- 1Department of Veterinary Science and Public Health, Faculty of Natural Resources, Catholic University of Temuco, Temuco, Chile
- 2Nucleus of Research in Food Production, Faculty of Natural Resources, Catholic University of Temuco, Temuco, Chile
- 3Institute of Animal Science, Faculty of Veterinary Science, Austral University, Valdivia, Chile
The objective was to determine the effect of energy diet restriction on energy balance, systemic leptin and corpus luteum (CL) vascularization, development, and function in South American camelids. In experiment 1, adult llamas were randomly assigned to receive a diet of 70% of their maintenance energy requirements (MER) (Restricted group, n = 7) or fed ad libitum (Control group, n = 7) during 28 days. Body live weight (BLW) and body condition score (BCS) were recorded, blood samples were collected every 2 weeks to measure plasma leptin concentrations, and energy metabolites were quantified. In experiment 2, adult alpacas were randomly assigned to receive a diet of 40% MER for 21 days (Restricted group, n = 7) or fed ad libitum (Control group, n = 7). Then, ovulation was induced with gonadorelin acetate (day = 0), and trans-rectal ultrasonography (7.5 MHz) was performed using B and Doppler mode to record the diameter of the pre-ovulatory follicle, ovulation, CL diameter, and vascularization from Days 0 to 13. Blood samples were collected every 48 h from Days 1 to 13 to quantify plasma leptin and progesterone concentrations. In experiment 1, energy diet restriction of 70% MER did not affect plasma leptin concentration and metabolic parameters of the Restricted group. In experiment 2, the Restricted group had a lower BCS (p < 0.001), a smaller diameter of the CL on Days 5 and 7 (p < 0.05), and a smaller maximum diameter of the CL (10.2 ± 0.6 mm) than the Control group (12.1 ± 0.6 mm; p = 0.04). Low energy restriction of 70% MER for 28 days did not affect the energy balance of llamas (Experiment 1). Moderate energy restriction of 40% MER for 21 days negatively affected energy balance (BCS), and CL development but not its vascularization, leptin, and progesterone concentrations. These species must be submitted to longer periods or a higher level of energy restriction to impair ovarian function.
Introduction
Members of the Camelidae family are recognized for their ability to survive and reproduce in extreme environments (1). In Chile, the arrival of ruminants during the Spanish conquest decimated the population of camelids and displaced the domestic (Lama glama, Vicugna pacos) and wild species (Vicugna vicugna, Lama guanicoe) toward the marginal lands of the country (2, 3). Low fertility rates of highland camelid herds have been described [about 50%, (4)]. In a previous alpaca study (5), we have documented an overall embryo loss of 45.4% (49/108), during the 35 days after mating, which was close to the 50–58% reported in earlier studies (4, 6). Although the main factors related to embryo loss in these species are unknown, the poor nutritional status of the High Andean herds could be related (7).
Leptin, an adipocyte-derived hormone, acts as a critical metabolic signal linking nutrition and reproductive function (8). The rapid decrease in leptinemia in underfed animals could be translated as an acute signal to stimulate re-feeding behavior and glucocorticoid secretion; to decrease thyroid activity, energy expenditure, insulin sensitivity, and protein synthesis; and to block reproduction (9). Leptin modulates GnRH secretion in the hypothalamus indirectly through stimulation of neuropeptide Y and kisspeptin (10, 11). Systemic administration of leptin increased gonadotropin secretion in fasted ruminants and rats (12, 13). Leptin receptor, OB-R, has been characterized in the granulosa and theca cells, luteal cells, the ovarian stroma, and in endothelial cells in various species (14, 15) and recently in alpacas (16). These evidences support the notion that leptin may have a potential role in the maturation and selection of developing follicles, corpus luteum (CL) formation, function, and regression during estrus cycle via an autocrine/paracrine mechanism in several species (15).
In a previous study (17), severe long-term nutritional restriction negatively affected leptin concentration, the diameter of the pre-ovulatory follicle, and corpus luteum (CL) and progesterone production in llamas. The day-by-day profile of plasma leptin concentration was correlated with the CL growth during the entire luteal phase suggesting a potential local role of leptin on CL development in llamas. Kumar et al. (18) demonstrated that the leptin mRNA highest levels were in mid and late luteal stages consistent with in vivo luteinization of buffalo CL and declined coincidental to luteal regression. Also, the increased expression of steroidogenic enzymes (StAR, P450scc, HSD) has been correlated with the OB gene and OBR receptor activity in mid luteal phase suggesting a key role of leptin in progesterone production (18).
The mechanism of action of leptin on CL development is unknown. Wiles et al. (19) have demonstrated that the addition of leptin in an in vitro culture of goats luteal cells increased the expression of angiogenic factors such as angiotensin I (Ang1), fibroblast growth factor 2 (FGF2), and vascular endothelial growth factor (VEGF). In fact, CL vascularization did increase in alpacas previously treated with leptin during follicular phase (pre-ovulatory follicle > 7 mm of diameter) and given GnRH for ovulation induction, however, CL diameter, plasma luteinizing hormone (LH), and progesterone concentration were similar between leptin and non-leptin-treated females (20).
The objectives of this study were to determine the effect of low energy diet restriction on energy balance in llamas (Experiment 1) and to evaluate the effect of moderate energy restriction on energy balance on leptin concentration and CL vascularization, development, and function in alpacas (Experiment 2).
Materials and Methods
Experimental procedures were reviewed and approved by the Bioethical Committees of the Universidad Austral de Chile and Universidad Católica de Temuco and were performed in accordance with the animal care protocols established by the same institutions and in accordance with Chilean Animal Protection Act (2009).
Experiment 1: Effect of Low Energy Diet Restriction (70% of the Maintenance Energy Requirements) on Energy Balance in Llamas
Animals and Nutrition Management
This study was conducted in the llama farm at the University Austral of Chile located in Valdivia (39°38′S, 72°35′W). Mature non-lactating, non-pregnant, female llamas (n = 14), 6–9 years old, with a body condition score (BCS) of 3–3.5 [scale 1–5, (21)], were randomly assigned to an energy Restricted or a Control group (n = 7 per group). The Control group, weighting 149 ± 6 kg (mean ± SEM), was fed ad libitum with Ballica sp. hay [4.4% crude protein (CP); 5.7% total ashes (TA), and 1.8 Mcal/kg metabolic energy (ME), on dry matter base] and 300 g day−1 of commercial pelleted concentrate (16% CP; 5.4% TA, and 3.0 Mcal/kg ME; Cosetán®, Suralim, Osorno). Llamas of the Restricted group, weighting 146 ± 4 (mean ± SEM), were individually fed with the same diet as that of the Control group (3 hay:1 pellet, on the basis of total energy intake), but the amount of food provided 70% of maintenance energy requirements (MER) of the initial body live weigh (BLW). MER was calculated for each female using the following equation: 61.2 * BLW0.75 (22). Llamas were kept indoors and fed under nutritional management during 28 days.
Llamas received water and mineral salts (Usablock®, Sweetlix) ad libitum, and they were dewormed using Sofomax® (Intervet). BLW (kg) and BCS were assessed weekly. Blood samples (4 ml) were collected by jugular venipuncture into heparinized tubes every 2 weeks to determine metabolic markers and plasma leptin concentration. Plasma was separated by centrifugation at 1,500g for 15 min and stored at −80°C until assayed.
Metabolic Markers
Metabolites such as glucose, triglycerides, non-esterified fatty acids, beta-hydroxybutyrate, cholesterol, and urea were analyzed as previously described (17, 23). In brief, glucose concentration was measured using a portable equipment (One touch®, LifescanInc, USA). Triglycerides plasma concentration (GPO-PAD enzymatic method), non-esterified fatty acids (NEFA; ASC-ACOD enzymatic colorimetric method), beta-hydroxibutirato (BHB; 3 HBDH dependent enzymatic method), cholesterol (CHOD-PAD enzymatic method), and urea (kinetic glutamic-dehydrogenase method) were determined using Cobas-Miras-Plus® autoanalyzer (Roche D-10587, Berlin, Germany).
Leptin Concentration Analysis
Plasma leptin concentration was determined by radioimmunoassay (RIA; multi-species leptin kit®, Millipore, USA), using a 1470 Wallac Wizard Gamma counter (PerkinElmer Inc, USA). The limit of sensitivity and intra-assay coefficient of variation were 2.5 ng/ml and 10%, respectively.
Experiment 2: Effect of Moderate Energy Diet Restriction (40% of the Maintenance Energy Requirements) on Energy Balance and Luteal Function in Alpacas
Animals, Nutrition Management, and Experimental Design
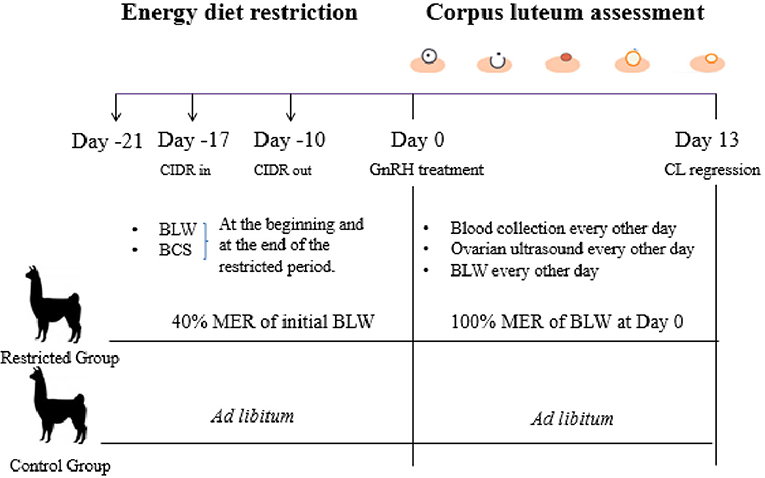
The experiment was conducted in the alpaca farm at the Universidad Católica de Temuco, located in Temuco (38°46′S, 72°38′W). Non-pregnant non-lactating adult alpacas (n = 14) aged 3 to 9 years old with a BCS of 3 to 3.5 (scale 1–5; 20) were used in the study. Females were randomly assigned to the Restricted or Control group (n = 7 per group). The Control group weighting was fed ad libitum with Ballica sp. hay (4.5% CP, 1.2% TA, 2.6 Mcal/kg ME, on dry matter base) and 200 g day−1 of commercial pelleted concentrate (17% CP, 6% TA, 3 Mcal/kg ME on dry matter base; Cosetán®, Iansagro SA, Chile). Alpacas in the Restricted group were individually fed twice daily, with hay and pellets (3:1 on the basis of total energy intake), but the amount of food was reduced to 40% MER of each alpaca. The MER was calculated using the equation 66* initial BLW0.75 (7). Alpacas were kept indoors and fed under nutritional management for 21 days (Day −21 to Day −1, Figure 1). They received water and mineral salts (Usablock®, Sweetlix) ad libitum, and they were dewormed using Sofomax® (Intervet). On the fourth day after the starting of the diet, alpacas received an intravaginal progesterone device (CIDR-B®, Easy-Breed, 0.3 g, Lab. Pfizer NZ) for 7 days to synchronize follicular wave emergence as previously described (24). All alpacas were examined daily by transrectal ultrasonography using a 7.5 MHz linear array transducer coupled to a monitor (Sonovet r3, Samsung Madison, USA) after the day of progesterone removal to determine the diameter of the pre-ovulatory follicle. Ovulation was induced 10 days after progesterone removal with an intramuscular administration of 50 μg of gonadorelin acetate (GnRH, Gonasyl®, Virbac, Day 0 = GnRH treatment, Figure 1). The ovaries were examined every other day from Day 1 to Day 13 to determine ovulation, CL diameter, and vascularization using B and Doppler mode. Ovulation was defined as the disappearance of a large follicle (≥7 mm) that had been detected in the previous examination.
Alpacas from the Restricted group were switched from 40% MER of the initial BLW to 100% MER of the BLW recorded at Day 0 (GnRH treatment, see Figure 1). This change of diet restriction was conducted in order to maintain the new BLW recorder at Day 0 until Day 13 during CL assessment. BLW and BCS were recorded twice in alpacas of both groups, at the beginning and at the end of the restricted period. BLW was recorded every other day from Day 1 to Day 13 using a digital scale (±0.1 kg).
B and Power Doppler Ultrasonography and Image Analysis
CL diameter and vascularization were recorded using B and Power Doppler mode from Day 1 to Day 13 by an operator who was blinded to the treatments and according to previous South American camelid studies (25, 26). In brief, the echotexture of the CL was measured in B mode at its maximum diameter using horizontal and vertical calipers. In Power Doppler mode, cineloops (10 s in length) of the CL vascularization were recorded during Power Doppler imaging and downloaded into VLC media player (www.videolan.org, Version 2.0, Boston, USA). Cineloops were examined frame by frame to select three images that represented the maximum vascular signal at the maximum cross-sectional area of the CL. Images were saved in JPG format with minimal compression and analyzed by ImageJ software (National Institute of Health, Maryland, Washington DC, USA) to determine the area of vascularization of the CL. The area of vascularization was estimated by measuring the area (cm2) of the vascular flow signals (Power-Doppler) overlaying the B-mode image of the CL; the average of the three images was taken as the value for a given animal on a given day.
Leptin and Progesterone Concentration Analysis
Blood samples (4 ml) were collected into heparinized tubes to determine plasma leptin and progesterone concentration from Day 1 to Day 11 (Day 0 = GnRH treatment) and Day 1 to Day 13, respectively. Plasma leptin concentration was determined using the Sheep Leptin ELISA Kit® (MyBiosource, USA) which was validated previously (20). In brief, serially diluted plasma from alpacas containing high concentrations of leptin produced a displacement curve parallel to the standard curve. Intra- and inter-coefficients of variation were 5.0 and 7.2%, respectively, and the limit of sensitivity was 2.5 ng/ml. Plasma progesterone concentration was determined using a commercial, double-antibody radioimmunoassay kit (COAT-A COUNT 17A-OH Progesterone, Siemens, USA). The intra-assay coefficient of variation varied between 0.5 and 3.5%, and the inter-assay coefficient of variation was between 0.5 and 2.1%. The limit of sensitivity was 0.61 ng/ml. Analyses were performed at the Universidad de Concepción, Chile.
Statistical Analyses
Normality and homoscedasticity were assessed by Kolmogorov-Smirnov and Levene test, respectively, Log10− transformations were performed whenever necessary. Paired or unpaired Student t test were used to compare non-serial data as BLW, BCS, pre-ovulatory follicle diameter, and maximum CL diameter between groups. A mixed ANOVA with time points as repeated measurements was used to compare serial data (BLW, leptin and progesterone plasma concentration, CL diameter, and vascularization area). If significant main effects or interactions were detected (p < 0.05), ANOVA pairwise comparisons with Bonferroni adjustment were used. Pearson's correlation was used to determine relationships between CL diameter, vascularization area, and plasma progesterone concentration. Statistical analyses were done using SPSS Program (V 20). Data are reported as mean ± SEM.
Results
Experiment 1
Plasma leptin concentration, metabolite markers, BLW, and BCS did not differ between the Restricted and Control groups (Table 1). The initial and final BLW and BCS of the Restricted group did not differ (p = 0.06 and p = 0.9, respectively). The Restricted group lost 9 kg, equivalent to 6% of its initial BLW. Initial and final BLW and BCS of Control group also were similar (p = 0.4 and p = 0.9, respectively).
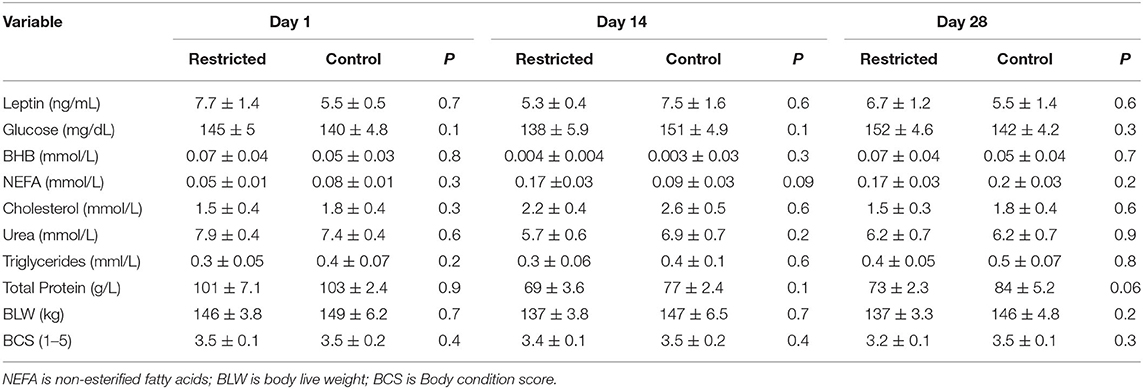
Table 1. Plasma leptin concentration, metabolites, body weight and body condition score (Mean ± SEM) of llamas fed 70% energy of maintenance requirements (Restricted group; n = 7), or fed ad libitum (Control group, n = 7) during 28 days.
Experiment 2
The Restricted and Control groups had similar BLW and BCS at the beginning of the diet restricted period (Table 2). Although BLW was not different between groups, BCS was higher in the Control than that of the Restricted group after 21 days of 40% MER. The Restricted group reduced the BLW and BCS significantly during the metabolic trial, but Control group did not (Table 2). There were no significant differences in BLW between groups during the corpus luteum assessment (p > 0.05). The diameter of the preovulatory follicle at the time of GnRH treatment was similar between groups: 8.4 ± 0.7 and 10 ± 0.8 mm for Restricted and Control groups, respectively (p = 0.9). Similarly, the proportion of ovulated animals (7/7 and 7/7, p = 0.9) was not different between Restricted and Control groups, respectively. There was an effect of treatment (p = 0.01) and day (p = 0.001) but not interaction (p = 0.5) on CL diameter between groups. Diameter of CL was greater in the Control than that of the Restricted group at Days 5 and 7 after GnRH treatment (Figure 2A). Maximum CL diameter was lower (p < 0.04) in the Restricted (10.2 ± 0.6) than that of the Control group (12.1 ± 0.6 mm). Maximum CL vascularization was not different between Restricted and Control groups (0.37 ± 0.26 vs. 0.42 ± 0.25 cm2, respectively; p = 0.3, Figure 2B). However, there was an effect of time on CL vascularization (p < 0.001). Plasma progesterone and leptin concentrations were not affected by the treatment (p = 0.7 and p = 0.7, respectively, Figures 2C,D). Daily mean values of plasma leptin concentration during the luteal phase ranged from 6.9 ± 1.7 to 8.0 ± 1.6 ng/ml in both groups. There was no correlation between plasma leptin concentration on CL diameter, vascularization area, and plasma progesterone concentration (p > 0.05). The diameter of CL was positively correlated with vascularization area (r = 0.6; p < 0.001) and plasma progesterone concentration (r = 0.3; p < 0.01).
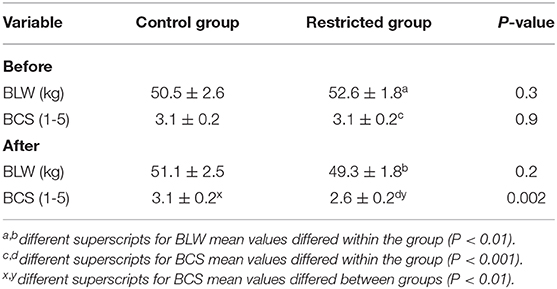
Table 2. Values (Mean ± SEM) of body live weight (BLW) and body condition score (BCS) of alpacas fed 40% of maintenance energy requirements (restricted group; n = 7) or fed ad libitum (control group; n = 7) before and after the nutrition management for 21 days.
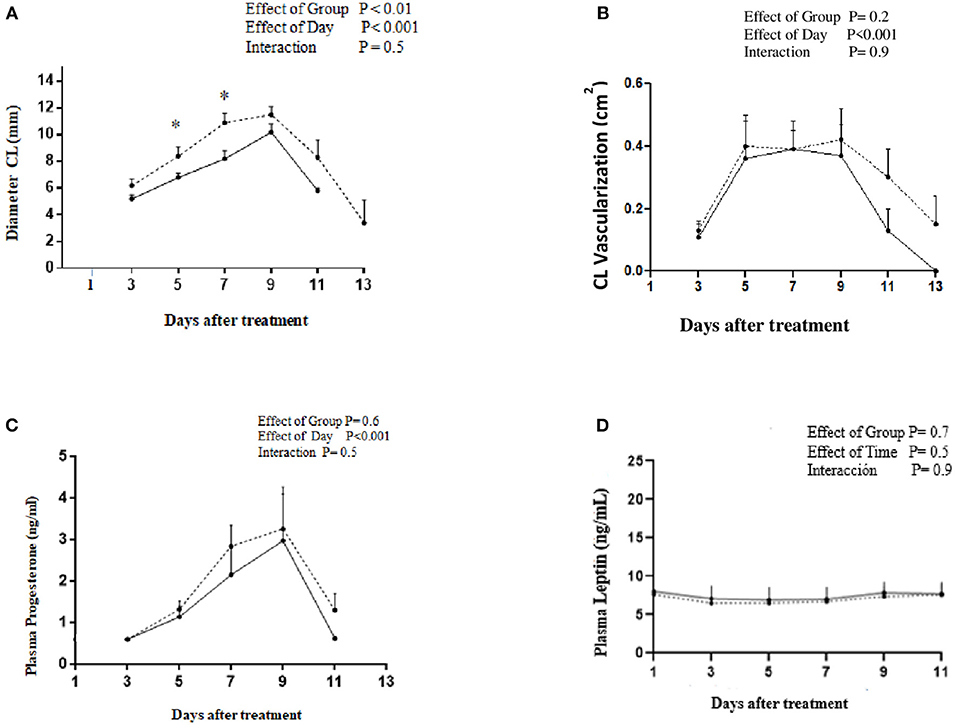
Figure 2. Profiles of (A) CL diameter, (B) CL vascularization, (C) plasma progesterone concentration, and (D) plasma leptin concentration after treatment with GnRH to induce ovulation in alpacas under energy restriction 40% (______, n = 7) or fed ad libitum (-------, n = 7).
Discussion
Based on the results of the present study, low energy restriction at 70% MER in llamas did not affect BLW, BCS, plasma leptin concentration, and metabolic parameters that may indicate a negative energy balance (Experiment 1). Moderate energy restriction at 40% MER in alpacas only reduced BCS and CL diameter, but no changes were detected in CL vascularization and plasma progesterone and leptin concentrations (Experiment 2).
Several studies have shown that energy status affects fertility in ruminant herds (27, 28). It has been described that nutritional restriction caused the absence or dysfunction of GnRH and gonadotrophic hormones secretion in sheep and cows (29–31). Hormonal dysfunction can result in the formation of a dominant follicle of smaller size and lower secretion of estradiol affecting estrus behaviors and the ovulatory process in cows (32, 33). Moreover, nutritional restriction reduced the diameter of the corpus luteum resulting in a lower production of progesterone which is associated with a higher rate of embryonic mortality and implantation /conception failures (32, 34). Negative energy balance has been associated to a dysfunction of various hormones and neuropeptides such as leptin, IGF-1, insulin, and neuropeptide Y that act at hypothalamic-pituitary-ovarian axis modulating GnRH secretion (11, 35).
In the present study, nutritional restriction at 70% MER for 28 days failed to induce negative energy balance in llamas (Experiment 1). However, females from the Restricted group lost 6% of their BLW; this loss can be partially attributed to a reduced gastrointestinal content that represents up to 11% of the BLW in llamas (36). Similar to our results, Bengoumi et al. (37) did not find any differences on body mass, hump volume, and lipid content after 2 months of restricted feeding at 68% MER in Camelus dromedarius. It has been described that the dromedary camel has the capability to reduce their energy expenditure up to 21% under fasting conditions (38). Llamas from the Restricted group maintained the homeostasis of proteins, lipids, carbohydrates, and energy; the values of metabolic markers were according to the referential values reported for camelids (39, 40). Negative energy balance (NEB) increased plasma concentrations of NEFA, BHB, or urea in previous studies conducted in llamas, alpacas, and vicuñas (17, 23, 41, 42). Starvation or dietary insufficiency results in mobilization of adipose stores, characterized by an increase in blood NEFA (over 0.6 mmol/L), and a mild increase in blood BHB (over 0.1 to 0.19 mmol/L) in camelids (43, 44). NEB induces amino acid breakdown to be used as energy substrate and possible gluconeogenesis that increases circulating urea (1, 17). The NEFA, BHB, and urea values reported in the study were lower than those cited before.
Sheep under energy diet restriction of 82% MER suffered a negative energy balance at Day 30 (45); apparently there must be a specific species response to the dietary restriction that also could be associated to the physiological and productive status of the animal.
In Experiment 2, although, alpacas fed 40% MER reduced their BLW up to 8%, it did not differ from the Control group. Nonetheless, BCS in alpacas from the Restricted group was lower than that observed in the Control Group indicating that negative energy balance caused lipolysis of fat reserves. Chagas et al. (35) suggested that measurement of BCS, unlike BLW, minimizes the influence of body size and gastrointestinal content. Leptin regulates energy balance, and its plasma concentration level correlates with fat reserves in several species (46) including BCS in llamas [r = 0.8, (23)]. Leptin exerts its effects through the leptin receptor (OB-R) which is highly expressed in the hypothalamus, where it is primarily responsible for suppression of food intake and stimulation of energy expenditure (47). Nonetheless, the restricted period of 21 days was not enough to induce some changes in leptin concentration as mean values observed by Day 1 (Day 0 = GnRH treatment) were similar between groups (7.6 ± 1.5 vs. 8.0 ± 1.5 ng/ml for Restricted and Control groups, respectively, Figure 2D). Leptin values observed in the present study were lower than that previously reported (~12 ng/ml) during the luteal phase in alpacas submitted to a fasting period during the pre-ovulatory stage (20).
The diameter of the pre-ovulatory follicle was similar between groups, and their sizes are similar to the mean values reported for non-pregnant, non-lactating alpacas (48). CL diameter in the Control group was significantly greater than that of the Restricted group. Indeed, CL diameter in the Restricted group was smaller than mean values (12–14 mm) reported in another study conducted in camelids (49). Corpus luteum formation involves the luteinization of granulosa and theca cells, increment of the ovarian blood flow, and the stimulation of a proliferation and differentiation process of endothelial and steroidogenic cells (50, 51). Luteinizing (LH) and growth hormones (GH) are the main luteotrophic hormones in bovines and along with insulin-like growth factor I (IGF-I) promote angiogenesis, mitosis, and progesterone production (52). Taking into account that 85% of the cells that proliferate in a developing CL are endothelial cells, it could be speculated that the greater size of CL observed in the Control group could be attributed to an increase of CL vascularization area (52), but although CL vascularization area was numerically greater in the Control than that of the Restricted group, these were not significantly different and so failed to explain CL size differences. The vascularization values observed in the present study were similar to those reported in other studies in alpacas and llamas (20, 25, 26).
It is well-known that negative energy balance (NEB) reduces systemic LH secretion in ruminants influencing CL development and progesterone production (32, 53). NEB also negatively affects the plasma concentration of IGF-I, a hormone that stimulates LH secretion at the hypothalamic and pituitary levels (11, 54). In addition, Insulin-like growth factor receptor has been detected in most of the luteal cells in alpacas suggesting that IGF-I may be involved in the CL steroid synthesis in camelids (16). It has been described that LH and IGF-I hormones also act synergistically stimulating the proliferation and differentiation of granulosa cells, as well as promoting steroidogenesis, vascularization, and oocyte health (16, 55). Nevertheless, the diameter of the pre-ovulatory follicles as well as the ovulatory rate of the Restricted alpacas were not affected by NEB, which rules out a dysfunction of these hormones.
Despite the differences in CL diameter between groups, progesterone concentration was similar in both Restricted and Control groups; these values were similar to those reported in other studies in alpacas (56, 57). A positive correlation between CL vascularization and plasma progesterone concentration has been reported in camelid studies (20, 25). Then, the stability of the leptin plasma concentration of the Restricted group could stimulate an adequate vascularization of the CL to provide the precursors needed for progesterone production (19, 20).
The CL diameter and plasma progesterone concentration were positively correlated in the present study. Probably, a higher energy challenge could affect both parameters simultaneously, similarly as described in restricted llamas that lost near to 20% of BLW (17). Similarly, in ruminants, a decline in CL diameter and progesterone plasma concentration was observed in the two estrous cycles preceding anovulation, in heifers that lost 22% of BLW (53).
In ruminants, diets 40% MER for 14 days reduced leptinemia in sheep (58), and 60% MER for 21 days did it in a group of cows when compared to 130% MER (59). In addition, dietary restriction of 40% MER during 15 or 21 days induced anestrus in 60% of heifers, with reduced pre-ovulatory follicles or CL (29, 34). In this sense, the ability for camelids to maintain the energy balance and leptinemia may have comparative advantages of these species with respect to ruminants in terms of reproductive performances in the High Andes. The differential response between ruminants and camelids under nutritional restriction could be based on the following: (i) camelids are more efficient in reducing their basal metabolic rate during fasting (38), (ii) apparently, camelids use, to a greater extent, proteins as substrate for energy production and gluconeogenesis, being more efficient with the urea recycling (1, 7, 43).
We conclude that low energy restriction at 70% MER during 28 days in llamas did not affect BLW, BCS, plasma leptin concentration, and metabolic parameters that may indicate a negative energy balance (Experiment 1). The moderate energy restriction at 40% MER during 21 days in alpacas reduced BCS, BLW, and the CL diameter, but no changes were detected in CL vascularization, plasma progesterone, and leptin concentrations (Experiment 2). The results of the present study suggest that these species must be submitted to longer periods of nutritional restriction to induce a significant effect on systemic leptin concentration in order to impair follicular growth, CL vascularization, and progesterone production.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by Bioethic Committee from Universidad Catolica de Temuco and Universidad Austral de Chile.
Author Contributions
CN design the study and execute all the experiments, participate in data collection, data interpretation and writing the manuscript. FH, JA,GS, and ST collaboration in saplles collection, ultrasonography, and animal management. MR experimental design and writing of the manuscript, and direction of the study. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported by the grant Fondecyt Iniciación 11130206 (Dr. Norambuena), Government of Chile and by the Nucleus of Research in Food Production, Faculty of Natural Resources, Catholic University of Temuco, Chile.
References
1. Wenswoort J, Kyle D, Orskov E, Bourke D. Biochemical adaptation of camelids during periods where feed is withheld. Revista Rangifer. (2001) 21:45–8. doi: 10.7557/2.21.1.1527
2. Raggi SA, Ferrando R. Avances en fisiología y adaptación de camélidos sudamericanos. Avances en Ciencias Veterinarias. (1998) 13:1. doi: 10.5354/0719-5273.1998.4806
3. Marín JC, Zapata B, Gonzalez B, Bonacic C, Wheeler J, Casey C, et al. Sistemática, taxonomía y domesticación de alpacas y llamas: nueva evidencia cromosómica y molecular. Revista Chilena de Historia Natural. (2007) 80:121–40. doi: 10.4067/S0716-078X2007000200001
4. Fernández Baca S, Novoa C, Sumar J. Embryonic mortality in the Alpaca. Biol Reprod. (1970) 3:243–51. doi: 10.1093/biolreprod/3.2.243
5. Ratto M, Cervantes M, Norambuena MC, Silva M, Miragaya M, Huanca W. Effect of location and stage of development of dominant follicle on ovulation and embryo survival rate in alpacas. Anim Reprod Sci. (2011) 127:100–5. doi: 10.1016/j.anireprosci.2011.07.003
6. Bravo PW, Sumar J. Factors that Determine Fertility in Alpacas. In: Proceedings of the International Convention on SAC. Cusco, Perú (1985).
7. San Martin F, Van Saun R. Feeding management systems. In: Cebra C, Anderson DE, Tibary A, Van Saun RJ, Johnson LW, editors. Alpaca and Llama Care. Philadelphia, PA: Elsevier Inc. (2014). p. 91–100.
8. Donato J, Silva RJ, Sita LV, Lee S, Lee C, Lachinni S, et al. The ventral premammillary nucleus links fasting-induced changes in leptin levels and coordinated luteinizing hormone secretion. J Neurosci. (2009) 29:5240–50. doi: 10.1523/JNEUROSCI.0405-09
9. Chilliard Y, Delavaud C, Bonnet M. Leptin expression in ruminants: nutritional and physiological regulations in relation with energy metabolism. Domest Anim Endocrinol. (2005) 29:3–22. doi: 10.1016/j.domanied.2005.02.026
10. Henry BA, Goding JW, Tilbrook AJ, Dunshea FR, Clarke IJ. Intracerebroventricular infusion of leptin elevates the secretion of luteinising hormone without affecting food intake in long-term food-restricted sheep, but increases growth hormone irrespective of bodyweight. J Endocrinol. (2001) 168:67–77. doi: 10.1677/joe.0.1680067
11. García-García RM. Integrative control of energy balance in the female. ISRN Vet Sci. (2012) 1:1–13. doi: 10.5402/2012/121389
12. Amstalden M, García M, Stanko R, Nizielski S, Morrison C, Keisler D, et al. Central infusion of recombinant ovine leptin normalizes plasma insulin and stimulates a novel hypersecretion of luteinizing hormone after short-term fasting in mature beef cows. Biol Reprod. (2002) 66:1555–61. doi: 10.1095/biolreprod66.5.1555
13. Zieba D, Amsteldan M, Maciel M, Keisle D, Raver N, Gertler A, et al. Divergent effects of leptin on luteinizing hormone and insuline secretion are dose dependent. Exp Biol Med. (2003) 228:325–30. doi: 10.1177/153537020322800312
14. Di Yorio MP, Bilbao MG, Pustovrh MC, Prestifilippo JP, Faletti AG. Leptin modulates the expression of its receptors in the hypothalamic-pituitary-ovarian axis in a differential way. J Endocrinol. (2008) 198:355–66. doi: 10.1677/JOE-07-0622
15. Sarkar M, Schilffarth S, Schams D, Meyer H, Berisha B. The expression of leptin and its receptor during different physiological stage in the bovine ovary. Mol Reprod Dev. (2010) 77:174–81. doi: 10.1002/mrd.21129
16. Gallelli MF, Bianchi C, Lombardo D, Rey F, Rodriguez FM, Castillo VA, et al. Leptin and IGF1 receptors in alpaca (Vicugna pacos) ovaries. Anim Reprod Sci. (2019) 200:96–104. doi: 10.1016/j.anireprosci.2018.12.001
17. Norambuena MC, Silva M, Urra F, Ullloa-Leal C, Fernández A, Adams G, et al. Effects of nutritional restriction on metabolic, endocrine, and ovarian function in llamas (Lama glama). Anim Reprod Sci. (2013) 138:252–60. doi: 10.1016/j.anireprosci.2013.01.019
18. Kumar L, Panda R, Hydera I, Yadav V, Sastry K, Sharma G, et al. Expression of leptin and its receptor in corpus luteum during estrous cycle in buffalo (Bubalus bubalis). Anim Reprod Sci. (2012) 135:8–17. doi: 10.1016/j.anireprosci.2012.08.030
19. Wiles JR, Katchko RA, Benavides EA, O'Gorman CW, Escudero JM, Keisler DH, et al. The effect of leptin on luteal angiogenic factors during the luteal phase of the estrous cycle in goats. Anim Reprod Sci. (2014) 148:121–9. doi: 10.1016/j.anireprosci.2014.05.002
20. Norambuena MC, Hernández F, Maureira J, Rubilar C, Alfaro J, Silva G, et al. Effects of leptin administration on development, vascularization and function of Corpus luteum in alpacas submitted to pre-ovulatory fasting. Anim Reprod Sci. (2017) 182:28–34. doi: 10.1016/j.anireprosci.2017.04.006
21. Van Saun RJ. Nutritional requirements and assessing nutritional status in camelids. Vet Clin Anim Food Anim Pract. (2009) 25:265–79. doi: 10.1016/j.cvfa.2009.03.003
22. Lopez A, Raggi LA. Requerimientos nutritivos de camélidos sudamericanos: llamas (Lama glama) y Alpacas (Lama pacos). Archivos de Medicina Veterinaria. (1992) 24:121–30.
23. Norambuena MC, Gómez Y, Ulloa-Leal C, Fernández A, Von Baer A, Ratto M. Relationship between systemic leptin concentration and reproductive state in llamas (Lama glama) from southern Chile. Small Ruminant Res. (2013) 113:402–4. doi: 10.1016/j.smallrumres.2013.04.008
24. Cavilla MV, Bianchi CP, Aguilera F, Hermida M, Aba MA. Hormonal changes and follicular activity after treatment with intravaginal progesterone-releasing devices in llamas. Reprod Domest Anim. (2016) 51:930–9. doi: 10.1111/rda.12762
25. Fernández A, Ulloa-Leal C, Silva M, Norambuena C, Adams G, Guerra M, et al. The effect of repeated administrations of llama ovulation-inducing factor (OIF/NGF) during the peri-ovulatory period on corpus luteum development and function in llamas. Anim Reprod Sci. (2014) 148:345–52. doi: 10.1016/j.anireprosci.2014.08.001
26. Ulloa-Leal C, Bogle OA, Adams GP, Ratto MH. Luteotrophic effect of ovulation-inducing factor/nerve growth factor present in the seminal plasma of llamas. Theriogenology. (2014) 81:1101–7. doi: 10.1016/j.theriogenology.2014.01.038
27. Patton J, Kenny DA, McNamara S, Mee JF, O'Mara FP, Diskin MG, et al. Relationships among milk production, energy balance, plasma analytes, and reproduction in Holstein-Friesian cows. J Dairy Sci. (2007) 90:649–58. doi: 10.3168/jds.S0022-0302(07)71547-3
28. Kim IH, Jeong JK. Risk factors limiting first service conception rate in dairy cows and their economic impact. Asian-Australas J Anim Sci. (2019) 32:519–26. doi: 10.5713/ajas.18.0296
29. Rhodes FM, Entwistle KW, Kinder JE. Changes in ovarian function and gonadotropin secretion preceding the onset of nutritionally induced anestrus in Bos indicus heifers. Biol Reprod. (1996) 55:1437–43. doi: 10.1095/biolreprod55.6.1437
30. Kiyma Z, Alexander BM, Van Kirk EA, Murdoch WJ, Hallford DM, Moss GE. Effects of feed restriction on reproductive and metabolic hormones in ewes. J Anim Sci. (2004) 82:2548–57. doi: 10.2527/2004.8292548x
31. Schneider J. Energy balance and reproduction. Physiol Behav. (2004) 81:289–317. doi: 10.1016/j.physbeh.2004.02.007
32. Diskin MG, Mackey DR, Roche JF, Sreenan JM. Effects of nutrition and metabolic status on circulating hormones and ovarian follicle development in cattle. Anim Reprod Sci. (2003) 78:345–70. doi: 10.1016/s0378-4320(03)00099-x
33. Diskin M. Reproductive management of dairy cows: a review (part I). Ir Vet J. (2008) 61:326–32. doi: 10.1186/s13620-017-0112-y
34. Mackey DR, Sreenan JM, Roche JF, Diskin MG. Effect of acute nutritional restriction on incidence of anovulation and periovulatory estradiol and gonadotropin concentrations in beef heifers. Biol Reprod. (1999) 61:1601–7. doi: 10.1095/biolreprod61.6.1601
35. Chagas LM, Bass JJ, Blache D, Burke CR, Kay JK, Lindsay DR, et al. Invited review: new perspectives on the roles of nutrition and metabolic priorities in the subfertility of high-producing dairy cows. J Dairy Sci. (2007) 90:4022–32. doi: 10.3168/jds.2006-852
36. Cristofanelli MA, Torres AD, Polidori C. Carcass characteristics of peruvian llama (Lama glama) and alpaca (Lama pacos) reared in the Andean highlands. Small Ruminant Res. (2005) 58:219–22. doi: 10.1016/j.smallrumres.2004.10.004
37. Bengoumi M, Faulconnier Y, Tabarani A, Sghiri A, Faye B, Chilliard Y. Effect of feeding level on body weight, hump size, lipid content and adiposyte volume in the dromedary camel. Anim Res. (2005) 54:383–93. doi: 10.1051/animres:2005029
38. Guerouali A, Zine-Filali R, Vermorel M, Wardeh M. Maintenance energy requirements and energy utilization by dromedary at rest. Actes du Séminaire sur l'élevage et l'alimentation du dromadaire Douz Options Méditerranéennes, Série B. (1995) 13:59–69.
39. Foster A, Bidewell C, Barnett J, Sayers R. Haematology and biochemistry in alpacas and llamas. In Pract. (2009) 31:276–81. doi: 10.1136/inpract.31.6.276
40. Husakova T, Pavlata L, Pechova A, Hauptmanova K, Pitropovska E, et al. Reference values for biochemical parameters in blood serum of young and adult alpacas (Vicugna pacos). Animal. (2014) 8:1448–55. doi: 10.1017/S1751731114001256
41. Norambuena MC, Hernández F, Alfaro J, Cárcamo G, Olavarría A, Velasco M. Relationship between the nutritional state before the breeding period and the reproductive success in alpacas (Vicugna pacos) from the Chilean Puna. Austral J Vet Sci. (2018) 50:55–7. doi: 10.4067/S0719-81322018000100110
42. Norambuena C, Mussa K, Hernández F, Alfaro J, Velasco M. Energy balance of pregnant vicuñas (Vicugna vicugna) in the Chilean High Andes. Austral J Vet Sci. (2019) 51:33–36. doi: 10.4067/S0719-81322019000100106
43. Cebra C. Hepatic, pancreatic, and metabolic disorders. In: Cebra C, Anderson DE, Tibary A, Van Saun RJ, Johnson LW, editors. Alpaca and Llama Care. Philadelphia, PA: Elsevier Inc. (2014). p. 537–52.
44. Van Saun R, Cebra C. Nutritional Diseases. In: Cebra C, Anderson DE, Tibary A, Van Saun RJ, Johnson LW, editors. Alpaca and llama Care. Philadelphia, PA: Elsevier Inc. (2014). p.124–39.
45. Sano H, Takebayashi Y, Kodama Y, Nakamura K, Ito H, Arino Y, et al. Effect of feed restriction and cold exposure on glucose metabolism in response to feeding and insulin in sheep. J Anim Sci. (1999) 77:2564–73. doi: 10.2527/1999.7792564x
46. Bonnet M, Delavaud C, Faulconnier Y, Leroux C, Djiane J, Bocquier F. Leptin in ruminants. Gene expression in adipose tissue and mammary gland, and regulation of plasma concentration. Domest Anim Endocrinol. (2001) 21:271–95. doi: 10.1016/s0739-7240(01)00124-2
47. Park H, Ahima R. Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism. (2015) 64:24–34. doi: 10.1016/j.metabol.2014.08.004
48. Vaughan J, Macmillan K, D'Occhio MD. Ovarian follicular wave characteristics in alpacas. Anim Reprod Sci. (2004) 80:353–61. doi: 10.1016/j.anireprosci.2003.08.002
49. Abba M. Anatomy and physiology of Reproduction in the female alpaca. In: Cebra C, Anderson DE, Tibary A, Van Saun RJ, Johnson LW, editors. Alpaca and Llama Care. Philadelphia, PA: Elsevier Inc. (2014). p 140–150.
50. Acosta TJ, Miyamoto A. Vascular control of ovarian function: ovulation, corpus luteum formation and regression. Anim Reprod Sci. (2004) 82:127–40. doi: 10.1016/j.anireprosci.2004.04.022
51. Wiltbank MC, Salih SM, Atli MO, Luo W, Bormann CL, Ottobre JS, et al. Comparison of endocrine and cellular mechanisms regulating the corpus luteum of primates and ruminants. Anim Reprod. (2012) 9:242–59.
52. Schams D, Berisha B. Regulation of corpus luteum in cattle—an overview. Reprod Domest Anim. (2004) 39:241–51. doi: 10.1111/j.1439-0531.2004.00509.x
53. Bossis I, Welty SD, Wettemann RP, Vizcarra JA, Spicer LJ, Diskin MG. Nutritionally induced anovulation in beef heifers: ovarian and endocrine function preceding cessation of ovulation. J Anim Sci. (1999) 77:1536–46. doi: 10.2527/1999.7761536x
54. Vandehahar MJ, Sharma B-K, Fogwell RL. Effect of energy diet restriction on the expression of Insulin-like growth factor 1 in livers and corpus luteum of heifers. J Dairy Sci. (1995) 78:10–20. doi: 10.3168/jds.S0022-0302(95)76695-4
55. Lucy MC. Regulation of ovarian follicular growth by somatotropin and insulin-like growth factors in cattle. J Dairy Sci. (2001) 83:1635–47. doi: 10.3168/jds.S0022-0302(00)75032-6
56. Bravo W, Pezo D, Alarcon B. Evaluation of early reproductive performance in the postpartum alpaca by progesterone concentrations. Animal Reproduction Science. (1995) 39:71–7.doi: 10.1016/0378-4320(94)01374-U
57. Echavarria L, Mendoza G, Evaristo R, Hinostroza E, Delgado A, Morán A, et al. Vías de señalización asociadas a la esteroidogénesis. Revista Especializada en Ciencias Químico-Biológicas. (2012) 15:24–36.
58. Delavaud C, Bocquier F, Baumont R, Chaillou E, Ban-Tokuda T, Chilliard Y. Body fat content and feeding level interact strongly in the short- and medium-term regulation of plasma leptin during underfeeding and re-feeding in adult sheep. Br J Nutr. (2007) 98:106–15. doi: 10.1017/S0007114507704968
Keywords: diet restriction, leptin, corpus luteum, llama, alpaca, progesterone
Citation: Norambuena C, Hernandez F, Alfaro J, Silva G, Topp S and Ratto M (2020) Effect of Different Levels of Energy Diet Restriction on Energy Balance, Leptin and CL Development, Vascularization, and Function in South American Camelids. Front. Vet. Sci. 7:598147. doi: 10.3389/fvets.2020.598147
Received: 23 August 2020; Accepted: 13 November 2020;
Published: 16 December 2020.
Edited by:
Rosa Maria Garcia-Garcia, Complutense University of Madrid, SpainReviewed by:
Jamie L. Stewart, Virginia-Maryland College of Veterinary Medicine, United StatesMaria Florencia Gallelli, University of Buenos Aires, Argentina
Copyright © 2020 Norambuena, Hernandez, Alfaro, Silva, Topp and Ratto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cecilia Norambuena, bWNub3JhbWJ1ZW5hQHVjdC5jbA==
 Cecilia Norambuena
Cecilia Norambuena Francisca Hernandez1
Francisca Hernandez1 Marcelo Ratto
Marcelo Ratto