Cao G, Yang Q, et al. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Different. (2016) 23:1128–39. doi: 10.1038/cdd.2015.168
27. Zheng C, Zou X, Zhao B, Zhang M, Lin H, Luo C, et al. miRNA-185 regulates retained fetal membranes of cattle by targeting STIM1. Theriogenology. (2019) 126:166–71. doi: 10.1016/j.theriogenology.2018.11.030
28. Sun X, Luo S, Jiang C, Tang Y, Cao Z, Jia H, et al. Sodium butyrate reduces bovine mammary epithelial cell inflammatory responses induced by exogenous lipopolysaccharide, by inactivating NF-κB signaling. J Dairy Sci. (2020) 103:8388–97. doi: 10.3168/jds.2020-18189
29. Zhao C, Liu G, Li X, Guan Y, Wang Y, Yuan X, et al. Inflammatory mechanism of Rumenitis in dairy cows with subacute ruminal acidosis. BMC Vet Res. (2018) 14:135. doi: 10.1186/s12917-018-1463-7
30. Kowsar R, Hambruch N, Liu J, Shimizu T, Pfarrer C., Miyamoto A. Regulation of innate immune function in bovine oviduct epithelial cells in culture:the homeostatic role of epithelial cells in balancing Th1/Th2 response. J Reprod Dev. (2013) 59:470–8. doi: 10.1262/jrd.2013-036
31. Campagnoli C, Roberts I, Kumar S, Bennett P, Bellantuono I, Fisk N. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. (2001) 98:2396–402. doi: 10.1182/blood.V98.8.2396
32. De Bari C, Dell'Accio F, Tylzanowski P, Luyten F. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheumat. (2001) 44:1928–42. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P
33. Zuk P, Zhu M, Mizuno H, Huang J, Futrell J, Katz A, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. (2001) 7:211–28. doi: 10.1089/107632701300062859
34. Figliuzzi M, Bonandrini B, Silvani S, Remuzzi A. Mesenchymal stem cells help pancreatic islet transplantation to control type 1 diabetes. World J Stem Cells. (2014) 6:163–72. doi: 10.4252/wjsc.v6.i2.163
35. Zheng G, Huang L, Tong H, Shu Q, Hu Y, Ge M, et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respiratory Res. (2014) 15:39. doi: 10.1186/1465-9921-15-39
36. Ntege E, Sunami H, Shimizu Y. Advances in regenerative therapy: a review of the literature and future directions. Regenerative Therapy. (2020) 14:136–53. doi: 10.1016/j.reth.2020.01.004
37. Zheng G, Ge M, Qiu G, Shu Q, Xu J. Mesenchymal stromal cells affect disease outcomes via macrophage polarization. Stem Cells Int. (2015) 2015:989473. doi: 10.1155/2015/989473
38. Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nature reviews. Immunology. (2010) 10:170–81. doi: 10.1038/nri2711
39. Fioranelli M. Twenty-five years of studies and trials for the therapeutic application of IL-10 immunomodulating properties. From High Doses administration to low dose medicine new paradigm. J Integr Cardiol. (2015) 1:2–6. doi: 10.15761/JIC.100010
40. Kim D, Jang I, Lee M, Ko Y, Lee D, Lee J, et al. Enhanced immunosuppressive properties of human mesenchymal stem cells primed by interferon-γ. EBioMed. (2018) 28:261–73. doi: 10.1016/j.ebiom.2018.01.002
41. Carrion B, Janson I, Kong Y, Putnam A. A safe and efficient method to retrieve mesenchymal stem cells from three-dimensional fibrin gels. Tissue Eng. (2014) 20:252–63. doi: 10.1089/ten.tec.2013.0051
42. Pers Y, Ruiz M, Noël D, Jorgensen C. Mesenchymal stem cells for the management of inflammation in osteoarthritis: state of the art and perspectives. Osteoarthritis Cartilage. (2015) 23:2027–35. doi: 10.1016/j.joca.2015.07.004
43. Fan X, Zhang Z, Ma C, Fu Q. Mesenchymal stem cells for inflammatory airway disorders: promises and challenges. Biosci Rep. (2019) 39:BSR20182160. doi: 10.1042/BSR20182160
44. Luu N, McGettrick H, Buckley C, Newsome P, Rainger G, Frampton J, et al. Crosstalk between mesenchymal stem cells and endothelial cells leads to downregulation of cytokine-induced leukocyte recruitment. Stem Cells. (2013) 31:2690–702. doi: 10.1002/stem.1511
45. Denewar M, Amin L. Role of bone marrow-derived mesenchymal stem cells on the parotid glands of streptozotocin induced diabetes rats. J Oral Biol Craniofacial Res. (2020) 10:33–7. doi: 10.1016/j.jobcr.2020.02.003
46. Saini P, Singh M, Kumar P. Fungal endometritis in bovines. Open Vet J. (2019) 9:94–8. doi: 10.4314/ovj.v9i1.16
47. Jhamat N, Niazi A, Guo Y, Chanrot M, Ivanova E, Kelsey G, et al. LPS-treatment of bovine endometrial epithelial cells causes differential DNA methylation of genes associated with inflammation and endometrial function. BMC Genomics. (2020) 21:385. doi: 10.1186/s12864-020-06777-7
48. Magata F, Horiuchi M, Miyamoto A, Shimizu T. Lipopolysaccharide (LPS) inhibits steroid production in theca cells of bovine follicles in vitro:distinct effect of LPS on theca cell function in pre- and post-selection follicles. J Reprod Dev. (2014) 60:280–7. doi: 10.1262/jrd.2013-124
49. Lei C, Wu X, Zhong X, Jiang L, Zhong B, Shu H. USP19 Inhibits TNF-α- and IL-1β-triggered NF-κB activation by Deubiquitinating TAK1. J Immunol. (2019) 203:259–268. doi: 10.4049/jimmunol.1900083
50. Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz D. JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs. (2017) 77:521–46. doi: 10.1007/s40265-017-0701-9
51. Daniella S, Kanno Y, Villarino A, Ward M, Gadina M, O'Shea JJ, et al. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov. (2017) doi: 10.1038/nrd.2017.201
52. Costa-Pereira AP, Bonito NA, Seckl MJ. Dysregulation of janus kinases and signal transducers and activators of transcription in cancer. Am J Cancer Res. (2011) 1:806–16.
53. Imada K, Leonard W. The Jak-STAT pathway. Mol Immunol. (2000) 37:1–11. doi: 10.1016/S0161-5890(00)00018-3
54. Xaus J, Comalada M, Valledor AF, Lloberas J, López-Soriano F, Argilés JM, et al. LPS induces apoptosis in macrophages mostly through the autocrine production of TNF-alpha. Blood. (2000) 95:3823–31. doi: 10.1182/blood.V95.12.3823.012k07_3823_3831
Keywords: bovine adipose-derived mesenchymal stem cell, inflammation, lipopolysaccharide, endometritis, dairy cows
Citation: Lu W, Xu Z-M, Liu Q, Yu N-N, Yu J-B, Li W-L, Mao Y-Y, Du Z, Si L, Yuan S, Jin J, Fu S, Sun D and Han Y-H (2021) Inhibitory Effect of Bovine Adipose-Derived Mesenchymal Stem Cells on Lipopolysaccharide Induced Inflammation of Endometrial Epithelial Cells in Dairy Cows. Front. Vet. Sci. 8:726328. doi: 10.3389/fvets.2021.726328
Received: 16 June 2021; Accepted: 29 September 2021;
Published: 21 October 2021.
Edited by:
Tereza Cristina Cardoso, Universidade Estadual de São Paulo, BrazilReviewed by:
Andrea Verna, Instituto Nacional de Tecnología Agropecuaria, ArgentinaOscar Peralta, University of Chile, Chile
Copyright © 2021 Lu, Xu, Liu, Yu, Yu, Li, Mao, Du, Si, Yuan, Jin, Fu, Sun and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying-Hao Han, aHloYnluZEAxNjMuY29t; Dongbo Sun, ZG9uZ2Jvc3VuQDEyNi5jb20=
 Wengeng Lu
Wengeng Lu Zheng-Mei Xu
Zheng-Mei Xu Qing Liu
Qing Liu Nan-Nan Yu
Nan-Nan Yu Jia-Bin Yu
Jia-Bin Yu Wei-Long Li
Wei-Long Li Ying-Ying Mao
Ying-Ying Mao Zhenzhen Du
Zhenzhen Du Linqing Si1
Linqing Si1 Siqi Yuan
Siqi Yuan Jidong Jin
Jidong Jin Dongbo Sun
Dongbo Sun