- 1Veterinary Medicine Teaching and Research Center, School of Veterinary Medicine, University of California, Davis, Tulare, CA, United States
- 2Department of Population Health and Reproduction, School of Veterinary Medicine, University of California, Davis, Davis, CA, United States
- 3Department of Internal Medicine and Infectious Diseases, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt
- 4Antimicrobial Use and Stewardship Program, California Department of Food and Agriculture, Sacramento, CA, United States
The California (CA) dairy industry was surveyed in July 2017 to evaluate producers' knowledge and perceptions and antimicrobial drug (AMD) use in preweaned dairy calves following the implementation of the nationwide veterinary feed directive final rule (VFD) in January 2017 and prior to statewide implementation of CA Senate Bill (SB) 27 in January 2018. Together, these regulations require veterinary oversight for all uses of medically important antimicrobial drugs (MIADs) administered to livestock in CA. Survey questionnaire was mailed to 1,361 CA Grade A milk producing dairies and calf ranches across CA resulting in a 12% (169) response. Most respondents (83%) were aware of the VFD and SB 27 changes. Use of antibiotics was perceived as important (77%) in raising preweaned dairy calves and judicious use of antibiotics was ranked as the most important antimicrobial stewardship practice, amongst record keeping, observing withdrawal periods, having a valid Veterinarian-Client-Patient-Relationship (VCPR), and use of alternatives to antibiotics. Treating sick calves was the major indication for AMD use (90.5%); however, few producers reported use of antibiotics to control (12.7%) or prevent disease (11%). Neomycin sulfate, chlortetracycline, oxytetracycline and sulfamethazine were the most used AMD. The respondents reported a decreased use of AMD in milk (10%) and in solid feed (5%), and discontinuation of one or more AMDs used in milk (18.6%) or in solid feed (5%) post-VFD rule implementation in 2017. Most respondents reported keeping treatment records and the information recorded included date (82%), dose (44%) and route (15%) of AMD used. A few respondents reported they had initiated use of alternatives to AMDs, such as vitamins (32.6%), minerals (25.6%), herbal remedies (11.6%) and pathogen specific antibodies (7%), post-VFD. The limited changes noted in AMD use could be attributed to the short period between the implementation of the VFD and the time of the survey. Our study outcomes identified opportunities to improve AMD use practices, including record keeping and use of AMD alternatives, and provides a baseline for future evaluation of the impact of these regulatory changes, as well as guidance for the future recommendations on best practices to promote judicious AMD use.
Introduction
Antimicrobial drugs (AMD) are important compounds used in food animal production for treatment, control, and prevention of bacterial diseases. However, use of AMD is associated with development of antimicrobial resistance (AMR) (1, 2), which is a major concern for both human and animal health worldwide. Many countries have formulated and implemented surveillance programs to monitor AMD use in food animals, as the first step toward promoting their judicious use (3–9). In the United States, cattle production leads the utilization of AMD among the different livestock species. The 2018 FDA report on domestic sales and distribution of AMD for use in cattle (dairy and beef) accounted for the highest percentage (42%) of the total species-specific sales (kg) estimates for antimicrobial drugs approved for use in food animals in the United States (10). Although the U.S. does not currently have an antimicrobial drug use report in livestock that accounts for individuals at risk of being treated and a standard body weight at treatment, such as the “defined daily dose for animals,” the annual sales and distribution report has indicated decreased consumption of AMDs among the different livestock species.
In dairy cattle, the most common use of AMD are to prevent and treat mastitis in lactating and dry cows (11, 12), and for treatment or control of enteritis and respiratory diseases in calves (13). Besides direct exposure of dairy calves to AMD for treatment and prevention purposes, additional indirect exposure occurs through feeding non-saleable (waste or hospital) milk that may contain low concentrations of drug residues (14). Waste milk is milk harvested from cows treated with intramammary, oral, or injectable antimicrobials during the withholding period when such milk cannot be sold for human consumption. Waste milk may also contain milk from recently calved cows while they transition from colostrum to normal milk secretion. The practice of feeding waste milk is widespread in the dairy industry, despite research evidence which indicates that it is associated with changes in microbial populations and increased presence of AMR bacteria in calves, compared to calves fed saleable bulk tank milk (15–19).
Understanding the ways in which AMD are used in the dairy industry and estimating the associated risks for AMR is critical for understanding hurdles encountered by the dairy industry to adopt judicious drug use practices necessary to ensure the well-being of food animals and protect both veterinary and public health (20). Previous research has shown that both veterinarians and producers play crucial roles in the use of AMD; among veterinarians, prescription decision-making habits are the major factors that influence the use of AMD (21, 22), while the major drivers for AMD use among producers included the type of cattle operation, disease and animal welfare, economic factors, veterinary consultation, producer's experience and peer support, perceived drug efficacy and drug use regulations (23). Furthermore, raising food animals in a production setting sustainably should employ preventative management practices that modify the environment and host to reduce the risk of disease as a priority before using AMD. Specifically, these preventative strategies should include modifying the environment to reduce stress using proper housing, optimum colostrum management and nutrition, and use of effective vaccines (24, 25). An approach that allows producers to raise and manage food animals sustainably with emphasis on addressing the environment and host factors includes the risk assessment tool for bovine respiratory disease (26).
In the United States, recent regulatory changes were made by the Food and Drugs Administration (FDA) to improve the regulatory oversight of Veterinary Feeds Directive (VFD) drugs while continuing to protect human and animal health. The VFD drugs refer to new animal drugs intended for use in or on animal feed which are limited to use under the professional supervision of a licensed veterinarian (27). The VFD final rule was implemented effective January 1, 2017. Locally in California, Senate Bill (SB) 27 was approved and passed in 2015 as the Livestock: Use of Antimicrobial Drugs Law (California Food and Agriculture Code Sections 14400-14408) (28), here onwards referred to as SB 27. Effective January 1, 2018, SB 27 regulations restricted all uses of medically important antimicrobial drugs (MIADs) to veterinary prescription or VFD only. The term MIADs refers to antimicrobial drugs listed in Appendix A of the federal Food and Drug Administration's Guidance for Industry #152, and include critically important, highly important, and important antimicrobial drugs such as norfloxacin, cephalexin, cefaclor, penicillin, oxacillin, ampicillin, streptomycin, erythromycin, clarithromycin, tetracyclines, vancomycin, chloramphenicol and trimeth/sulfameth (29). Jointly, these regulatory changes increased veterinary oversight in the distribution and use of MIADs in livestock by changing the availability of MIADs from over-the-counter (OTC) to prescription or VFD only.
The purpose of the statewide survey described here was to document AMD use practices for preweaned dairy calves, evaluate producers' knowledge and perception on AMD use, and document any changes in management and AMD use practices following the implementation of VFD rule and prior to the implementation SB-27. In California, newborn dairy calves are either raised on-site at their source dairy or off-site in a calf nursery, commonly known as calf ranches (30). Dairies and calf ranches raise replacement dairy heifers or bulls for dairy beef. The survey targeted both dairies and calf ranches that were raising preweaned calves. The outcome of this survey furthers our understanding of the industry's AMD use practices and perceptions and provides baseline data to guide future evaluation of the rule changes as well as recommendations on best practices to promote judicious use of AMDs.
Materials and Methods
Study Design
A survey of the California dairy producers was conducted in July 2017. The questionnaire was pre-tested by the coauthors and several collaborators with in-depth knowledge of the CA dairy industry. The survey questionnaire was comprised of 54 questions grouped into four sections (Supplementary Material). Section 1 included questions on the respondent's role on the farm and herd demographics including location, herd size, cow breeds raised and participation in welfare audit programs; Section 2 was comprised of questions about preweaned calf management practices including housing types, feeding practices, health management protocols such as adding AMD in feed, milk or water and vaccination; Section 3 questions sought information on AMD use in preweaned calves including information sources and decision making on AMD purchased and used on the farm, availability and use of treatment protocols including dose estimation and extra-label AMD use, drugs and health records system, choice of AMD used to treat sick calves, and the extent of veterinarian involvement in on-farm AMD use practices. Section 4 assessed the knowledge and perceptions on AMD stewardship practices as well as awareness of regulations. The study materials were reviewed by the University of California, Davis, Institutional Review Board and granted IRB review exemption approval (IRB ID: 1709653-1).
Surveys and Data Collection
The survey questionnaire was mailed to 1,361 licensed Grade A milk producing dairies and calf ranches in California. In the United States, Grade A milk is the category of milk produced under higher farm sanitary conditions (compared to Grade B) to qualify for sale or consumption as fluid beverage (31). A sample size was not conducted prior to the survey. However, a post-hoc sample size and power analysis based on the question on respondent knowledge of AMD regulatory changes was estimated. The mailed survey included a cover letter, questionnaire, an additional form for producers to share their comments about AMD use in preweaned calves or other dairy cattle, a follow up request form, and postage-paid return envelope. The cover letter also provided a web link to the online version of the survey as an alternate response option. Dairies and calf ranches identified to receive the survey were each assigned a confidential index number used only to identify respondents for a second mailing if they had not responded to the first mailing, and to verify the county location of their premises. The second mailing was 4 weeks after the initial mailing and was sent to addresses that did not respond the first time. Similarly, a reminder card was sent 2-weeks after each mailing to those who had not responded at the time of mailing.
Statistical Analyses
Descriptive Statistics
Survey data were analyzed using Stata IC 16 (Stata Corp LLC, College Station, TX USA) and (32). Responses to questions were summarized using descriptive statistics and reported as proportions for categorical variables or means for continuous variables. Uncertainty measures including standard errors and 95% Confidence Intervals (CI) were also reported.
Multiple Factor Analysis
Multiple Factor Analysis (MFA), an extension of the Principle Components Analysis (PCA) was used to explain the variability of the producers responses to the survey on AMD use in preweaned calves on California dairies (33). Principle Components Analysis was achieved through dimensionality reduction of the dataset's variables, both quantitative and categorical (34), through the FactoMineR package in R (35). The MFA was performed based on a subset of 66 variables classified into 12 groups, 11 qualitative groups (65 categorical variables) and one quantitative group (one continuous variables). The first three dimensions of the MFA were used to interpret the percentage of the explained dataset's variance explained. Groups with variance of 0.4 or greater on any of the first three principal components dimensions were retained for interpretation, and within each group, variables with correlation coefficients (coordinates) of 0.4 or greater were retained for interpretation of variability.
Results
Respondents and Their Herd Characteristics
A post-hoc sample size based on the question on respondent knowledge of AMD regulations showed that a sample size of 136 respondents is needed assuming that 50% answer yes, 5% level of significance and 80% power in a two-way test, and a 10% response rate out of a total of 1,361 mailed surveys. The power analysis based on the 139 surveys returned and 115 responses indicating knowledge of AMD regulations (82.7%) resulted in a P-value of 0.04 and power >99%.
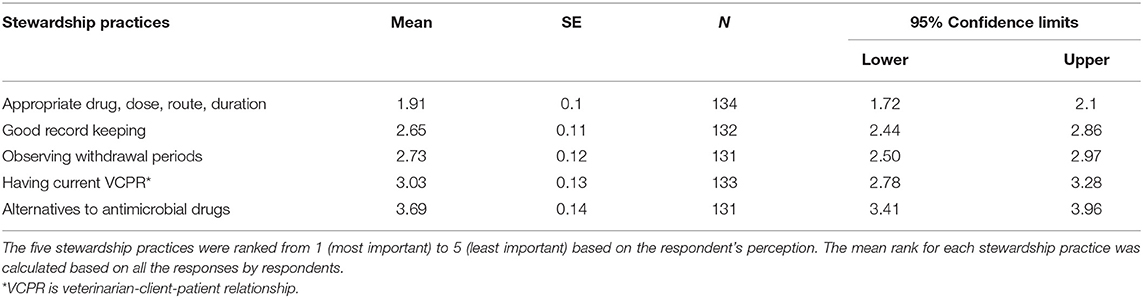
Of the 1,361 mailed surveys, 169 (12%) responses were received including five responses completed online. Among the 169 responses received, 23 were excluded from the analysis either because they were not completed but returned or the premise responded twice, in which case only the first response was considered. Because some respondents did not answer all the questions of the surveys included in the analysis (n = 146), each question was analyzed based on the number of respondents (n) who answered the specific question. During analysis, the respondents' locations were categorized as one of the three milk shed regions of California; Northern California (NCA), Northern San Joaquin Valley (NSJV) and Greater Southern California (GSCA), with the latter being a merger of dairies in the Southern San Joaquin Valley and the limited number of dairies in Southern California (36). Most of the respondents (84 responses; 49.7%) were from GSCA, followed by NSJV (58 responses; 34.2%) and lastly NCA (27; 16%). Among the responses received, five were from calf ranches located in the GSCA. Most of the dairy survey respondents were dairy owners (83.8%, n = 146), and only a smaller proportion were individuals with various roles on the dairy such as herd manager (32%, n = 146), calf manager (12.2%, n = 146) and/or calf feeder (6.2%, n = 146). Responses from certified organic dairy farms (8.9%, n = 146) were excluded from analyses on AMD use, except for the questions on availability and type of VCPR. The mean calf herd size of respondents was 246 preweaned calves, with the predominant breeds being Holsteins (79.2%). Table 1 summarizes the demographics of the respondent dairies.
Knowledge, Perception, and Impact of VFD and SB 27 Regulatory Changes
Knowledge on Regulatory Changes
Most of the producers were aware of the VFD rule changes and the then soon-to-be-implemented SB 27. A total of 82.7% (n = 139) respondents reported knowledge of the requirement for veterinary prescription for all OTC AMD starting January 1, 2018. This regulatory change was projected by the respondents to affect the producers who were reportedly using OTC AMD according to label (35.7%, n = 129) or extra-label (6.6%, n = 129). Among the calf ranches, 3 out of the 5 respondents reported knowledge of regulatory changes that were set to start on January 1, 2018 at the time of the survey, and one respondent reported using OTC AMD according to label.
Knowledge and Perception of Antimicrobial Stewardship Practices
The producers' knowledge and perceptions of AMD stewardship practices were assessed by asking respondents to rank the relative importance of five key stewardship practices ranging from most important (1) to least important (5). The responses for each of these practices were then ranked in order based on their mean values. Respondents ranked administration of appropriate AMD, dose, route and duration as the most important (1st), followed by good record keeping (2nd), observing withdrawal periods and drug residue avoidance (3rd), having a current veterinary-client-patient-relationship (VCPR) (4th), and the least important was the use of alternatives to antibiotics (5th). Details of the ranking are summarized in Table 2. The same ranking order was observed among the 5 calf ranch respondents.
Perception of Antimicrobial Drugs
The respondents perceived the use of AMD to be extremely important (39%; n = 64; CI: 27.67, 51.78) or important (37.5%; n = 64; CI: 26.29,50.23) in raising preweaned dairy calves, and only a small percentage indicated that AMD were somewhat important (17.19%; n = 64; CI: 9.62, 28.8) or not important (10.9%; n = 64; CI: 5.2, 21.58). In the calf ranch responses, AMD use was perceived by respondents as extremely important (two respondents), important (one respondent) or somewhat important (two respondents). The importance of AMD in raising preweaned dairy calves was further emphasized by the fact that 62.5% (n = 65; CI: 49.77, 73.71) of respondent dairies predicted increased disease prevalence if AMD use for dairy calves was ceased completely. Similarly, 73.4% (n = 64; CI: 60.99, 83.02) and 54.7% (n = 64; CI: 42.12, 66.81) of respondent dairies predicted poor animal welfare and decreased performance, respectively, if the use of AMD in preweaned calves was stopped. Only a small proportion of the respondents indicated there would be no effect if AMD use in preweaned calves was stopped (14.1%; n = 64; CI: 7.39, 25.24) or were organic producers who were not using antibiotics (7.8%; n = 64; CI: 3.21, 17.8). The same response pattern was seen among calf ranches with four in five respondents predicting increased disease prevalence, poor growth, and compromised animal welfare, and only one respondent predicting no effect.
Change in Cost of Antimicrobials
Cost-wise, most of the respondents reported no change in the cost of AMD in their operations following the implementation of VFD regulatory changes (69.7%; n = 59; CI: 56.28, 80.12). Majority of the respondents reported no change in the cost of antibiotics following the implementation of VFD changes (70.5%; n = 61; CI: 56.28, 80.12), while only a small proportion of the respondents indicated that the cost of AMD had either increased (13.1%; n = 61; CI: 6.56, 24.49) or decreased (16.4%; n = 61; CI: 8.89, 28.26). Two calf ranch respondents reported an increase in cost of AMD while three respondents reported a decrease in cost.
Change in Antimicrobial Drug Use
Most of the producers reported no change in the use of AMD in milk (61%, n = 59), solid feed (81.7%, n = 60) or water (8.6%, n = 61) for preweaned calves. The details of the specific changes reported in AMD use are summarized in Table 3. A small proportion of respondents (10.9%; n = 55) reported a decrease in use of OTC AMD labeled for feed that do not fall under VFD requirements (e.g., amprolium); this decrease was mainly due to reduced use of these AMDs in milk. Respondents that reported a decrease in OTC AMD use also reported an increase in calf mortality. A single respondent (1.9%, n = 54) reported increased use of OTC AMD labeled for feed that do not fall under the VFD, explaining that reduced use of OTC AMD in milk “resulted in more aggressive use through other routes.” Some respondents reported they had initiated use of alternatives to AMD after the VFD requirements were implemented such as herbal remedies (11.6%, n = 43) and pathogen specific antibodies derived from sources such as eggs (7%, n = 43).
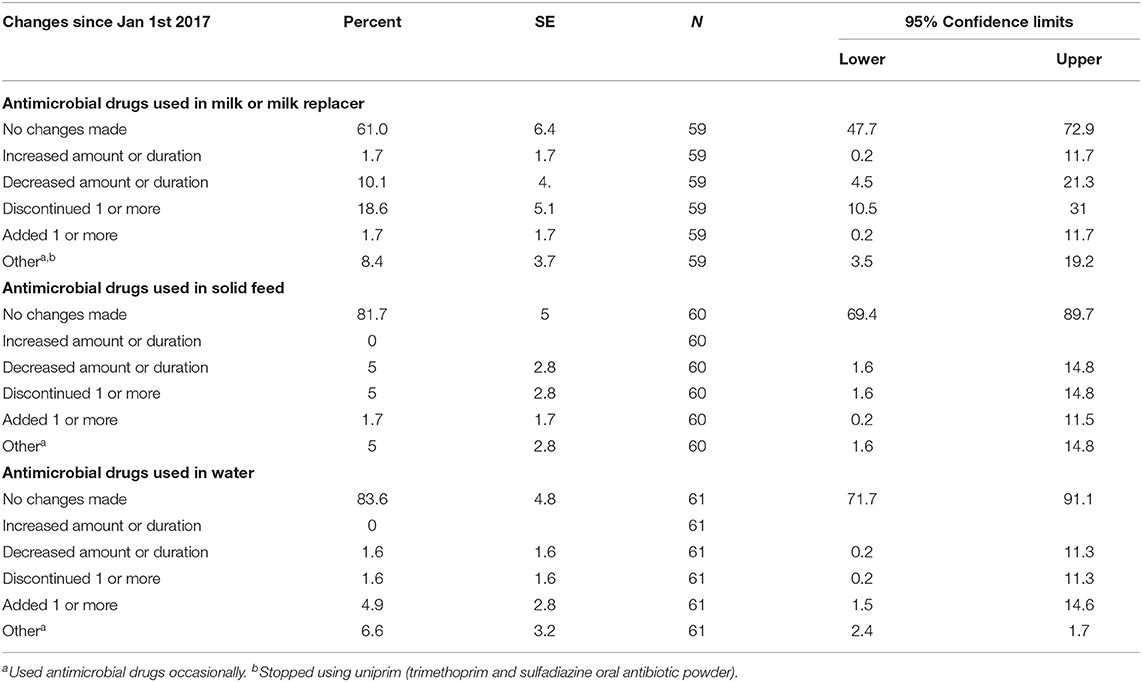
Table 3. Changes in the use of antimicrobial drugs in preweaned calves on California dairies following the implementation of the Veterinary Feed Directive (VFD) final rule on January 1, 2017 compared to practices during the year 2016.
Antimicrobial Drug Use Practices in Preweaned Dairy Calves
Preweaned dairy calves were reportedly exposed directly to AMD for treatment, control, or prevention purposes through parenteral or oral administration, or by adding in milk, solid feed, or water. Indirect exposure to AMDs reportedly occurred through feeding non-saleable or waste milk containing drug residues. Sources of non-saleable milk included recently calved cows that were previously treated with long acting intramammary AMD infusion at dry-off, mastitis cows treated with intramammary or other parenteral AMD or other lactating cows treated with systemic AMD as treatment for other health conditions.
Exposure of Preweaned Dairy Calves to Antimicrobial Drugs
Indirect exposure of preweaned dairy calves to AMD occurred mainly through feeding of milk sources that presumably contained AMD residues. The mean proportion of liquid diet fed to calves by the responding dairies included non-saleable or waste milk (44.2%; n = 68; CI: 34.34, 53.96), saleable or bulk tank milk (28.31%; n = 68; CI: 18.73, 37.89), milk replacer (20.56%; n = 68; CI: 12.8, 28.32), and other minor sources such as transition cow milk, fortified non-saleable milk and non-fat dry milk powder (3.94%; n = 68; CI: −0.21, 8.09). Sources of milk containing AMD residues could have included waste (hospital) milk, as well as colostrum and milk from transition cows treated at dry-off with long acting intramammary (IMM) AMD. Most of the responding dairies treated all cows (77.5%; n = 142; CI: 69.77, 83.66) while a few dairies treated cows selectively (4.9%; n = 142; CI: 2.35, 10.06) at dry-off with long acting IMM AMD. Dairy reported sources of colostrum fed to calves included pooled colostrum (51.2%; n = 141; CI: 43.74, 58.6), individual cow colostrum (34.6%; n = 142; CI: 27.47, 41.74) or direct nursing from the dam (5.5%; n = 139; CI: 2.28, 8.65). A few respondents reported feeding transition cow milk to preweaned calves 5.42%; n = 48; CI: 0.88, 10). Among the calf ranches, 2 out of 5 respondents reported feeding preweaned calves with a liquid diet comprised of either 75 or 100% saleable milk.
Direct exposure of preweaned dairy calves to AMD occurred parenterally or orally in milk, grain, or water. More than half of the respondents (64%; n = 61) had a treatment protocol developed by either a veterinarian (50%, n = 34), farm owner (17.6%, n = 34) or both veterinarian and owner (30.3%, n = 33). The availability, content and access to the treatment protocols are summarized in Table 4. Only 27.8% (n = 61) respondents reported submission of calves for diagnosis, while 30.2% (n = 61) used other diagnostic techniques to guide antimicrobial treatment of preweaned calves. Treatment of calves using AMD was reported to mainly follow label-use (78.3%, n = 60) and only a small proportion of respondents reported extra-label use (16.7%, n = 60) or did not know if antibiotics were being used extra-label (5%, n = 60). Generally, most of the respondents reported following label recommendations when estimating treatment dosage (87.1%, n = 62) and treatment duration for both parenteral or oral AMD administration (88.1%, n = 59) or AMDs added in feed (74.6%, n = 55). The major indications for AMD use in calves, methods for estimating treatment dosage and treatment duration are summarized in Table 5. Individual treatment of sick calves was the single most important indication for AMD use (90.5%, n = 63), whereas use for control of ongoing diseases (12.7%, n = 63) or prevent disease in high-risk calves (11.1%, n = 63) were minor indications. Table 6 shows the mean percentage of calves that received different AMD administrations between birth and weaning. Neomycin and oxytetracycline were the most used AMDs administered to more than half of the calves during the preweaning period. The list of common AMDs of choice for treating respiratory diseases and diarrhea or enteritis in preweaned calves is shown in Table 7. The most common antimicrobials reportedly used by respondents as first choice treatment for respiratory disease and enteritis were florfenicol (43% respondents) and sulfonamide (24.4% respondents), respectively.
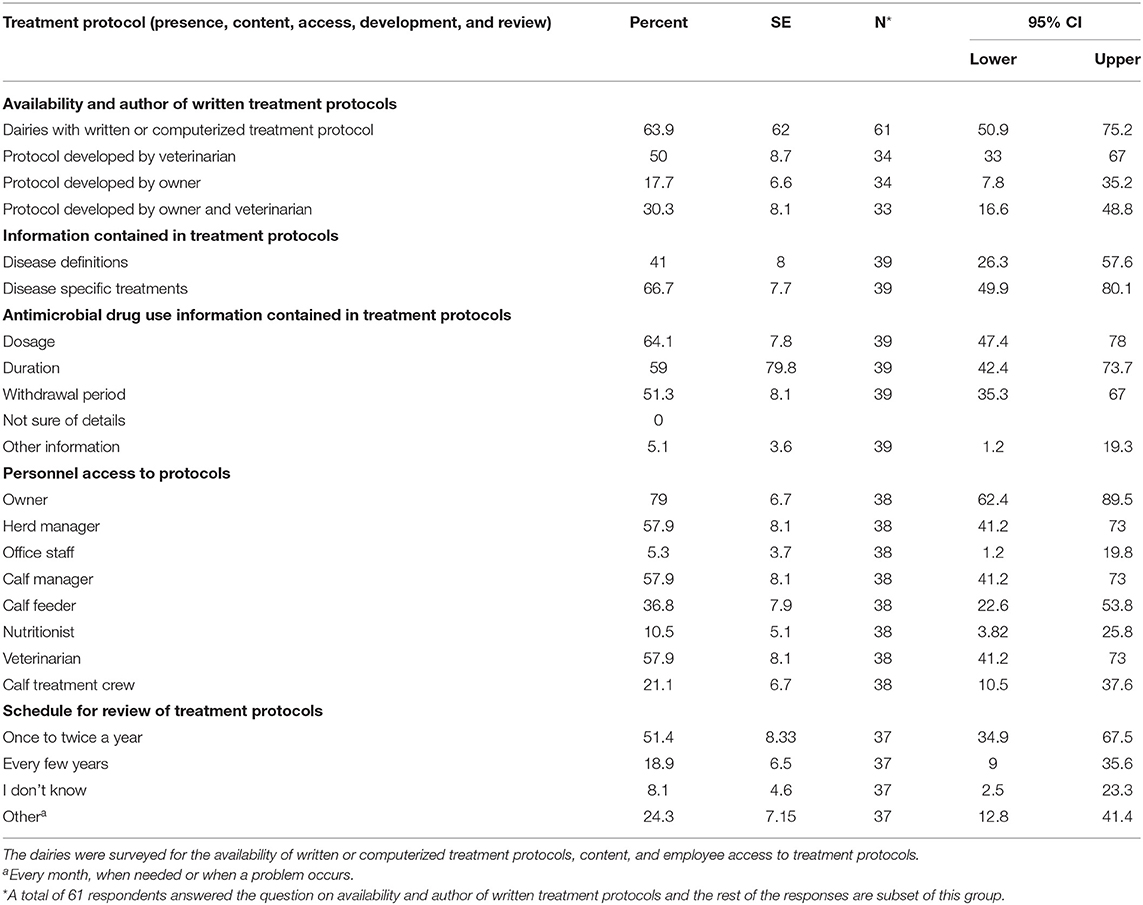
Table 4. Use of treatment protocols for health management in preweaned calves on California dairies.
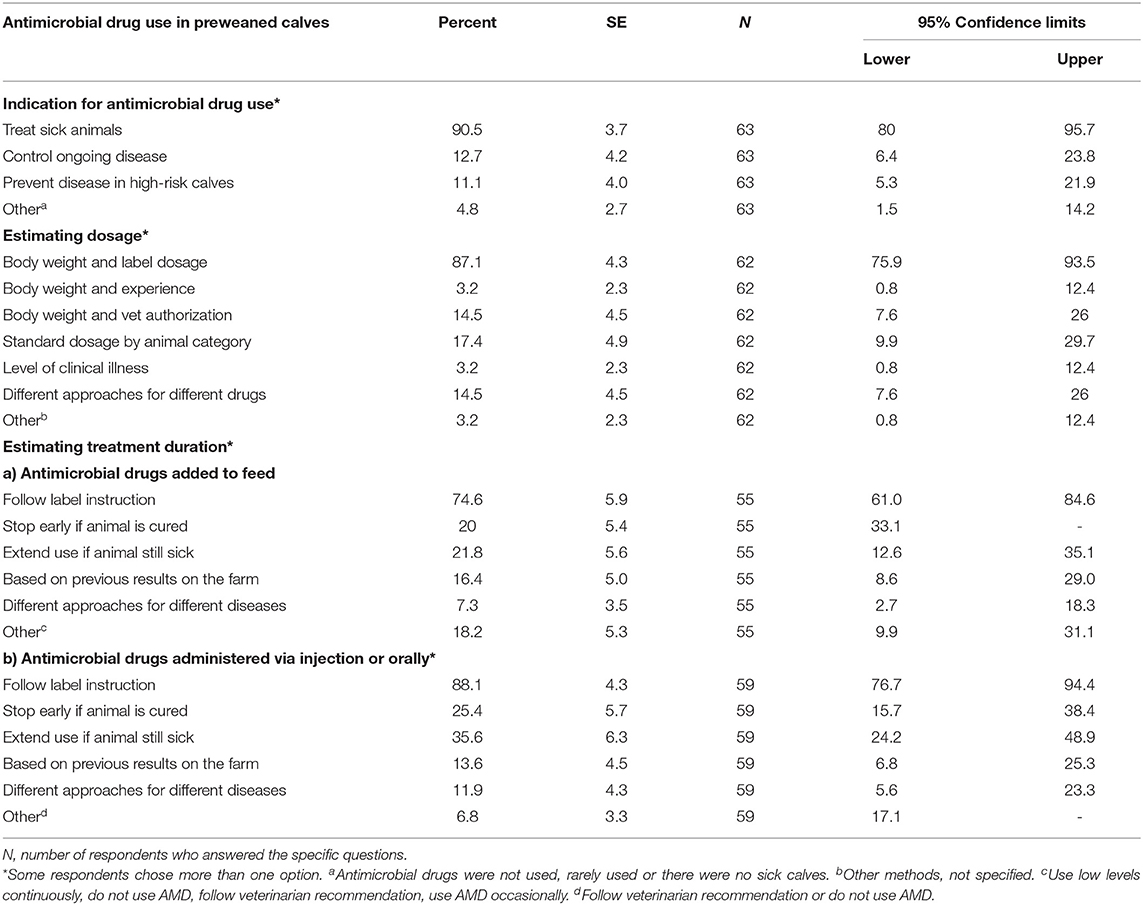
Table 5. Indications, estimation of dosage and treatment duration for antimicrobial drugs administered to preweaned dairy calves on California dairies.
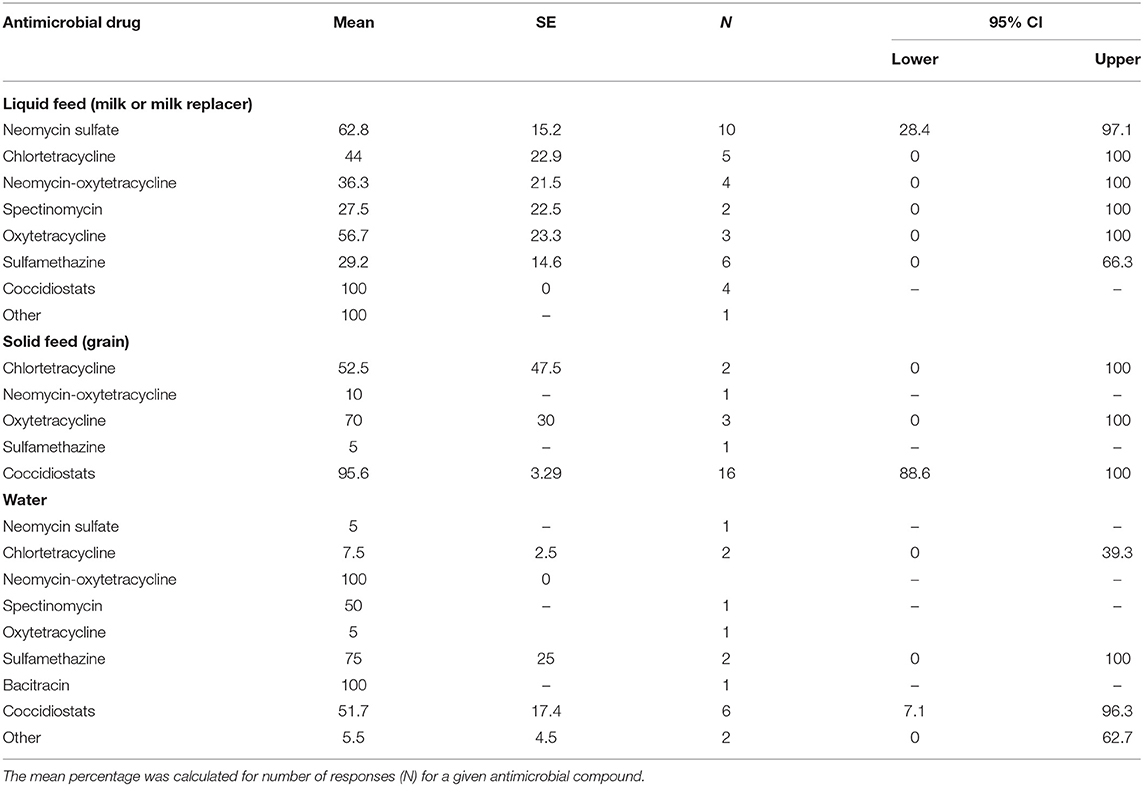
Table 6. Mean percentage of preweaned dairy calves receiving antimicrobial therapy between birth and weaning with different antimicrobial drugs.
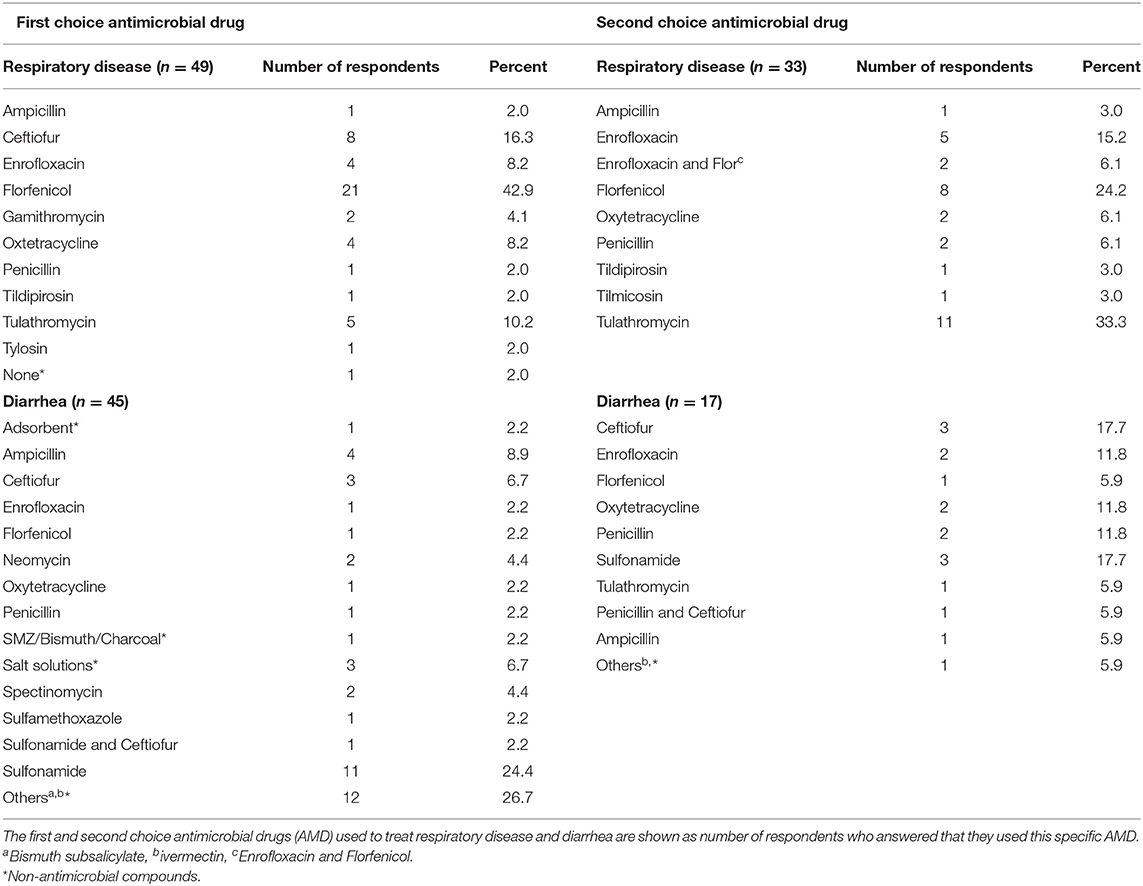
Table 7. Antimicrobial drugs used to treat respiratory disease and diarrhea in preweaned calves after January 1, 2017 on California dairies.
Drug and Treatment Records
Only 32.31% (n = 65; CI: 21.86, 44.88) of the respondents kept a drug inventory log on the dairy, but respondents recorded AMD treatment information such as treatment date (82.3%; n = 62; CI: 70.34, 90.06), dose (43.6%; n = 62; CI: 31.52,56.37), and route (14.5%; n = 62; CI: 7.59, 25.99) of administration. Forty percent (n = 60, CI: 28.15, 53.15) of the respondents reportedly did not track the antibiotic withdrawal interval in treated calves. The record systems used by respondents who tracked AMD withdrawal, included paper records (31%; n = 58; CI: 20.23, 44.39), computer software such as DC 305® (Valley Ag Software, Tulare, CA 93274) (22.4%; n = 58; CI: 13.26, 35.3), marking the calf hutch (12.1%; n = 58; CI: 5.73,23.65), memory (8.6%; n = 58; CI: 3.54, 19.53), or used other methods including chalk and phone (3.6%; n = 56; CI: 0.86, 13.71). Besides withdrawal period, other drug related information tracked included drug name (60%; n = 60; CI: 46.85, 71.85) quantity at hand (56%; n = 59; CI: 42.78, 68.31), supplier (22%; n = 59; CI: 13.04, 34.76), expiration date (33%; n = 60; 22.34, 46.49), cost (27%; n = 59; CI: 170.8, 40.19), manufacturer (22%; n = 60; 12.81, 34.24), and purchase date (15%; n = 59; CI: 7.97, 27.21).
Veterinarian Client Patient Relationship (VCPR) and Antimicrobial Drug Use
Most of the respondents had a VCPR with a practicing veterinarian (89.2%; n = 65; CI: 78.73,94.88) and a minor proportion had a VCPR with a technical services or consulting veterinarian (4.6%; n = 65; CI: 1.45,13.72). Three dairies (4.6%; n = 65; CI: 1.45,13.72) reported having no VCPR; among these dairies, one was an organic dairy and the remaining two showed evidence of working with a local veterinarian or having a written VCPR which indicated that their response of not having a VCPR was in error or the question was misunderstood. The nature of the VCPR included a written signed agreement (47.5%; n = 61; CI: 38.08,60.32), verbal agreement (42.6%; n = 61; CI: 30.59,55.6) or being assumed based on veterinary care provided (14.8%; n = 61; CI: 7.71, 26.39) or based on a longtime acquaintance between the veterinarian and the producer (1.6%; n = 61; CI: 0.22, 11.3). All respondent calf ranches reported having a VCPR which was either written (2 out of 5), verbal (2 out of 5) or not discussed (1 out of 5).
Decision Making on Antimicrobial Drugs Purchased and Used on Farms
The higher proportion of respondents reported that owners made decisions for purchase and stocking of AMD added to feed or water on the dairy (69.4%, n = 63), compared to the veterinarian (49.2%; n = 63), herd manager (25.4%, n = 63), calf manager (20.6%, n = 63) and the nutritionist (3.2%, n = 63) (Table 8). A similar pattern was reported in the decision making to purchase injectable or oral AMD. The calf manager, however, was more commonly the decision maker to treat preweaned calves on their 1st day of illness compared to the veterinarian. The information sources that guided the producer's decision in treating preweaned calves were mainly the veterinarians (84.6%, n = 65), previous experience with the drug (66.2%, n = 65), pharmaceutical company representative (32.3%, n = 65), or product label (27.7%, n = 65). Other minor sources of information on AMD included magazines, journals, promotional materials, other producers, local/national meeting, and online materials. Cooperative extension and FARAD (Food Animal Residue Avoidance Databank) were not among the information sources reported (Table 9).
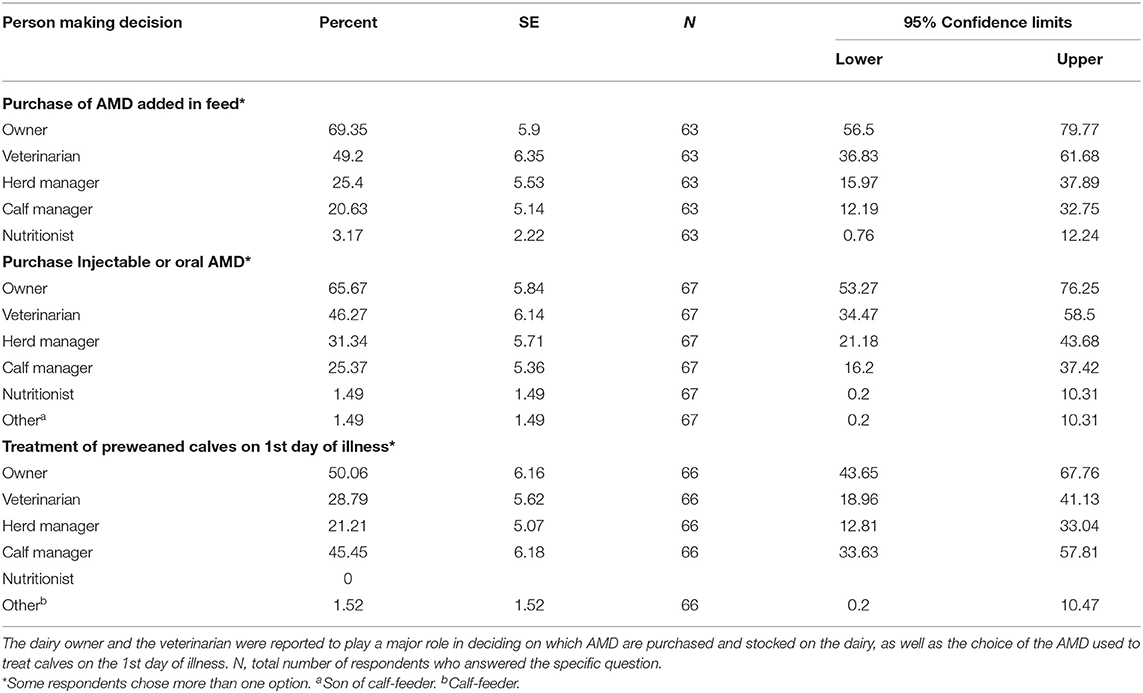
Table 8. Decision making on antimicrobial drugs (AMD) purchased and used to treat sick calves on California dairies.
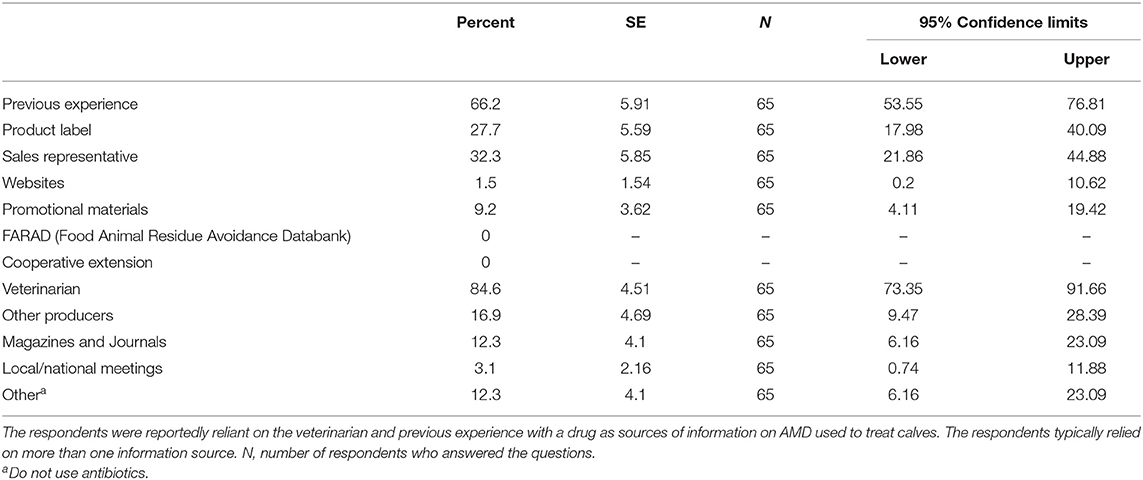
Table 9. Information sources for antimicrobial drugs used to treat preweaned calves on California dairies.
The majority of dairy producers who responded to the survey reported that that they consulted veterinarians on disease management decisions (96.8%). Similarly, veterinarians were the main prescribers for AMD used in feed or water (71.4%), besides other personnel such as nutritionists and pharmaceutical veterinarians and sales representatives. Similarly, all the calf ranches consulted the veterinarians on disease management and antimicrobial use. Table 10 summarizes the role of veterinarians and other livestock health professionals in providing consultancy services on animal disease management and prescription for AMD use.
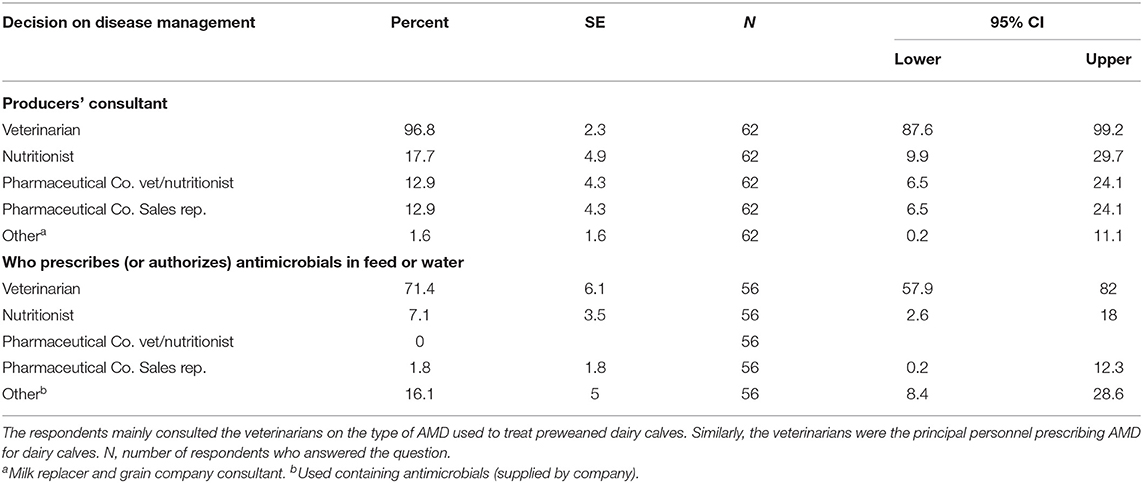
Table 10. Consultation and prescription of antimicrobial drugs used to manage diseases in preweaned calves on California dairies.
Health Management Practices
Health management practices explored included colostrum management and vaccination practices. Most of the responding premises did not heat-treat colostrum fed to calves (87.1%; n = 139; CI: 80.28, 91.73). Intranasal vaccination against respiratory pathogens was the most used form of vaccine delivery in calves (77.6%; n = 58; 64.70, 86.74), administered at a mean age of 5.5 days (n = 41); other vaccine types reported included modified live vaccines against pneumonia or diarrhea causing pathogens (52.5%; n = 58; CI: 39.54, 65.21) administered at a mean age of 35.5 days (n = 31) days, and killed vaccines against pneumonia or diarrhea pathogens (20.7%; n = 58; CI: 11.94, 33.43) given at mean age of 19.3 days (n = 15).
Multiple Factor Analysis
Multiple factor analysis of responses to 66 survey questions identified six components from which 25 variables explained most of the variability in the survey responses (Table 11). The first three dimensions described 21.46% of the variability in the data set. The first dimension explained 9.75% of the variability with most of the variability (65.23%) explained by use of diagnostics to guide treatment with AMD (18.60%), the source of information and decision on AMD (17.95%), treatment protocols and records (17.64%), and the common drugs used for treatment of diarrhea and pneumonia (11.06%). The second dimension explained 6.56% of the variability with calf management practices explaining 16.70% of the variability with the highest correlation 0.642 to pasteurization of milk (yes/no) for pre-weaned calves. The highest variation explained on the third dimension (37.13%) was related to the changes made in AMD use on dairies post-VFD final rule change, with high correlation determined for changes in AMD administered to pre-weaned calves in grain, water, injectables, and milk or milk replacer (0.670, 0.633, 0.629, 0.623, respectively).
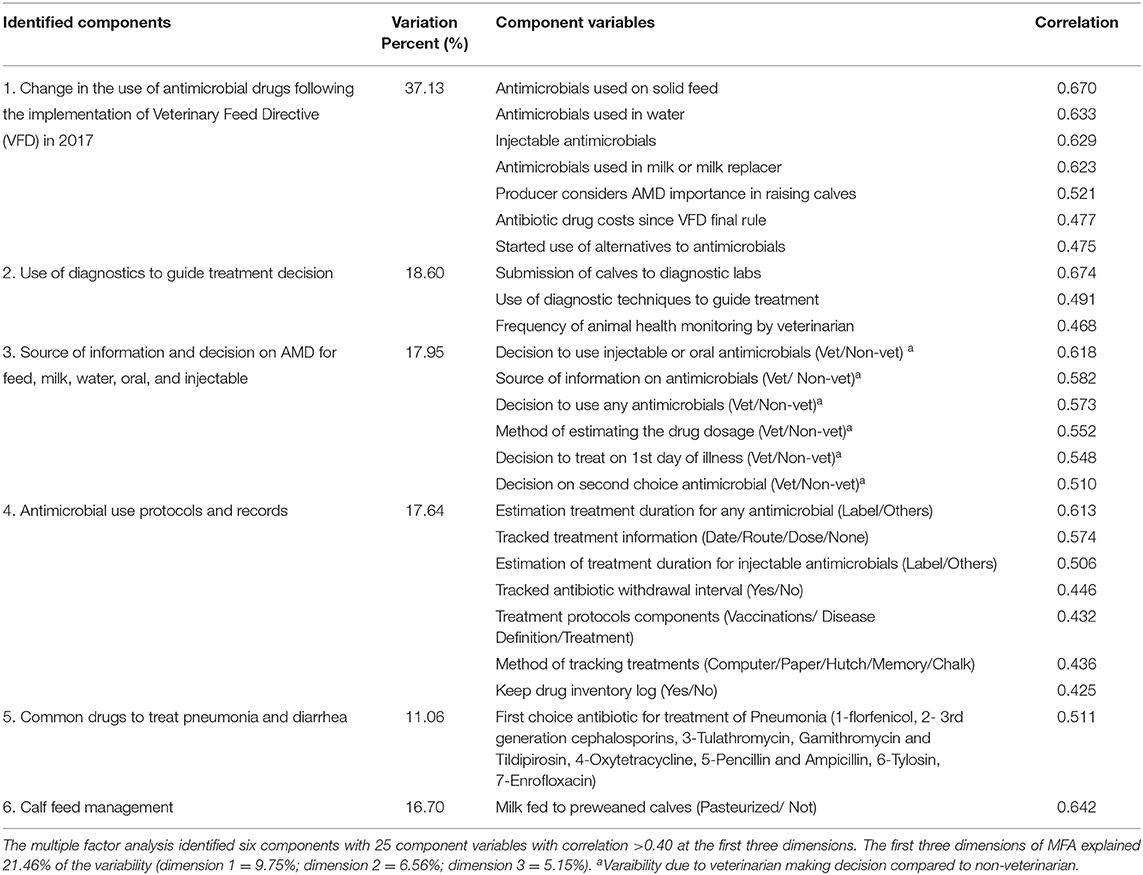
Table 11. Component variables explaining variability in antimicrobial drugs (AMD) in preweaned dairy calves on California dairies.
Discussion
The survey response rate of 12% in this study was comparable to the 15% response outcome from a previous mailed survey of the same demographics (37). The mean characteristics of the respondent dairies (herd size, rolling herd average, and breed composition) mirrored the state averages for the year 2016 (38), indicating the respondents were representative sample of the dairy farmers in the area. Similarly, majority of the respondents were from GSCA which has the highest number of dairy farms within the state. Most of the producers (82.7%) were aware of the recently implemented regulatory changes in the VFD rules which could have been due to extensive awareness campaigns at both the national and state levels through online resources, fact sheets, and other materials as well as workshop presentations at producer and veterinary meetings. Use of AMD was generally perceived as important in raising calves and the respondents thought calf health and welfare would be negatively affected if AMD were no longer available. The same opinion was expressed among dairy farmers in Tennessee during focus group discussions on the impact of VFD changes on the ability of producers to prevent disease on their herds; these producers indicated that the VFD regulation had limited access to essential AMD which led to increased disease occurrence and deaths particularly among calves, and reduced growth rate (39). It is worth noting that the outcome of this study could have been influenced by the negative perception of surveyed producers toward regulatory changes and may not correlate to actual increase in disease occurrences. Comparatively, the European ban on the use of growth-promoting antimicrobials was mainly associated with increased early postweaning diarrhea in piglets and enteritis in broiler chicken, while minimal or no negative clinical effects on the ban was reported in other animal species (40–42).The increased disease burden in affected animal species resulted in increased use of antimicrobials for treatment and prevention, a challenge that was later addressed by improvements in animal health management and housing (41).
Among the five key AMD stewardship practices stated in the survey (judicious AMD use, good record keeping, having a valid VCPR, observing withdrawal period and using alternatives to AMD), the respondents ranked judicious drug use (appropriate drug, dose, route, and duration of use) as the most important, and the use of alternatives to AMD as the least important. This finding indicates that the respondents appreciated the concept of judicious drug use and highlights other aspects of stewardship, such as disease prevention and the use of alternatives to AMD, which should be the focus of future outreach efforts. Having a valid VCPR was ranked 4th (low), although up to 90% of the respondents had a valid VCPR. It is possible the respondents considered having a valid VCPR to be a regulatory requirement for access to AMDs, rather than a stewardship practice. Most of the respondents reported no change in the use of AMD post-VFD rule changes, although a small proportion indicated a decrease in the use of AMD, primarily for those administered in milk fed to calves. The limited changes following the implementation of the VFD final rule may be attributed to the short time lapse (6 months) between the implementation date and administration of this survey. It is worthwhile though that the key change noted among a few respondents was a reduction in use of antimicrobials. However, the producers that reported decreased use of OTC drugs due to the VFD requirement also indicated a resultant increase in calf mortality. It is possible that respondents who reported increased calf mortality post-VFD were partly reliant on AMD prophylaxis for disease prevention prior to VFD implementation. In addition, the survey respondents could have been biased in their responses by a perceived negative effect of the regulatory changes on AMD use. Opportunities exist to improve and manage calf health through vaccination, use of diagnostics to guide treatment decisions, and use of supportive therapy when AMD use is not justified, such as cases of viral enteritis. Indeed, very respondents reported use of salt solutions as the first-choice treatment for diarrhea, and less than one-third of the respondents either reported submission of calves for diagnosis or use of other diagnostic methods to guide treatment of preweaned calves. Furthermore, there is need for future on-farm studies to generate data on-farm changes in AMD use in preweaned calves post-VFD and the associated impact on calf health and mortality. In addition, longitudinal studies that investigate implementation of stewardships practices that reduce unnecessary use of AMDs and the animal health and welfare outcomes are needed.
Feeding calves colostrum or non-saleable (waste) milk containing AMD residues, as reported by most respondents, constituted potential sources for indirect exposure of preweaned calves to AMD. Previous studies on California dairies reported detectable concentrations of at least one AMD compound in 15 out of 25 waste milk samples tested (43). The presence of AMD in waste milk could potentially contribute to development of AMR (2). Since waste milk is a valuable feed source for calves (44), stewardship efforts should focus on strategies to reduce residues in waste milk to mitigate the potential risk of AMR development. Although most dairies pasteurized waste milk prior to feeding to calves, pasteurization is not effective in removing residues, and calves fed pasteurized waste milk were shown to have increased presence of AMR gut bacteria compared to calves fed milk replacers (16, 45). Recent research evidence shows that alkalinization of milk to pH 10 and spiked with ceftiofur sodium resulted in 96% degradation of the initial drug concentration (46), and is thus a potential strategy to treat waste milk before feeding to calves. Such a strategy would increase wider use of waste milk for feeding calves as a low-cost diet alternative and reduce costs associated with its disposal otherwise.
Treatment of calf diseases was the main cause of direct exposures of calves to AMD. This finding is consistent with the outcome of a previous survey of Tennessee cattle producers which showed that treatment of clinical disease and animal welfare were some of the key drivers for AMD use (23). With regards to the specific AMD types, the highest mean percentage of calves that were administered a given AMD type was reported for tetracycline and neomycin added in milk, grain, and water. In the United States, tetracycline and neomycin are among the drugs currently labeled for treatment of diarrhea (scours) (47, 48), which is the most common preweaning calfhood disease (49). Some respondents listed non-antimicrobial compounds amongst AMD administered treat preweaned calves indicating the need for awareness to correctly identify drug classes. Whereas, most of the respondents reported keeping treatment records, treatment date was the only common information recorded, and only a few respondents recorded additional information such as the drug dose, duration of treatment and route of administration. In addition, only half of the respondents used permanent computer software or paper records, the remaining respondents relied on hutch markings, memory, used chalk or did not keep records. Record keeping is one of the key elements of AMD stewardship, and future efforts to promote antimicrobial stewardship for preweaned calves should address this area.
Some of the reported uses of AMD in preweaned calves indicated usages that are not permitted by FDA. Among these uses were a few responses that indicated administration of spectinomycin or coccidiostats in milk or milk replacer. None of these products has label directions for use in milk or milk replacer, and extra-label use (ELDU) of any animal or human drug in or on animal feed is not permitted by FDA (50). By contrast, ELDU of AMD administered in water is permissible provided that the other requirements for ELDU established by FDA are properly met, which includes supervision of such use by a licensed veterinarian with a VCPR. The single reported use of enrofloxacin as an AMD choice for treatment of scours or diarrhea would be a violation of a specific FDA regulation which prohibits ELDU of this AMD in food-producing animals (51). The few responses for these uses associated with regulatory violations may be attributed to the tendency of written survey respondents to give answers they feel is correct rather than the actual practice or may represent errors by respondents in completing the survey items; otherwise, these responses indicate the ongoing need for veterinarians and producers to be vigilant of current regulations and to be in compliance with those requirements to ensure a safe food supply of animal origin.
Having a valid VCPR constitutes the regulatory and operational basis of interaction between veterinarians and their clients in provision of health care for their animals (52). Up to 90% of responding dairies reported having a VCPR. Dairies that reported not having a VCPR indicated otherwise in their responses to other questions. It is therefore most likely that 100% of the respondents had a VCPR; however, outreach efforts are further needed to inform a small percent of CA dairies on what constitutes the creation and maintenance of a VCPR with a veterinarian. Maintaining a valid VCPR allows the veterinarian to be in the best position to provide advice on AMD use decisions on farms (53). The decisions to purchase AMD for the dairy and treat sick animals with a specific AMD influence drug use on farms. In this survey, producers had a greater influence on the decision to purchase drugs, followed by veterinarians. However, the veterinarians were the major source of information that guided the producer's decisions, besides producers' experience with the drug, pharmaceutical sales representatives, and drug label information. Our finding is in concordance with previous studies that identified veterinary advice was the primary reason for choosing AMD by farmers in New Zealand (21).
Six components explained most of the variability in the survey responses. Knowledge of these components provide insights into management practices that can be the focus for stewardship interventions and outreach. The first major component identified pertained to the changes made in the use of antimicrobials following implementation of the VFD final rule, including discontinued use of one or more AMD or reduced the amount or duration of use. Such changes could have been the direct consequence of the VFD rule restricting use of AMD. Only a small proportion of respondents had started the use of alternatives to AMD, and as such future studies on stewardship should explore barriers and motivations for use of AMD alternatives. The rest of the identified components were key features of AMD management practices (disease diagnosis, AMD use practices, record keeping, information sources and decision of AMD use, and health management practices). In the health management component, heat treatment of colostrum fed to calves was the single most important variable to explain the variability between dairies. Variation in the colostrum management was possibly associated with farm size as shown in a previous survey of Pennsylvania dairy farmers in which larger farms were more likely to have the equipment for colostrum heat treatment (54).
One limitation of the current study was the small number of responses (n = 5) received form the calf ranch producers. Similarly, the number dairy respondents were relatively low, although the response rate was comparable to the outcome of previous survey conducted among the same demographics. The low response could be attributed to the survey fatigue and challenges inherent in the discussions and reporting of antimicrobial drug use among food animal producers in general.
Conclusion
Following the implementation of the VFD final rule on January 1, 2017, more than one third of the producers had made changes in the use of AMD, most notably by reducing the amount or duration of use or discontinuing the use of one or more AMD added in liquid diet or on solid feeds. The limited changes noted in AMD use could have been due to the short period between the implementation of VFD and conducting the survey. Most respondents reported a greater involvement of the herd veterinarian, compared to nutritionists or pharmaceutical sales representatives, in informing producers about the use of AMDs. Whereas, most producers had knowledge of the VFD and SB 27, opportunities exist to improve AMD use practices, including record keeping, using AMD alternatives, and improved farm management practices to reduce disease burden and need for AMD use.
The current survey outcomes allow immediate assessment of the impact of VFD final rule implementation and provides baseline data for future evaluation of the impact of VFD as well as SB 27 regulatory changes. The knowledge gained from this study is a valuable resource that could guide future recommendations for best health management practices and promote antimicrobial stewardship efforts.
Data Availability Statement
Data collected for this study is protected under California Food and Agriculture Code 14407. Requests for raw data may be made to the authors, who will consult with the California Department of Food and Agriculture (CDFA) on fulfilling the request.
Author Contributions
SA and EO designed the study, authored the first draft of the survey questions, and conducted the survey. SA, EO, DW, TL, RP, and JA reviewed and edited the survey. EO, SA, and WE analyzed the survey data. EO wrote the first draft of the manuscript. All authors edited and approved the final manuscript.
Funding
This study was supported by research contract award from the California Department of Food and Agriculture (PI: SA; Contract# 16-0342).
Conflict of Interest
JA is an employee of the California Department of Food and Agriculture.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors express great appreciation to all the dairies and calf ranches that participated in the survey. We thank Dr. Tapakorn Chamchoy for his help in data housing.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2021.636670/full#supplementary-material
References
1. Langford FM, Weary DM, Fisher L. Antibiotic resistance in gut bacteria from dairy calves: a dose response to the level of antibiotics fed in milk. J Dairy Sci. (2003) 86:3963–6. doi: 10.3168/jds.S.0022-0302(03)74006-5
2. Pereira RVV, Siler JD, Bicalho RC, Warnick LD. In vivo selection of resistant E. coli after ingestion of milk with added drug residues. PLoS ONE. (2014) 9:e115223. doi: 10.1371/journal.pone.0115223
3. Loo E, Song Lai K, Mansor R. Antimicrobial usage and resistance in dairy cattle production. In: Veterinary Medicine and Pharmaceuticals. IntechOpen (2020). p. 5–10. doi: 10.5772/intechopen.81365
4. Hammerum AM, Heuer OE, Emborg HD, Bagger-Skjøt L, Jensen VF, Rogues AM, et al. Danish integrated antimicrobial resistance monitoring and research program. Emerg Infect Dis. (2007) 13:1632–9. doi: 10.3201/eid1311.070421
5. Pol M, Ruegg PL. Treatment practices and quantification of antimicrobial drug usage in conventional and organic dairy farms in Wisconsin. J Dairy Sci. (2007) 90:249–61. doi: 10.3168/jds.S0022-0302(07)72626-7
6. Zwald AG, Ruegg PL, Kaneene JB, Warnick LD, Wells SJ, Fossler C, et al. Management practices and reported antimicrobial usage on conventional and organic dairy farms. J Dairy Sci. (2004) 87:191–201. doi: 10.3168/jds.S0022-0302(04)73158-6
7. Carmo LP, Schüpbach-Regula G, Müntener C, Chevance A, Moulin G, Magouras I. Approaches for quantifying antimicrobial consumption per animal species based on national sales data: a Swiss example, 2006 to 2013. Eurosurveillance. (2017) 22:30458. doi: 10.2807/1560-7917.ES.2017.22.6.30458
8. Sanders P, Vanderhaeghen W, Fertner M, Fuchs K, Obritzhauser W, Agunos A, et al. Monitoring of farm-level antimicrobial use to guide stewardship: overview of existing systems and analysis of key components and processes. Front Vet Sci. (2020) 7:540. doi: 10.3389/fvets.2020.00540
9. Varona OM, Chaintarli K, Muller-Pebody B, Anjum MF, Eckmanns T, Norström M, et al. Monitoring antimicrobial resistance and drug usage in the human and livestock sector and foodborne antimicrobial resistance in six European countries. Infect Drug Resist. (2020) 13:957–93. doi: 10.2147/IDR.S237038
10. FDA-USDA. Antimicrobials Sold or Distributed for Use in Food-Producing Animals. (2019). Available online at: https://www.fda.gov/media/144427/download
11. Stevens M, Piepers S, Supré K, Dewulf J, de Vliegher S. Quantification of antimicrobial consumption in adult cattle on dairy herds in Flanders, Belgium, and associations with udder health, milk quality, and production performance. J Dairy Sci. (2016) 99:2118–30. doi: 10.3168/jds.2015-10199
12. Thomson K, Rantala M, Hautala M, Pyörälä S, Kaartinen L. Cross-sectional prospective survey to study indication-based usage of antimicrobials in animals: results of use in cattle. BMC Vet Res. (2008) 4:1–6. doi: 10.1186/1746-6148-4-15
13. González Pereyra V, Pol M, Pastorino F, Herrero A. Quantification of antimicrobial usage in dairy cows and preweaned calves in Argentina. Prev Vet Med. (2015) 122:273–9. doi: 10.1016/j.prevetmed.2015.10.019
14. Pereira RV, Siler JD, Bicalho RC, Warnick LD. Multiresidue screening of milk withheld for sale at dairy farms in central New York State. J Dairy Sci. (2014) 97:1513–9. doi: 10.3168/jds.2013-7421
15. Brunton LA, Reeves HE, Snow LC, Jones JR. A longitudinal field trial assesing the impact of feeding waste milk containing antibiotic residues on the prevalence of ESBL-producing Escherichia coli in calves. Prev Vet Med. (2014) 117:403–12. doi: 10.1016/j.prevetmed.2014.08.005
16. Aust V, Knappstein K, Kunz HJ, Kaspar H, Wallmann J, Kaske M. Feeding untreated and pasteurized waste milk and bulk milk to calves: effects on calf performance, health status and antibiotic resistance of faecal bacteria. J Anim Physiol Anim Nutr (Berl). (2013) 97:1091–103. doi: 10.1111/jpn.12019
17. Maynou G, Chester-Jones H, Bach A, Terré M. Feeding pasteurized waste milk to preweaned dairy calves changes fecal and upper respiratory tract microbiota. Front Vet Sci. (2019) 6:159. doi: 10.3389/fvets.2019.00159
18. Pereira RVV, Carroll LM, Lima S, Foditsch C, Siler JD, Bicalho RC, et al. Impacts of feeding preweaned calves milk containing drug residues on the functional profile of the fecal microbiota. Sci Rep. (2018) 8:554. doi: 10.1038/s41598-017-19021-2
19. Ricci A, Allende A, Bolton D, Chemaly M, Davies R, Fernández Escámez PS, et al. Risk for the development of Antimicrobial Resistance (AMR) due to feeding of calves with milk containing residues of antibiotics. EFSA J. (2017) 15:e04665. doi: 10.2903/j.efsa.2017.4665
20. Hoelzer K, Wong N, Thomas J, Talkington K, Jungman E, Coukell A. Antimicrobial drug use in food-producing animals and associated human health risks: what, and how strong, is the evidence? BMC Vet Res. (2017) 13:211. doi: 10.1186/s12917-017-1131-3
21. McDougall S, Compton CWR, Botha N. Factors influencing antimicrobial prescribing by veterinarians and usage by dairy farmers in New Zealand. N Z Vet J. (2017) 65:84–92. doi: 10.1080/00480169.2016.1246214
22. De Briyne N, Atkinson J, Pokludová L, Borriello SP, Price S. Factors influencing antibiotic prescribing habits and use of sensitivity testing amongst veterinarians in Europe. Vet Rec. (2013) 173:475. doi: 10.1136/vr.101454
23. Ekakoro JE, Caldwell M, Strand EB, Okafor CC. Drivers of antimicrobial use practices among tennessee dairy cattle producers. Vet Med Int. (2018) 2018:1836836. doi: 10.1155/2018/1836836
24. Dubrovsky SA, Van Eenennaam AL, Karle BM, Rossitto PV, Lehenbauer TW, Aly SS. Epidemiology of bovine respiratory disease (BRD) in preweaned calves on California dairies: the BRD 10K study. J Dairy Sci. (2019) 102:7306–19. doi: 10.3168/jds.2018-14774
25. Maier GU, Love WJ, Karle BM, Dubrovsky SA, Williams DR, Champagne JD, et al. Management factors associated with bovine respiratory disease in preweaned calves on California dairies: the BRD 100 study. J Dairy Sci. (2019) 102:7288–305. doi: 10.3168/jds.2018-14773
26. Maier GU, Love WJ, Karle BM, Dubrovsky SA, Williams DR, Champagne JD, et al. A novel risk assessment tool for bovine respiratory disease in preweaned dairy calves. J Dairy Sci. (2020) 103:9301–17. doi: 10.3168/jds.2019-17650
27. FDA. Federal Register - Veterinary Feed Directive. Final Rule, Federal Register (2015). Available online at: https://www.federalregister.gov/documents/2015/06/03/2015-13393/veterinary-feed-directive
28. FAC Food and Agricultural Code. Livestock: Use of Antimicrobial Drugs [14400–14408]. Sacramento CA: FAC Food and Agricultural Code (2015). Available online at: https://leginfo.legislature.ca.gov/faces/codes_displayText.xhtml?lawCode=FAC&division=7.&title=&part=&chapter=4.5.&article
29. FDA Center for Veterinary Medicine (CVM). Guidance for Industry #152: Guidance for Industry: Evaluating the Safety of Antimicrobial New Animal Drugs With Regard to Their Microbiological Effects on Bacteria of Human Health Concern. Federal Register (2003). Available online at: https://www.fda.gov/files/animal%20&%20veterinary/published/CVM-GFI–152-Evaluating-the-Safety-of-Antimicrobial-New-Animal-Drugs-with-Regard-to-Their-Microbiological-Effects-on-Bacteria-of-Human-Health-Concern.pdf
30. Aly SS, Gardner IA, Adaska JM, Anderson RJ. Off-site rearing of heifers reduces the risk of Mycobacterium avium ssp. paratuberculosis ELISA seroconversion and fecal shedding in a California dairy herd. J Dairy Sci. (2015) 98:1–10. doi: 10.3168/jds.2014-8759
31. Buxton BM. The Disappearance of the Grade B Milk Market — A Matter of Policy Choice? Agricultural Economic Report No. 416 (1978). p. 1–2. Available online at: https://naldc.nal.usda.gov/download/CAT87202101/PDF
32. R Core Team (2020). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: https://www.R-project.org/.
33. Bécue-Bertaut M, Pagès J. Multiple factor analysis and clustering of a mixture of quantitative, categorical and frequency data. Comput Stat Data Anal. (2008) 52:3255–68. doi: 10.1016/j.csda.2007.09.023
34. Abdi H, Williams LJ, Valentin D. Multiple factor analysis: principal component analysis for multitable and multiblock data sets. Wiley Interdiscip Rev Comput Stat. (2013) 5:149–79. doi: 10.1002/wics.1246
35. Lê S, Josse J, Husson F. FactoMineR: an R package for multivariate analysis. J Stat Softw. (2008) 25:1–18. doi: 10.18637/jss.v025.i01
36. Love WJ, Lehenbauer TW, Karle BM, Hulbert LE, Anderson RJ, Van Eenennaam AL, et al. Survey of management practices related to bovine respiratory disease in preweaned calves on California dairies. J Dairy Sci. (2016) 99:1483–94. doi: 10.3168/jds.2015-9394
37. Aly SS, Rossow HA, Acetoze G, Lehenbauer TW, Payne M, Meyer D, et al. Survey of beef quality assurance on California dairies. J Dairy Sci. (2014) 97:1348–57. doi: 10.3168/jds.2013-6856
38. Brown EG. California Agricultural Statistics Review 2017-2018, California. Sacramento, CA: Office of Public Affairs. Available online at: https://www.cdfa.ca.gov/statistics/PDFs/2017-18AgReport.pdf
39. Ekakoro JE, Caldwell M, Strand EB, Okafor CC. Perceptions of Tennessee cattle producers regarding the veterinary feed directive. PLoS ONE. (2019) 14:e0217773. doi: 10.1371/journal.pone.0217773
40. Casewell M, Friis C, Marco E, McMullin P, Phillips I. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J Antimicrob Chemother. (2003) 52:159–61. doi: 10.1093/jac/dkg313
41. McEwen SA, Angulo FJ, Collignon PJ, Conly JM. Unintended consequences associated with national-level restrictions on antimicrobial use in food-producing animals. Lancet Planet. Heal. (2018) 2:e279–82. doi: 10.1016/S2542-5196(18)30138-4
42. Wierup M. The Swedish experience of the 1986 year ban of antimicrobial growth promoters, with special reference to animal health, disease prevention, productivity, and usage of antimicrobials. Microb Drug Resist. (2001) 7:183–90. doi: 10.1089/10766290152045066
43. Tempini PN, Aly SS, Karle BM, Pereira RV. Multidrug residues and antimicrobial resistance patterns in waste milk from dairy farms in Central California. J Dairy Sci. (2018) 101:8110–22. doi: 10.3168/jds.2018-14398
44. Jamaluddin AA, Carpenter TE, Hird DW, Thurmond MC. Economics of feeding pasteurized colostrum and pasteurized waste milk to dairy calves. J Am Vet Med Assoc. (1996) 209:751–6.
45. Maynou G, Migura-Garcia L, Chester-Jones H, Ziegler D, Bach A, Terré M. Effects of feeding pasteurized waste milk to dairy calves on phenotypes and genotypes of antimicrobial resistance in fecal Escherichia coli isolates before and after weaning. J. Dairy Sci. (2017) 100:7967–79. doi: 10.3168/jds.2017-13040
46. Garzon A, Pandey P, Tell L, Aly SS, Poppenga R, Pereira R. Evaluation of heat and pH treatments on degradation of ceftiofur in whole milk. Front Vet Sci. (2020) 7:288. doi: 10.3389/fvets.2020.00288
47. Constable PD. Antimicrobial use in the treatment of calf diarrhea. J Vet Intern Med. (2004) 18:8–17. doi: 10.1111/j.1939-1676.2004.tb00129.x
48. Smith G. Antimicrobial decision making for enteric diseases of cattle. Vet Clin North Am Food Anim Pract. (2015) 31:47–60. doi: 10.1016/j.cvfa.2014.11.004
49. USDA-APHIS-VS-CEAH-NAHMS. Dairy 2014: Health and Management Practices on U.S. Dairy Operations. (2018). Available online at: https://www.aphis.usda.gov/animal_health/nahms/dairy/downloads/dairy14/Dairy14_dr_PartIII.pdf
50. FDA. Animal Medicinal Drug Use Clarification Act of 1994 (AMDUCA) [WWW Document]. (2020). Available online at: https://www.fda.gov/animal-veterinary/guidance-regulations/animal-medicinal-drug-use-clarification-act-1994-amduca (accessed November 4, 2020).
51. FARAD. Food Animal Residue Avoidance Databank: Restricted and prohibited drugs in food animals [WWW Document]. (2018). Available online at: http://www.farad.org/prohibited-and-restricted-drugs.html (accessed November 4, 2020).
52. Léger DF, Newby NC, Reid-Smith R, Anderson N, Pearl DL, Lissemore KD, et al. Antimicrobial dispensing by Ontario dairy veterinarians. Can Vet J. (2015) 56:723–9.
53. AABP. Establishing and maintaining the veterinarian-client-patient-relationship in bovine practice [WWW Document]. (2020). Available online at: http://www.aabp.org/resources/aabp_guidelines/VCPRGuideline_032020.pdf (accessed November 8, 2020).
Keywords: antimicrobial drug use, veterinary feed directive, perception, preweaned dairy calves, California Senate Bill 27
Citation: Okello E, Williams DR, ElAshmawy WR, Adams J, Pereira RV, Lehenbauer TW and Aly SS (2021) Survey on Antimicrobial Drug Use Practices in California Preweaned Dairy Calves. Front. Vet. Sci. 8:636670. doi: 10.3389/fvets.2021.636670
Received: 01 December 2020; Accepted: 15 March 2021;
Published: 22 April 2021.
Edited by:
Marta Hernandez-Jover, Charles Sturt University, AustraliaReviewed by:
Angel Abuelo, Michigan State University, United StatesClair L. Firth, University of Veterinary Medicine Vienna, Austria
Copyright © 2021 Okello, Williams, ElAshmawy, Adams, Pereira, Lehenbauer and Aly. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sharif S. Aly, c2FseUB1Y2RhdmlzLmVkdQ==
 Emmanuel Okello
Emmanuel Okello Deniece R. Williams
Deniece R. Williams Wagdy R. ElAshmawy
Wagdy R. ElAshmawy Jaymes Adams
Jaymes Adams Richard V. Pereira
Richard V. Pereira Terry W. Lehenbauer1,2
Terry W. Lehenbauer1,2 Sharif S. Aly
Sharif S. Aly