
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 28 September 2021
Sec. Veterinary Pharmacology and Toxicology
Volume 8 - 2021 | https://doi.org/10.3389/fvets.2021.695375
 Alessandro Di Cerbo1*
Alessandro Di Cerbo1* Luca Roncati2
Luca Roncati2 Carlotta Marini1
Carlotta Marini1 Gianluca Carnevale3
Gianluca Carnevale3 Manuela Zavatti4
Manuela Zavatti4 Rossella Avallone5
Rossella Avallone5 Lorenzo Corsi5,6*
Lorenzo Corsi5,6*Objective: Hepatic encephalopathy (HE) is a neuropsychiatric syndrome caused by liver failure and by an impaired neurotransmission and neurological function caused by hyperammonemia (HA). HE, in turn, decreases the phosphorylation of protein kinase C epsilon (PKCε), contributing to the impairment of neuronal functions. Dehydroepiandrosterone (DHEA) exerts a neuroprotective effect by increasing the GABAergic tone through GABAA receptor stimulation. Therefore, we investigated the protective effect of DHEA in an animal model of HE, and the possible modulation of PKCε expression in different brain area.
Methods: Fulminant hepatic failure was induced in 18 male, Sprague–Dawley rats by i.p. administration of 3 g/kg D-galactosamine, and after 30 min, a group of animals received a subcutaneous injection of 25 mg/kg (DHEA) repeated twice a day (3 days). Exploratory behavior and general activity were evaluated 24 h and 48 h after the treatments by the open field test. Then, brain cortex and cerebellum were used for immunoblotting analysis of PKCε level.
Results: DHEA administration showed a significant improvement of locomotor activity both 24 and 48 h after D-galactosamine treatment (****p < 0.0001) but did not ameliorate liver parenchymal degeneration. Western blot analysis revealed a reduced immunoreactivity of PKCε (*p < 0.05) following D-galactosamine treatment in rat cortex and cerebellum. After the addition of DHEA, PKCε increased in the cortex in comparison with the D-galactosamine-treated (***p < 0.001) and control group (*p < 0.05), but decreased in the cerebellum (*p < 0.05) with respect to the control group. PKCε decreased after treatment with NH4Cl alone and in combination with DHEA in both cerebellum and cortex (****p < 0.0001). MTS assay demonstrated the synergistic neurotoxic action of NH4Cl and glutamate pretreatment in cerebellum and cortex along with an increased cell survival after DHEA pretreatment, which was significant only in the cerebellum (*p < 0.05).
Conclusion: An association between the DHEA-mediated increase of PKCε expression and the improvement of comatose symptoms was observed. PKCε activation and expression in the brain could inhibit GABA-ergic tone counteracting HE symptoms. In addition, DHEA seemed to ameliorate the symptoms of HE and to increase the expression of PKCε in cortex and cerebellum.
Hepatic encephalopathy (HE) is a reversible neuropsychiatric syndrome characterized by an impaired brain function that generally occurs in patients suffering from chronic liver disease or acute liver failure (1, 2). Three different forms of HE have been identified: type A (associated with acute liver failure resulting from severe inflammatory and/or necrotic liver disease of rapid onset), Type B (resulting from portacaval shunt in absence of parenchymal liver disease) and Type C (accompanying chronic liver failure) (3).
Although the pathophysiological process of HE is poorly understood, the scenario of an impaired neurotransmission due to metabolic changes in liver and brain with a consequent systemic inflammatory response and blood–brain barrier alterations has been hypothesized (1). In addition, non-specific neurological and psychiatric manifestations ranging from attention, working memory, psychomotor speed, and visuospatial ability alteration to disorientation to time and space, inappropriate behavior, and acute confusional state with agitation or somnolence have also been documented in patients suffering from HE (4, 5). A poor survival and a high risk of recurrence strongly characterize HE if liver disease is not successfully treated (6). Nevertheless, also the mildest form is correlated to a reduced quality of life as well as to the increased risk to develop severe HE (1, 7). One of the main contributors to the alteration in neurotransmission and neurological function in HE is hyperammonemia (1, 8). In this sense, blood ammonia increase following impaired liver function can cause muscle wasting, neuronal electric activity, and astrocyte swelling (1, 9–12). Astrocyte swelling, due to an increased intracellular osmolarity caused by impaired ammonia metabolism and glutamine production, is supposed to be one of the possible explanations of brain edema that generally characterizes HE (11, 13).
Neurochemical, neurobehavioral, and electrophysiological studies revealed the involvement of GABA-ergic (8, 14), dopaminergic (15), and serotoninergic and glutamatergic (14) neurotransmitter systems in the neurotransmission and neurological impairment that characterize HE. It is well-known that neurosteroids play pivotal functions in CNS. In particular, dehydroepiandrosterone (DHEA) has different positive effects on the central nervous system (CNS) ranging from neuronal plasticity and survival to cognition and behavior (16). As far as type A and type C HE are concerned, it seems that the GABAA-ergic neurotransmission is activated by benzodiazepines (diazepam and N-desmethyldiazepam) and neuroactive steroids (allopregnanolone and pregnenolone) present in the brain, sera, and cerebrospinal fluid of patients (17, 18). Conversely, recent neurophysiological studies on HE revealed that reduced brain levels of DHEA, which is a negative allosteric modulator of GABAA receptors, induced the stimulation of these latter with a consequent increase in GABAergic tone and neuroinhibition prevention (19).
Although these findings described a neuroprotective role of DHEA and partially explain its non-genomic molecular mechanism, its potential neuroprotection is still a matter of investigation in HE. Another potent modulator of GABAA and glutamate (in particular NMDA) receptor activity involved in HE is the protein kinase C (PKC) (20, 21). In fact, hyperammonemia decreases PKC-mediated phosphorylation and alters the function of key proteins involved in the neurotransmission (Na+/K+-ATPase and NMDA receptors) (22) and glutamine transporter [system N (SN)1 and SN2] (23) contributing to the impairment of neuronal functions (21). In addition, the PKC epsilon (PKCε) is involved in the molecular events leading to the decrease in the number of GABAA receptors through phosphorylation and activation of ATPase that regulates membrane fusion events (24). This finding is even more interesting if we consider that DHEA was able to interact with the PKC containing α, β, and γ isoforms and promote the translocation of PKC-β and -ζ from the cytosol to the membrane in rat adipocytes (25).
Given this information, in the present research project, we investigated the protective effect of DHEA in an animal model of HE, and the possible modulation of PKCε expression in different brain area.
Twenty-four, 7-week-old, male Sprague–Dawley rats (Charles River, Calco, Italy), with a body weight ranging from 200 and 220 g, were used. The animals were housed in an air-conditioned environment at 22 ± 1°C, with controlled humidity (60%), and a 12-h light/12-h dark cycle. Food and water were available ad libitum. Animal care and maintenance were conducted in accordance with the Italian law (D.L. n. 116/1992) and European legislation (EEC n. 86/609). The experimental designs and procedures received the approval of the Bioethical Committee of the Italian Institute of Health.
Eighteen rats were randomly divided into three groups of six animals each as given below:
Group I: control (saline only)
Group II: D-galactosamine
Group III: D-galactosamine + DHEA
D-galactosamine (Sigma-Aldrich, Milan, Italy) was solubilized in physiological solution and administered at a single dose of 2.5 g/kg by intraperitoneal injection. DHEA (Sigma-Aldrich, Milan, Italy) was solubilized in peanut oil and subcutaneously injected at the dose of 25 mg/kg twice a day.
It is well-known that the administration of D-galactosamine at the dose of 2.5 g/kg induced fulminant hepatic encephalopathy after 48 h from injection (14). For this reason, 48 h after the galactosamine treatment, all animals were sacrificed and the brains were isolated.
The endpoints of functional neurotoxicity were investigated with the open field test. Particularly, the spontaneous motor activity expressed as the total distance traveled (cm), and the number of rears (when the rat reared upon its hind feet) were recorded at two different times:
(1) 24 h after the D-galactosamine treatment
(2) 48 h after the D-galactosamine treatment
For the test, a black painted arena of 100 × 100 cm square floor and 50 cm high walls was used. The test started by gently placing each animal in the center of the arena. Rats were continuously filmed for 10 min with a camera connected at the video tracking system (SMART 2.5 version, PanLab, Barcelona, Spain). Each rat was tested only once, and at the end of each trial, the open field was wiped clean with 10% ethanol solution to remove traces of the previous assay.
For primary rats, cerebellar granule neurons were prepared from 8-day-old Sprague–Dawley rat cerebella as previously described (26). Instead, primary cortical neurons were prepared from 0 to 2 days postnatal brain pups. For cerebellar granule neurons, the brains were extracted from the skull and the cerebellum was excised, minced, and treated as previously reported (26). Both primary granule cells and cortical neurons were dispersed with trypsin (0.25 mg/ml; Sigma, Italy) and plated at a density of 100–105 cells/cm2 on 35-mm petri dishes or 96 multi-well plates coated with poly-L-lysine (10 mg/ml; Merck Life Science S.r.l., Milano, Italy). Cells were cultured in basal Eagle's medium (BME) (Euroclone, Milano, Italy) supplemented with 10% bovine calf serum, 2 mM glutamine, and 100 mg/ml of gentamycin (Euroclone) and maintained at 37°C in 6% CO2.
After 24 h in vitro, in the cortical neurons, the medium was replaced with 1:1 mixture of BME and Neurobasal medium (Thermofisher, GIBCO, Monza, Italy) containing 2% B27 supplement, 1% antibiotic, and 0.25% glutamine [H2NCOCH2CH2CH(NH2)CO2H, molecular weight 146.14, Thermofisher Invitrogen, Monza, Italy]. At 5 days in culture (DIV 5), cytosine arabinofuranoside (Ara-C; C9H13N3O5, ≥ 90.0% purity, molecular weight 243.22, Merck Life Science S.r.l., Milano, Italy) was added at a final concentration of 1 mM, whereas in the cerebellar granule cells, Ara-C (1 mM) was added to all cultures 18–24 h after plating to inhibit glial proliferation. Cerebellar granule cells and cortical neurons were then pre-treated with DHEA (1 mM, C19H28O2, molecular weight 288.42) alone or in combination with PKCε inhibitor peptide (1 mM; C37H65N9O13, molecular weight 843.96, Santa Cruz, USA) and PMA (50 nM; C36H56O8, molecular weight 616.83, Merck Life Science S.r.l., Milano, Italy) on days 5 and 7, respectively, after plating. Based on previous dose/response (data not shown) on the same cell line, on days 6 and 8, the cells were treated with Glutamate (100 mM; C10H16MgN2O8 · 4H2O, ≥ 98.0% purity, molecular weight 388.61, Merck Life Science S.r.l., Milano, Italy) + ammonium chloride (10 mM; NH4Cl, ≥ 99.998% purity, molecular weight 53.49, Merck Life Science S.r.l., Milano, Italy) for further 24 h. The drugs were diluted in culture medium from stock solutions prepared in water or dimethyl sulfoxide [DMSO, (CH3)2SO, ≥99.9% purity, molecular weight 78.13, Sigma Aldrich]. DMSO at the same final dilution (0.2%) was used for control cells.
Proteins from brain tissues, cerebellar granule cells, and cortical neurons of control and treated samples were obtained by lysing and homogenizing (in ice) the samples in RIPA buffer [50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% sodium deoxycolate, 1% Triton X-100, 2 mM phenylmethylsulfonyl fluoride (PMSF)] (C7H7FO2S, molecular weight 174.19, Merck Life Science S.r.l., Italy). The samples were then quantified using Bradford colorimetric method (Pierce, Rockford, USA) according to the manufacturer's protocol. Equal amount of protein, 0.4 μg/μl for each sample, was loaded onto a pre-cast 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) (Thermofisher Invitrogen, Monza, Italy) and electrophoretically transferred to nitrocellulose membrane (Thermofisher Invitrogen, Monza, Italy). Membrane was blocked in tris-buffered saline and Tween 20 (TBST) buffer (20 mM Tris- HCl, 0.5 M NaCl, and 0.05% Tween 20) containing 5% non-fat, dried, and incubated with primary antibody anti-PKCε (1 mg/ml, molecular weight 83 kDa, Upstate, USA) at 5°C overnight under gentle agitation. Membrane was then washed three times in TBST, incubated for 1.5 h with HRP-conjugated anti-mouse antibody (Cell Signaling, USA), and visualized using chemiluminescence method (Amersham, GE Healthcare Europe GmbH, Milan, Italy). The determination of relative protein expression was performed using densitometric analysis using a BioRad GS 690 Imaging densitometer with molecular analysis software (Life science, Milan, Italy). β-actin was used a loading control.
After 8 days since the plating in the 96-well microplates, both cell cultures were pre-challenged with DHEA 1 μM and PMA (Merck Life Science S.r.l., Milano, Italy) 50 nM in aqueous solution. Cells were incubated for 24 h and then challenged with glutamate 100 μM (solubilized in HCl) and ammonium chloride 10 mM. The cell viability assay was carried out following another 24 h of incubation using the 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTS) assay (Life Technologies, Monza, Italy). Viable cells were spectrophotometrically read at 490 nm.
The rat liver samples were washed with PBS, fixed with formalin, and serially sectioned into 5-μm slices after paraffin embedding. The slides were then dewaxed and stained using hematoxylin–eosin (HE) reagent. The histological changes of rat liver tissues were investigated under a light microscope.
Data were analyzed using GraphPad Prism8 software (GraphPad Software, Inc., La Jolla, CA, USA) and reported as the mean ± standard error of the mean. Differences in total distance traveled and rears were analyzed using a two-way analysis of variance (ANOVA) followed by Tukey's multiple comparisons test, with individual variances computed for each comparison.
Differences in PKCε expression in protein extract from control (n = 6), D-galactosamine-treated (n = 6), and D-galactosamine + DHEA-treated animals (n = 6) were performed in triplicate and analyzed by a one-way ANOVA followed by Tukey's multiple comparisons test, with a single pooled variance.
At the same time, differences between cortical neuronal cells and cerebellar granule neurons were performed in quadruplicate in three different experiments analyzed by a one-way ANOVA followed by Tukey's multiple comparisons test, with a single pooled variance.
As far as cell viability assays are concerned, each experimental condition is reported by the mean ± standard error of the mean of three different experiments done in quintuplicate and were analyzed by a one-way ANOVA followed by Tukey's multiple comparisons test, with a single pooled variance. *p < 0.05 was considered significant.
In the evaluation of horizontal activity following the open field test, the groups of animals treated with D-galactosamine alone and treated with D-galactosamine + DHEA showed a significant reduction of the total distance traveled at both 24 and 48 h (****p < 0.0001) when compared with the control group (Figure 1).
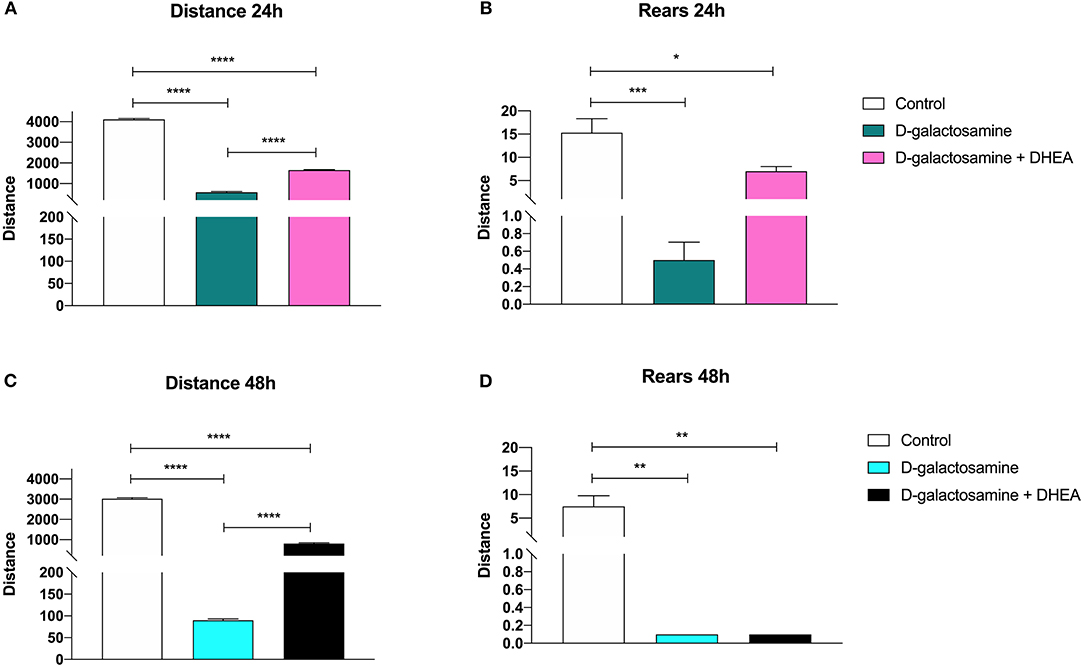
Figure 1. Effect of the administration of D-galactosamine alone and of D-galactosamine + DHEA on the total distance traveled and the number of rears in the open field test after (A,B) 24 h and (C,D) 48 h of treatment; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
However, the animals treated with D-galactosamine + DHEA showed a significant increase of the total distance traveled at both times 24 and 48 h (****p < 0.0001) in comparison with those treated with the D-galactosamine alone (Figure 1A).
The number of rears in the animals treated with D-galactosamine and D-galactosamine + DHEA were significantly lower in comparison with the control group at both 24 (****p < 0.0001 and **p < 0.01, respectively) and 48 h (**p < 0.01) (Figure 1B).
A significant increase in the number of rears of the animals treated with D-galactosamine + DHEA with respect to the D-galactosamine-treated group (*p < 0.05) was observed at 24 h (Figure 1B). After 48 h of treatment, DHEA was unable to increase the number of rears in comparison to the D-galactosamine alone; this could be due to the degenerative effect promoted by D-galactosamine on liver parenchyma, where DHEA did not exert any significant protective effect (Figures 2B,C).
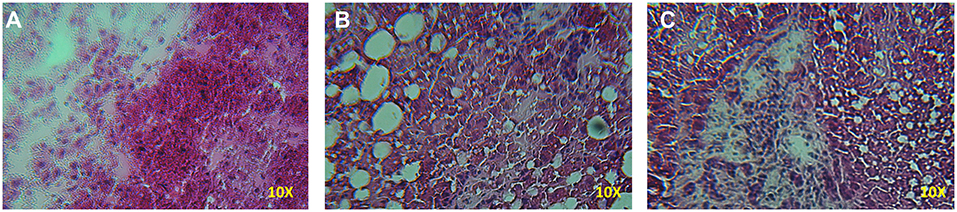
Figure 2. Histopathology of the liver samples stained with HE from rats treated with D-galactosamine and D-galactosamine + DHEA for 48 h. (A) Normal control group with proper architecture of the liver, (B) D-galactosamine-induced hepatotoxicity characterized by marked steatosis and hepatocyte degeneration, and (C) D-galactosamine + DHEA with steatosis and altered parenchymal structure.
Indeed, as shown in Figure 2, D-galactosamine was able to alter the architecture of the liver by inducing hepatocyte degeneration and a dramatic increase of steatosis. The treatment with DHEA did not ameliorate the parenchymal degeneration, and the samples present steatosis and an altered cellular architecture.
Immunoblot analysis of PKCε protein expression in rat cortex and cerebellum tissue samples, revealed the presence, in both membrane preparations, of the 95-kDa protein, corresponding to PKCε (Figures 3A,C). In both cortex and cerebellum, D-galactosamine-treated rats significantly reduced (*p < 0.05) the immunoreactivity of PKCε compared to that of the control (Figures 3B,D).
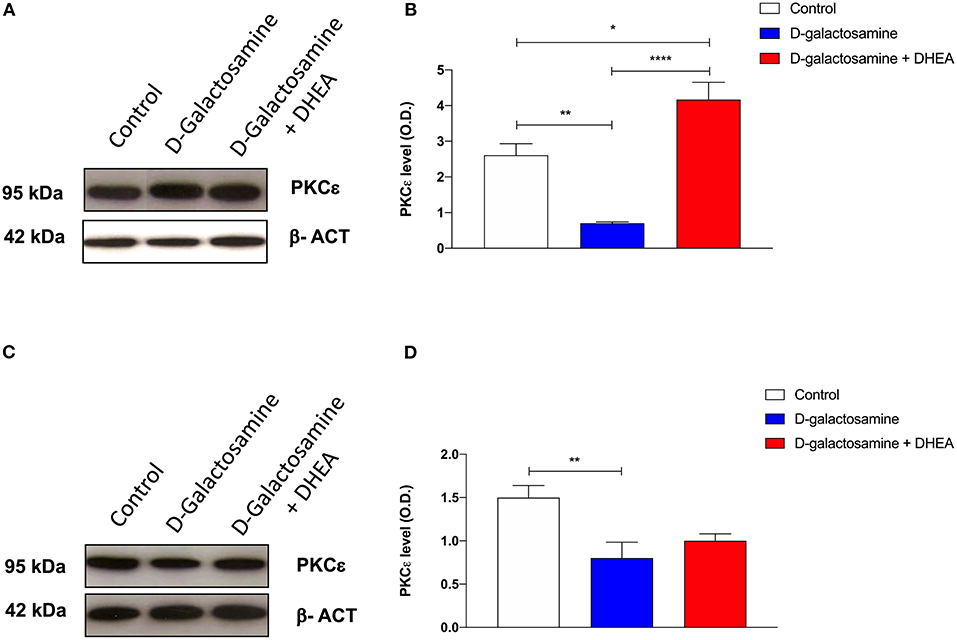
Figure 3. Western blot analysis of cortex (A) and cerebellum (C) PKCε expression after 48 h of treatment with saline (control), D-galactosamine, and D-galactosamine + DHEA (β-actin was used as internal standard) and their respective representative blots (B,D); *P < 0.05, **P < 0.01, ****P < 0.0001. Western blot pictures were sliced since cortex and cerebellum samples were loaded in wells far from the control.
In particular, band spectrophotometric analysis of the protein showed a one-fold expression reduction in cortex and a 0.75-fold in cerebellum. As far as cortex tissue samples are concerned, after the addition of the DHEA, the protein level of PKCε significantly increased in comparison with both the D-galactosamine (***p < 0.001) and control group (*p < 0.05) (Figure 3B). Indeed, D-galactosamine + DHEA treatment expression value showed a two-fold increase with respect to the control and a three-fold increase with respect to the D-galactosamine group, thus demonstrating that DHEA was able to stimulate PKCε expression in brain cortex area.
On the contrary, densitometric analysis performed on cerebellum protein tissue extracts showed that DHEA treatment failed to restore the protein concentration with respect to that of the control group, although a 0.25-fold increase with respect to the D-galactosamine group was observed.
The results obtained on the HE-animal model prompted us to investigate the role of PKCε in neuroprotection of cortical and cerebellar granular neurons challenged with a combined treatment with glutamate and ammonium chloride. The reason for the choice of ammonia and glutamate plus ammonia treatments was based on a previous report, where researchers observed a dramatic increase in ammonia in CNS in D-galactosamine-induced HE in rats (27), along with an increase in glutamate in the intersynpatic cleft (8, 11).
As expected, the signal of PKCε decreased significantly after treatment with NH4Cl alone (0.75- and 10-fold) and in combination with DHEA (0.75- and 1.5-fold) (1.42 ± 0.01 vs. 0.81 ± 0.01 and 0.78 ± 0.01, respectively; ****p < 0.0001) in both cerebellum (Figures 4C,D) and cortex tissue extracts (2.45 ± 0.26 vs. 0.06 ± 0.02 and 0.61 ± 0.02, respectively; ****p < 0.0001) (Figures 4A,B). As observed in the animal model, the effect of NH4Cl in the cerebellar cortical cells produced a decrease in the PKCε expression, which was not reverted by DHEA. In the cortical neurons instead, the NH4Cl + DHEA challenge improved the PKCε expression with respect to the NH4Cl alone. We hypothesized that this could be due to an increase in transcription elicited by DHEA.
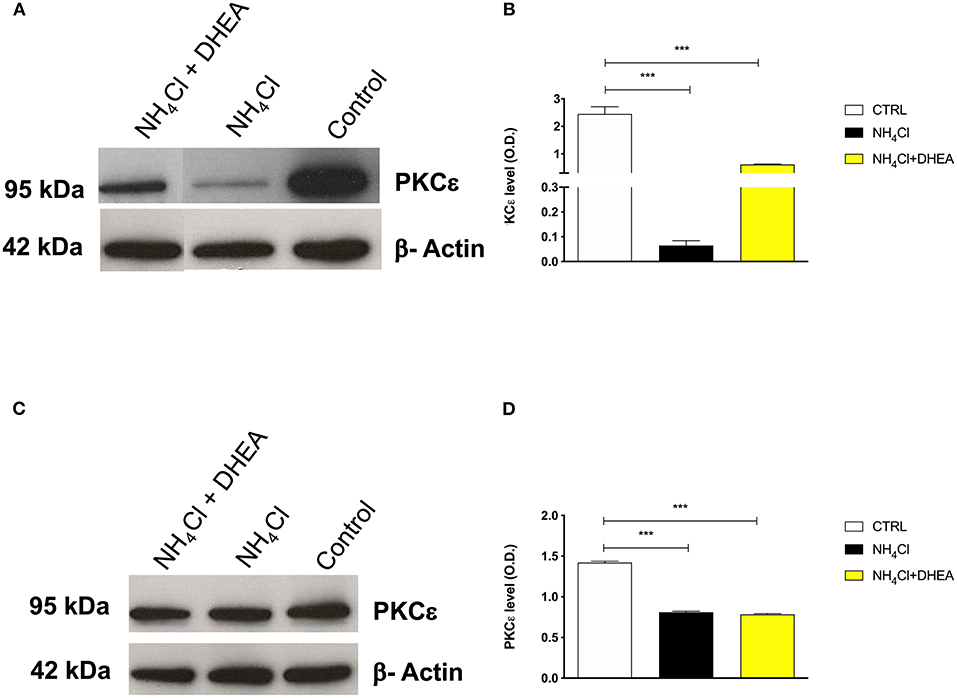
Figure 4. Western blot analysis of cortex (A) and cerebellum (C) PKCε expression after 48 h of treatment with saline (control), NH4Cl, and NH4Cl + DHEA (β-actin was used as internal standard) and their respective representative blots (B,D); ***p < 0.001. Western blot pictures were spliced since cortex and cerebellum samples were loaded in wells far from the control.
We then treated the cells with the combination of glutamate (100 mM) and ammonium chloride (10 mM) for 24 h, with or without DHEA (1 mM) and PMA (50 nM) pretreatment and with a PKCε-specific inhibitor peptide (1 mM) to better characterize and describe further possible synergistic combinations. The results obtained by MTS assay in both cerebellum and cortex demonstrated the synergistic neurotoxic action of ammonium chloride and glutamate (N + G). In fact, the cells treated with this combination showed a significantly reduced cell viability characterized by a decreased absorbance compared to the control cells (untreated). Conversely, pretreatment of cells with glutamate, ammonium chloride, and DHEA (G + N + D) significantly increased (*p < 0.05) the cell survival with respect to N + G, thus demonstrating the protective effect of DHEA (Figure 5).
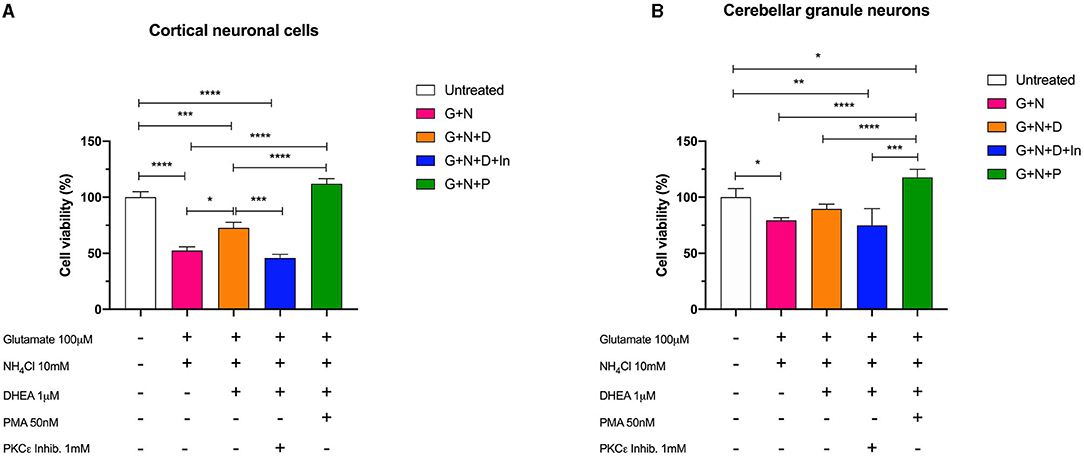
Figure 5. Cell viability assay of (A) cerebellar cells and (B) cortical cells following 24 h of incubation with glutamate (G), ammonium chloride (N), DHEA (D), PMA (P), and PKCε inhibitor (In); *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Furthermore, a significant decrease (***p < 0.001) in the cell viability was observed in cells treated with ammonium chloride, glutamate, and PKCε inhibitor (G + N + D + In), possibly indicating a synergistic activity due to the neurotoxic effect of glutamate and ammonia, and the reduction of metabolic activity of PKCε. Interestingly, cell viability of cortical neuronal cells pretreated with glutamate, ammonium chloride, and PMA (G + N + P) was significantly increased compared to the other treatments (****p < 0.0001) and slightly, but not significantly, increased with respect to the control (untreated). In cerebellar granule neurons, a similar trend for the (G + N + P) treatment with respect to the other treatments was observed; however, a significant increase (*p < 0.05) with respect to the control (untreated) was further observed. This result is compatible with the intrinsic activity of PMA, which is an activator of PKC, including the γ isoform, and acts by stimulating the metabolic activity of cells. Moreover, the co-treatment with DHEA and PKCε inhibitor brings the value of cell viability back to that of the cells treated with glutamate and ammonia, in both cortical and granule cells. These latter data confirmed the neuroprotective role played by DHEA through the involvement of the modulation of PKCε.
As previously described, HE is a severe neuropsychiatric syndrome that arises during the presence of severe liver damage and is characterized by a general depression of the CNS induced by an increase of the GABA-ergic tone. In particular, this hypertonia of the GABA-ergic system can be interpreted as the final result of a series of enzymatic, metabolic, and degenerative modifications that occur in astrocytes and in neurons as well.
Although the pathogenesis of HE is not yet fully known, the accumulation of ammonium in the brain has been identified as the main factor able to trigger biochemical alterations that lead to CNS depression. The increase in GABA-ergic tone along with the degeneration of astrocytic cells contributes to the onset of HE. The neuronal alterations seem to be identified in an imbalance between inhibitory and excitatory systems.
It is widely demonstrated that the neurosteroid DHEA performs a neuro-protective action in the various models of insult (28). This action seems to take place with multiple action mechanisms both at ion channels and cell metabolism level. However, despite this evidence, it has not been elucidated yet whether there is a single pathway modulated by DHEA depending on the experimental insult.
The so-called novel ε isoform of PKC is particularly present within the CNS, more specifically at the level of the nerve endings of the cerebellum, hippocampus, and cortex where it is mostly expressed (29, 30). In particular, in these brain areas, the PKCε, through its catalytic activity, negatively modulates the activity of the GABAA receptor complex through a decrease in the Cl− currents induced by GABA. Since it has been reported that DHEA was able to activate typical and atypical PKC isoforms such α, β, and ζ in different tissues (31, 32), we sought a potential modulation of PKCε exerted by DHEA in our HE model, therefore unveiling a possible new molecular mechanism of DHEA neuroprotection.
Our results revealed that DHEA action significantly improved locomotor activity after 24 h with respect to the animals treated with D-galactosamine-induced hepatic encephalopathy.
Indeed, in animals treated with the DHEA, the ability of the animals to increase the total distance traveled was increased significantly. This result is in agreement with a decrease of rat's stress/anxiety level, since it is well-known that benzodiazepine, such as diazepam and 5-HT1A agonist, was able to increase the locomotion in the open field experimental model (33). It is noteworthy that this parameter is of importance in HE since it has been demonstrated that HE mimics all the major psychiatric syndromes (34).
This result, together with the finding that DHEA protects the cultured neurons from the toxicity elicited by NH4Cl, suggests that the effect of the neurosteroid could be ascribed to both its neuroprotective effect and to an increase in PKCε expression.
Indeed, the PKCε protein expression increased in both cerebellar and cortical tissue in DHEA-treated animals compared to D-galactosamine although to different extents. The effect was particularly relevant in cerebral cortex tissue extracts, where the DHEA stimulated the expression of PKCε exceeding the level of the non-treated animals. These findings strongly suggest a direct modulation elicited by the neurosteroid on the PKCε activity and/or expression.
In fact, although the in vitro results seem to confirm the data obtained in the HE animal model, regarding the reduction of the PKCε expression in both cortical and cerebellar granule neuron cells following the glutamate + ammonium chloride treatment, the treatment with DHEA did not reflect precisely the results obtained in the animal model. Indeed, whereas we found an increase in the protein expression following the DHEA treatment in cortical neuronal cells, in the cerebellar granule neurons, this increment was not detectable. This discrepancy could be addressed to the intrinsic difference between the two models, although a post-translational regulation, rather than an PKCε gene expression modulation, cannot be excluded.
Although this finding needs further investigations, the results obtained on cell viability indicate a neuroprotection elicited by DHEA, through the involvement of PKCε.
Indeed, pretreatment of cerebellar neurons and granule cells with DHEA determined an increase of survival in comparison to the effect exerted by ammonium chloride and glutamate neurotoxins. Moreover, the use of a PKC activator, PMA, also led to a cell survival increase supporting the idea of a direct involvement of PKC activation. Interestingly, the treatment with combination of DHEA and PKCε inhibitor led to a decreased cell survival, reinforcing the hypothesis that the neuroprotective action of DHEA could occur through the involvement of the PKCε. In this context, it is reasonable to hypothesize that DHEA might decrease the GABA-ergic hypertonia by increasing the PKCε.
In fact, it has been shown that in knockout experiments for the gene encoding PKCε, there was an increase in the sensitivity of the GABAA receptor to its allosteric modulators such as benzodiazepines, barbiturates, and neurosteroids, in brain areas including cerebellum and cortex (35).
Although more experiments are necessary to better understand the molecular mechanism, which involves DHEA in the PKCe translocation, our results showed, for the first time, a neuroprotective effect of DHEA through the stimulation of the PKCε, suggesting that molecules able to promote the expression of such protein could be useful for the symptomatic treatment of HE.
In summary, the results obtained show an association between the DHEA-mediated increase in PKCε expression and the improvement of comatose symptoms of the HE animal model. This finding supports the idea that PKCε activation and expression at the brain level significantly inhibit GABA-ergic tone counteracting HE symptoms. In addition, our results have clearly shown that DHEA was able to ameliorate the symptoms of HE in animal models and also to increase the expression of PKCε in rat brain cortex and cultured primary neuronal cells as well.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by Bioethical Committee of the Italian Institute of Health.
LC contributed to the conception and design of the study. LC, MZ, RA, LR, and GC performed the experiments. AD and CM performed the statistical analyses. AD and LC wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2021.695375/full#supplementary-material
1. Ferenci P. Hepatic encephalopathy. Gastroenterol Rep. (2017) 5:138–47. doi: 10.1093/gastro/gox013
2. Rose CF, Amodio P, Bajaj JS, Dhiman RK, Montagnese S, Taylor-Robinson SD, et al. Hepatic encephalopathy: novel insights into classification, pathophysiology and therapy. J Hepatol. (2020) 73:1526–47. doi: 10.1016/j.jhep.2020.07.013
3. Butterworth RF, Norenberg MD, Felipo V, Ferenci P, Albrecht J, Blei AT, et al. Experimental models of hepatic encephalopathy: ISHEN guidelines. Liver Int. (2009) 29:783–8. doi: 10.1111/j.1478-3231.2009.02034.x
4. Lizardi-Cervera J, Almeda P, Guevara L, Uribe M. Hepatic encephalopathy: a review. Ann Hepatol. (2003) 2:122–30. doi: 10.1016/S1665-2681(19)32137-4
5. Córdoba J. New assessment of hepatic encephalopathy. J Hepatol. (2011) 54:1030–40. doi: 10.1016/j.jhep.2010.11.015
6. Kaplan PW, Rossetti AO. EEG patterns and imaging correlations in encephalopathy: encephalopathy part II. J Clin Neurophysiol. (2011) 28:233–51. doi: 10.1097/WNP.0b013e31821c33a0
7. Arguedas MR, Delawrence TG, Mcguire BM. Influence of hepatic encephalopathy on health-related quality of life in patients with cirrhosis. Dig Dis Sci. (2003) 48:1622–6. doi: 10.1023/A:1024784327783
8. Zhou Y, Eid T, Hassel B, Danbolt NC. Novel aspects of glutamine synthetase in ammonia homeostasis. Neurochem Int. (2020) 140:104809. doi: 10.1016/j.neuint.2020.104809
9. Raabe W. Effects of hyperammonemia on neuronal function: NH4+, IPSP and Cl(-)-extrusion. Adv Exp Med Biol. (1993) 341:71–82. doi: 10.1007/978-1-4615-2484-7_7
10. Haussinger D, Kircheis G, Fischer R, Schliess F, Vom Dahl. S. Hepatic encephalopathy in chronic liver disease: a clinical manifestation of astrocyte swelling and low-grade cerebral edema? J Hepatol. (2000) 32:1035–38. doi: 10.1016/S0168-8278(00)80110-5
11. Jover R, Rodrigo R, Felipo V, Insausti R, Saez-Valero J, Garcia-Ayllon MS, et al. Brain edema and inflammatory activation in bile duct ligated rats with diet-induced hyperammonemia: a model of hepatic encephalopathy in cirrhosis. Hepatology. (2006) 43:1257–66. doi: 10.1002/hep.21180
12. Hadjihambi A, De Chiara F, Hosford PS, Habtetion A, Karagiannis A, Davies N, et al. Ammonia mediates cortical hemichannel dysfunction in rodent models of chronic liver disease. Hepatology. (2017) 65:1306–18. doi: 10.1002/hep.29031
13. Gorg B, Schliess F, Haussinger D. Osmotic and oxidative/nitrosative stress in ammonia toxicity and hepatic encephalopathy. Arch Biochem Biophys. (2013) 536:158–63. doi: 10.1016/j.abb.2013.03.010
14. Baraldi M, Zeneroli ZL. Experimental hepatic encephalopathy: changes in the binding of gamma-aminobutyric acid. Science. (1982) 216:427–9. doi: 10.1126/science.6280279
15. Nasehi M, Mafi F, Ebrahimi-Ghiri M, Zarrindast MR. Function of opioidergic and dopaminergic antagonists on both spatial and object novelty detection deficits induced in rodent model of hepatic encephalopathy. Behav Brain Res. (2016) 313:58–66. doi: 10.1016/j.bbr.2016.07.011
16. Quinn TA, Robinson SR, Walker D. Ehydroepiandrosterone (DHEA) and DHEA sulfate: roles in brain function and disease. In: Drevenšek G, editor. Sex Hormones in Neurodegenerative Processes and Diseases. London: IntechOpen (2017).
17. Basile AS, Hughes RD, Harrison PM, Murata Y, Pannell L, Jones EA, et al. Elevated brain concentrations of 1,4-benzodiazepines in fulminant hepatic failure. N Engl J Med. (1991) 325:473–8. doi: 10.1056/NEJM199108153250705
18. Butterworth RF. Neurosteroids in hepatic encephalopathy: novel insights and new therapeutic opportunities. J Steroid Biochem Mol Biol. (2016) 160:94–7. doi: 10.1016/j.jsbmb.2015.11.006
19. Ahboucha S, Talani G, Fanutza T, Sanna E, Biggio G, Gamrani H, et al. Reduced brain levels of DHEAS in hepatic coma patients: significance for increased GABAergic tone in hepatic encephalopathy. Neurochem Int. (2012) 61:48–53. doi: 10.1016/j.neuint.2012.03.020
20. Krishek BJ, Xie X, Blackstone C, Huganir RL, Moss SJ, Smart TG. Regulation of GABAA receptor function by protein kinase C phosphorylation. Neuron. (1994) 12:1081–1095. doi: 10.1016/0896-6273(94)90316-6
21. Giordano G, Sanchez-Perez AM, Burgal M, Montoliu C, Costa LG, Felipo V. Chronic exposure to ammonia induces isoform-selective alterations in the intracellular distribution and NMDA receptor-mediated translocation of protein kinase C in cerebellar neurons in culture. J Neurochem. (2005) 92:143–57. doi: 10.1111/j.1471-4159.2004.02852.x
22. Grau E, Marcaida G, Montoliu C, Minana MD, Grisolia S, Felipo V. Effects of hyperammonemia on brain protein kinase C substrates. Metab Brain Dis. (1996) 11:205–16. doi: 10.1007/BF02237958
23. Leke R, Escobar TD, Rao KV, Silveira TR, Norenberg MD, Schousboe A. Expression of glutamine transporter isoforms in cerebral cortex of rats with chronic hepatic encephalopathy. Neurochem Int. (2015) 88:32–7. doi: 10.1016/j.neuint.2015.03.005
24. Chou WH, Wang D, Mcmahon T, Qi ZH, Song M, Zhang C, et al. GABAA receptor trafficking is regulated by protein kinase C(epsilon) and the N-ethylmaleimide-sensitive factor. J Neurosci. (2010) 30:13955–65. doi: 10.1523/JNEUROSCI.0270-10.2010
25. Ishizuka T, Kajita K, Miura A, Ishizawa M, Kanoh Y, Itaya S, et al. DHEA improves glucose uptake via activations of protein kinase C and phosphatidylinositol 3-kinase. Am J Physiol. (1999) 276:E196–204. doi: 10.1152/ajpendo.1999.276.1.E196
26. Novelli A, Reilly JA, Lysko PG, Henneberry RC. Glutamate becomes neurotoxic via the N-methyl-d-aspartate receptor when intracellular energy levels are reduced. Brain Research. (1988) 451:205–12. doi: 10.1016/0006-8993(88)90765-2
27. Zeneroli ML, Ventura E, Baraldi M, Penne A, Messori E, Zieve L. Visual evoked potentials in encephalopathy induced by galactosamine, ammonia, dimethyldisulfide, octanoic acid. Hepatology. (1982) 2:532–8. doi: 10.1002/hep.1840020504
28. Borowicz KK, Piskorska B, Banach M, Czuczwar SJ. Neuroprotective actions of neurosteroids. Front Endocrinol. (2011) 2:50. doi: 10.3389/fendo.2011.00050
29. Saitoh N, Hori T, Takahashi T. Activation of the epsilon isoform of protein kinase C in the mammalian nerve terminal. Proc Natl Acad Sci USA. (2001) 98:14017–21. doi: 10.1073/pnas.241333598
30. Vetri F, Chavez R, Xu HL, Paisansathan C, Pelligrino DA. Complex modulation of the expression of PKC isoforms in the rat brain during chronic type 1 diabetes mellitus. Brain Res. (2013) 1490:202–9. doi: 10.1016/j.brainres.2012.10.032
31. Kajita K, Ishizuka T, Miura A, Ishizawa M, Kanoh Y, Yasuda K. The role of atypical and conventional PKC in dehydroepiandrosterone-induced glucose uptake and dexamethasone-induced insulin resistance. Biochem Biophys Res Commun. (2000) 277:361–7. doi: 10.1006/bbrc.2000.3677
32. Aoki K, Tajima K, Taguri M, Terauchi Y. Effect of dehydroepiandrosterone (DHEA) on Akt and protein kinase C zeta (PKCζ) phosphorylation in different tissues of C57BL6, insulin receptor substrate (IRS)1–/–, and IRS2–/– male mice fed a high-fat diet. J Steroid Biochem Mol Biol. (2016) 159:110–20. doi: 10.1016/j.jsbmb.2016.03.011
33. Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. (2003) 463:3–33. doi: 10.1016/S0014-2999(03)01272-X
34. Telles-Correia D, Joao Freire M, Mega I, Barreiras D, Cortez Pinto. H. Anxiety and depression symptoms in hepatic encephalopathy: are they psychiatric or organic? Transplant Proc. (2015) 47:1005–7. doi: 10.1016/j.transproceed.2015.03.011
Keywords: hepatic encephalopathy, hyperammonemia, protein kinase C (PKC), DHEA, animal model
Citation: Di Cerbo A, Roncati L, Marini C, Carnevale G, Zavatti M, Avallone R and Corsi L (2021) Possible Association Between DHEA and PKCε in Hepatic Encephalopathy Amelioration: A Pilot Study. Front. Vet. Sci. 8:695375. doi: 10.3389/fvets.2021.695375
Received: 14 April 2021; Accepted: 25 August 2021;
Published: 28 September 2021.
Edited by:
Maria Aranzazu Martinez, Complutense University of Madrid, SpainReviewed by:
Julio César Morales-Medina, Instituto Politécnico Nacional de México (CINVESTAV), MexicoCopyright © 2021 Di Cerbo, Roncati, Marini, Carnevale, Zavatti, Avallone and Corsi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alessandro Di Cerbo, YWxlc3NhbmRyby5kaWNlcmJvQHVuaWNhbS5pdA==; Lorenzo Corsi, TG9yZW56by5jb3JzaUB1bmltb3JlLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.