- 1School of Veterinary Science, Massey University, Palmerston North, New Zealand
- 2Hopkirk Laboratory, AgResearch, Palmerston North, New Zealand
- 3Orthopaedic Research Center, C. Wayne McIlwraith Translational Medical Institute, Colorado State University, Fort Collins, CO, United States
Despite significant immunosuppressive activity, allogeneic mesenchymal stromal cells (MSCs) carry an inherent risk of immune rejection when transferred into a recipient. In naïve recipients, this immune response is initially driven by the innate immune system, an immediate reaction to the foreign cells, and later, the adaptive immune system, a delayed response that causes cell death due to recognition of specific alloantigens by host cells and antibodies. This review describes the actions of MSCs to both suppress and activate the different arms of the immune system. We then review the survival and effectiveness of the currently used allogeneic MSC treatments.
Introduction
Bone marrow-derived mesenchymal stromal cells (MSCs) possess immense potential for the treatment of many diseases (1, 2), and there has been rapid acceleration in the clinical use of MSCs (2). Bone marrow-derived MSCs have become the “gold standard” MSC for use in musculoskeletal therapies (3, 4), though adipose-derived and umbilical cord-derived MSC products are also commonly available (3). The use of allogeneic MSCs for treatment is less costly as it can be prepared for multiple animals and is immediately available for treatment (5, 6). An additional benefit is for older patients whose MSCs are known to have lower proliferation rates as compared to MSCs from younger donors (7). Allogeneic MSCs as an off the shelf product will likely be the main mode of MSC treatment in the future.
When treating an individual, be it human or equine, with allogeneic stem cell therapy, prevention of allorecognition of the recipient to the transplanted foreign antigens is an important component of achieving a persistent and potent effect. Medium- or long-term survival of the MSCs to exert their desired anabolic effects is likely to promote their effectiveness as a treatment as compared responses associated with short-term survival. Certainly, short-lived therapy with MSCs can be the catalyst for improvement of disease processes, as several studies have reported that the number of implanted MSCs detected in target tissue was too low to explain the improvement in disease state (1). However, without survival of the MSCs, there would be no source of ongoing therapeutic effect nor involvement of the MSC in the structural integrity of repair. Another concern regarding allorecognition is the described side effects of intra-articular allogeneic MSC injection in people (5) and horses (8). These include pain, swelling of the joint, and urticarial (5, 8). For these reasons, a complete understanding of the interaction of the MSCs with the immune system is necessary to foresee the risks and predict the effectiveness of allogeneic MSCs as a treatment.
Many studies have found that bone-marrow-derived MSCs are capable of substantial anti-inflammatory effects (9–13). The immunomodulation caused by MSCs is dependent on inhibitory molecule secretion, direct cell contact, and induction of regulatory leukocyte populations (14–17). Over 350 human studies are currently underway that investigate the ability of MSCs to limit immune reactions related to auto-immunity and tissue transplantation (18). Previous studies have shown that allogeneic MSCs suppress immune reactions in graft-vs.-host disease and organ transplantation even when steroids are unable to provide suppression (19).
From understanding the published literature, we know that allogeneic MSCs have both immunostimulatory and also immunosuppressive actions. What we must determine is the overall effect. Are allogeneic MSCs used in the equine patient able to provide anti-inflammatory and anabolic effects or does immune recognition negate these therapeutic benefits?
The Interaction of the Innate Immune System With Allogeneic MSCs
The cascade of events that occurs when MSCs encounter the immune system can be broken down into phases of the immune system response. These include the acute reaction by the innate immune system, and then the slightly delayed specific adaptive immunity of both cell-mediated and humoral (antibody) responses that result in long-term memory cells (20). It is important to understand how the MSCs are affected through each of these steps in order to determine the potential for efficacy and the side effects of allogeneic treatment.
The innate immune system responds quickly and non-specifically to foreign antigens. This involves the release of anti-microbial enzymes and peptides, complement activation, recruitment of inflammatory cells, phagocytosis and destruction of foreign pathogens, and cells (20). Endothelial cells are one of the first cells to detect foreign pathogens, resulting in release of chemokines which allow the blood vessels to dilate leading to the extravasation and migration of phagocytes such as neutrophils and macrophages (20).
Complement
The complement system is an important part of innate immunity. Complement is released from the liver into the blood in its inactive form and is cleaved to create its activated form by proteases derived from inflammation. Complement components can bind directly to alloantigens or utilize antibodies to mark the antigen for removal (21). The foreign cell is then removed by forming a membrane attack complex or by facilitating leukocyte phagocytosis (21, 22).
When the effects of complement are considered alone without accounting for the actions of other immune cells, this non-cellular agent has been shown to cause a decrease in viability of human allogeneic MSCs (22–24). Two studies found >40% of human adipose-derived MSCs were damaged upon culture with naïve human serum containing activated complement (23, 24). Another study found minimal damage to MSCs when complement alone was added, but complement-mediated phagocytosis caused MSC death when monocytes were added in vitro (22). Means of resolving complement-mediated cytotoxicity have been created, but thus far each requires manipulation of the MSCs by means of application of complement-inhibiting materials (Factor H or N-glycolylneuraminic acid) to the cells' surface which is likely impractical from a licensing perspective at this point in time (24, 25). CD59, a molecule found on some MSCs can prevent complement opsonization (22). Sourcing MSCs with high surface expression of CD59 may also be a potential means to mitigate complement-mediated MSC death (22).
The effects of the complement system on equine MSCs have not yet been reported in the horse.
Neutrophils
Neutrophils are the most numerous cell of the innate response and often the first leukocyte to infiltrate an allogeneic tissue (20, 26). Neutrophils are recruited to areas of inflammation by vascular endothelium and likely recruited to MSCs by chemokine proteins such as CXCL8 (IL-8) (26, 27). Once extravasated into allogeneic tissue, neutrophil infiltration leads to increased antigenicity and reduced allograft function (28). This may not occur when MSCs are administered as MSCs cause minimal activation of neutrophils in vivo by allogeneic MSCs (29). Allogeneic MSCs appear to be immunomodulatory in that they can suppress neutrophil activation by causing a significant reduction in ROS when neutrophils were activated prior to the addition of MSCs (28–31).
Although neutrophils in isolation are not activated by MSCs, one of the most concerning effects of the innate immune system in the horse is the rapid influx of neutrophils following intra-articular (both autologous and allogeneic) MSC injection (8, 32). Numerous studies investigating the effect of MSC injection into equine joints show an increase in neutrophil count in synovial fluid lasting 48–72 h after administration of autologous and allogeneic stem cells (8, 32–34). An increase in effusion (as measured by joint circumference) with or without a mild increase in lameness also occurs at similar time points (8, 32–34). There are several confounding factors for this neutrophil invasion. Joswig et al. (32) showed this increase in cell infiltration and swelling occurs to the same degree when MSC freeze media (autologous serum and 5% DMSO) is injected alone without MSCs, as when freeze media is injected with autologous or allogeneic MSCs. The authors determined that in these cases, MSCs may not be the primary cause of neutrophil infiltration (32). Another contributor to neutrophil activation found in earlier studies is the use of FBS in MSC media (32). There is a significant increase in nucleated cell counts in the synovial fluid of joints injected with FBS-cultured autologous MSCs as compared to autologous or allogeneic MSCs cultured in equine serum during the final 48 h of incubation (32). Because of this finding, where possible, studies are performed without this confounding factor.
Another possible cause of neutrophil influx may be due to a small proportion of MSCs in a cryopreserved or fresh MSC sample that become non-viable prior to administration (35). Activated neutrophils participate in the clearance of apoptotic cells; therefore, neutrophils enter the joint following an injection of dead cells. Interestingly, because apoptotic cells inhibit the proinflammatory functions of neutrophils, uptake of apoptotic cells by neutrophils can contribute to the resolution of inflammation in areas where dead cells are present (36). The degree to which dead MSCs cause neutrophil influx as compared to live MSCs is unknown.
In a different type of study, MSCs had immunosuppressive effects on neutrophils in an inflamed equine joint (37). In this study lipopolysaccharide (LPS) was injected into one joint to stimulate an inflammatory response, and LPS and umbilical cord-derived MSCs were injected into the contralateral joint. This study saw a significant decrease in neutrophil influx into the joint after injection of both MSCs and LPS compared to the injection of LPS alone (37). The interpretation of these findings is that the presence of MSCs suppresses the activation of innate immune system.
Overall, there is concern when a horse is treated with either autologous or allogeneic MSCs and the joint then becomes acutely swollen and/or lame. In layman's terms this reaction is called a “flare”; a short-lived inflammatory response that resolves without treatment or with anti-inflammatory medication. Flares in clinical cases have been reported to occur in between 1.8 and 9% of equine cases receiving autologous or allogeneic MSCs (38, 39). No long-term negative effects were seen in either of these studies. Human studies using allogenic MSCs and hyaluronic acid had a 25–53% rate of significant effusion after intra-articular treatment of the knee (40, 41), while administration of autologous MSCs and hyaluronic acid had a 45% rate of effusion (42). When, hyaluronic acid was used alone, 60% of human patients suffer from significant effusion (40).
Although these brief incidents of soreness and swelling can be worrying to the client, there is no evidence of long-term negative effects nor lack of response to treatment (39, 40). Additionally, as laboratories replace FBS during the final 48 h of culture, these “flares” should be less common. Therefore, neutrophil influx after allogeneic MSC treatment in the horse does not appear to be an impediment to the use of allogeneic MSCs.
Macrophages
Macrophages are the most efficient type of phagocyte and are able to eliminate a large variety of pathogens, including foreign cells (43). When human allogeneic MSCs are cultured with macrophages, the macrophages become immunosuppressive, inhibiting natural killer (NK) cells and pushing T lymphocytes down a regulatory pathway (44). At this time there are only two equine studies that have reported the reciprocal effects of MSCs and macrophages. Cassano et al. (45) found minimal effect of MSCs on activated macrophages in vitro showing that MSCs may not have a strong immunoregulatory ability to deactivate macrophages. Those MSC exposed to activated macrophages, though, then became immunosuppressive in an activated T lymphocyte proliferation assay (46). Although data in this area are extremely limited, allogeneic MSCs may be less capable of immunomodulation of activated macrophages (45).
Natural Killer Cells
Natural killer cells are a part of the innate immune system that can cause cell death through the targeted release of cytotoxins (47). NK cells can attack cells lacking major histocompatibility complex (MHC) I on the surface of cells (47). As bone marrow-derived equine MSCs express MHC I (48, 49), NK cells may be less likely to pose a threat for these MSCs. Any hypothesizing on this issue is debatable at this point as appropriate antibodies for recognition of NK cells in the horse are lacking. MSCs have been found capable of suppressing NK cytotoxic activity in a murine hepatotoxicity model and using human cells in vitro (50, 51).
Dendritic Cells
Dendritic cells capture and process alloantigens and serve to activate the adaptive immune system by presenting the alloantigens to B and T lymphocytes (52). Dendritic cells cultured with murine allogeneic MSCs cause the dendritic cells to decrease their surface expression of stimulatory molecules including CD80, CD83, CD86, and MHC II (53). In response to pathogens, these molecules are normally up-regulated to aid in activation of cell-mediated immunity. After interaction of the dendritic cells with murine allogeneic MSCs, the dendritic cells then cause a decrease in lymphocyte proliferation in mixed lymphocyte reactions (53). Here we see evidence of the inhibition of adaptive immune system through MSC effects on the innate responses.
The Interaction of the Adaptive Immune System With Allogeneic MSCs
As previously mentioned, the adaptive immune response consists of two primary pathways; one is cell-mediated and the other is antibody-mediated (i.e., humoral immunity). T lymphocytes are needed for both pathways. In the humoral response of the adaptive immune system, B cells or antigen presenting cells bound with alloantigens in association with major histocompatibility type II (MHC II) receptor interact with helper T cells (i.e., CD4 T lymphocytes) (54, 55). Upon interaction with CD4 lymphocytes, B cells then are activated to differentiate into plasma cells which secrete antibodies to the alloantigen (55). The earliest antibodies are seen in circulation after invasion of the organism is just less than 1 week (48, 56), and these antibodies can circulate for a long duration (56, 57). This may be important in clinical scenarios where repeat treatments with allogeneic equine MSCs are warranted.
The cell-mediated component of the adaptive immune response requires cytotoxic T cells (i.e., CD8 T lymphocytes). Cytotoxic T cells take part in both direct and indirect alloimmunity with cells bearing MHC I receptors that are bound with an alloantigen. In this way, cytotoxic T cells attack those cells that are foreign to the organism or cells that have taken up a foreign antigen. After a pathogen is recognized, a subset of CD8 cytotoxic T cells mature to form memory T cells (58). Memory T cells rapidly respond upon subsequent antigen recognition, triggering the removal of the foreign antigens even many years later (58). Both CD4+ and CD8+ lymphocytes are important when considering the use of allogeneic MSCs as these immune cells may recognize allogeneic MSCs due to their expression of MHC I and II.
MHC I and II Expression on MSCs
After some debate about the presence of major histocompatibility markers on equine MSCs, it is now known that the cell surface expression of MHC I and II on MSCs is variable from one donor to another and even one MSC sample to another (48, 49, 59). MHC I is expressed on all equine bone marrow-derived MSCs though the degree of expression varies (48). Conversely, some MSCs do not express MHC II antigens, while others have a strong positive expression (49, 59). Most problematically, MHC I and II expression is increased in the face of culture with foreign lymphocytes, when MSCs are cultured with inflammatory cytokines, or as the MSCs differentiate (46, 60–62). The expression of MHC I and II motifs on MSCs are important in that they are the key cell markers utilized for alloimmunity by the host's immune system, and expression of these markers identifies the MSCs as targets for destruction. Not only is the expression of these molecules important, but the degree to which these molecules are similar between the donor and recipient is also critical. The structure of each MHC molecule is defined by the human leukocyte antigen (HLA) or equine leukocyte antigen (ELA) haplotype (63). Horses are haplotyped using microsatellites to the ELA gene (63). The ELA haplotype and degree of mis-matching determines the recognizability of donor cell to the recipient's immune system. Therefore, an MSC that expresses MHC I or II would be minimally immunogenic if the ELA haplotype is “matched” to the recipient (61, 64).
T Lymphocyte Responses to MSCs
What is the overlying result when allogeneic MSCs are exposed to lymphocytes? Are the lymphocytes activated or suppressed? When suppression of activated lymphocytes is considered, studies have overwhelmingly shown that allogeneic equine MSCs are capable of preventing lymphocyte proliferation in response to an activating agent (phytohaemaglutinin, foreign leukocytes, etc), thereby quelling an immune response (10, 11, 13, 65). This immunosuppression occurs subsequent to the MSC-mediated increase in regulatory T lymphocytes (Tregs) which serve to dampen the adaptive immune response and can prevent rejection of foreign cells by the host (66). MSCs secrete immunomodulatory cytokines, including transforming growth factor beta (TGF-β), indoleamine 2,3-deoxygenase 1, IL-2, IL-10, IL-1beta receptor antagonist, hepatocyte growth factor and PGE2 (11, 67–70). These cytokines serve to push the T lymphocytes down the path to create more T regulatory cells and to suppress leukocyte activation (11, 70).
Many in vitro studies have been performed looking into lymphocyte behavior after interaction with MSCs. Two studies using equine MSCs, showed that both autologous and allogeneic MSCs have an equal immunosuppressive capacity when MSCs are cultured with activated lymphocytes (11, 13). This may indicate that immunosuppression is the predominant response when compared with immunoactivation by allogeneic MSCs. Another study examined activated lymphocytes and how they interacted with different types of allogeneic equine MSCs (59). Suppression of the lymphocytes occurred when MSCs expressing low levels of MHC II were co-cultured, but increased activation occurred when MSCs expressing high levels of MHC II were co-cultured (59). A study using 11 different human allogeneic MSC products found that every product tested was capable of immunosuppression when cultured with activated lymphocytes (65). These studies indicate allogeneic MSCs are repeatedly shown to be capable of suppressing activated T lymphocytes. It must be acknowledged that each of these studies were performed in vitro, and previous studies in the horse have shown a lack of correlation in immunomodulatory properties between in vitro and in vivo results (59, 71).
Do allogeneic MSCs cause activation of unactivated lymphocytes? Colbath et al. (11) has shown that allogeneic and autologous equine MSCs cause mild lymphocyte proliferation in vitro, the extent of which was similar for both groups. Similarly, in humans, lymphocyte proliferation occurs when lymphocytes are co-cultured with allogeneic MSCs (72). Interestingly, several human studies found an immunosuppressive form of the MHC I antigen, called HLA-G, which is expressed on some human MSCs (9, 72, 73). Nasef et al. (73) found that by adding an antibody against HLA-G, effectively inhibiting it from performing its function, activated lymphocytes proliferate when mixed with allogeneic MSCs. Without the neutralizing antibody, human allogeneic MSCs prevent lymphocyte activation. Other work has shown HLA-G causes lymphocyte suppression and increases the number of immunosuppressive Tregs (9). This HLA-G form of the MHC I molecule, which provides an innate ability to prevent the recognition of foreign cells, has likely evolved from the need to prevent fetal attack during gestation (9, 73). This immunosuppressive isoform of MHC I is likely to exist in the ELA system, though no evidence has yet been published for the horse.
Does repeat exposure of the T lymphocytes to an allogeneic MSC cause lymphocyte activation? Piggot et al. (74) co-cultured allogeneic MSCs with lymphocytes from horses that had previous exposure to the allogeneic MSCs and found no CD4+ lymphocyte proliferation signifying a lack of CD4+ memory cells. Koi et al. (75) found that the systemic CD8+ population of lymphocytes, not the CD 4+ lymphocytes, increased when horses were treated for a second time with intravenous allogeneic MSCs. This suggests that CD8+ memory T cells are generated upon original exposure leading to cytotoxic lymphocyte proliferation upon re-injection with MSCs (75).
B Cells and Alloantibody Responses to MSCs
Antibody production has been shown to be a limitation for allogeneic MSC survival. There is significant antibody production to allogeneic MSCs across species (71, 76, 77). Barrachina et al. (62) found that all equine patients receiving intra-articular allogeneic mis-matched MSCs formed antibodies after injection. Pezzanite et al. (71) used MSCs of a mis-matched ELA haplotype and injected these cells intradermally in horses. After 21 days, all horses had synthesized antibodies against the ELA type of the MSC that had been administered (71). These antibodies are capable of targeting the MSCs for destruction (64). Of the six horses tested, one also created an antibody response to another ELA type (71). This cross reactivity has been reported previously in the human literature (78, 79).
The synthesis of antibodies capable of destruction of the MSCs after allogeneic treatment may limit the survival of the MSCs and therefore decrease the potency of therapeutic effect. Overcoming the undesirable consequences of the adaptive immune response is important when repeat MSC treatment is required as antibodies to the MSC may be present on administration (59). There are several methods to mitigate alloantibody production. One way forward is to ELA type donors and recipients to find a “matched” pair. This is challenging as there are at least 50 variations in ELA haplotypes (63). Another strategy is to ELA type the donor horses of the MSCs and give subsequent treatments with MSCs of a different haplotype. Using this technique, only the horses that have cross-reactive antibodies would carry antibodies against the MSCs at the time of treatment. A third possible technique relies upon the manipulation the MSCs to prevent expression of MHC I and II. The reduction of MHC I and II expression has been successfully performed in human and murine MSCs using molecular biologic techniques (6, 80). The addition of TGF β2 has also been shown to reduce MHC I and II expression (48).
Even without these techniques to decrease the effects of the major histocompatibility molecules, the MSCs that are currently being utilized provide beneficial treatment effects despite alloimmunity being present (38, 81–86).
Allogeneic MSC Survival in vivo
There is some controversy as to whether there is a considerable beneficial effect of longer-term MSC survival in damaged tissue as compared to a short-lived effect. One study found that dead MSCs used to treat cardiac ischemia-reperfusion injury in mice had the same beneficial effect as viable MSCs (87). This study determined that the effect of MSCs on macrophages caused the improvement in cardiac output. Another study with the same method of cardiac insult found a significant effect between MSC survival and improved cardiac function (88). The MSCs in this second study were tracked over 30 days and were found to be present in the myocardium throughout the study period. These studies seem to conflict with one another, but perhaps this is due to the method of improvement in function seen in the different studies. An immune-mediated effect may not necessitate long term MSC survival as some reports suggest (1, 88, 89), while a structural effect may require long-term MSC incorporation.
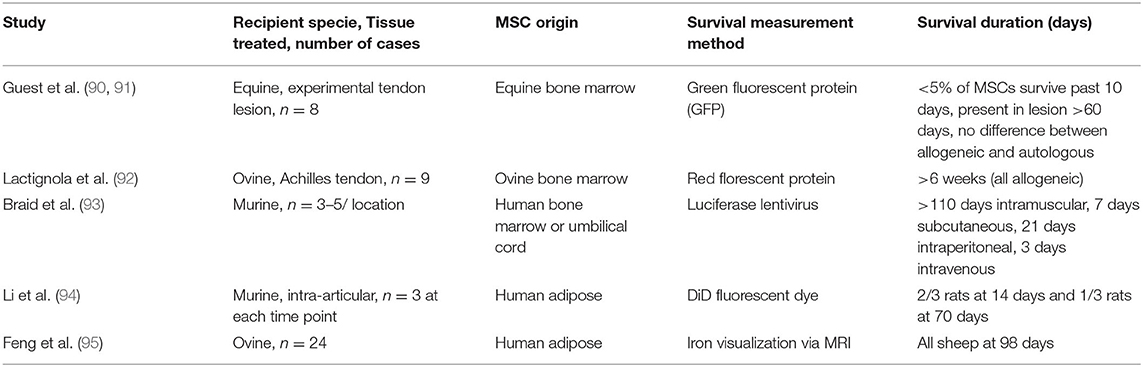
Few equine studies focusing on the duration of survival of allogeneic MSCs have yet been published. Furthermore, it is largely unknown what percent of the original dose of MSCs that is given to a patient survives long term, but generally this is believed to be a very small proportion for both autologous and allogeneic MSCs (90, 91). Guest et al. (90, 91) found that ~2% of the originally injected equine bone marrow-derived allogeneic MSCs survived to 30 days in the lesion and 1% survived to 60 days in the lesion (Table 1). Ovine bone marrow-derived allogeneic MSCs survive at least 6 weeks after intra-tendinous injection though the percent survival was not measured (Table 1) (92). Human MSCs injected into mice survive longer than 5 months when injected intramuscularly, 1–4 weeks when injected subcutaneously or intraperitoneally, but only a few days when injected intravenously (Table 1) (93). When allogenic adipose-derived MSCs were used intra-articularly after disease induction in the femorotibial joint, MSCs survived 10 weeks in the rat and 14 weeks in sheep (Table 1) (94, 95). By extrapolating the data in these studies, it appears that allogeneic MSCs survive for a longer period in areas of lower vascularity.
Results of Allogeneic MSC Therapy for Musculoskeletal Disease
Above and beyond the possible mechanisms for deleterious effects on MSCs by the immune system, the results of in vivo clinical trials and experimental studies must be considered. The use of bone marrow-derived allogeneic MSCs for joint disease has gained popularity, likely due to largely positive results (2, 96). A large equine clinical trial of 165 horses treated with allogeneic MSCs and platelet rich plasma has been described (38). In this report 45% of cases at 6 weeks post-treatment, and 78% of cases by 18 weeks returned to athleticism, though this study lacked a control population (Table 2) (38). A study using a chemically induced- model of arthritis in the horse showed significant upregulation of type 2 collagen and significantly decreased expression of inflammatory mediators in cartilage at 6 months post-treatment when allogeneic MSC-treated joints were compared to untreated joints, though no significant gross nor histologic improvement was seen (Table 2) (33). In a similar study, allogeneic MSCs did not cause significant clinical improvement in IL-1beta- induced arthritis, however, this was a very acute and severe inflammatory model (Table 2) (97). Additional allogeneic MSC studies focusing on joint disease in the horse have shown beneficial clinical and histologic results using blood-derived (81), neonatal-derived (82), or adipose-derived MSCs (Table 2) (83). In people with severe knee osteoarthritis, Vega et al. (40) showed improved function and cartilage grade on MRI as compared to hyaluronic acid when MSCs were used intra-articularly. Experimentally created knee arthritis in rabbits was improved when treated intra-articularly, but only when the animals were treated on three occasions as one injection was insufficient to improve outcomes (98). The use of allogeneic MSCs in joint disease appears to be beneficial though in some studies, this benefit was not clinically relevant.
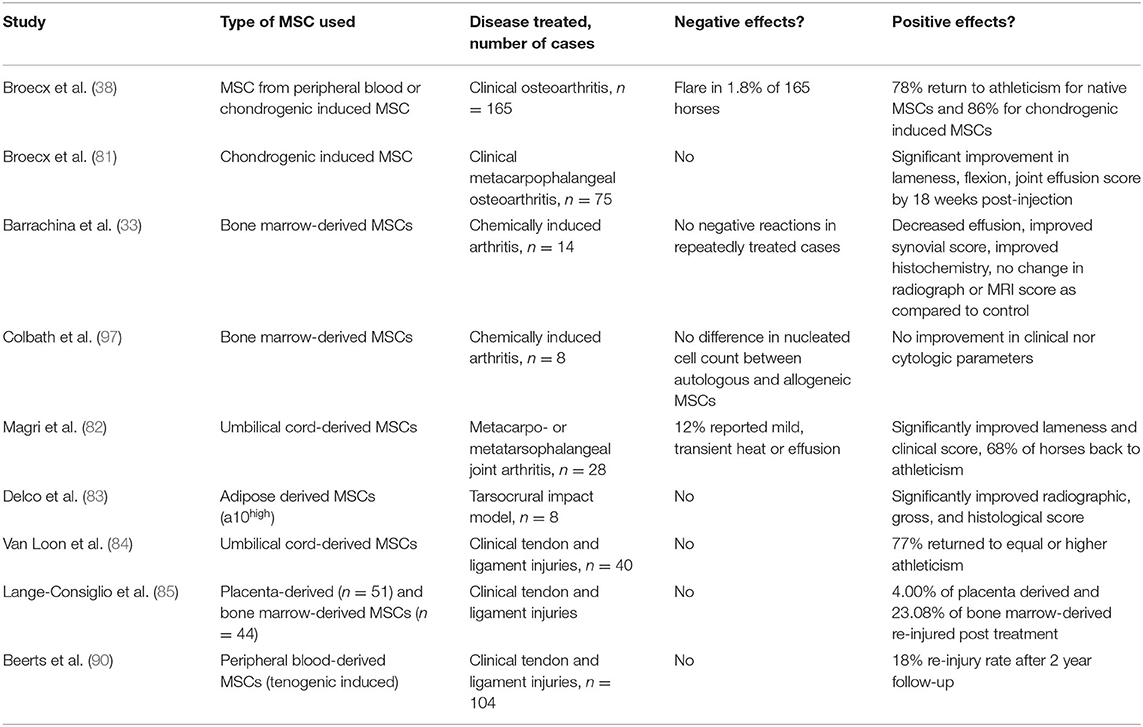
Table 2. Relevant equine studies evaluating the use of allogeneic MSCs in clinical and experimental musculoskeletal disease.
The use of bone marrow-derived allogeneic MSCs for soft tissue lesions show promise when the treatment is administered directly into the injured tissue. A clinical study of 40 horses treated with adipose-derived MSCs for tendon lesions concluded that 77% of those horses returned to full athletic function of equal or higher levels than prior to the injury (Table 2) (84). Another study using 44 clinical cases of tendon or ligament lesions showed a similar proportion of horses returning to athleticism after bone marrow-derived allogeneic MSC therapy (Table 2) (85). A recent large clinical equine study on soft tissue lesions found 18% of horses reinjuring within 2 years of follow up (Table 2) (86). These data appear favorable in comparison to the 44% re-injury rates among horses treated with rest and simple rehabilitation techniques alone (99).
When evaluating the therapeutic potential of allogeneic MSCs in experimental models of soft tissue lesions, laboratory animals were the only populations examined to date. Direct injection into a rat Achilles tendon rupture model results in improved elasticity and strength of treated tendons as compared to untreated tendons at 30 days post-treatment (100). Intrathecal injection of bone marrow-derived MSCs to treat a surgically created defect in the intra-synovial portion of the Achilles tendon in sheep does not improve healing of the treated tendons at 24 weeks post-injury (101). A study using adipose-derived allogeneic MSCs in a rat Achilles tendon tear model showed improved strength of the injured tendon when treated into the lesion with MSCs (102). Based on the evidence to date, tendons appear to have improved healing when treated with allogeneic MSCs, and the use of these treatments in equine tendon and ligament lesions is warranted.
Repeated Allogeneic MSC Administration for Treatment of Disease
Few studies have been completed to determine if repeat administration of allogeneic MSCs is more beneficial than a single treatment. As we have detailed, there would likely be antibody presence in the animal upon repeat treatment along with memory Tcells (62, 71, 75). Repeat treatment using allogeneic MSCs has shown to cause an increase in leukocyte recruitment when used intra-articularly (32). One study using umbilical-derived MSCs showed no improvement in therapeutic efficacy when clinical cases of equine joint disease were treated twice in a 1 month interval as compared to those joints that were only treated once (82). As previously discussed, one rabbit study saw no improvement in arthritis when only one treatment of bone marrow-derived MSCs was given, while repeat therapy proved beneficial (98). In contrast, a study using mouse model of colitis showed that allogeneic MSCs improved the disease upon initial treatment, but when mice were again inflicted with colitis, only syngenic MSCs were beneficial, not the allogeneic MSCs that had provided therapy upon initial treatment (103). It is a common concern that repeat allogeneic therapy may lead to reduced therapeutic benefit in the horse, and we have yet to fully answer this question. Judging from the great amount of research showing immune response to interaction of MSCs and leukocytes, adaptive immunity likely will limit the functional ability of allogeneic MSCs upon repeat administration unless a means to mitigate MHC expression has been reconciled.
Conclusion
Allogeneic MSCs have both immunostimulatory and immunosuppressive effects. Resounding immunosuppressive effects are seen when MSCs are mixed with activated neutrophils or activated lymphocytes (10, 11, 13, 28–31, 65). Allogeneic MSCs are recognized by the innate and adaptive arms of the immune system and their viability may be decreased following immune recognition (62, 64, 71). An antibody response is generated post-injection in the horse which likely would inhibit their therapeutic efficacy upon repeat treatment (32, 62, 64, 103). Allogeneic bone marrow- derived MSCs can survive in the recipient long term when delivered into low vascularity regions such as tendons and muscle (88, 90, 93).
There is evidence that use of allogeneic MSC therapy is beneficial to the patient (38, 81–86, 103). Results of several studies have shown allogeneic MSCs carry no greater rate of short-term complications when used as a one-off therapy as compared to other biologic therapies (8, 32–34), and improving laboratory techniques will continue to lower the occurrence of side effects (32). These side effects seen thus far have no relation to the level of success of the treatment (38, 40). The response generated from current allogeneic MSC therapies that may not survive long-term is substantial and should not be disregarded. Potentially, a more potent response will be generated from an MSC that is minimally recognized by the recipient immune system and allowed to have a longer time frame to exert a therapeutic effect (45). Methods to mitigate alloantibody production are being researched. ELA matching can be performed between recipient and donor. Molecular manipulation the MSCs to prevent expression of MHC I and II would decrease immune recognition. If repeat MSC therapy is given, variation of the donor MSC haplotype could minimize the immediate adaptive immune response. These options deserve continued investigation to improve upon the therapeutic benefits of allogeneic MSC therapy.
Author Contributions
JLK constructed the manuscript. CR, NP, EG, and CWM edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Funding has been provided by a grant from the New Zealand Equine Trust.
Conflict of Interest
JLK and CWM are partners in Advanced Regenerative Therapies New Zealand.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Squillaro T, Peluso G, Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant. (2016) 25:829–48. doi: 10.3727/096368915X689622
2. Chahal J, Gómez-Aristizábal A, Shestopaloff K. Bone marrow mesenchymal stromal cell treatment in patients with osteoarthritis results in overall improvement in pain and symptoms and reduces synovial inflammation. Stem Cells Transl Med. (2019) 8:746–57. doi: 10.1002/sctm.18-0183
3. Wilson A, Hodgson-Garms M, Frith JE, Genever P. Multiplicity of mesenchymal stromal cells: finding the right route to therapy. Front Immunol. (2019) 10:1112. doi: 10.3389/fimmu.2019.01112
4. Frisbie DD, Smith RK. Clinical update on the use of mesenchymal stem cells in equine orthopaedics. Equine Vet J. (2010) 42:86–9. doi: 10.2746/042516409X477263
5. Peeters CM, Leijs MJ, Reijman M, van Osch GJVM, Bos PK. Safety of intra-articular cell-therapy with culture-expanded stem cells in humans: a systematic literature review. Osteoarthr Cartil. (2013) 21:1465–73. doi: 10.1016/j.joca.2013.06.025
6. Huang XP, Ludke A, Dhingra S, Guo J, Sun Z, Zhang L, et al. Class II transactivator knockdown limits major histocompatibility complex II expression, diminishes immune rejection, and improves survival of allogeneic bone marrow stem cells in the infarcted heart. FASEB J. (2016) 30:3069–82. doi: 10.1096/fj.201600331R
7. Brohlin M, Kingham PJ, Novikova LN, Novikov LN, Wiberg M. Aging effect on neurotrophic activity of human mesenchymal stem cells. PLoS ONE. (2012) 7:e45052. doi: 10.1371/journal.pone.0045052
8. Ardanaz N, Vázquez FJ, Romero A, Remacha AR, Barrachina L, Sanz A, et al. Inflammatory response to the administration of mesenchymal stem cells in an equine experimental model: effect of autologous, and single and repeat doses of pooled allogeneic cells in healthy joints. BMC Vet Res. (2016) 12:65. doi: 10.1186/s12917-016-0692-x
9. Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. (2008) 26:212–22. doi: 10.1634/stemcells.2007-0554
10. Remacha AR, Barrachina L, Álvarez-Arguedas S, Ranera B, Romero A, Vázquez FJ, et al. Expression of genes involved in immune response and in vitro immunosuppressive effect of equine MSCs. Vet Immunol Immunopathol. (2015) 165:107–18. doi: 10.1016/j.vetimm.2015.04.004
11. Colbath AC, Dow SW, Phillips JN, McIlwraith CW, Goodrich LR. Autologous and allogeneic equine mesenchymal stem cells exhibit equivalent immunomodulatory properties in vitro. Stem Cells Dev. (2017) 26:503–11. doi: 10.1089/scd.2016.0266
12. Ge W, Jiang J, Arp J, Liu W, Garcia B, Wang H. Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation. (2010) 90:1312–20. doi: 10.1097/TP.0b013e3181fed001
13. Ranera B, Antczak D, Miller D, Doroshenkova T, Ryan A, McIlwraith CW, et al. Donor-derived equine mesenchymal stem cells suppress proliferation of mismatched lymphocytes. Equine Vet J. (2016) 48:253–60. doi: 10.1111/evj.12414
14. Consentius C, Reinke P, Volk HD. Immunogenicity of allogeneic mesenchymal stromal cells: what has been seen in vitro and in vivo. Regen Med. (2015) 10:305–31. doi: 10.2217/rme.15.14
15. Moravej A, Karimi MH, Geramizadeh B, Hossein Aghdaie M, Kohi-Hoseinabadi O, Ebrahimnezhad S. Effect of mesenchymal stem cells on ILT3 expression in the splenocytes of skin graft recipient mice. Iran J Immunol. (2016) 13:274–88.
16. Khosravi M, Karimi MH, Aghdaie MH, Kalani M, Naserian S, Bidmeshkipour A. Mesenchymal stem cells can induce regulatory T cells via modulating miR-126a but not miR-10a. Gene. (2017) 627:327–36. doi: 10.1016/j.gene.2017.06.012
17. Girdlestone J. Mesenchymal stromal cells with enhanced therapeutic properties. Immunotherapy. (2016) 8:1405–16. doi: 10.2217/imt-2016-0098
18. Clinicaltrials.gov, terms MSC, auto-immune, graft vs host, transplant, (2020).
19. Dunavin N, Dias A, Li M, McGuirk J. Mesenchymal stromal cells: what is the mechanism in acute graft-versus-host disease? Biomedicines. (2017) 5:39. doi: 10.3390/biomedicines5030039
20. Murphy, KA. Basic concepts in immunology. In: Janeways Immunobiology, 8th ed, New York, NY: Garland Science (2012). p. 1–36.
21. Murphy, KA. The complement system and innate immunity. In: Janeways Immunobiology, 8th ed. New York, NY: Garland Science (2012) p. 48–65.
22. Gavin C, Meinke S, Heldring N, Heck KA, Achour A, Iacobaeus E, et al. The complement system is essential for the phagocytosis of mesenchymal stromal cells by monocytes. Front Immunol. (2019) 10:2249. doi: 10.3389/fimmu.2019.02249
23. Li Y, Lin F. Mesenchymal stem cells are injured by complement after their contact with serum. Blood. (2012) 120:3436–43. doi: 10.1182/blood-2012-03-420612
24. Li Y, Fung J, Lin F. Local inhibition of complement improves mesenchymal stem cell viability and function after administration. Mol Ther. (2016) 24:1665–74. doi: 10.1038/mt.2016.142
25. Li Y, Qiu W, Zhang L, Fung J, Lin F. Painting factor H onto mesenchymal stem cells protects the cells from complement- and neutrophil-mediated damage. Biomaterials. (2016) 102:209–19. doi: 10.1016/j.biomaterials.2016.05.055
26. Scozzi D, Ibrahim M, Menna C, Krupnick AS, Kreisel D, Gelman AE. The role of neutrophils in transplanted organs. Am J Transplant. (2017) 17:328–35. doi: 10.1111/ajt.13940
27. Mardpour S, Hamidieh AA, Taleahmad S, Sharifzad F, Taghikhani A, Baharvand H. Interaction between mesenchymal stromal cell-derived extracellular vesicles and immune cells by distinct protein content. J Cell Physiol. (2019) 234:8249–58. doi: 10.1002/jcp.27669
28. Mumaw JL, Schmiedt CW, Breidling S, Sigmund A, Norton NA, Thoreson M, et al. Feline mesenchymal stem cells and supernatant inhibit reactive oxygen species production in cultured feline neutrophils. Res Vet Sci. (2015) 103:60–9. doi: 10.1016/j.rvsc.2015.09.010
29. Mittal SK, Mashaghi A, Amouzegar A, Li M, Foulsham W, Sahu SK, Chauhan SK. Mesenchymal stromal cells inhibit neutrophil effector functions in a murine model of ocular inflammation. Invest Ophthalmol Vis Sci. (2018) 59:1191–8. doi: 10.1167/iovs.17-23067
30. Salami F, Tavassoli A, Mehrzad J, Parham A. Immunomodulatory effects of mesenchymal stem cells on leukocytes with emphasis on neutrophils. Immunobiology. (2018) 223:786–91. doi: 10.1016/j.imbio.2018.08.002
31. Jiang D, Muschhammer J, Qi Y, Kügler A, de Vries JC, Saffarzadeh M, et al. Suppression of neutrophil-mediated tissue damage-a novel skill of mesenchymal stem cells. Stem Cells. (2016) 34:2393–406. doi: 10.1002/stem.2417
32. Joswig AJ, Mitchell A, Cummings KJ, Levine GJ, Gregory CA, Smith R III, et al. Repeated intra-articular injection of allogeneic mesenchymal stem cells causes an adverse response compared to autologous cells in the equine model. Stem Cell Res Ther. (2017) 8:42. doi: 10.1186/s13287-017-0503-8
33. Barrachina L, Remacha AR, Romero A, Vitoria A, Albareda J, Prades M, et al. Assessment of effectiveness and safety of repeat administration of proinflammatory primed allogeneic mesenchymal stem cells in an equine model of chemically induced osteoarthritis. BMC Vet Res. (2018) 14:241. doi: 10.1186/s12917-018-1556-3
34. Colbath AC, Dow SW, Hopkins LS, Phillips JN, McIlwraith CW, Goodrich LR. Allogeneic vs. autologous intra-articular mesenchymal stem cell injection within normal horses: clinical and cytological comparisons suggest safety. Equine Vet J. (2020) 52:144–51. doi: 10.1111/evj.13136
35. Chatzistamatiou TK, Papassavas AC, Michalopoulos E, Gamaloutsos C, Mallis P, Gontika I, et al. Optimizing isolation culture and freezing methods to preserve Wharton's jelly's mesenchymal stem cell (MSC) properties: an MSC banking protocol validation for the Hellenic Cord Blood Bank. Transfusion. (2014) 54:3108–20. doi: 10.1111/trf.12743
36. Esmann L, Idel C, Sarkar A, Hellberg L, Behnen M, Möller S, et al. Phagocytosis of apoptotic cells by neutrophil granulocytes: diminished proinflammatory neutrophil functions in the presence of apoptotic cells. J Immunol. (2010) 184:391–400. doi: 10.4049/jimmunol.0900564
37. Williams LB, Koenig JB, Black B, Gibson TW, Sharif S, Koch TG. Equine allogeneic umbilical cord blood derived mesenchymal stromal cells reduce synovial fluid nucleated cell count and induce mild self-limiting inflammation when evaluated in an lipopolysaccharide induced synovitis model. Equine Vet J. (2016) 48:619–25. doi: 10.1111/evj.12477
38. Broeckx S, Suls M, Beerts C, Vandenberghe A, Seys B, Wuertz-Kozak K, et al. Allogeneic mesenchymal stem cells as a treatment for equine degenerative joint disease: a pilot study. Curr Stem Cell Res Ther. (2014) 9:497–503. doi: 10.2174/1574888X09666140826110601
39. Ferris DJ, Frisbie DD, Kisiday JD, McIlwraith CW, Hague BA, Major MD, et al. Clinical outcome after intra-articular administration of bone marrow derived mesenchymal stem cells in 33 horses with stifle injury. Vet Surg. (2014) 43:255–65. doi: 10.1111/j.1532-950X.2014.12100.x
40. Vega A, Martin-Ferrero MA, Del Canto F, Alberca M, Garcia V, Munar A, et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. (2015) 99:1681–90. doi: 10.1097/TP.0000000000000678
41. Gupta PK, Chullikana A, Rengasamy M, Shetty N, Pandey V, Agarwal V, et al. Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel(R)): preclinical and clinical trial in osteoarthritis of the knee joint. Arthr Res Ther. (2016) 18:301. doi: 10.1186/s13075-016-1195-7
42. Lamo-Espinosa JM, Mora G, Blanco JF, Granero-Molto F, Nunez-Cordoba JM, Sanchez-Echenique C, et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: multicenter randomized controlled clinical trial (phase I/II). J Transl Med. (2016) 14:246. doi: 10.1186/s12967-016-0998-2
43. Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. (2011) 11:723–37. doi: 10.1038/nri3073
44. Chiossone L, Conte R, Spaggiari GM, Serra M, Romei C, Bellora F, et al. Mesenchymal stromal cells induce peculiar alternatively activated macrophages capable of dampening both innate and adaptive immune responses. Stem Cells. (2016) 34:1909–21. doi: 10.1002/stem.2369
45. Cassano JM, Schnabel LV, Goodale MB, Fortier LA. Inflammatory licensed equine MSCs are chondroprotective and exhibit enhanced immunomodulation in an inflammatory environment. Stem Cell Res Ther. (2018) 9:82. doi: 10.1186/s13287-018-0840-2
46. Cassano JM, Schnabel LV, Goodale MB, Fortier LA. The immunomodulatory function of equine MSCs is enhanced by priming through an inflammatory microenvironment or TLR3 ligand. Vet Immunol Immunopathol. (2018) 195:33–9. doi: 10.1016/j.vetimm.2017.10.003
47. Murphy, KA. The induced responses of innate immunity. In: Janeways Immunobiology, 8th ed, New York, NY: Garland Science (2012). p. 75–98.
48. Berglund AK, Fisher MB, Cameron CA, Poole1 EJ, Schnabel LV. Transforming growth factor-β2 downregulates major histocompatibility complex (MHC) I and MHC II surface expression on equine bone marrow-derived mesenchymal stem cells without altering other phenotypic cell surface markers. Frontiers Vet Sci. (2017) 4:84. doi: 10.3389/fvets.2017.00084
49. Kamm JL, Parlane NA, Riley CB, Gee EK, Dittmer KE, McIlwraith CW. Blood type and breed-associated differences in cell marker expression on equine bone marrow-derived mesenchymal stem cells including major histocompatibility complex class II antigen expression. PLoS ONE. (2019) 14:e0225161. doi: 10.1371/journal.pone.0225161
50. Milosavljevic N, Gazdic M, Simovic Markovic B, Arsenijevic A, Nurkovic J, Dolicanin Z, et al. Mesenchymal stem cells attenuate acute liver injury by altering ratio between interleukin 17 producing and regulatory natural killer T cells. Liver Transpl. (2017) 23:1040–50. doi: 10.1002/lt.24784
51. Li Y, Qu YH, Wu YF, Liu L, Lin XH, Huang K, et al. Bone marrow mesenchymal stem cells suppressing activation of allogeneic cytokine-induced killer/natural killer cells either by direct or indirect interaction. Cell Biol Int. (2015) 39:435–45. doi: 10.1002/cbin.10404
52. Eisenbarth SC. Dendritic cell subsets in T cell programming: location dictates function. Nat Rev Immunol. (2019) 19:89–103. doi: 10.1038/s41577-018-0088-1
53. Zhang Y, Ge XH, Guo XJ, Guan SB, Li XM, Gu W. Bone marrow mesenchymal stem cells inhibit the function of dendritic cells by secreting Galectin-1. BioMed Res Int. (2017) 2017:3248605. doi: 10.1155/2017/3248605
54. Haabeth OA, Tveita AA, Fauskanger M, Schjesvold F, Lorvik KB, Hofgaard PO, et al. How Do CD4(+) T cells detect and eliminate tumor cells that either lack or express MHC class II molecules? Front Immunol. (2014) 5:174. doi: 10.3389/fimmu.2014.00174
55. Hickey MJ, Valenzuela NM, Reed EF. Alloantibody generation and effector function following sensitization to human leukocyte antigen. Front Immunol. (2016) 7:30. doi: 10.3389/fimmu.2016.00030
56. Pei J, Collisson EW. Specific antibody secreting cells from chickens can be detected by three days and memory B cells by three weeks post-infection with the avian respiratory coronavirus. Dev Comp Immunol. (2005) 29:153–60. doi: 10.1016/j.dci.2004.06.009
57. Wood BA, Carver S, Troyer RM, Elder JH, VandeWoude S. Domestic cat microsphere immunoassays: detection of antibodies during feline immunodeficiency virus infection. J Immunol Methods. (2013) 396:74–86. doi: 10.1016/j.jim.2013.08.001
58. Akondy RS, Fitch M, Edupuganti S, Yang S, Kissick HT, Li KW, et al. Origin and differentiation of human memory CD8 T cells after vaccination. Nature. (2017) 552:362–7. doi: 10.1038/nature24633
59. Schnabel LV, Pezzanite LM, Antczak DF, Felippe MJ, Fortier LA. Equine bone marrow-derived mesenchymal stromal cells are heterogeneous in MHC class II expression and capable of inciting an immune response in vitro. Stem Cell Res Ther. (2014) 5:13. doi: 10.1186/scrt402
60. Hill JA, Cassano JM, Goodale MB, Fortier LA. Antigenicity of mesenchymal stem cells in an inflamed joint environment. Am J Vet Res. (2017) 78:867–75. doi: 10.2460/ajvr.78.7.867
61. Barrachina L, Remacha AR, Romero A, Zaragozaa P, Vázqueza FJ, Rodellara C. Differentiation of equine bone marrow derived mesenchymal stem cells increases the expression of immunogenic genes. Vet Immun. (2018) 200:1–6. doi: 10.1016/j.vetimm.2018.04.004
62. Barrachina L, Cequier A, Romero A, Vitoria A, Zaragoza P, Vazquez FJ. Allo-antibody production after intraarticular administration of mesenchymal stem cells (MSCs) in an equine osteoarthritis model: effect of repeated administration, MSC inflammatory stimulation, and equine leukocyte antigen (ELA) compatibility. Stem Cell Res Ther. (2020) 11:52. doi: 10.1186/s13287-020-1571-8
63. Miller D, Tallmadge RL, Binns M, Zhu B, Mohamoud YA, Ahmed A, et al. Polymorphism at expressed DQ and DR loci in five common equine MHC haplotypes. Immunogenetics. (2017) 69:145–56. doi: 10.1007/s00251-016-0964-4
64. Berglund AK, Schnabel LV. Allogeneic major histocompatibility complex-mismatched equine bone marrow-derived mesenchymal stem cells are targeted for death by cytotoxic anti-major histocompatibility complex antibodies. Equine Vet J. (2017) 49:539–44. doi: 10.1111/evj.12647
65. Bloom DD, Centanni JM, Bhatia N, Emler CA, Drier D, Leverson GE, et al. A reproducible immunopotency assay to measure mesenchymal stromal cell-mediated T-cell suppression. Cytotherapy. (2015) 17:140–51. doi: 10.1016/j.jcyt.2014.10.002
66. Wang Y, Zhang A, Ye Z, Xie H, Zheng S. Bone marrow derived mesenchymal stem cells inhibit acute rejection of rat liver allografts in association with regulatory T-cell expansion. Transplant Proc. (2009) 41:4352–6. doi: 10.1016/j.transproceed.2009.08.072
67. Prasanna SJ, Gopalakrishnan D, Shankar SR, Vasandan AB. Pro-inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PLoS ONE. (2010) 5:e9016. doi: 10.1371/journal.pone.0009016
68. Klinker MW, Marklein RA, Lo Surdo JL, Wei CH, Bauer SR. Morphological features of IFN-γ-stimulated mesenchymal stromal cells predict overall immunosuppressive capacity. Proc Natl Acad Sci USA. (2017) 114:E2598–607. doi: 10.1073/pnas.1617933114
69. Liu F, Qiu H, Xue M, Zhang S, Zhang X, Xu J. MSC-secreted TGF-beta regulates lipopolysaccharide-stimulated macrophage M2-like polarization via the Akt/FoxO1 pathway. Stem Cell Res Ther. (2019) 10:345. doi: 10.1186/s13287-019-1447-y
70. Darlan DM, Munir D, Putra A, Jusuf NK. MSCs-released TGFβ1 generate CD4+CD25+Foxp3+ in T-reg cells of human SLE PBMC. J Formos Med Assoc. (2021) 120:602–8. doi: 10.1016/j.jfma.2020.06.028
71. Pezzanite LM, Fortier LA, Antczak DF, Cassano JM, Brosnahan MM, Miller D, et al. Equine allogeneic bone marrow-derived mesenchymal stromal cells elicit antibody responses in vivo. Stem Cell Res Ther. (2015) 6:54. doi: 10.1186/s13287-015-0053-x
72. Montespan F, Deschaseaux F, Sensébé L, Carosella ED, Rouas-Freiss N. Osteodifferentiated mesenchymal stem cells from bone marrow and adipose tissue express HLA-G and display immunomodulatory properties in HLA-mismatched settings: implications in bone repair therapy. J Immunol Res. (2014) 2014:230346. doi: 10.1155/2014/230346
73. Nasef A, Mathieu N, Chapel A, Frick J, François S, Mazurier C, et al. Immunosuppressive effects of mesenchymal stem cells: involvement of HLA-G. Transplantation. (2007) 84:231–7. doi: 10.1097/01.tp.0000267918.07906.08
74. Pigott, JH, Ishihara A, Wellman ML, Russell DS, Bertone AL. Investigation of the immune response to autologous, allogeneic, and xenogeneic mesenchymal stem cells after intra-articular injection in horses. Vet Immunol Immunopathol. (2013) 156:99–106. doi: 10.1016/j.vetimm.2013.09.003
75. Kol A, Wood JA, Carrade Holt DD, Gillette JA, Bohannon-Worsley LK, Puchalski SM, et al. Multiple intravenous injections of allogeneic equine mesenchymal stem cells do not induce a systemic inflammatory response but do alter lymphocyte subsets in healthy horses. Stem Cell Res Ther. (2015) 6:73. doi: 10.1186/s13287-015-0050-0
76. Gu LH, Zhang TT, Li Y, Yan HJ, Qi H, Li FR. Immunogenicity of allogeneic mesenchymal stem cells transplanted via different routes in diabetic rats. Cell Mol Immunol. (2015) 12:444–55. doi: 10.1038/cmi.2014.70
77. Owens SD, Kol A, Walker N, Borjesson DL. Allogeneic mesenchymal stem cell treatment induces specific alloantibodies in horses. Stem Cells Int. (2016) 2016:583010. doi: 10.1155/2016/5830103
78. Sernee MF1, Ploegh HL, Schust DJ. Why certain antibodies cross-react with HLA-A and HLA-G: epitope mapping of two common MHC class I reagents. Mol Immunol. (1998) 35:177–88. doi: 10.1016/S0161-5890(98)00026-1
79. Gao B, Rong C, Porcheray F, Moore C, Girouard TC, Saidman SL, et al. Evidence to support a contribution of polyreactive antibodies to HLA serum reactivity. Transplantation. (2016) 100:217–26. doi: 10.1097/TP.0000000000000840
80. Soland MA, Bego MG, Colletti E, Porada CD, Zanjani ED, St Jeor S, et al. Modulation of human mesenchymal stem cell immunogenicity through forced expression of human cytomegalovirus us proteins. PLoS ONE. (2012) 7:e36163. doi: 10.1371/journal.pone.0036163
81. Broeckx SY, Seys B, Suls M, Vandenberghe A, Marien T, Adriaensen E, et al. Equine allogeneic chondrogenic induced mesenchymal stem cells are an effective treatment for degenerative joint disease in Horses. Stem Cells Dev. (2019) 28:410–22. doi: 10.1089/scd.2018.0061
82. Magri C, Schramme M, Febre M, Cauvin E, Labadie F, Saulnier N. Comparison of efficacy and safety of single versus repeated intra-articular injection of allogeneic neonatal mesenchymal stem cells for treatment of osteoarthritis of the metacarpophalangeal/metatarsophalangeal joint in horses: a clinical pilot study. PLoS ONE. (2019) 14:e0221317. doi: 10.1371/journal.pone.0221317
83. Delco ML, Goodale M, Talts JF, Pownder SL, Koff MF, Miller AD. Integrin alpha10beta1-selected mesenchymal stem cells mitigate the progression of osteoarthritis in an equine talar impact model. Am J Sports Med. (2020) 48:612–23. doi: 10.1177/0363546519899087
84. Van Loon VJ, Scheffer CJ, Genn HJ, Hoogendoorn AC, Greve JW. Clinical follow-up of horses treated with allogeneic equine mesenchymal stem cells derived from umbilical cord blood for different tendon and ligament disorders. Vet Q. (2014) 34:92–7. doi: 10.1080/01652176.2014.949390
85. Lange-Consiglio A, Tassan S, Corradetti B, Meucci A, Perego R, Bizzaro D, et al. Investigating the efficacy of amnion-derived compared with bone marrow-derived mesenchymal stromal cells in equine tendon and ligament injuries. Cytotherapy. (2013) 15:1011–20. doi: 10.1016/j.jcyt.2013.03.002
86. Beerts C, Suls M, Broeckx SY, Seys B, Vandenberghe A, Declercq J, et al. Tenogenically induced allogeneic peripheral blood mesenchymal stem cells in allogeneic platelet-rich plasma: 2-year follow-up after tendon or ligament treatment in Horses. Front Vet Sci. (2017) 4:158. doi: 10.3389/fvets.2017.00158
87. Vagnozzi RJ, Maillet M, Sargent MA, Khalil H, Johansen AKZ, Schwanekamp JA, et al. An acute immune response underlies the benefit of cardiac stem cell therapy. Nature. (2020) 577:405–9. doi: 10.1038/s41586-019-1802-2
88. Xia C, Cao J. Imaging the survival and utility of pre-differentiated allogeneic MSC in ischemic heart. Biochem Biophys Res Commun. (2013) 438:382–7. doi: 10.1016/j.bbrc.2013.07.084
89. Tasso R, Ilengo C, Quarto R, Cancedda, R, Caspi RR, Pennesi G. Mesenchymal stem cells induce functionally active T-regulatory lymphocytes in a paracrine fashion and ameliorate experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. (2012) 53:786–93. doi: 10.1167/iovs.11-8211
90. Guest DJ, Smith MRW and Allen WR. Monitoring the fate of autologous and allogeneic mesenchymal progenitor cells injected into the superficial digital flexor tendon of horses: preliminary study. Equine Vet J. (2008) 40:178–81. doi: 10.2746/042516408X276942
91. Guest DJ, Smith MRW, Allen WR. Equine embryonic stem-like cells and mesenchymal stromal cells have different survival rates and migration patterns following their injection into damaged superficial digital flexor tendon. Equine Vet J. (2010) 42:636–42. doi: 10.1111/j.2042-3306.2010.00112.x
92. Lacitignola L, Staffieri F, Rossi G, Francioso E, Crovace A. Survival of bone marrow mesenchymal stem cells labelled with red fluorescent protein in an ovine model of collagenase-induced tendinitis. Vet Comp Orthop Trauma. (2014) 27:204–9. doi: 10.3415/VCOT-13-09-0113
93. Braid LR, Wood CA, Wiese DM, Ford BN. Intramuscular administration potentiates extended dwell time of mesenchymal stromal cells compared to other routes. Cytotherapy. (2018) 20:232–44. doi: 10.1016/j.jcyt.2017.09.013
94. Li M, Luo X, Lv X, Liu V, Zhao G, Zhang X, et al. In vivo human adipose-derived mesenchymal stem cell tracking after intra-articular delivery in a rat osteoarthritis model. Stem Cell Res Ther. (2016) 7:160. doi: 10.1186/s13287-016-0420-2
95. Feng C, Luo X, He N, Xia H, Lv X, Zhang X, et al. Efficacy and persistence of allogeneic adipose-derived mesenchymal stem cells combined with hyaluronic acid in osteoarthritis after intra-articular injection in a sheep model. Tissue Eng Part A. (2018) 24:219–33. doi: 10.1089/ten.tea.2017.0039
96. Wang AT, Feng Y, Jia HH, Zhao M, Yu H. Application of mesenchymal stem cell therapy for the treatment of osteoarthritis of the knee: a concise review. World J Stem Cells. (2019) 11:222–35. doi: 10.4252/wjsc.v11.i4.222
97. Colbath AC, Dow SW, Hopkins LS, Phillips JN, McIlwraith CW, Goodrich LR. Single and repeated intra-articular injections in the tarsocrural joint with allogeneic and autologous equine bone marrow-derived mesenchymal stem cells are safe, but did not reduce acute inflammation in an experimental interleukin-1β model of synovitis. Equine Vet J. (2019) 52:601–12. doi: 10.1111/evj.13222
98. Mahmoud EE, Adachi N, Mawas AS, Deie M, Ochi M. Multiple intra-articular injections of allogeneic bone marrow-derived stem cells potentially improve knee lesions resulting from surgically induced osteoarthritis: an animal study. Bone Joint J. (2019) 101B:824–31. doi: 10.1302/0301-620X.101B7.BJJ-2018-1532.R1
99. Dyson SJ. Medical management of superficial digital flexor tendonitis: a comparative study in 219 horses (1992-2000). Equine Vet J. (2004) 36:415–9. doi: 10.2746/0425164044868422
100. Yuksel S, Guleç MA, Gultekin MZ, Adanir O, Caglar A, Beytemur O, et al. Comparison of the early period effects of bone marrow-derived mesenchymal stem cells and platelet-rich plasma on the Achilles tendon ruptures in rats. Connect Tissue Res. (2016) 57:360–73. doi: 10.1080/03008207.2016.1189909
101. Khan MR, Dudhia J, David FH, De Godoy R, Mehra V, Hughes G, et al. Bone marrow mesenchymal stem cells do not enhance intra-synovial tendon healing despite engraftment and homing to niches within the synovium. Stem Cell Res Ther. (2018) 9:169. doi: 10.1186/s13287-018-0900-7
102. Lee SY, Kwon B, Lee K, Son YH, Chung SG. Therapeutic mechanisms of human adipose-derived mesenchymal stem cells in a rat tendon injury model. Am J Sports Med. (2017) 45:1429–39. doi: 10.1177/0363546517689874
Keywords: allogeneic, mesenchymal stromal cell, equine, immune, lymphocyte
Citation: Kamm JL, Riley CB, Parlane N, Gee EK and McIlwraith CW (2021) Interactions Between Allogeneic Mesenchymal Stromal Cells and the Recipient Immune System: A Comparative Review With Relevance to Equine Outcomes. Front. Vet. Sci. 7:617647. doi: 10.3389/fvets.2020.617647
Received: 15 October 2020; Accepted: 02 December 2020;
Published: 13 January 2021.
Edited by:
Lauren Virginia Schnabel, North Carolina State University, United StatesReviewed by:
Alix Kay Berglund, North Carolina State University, United StatesLaura Barrachina, Universidad de Zaragoza, Spain
Copyright © 2021 Kamm, Riley, Parlane, Gee and McIlwraith. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: J. Lacy Kamm, bGFjeWthbW1AZ21haWwuY29t
 J. Lacy Kamm
J. Lacy Kamm Christopher B. Riley
Christopher B. Riley Natalie Parlane
Natalie Parlane Erica K Gee1
Erica K Gee1 C. Wayne McIlwraith
C. Wayne McIlwraith