
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 26 August 2021
Sec. Zoological Medicine
Volume 8 - 2021 | https://doi.org/10.3389/fvets.2021.694354
 Steven Barajas-Valero1,2*†
Steven Barajas-Valero1,2*† Cristian Rodríguez-Almonacid1*†
Cristian Rodríguez-Almonacid1*† Zulma Rojas-Sereno3
Zulma Rojas-Sereno3 Carlos Moreno-Torres2
Carlos Moreno-Torres2 Nubia E. Matta1
Nubia E. Matta1The Orinoco crocodile (Crocodylus intermedius, Graves, 1918) is the most threatened crocodilian of South America. There is only scarce information available about the physiology of this neotropical crocodile. This study aimed to propose baseline hematological and biochemistry reference data and intervals and a morphological description of the peripheral blood cells of captive C. intermedius. Blood was collected from 318 clinically healthy individuals maintained in captivity at Villavicencio, Colombia. Eight of these individuals were sampled and resampled, and these data were compared. Reference intervals were proposed for hematological values [packed cell volume (PCV), red blood cell count, white blood cell count, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, hemoglobin, and white blood cell count differential counts] and biochemistries [total solids, alanine aminotransferase (ALT), aspartate aminotransferase, alkaline phosphatase, lactate dehydrogenase, creatine kinase, glucose, albumin, cholesterol, uric acid, creatinine, and lactate] including adults and juveniles, males and females' crocodiles. Blood cell morphology for the species is described. Significant differences between sex and age were observed. The intraindividual analysis concluded differences for total solids (P ≤ 0.01) and red blood cell counts (P ≤ 0.01). Some biochemical analytes showed a moderate correlation between them, such as ALT–alkaline phosphatase and ALT–uric acid. We present here novel and baseline data with special importance for the clinical diagnosis, improving the national reintroduction programs from either in situ and ex situ populations.
The Orinoco crocodile (Crocodylus intermedius) is distributed in the Orinoquean basin in the wetlands of Colombia and Venezuela and is one of the six crocodilian species found in Colombia (1, 2). This species is the most threatened neotropical crocodilian due to historical overexploitation and other anthropic interventions (3–5). It is classified as critically endangered in the International Union for Conservation of Nature's endangered species categories and in Appendix I by Convention on International Trade in Endangered Species of Wild Fauna and Flora (6, 7).
The national ex situ conservation program has been partially successful with reintroduction into various Orinoquean watersheds (6, 8, 9). However, research on C. intermedius has focused primarily on ecology and population status with few veterinary health investigations for the species in Colombia (10–12).
Veterinary diagnostics for crocodilian species rely mainly on postmortem examination, although they are globally maintained in controlled facilities for conservation and other purposes (13). Because reptiles and especially crocodilians do not exhibit early signs of discomfort or disease, routine observations are not practical or neither recommended. However, blood testing could offer more information and be performed twice per year for preventive medical programs (14, 15).
This leads to a frequent and tedious issue with reptiles; the lack of baseline information limits the application of diagnostic tools (16). The establishment of hematological and cytological reference data and intervals is essential for obtaining baseline criteria for a health assessment (17). Such testing is useful for monitoring of reintroductions and immunological and/or environmental toxicology studies (18–20).
This study aimed to propose baseline reference data and reference intervals for hematology and serum biochemistry and provide a peripheral blood cell description for captive C. intermedius in Colombia.
The Science Faculty ethics committee of the Universidad Nacional de Colombia approved the methodology for this research through N° 03-2019.
Blood samples from 318 clinically healthy C. intermedius were collected during two periods that cover 6 years (2010–2013 and 2019–2020). All individuals were held in captivity at the Estación Biológica Tropical Roberto Franco of the Universidad Nacional de Colombia, located in Villavicencio, Meta, Colombia (latitude 4.13°, longitude 73.63°), at 419 meters above mean sea level. Local temperature oscillates between 20 and 32°C with a mean relative humidity of 76%, an average annual rainfall of 4.008 mm, and a unimodal rainfall regime. Sex and age were recorded for all individuals. Juvenile and subadults were sampled during the first phase; only subadults were sampled in the second phase. Individuals did not receive any medical treatment or intervention 3 months before sampling. Morphometry and weight data were documented for 41 individuals in the second phase. Eight individuals were sampled twice during the 6-year period.
Individuals were captured and physically restrained (21). To diminish lymphatic hemodilution possibility, blood was obtained from the ventral coccygeal vein using a 5-ml syringe without anticoagulant, with a 21 G*1½ -inch needle. Immediately after sample obtention, four thin blood smears per individual were prepared with fresh non-heparinized blood using the slide-to-slide technique. Blood smears were dried using low airflow at environmental temperature and fixed with absolute methanol for 5 min. Whole blood was placed in sodium heparin vials (Liquemine, Roche) and carefully mixed. These vials were stored at 4°C and processed within the next 8 h after sampling. Serum was obtained using separating gel vials (Liu yang Sanli Medical Technology Development Co Ltd., China), centrifugated at 4,000 rpm per 10 min, and then stored at −20°C for subsequent analysis.
Blood smears were stained using Wright and Giemsa. Microscopic evaluation was carried out with an Olympus CX41 microscope (Olympus Corp., Tokyo, Japan). Red blood cell counts (RBC) and white blood cell counts (WBC) were performed manually with Neubauer chamber and Natt–Herrick solution [1:100 dilution; ((22), p. 735–738)]. The corner squares and central one (0.4 mm each) in the center (1 mm) of the Neubauer chamber were considered for RBC. Differential leukocyte counts were performed on Wright-stained blood smears based on 100 counted leukocytes in the 100× objective (23–32). The PCV was determined using the microhematocrit method [5 min at 12,000 rpm; ((33), p. 131–141)]. The hemoglobin (HGB) concentration was measured with spectrophotometry using the BTS-350 equipment (Biosystem, Spain). Mean corpuscular volume, mean corpuscular HGB, and mean corpuscular HGB concentration (MCHC) indices were calculated following Eatwell et al. (23).
Total solids (TSs) were determined using hand refractometry (Scientific, China). Serum biochemistry analyses were performed by spectrophotometry using Biosystem BTS-350 (Biosystem, Spain) and Spinreact kits (Spinreact, Spain). Measured analytes included glucose, aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, alkaline phosphatase (ALP), cholesterol, uric acid (UA), creatinine (CREA), creatine kinase (CK), lactate, and lactate dehydrogenase (LDH).
To determine if there were differences between hematological and serum biochemistry parameters within the same individual in juvenile and adult stages (5-year interval), intraindividual comparisons were made among PCV, TS, mean corpuscular volume, ALT, RBC's, and WBC's relative and absolute counts.
Digital microphotographs of blood cells were obtained at 100× objective using an Olympus DP27 digital camera and processed with cellSens Standard 1.13 software (Olympus, Tokyo, Japan). Measurements were processed with ImageJ® software [(34), p. 671–675] for mature erythrocytes, polychromatophils, thrombocytes, heterophiles, lymphocytes, eosinophils, monocytes, and basophils.
Reference intervals (RIs) for hematology and serum biochemistry data were generated in accordance with the American Society of Veterinary Clinical Pathology guidelines (35). To verify data distribution, Shapiro–Wilk and Kolmogorov–Smirnov with Lilliefors correction tests were applied according to the sample size. Outlier detection was performed through the Tukey test with the Carling modification and robust kernel-based local outlier detection index. The values 2.5 and 97.5% were considered as lower and upper limits for the RI, respectively. Ninety percent confidence intervals for each one of the limits were calculated. The Mann–Whitney test for independent samples was implemented for sex and age (juvenile and adult) group comparison.
Intraindividual comparisons were developed by Wilcoxon paired samples testing for non-parametric data or a T-paired test for parametric data. Correlation analyses were made between the following paired analytes: CK–AST, CK–LDH, CREA–UA, ALT–LDH, ALT–ALP, ALT–UA, and ALT–cholesterol, using Spearman's rho or Pearson tests. Simple and multiple linear regression for hematology and biochemistries vs. weight and total length morphometrics were explored. All analyses were carried out with RStudio® (v4.0.0) software. Statistical significance for all analyses was set at P < 0.05.
A total of 318 clinically healthy individuals (249 adults and 69 juveniles; 254 of them females, 52 males, and 12 not sex-identified) were sampled. Bodyweight (50.14 ± 10.6 kg) and total length (224.03 ± 20 cm) showed a direct proportional correlation (P = ≤ 0.01, rho = 0.77; Supplementary Figure 1).
Hematological and serum biochemistry RIs for all C. intermedius were established (Table 1). Heterophils were the most frequent leukocyte (33–81%), followed by lymphocytes (up to 50%) and basophils (till 16%). Significant differences between sexes were observed, where females had higher values for PCV (P ≤ 0.01), CK (P = 0.03), MCHC (P = 0.04), HGB (P ≤ 0.01), ALP (P = 0.03), CREA (P = 0.01), RBC (P = 0.04) and WBC (P = 0.01; Supplementary Table 1). Significant differences between ages for TS (P ≤ 0.01) and RBC (P = 0.02; Supplementary Table 1) were observed, where adults had higher values for both analytes. Most of those differences were within the established ranges in this study; groups with substantial differences were presented separately (Table 2).
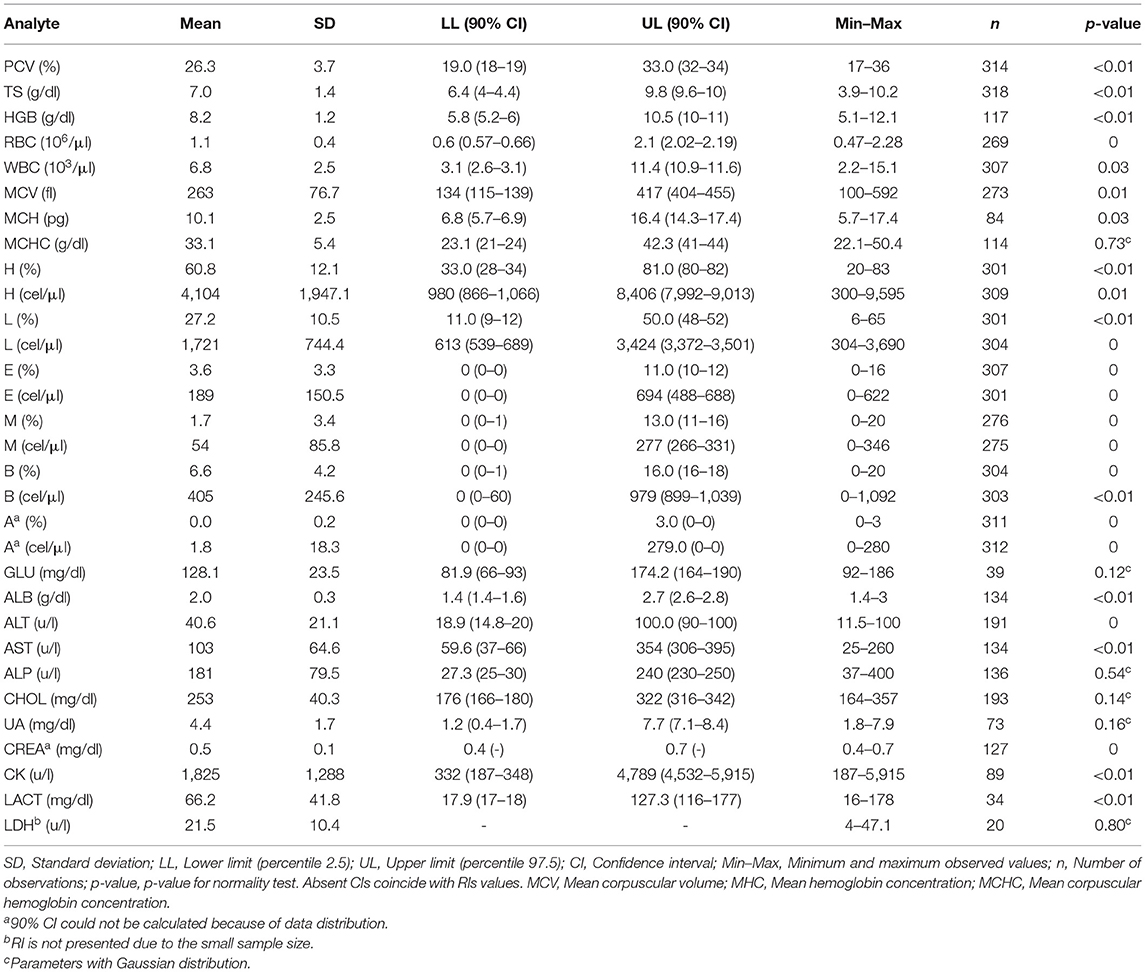
Table 1. Hematological and biochemical reference values and intervals (90% CI) for captive Crocodylus intermedius.
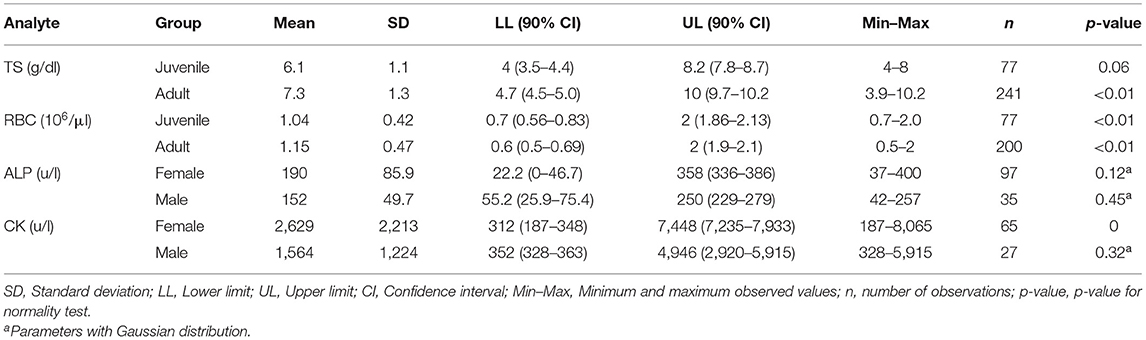
Table 2. Hematological and biochemical reference values and intervals for captive Crocodylus intermedius' parameters with statistical differences according to sex/age groups.
Morphological description of peripheral blood cells of C. intermedius using Giemsa (G) and Wright (W) stains are shown in Table 3 and Figure 1. Morphometric parameters are shown in Table 4. Polychromatophils appeared as larger cells than mature erythrocytes (148.1 ± 8.87 μm vs. 108.8 ± 14.4 μm cell area). Azurophils were rarely seen, and they could be easily mistaken or missed in deficient smears (under or overstained). One adult exhibited vacuolated thrombocytes with slight nucleus displacement (Figure 2). All the blood smears were deposited in the GERPH Biological Collection – Biology Department, Universidad Nacional de Colombia.

Table 3. Morphology description of Crocodylus intermedius' peripheral blood cells on light microscopy.
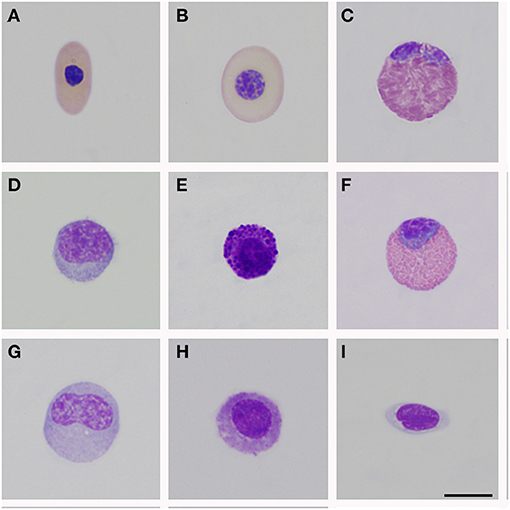
Figure 1. Peripheral blood cells of Crocodylus intermedius. (A) Mature erythrocyte; (B) Polychromatophil; (C) Heterophil; (D) Mature lymphocyte; (E) Basophil; (F) Eosinophil; (G) Monocyte; (H) Azurophil-like; (I) Thrombocytes. Bar: 10 μm.
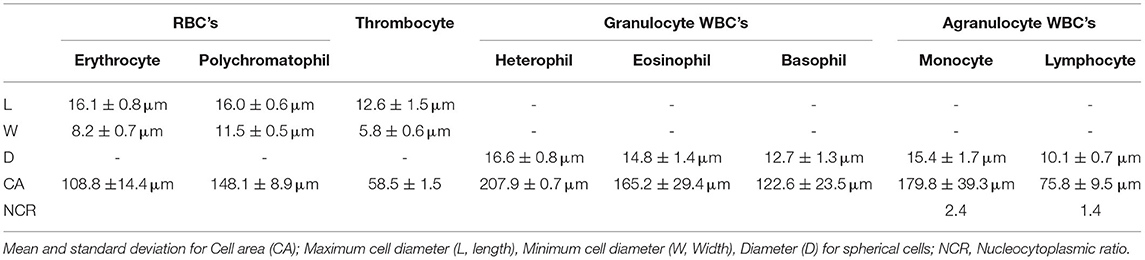
Table 4. Basic morphometric aspects on light microscopy for peripheral blood cells of Crocodylus intermedius.
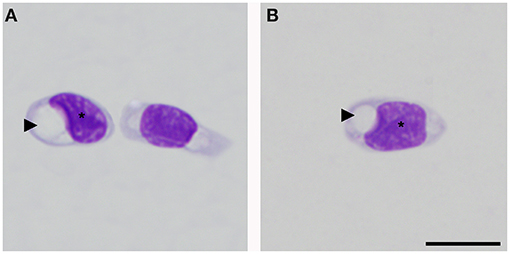
Figure 2. Thrombocytes of Crocodylus intermedius that showed an irregular appearance. Cytoplasm with the large colorless vacuole (arrowhead), note the deformed and slightly displaced nucleus (asterisk), and the normal coloration of thrombocyte cytoplasm (right side cell on A). Bar: 10 μm.
Intraindividual analysis showed significant differences for TS (P ≤ 0.01) and RBC (P ≤ 0.01), although all parameters were within the established range. For both parameters, values were higher when individuals were juvenile (Supplementary Table 2).
Moderate correlations were found between ALT–ALP (P ≤ 0.01, rho = 0.63) and ALT–UA (P ≤ 0.01, rho = 0.47; Figure 3). No lineal model could explain the hematological or biochemical variables as a function of bodyweight or total length.
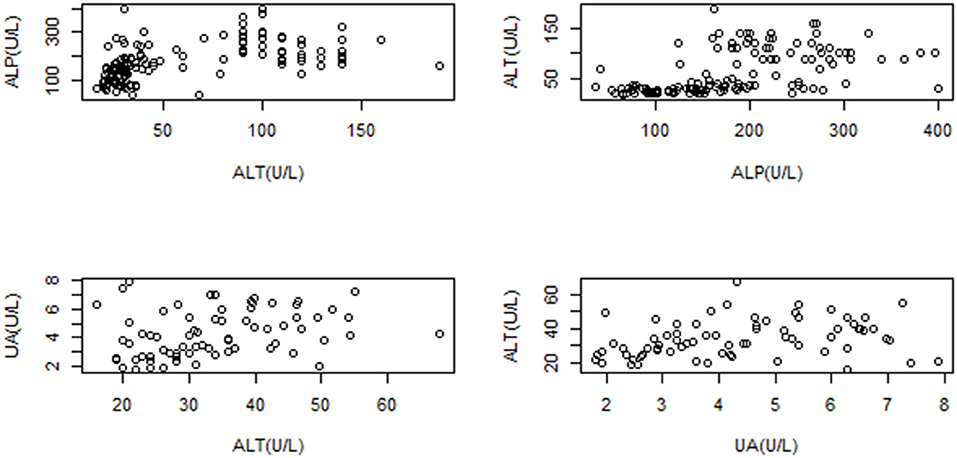
Figure 3. Dispersion correlation displayed for ALT-ALP (P ≤ 0.05, rho = 0.6353); and ALT-UA (P ≤ 0.01, rho = 0.4716) of Crocodylus intermedius, showing moderate correlation.
This study represents the most extensive sampling of a captive population of C. intermedius. Here, we report baseline data for hematology, serum biochemistry, and blood cell morphometrics.
The C. intermedius' WBC showed similar ranges to captive individuals of Crocodylus niloticus and Crocodylus moreletti; however, the values were lower than those observed in wild individuals of the same species (Supplementary Table 4). Lower frequency of pathogen exposure and aggressions, as product of controlled settings, and reduced competition and then seasonal induced stress could be determinant factors for the lower WBC in captive crocodiles; in contrast, turtles and other reptiles generate higher leukocyte counts secondary to captivity stress (19, 36, 37). Heterophils were the most frequent leukocyte, agreeing with the Manzanilla et al. (11). However, this differed from other crocodilians with predominant lymphocyte leukograms (ranging up to 80%; Supplementary Table 4). Species-specific understanding of the baseline data of diagnostic tools is essential for posterior applicability (38). Based on this, the relationship between maintenance conditions and the leukogram spectrum among crocodilians must be studied to clarify this species' actual findings.
Higher RBC, PCV, HGB, and MCHC concentrations of females recall C. niloticus and C. moreletti findings, whereas the higher WBC observed in females differs from those species and Crocodylus palustris, where males show slight to markedly higher values [(39); Supplementary Tables 1, 4]. Intraindividual analyses showed significant differences in TS, RBC, and PCV; higher values were found in juveniles. Statistical differences for the same parameters were observed on the general sample, but higher values corresponded to adults. Previous reports of C. niloticus show significant differences for PCV, HGB, total protein, globulins, and AST concentrations according to the age category; likewise, higher values correspond to adult samples (13). According to this, we determined that certain analytes changed during the covered period for crocodiles, as it has been reported for other reptiles (40). Furthermore, we also recognized an absence of strict linearity for age-related analytes' shifts. Actually, one must consider that changes across time could be influenced by other intrinsic and/or extrinsic factors (41). Further studies focusing on intraindividual analyzes are desirable and should give an overview of analytes' behavior over time.
They normally proposed ALT values for reptiles are lower or equal to 20 U/L, whereas previous reports for the genus Crocodylus sp. document upper limits near 60 U/L (13). However, our results showed an upper limit of 100 U/L. Although ALT is a non-organ-specific enzyme, high values should not be taken as indicators of hepatic pathology by themselves; neither can they be strictly associated with specific pathologies without further clinical information and analyses (23). UA values varied across upper and lower limits against the previous reports for Crocodylus sp. (Supplementary Table 4). Feeding habits and husbandry conditions influence both ALT and UA and other analytes. Further studies might consider including wild and captive populations to determine how seasonality, feeding frequencies, quality, and variety of diet interact with biochemistries or hematology in the species.
Some biochemistries by themselves might not be tissue-specific on normal or pathological conditions, as we mentioned. However, when correlated analyses are carried out, a concerted behavior among analytes could provide more information about a target tissue (e.g., ALT with ALP or UA; could offer evidence about either liver or kidney tissue, respectively). Then, we proposed a correlation analysis between biochemistries as a possibility for a wider comprehension of available analytes, whose primary utility is still not clearly elucidated on reptile clinical pathology. This could be considered preliminary data for further controlled and extensive studies to understand their clinical applicability.
Sex-related differences for biochemistries such as CK, ALP, and CREA have not been reported previously; either way, formerly cited factors, and additional ones, such as the amount of displacement and enclosure type/size, could be related to higher values on females and/or additional variations.
Our limited body morphometric data (sub-adult sample) could be the reason for absent correlations between body morphometry and hematological or biochemical values in the current study. In contrast, Manzanilla et al. (11) report negative correlations for total length vs. HGB and total length vs. WBC; nevertheless, Scheelings et al. (42) find no correlations.
Peripheral blood cell morphology was akin to that reported for crocodilians and other reptile species (24–26, 43). Usually, polychromatophils are described as similar-sized or smaller than mature erythrocytes for birds and reptiles (27, 28). However, an inverse size proportion for C. intermedius was observed (Table 4). Azurophil-like cells were infrequently seen. Colorless and large vacuoles were observed in one individual's thrombocytes. Clear vacuoles have been described in lizard thrombocytes as glycogen storage with positive staining with Schiff's periodic acid (29). Also, Progarnia infection is described as thrombocyte-related parasites in crocodilians, causing cell morphological variations (30). However, we did not see chromatin nor other parasitic-like morphology. Supplementary techniques must be performed to clarify this isolated finding. We endorse cytochemical analysis to distinguish these and other unclassified cells and for elucidating morphological alterations.
This is the first study of hematology, biochemistry, and blood cell morphometrics of Colombian populations of C. intermedius. Intraindividual analyses could offer some valuable physiological data and deserves further studies. Sex- and age-based variations for both hematological and biochemistries on the species must be considered for routine evaluations. By preference, biochemical parameters and their correlations should be in-depth studied for assumptions about their diagnostic value among crocodiles and reptile species (38). Displayed data provide baseline information for health assessment. Likely, due to the influence of environmental, diet, husbandry, and stress factors, the reference intervals obtained were wide, as it is frequently observed in reptiles (16, 25). This work contributes as a useful tool for veterinary health assessment in reproduction and reintroduction programs for the species.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by Science Faculty Ethics Committee, Universidad Nacional de Colombia.
Manuscript writing and editing, statistical analyses, and graphics and tables assembling developed by SB-V and CR-A. Statistical design and database arrangement accomplished by ZR-S and SB-V. Project scheme in charge of NM and CM-T. Literature compilation, morphological cells studies, and microphotographs developed by SB-V. Second sampling phase (2019-2020, sampling, processing, storage, and database transcription) was carried out by CR-A and CM-T. CM-T collected first phase samples (2010-2013) and offered the dataset. All authors contributed to the article and approved the submitted version.
This work was funded by the Research Division of the Universidad Nacional de Colombia under project N° 42105 and 50839. The National Agency for Research and Development (ANID) thorough Beca Doctorado Nacional No. 21201076, awarded by ZR-S.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank the Estación Biológica Tropical Roberto Franco members, Grupo de Estudio Relación Parásito-Hospedero members, statistical department (Gabriel Alfonso Patron Herrera, Joan Gabriel Bofill Barrera, Laura Daniela Villamarin Trejos, and Luis Alejandro Jiménez Avendaño), and Veterinary Clinic Laboratory of the Universidad Nacional de Colombia for their collaboration during the development.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2021.694354/full#supplementary-material
ALT, Alanine aminotransferase; ALP, Alkaline phosphatase; UA, Uric acid; RBC, Red blood cell count; WBC, White blood cell count; PCV, Packed cell volume; HGB, Hemoglobin; MCHC, Mean corpuscular hemoglobin concentration; TS, Total solids; AST, Aspartate aminotransferase; CREA, Creatinine; CK, Creatine kinase; LDH, Lactate dehydrogenase; RI, Reference interval.
1. Barahona-Buitrago S, Bonilla-Centeno O. Evaluación del estatus poblacional del Caimán Llanero (Crocodylus intermedius Graves, 1819) en un subáreal de distribución en el Departamento de Arauca (Colombia). Acad Colomb Cien. (1999) 23:445–51.
2. Medem F. Los Crocodylia de Suramérica: Los Crocodylia de Colombia, Volumen II. Bogotá DC: Colciencias-Universidad Nacional de Colombia (1983).
3. Clavijo J, Anzola F. Elementos claves para la conservación in situ de Crocodylus intermedius derivados del seguimiento de metapoblaciones y hábitats en Arauca, Colombia. Rev Colomb Ciencia Animal. (2013) 5:560–573. doi: 10.24188/recia.v5.n2.2013.465
4. Medem F. Los Crocodylia de Suramérica: Los Crocodylia de Colombia, Volumen I. Bogotá DC: Colciencias (1981).
5. Morales-Betancourt M, Lasso C, De La Ossa J, Fajardo-Patiño A. Biología y Conservación de los Crocodylia de Colombia. Bogotá D.C: Serie Editorial Recursos Hidrobiológicos y Pesqueros Continentales de Colombia. Instituto de Investigación de Recursos Biológicos Alexander von Humboldt (IAvH) (2013). doi: 10.21068/B001.2014.204
6. Balaguera-Reina SA, Espinosa-Blanco A, Antelo R, Morales-Betancourt M, Seijas A. Crocodylus intermedius. IUCN Red List of Threatened Species (2018). doi: 10.2305/IUCN.UK.2018-1.RLTS.T5661A3044743.en
7. Morales-Betancourt M, Lasso C, Martínez W, Ardila-Robayo C, Bloor P. Caimán llanero, Crocodylus intermedius (Graves, 1819). In: Morales-Betancourt MA, Lasso CA, Páez y VP, Bock BC, editors. Libro rojo de reptiles de Colombia edited by Morales-Betancourt et al. 186-190. Bogotá, DC: Instituto de Investigación de Recursos Biológicos Alexander Von Humboldt (IAvH). Universidad de Antioquia (2015).
8. Anzola F, Antelo R. First data of natural recovery of any Orinoco crocodile Crocodylus intermedius population: evidence from nesting. Herpetol Bull. (2015) 134:10–4. Available online at: https://www.thebhs.org/publications/the-herpetological-bulletin/issue-number-134-winter-2015/819-03-first-data-of-natural-recovery-of-any-orinoco-crocodile-i-crocodylus-intermedius-i-population-evidence-from-nesting?format=html
9. Moreno-Arias R, Ardila-Robayo C. Journeying to freedom: the spatial ecology of a reintroduced population of Orinoco crocodiles (Crocodylus intermedius) in Colombia. Animal Biotelemetry. (2020) 8:15. doi: 10.1186/s40317-020-00202-2
10. Boede E, Sogbe E. Enfermedades en caimanes del Orinoco (Crocodylus intermedius) y caimanes de la costa (Crocodylus acutus) mantenidos en zoocriaderos venezolanos. Rev Científ Facult Ciencias Vet. (2000) 10:328–339. Available online at: https://produccioncientificaluz.org/index.php/cientifica/article/view/14718
11. Manzanilla A, Seijas A, Rossini M. Valores hematológicos en ejemplares jóvenes de caimán del Orinoco (Crocodylus intermedius) en Venezuela. Rev Científ. (2011) 4:360–56. Available online at: https://produccioncientificaluz.org/index.php/cientifica/article/view/15660/15634
12. Pachón D, Pulido A, Moreno C. Aislamiento e identificación de microorganismos entéricos en muestras ambientales y cloacales en Crocodylus intermedius y testudines de la Estación de Biología Tropical Roberto Franco en Villavicencio, Colombia. Rev Facult Med Vet Zoo. (2010) 57:23–24. Available online at: https://repositorio.unal.edu.co/handle/unal/30926
13. Lovely CJ, Pittman J, Leslie A. Normal haematology and blood biochemistry of wild Nile crocodiles (Crocodylus niloticus) in the Okavango Delta, Botswana. J South Afr Vet Assoc. (2007) 78:137–44. doi: 10.4102/jsava.v78i3.305
14. Fleming GJ, Fontenot D. Crocodilians (crocodiles, alligators, caiman, gharial). Fowler Zoo Wild Animal Med. (2015) 8:38–48. doi: 10.1016/B978-1-4557-7397-8.00005-0
15. Nevarez JG. Differential diagnoses by clinical signs - crocodilians. In: Mader's Reptile and Amphibian Medicine and Surgery Third edition. Divers SJ, Stahl SJ, editors. Riverport Lane: Elsevier Health Sciences (2019). doi: 10.1016/B978-0-323-48253-0.00136-7
16. Martínez-Silvestre A, Lavín S, Cuenca R. La bioquímica sanguínea en clínica de reptiles. Consult Difus Vet. (2013) 200:31–40. Available online at: http://www.amasquefa.com/uploads/136._La_bioqu_mica_sangu_nea_en_la_cl_nica_de_reptiles36.pdf
17. Maceda-Veiga A, Figuerola J, Martínez-Silvestre A, Viscor G, Ferrari N, Pacheco M. Inside the Redbox: applications of haematology in wildlife monitoring and ecosystem health assessment. Sci Total Environ. (2015) 514:322–32. doi: 10.1016/j.scitotenv.2015.02.004
18. Chung C, Cheng C, Chin S, Lee A, Chi C. Morphologic and cytochemical characteristics of asian yellow pond turtle (ocadia sinensis) blood cells and their hematologic and plasma biochemical reference values. J Zoo Wildlife Med. (2009) 40:76–85. doi: 10.1638/2008-0023.1
19. Davis AK, Maney D, Maerz J. The use of leukocyte profiles to measure stress in vertebrates: A review for ecologists. Funct Ecol. (2008) 22:760–72. doi: 10.1111/j.1365-2435.2008.01467.x
20. Dzul-Caamal R, Hernández-López A, Gonzalez-Jáuregui M, Padilla S, Girón-Pérez M, Vega-López A. Usefulness of oxidative stress biomarkers evaluated in the snout scraping, serum and Peripheral Blood Cells of Crocodylus moreletti from Southeast Campeche for assessment of the toxic impact of PAHs, metals and total phenols. Compar Biochem Physiol Part A. (2016) 200:35–46. doi: 10.1016/j.cbpa.2016.05.006
21. Vliet KA. Crocodilian capture and restraint. In: Zoo Animal and Wildlife Immobilization and Anesthesia. West, editor. Ames: John Wiley & Sons (2014). p. 313–20. doi: 10.1002/9781118792919.ch18
22. Natt MP, Herrick C. A new blood diluent for counting the erythrocytes and leucocytes of the chicken. Poultry Sci. (1952) 31:735–8. doi: 10.3382/ps.0310735
23. Eatwell K, Hedley J, Barron R. Reptile haematology and biochemistry. Practice. (2014) 36:34–42. doi: 10.1136/inp.f7488
24. Rossini M, García G. Descripción Morfológica de las Células Sanguíneas de la Baba (Caiman crocodilus crocodilus) en Vida Silvestre. Rev Facult Ciencias Vet. (2010) 51:63–70. Available online at: https://www.redalyc.org/articulo.oa?id=373139076001
25. Stacy NI, Alleman R, Sayler K. Diagnostic hematology of reptiles. Clin Lab Med. (2011) 31:87–108. doi: 10.1016/j.cll.2010.10.006
26. Zayas MA, Rodríguez H, Galoppo G, Stoker C, Durando M, Luque E, et al. Hematology and blood biochemistry of young healthy broad-snouted caimans (Caiman latirostris). J Herpetol. (2011) 45:516–24. doi: 10.1670/10-158.1
27. Campbell T. Exotic Animal Hematology and Cytology. Ames: John Wiley & Sons (2015). doi: 10.1002/9781118993705
28. Sykes JM, Klaphake E. Reptile hematology. Vet Clin North Am. (2008) 11:481–500. doi: 10.1016/j.cvex.2008.03.005
29. Heard D, Harr K, Wellehan J. Diagnostic sampling and laboratory tests. In: Girling S. and Raiti P, editors. BSAVA Manual of Reptiles Second edition. Gloucester: British Small Animal Veterinary Association (2014).
30. Lainson R. Atlas of protozoan parasites of the Amazonian fauna of Brazil. Haemosporida Reptiles. (2012) 1:49–73.
31. Millan JM, Janmaat A, Ichardson K, Chambers L, Fomiatti K. Reference ranges for biochemical and haematological values in farmed saltwater crocodile (Crocodylus porosus) yearlings. Austr Vet J. (1997) 75:814–7. doi: 10.1111/j.1751-0813.1997.tb15660.x
32. Padilla SE, Weber M, Jacobson E. Hematologic and plasma biochemical reference intervals for Morelet's crocodiles (Crocodylus moreletti) in the northern wetlands of Campeche, Mexico. J Wildlife Dis. (2011) 47:511–22. doi: 10.7589/0090-3558-47.3.511
33. Martínez-Silvestre A, Cuenca R, Lavín S. Hematología y citología sanguínea en reptiles. Clín Vet Pequeños Anim. (2011) 31:131–41. Available online at: https://core.ac.uk/download/pdf/78524277.pdf
34. Schneider CA, Rasband W, Eliceiri K. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. (2012) 9:671–5. doi: 10.1038/nmeth.2089
35. Friedrichs KR, Harr K, Freeman K, Szladovits B, Walton R, Barnhart K, et al. ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Vet Clin Pathol. (2012) 41:441–53. doi: 10.1111/vcp.12006
36. Page-Karjian A, Rafferty K, Xavier C, Stacy NI, Moore J, Hirsch S, et al. Comprehensive health assessment and blood analyte reference intervals of gopher tortoises (Gopherus polyphemus) in southeastern FL, USA. Conserv Physiol. (2021) 9:coab015. doi: 10.1093/conphys/coab015
37. Carrascal Velásquez J, Negrete H, Rojano C, Álvarez G, Chacón J, Linares J. Caracterización hematológica de hicoteas (Trachemys callirostris Gray, 1856) en Córdoba, Colombia. Rev Med Vet. (2014) 28:43–55. doi: 10.19052/mv.3180
38. Rosenberg JF, Wellehan J, Crevasse S, Cray C, Stacy NI. Reference intervals for erythrocyte sedimentation rate, lactate, fibrinogen, hematology, and plasma protein electrophoresis in clinically healthy captive gopher tortoises (Gopherus polyphemus). J Zoo Wildlife Med. (2018) 49:520–7. doi: 10.1638/2017-0183.1
39. Stacy B, Whitaker N. Hematology and blood biochemistry of captive mugger crocodiles (Crocodylus palustris). J Zoo Wildlife Med. (2000) 31:339–47. doi: 10.1638/1042-7260(2000)031<0339:HABBOC>2.0.CO;2
40. Perrault JR, Arendt M, Schwenter J, Byrd J, Harms C, Cray C, et al. Blood analytes of immature Kemp's ridley sea turtles (Lepidochelys kempii) from Georgia, USA: reference intervals and body size correlations. Conserv Physiol. (2020) 8:coaa091. doi: 10.1093/conphys/coaa091
41. Sacchi R, Mangiacotti M, Scali S, Coladonato A, Pitoni S, Falaschi M, et al. Statistical methodology for the evaluation of leukocyte data in wild reptile populations: a case study with the common wall lizard (Podarcis muralis). PLoS ONE. (2020) 15:e0237992. doi: 10.1371/journal.pone.0237992
42. Scheelings TF, Williamson S, Reina R. Hematology and serum biochemistry for free-ranging freshwater crocodiles (Crocodylus johnstoni) in Western Australia. J Wildlife Dis. (2016) 52:959–61. doi: 10.7589/2015-03-064
Keywords: captive orinoco crocodile, Crocodylus intermedius, reference intervals, hematology, RBC, Colombia
Citation: Barajas-Valero S, Rodríguez-Almonacid C, Rojas-Sereno Z, Moreno-Torres C and Matta NE (2021) Hematology, Biochemistry Reference Intervals, and Morphological Description of Peripheral Blood Cells for a Captive Population of Crocodylus intermedius in Colombia. Front. Vet. Sci. 8:694354. doi: 10.3389/fvets.2021.694354
Received: 13 April 2021; Accepted: 25 June 2021;
Published: 26 August 2021.
Edited by:
Michele Ann Miller, Stellenbosch University, South AfricaReviewed by:
Jan Myburgh, University of Pretoria, South AfricaCopyright © 2021 Barajas-Valero, Rodríguez-Almonacid, Rojas-Sereno, Moreno-Torres and Matta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Steven Barajas-Valero, aHNiYXJhamFzdkB1bmFsLmVkdS5jbw==; Cristian Rodríguez-Almonacid, Y3Jyb2RyaWd1ZXphQHVuYWwuZWR1LmNv
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.