- 1Faculty of Veterinary Medicine, University of Teramo, Teramo, Italy
- 2Faculty of Veterinary Medicine, University of Turin, Turin, Italy
Canine tumors are valuable comparative models for human counterparts, especially to explore novel biomarkers and to understand pathways and processes involved in metastasis. Vasculogenic mimicry (VM) is a unique property of malignant cancer cells which promote metastasis. Thus, it represents an opportunity to investigate both the molecular mechanisms and the therapeutic targets of a crucial phenotypic malignant switch. Although this biological process has been largely investigated in different human cancer types, including osteosarcoma, it is still largely unknown in veterinary pathology, where it has been mainly explored in canine mammary tumors. The presence of VM in human osteosarcoma is associated with poor clinical outcome, reduced patient survival, and increased risk of metastasis and it shares the main pathways involved in other type of human tumors. This review illustrates the main findings concerning the VM process in human osteosarcoma, search for the related current knowledge in canine pathology and oncology, and potential involvement of multiple pathways in VM formation, in order to provide a basis for future investigations on VM in canine tumors.
Introduction
Vasculogenic mimicry (VM) is a unique ability of malignant cancer cells to create their own fluid-conducting microvascular channels without the involvement of endothelial cells. It was firstly described in human uveal melanomas as periodic acid–Schiff (PAS)-positive microvascular channel networks (1). Since then, VM has been observed in a variety of human malignant tumors, including osteosarcoma (OSA), glioblastoma and gallbladder, ovarian, prostate, lung, gastric, hepatocellular, and breast cancer (2, 3). In addition, the presence of VM has been associated with high tumor grade, invasion, metastasis, and poor prognosis in cancer patients (4, 5). Thus, VM has emerged as a potential target for anti-tumor therapy (2, 3, 6).
In veterinary pathology, the VM process has been demonstrated in canine inflammatory mammary carcinomas and in a palpebral melanocytoma (7, 8). Rasotto et al. explored the presence of VM in primary canine mammary tumors, revealing no relation with lymphatic infiltration (9). As well, primary cell lines from canine mammary tumors, showing ability to form VM in vitro and in vivo, have been recently established and characterized (10–12). Moreover, canine inflammatory mammary carcinomas were analyzed for the presence of VM by transmission and scanning electron microscopy (13). In addition, as far as canine OSA is concerned, the presence of vessel-like structures in a long-term canine D17 OSA cell cultured on type I collagen has been recently described (14). As well, treatment with the heat shock protein 90 (Hsp90) inhibitor 17-N-allylamino-17-demethoxygeldanamycin (17-AAG) inhibited the migration of D17 OSA cells, also decreasing VM markers in vitro and inducing a reduction of hypoxia-inducible factor 1α (HIF1α) transcript and protein expression (14). Notwithstanding this, information regarding VM formation, molecular features, and prognostic implications in canine oncology is still limited.
Since VM has been known from a relatively short time, the molecular mechanisms involved in this process remain largely unknown. Aggressive tumor cells capable of VM display a varied gene profile which includes that of fibroblasts and epithelial and endothelial cells (15). Hypoxia, epithelial-mesenchymal transition (EMT) and in particular epithelial-endothelial transition (EET), response to extracellular matrix (ECM), and the presence of cancer stem cells (CSCs) are considered the key regulators of VM (16, 17). Various signaling pathways, promoting tumor migration and invasion, have been reported to participate in VM formation, including those involved in vasculogenesis such as vascular endothelial (VE)-cadherin, vascular endothelial growth factor (VEGF)/VEGF receptor (VEGFR) and platelet-derived growth factor (PDGF)/PDGF receptor (PDGFR) axis, and HIF1α (3). VM progression is also mediated by pathways involved in ECM adhesion and cell migration, such as focal adhesion kinase (FAK) and migration inducting gene 7 (Mig7) encoding for breast cancer anti-estrogen resistance protein 3 (BCARP 3), matrix metalloproteinases (MMPs), integrins and erythropoietin-producing hepatocellular receptorA2 (EphA2), as well as multiple signaling pathways including mechanistic target of rapamycin (mTOR) and Rho-associated coiled-coil kinase (RhoA/ROCK) (3). Finally, increasing evidence showed that VM can be affected by microRNA (miRNA), long non-coding RNA (lncRNA), and circular RNA (circRNA) (18).
Thus, the aim of this review is to illustrate the main findings concerning the VM process in human OSA (Figures 1, 2), as well as the current knowledge on the molecular pathways potentially involved in VM formation in canine pathology and oncology (Supplementary Table 1), in order to provide a basis for establishing further investigations on VM in canine tumors in the future.
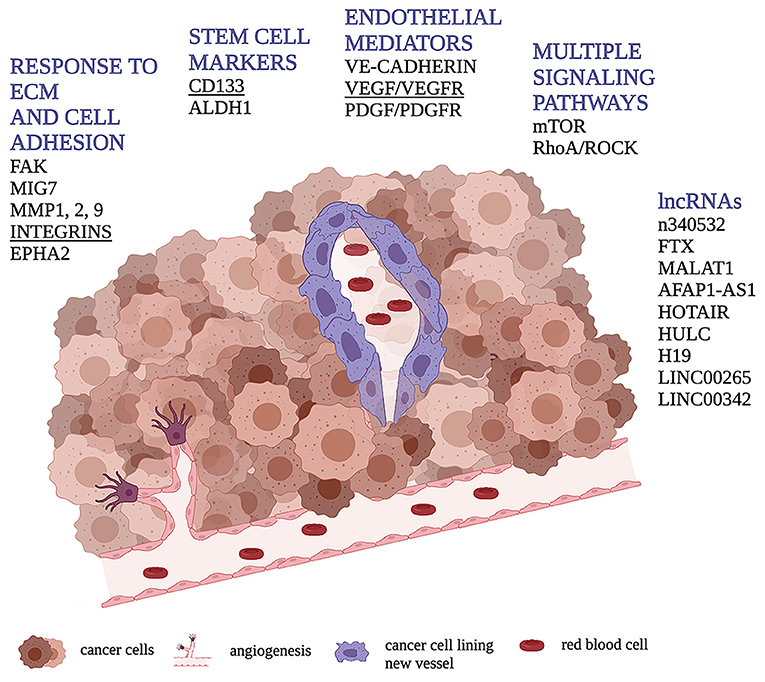
Figure 1. Schematic representation of VM through cancer cells (in purple) forming a vessel containing red blood cells. Figure shows the main molecular pathways involved in the VM process in human osteosarcoma highlighting, in underlined bold, those found to be related with VM presence or tubular/vessel-like formation in vitro in dog.
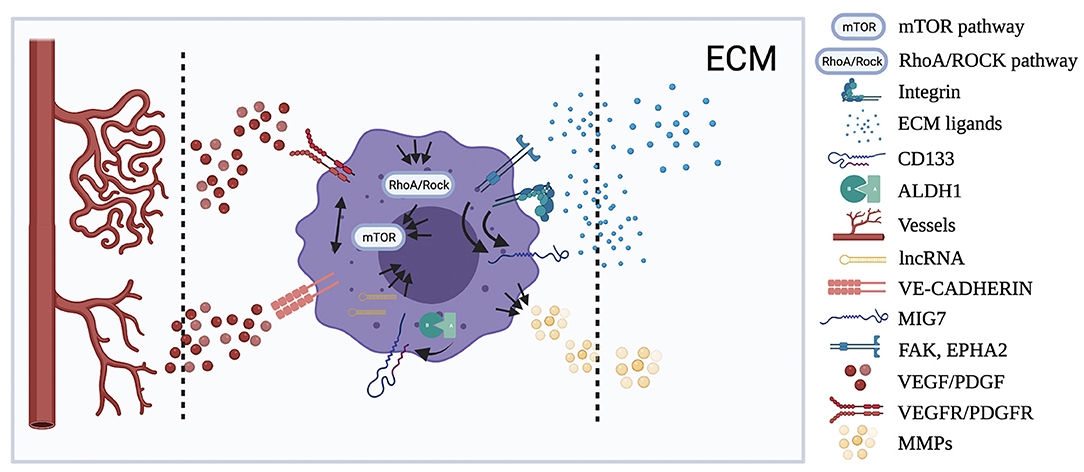
Figure 2. Localization of the principal molecular pathways involved in VM. Figure shows the cellular and tumor microenvironmental distribution of the human OSA pathways resumed in the review showing, when known, the possible interactions (black arrow) between them. Multiple arrows show multiple interactions between pathways.
CSCs Markers: CD133 and Aldehyde Dehydrogenase 1 (ALDH1)
CSCs represent an important feature of VM progression for their ability to differentiate in endothelial cells forming new microvessels (19). Stemness and differentiation potential of CSCs are enhanced under hypoxic microenvironments, through hypoxia-induced EET and ECM remodeling, thus determining the formation of the specific features of VM (17). Bao et al. (30) described a positive correlation between CD133 expression and presence of VM in OSA, which was, in turn, positively associated with ALDH1 expression. CD133, also called prominin-1, is a common biomarker of CSCs, which encodes a 120-kDa five-transmembrane domain glycoprotein. Its dysregulation has been considered as a CSC biomarker in various human cancers including OSA (20, 21), and it is correlated with VM, presence of metastasis, and poor prognosis in different tumors (21, 22). Little is still known about the mechanisms used by CSCs for promoting angiogenesis and VM (23). In this respect, the ability of CD133 to activate the Wingless-related integration site (Wnt) signaling pathway, thus increasing the expression of VEGFα and interleukin-8 (IL-8) (24), and its mechanistic link with cell motility (25), may be involved in the VM process.
ALDH1 is another common biomarker of dysregulated CSCs in a variety of human cancers (26, 27), the inhibition of which could represent a target in OSA therapy (28, 29). In the study of Bao et al. (30) multivariate analysis data showed that the expression of CD133, ALDH1, and VM; grade of differentiation; recurrence; as well as Enneking stages were independent prognostic factors for OSA patients. Despite the identified correlation with prognosis, the presence of CSC markers lining VM-dependent vessels has not been demonstrated in OSA tissues, even though CD133+ stem-like cell accumulation has been observed in the melanoma perivascular niche (30, 31).
CD133 and ALDH1 as Canine CSC Markers and Their Expression in Madin–Darby Canine Kidney Cells
The general structure of prominin-1, including its membrane topology, has been conserved throughout the animal kingdom (32). Non-tumor canine cells, in particular MDCK cells, have been widely used for understanding the mechanisms on the basis of cell motility. In fact, considering that prominin-1 is associated with plasma membrane protrusions, the overexpression of Prom1 gene increased the number of MDCK microvilli, while the overexpression of a dominant-negative mutant variant significantly decreased ciliary length (25). The involvement of CD133 in cell motility was also demonstrated by Liu et al., showing the ability of isolated canine CD133+ epithelial cells to form a tubular-like structure when cultured on Matrigel (33). Likewise, endothelial progenitor cells isolated from canine bone marrow CD133+ are capable of forming a capillary structure on Matrigel after 24 h of culture and can be transplanted in ischemic injured tissues to enable neovascularization (34, 35).
In cancer, CD133 staining, together with functional properties including ALDH enzyme activity and spheroids formation in vitro, is commonly used to characterize potential CSCs in canine OSA and others types of spontaneous canine cancer, not only those deriving from a hematopoietic lineage (36–40). Immunohistochemical investigations revealed that CD133 was expressed in all grades of OSA, glioma, melanoma, hepatocellular carcinoma, B-cell lymphoma, and granular cell tumor, with a higher proportion of positive cells in high-grade tumors (41–45).
Although a direct association between CD133 and VM has not been investigated in canine tumors, CD133+ cancer cells showed different features linked to VM both in vivo and in vitro. Highly invasive and tumorigenic canine insulinoma CSC-like cells and canine prostate cancer cellsCD133+ showed an invasive and tumorigenic phenotype in vivo, similar to hepatocellular carcinoma and lung adenocarcinoma cell lines that were also capable of forming spheroids in culture (46–49). CD133+ hemangiosarcoma cell lines cultured under normal and sphere-forming conditions generated three distinct tumor subtypes in vitro, associated with angiogenesis, inflammation, and adipogenesis (50). CD133+ canine cell lines derived from OSA, melanoma, transitional cell carcinoma, and lung adenocarcinoma resulted to be significantly resistant against X-ray irradiation (38). Gatti et al. observed that canine OSA primary cultures containing CD133+ CSCs exhibited distinctive sensitivity to anticancer agents (51), as well as spheroids derived from canine mammary gland adenocarcinoma (52).
As far as ALDH1 is concerned, despite ALDH enzymatic activity being also considered a cancer marker in canine samples (37, 53, 54), to the best of our knowledge, its protein and gene expression has not been directly investigated in canine tumors, as well as in normal tissues or in other canine pathological conditions (55–57).
Endothelial Mediators
VM takes place independently of angiogenesis or endothelial cell proliferation, although it is often associated with endothelial marker expression (15). Gene expression analysis showed that aggressive tumor cells capable of VM display a diversified gene profile, expressing genes from multiple cell types including those of endothelial cells (58). In fact, the concept of “embryonic-like and vascular phenotype in the absence of endothelial markers,” referred to as the first histological definition of VM (1), is controversial. In this respect, it has been demonstrated that primary and established sarcoma cell lines, after prolonged stimulation with post-surgery fluids from a cohort of patients affected by giant cell tumors of bone, transdifferentiated into VE-Cadherin+ and CD31+tubular-like structures (59). For this reason, the term “endothelial mediators” (and not endothelial markers) is preferred, to avoid controversy concerning the attribution of specific endothelial markers to highly aggressive cells that undergo VM. In several tumors, especially melanoma, an important group of endothelial mediators has been found in association with VM, including VE-cadherin (60–62) and VEGFR1 (63).
In MG63 OSA cells, the inhibition of Cdh5 gene encoding for VE-cadherin with small interfering RNA (siRNA) reduced the ability of cells to form endothelial-like networks when cultured on type I collagen or Matrigel (64), and the same phenomenon has been observed in silencing the Vegf gene (65). In fact, autocrine VEGF/VEGFR1 signaling, associated with increased tumor growth and tumor vascularity, may possibly confer the capacity to develop vasculogenic properties to OSA cells (66). In recent studies, differentially expressed genes (DEGs) were investigated between different OSA cells cultured on Matrigel for profiling the molecular patterns involved in VM phenotypes. Results from these studies showed that the endothelial mediators PDGFRα and PDGFRβ were correlated with malignancy and tubular-like structure formation in vitro (67, 68).
VE-Cadherin as Regulator of EMT and Vascular Integrity in Canine Pathology
VE-cadherin is an endothelial cell-specific cadherin that functions to stabilize cell structure because of its involvement in calcium-dependent intercellular adhesion (69). In dog, it has not been linked with VM, nor investigated in MDCK cells, although its role in EMT, a process closely related to VM and vascular integrity, has been explored (70, 71). In fact, VE-cadherin gene expression and immunohistochemical staining was evaluated in canine myxomatous mitral valve disease to investigate the role of EMT in chronic valvulopathies, showing a significant cdh5 gene dysregulation (72).
In cancer, VE-cadherin protein expression was observed at intercellular junctions in both normal canine tissue-derived cells (NECs) and in canine tumor-derived cells (TECs), isolated from thyroid carcinoma and perianal gland epithelioma. The observed zigzag pattern in TECs, with respect to the linear in NECs, may be indicative of VE-cadherin dysfunction and increased vascular permeability, probably dependent on the high concentration of VEGF in the tumor microenvironment in vivo. In fact, an abnormal VE-cadherin expression pattern was observed in 100% confluent NECs, following culture in a tumor-conditioned medium containing excessive VEGF (73). Moreover, this study showed that Combretastatin A-4 phosphate (CA4P) has selective effects on TEC morphology and NECs in tumor culture conditions, also disrupting vasculature in canine OSA xenografted into mice (74). Furthermore, genome-wide methylation analysis performed in canine mammary tumors showed a significant hypermethylation at the PAX5 (paired box protein 5) motifs in the intron regions of cdh5 gene and a consequent gene down-regulation (75).
VEGF/VEGFR Axis in Relation to VM in Canine Osteosarcoma and Mammary Tumors
As far as VEGF/VEGFR axis in veterinary oncology is concerned, VEGF family members were identified in several canine cancers (76), as well as OSA tissue, serum, and cultured cells (77–79). A relation between VM and VERGFR was found in D17 canine OSA cells cultured on type I collagen where malignant cancer cells with endothelial morphology express VEGFR1 (14). Correlation between VM and VEGF axis has been firstly investigated in dogs with mammary tumors (7). VEGFα, VEGFγ, and VEGFR3 were expressed in spontaneous canine mammary tumor and xenograft models (80), showing increased expression in the inflammatory mammary carcinoma (IMC) model compared to non-IMC and mammary OSA (80, 81). VM has been shown to occur more frequently in IC compared with other types of canine mammary tumors (7). Furthermore, overexpression of VEGFα, VEGFγ, and VEGFR3 was observed in canine malignant non-IMC, and it was correlated with cyclooxygenase 2 (COX2) immunoexpression, which is particularly related to VM progression (82, 83).
Another indirect relation to VM can be found in the study of Cam et al. in which VEGFα expression in different OSA cell lines and its correlation with ΔNp63 and cell migration on Matrigel was described, demonstrating that ΔNp63 exerts its angiogenesis and invasion property through VEGFα (84). It has been also demonstrated that VEGFα is the direct target of miR34a, which is less expressed in OSA cell lines with respect to normal osteoblast; OSA cells that have been induced to overexpressing miR34a show decreased motility and invasion ability on Matrigel and increased levels of VEGFα (85). On the other hand, a correlation between VEGFα transcript and chemical hypoxia was not observed (77).
As far as OSA ex vivo samples are concerned, literature data regarding VEGF axis and VM are lacking. Moreover, no correlation was observed between VEGF expression and clinicopathological parameters or hypoxia markers, which are often related to VM (77). On the contrary, its serum concentration has been previously correlated with poor prognosis in canine OSA (86). Increased levels of VEGF in serum or in cell supernatant were also observed after treatment of canine OSA with tyrosine kinase inhibitors such as Toceranib, Erlotinib, and Masitinib mesylate, probably due to a mechanism of feedback response to VEGFR2 inhibition (87–90).
PDGF/PDGFR Axis in Osteosarcoma and Other Canine Tumors
An extensive knowledge concerning PDGF/PDGFR axis is available in veterinary literature. This molecular axis has been investigated as endothelial marker (91), in wound healing (92), spontaneous canine astrocytoma (93), fibrosarcoma (94), squamous cell carcinoma (95), lymphoma (96), prostate cancer (97), hemangioma and hemangiosarcoma (98), melanoma (99), mast cell tumors (100), hepatocellular carcinoma (101), mammary tumors (102), and nervous system tumors (103, 104).
PDGFs and PDGFRs were also found to be coexpressed and overexpressed in canine OSA, suggesting an autocrine and/or paracrine loop. In particular, in the study of Maniscalco et al. (105) all evaluated canine OSA cell lines overexpressed PDGFRα, while 6/7 overexpressed PDGFRβ, when compared to a normal osteoblastic cell line (106). The involvement of an autocrine loop of PDGF signaling pathway in the pathogenesis of canine OSA was confirmed in other studies, showing the overexpression of cis, the coding gene of PDGFRβ, in a OSA cell line (CO8), and the ability of its supernatant to induce tyrosine phosphorylation and therefore the activation of the PDGFRα and PDGFRβ on murine 3T3 cells (107, 108). Meyer et al. demonstrated that, in addition to tumor cells, giant cells and osteoblasts in canine OSA were positive for PDGFBB immunostaining, composed of two subunits β, also showing the detection of its mRNA in all study cases (109). Finally, the dysregulation of the expression levels of PDGFRβ in canine OSA has been attributed to the strong demethylation of CpG sites within the promoter (110).
No evidence currently exists concerning a relationship between VM and PDGF/PDGFR axis in canine oncology. Furthermore, no significant correlation was observed between the expression of these molecules and survival or histological grading in canine OSA (105). Despite this, the significant relation of this axis with malignant features of canine OSA has been observed both in vivo and in vitro (111). In fact, treating OSA cells with Toracenib, a potent inhibitor of PDGFRs, has been shown to induce a decrease in cell growth, migration, motility, and colony formation, as well as a significant blunting of tumor growth and proliferation index in an orthotopic xenograft model (111). These findings suggest that PDGF/PDGFR axis can represent a target therapy more than a diagnostic tool. With the coming of new technologies linked to miRNA, miR34a was tested on OSA cell lines and xenograft mouse models, showing PDGFRα reduction, together with decrease in cell proliferation and migration in vitro and tumor growth in vivo (112).
Response to ECM Environment and Cell Adhesion
Among the myriad of microenvironmental factors affecting cancer cell resistance, cell adhesion to the ECM has been recently identified as a key determinant (113). FAK is a non-receptor tyrosine kinase that mediates signaling events downstream of integrin engagement of the ECM, regulating cell survival, proliferation, and migration and supporting neovascularization and maintenance of CSCs (114). FAK is expressed in different cancer types, where it is involved in the progression of tumor aggressiveness. Small molecule FAK inhibitors in clinical phase trials demonstrated to be effective in cancer by inducing tumor cell apoptosis in addition to reducing metastasis and angiogenesis (115). Association between FAK and VM or invasive behavior has been observed in different cancer types, including OSA. Ren et al. showed FAK staining in the cytoplasm of OSA tissue cells with high intensity around VM vessels (116). Similarly, Mig7 gene was expressed in the cytoplasm with higher percentage of positivity in the VM with respect to non-VM group, suggesting an association between Mig7expression and VM formation and identifying in VM a prognostic marker of OSA (116). Mig7 protein is enriched in embryonic cytotrophoblast cells during placental development and in more than 80% of tumors compared to normal tissue samples and blood from normal subjects (117). It was found to colocalize with VE-cadherin in cells lining VM structures in a lymph node metastasis (118) and to initiate a signaling cascade that results in tumor VM (119, 120). Moreover, Mig7 knockdown inhibited tubular-like vessel formation and invasion of MG63 and 143B OSA cells cultured on Matrigel, as well as growth and metastasis of OSA cells in a mouse model (121). Parispolyphylla, from traditional Chinese medicine, inhibited cell migration, invasion, and VM formation in vitro and in vivo by reducing expression of FAK, Mig7, MMP2 (gelatinase A), and MMP9 (gelatinase B) (122). MMP1 (interstitial collagenase) also resulted to be the first upregulated gene among the DEGs of the abovementioned studies performed on OSA cells cultured on Matrigel (67, 68).
Among the plethora of membrane proteins interacting with the ECM, integrin-α2 (ITGA2) has acquired an important role for its involvement in tumor cell proliferation, invasion, metastasis, and angiogenesis. In fact, its abnormal expression correlates with unfavorable prognosis in multiple types of cancer (123). Itga2 gene overexpression has been reported to be related to increased OSA metastasis and invasion (124) and was upregulated in malignant OSA cells in vitro (68). In the study of Yao et al., gene signal transduction networks (Signal-net) were performed to identify the key genes involved in VM formation in OSA and the top-ranked ones resulted to be Itga2, integrin subunit alpha 1 (Itga1) and integrin subunit alpha 6 (Itga6) together with protein kinase cAMP-activated catalytic subunit beta (Prkacb), actinin alpha 1(Actn1), actinin alpha 4 (Actn4), phospholipase C beta 4 (Plcb4), gap junction protein alpha 1(Gja1), and the already mentioned gene encoding for PDGFRβ and PDGFα. Finally, this study demonstrated that Itga1 knockdown inhibited VM formation by 143B cells in vitro and in vivo (68).
In addition, the tyrosine kinase EphA2, which belongs to the family of Eph tyrosine kinase receptors, is highly expressed in tumors, while it has been found at relatively low levels in most normal adult tissues, indicating its potential application in cancer treatment (125). Recent evidence suggests that VM occurrence is positively correlated with high expression of EphA2 and that its gene silencing inhibits VM formation (126). Interesting is also the correlation with Epstein–Barr virus (EBV) infection that stimulates plasticity in epithelial cells to express an endothelial phenotype (127). As well, Zhang et al. demonstrated that Epha2 gene silencing inhibited VM formation in MG63 OSA cells (128).
FAK Protein in Canine Tumor Progression
Interactions between tumor cells and tumor microenvironment are considered critical in carcinogenesis, tumor invasion, and metastasis (129). The involvement of adhesion proteins in canine OSA has been demonstrated through an expression profiling comparison between dogs with disease-free intervals (DFI) of <100 and >300 days (130).
The study of Brachelente et al. exploring the differential expression between melanomas and melanocytomas, identified differentially expressed gene clusters including nine genes belonging to the focal adhesion family (129). As far as FAK protein in humans is concerned, it is well-established that FAK serves as a scaffold for multiple protein signaling complexes, and its scaffolding function is very important for tumor progression (131). In canine oncology, interesting results were shown by Rizzo et al., demonstrating that the treatment of highly invasive D17 cells and other two OSA cell lines with Sulforaphane significantly decreased the phosphorylated state of FAK, also diminishing the invasion ability of cells cultured on Matrigel (132). These findings indirectly suggest a correlation between FAK activity and VM, considering that the inhibition of D17 OSA cell invasiveness corresponds to a decrease of VM features in vitro (14). Moreover, inhibition of FAK phosphorylation improved migration of canine hemangiosarcoma cells (133). FAK-mediated signaling was induced by numerous microenvironmental inputs and plays a central role in tumor-associated EMT and epithelial cells extrusion, migration, and response to the transforming growth factorβ (TGFβ) and the hepatocyte growth factor (HGF), as often demonstrated on MDCK cells (134–140). The use of these cells has also allowed understanding the involvement of FAK in the EMT induced by latent membrane protein 1 (LMP1) of EBV (141). Finally, the FAK inhibitor Masitinib mesylate (AB1010) has been the first anticancer therapy approved in veterinary medicine for the treatment of unresectable canine mast cell tumors (142).
MMPs in Canine Tumors
In veterinary literature, current knowledge on the activity and function of proteases and stroma and their relationship with canine cancer malignancy is still limited (143), despite the fact that MMPs have been widely explored in several human cancers and are strictly related to the VM process (144, 145). Inhibition of extracellular proteolysis, in particular of collagenases MMP1, MMP2, and MMP9, is recognized as a valid approach to canine cancer therapy including OSA (146). In fact, Doxycycline at doses >5 μg/ml significantly decreased OSA cell proliferation and MMP1 activity in vitro (147).
Mmp1 is the most significantly downregulated gene in Hsp70 knockdown canine OSA cells, and increased expression of mmp2 and mmp9 was linked to increased invasive capability in canine OSA (78, 148, 149).
Furthermore, MMP2 and MMP9 enzyme activity was found by means of zymography in three high malignant OSA cell lines (150).
The association between collagenase expression and activity and histological grade has also been demonstrated in canine mast cell tumor and lymphoma, together with VEGF dysregulation (151, 152), in mammary tumors, in relation to E-cadherin (153, 154), and in chondrosarcoma (153, 155–160). No differences in MMP9 expression were observed between IMC and non-IMC, although its expression was associated with higher nuclear grade in IMC tumors (161). As well, MMP2 and MMP9 dysregulation was found in canine oronasal tumors, hemangiosarcomas, and meningiomas, not always in association with malignant morphological patterns (143).
Integrin Signaling in MDCK Cells and Canine Cancers
Integrin subunits may combine each other to affect the characteristics of cancer cells and the progression of tumors, both binding with proteins that directly regulate the actin cytoskeleton of cells and by phosphorylating the relative kinases, including FAKs (162). It is well-known that integrin complexes bind ECM components to promote cell adhesion and invasion, also mediating tissue tropism (163, 164). MDCK cells were used to demonstrate that α2β1 integrin mediates adhesion to types I and IV collagen in an Mg2+-dependent manner, thus improving cell survival, EMT, cell spreading, and brunching morphogenesis. Furthermore, overexpression of Galectin8, which activates selective β1-integrins involved in EMT, promotes oncogenic-like transformation of MDCK cells (134, 154, 165, 166).
In veterinary oncology, a deregulation of integrin pathway, together with Wnt and chemokine/cytokine signaling, has been found in relation to short survival in canine OSA (167). The expressions of β1 integrin and α5β1 complex were immunohistochemically evaluated in a series of normal, dysplastic, and neoplastic canine mammary glands, and in lymph node metastases (168, 169), while β2 integrin was found in canine cutaneous histiocytoma (170). Finally, canine hemangiosarcoma cell lines expressing several endothelial mediators including VEGF and αvβ3 integrin recapitulate features of mitotically activated endothelia and stimulate robust angiogenic responses in mice, forming tumor masses composed of aberrant vascular channels. Furthermore, they showed anchorage-independent growth and were motile and invasive, forming vessel-like structures when cultured on a basement membrane matrix (171, 172).
EphA2 Inhibition in Canine Tumor Therapy and Its Mechanisms of Action
Targeting EphA2 represents an important goal in the development of recent anti-cancer drugs also in veterinary medicine, as shown by the attempt to evaluate the mechanism of Desanitib in the treatment of canine histiocytic sarcoma and the development of a cytotoxic compound that targets EphA2, EphA3, EphAB2, and interleukin 31 receptor A2 (IL31RA2) in canine high-grade gliomas (173, 174). The inhibition of EphA2 and IL31RA activity reduced up to 94% of tumor volume in 50% of dogs in the cohort (175). Furthermore, dogs were used to test the performance of a nanotherapeutic encapsulating a hydrolytically sensitive Docetaxel prodrug and conjugated to an antibody specific for EphA2, demonstrating an improvement in tumor penetration and antitumor activity (174). In ex vivo specimens, EphA2 resulted to be highly overexpressed in neoplastic cells of canine appendicular OSA, together with EphA3 (176). In vitro, ephA2 expression was increased by up to 60-fold in canine prostate carcinoma lines derived from lung or bone metastases (177). MDCK cells were used to demonstrate the role of EphA2 in the epithelial morphogenesis in 3D culture and in the apical extrusion of transformed epithelial cells as a protective event. MDCK cells were also used to investigate EphA2 role in the decreased integration of claudin4 into sites of cell–cell contact as tumorigenic trigger and in the anoikis resistance process (178–181).
mTOR and RhoA/ROCK Pathways
DEP domain-containing mTOR-interacting protein (DEPTOR) is an important modulator of mTOR, a kinase at the center of two important protein complexes named mTORC1 and mTORC2 (182). DEPTOR is able to interact with mTOR, thus inhibiting its kinase activity. It is involved in several molecular pathways controlling cellular homeostasis and it can behave either as an oncogene or oncosuppressor, depending on the cell or tissue type (183). It has been demonstrated that DEPTOR knockdown significantly decreased the number of tube-like structures and the invasion ability of the methylnitronitrosoguanidine transformed human OSA cells (MNNG/HOS) (184).
RhoA/ROCK pathway is a versatile regulator of multiple cellular processes, and it is dysregulated in several cancers. Recently, ROCK has attracted attention for its crucial role in angiogenesis, in regulating permeability, migration, proliferation, and tubulogenesis of endothelial cells (185). RhoA/ROCK stabilizes HIF1α during hypoxia inducing VM in hepatocellular carcinoma (186). Moreover, RhoA/ROCK expression was found to be higher in human OSA tissues and in the human OSA cell line U2OS with respect to control. Inhibition of RhoA/ROCK signaling pathway by the pharmacological inhibitor Fasudil reduced vascular-like channels in U2OS and melanoma cells cultured on Matrigel, decreasing cell plasticity and motility, both of which play key roles in VM formation (187, 188).
Role of mTOR Pathway in Canine MDCK Cells and Cancers
mTOR pathway belongs to the series of conserved pathways that impact upon longevity and aging-related diseases such as cancer (189). Phosphatidyl inositol 3-kinase (PI3K)-AKT-mTOR was identified as one of the most relevant pathways involved in OSA progression both in humans and canines (190). The screening of protein kinase inhibitor compounds, particularly against PI3K-AKT-mTOR activity, represents an important topic of canine OSA therapy (191–193). Although the effect of the aberrant PI3K-AKT-mTOR signaling on tumor cell proliferation and apoptosis is well-known in canine OSA, the relation between mTOR and migration, invasion, and angiogenesis properties has been better explored in other types of canine cancer including hemangiosarcoma (194), prostate cancer (195), mammary tumors (196, 197), melanoma (198), and mast cell tumors (199).
Of relevance, MDCK cell model was used to demonstrate that mTOR signaling plays important roles in the regulation of epithelial tubule formation on Matrigel. It was observed that PI3-kinase regulates early epithelial remodeling stages, while mTOR modulates latter stages of tubule development (200), suggesting a possible involvement of mTOR pathway in VM progression. To the best of our knowledge, there are no studies investigating mTOR modulation mediated by the DEPTOR domain in dog.
RhoA/ROCK in Canine MDCK Cells
Considering that cell migration plays crucial roles in cancer cell invasion, the study of mechanisms of junction and cytoskeletal organization mediated by guanosine triphosphatases (GTPases) of the Rho family has acquired great importance (201, 202). RhoA/ROCK pathway has been widely investigated in MDCK cells as a model of cell migration, cell-cell interaction and adhesion, EMT promotion, and virus entry (201–204).
In Moloney sarcoma virus-(MDCK)-invasive (MSV-MDCK-INV) variant tumor cells, it has been observed that Rho/ROCK activation may affect tumor cell migration and metastasis by stimulating the pseudopodal translocation of mRNAs and thereby regulating the expression of local signaling tumorigenic cascades (205, 206). RhoA hyperactivation can also influence normal MDCK cell polarity (Yu et al., 2008). The inhibition of RhoA pathway leads to a decrease of anchorage-independent growth of MDCK cells in vitro and in syngeneic mice, also downregulating Cox2gene (207, 208).
LncRNAs
Non-coding RNAs, especially miRNAs and lncRNAs, have been widely investigated due to their roles as key players in regulating various biological and pathological processes involved in OSA progression, including cancer cell migration, invasion, angiogenesis, and metastasis (209, 210). LncRNAs are non-coding transcripts >than 200 bp in length, and different studies demonstrated the influence of these molecules in gene expression at the epigenetic, transcriptional, and post-transcriptional levels. One of the most classical mechanisms through which lncRNAs regulate gene expression involves their association with chromatin modeling complexes and transcription factors, influencing transcriptional repression and activation of gene promoters (211).
Ren et al. profiled the expression of lncRNAs in highly aggressive OSA cell line 143B in comparison with its parental poorly aggressive cell line HOS, both plated on Matrigel. The top five upregulated lncRNAs were n337322, n333984, n381586, n338209, and TCONS_l2_00028738-XLOC_l2_014777, while the five downregulated lncRNAs were n334144, n342556,n410003, n335665, and ENST00000442174, also indicating that the top-ranked hub lncRNA that had the highest connections with the majority of the others in the network was n340532 (67). Through VM assay, this study also showed that VM ability of 143B cells strongly decreased following n340532 knockdown, as well as the number of metastatic nodules after injection of 143B cells stably transfected with sh-n340532 into nude mice. Tumor tissues collected from the sh-n340532 group exhibited a decreased number of VM channels compared to the control group (67). FTX and MALAT1 were also strongly upregulated in this study. As far as FTX is concerned, its involvement in migration and metastasis was also previously demonstrated, as well as the induction of VM by MALAT1 (16, 212).
Among others, lncRNA AFAP1-AS1 was found to be aberrantly expressed in OSA together with HOTAIR, HULC, and H19 that were upregulated in human OSA tissues and cell lines. Shi et al. also performed an in-depth investigation to explore the role and the mechanism of AFAP1-AS1 in OSA progression, demonstrating that the stable transfection of different OSA cell lines with siRNA AFAP1-AS1 strongly reduced their ability to form tube-like structures in vitro. In the same work, a concomitant decrease of EMT and RhoC/ROCK1/p38MAPK/Twist1 signaling pathway was also observed (213).
Moreover, differences between non-VM and VM cells compared in a microarray highlighted the significant overexpression of the lncRNAs LINC00265 and LINC00342 in the VMOSA cell line with respect to control. The study also confirmed that both LINC00265 and LINC00342 were upregulated in OSA tissues and that the high expression of LINC00265 was positively correlated with Spermine N1-Acetyltransferase 1 (Sat1) and Vav Guanine Nucleotide Exchange Factor 3 (Vav3) gene expression, as well as with poor prognosis. LINC00265 was also demonstrated to promote proliferation, migration, invasion, and tube formation via miR3825p targeting Sat1 and Vav3 genes in OSA cells cultured on Matrigel. SAT1 is a polyamine acetyltransferase that has a controversial role among different tumors, although it has been demonstrated to promote proliferation and metastasis of OSA cells both in vitro and in vivo (214). VAV3 is an important factor regulating angiogenesis and regulates the Rho/Rac family of GTPases involved in cell growth and motility (214).
LncRNA in Dogs
Among the multiple epigenetic mechanisms found in canine cancer, DNA methylation and histone modification have been identified on the basis of OSA progression (211). Le Beguec et al. characterized the expression profiles of 10.444 canine lncRNAs in 26 distinct tissue types. Their study showed that lncRNA expression is mainly clustered by tissue type, highlighting that 44% of canine lncRNAs are expressed in a tissue-specific manner and also identifying more than 900 conserved dog-human lncRNAs (215). An alignment-free program that accurately annotates lncRNAs (FEELnc) was used on a real data set of 20 RNA-Seq from 16 different canine tissues, produced by the European LUPA consortium to expand the canine genome annotation, including 10.374 novel lncRNAs and 58.640 mRNAs transcripts (216). This work allowed identifying three new cancer susceptibility candidate lncRNAs in dogs, which are well-described in human cancer, including MALAT1, that is associated with human VM and metastasis (16, 217). Other studies observed more than 900 dog-human conserved lncRNAs using comparative genomics, confirming the presence of well-studied lncRNAs in dogs, such as HOTAIR and MALAT1 in canine B cell lymphoma and identifying lncRNAs differential expression as a prognostic tool (218–220). Of relevance, 417 differentially expressed lncRNAs were identified in canine oral melanomas in comparison with control samples, including the well-studied lncRNA ZEB2-AS, a lncRNA involved in the regulation of the transcription factor Zinc Finger E-Box Binding Homeobox 2 (Zeb2) during EMT in human colon, pancreatic, and breast cancer cell lines, as well as SOX21 Antisense Divergent Transcript 1(Sox21-as1) and Cancer Susceptibility 15(Casc15) (211, 221, 222). Finally, long non-coding transcripts from telomeres, called telomeric repeat-containing RNA (TERRA), were identified as blocking telomerase activity in canine tumor cell lines originated from soft tissue sarcomas (223). MDCK cells were also tested for the presence of tumorigenic lncRNAs, with the aim of preparing a safer and more reliable non-neoplastic MDCK cell line for vaccine production, founding several tumor-associated lncRNAs (224). Furthermore, a highly upregulated lncRNA in liver cancer was demonstrated to be a promoter during the epithelial and smooth-muscle-like differentiation of adipose-derived stem cells (ADSCs) via the bone morphogenetic protein 9(BMP9)/Wnt/β-catenin/Notch network (225). Genome-wide association studies (GWAS) identified a set of variants within the intron of a lncRNA upstream of the adrenoceptor beta 1(Adrb1) gene which is strongly associated with coat color. Two variants were found at high frequency in single-coated dogs and are rare in wolves (226).
Therapeutic Potential and Current Limitations
Both western and traditional Chinese medicines were used to evaluate a potential VM inhibition. Current anti-angiogenic drugs are often useless in the dampening of VM, inhibiting directly endothelial cell proliferation. At the same time, the consequent vascular density decrease can cause hypoxia in the tissue triggering VM as a compensatory stimulus (2). The combination of drugs targeting VM and classical tumor angiogenesis can definitively reduce the blood and nutrient supply of tumors (227). Furthermore, in the era of chimeric antigen receptor (CAR)-T cell therapy, it is increasingly urgent to find specific markers for cancer management, and VM can represent an opportunity to find a cancer selective therapeutic target. In fact, in the VM process, multipotent tumor cells with CSC-like phenotype can transdifferentiate, generating ECM-rich, CD31-negative, and PAS-positive vascular networks, but CD31+, PAS-negative tubular-like structures have also been observed (6, 59). This evidence demonstrates that the mechanism of endothelial transdifferentiation of cancer cells within the tumor is still unclear, and this issue complicates the identification of specific cancer biomarkers. Recently, increasingly advanced in vitro models have been developed for the deeper investigation of this relative new process.
Conclusion and Perspectives
A growing body of evidence indicates that VM plays fundamental roles in tumor invasion, metastasis, and poor prognosis in human patients with malignant tumors, including OSA. Thus, VM may represent a potential novel target of anti-tumor therapy, even though the cellular mechanisms and molecular pathways by which VM is promoted have not been fully clarified. Endothelial mediators have been especially explored in human OSA and in veterinary oncology, together with the presence of CSC markers and the pathways involved in ECM interaction and cell adhesion. The molecular pathways involving VEGF/VEGFR and integrins have been found to be related to VM and vessel-like formation in vitro in canine oncology, while CD133 resulted to be determinant for tubular-like structure formation in vitro of canine normal cells (Supplementary Table 1). Information concerning the VM process and its biological implications in cancer is still limited in veterinary literature, despite the importance of canine tumor models in comparative oncology. The current knowledge concerning VM findings in human OSA, summarized in the present review, may provide a basis for stimulating future studies investigating VM in canine oncology as a possible target with great promise in cancer therapy.
Author Contributions
MM, MR, and LDS conceived and designed the review. MM and MR wrote the review. LDS and RDM supervised and guided the entire project. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2021.722432/full#supplementary-material
References
1. Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LMG, Pe'er J, et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. (1999) 155:739–52. doi: 10.1016/S0002-9440(10)65173-5
2. Qiao L, Liang N, Zhang J, Xie J, Liu F, Xu D, et al. Advanced research on vasculogenic mimicry in cancer. J Cell Mol Med. (2015) 19:315–26. doi: 10.1111/jcmm.12496
3. Luo Q, Wang J, Zhao W, Peng Z, Liu X, Li B, et al. Vasculogenic mimicry in carcinogenesis and clinical applications. J Hematol Oncol. (2020) 13:19. doi: 10.1186/s13045-020-00858-6
4. Liu R, Yang K, Meng C, Zhang Z, Xu Y. Vasculogenic mimicry is a marker of poor prognosis in prostate cancer. Cancer Biol Ther. (2012) 13:527–33. doi: 10.4161/cbt.19602
5. Yang JP, Liao YD, Mai DM, Xie P, Qiang YY, Zheng LS, et al. Tumor vasculogenic mimicry predicts poor prognosis in cancer patients: a meta-analysis. Angiogenesis. (2016) 19:191–200. doi: 10.1007/s10456-016-9500-2
6. Ge H, Luo H. Overview of advances in vasculogenic mimicry – a potential target for tumor therapy. Cancer Manag Res. (2018) 10:2429–37. doi: 10.2147/CMAR.S164675
7. Clemente M, Pérez-Alenza MD, Illera JC, Peña L. Histological, immunohistological, and ultrastructural description of vasculogenic mimicry in canine mammary cancer. Vet Pathol. (2010) 47:265–74. doi: 10.1177/0300985809353167
8. Nordio L, Fattori S, Vascellari M, Giudice C. Evidence of vasculogenic mimicry in a palpebral melanocytoma in a dog. J Comp Pathol. (2018) 162:43–6. doi: 10.1016/j.jcpa.2018.06.003
9. Rasotto R, Zappulli V, Castagnaro M, Goldschmidt MH. A retrospective study of those histopathologic parameters predictive of invasion of the lymphatic system by canine mammary carcinomas. Vet Pathol. (2012) 49:330–40. doi: 10.1177/0300985811409253
10. de Faria Lainetti P, Brandi A, Leis Filho AF, Prado MCM, Kobayashi PE, Laufer-Amorim R, et al. Establishment and characterization of canine mammary gland carcinoma cell lines with vasculogenic mimicry ability in vitro and in vivo. Front Vet Sci. (2020) 7:583874. doi: 10.3389/fvets.2020.583874
11. Prado MCM, Macedo S de AL, Guiraldelli GG, de Faria Lainetti P, Leis-Filho AF, Kobayashi PE, et al. Investigation of the prognostic significance of vasculogenic mimicry and its inhibition by sorafenib in canine mammary gland tumors. Front Oncol. (2019) 9:1445. doi: 10.3389/fonc.2019.01445
12. Caceres S, Peña L, DeAndres PJ, Illera MJ, Lopez MS, Woodward WA, et al. Establishment and characterization of a new cell line of canine inflammatory mammary cancer: IPC-366. PLoS ONE. (2015) 10:e0122277. doi: 10.1371/journal.pone.0122277
13. Barreno L, Cáceres S, Alonso-Diez Á, Vicente-Montanã A, Garciá ML, Clemente M, et al. Vasculogenic mimicry-associated ultrastructural findings in human and canine inflammatory breast cancer cell lines. BMC Cancer. (2019) 19:750. doi: 10.1186/s12885-019-5955-z
14. Massimini M, De Maria R, Malatesta D, Romanucci M, D'Anselmo A, Della Salda L. Establishment of three-dimensional canine osteosarcoma cell lines showing vasculogenic mimicry and evaluation of biological properties after treatment with 17-AAG. Vet Comp Oncol. (2019) 17:376–84. doi: 10.1111/vco.12482
15. Fernández-Cortés M, Delgado-Bellido D, Javier Oliver F. Vasculogenic mimicry: become an endothelial cell “but not so much.” Front Oncol. (2019) 9:803. doi: 10.3389/fonc.2019.00803
16. Hernández de la Cruz ON, López-González JS, García-Vázquez R, Salinas-Vera YM, Muñiz-Lino MA, Aguilar-Cazares D, et al. Regulation networks driving vasculogenic mimicry in solid tumors. Front Oncol. (2020) 9:1419. doi: 10.3389/fonc.2019.01419
17. Wei X, Chen Y, Jiang X, Peng M, Liu Y, Mo Y, et al. Mechanisms of vasculogenic mimicry in hypoxic tumor microenvironments. Mol Cancer. (2021) 20:7. doi: 10.1186/s12943-020-01288-1
18. Zhang X, Zhang J, Zhou H, Fan G, Li Q. Molecular mechanisms and anticancer therapeutic strategies in vasculogenic mimicry. J Cancer. (2019) 10:6327–40. doi: 10.7150/jca.34171
19. Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. (2010) 468:829–35. doi: 10.1038/nature09624
20. Barzegar Behrooz A, Syahir A, Ahmad S. CD133: beyond a cancer stem cell biomarker. J Drug Target. (2019) 27:257–69. doi: 10.1080/1061186X.2018.1479756
21. Czarnecka AM, Synoradzki K, Firlej W, Bartnik E, Sobczuk P, Fiedorowicz M, et al. Molecular biology of osteosarcoma. Cancers. (2020) 12:1–27. doi: 10.3390/cancers12082130
22. Sun H, Yao N, Cheng S, Li L, Liu S, Yang Z, et al. Cancer stem-like cells directly participate in vasculogenic mimicry channels in triple-negative breast cancer. Cancer Biol Med. (2019) 16:299–311. doi: 10.20892/j.issn.2095-3941.2018.0209
23. Lizárraga-Verdugo E, Avendaño-Félix M, Bermúdez M, Ramos-Payán R, Pérez-Plasencia C, Aguilar-Medina M. Cancer stem cells and its role in angiogenesis and vasculogenic mimicry in gastrointestinal cancers. Front Oncol. (2020) 10:413. doi: 10.3389/fonc.2020.00413
24. Schömig K, Busch G, Steppich B, Sepp D, Kaufmann J, Stein A, et al. Interleukin-8 is associated with circulating CD133+ progenitor cells in acute myocardial infarction. Eur Heart J. (2006) 27:1032–7. doi: 10.1093/eurheartj/ehi761
25. Jászai J, Thamm K, Karbanová XJ, Janich P, Fargeas CA, Huttner WB, et al. Prominins control ciliary length throughout the animal kingdom: new lessons from human prominin-1 and zebrafish prominin-3. J Biol Chem. (2020) 295:6007–22. doi: 10.1074/jbc.RA119.011253
26. Tomita H, Tanaka K, Tanaka T, Hara A. Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget. (2016) 7:11018–32. doi: 10.18632/oncotarget.6920
27. Li W, Ma H, Zhang J, Zhu L, Wang C, Yang Y. Unraveling the roles of CD44/CD24 and ALDH1 as cancer stem cell markers in tumorigenesis and metastasis. Sci Rep. (2017) 7:13856. doi: 10.1038/s41598-017-14364-2
28. Cheng M, Duan PG, Gao ZZ, Dai M. MicroRNA-487b-3p inhibits osteosarcoma chemoresistance and metastasis by targeting ALDH1A3. Oncol Rep. (2020) 44:2691–700. doi: 10.3892/or.2020.7814
29. Wang X, Li C, Yao W, Tian Z, Liu Z, Ge H. MicroRNA-761 suppresses tumor progression in osteosarcoma via negatively regulating ALDH1B1. Life Sci. (2020) 262:118544. doi: 10.1016/j.lfs.2020.118544
30. Bao Z, Cheng Z, Chai D. The expressions of CD133, ALDH1, and vasculogenic mimicry in osteosarcoma and their clinical significance. Int J Clin Exp Pathol. (2018) 11:3656–63.
31. Schnegg CI, Yang MH, Ghosh SK, Hsu MY. Induction of vasculogenic mimicry overrides VEGF-A silencing and enriches stem-like cancer cells in melanoma. Cancer Res. (2015) 75:1682–90. doi: 10.1158/0008-5472.CAN-14-1855
32. Thamm K, Graupner S, Werner C, Huttner WB, Corbeil D. Monoclonal antibodies13A4 and AC133 do not recognize the canine ortholog of mouse and human stem cell antigen prominin-1 (CD133). PLoS ONE. (2016) 11:e0164079. doi: 10.1371/journal.pone.0164079
33. Chen TC, Neupane M, Chien SJ, Chuang FR, Crawford RB, Kaminski NE, et al. Characterization of adult canine kidney epithelial stem cells that give rise to dome-forming tubular cells. Stem Cells Dev. (2019) 28:1424–33. doi: 10.1089/scd.2019.0049
34. Chen C, Dai P, Nan L, Lu R, Wang X, Tian Y, et al. Isolation and characterization of endothelial progenitor cells from canine bone marrow. Biotech Histochem. (2020) 96:85–93. doi: 10.1080/10520295.2020.1762001
35. Kasimanickam V, Kasimanickam R. A method to isolate cd34+ mononuclear cells from canine peripheral blood. Curr Protoc Stem Cell Biol. (2019) 49:e84. doi: 10.1002/cpsc.84
36. Liu W, Moulay M, Willenbrock S, Roolf C, Junghanss C, Ngenazahayo A, et al. Comparative characterization of stem cell marker expression, metabolic activity and resistance to doxorubicin in adherent and spheroid cells derived from the canine prostate adenocarcinoma cell line CT1258. Anticancer Res. (2015) 35:1917–27.
37. Blacking TM, Waterfall M, Samuel K, Argyle DJ. Flow cytometric techniques for detection of candidate cancer stem cell subpopulations in canine tumour models. Vet Comp Oncol. (2012) 10:252–73. doi: 10.1111/j.1476-5829.2011.00293.x
38. Deguchi T, Hosoya K, Murase Y, Koangyong S, Kim S, Okumura M. Analysis of radiosensitivity of cancer stem-like cells derived from canine cancer cell lines. Vet Comp Oncol. (2019) 17:119–29. doi: 10.1111/vco.12452
39. Ito D, Childress M, Mason N, Winter A, O'Brien T, Henson M, et al. A double blinded, placebo-controlled pilot study to examine reduction of CD34+/CD117+/CD133+ lymphoma progenitor cells and duration of remission induced by neoadjuvant valspodar in dogs with large B-cell lymphoma. F1000Research. (2017) 4:42. doi: 10.12688/f1000research.6055.2
40. Lamerato-Kozicki AR, Helm KM, Jubala CM, Cutter GC, Modiano JF. Canine hemangiosarcoma originates from hematopoietic precursors with potential for endothelial differentiation. Exp Hematol. (2006) 34:870–8. doi: 10.1016/j.exphem.2006.04.013
41. Fernández F, Deviers A, Dally C, Mogicato G, Delverdier M, Cauzinille L, et al. Presence of neural progenitors in spontaneous canine gliomas: a histopathological and immunohistochemical study of 20 cases. Vet J. (2016) 209:125–32. doi: 10.1016/j.tvjl.2015.10.039
42. Fujimoto A, Neo S, Ishizuka C, Kato T, Segawa K, Kawarai S, et al. Identification of cell surface antigen expression in canine hepatocellular carcinoma cell lines. J Vet Med Sci. (2013) 75:831–5. doi: 10.1292/jvms.12-0549
43. Guth AM, Deogracias M, Dow SW. Comparison of cancer stem cell antigen expression by tumor cell lines and by tumor biopsies from dogs with melanoma and osteosarcoma. Vet Immunol Immunopathol. (2014) 161:132–40. doi: 10.1016/j.vetimm.2014.07.006
44. Ito D, Endicott MM, Jubala CM, Helm KM, Burnett RC, Husbands BD, et al. A tumor-related lymphoid progenitor population supports hierarchical tumor organization in canine B-cell lymphoma. J Vet Intern Med. (2011) 25:890–6. doi: 10.1111/j.1939-1676.2011.0756.x
45. Suzuki S, Uchida K, Harada T, Nibe K, Yamashita M, Ono K, et al. The origin and role of autophagy in the formation of cytoplasmic granules in canine lingual granular cell tumors. Vet Pathol. (2015) 52:456–64. doi: 10.1177/0300985814546051
46. Capodanno Y, Buishand FO, Pang LY, Kirpensteijn J, Mol JA, Argyle DJ. Notch pathway inhibition targets chemoresistant insulinoma cancer stem cells. Endocr Relat Cancer. (2018) 25:131–44. doi: 10.1530/ERC-17-0415
47. Michishita M, Ezaki S, Ogihara K, Naya Y, Azakami D, Nakagawa T, et al. Identification of tumor-initiating cells in a canine hepatocellular carcinoma cell line. Res Vet Sci. (2014) 96:315–22. doi: 10.1016/j.rvsc.2014.01.004
48. Tanabe A, Deguchi T, Sato T, Nemoto Y, Maruo T, Madarame H, et al. Radioresistance of cancer stem-like cell derived from canine tumours. Vet Comp Oncol. (2016) 14:e93–e101. doi: 10.1111/vco.12110
49. Elshafae SM, Kohart NA, Altstadt LA, Dirksen WP, Rosol TJ. The effect of a histone deacetylase inhibitor (AR-42) on canine prostate cancer growth and metastasis. Prostate. (2017) 77:776–93. doi: 10.1002/pros.23318
50. Gorden BH, Kim JH, Sarver AL, Frantz AM, Breen M, Lindblad-Toh K, et al. Identification of three molecular and functional subtypes in canine hemangiosarcoma through gene expression profiling and progenitor cell characterization. Am J Pathol. (2014) 184:985–95. doi: 10.1016/j.ajpath.2013.12.025
51. Gatti M, Solari A, Pattarozzi A, Campanella C, Thellung S, Maniscalco L, et al. In vitro and in vivo characterization of stem-like cells from canine osteosarcoma and assessment of drug sensitivity. Exp Cell Res. (2018) 363:48–64. doi: 10.1016/j.yexcr.2018.01.002
52. Michishita M, Akiyoshi R, Yoshimura H, Katsumoto T, Ichikawa H, Ohkusu-Tsukada K, et al. Characterization of spheres derived from canine mammary gland adenocarcinoma cell lines. Res Vet Sci. (2011) 91:254–60. doi: 10.1016/j.rvsc.2010.11.016
53. Michishita M, Akiyoshi R, Suemizu H, Nakagawa T, Sasaki N, Takemitsu H, et al. Aldehyde dehydrogenase activity in cancer stem cells from canine mammary carcinoma cell lines. Vet J. (2012) 193:508–13. doi: 10.1016/j.tvjl.2012.01.006
54. Wilson-Robles HM, Daly M, Pfent C, Sheppard S. Identification and evaluation of putative tumour-initiating cells in canine malignant melanoma cell lines. Vet Comp Oncol. (2015) 13:60–9. doi: 10.1111/vco.12019
55. Wilke VL, Nettleton D, Wymore MJ, Gallup JM, Demirkale CY, Ackermann MR, et al. Gene expression in intestinal mucosal biopsy specimens obtained from dogs with chronic enteropathy. Am J Vet Res. (2012) 73:1219–29. doi: 10.2460/ajvr.73.8.1219
56. Yang K, Adin C, Shen Q, Lee LJ, Yu L, Fadda P, et al. Aldehyde dehydrogenase 1 a1 regulates energy metabolism in adipocytes from different species. Xenotransplantation. (2017) 24:e12318. doi: 10.1111/xen.12318
57. Kasimanickam VR, Kasimanickam RK. Retinoic acid signaling biomarkers after treatment with retinoic acid and retinoic acid receptor alpha antagonist (Ro 41-5253) in canine testis: an in vitro organ culture study. Theriogenology. (2013) 79:10–16. doi: 10.1016/j.theriogenology.2012.09.001
58. Bittner M, Meltzer P, Chen Y, Jiang Y, Seftor E, Hendrix M, et al. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. (2000) 406:536–40. doi: 10.1038/35020115
59. Fazioli F, Colella G, Miceli R, Di Salvatore MG, Gallo M, Boccella S, et al. Post-surgery fluids promote transition of cancer stem cellto- endothelial and AKT/mTOR activity, contributing to relapse of giant cell tumors of bone. Oncotarget. (2017) 8:85040–53. doi: 10.18632/oncotarget.18783
60. Hendrix MJC, Seftor EA, Meltzer PS, Gardner LMG, Hess AR, Kirschmann DA, et al. Expression and functional significance of VE-cadherin in aggressive human melanoma cells: role in vasculogenic mimicry. Proc Natl Acad Sci USA. (2001) 98:8018–23. doi: 10.1073/pnas.131209798
61. Laporta CA, De Biasi S, Rao S, Fascio U, Mihm MC, Clemente C. Molecular mechanism of vasculogenic mimicry. Cancer Res. (2004) 64.
62. Folberg R, Maniotis AJ. Vasculogenic mimicry. APMIS. (2004) 112:508–25. doi: 10.1111/j.1600-0463.2004.apm11207-0810.x
63. Liu H, Gao M, Gu J, Wan X, Wang H, Gu Q, et al. VEGFR1-targeted contrast-enhanced ultrasound imaging quantification of vasculogenic mimicry microcirculation in a mouse model of choroidal melanoma. Transl Vis Sci Technol. (2020) 9:4. doi: 10.1167/tvst.9.3.4
64. Zhang LZ, Mei J, Qian ZK, Cai XS, Jiang Y, Huang W. The role of VE-cadherin in osteosarcoma cells. Pathol Oncol Res. (2010) 16:111–7. doi: 10.1007/s12253-009-9198-1
65. Mei J, Gao Y, Zhang L, Cai X, Qian Z, Huang H, et al. VEGF-siRNA silencing induces apoptosis, inhibits proliferation and suppresses vasculogenic mimicry in osteosarcoma in vitro. Exp Oncol. (2008) 30:29–34.
66. Corre I, Verrecchia F, Crenn V, Redini F, Trichet V. The osteosarcoma microenvironment: a complex but targetable ecosystem. Cells. (2020) 9:976. doi: 10.3390/cells9040976
67. Ren K, Ni Y, Li X, Wang C, Chang Q, Li Y, et al. Expression profiling of long noncoding RNAs associated with vasculogenic mimicry in osteosarcoma. J Cell Biochem. (2019) 120:12473–88. doi: 10.1002/jcb.28514
68. Yao N, Ren K, Gu XJ, Wu SJ, Shi X, Chang Q, et al. Identification of potential crucial genes associated with vasculogenic mimicry in human osteosarcoma based on gene expression profile. Neoplasma. (2020) 67:286–95. doi: 10.4149/neo_2019_190414N329
69. Heimark RL, Degner M, Schwartz SM. Identification of a Ca2+-dependent cell-cell adhesion molecule in endothelial cells. J Cell Biol. (1990) 110:1745–56. doi: 10.1083/jcb.110.5.1745
70. Suda K, Rothen-Rutishauser B, Günthert M, Wunderli-Allenspach H. Phenotypic characterization of human umbilical vein endothelial (ECV304) and urinary carcinoma (T24) cells: endothelial versus epithelial features. Vitr Cell Dev Biol Anim. (2001) 37:505–14. doi: 10.1290/1071-2690(2001)037<0505:PCOHUV>2.0.CO;2
71. You X, Liu Q, Wu J, Wang Y, Dai J, Chen D, et al. Galectin-1 promotes vasculogenic mimicry in gastric cancer by upregulating EMT signaling. J Cancer. (2019) 10:6286–97. doi: 10.7150/jca.33765
72. Lu CC, Liu MM, Clinton M, Culshaw G, Argyle DJ, Corcoran BM. Developmental pathways and endothelial to mesenchymal transition in canine myxomatous mitral valve disease. Vet J. (2015) 206:377–84. doi: 10.1016/j.tvjl.2015.08.011
73. Izumi Y, Aoshima K, Hoshino Y, Takagi S. Effects of combretastatin A-4 phosphate on canine normal and tumor tissue-derived endothelial cells. Res Vet Sci. (2017) 112:222–8. doi: 10.1016/j.rvsc.2017.05.017
74. Izumi Y, Takagi S. Vascular disrupting effect of combretastatin A-4 phosphate with inhibition of vascular endothelial cadherin in canine osteosarcoma-xenografted mice. Res Vet Sci. (2019) 122:1–6. doi: 10.1016/j.rvsc.2018.10.017
75. Nam AR, Lee KH, Hwang HJ, Schabort JJ, An JH, Won SH, et al. Alternative methylation of intron motifs is associated with cancer-related gene expression in both canine mammary tumor and human breast cancer. Clin Epigenetics. (2020) 12:110. doi: 10.1186/s13148-020-00888-4
76. Tanabe A, Kobayashi D, Maeda K, Taguchi M, Sahara H. Angiogenesis-related gene expression profile in clinical cases of canine cancer. Vet Med Sci. (2019) 5:19–29. doi: 10.1002/vms3.127
77. Gola C, Iussich S, Noury S, Martano M, Gattino F, Morello E, et al. Clinical significance and in vitro cellular regulation of hypoxia mimicry on HIF-1α and downstream genes in canine appendicular osteosarcoma. Vet J. (2020) 264:105538. doi: 10.1016/j.tvjl.2020.105538
78. Fossey SL, Bear MD, Kisseberth WC, Pennell M, London CA. Oncostatin M promotes STAT3 activation, VEGF production, and invasion in osteosarcoma cell lines. BMC Cancer. (2011) 11:125. doi: 10.1186/1471-2407-11-125
79. Wergin MC, Kazer-Hotz B. Plasma vascular endothelial growth factor (VEGF) measured in seventy dogs with spontaneously occuring tumours. In Vivo. (2004) 18:15–20.
80. Camacho L, Peña L, Gil AG, Martín-Ruiz A, Dunner S, Illera JC. Immunohistochemical vascular factor expression in canine inflammatory mammary carcinoma. Vet Pathol. (2014) 51:737–48. doi: 10.1177/0300985813503568
81. Raposo TP, Pires I, Prada J, Queiroga FL, Argyle DJ. Exploring new biomarkers in the tumour microenvironment of canine inflammatory mammary tumours. Vet Comp Oncol. (2017) 15:655–66. doi: 10.1111/vco.12209
82. Clemente M, Sánchez-Archidona AR, Sardón D, Díez L, Martín-Ruiz A, Caceres S, et al. Different role of COX-2 and angiogenesis in canine inflammatory and non-inflammatory mammary cancer. Vet J. (2013) 197:427–32. doi: 10.1016/j.tvjl.2013.02.009
83. Queiroga FL, Pires I, Parente M, Gregório H, Lopes CS. COX-2 over-expression correlates with VEGF and tumour angiogenesis in canine mammary cancer. Vet J. (2011) 189:77–82. doi: 10.1016/j.tvjl.2010.06.022
84. Cam M, Gardner HL, Roberts RD, Fenger JM, Guttridge DC, London CA, et al. ΔNp63 mediates cellular survival and metastasis in canine osteosarcoma. Oncotarget. (2016) 7:48533–46. doi: 10.18632/oncotarget.10406
85. Lopez CM, Yu PY, Zhang X, Yilmaz AS, London CA, Fenger JM. MiR-34a regulates the invasive capacity of canine osteosarcoma cell lines. PLoS ONE. (2018) 13:e01900086 doi: 10.1371/journal.pone.0190086
86. Thamm DH, O'Brien MG, Vail DM. Serum vascular endothelial growth factor concentrations and postsurgical outcome in dogs with osteosarcoma. Vet Comp Oncol. (2008) 6:126–32. doi: 10.1111/j.1476-5829.2007.00153.x
87. Laver T, London CA, Vail DM, Biller BJ, Coy J, Thamm DH. Prospective evaluation of toceranib phosphate in metastatic canine osteosarcoma. Vet Comp Oncol. (2018) 16:E23–9. doi: 10.1111/vco.12328
88. Bernabe LF, Portela R, Nguyen S, Kisseberth WC, Pennell M, Yancey MF, et al. Evaluation of the adverse event profile and pharmacodynamics of toceranib phosphate administered to dogs with solid tumors at doses below the maximum tolerated dose. BMC Vet Res. (2013) 9:190. doi: 10.1186/1746-6148-9-190
89. Mantovani FB, Morrison JA, Mutsaers AJ. Effects of epidermal growth factor receptor kinase inhibition on radiation response in canine osteosarcoma cells. BMC Vet Res. (2016) 12:82. doi: 10.1186/s12917-016-0707-7
90. Fahey CE, Milner RJ, Kow K, Bacon NJ, Salute ME. Apoptotic effects of the tyrosine kinase inhibitor, masitinib mesylate, on canine osteosarcoma cells. Anticancer Drugs. (2013) 24:519–26. doi: 10.1097/CAD.0b013e32836002ba
91. Lutty GA, Merges C, Grebe R, Prow T, McLeod DS. Canine retinal angioblasts are multipotent. Exp Eye Res. (2006) 83:183–93. doi: 10.1016/j.exer.2005.09.025
92. Kim N, Choi KU, Lee E, Lee S, Oh J, Kim WK, et al. Therapeutic effects of platelet derived growth factor overexpressed-mesenchymal stromal cells and sheets in canine skin wound healing model. Histol Histopathol. (2020) 35:751–67. doi: 10.14670/HH-18-196
93. Higgins RJ, Dickinson PJ, Lecouteur RA, Bollen AW, Wang H, Wang H, et al. Spontaneous canine gliomas: overexpression of EGFR, PDGFRα and IGFBP2 demonstrated by tissue microarray immunophenotyping. J Neurooncol. (2010) 98:49–55. doi: 10.1007/s11060-009-0072-5
94. Ahmed I, Sozmen M. Expression of PDGF-A, PDGFRA, integrin subunit alpha V and selectin E is increased in canine cutaneous fibrosarcomas. Biotech Histochem. (2020) 9:1–9. doi: 10.1080/10520295.2020.1832256
95. Sözmen M, Devrim AK, Sudagidan M, Kabak YB, Yildirim F. Expression of angiogenic growth factors in canine squamous cell cancers. Biotech Histochem. (2020) 96:450–9. doi: 10.1080/10520295.2020.1818826
96. Holtermann N, Kiupel M, Kessler M, Teske E, Betz D, Hirschberger J. Masitinib monotherapy in canine epitheliotropic lymphoma. Vet Comp Oncol. (2016) 14:127–35. doi: 10.1111/vco.12157
97. Kobayashi PE, Lainetti PF, Leis-Filho AF, Delella FK, Carvalho M, Cury SS, et al. Transcriptome of two canine prostate cancer cells treated with toceranib phosphate reveals distinct antitumor profiles associated with the PDGFR pathway. Front Vet Sci. (2020) 7:561212. doi: 10.3389/fvets.2020.561212
98. Abou Asa S, Murai A, Murakami M, Hoshino Y, Mori T, Maruo K, et al. Expression of platelet-derived growth factor and its receptors in spontaneous canine hemangiosarcoma and cutaneous hemangioma. Histol Histopathol. (2012) 27:601–7. doi: 10.14670/HH-27.601
99. Iussich S, Maniscalco L, Di Sciuva A, Iotti B, Morello E, Martano M, et al. PDGFRs expression in dogs affected by malignant oral melanomas: correlation with prognosis. Vet Comp Oncol. (2017) 15:462–9. doi: 10.1111/vco.12190
100. Takeuchi Y, Fujino Y, Watanabe M, Nakagawa T, Ohno K, Sasaki N, et al. Screening of therapeutic targets for canine mast cell tumors from a variety of kinase molecules. J Vet Med Sci. (2011) 73:1295–302. doi: 10.1292/jvms.11-0093
101. Iida G, Asano K, Seki M, Sakai M, Kutara K, Ishigaki K, et al. Gene expression of growth factors and growth factor receptors for potential targeted therapy of canine hepatocellular carcinoma. J Vet Med Sci. (2014) 76:301–6. doi: 10.1292/jvms.13-0378
102. Li W, Guo M, Liu Y, Mu W, Deng G, Li C, et al. selenium induces an anti-tumor effect via inhibiting intratumoral angiogenesis in a mouse model of transplanted canine mammary tumor cells. Biol Trace Elem Res. (2016) 171:371–9. doi: 10.1007/s12011-015-0554-6
103. Whelan HT, Przybylski C, Bajic DM, Schmidt MH. Intracellular growth factor metabolism in proliferation of a brain tumor cell line - intracellular growth factors and brain tumor proliferation. J Neurooncol. (1993) 15:243–50. doi: 10.1007/BF01050070
104. Muscatello LV, Avallone G, Serra F, Seuberlich T, Mandara MT, Sisó S, et al. Glomeruloid microvascular proliferation, desmoplasia, and high proliferative index as potential indicators of high grade canine choroid plexus tumors. Vet Pathol. (2018) 55:391–401. doi: 10.1177/0300985817754124
105. Maniscalco L, Iussich S, Morello E, Martano M, Biolatti B, Riondato F, et al. PDGFs and PDGFRs in canine osteosarcoma: new targets for innovative therapeutic strategies in comparative oncology. Vet J. (2013) 195:41–7. doi: 10.1016/j.tvjl.2012.05.003
106. Xue Y, Lim S, Yang Y, Wang Z, Jensen LDE, Hedlund EM, et al. PDGF-BB modulates hematopoiesis and tumor angiogenesis by inducing erythropoietin production in stromal cells. Nat Med. (2012) 18:100–10. doi: 10.1038/nm.2575
107. Levine RA. Overexpression of the sis oncogene in a canine osteosarcoma cell line. Vet Pathol. (2002) 39:411–2. doi: 10.1354/vp.39-3-411
108. Kochevar DT, Kochevar J, Garrett L. Low level amplification of c-sis and c-myc in a spontaneous osteosarcoma model. Cancer Lett. (1990) 53:213–22. doi: 10.1016/0304-3835(90)90216-K
109. Meyer FRL, Steinborn R, Grausgruber H, Wolfesberger B, Walter I. Expression of platelet-derived growth factor BB, erythropoietin and erythropoietin receptor in canine and feline osteosarcoma. Vet J. (2015) 206:67–74. doi: 10.1016/j.tvjl.2015.06.003
110. Gentilini F, Capitani O, Tinto D, Rigillo A, Sabattini S, Bettini G, et al. Assessment of PDGFRβ promoter methylation in canine osteosarcoma using methylation-sensitive high-resolution melting analysis. Vet Comp Oncol. (2020) 18:484–93. doi: 10.1111/vco.12567
111. Sánchez-Céspedes R, Accornero P, Miretti S, Martignani E, Gattino F, Maniscalco L, et al. In vitro and in vivo effects of toceranib phosphate on canine osteosarcoma cell lines and xenograft orthotopic models. Vet Comp Oncol. (2020) 18:117–27. doi: 10.1111/vco.12562
112. Alegre F, Ormonde AR, Snider KM, Woolard K, Yu AM, Wittenburg LA. A genetically engineered microRNA-34a prodrug demonstrates anti-tumor activity in a canine model of osteosarcoma. PLoS ONE. (2018) 13:e0209941. doi: 10.1371/journal.pone.0209941
113. Eke I, Cordes N. Focal adhesion signaling and therapy resistance in cancer. Semin Cancer Biol. (2015) 31:65–75. doi: 10.1016/j.semcancer.2014.07.009
114. Wilton J, Kurenova E, Pitzonka L, Gaudy A, Curtin L, Sexton S, et al. Pharmacokinetic analysis of the FAK scaffold inhibitor C4 in dogs. Eur J Drug Metab Pharmacokinet. (2016) 41:55–67. doi: 10.1007/s13318-014-0233-6
115. Lv PC, Jiang AQ, Zhang WM, Zhu HL. FAK inhibitors in cancer, a patent review. Expert Opin Ther Pat. (2018) 28:139–45. doi: 10.1080/13543776.2018.1414183
116. Ren K, Yao N, Wang G, Tian L, Ma J, Shi X, et al. Vasculogenic mimicry: a new prognostic sign of human osteosarcoma. Hum Pathol. (2014) 45:2120–9. doi: 10.1016/j.humpath.2014.06.013
117. Ho MY, Liang CM, Liang SM. MIG-7 and phosphorylated prohibitin coordinately regulate lung cancer invasion/metastasis. Oncotarget. (2015) 6:381–93. doi: 10.18632/oncotarget.2804
118. Petty AP, Garman KL, Winn VD, Spidel CM, Lindsey JS. Overexpression of carcinoma and embryonic cytotrophoblast cell-specific Mig-7 induces invasion and vessel-like structure formation. Am J Pathol. (2007) 170:1763–80. doi: 10.2353/ajpath.2007.060969
119. Ayala-Domínguez L, Olmedo-Nieva L, Muñoz-Bello JO, Contreras-Paredes A, Manzo-Merino J, Martínez-Ramírez I, et al. Mechanisms of vasculogenic mimicry in ovarian cancer. Front Oncol. (2019) 9:998. doi: 10.3389/fonc.2019.00998
120. Robertson GP. Mig-7 linked to vasculogenic mimicry. Am J Pathol. (2007) 170:1454–6. doi: 10.2353/ajpath.2007.070127
121. Ren K, Zhang J, Gu X, Wu S, Shi X, Ni Y, et al. Migration-inducing gene-7 independently predicts poor prognosis of human osteosarcoma and is associated with vasculogenic mimicry. Exp Cell Res. (2018) 369:80–9. doi: 10.1016/j.yexcr.2018.05.008
122. Yao N, Ren K, Wang Y, Jin Q, Lu X, Lu Y, et al. Paris polyphylla suppresses proliferation and vasculogenic mimicry of human osteosarcoma cells and inhibits tumor growth in vivo. Am J Chin Med. (2017) 45:575–98. doi: 10.1142/S0192415X17500343
123. Ren D, Zhao J, Sun Y, Li D, Meng Z, Wang B, et al. Overexpressed ITGA2 promotes malignant tumor aggression by up-regulating PD-L1 expression through the activation of the STAT3 signaling pathway. J Exp Clin Cancer Res. (2019) 38:485. doi: 10.1186/s13046-019-1496-1
124. Liu X, Liang Z, Gao K, Li H, Zhao G, Wang S, et al. MicroRNA-128 inhibits EMT of human osteosarcoma cells by directly targeting integrin α2. Tumor Biol. (2016) 37:7951–57. doi: 10.1007/s13277-015-4696-0
125. Xiao T, Xiao Y, Wang W, Tang YY, Xiao Z, Su M. Targeting EphA2 in cancer. J Hematol Oncol. (2020) 13:114. doi: 10.1186/s13045-020-00944-9
126. Kim HS, Won YJ, Shim JH, Kim HJ, Kim J, Hong HN, et al. Morphological characteristics of vasculogenic mimicry and its correlation with EphA2 expression in gastric adenocarcinoma. Sci Rep. (2019) 9:3414. doi: 10.1038/s41598-019-40265-7
127. Xiang T, Lin YX, Ma W, Zhang HJ, Chen KM, He GP, et al. Vasculogenic mimicry formation in EBV-associated epithelial malignancies. Nat Commun. (2018) 9:5009. doi: 10.1038/s41467-018-07308-5
128. Zhang L, Cai X, Qian Z, Mei J, Ma X, Huang H, et al. Effect of EPHA2-siRNA plasmid on biological behavior of human osteosarcoma cells in vitro. Zhonghua Zhong Liu Za Zhi. (2007) 29:566–9.
129. Brachelente C, Cappelli K, Capomaccio S, Porcellato I, Silvestri S, Bongiovanni L, et al. Transcriptome analysis of canine cutaneous melanoma and melanocytoma reveals a modulation of genes regulating extracellular matrix metabolism and cell cycle. Sci Rep. (2017) 7:6386. doi: 10.1038/s41598-017-06281-1
130. O'Donoghue LE, Ptitsyn AA, Kamstock DA, Siebert J, Thomas RS, Duval DL. Expression profiling in canine osteosarcoma: identification of biomarkers and pathways associated with outcome. BMC Cancer. (2010) 10:506. doi: 10.1186/1471-2407-10-506
131. Cance WG, Kurenova E, Marlowe T, Golubovskaya V. Disrupting the scaffold to improve focal adhesion kinase-targeted cancer therapeutics. Sci Signal. (2013) 6:pe10. doi: 10.1126/scisignal.2004021
132. Rizzo VL, Levine CB, Wakshlag JJ. The effects of sulforaphane on canine osteosarcoma proliferation and invasion. Vet Comp Oncol. (2017) 15:718–30. doi: 10.1111/vco.12212
133. Marley K, Maier CS, Helfand SC. Phosphotyrosine enrichment identifies focal adhesion kinase and other tyrosine kinases for targeting in canine hemangiosarcoma. Vet Comp Oncol. (2012) 10:214–22. doi: 10.1111/j.1476-5829.2012.00325.x
134. Oyanadel C, Holmes C, Pardo E, Retamal C, Shaughnessy R, Smith P, et al. Galectin-8 induces partial epithelial–mesenchymal transition with invasive tumorigenic capabilities involving a FAK/EGFR/proteasome pathway in madin–darby canine kidney cells. Mol Biol Cell. (2018) 29:557–74. doi: 10.1091/mbc.E16-05-0301
135. Selvaggio G, Canato S, Pawar A, Monteiro PT, Guerreiro PS, Brás MM, et al. Hybrid epithelial-mesenchymal phenotypes are controlled by microenvironmental factors. Cancer Res. (2020) 80:2407–20. doi: 10.1158/0008-5472.CAN-19-3147
136. Kajita M, Hogan C, Harris AR, Dupre-Crochet S, Itasaki N, Kawakami K, et al. Interaction with surrounding normal epithelial cells influences signalling pathways and behaviour of Src-transformed cells. J Cell Sci. (2010) 123:171–80. doi: 10.1242/jcs.057976
137. Hong M, Wilkes MC, Penheiter SG, Gupta SK, Edens M, Leof EB. Non-Smad transforming growth factor-β signaling regulated by focal adhesion kinase binding the p85 subunit of phosphatidylinositol 3-kinase. J Biol Chem. (2011) 286:17841–50. doi: 10.1074/jbc.M111.233676
138. Gu Y, Shea J, Slattum G, Firpo MA, Alexander M, Golubovskaya VM, et al. Defective apical extrusion signaling contributes to aggressive tumor hallmarks. Elife. (2015) 4:e04069. doi: 10.7554/eLife.04069
139. Chan PC, Liang CC, Yu KC, Chang MC, Ho WL, Chen BH, et al. Synergistic effect of focal adhesion kinase overexpression and hepatocyte growth factor stimulation on cell transformation. J Biol Chem. (2002) 277:50373–9. doi: 10.1074/jbc.M204691200
140. Castelli M, De Pascalis C, Distefano G, Ducano N, Oldani A, Lanzetti L, et al. Regulation of the microtubular cytoskeleton by Polycystin-1 favors focal adhesions turnover to modulate cell adhesion and migration. BMC Cell Biol. (2015) 16:15. doi: 10.1186/s12860-015-0059-3
141. Morris MA, Laverick L, Wei W, Davis AM, O'Neill S, Wood L, et al. The EBV-encoded oncoprotein, LMP1, induces an epithelial-to-mesenchymal transition (EMT) via its CTAR1 domain through integrin-mediated ERK-MAPK signalling. Cancers. (2018) 10:130. doi: 10.3390/cancers10050130
142. Marech I, Patruno R, Zizzo N, Gadaleta C, Introna M, Zito AF, et al. Masitinib (AB1010), from canine tumor model to human clinical development: where we are? Crit Rev Oncol Hematol. (2014) 91:98–111. doi: 10.1016/j.critrevonc.2013.12.011
143. Pulz LH, Strefezzi RF. Proteases as prognostic markers in human and canine cancers. Vet Comp Oncol. (2017) 15:669–83. doi: 10.1111/vco.12223
144. Gobin E, Bagwell K, Wagner J, Mysona D, Sandirasegarane S, Smith N, et al. A pan-cancer perspective of matrix metalloproteases (MMP) gene expression profile and their diagnostic/prognostic potential. BMC Cancer. (2019) 19:581. doi: 10.1186/s12885-019-5768-0
145. Yang F, Wen M, Pan D, Lin X, Mo J, Dong X, et al. IL-33/ST2 axis regulates vasculogenic mimicry via ERK1/2-MMP-2/9 pathway in melanoma. Dermatology. (2019) 235:225–33. doi: 10.1159/000498857
146. Gieger TL, Nettifee-Osborne J, Hallman B, Johannes C, Clarke D, Nolan MW, et al. The impact of carboplatin and toceranib phosphate on serum vascular endothelial growth factor (VEGF) and metalloproteinase-9 (MMP-9) levels and survival in canine osteosarcoma. Can J Vet Res. (2017) 81:199–205.
147. Hadjimichael AC, Foukas AF, Savvidou OD, Mavrogenis AF, Psyrri AK, Papagelopoulos PJ. The anti-neoplastic effect of doxycycline in osteosarcoma as a metalloproteinase (MMP) inhibitor: a systematic review. Clin Sarcoma Res. (2020) 10:7. doi: 10.1186/s13569-020-00128-6
148. Lana SE, Ogilvie GK, Hansen RA, Powers BE, Dernell WS, Withrow SJ. Identification of matrix metalloproteinases in canine neoplastic tissue. Am J Vet Res. (2000) 61:111–4. doi: 10.2460/ajvr.2000.61.111
149. Nytko KJ, Thumser-Henner P, Russo G, Weyland MS, Rohrer Bley C. Role of HSP70 in response to (thermo)radiotherapy: analysis of gene expression in canine osteosarcoma cells by RNA-seq. Sci Rep. (2020) 10:12779. doi: 10.1038/s41598-020-69619-2
150. Loukopoulos P, O'Brien T, Ghoddusi M, Mungall BA, Robinson WF. Characterisation of three novel canine osteosarcoma cell lines producing high levels of matrix metalloproteinases. Res Vet Sci. (2004) 77:131–41. doi: 10.1016/j.rvsc.2004.01.006
151. Giantin M, Aresu L, Benali S, Aricò A, Morello EM, Martano M, et al. Expression of matrix metalloproteinases, tissue inhibitors of metalloproteinases and vascular endothelial growth factor in canine mast cell tumours. J Comp Pathol. (2012) 147:419–29. doi: 10.1016/j.jcpa.2012.01.011
152. Gentilini F, Calzolari C, Turba ME, Agnoli C, Fava D, Forni M, et al. Prognostic value of serum vascular endothelial growth factor (VEGF) and plasma activity of matrix metalloproteinase (MMP) 2 and 9 in lymphoma-affected dogs. Leuk Res. (2005) 29:1263–9. doi: 10.1016/j.leukres.2005.04.005
153. Nakaichi M, Yunuki T, Okuda M, Une S, Taura Y. Activity of matrix metalloproteinase-2 (MMP-2) in canine oronasal tumors. Res Vet Sci. (2007) 82:271–9. doi: 10.1016/j.rvsc.2006.07.007
154. Nowak M, Madej JA, Podhorska-Okolow M, Dziegiel P. Expression of extracellular matrix metalloproteinase (MMP-9), E-cadherin and proliferation-associated antigen Ki-67 and their reciprocal correlation in canine mammary adenocarcinomas. In Vivo. (2008) 22:463–70.
155. Miya K, Misumi K, Miyoshi N, Arai K, Fujiki M, Kubota C, et al. Interpreting gelatinase activity in tumor tissue and serum as a prognostic marker of naturally developing canine tumors. J Vet Med Sci. (2005) 67:769–75. doi: 10.1292/jvms.67.769
156. Loukopoulos P, Mungall BA, Straw RC, Thornton JR, Robinson WF. Matrix metalloproteinase-2 and−9 involvement in canine tumors. Vet Pathol. (2003) 40:382–94. doi: 10.1354/vp.40-4-382
157. Hirayama K, Yokota H, Onai R, Kobayashi T, Kumata T, Kihara K, et al. Detection of matrix metalloproteinases in canine mammary tumours: analysis by immunohistochemistry and zymography. J Comp Pathol. (2002) 127:249–56. doi: 10.1053/jcpa.2002.0590
158. Aresu L, Giantin M, Morello E, Vascellari M, Castagnaro M, Lopparelli R, et al. Matrix metalloproteinases and their inhibitors in canine mammary tumors. BMC Vet Res. (2011) 7:33. doi: 10.1186/1746-6148-7-33
159. Yokota H, Kumata T, Taketaba S, Kobayashi T, Moue H, Taniyama H, et al. High expression of 92 kDa type IV collagenase (matrix metalloproteinase-9) in canine mammary adenocarcinoma. Biochim Biophys Acta Gen Subj. (2001) 1568:7–12. doi: 10.1016/S0304-4165(01)00192-1
160. Kuroki K, Kreeger JM, Cook JL, Tomlinson JL, Johnson GC, Pace LW, et al. Immunohistochemical analysis of matrix metalloproteinase-1,−3, and−13 in naturally occuring cartilaginous tumors of dogs. Am J Vet Res. (2002) 63:1285–91. doi: 10.2460/ajvr.2002.63.1285
161. Raposo TP, Beirão BCB, Pires I, Prada J, Brilhante P, Argyle DJ, et al. Immunohistochemical expression of CCR2, CSF1R and MMP9 in canine inflammatory mammary carcinomas. Anticancer Res. (2016) 36:1805–13.
162. Pan B, Guo J, Liao Q, Zhao Y. β1 and β3 integrins in breast, prostate and pancreatic cancer: a novel implication (review). Oncol Lett. (2018) 15:5412–6. doi: 10.3892/ol.2018.8076
163. Moore PF, Rossitto P V, Danilenko DM. Canine leukocyte integrins: characterization of a CD 18 homologue. Tissue Antigens. (1990) 36:211–20. doi: 10.1111/j.1399-0039.1990.tb01831.x
164. Vandenberg P, Kern A, Ries A, Luckenbill-Edds L, Mann K, Kuhn K. Characterization of a type IV collagen major cell binding site with affinity to the α1β1 and the α2β1 integrins. J Cell Biol. (1991) 113:1475–83. doi: 10.1083/jcb.113.6.1475
165. Chen YS, Mathias RA, Mathivanan S, Kapp EA, Moritz RL, Zhu HJ, et al. Proteomics profiling of madin-darby canine kidney plasma membranes reveals Wnt-5a involvement during oncogenic H-Ras/TGF-β-mediated epithelial-mesenchymal transition. Mol Cell Proteomics. (2011) 10:M110.001131. doi: 10.1074/mcp.M110.001131
166. Yi-Chun YEH, Wang CZ, Tang MJ. Discoidin domain receptor 1 activation suppresses α2b 1 integrin-dependent cell spreading through inhibition of Cdc42 sctivity. J Cell Physiol. (2009) 218:146–56. doi: 10.1002/jcp.21578
167. Selvarajah GT, Kirpensteijn J, van Wolferen ME, Rao NAS, Fieten H, Mol JA. Gene expression profiling of canine osteosarcoma reveals genes associated with short and long survival times. Mol Cancer. (2009) 8:72. doi: 10.1186/1476-4598-8-72
168. Sánchez-Céspedes R, Millán Y, Guil-Luna S, Reymundo C, Espinosa de los Monteros A, Martín de las Mulas J. Myoepithelial cells in canine mammary tumours. Vet J. (2016) 207:45–52. doi: 10.1016/j.tvjl.2015.10.035
169. Peña L, Nieto A, Perez Alenza MD, Rodriguez A, Sanchez MA, Castaño M. Expression of fibronectin and its integrin receptor α5β1 in canine mammary tumours. Res Vet Sci. (1994) 57:358–64. doi: 10.1016/0034-5288(94)90131-7
170. Moore PF, Schrenzel MD, Affolter VK, Olivry T, Naydan D. Canine cutaneous histiocytoma is an epidermotropic langerhans cell histiocytosis that expresses CD1 and specific β2-integrin molecules. Am J Pathol. (1996) 148:1699–708.
171. Akhtar N, Padilla ML, Dickerson EB, Steinberg H, Breen M, Auerbach R, et al. Interleukin-12 inhibits tumor growth in a novel angiogenesis canine hemangiosarcoma xenograft model. Neoplasia. (2004) 6:106–16. doi: 10.1593/neo.03334
172. Fosmire SP, Dickerson EB, Scott AM, Bianco SR, Pettengill MJ, Meylemans H, et al. Canine malignant hemangiosarcoma as a model of primitive angiogenic endothelium. Lab Investig. (2004) 84:562–72. doi: 10.1038/labinvest.3700080
173. Ito K, Miyamoto R, Tani H, Kurita S, Kobayashi M, Tamura K, et al. Effect of dasatinib in a xenograft mouse model of canine histiocytic sarcoma and in vitro expression status of its potential target EPHA2. J Vet Pharmacol Ther. (2018) 41:e45–8. doi: 10.1111/jvp.12449
174. Kamoun WS, Kirpotin DB, Huang ZR, Tipparaju SK, Noble CO, Hayes ME, et al. Antitumour activity and tolerability of an EphA2-targeted nanotherapeutic in multiple mouse models. Nat Biomed Eng. (2019) 3:264–80. doi: 10.1038/s41551-019-0385-4
175. Rossmeisl JH, Herpai D, Quigley M, Cecere TE, Robertson JL, D'Agostino RB, et al. Phase I trial of convection-enhanced delivery of IL13RA2 and EPHA2 receptor targeted cytotoxins in dogs with spontaneous intracranial gliomas. Neuro Oncol. (2020) 23:422–34. doi: 10.1093/neuonc/noaa196
176. Laut MM, Akter SH, Irac SE, Palmieri C. Immunohistochemical expression of EphA2 and EphA3 receptors in canine osteosarcoma. J Comp Pathol. (2019) 166:124. doi: 10.1016/j.jcpa.2018.10.075
177. Walker-Daniels J, Coffman K, Azimi M, Rhim JS, Bostwick DG, Snyder P, et al. Overexpression of the EphA2 tyrosine kinase in prostate cancer. Prostate. (1999) 41:275–280. doi: 10.1002/(SICI)1097-0045(19991201)41:4<275::AID-PROS8>3.0.CO;2-T
178. Tanaka M, Kamata R, Sakai R. EphA2 phosphorylates the cytoplasmic tail of claudin-4 and mediates paracellular permeability. J Biol Chem. (2005) 280:42375–82. doi: 10.1074/jbc.M503786200
179. Harada K, Hiramoto-Yamaki N, Negishi M, Katoh H. Ephexin4 and EphA2 mediate resistance to anoikis through RhoG and phosphatidylinositol 3-kinase. Exp Cell Res. (2011) 317:1701–13. doi: 10.1016/j.yexcr.2011.05.014
180. Hill W, Hogan C. Normal epithelial cells trigger EphA2-dependent RasV12 cell repulsion at the single cell level. Small GTPases. (2019) 10:305–10. doi: 10.1080/21541248.2017.1324940
181. Harada K, Negishi M, Katoh H. HGF-induced serine 897 phosphorylation of EphA2 regulates epithelial morphogenesis of MDCK cells in 3D culture. J Cell Sci. (2015) 128:1912–21. doi: 10.1242/jcs.163790
182. Caron A, Briscoe DM, Richard D, Laplante M. DEPTOR at the nexus of cancer, metabolism, and immunity. Physiol Rev. (2018) 98:1765–803. doi: 10.1152/physrev.00064.2017
183. Catena V, Fanciulli M. Deptor: Not only a mTOR inhibitor. J Exp Clin Cancer Res. (2017) 36: doi: 10.1186/s13046-016-0484-y
184. Hu B, Lv X, Gao F, Chen S, Wang S, Qing X, et al. Downregulation of DEPTOR inhibits the proliferation, migration, and survival of osteosarcoma through PI3K/Akt/mTOR pathway. Onco Targets Ther. (2017) 10:4379–91. doi: 10.2147/OTT.S143518
185. Liu J, Wada Y, Katsura M, Tozawa H, Erwin N, Kapron CM, et al. Rho-associated coiled-coil kinase (ROCK) in molecular regulation of angiogenesis. Theranostics. (2018) 8:6053–69. doi: 10.7150/thno.30305
186. Zhang JG, Zhou HM, Zhang X, Mu W, Hu JN, Liu GL, et al. Hypoxic induction of vasculogenic mimicry in hepatocellular carcinoma: role of HIF-1 α, RhoA/ROCK and Rac1/PAK signaling. BMC Cancer. (2020) 20:32. doi: 10.1186/s12885-019-6501-8
187. Xia Y, Cai X, Fan J, Zhang L, Li Z, Ren J, et al. RhoA/ROCK pathway inhibition by fasudil suppresses the vasculogenic mimicry of U2OS osteosarcoma cells in vitro. Anticancer Drugs. (2017) 28:514–21. doi: 10.1097/CAD.0000000000000490
188. Xia Y, Cai XY, Fan JQ, Zhang LL, Ren JH, Chen J, et al. Rho kinase inhibitor fasudil suppresses the vasculogenic mimicry of B16 mouse melanoma cells both in vitro and in vivo. Mol Cancer Ther. (2015) 14:1582–90. doi: 10.1158/1535-7163.MCT-14-0523
189. Feng Z, Hu W, Rajagopal G, Levine AJ. The tumor suppressor p53: cancer and aging. Cell Cycle. (2008) 7:842–7. doi: 10.4161/cc.7.7.5657
190. Moriarity BS, Otto GM, Rahrmann EP, Rathe SK, Wolf NK, Weg MT, et al. A sleeping beauty forward genetic screen identifies new genes and pathways driving osteosarcoma development and metastasis. Nat Genet. (2015) 47:615–24. doi: 10.1038/ng.3293
191. Mauchle U, Selvarajah GT, Mol JA, Kirpensteijn J, Verheije MH. Identification of anti-proliferative kinase inhibitors as potential therapeutic agents to treat canine osteosarcoma. Vet J. (2015) 205:281–7. doi: 10.1016/j.tvjl.2014.08.006
192. Gordon IK, Ye F, Kent MS. Evaluation of the mammalian target of rapamycin pathway and the effect of rapamycin on target expression and cellular proliferation in osteosarcoma cells from dogs. Am J Vet Res. (2008) 69:1079–84. doi: 10.2460/ajvr.69.8.1079
193. Paoloni MC, Mazcko C, Fox E, Fan T, Lana S, Kisseberth W, et al. Rapamycin pharmacokinetic and pharmacodynamic relationships in osteosarcoma: a comparative oncology study in dogs. PLoS ONE. (2010) 5:e11013. doi: 10.1371/journal.pone.0011013
194. Pyuen AA, Meuten T, Rose BJ, Thamm DH. In vitro effects of PI3K/mTOR inhibition in canine hemangiosarcoma. PLoS ONE. (2018) 13:e0200634. doi: 10.1371/journal.pone.0200634
195. Rivera-Calderón LG, Fonseca-Alves CE, Kobayashi PE, Carvalho M, Vasconcelos RO, Laufer-Amorim R. p-mTOR, p-4EBP-1 and eIF4E expression in canine prostatic carcinoma. Res Vet Sci. (2019) 122:86–92. doi: 10.1016/j.rvsc.2018.11.006
196. Kim JW, Mahiddine FY, Kim GA. Leptin modulates the metastasis of canine inflammatory mammary adenocarcinoma cells through downregulation of lysosomal protective protein cathepsin a (Ctsa). Int J Mol Sci. (2020) 21:1–14. doi: 10.3390/ijms21238963
197. Zhao Y, Lin Z, Lin Z, Zhou C, Liu G, Lin J, et al. Overexpression of mucin 1 suppresses the therapeutical efficacy of disulfiram against canine mammary tumor. Animals. (2021) 11:1–13. doi: 10.3390/ani11010037
198. Wei BR, Hoover SB, Peer CJ, Dwyer JE, Adissu HA, Shankarappa P, et al. Efficacy, tolerability, and pharmacokinetics of combined targeted MEK and dual mTORC1/2 inhibition in a preclinical model of mucosal melanoma. Mol Cancer Ther. (2020) 19:2308–18. doi: 10.1158/1535-7163.MCT-19-0858
199. Rebuzzi L, Willmann M, Sonneck K, Gleixner K V, Florian S, Kondo R, et al. Detection of vascular endothelial growth factor (VEGF) and VEGF receptors Flt-1 and KDR in canine mastocytoma cells. Vet Immunol Immunopathol. (2007) 115:320–33. doi: 10.1016/j.vetimm.2006.11.009
200. Walid S, Eisen R, Ratcliffe DR, Dai K, Hussain MM, Ojakian GK. The PI 3-kinase and mTOR signaling pathways are important modulators of epithelial tubule formation. J Cell Physiol. (2008) 216:469–79. doi: 10.1002/jcp.21419
201. Isozaki Y, Sakai K, Kohiro K, Kagoshima K, Iwamura Y, Sato H, et al. The Rho-guanine nucleotide exchange factor solo decelerates collective cell migration by modulating the Rho-ROCK pathway and keratin networks. Mol Biol Cell. (2020) 31:741–52. doi: 10.1091/mbc.E19-07-0357
202. Citi S, Guerrera D, Spadaro D, Shah J. Epithelial junctions and Rho family GTPases: The zonular signalosome. Small GTPases. (2014) 5:973760. doi: 10.4161/21541248.2014.973760
203. Fernández-Muñoz B, Yurrita MM, Martín-Villar E, Carrasco-Ramírez P, Megías D, Renart J, et al. The transmembrane domain of podoplanin is required for its association with lipid rafts and the induction of epithelial-mesenchymal transition. Int J Biochem Cell Biol. (2011) 43:886–96. doi: 10.1016/j.biocel.2011.02.010
204. Soliman M, Cho EH, Park JG, Kim JY, Alfajaro MM, Baek Y Bin, et al. Rotavirus-Induced early activation of the RhoA/ROCK/MLC signaling pathway mediates the disruption of tight junctions in polarized MDCK cells. Sci Rep. (2018) 8:13931. doi: 10.1038/s41598-018-32352-y
205. Stuart HC, Jia Z, Messenberg A, Joshi B, Underhill TM, Moukhles H, et al. Localized Rho GTPase activation regulates RNA dynamics and compartmentalization in tumor cell protrusions. J Biol Chem. (2008) 283:34785–95. doi: 10.1074/jbc.M804014200
206. Ramírez De Molina A, Gallego-Ortega D, Sarmentero J, Bañez-Coronel M, Martín-Cantalejo Y, Lacal JC. Choline kinase is a novel oncogene that potentiates RhoA-induced carcinogenesis. Cancer Res. (2005) 65:5647–53. doi: 10.1158/0008-5472.CAN-04-4416
207. Benitah SA, Valerón PF, Lacal JC. ROCK and nuclear factor-κB-dependent activation of cyclooxygenase-2 by Rho GTPases: effects on tumor growth and therapeutic consequences. Mol Biol Cell. (2003) 14:3041–54. doi: 10.1091/mbc.e03-01-0016
208. Dohn MR, Brown M V, Reynolds AB. An essential role for p120-catenin in Src- and Rac1-mediated anchorage-independent cell growth. J Cell Biol. (2009) 184:437–50. doi: 10.1083/jcb.200807096
209. Wang J yan, Yang Y, Ma Y, Wang F, Xue A, Zhu J, et al. Potential regulatory role of lncRNA-miRNA-mRNA axis in osteosarcoma. Biomed Pharmacother. (2020) 121:109627. doi: 10.1016/j.biopha.2019.109627
210. Sanchez Calle A, Kawamura Y, Yamamoto Y, Takeshita F, Ochiya T. Emerging roles of long non-coding RNA in cancer. Cancer Sci. (2018) 109:2093–100. doi: 10.1111/cas.13642
211. Xavier PLP, Müller S, Fukumasu H. Epigenetic mechanisms in canine cancer. Front Oncol. (2020) 10:591843. doi: 10.3389/fonc.2020.591843
212. He X, Sun F, Guo F, Wang K, Gao Y, Feng Y, et al. Knockdown of long noncoding RNA FTX inhibits proliferation, migration, and invasion in renal cell carcinoma cells. Oncol Res. (2017) 25:157–66. doi: 10.3727/096504016X14719078133203
213. Shi D, Wu F, Mu S, Hu B, Zhong B, Gao F, et al. LncRNA AFAP1-AS1 promotes tumorigenesis and epithelial-mesenchymal transition of osteosarcoma through RhoC/ROCK1/p38MAPK/Twist1 signaling pathway. J Exp Clin Cancer Res. (2019) 38:375. doi: 10.1186/s13046-019-1363-0
214. Xiao Y, Li C, Wang H, Liu Y. LINC00265 targets miR-382-5p to regulate SAT1, VAV3 and angiogenesis in osteosarcoma. Aging. (2020) 12:20212–25. doi: 10.18632/aging.103762
215. Le Béguec C, Wucher V, Lagoutte L, Cadieu E, Botherel N, Hédan B, et al. Characterisation and functional predictions of canine long non-coding RNAs. Sci Rep. (2018) 8:13444. doi: 10.1038/s41598-018-31770-2
216. Wucher V, Legeai F, Hédan B, Rizk G, Lagoutte L, Leeb T, et al. FEELnc: A tool for long non-coding RNA annotation and its application to the dog transcriptome. Nucleic Acids Res. (2017) 45:e57. doi: 10.1093/nar/gkw1306
217. Gutschner T, Hämmerle M, Eißmann M, Hsu J, Kim Y, Hung G, et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. (2013) 73:1180–9. doi: 10.1158/0008-5472.CAN-12-2850
218. Verma A, Jiang Y, Du W, Fairchild L, Melnick A, Elemento O. Transcriptome sequencing reveals thousands of novel long non-coding RNAs in B cell lymphoma. Genome Med. (2015) 7:110. doi: 10.1186/s13073-015-0230-7
219. Cascione L, Giudice L, Ferraresso S, Marconato L, Giannuzzi D, Napoli S, et al. Long non-coding RNAs as molecular signatures for canine B-cell lymphoma characterization. Non Coding RNA. (2019) 5:47. doi: 10.3390/ncrna5030047
220. Hoeppner MP, Lundquist A, Pirun M, Meadows JRS, Zamani N, Johnson J, et al. An improved canine genome and a comprehensive catalogue of coding genes and non-coding transcripts. PLoS ONE. (2014) 9:e91172. doi: 10.1371/journal.pone.0091172
221. Beltran M, Puig I, Peña C, García JM, Álvarez AB, Peña R, Bonilla F, et al. A natural antisense transcript regulates Zeb2/Sip1 gene expression during Snail1-induced epithelial-mesenchymal transition. Genes Dev. (2008) 22:756–69. doi: 10.1101/gad.455708
222. Hitte C, Le Béguec C, Cadieu E, Wucher V, Primot A, Prouteau A, et al. Genome-wide analysis of long non-coding RNA profiles in canine oral melanomas. Genes. (2019) 10:477. doi: 10.3390/genes10060477
223. Qiao Z, Yang D, Liu L, Liu Z, Wang J, He D, et al. Genome-wide identification and characterization of long non-coding RNAs in MDCK cell lines with high and low tumorigenicities. Genomics. (2020) 112:1077–86. doi: 10.1016/j.ygeno.2019.08.002
224. Kreilmeier T, Mejri D, Hauck M, Kleiter M, Holzmann K. Telomere transcripts target telomerase in human cancer cells. Genes. (2016) 7:46. doi: 10.3390/genes7080046
225. Li Y, Shan Z, Yang B, Yang D, Men C, Cui Y, et al. LncRNA HULC promotes epithelial and smooth-muscle-like differentiation of adipose-derived stem cells by upregulation of BMP9. Pharmazie. (2018) 73:49–55. doi: 10.1691/ph.2018.7634
226. Whitaker DT, Ostrander EA. Hair of the dog: identification of a cis-regulatory module predicted to influence canine coat composition. Genes. (2019) 10:323. doi: 10.3390/genes10050323
Keywords: comparative oncology, dog, osteosarcoma, vasculogenic mimicry, molecular pathways
Citation: Massimini M, Romanucci M, De Maria R and Della Salda L (2021) An Update on Molecular Pathways Regulating Vasculogenic Mimicry in Human Osteosarcoma and Their Role in Canine Oncology. Front. Vet. Sci. 8:722432. doi: 10.3389/fvets.2021.722432
Received: 08 June 2021; Accepted: 23 August 2021;
Published: 23 September 2021.
Edited by:
Pablo Martín-Vasallo, University of La Laguna, SpainReviewed by:
Abdelhakim Salem, University of Helsinki, FinlandFelisbina Luisa Queiroga, University of Trás-os-Montes and Alto Douro, Portugal
Copyright © 2021 Massimini, Romanucci, De Maria and Della Salda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marcella Massimini, mmassimini@unite.it
 Marcella Massimini
Marcella Massimini