
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Vet. Sci. , 05 November 2020
Sec. Veterinary Epidemiology and Economics
Volume 7 - 2020 | https://doi.org/10.3389/fvets.2020.586919
In December 2019, a severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) that caused severe disease clusters was first reported in Wuhan, the capital of China's Hubei province. This viral disease, which is reported to originate from a seafood market where wild animals are illegally sold, has been transmitted among humans worldwide through close contact. Given the growing number of infected people worldwide and the disastrous consequences in all aspects of life, COVID-19 is a serious public health issue that requires special attention. In some countries, the epidemic curve of infection which was in the plateau phase or decreasing phase during the lockdown period increases day by day since the reopening, indicating the second phase of contamination. Therefore, the preventive measures recommended by the World Health Organization (WHO) must be respected to stop the spread of the disease. The international crisis due to the COVID-19 pandemic negatively affects many sectors, including animal production and its related industries. Indeed, with the cessation of imports and exports between countries, it is not possible to provide feeds that are considered as basic raw materials in livestock raising. This situation impairs animal movements, decreases production inputs availability, and negatively affects the economy. The sustainability of animal production is also affected by a shortage of workers due to the lockdown/curfew, the strong decrease in the purchasing power of the consumer, and the intensification of health care tasks. To prevent contamination of animal products and the spread of the disease with food, the U.S. Centers for Disease Control and Prevention (CDC) recommends frequent disinfection of food and human contact surfaces at production sites using an appropriate antiseptic. The purpose of this review article is to describe the current status of COVID-19 and investigate its effects on animal production. We propose potential approaches to keep animal products processing units and staff safe from SARS-CoV-2 infection and some strategies to improve animal production quantity and economy.
Virology is the science that studies viruses that can be transmitted by food, soil, air, and contact (1, 2). Viral infections whose cause of occurrence and their effects on human health are not fully known to have threatened mankind throughout history. The COVID 19 pandemic, which affects the whole world and infects 32.23 million people as of September 25, 2020, is a serious public health issue. Coronaviruses from the Corononaviridae family are enveloped positive polarity and single-chain RNA viruses that can infect both humans and animals (3, 4). However, coronaviruses are divided into four groups as alpha, beta, gamma, and delta coronaviruses. Coronaviruses that can be transmitted to humans are alpha and beta coronaviruses (5). To date, six coronaviruses (CoVs HCoVs-NL63, HCoVs-229E, HCoVs-OC43, HCoVs-HKU1, SARS-CoV, and MERS-CoV) which can infect humans have been identified (4, 6).
In December 2019, an unknown epidemic of lung infections occurred in Wuhan, China, and spread to the whole country in a short period of 2–3 weeks. It was determined that the pathogen causing this epidemic was a coronavirus and the International Virus Taxonomy Committee named the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), based on taxonomy (7, 8). This new type of beta coronavirus emerged in a market selling seafood in Wuhan and it was determined that 65% of the 41 confirmed cases were directly or indirectly associated with this market place (9). It is found that the disease is transmitted from person to person when it is also seen in people who have no connection with the market place, where the seafood is sold (10). The incubation period of COVID-19 is on average 5 to 6 days, however, can be up to 14 days in some cases (11). Clinical signs such as cough, fever, runny nose, and fatigue are observed in infected patients (12). However, asymptomatic patients have no symptoms of the disease. According to the studies, based on the previous coronavirus outbreaks and the genetic structure of the existing virus, it is thought that COVID-19 may have been transmitted from bats (13), or pangolins since the virus taken from pangolin are 99% of the genetic similarity of the virus taken from humans (14). The main way of transmission of COVID-19 is thought to be that the droplets that come out of the mouth of infected people during the speech, coughing, sneezing settle in the upper respiratory tract of other people, and then reach the lungs and cause infection. Another way of transmission is thought that people who touch the coronavirus-infected surfaces with their hands touch their hands to their mouths, noses, and eyes and take the disease factor (10, 15).
The COVID-19 pandemic has direct impacts on food systems, especially through changing the food supply-demand system, and indirect impacts through decreasing purchasing power and the capacity of food distribution and marketing, and increasing healthcare tasks (16). For instance, the disruptions of production and industrial supply chains due to the COVID-19 crisis are expected to reduce the global economic growth by 0.5% in 2020 (2.4% in 2020 vs. 2.9% in 2019) (17). These elements may increase the risk of food insecurity, malnutrition, and poverty in the countries most affected by the crisis (18). Given the growing number of infected people worldwide and the disastrous consequences in all aspects of life, COVID-19 is a serious public health issue, which requires special attention. The purpose of this review article is to describe the status (epidemiology, symptoms, diagnosis, and treatments) of COVID-19 and elucidate approaches to prevent contamination during animal production and boost the economy.
The epidemic curve of infection is generally divided into 3 parts: increasing, plateau, and declining phases (16). The 3 phases of the epidemic curve of all countries are different. In many countries such as USA, Brazil, and India, the peak of infection is not yet observed. Some countries like the United Kingdom, Canada, France, and Italy, which were in the decline phase of the disease on Jun 21, 2020 (19), have cumulative confirmed cases, which continue to increase as of July 21, 2020 (19). This difference could be due to many factors such as population size, the average age, the implementation of some preventive measures, the equipment of hospitals, and the percentage of peoples with chronic diseases which may increase contamination and mortality. It is important to note that the second phase of contamination is observed in some countries like Brazil, the United States, Turkey, France, Australia, UK, and Spain probably due to the reopening (19).
In general, the peak of infection and deaths in the world is not yet observed. The total confirmed COVID-19 cases and deaths are 14.89 million and 610097, respectively as of July 22, 2020 [(19); Figure 1]. The high mortality of infected patients in Europe and the North, America could be due to the high average age and associated chronic diseases, which may decrease the immunity system (20–22). Although the cumulative confirmed COVID-19 deaths are still increasing in the United Kingdom, France, United States, Brazil, Canada, and India, a plateau phase is observed in Germany (19). The lowest death rate in Germany could be justified by the effective response to COVID-19 by implementing mass testing and swift lockdown associated with the robust healthcare system of the country. To date (as of September 25, 2020), the current status of COVID-19 in the world is still worrying (32.23 million positive cases, 983 042 deaths, and 22.24 million recovered).
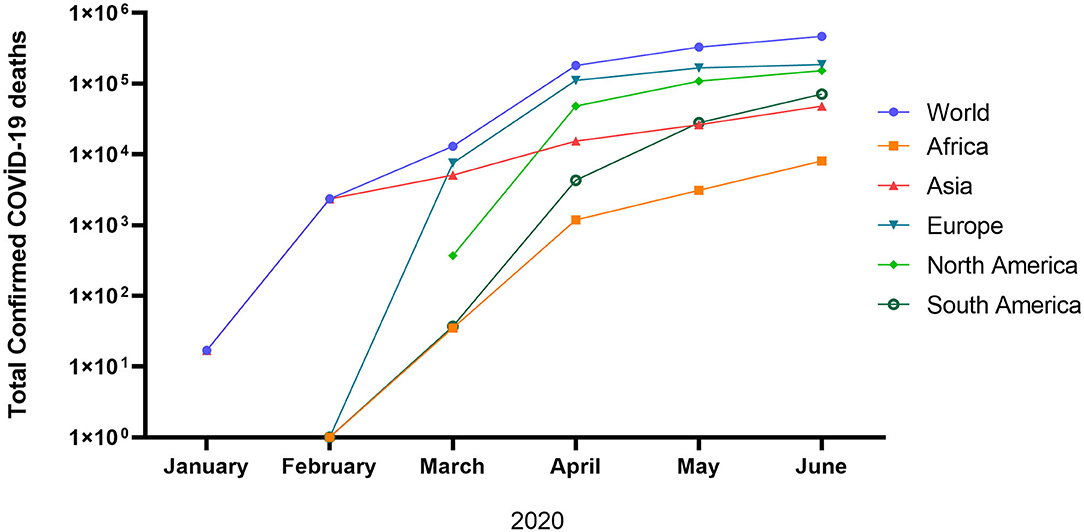
Figure 1. Total confirmed COVID-19 deaths (17).
People infected with COVID-19 can be asymptomatic or present various symptoms such as fever, cough, dyspnea, fatigue, muscle or body aches, headache, loss of taste or smell, congestion or runny nose, nausea, vomiting, and diarrhea (23, 24). COVID-19 can affect the neurological system and cause multiple organ failure such as kidney failure. In addition, pre-existing comorbidities such as heart disease, diabetes, hypertension, respiratory disorders, and cancers increase the severity of COVID-19 in patients (25, 26).
At the beginning of this pandemic, due to the lack of specific SARS-CoV-2 kits, the WHO has released a guideline on case surveillance of COVID-19 on January 31, 2020 (27), which recommended to first diagnose the most common respiratory diseases, followed by SARS-CoV-2 detection, if the previous exams are negative (28). Although quarantine of suspect patients with some symptoms of the disease was also recommended in many countries at the beginning of this pandemic, systematic diagnosis of SARS-CoV-2 should be a priority to control the spread of this pathogen. For SARS-CoV-2 testing, the World Health Organization (WHO) strongly recommends the collection of samples from respiratory tract specimens for the detection of viral nucleic acid (29). The real-time RT-PCR assay is the first line of molecular-based methods used, due to its high sensitivity (30–32). However, if a negative result is found in patients suspected of COVID-19 (presented classical symptoms of the disease), it is advisable to repeat the test (33). Although the effectiveness of the real-time RT-PCR assay is proven, it requires sophisticated equipment and highly trained personnel and is relatively time-consuming (about 1.5–2 h) (34). Recently, new rapid techniques such as rapid antigen testing serological tests, reverse transcription loop-mediated isothermal amplification (RT-LAMP), and CRISPR-Cas12-based assay called SARS-CoV-2 DNA endonuclease-targeted CRISPR Trans Reporter (DETECTR) have been developed for rapid and sensitive detection of SARS-CoV-2 (35).
There is no specific therapy for COVID-19 and no effective vaccine is available (36). However, depending on the stage of the disease and comorbidity factors, the treatment of COVID-19 patients included antimalarial drugs, antibiotic therapy, antiviral treatment, corticosteroids, immunoglobulin therapy, and convalescent plasma from patients recovered from COVID-19 (37–40).
A combination of hydroxychloroquine (an antimalarial drug) and azithromycin (an antibiotic agent) has been approved to be effective in many countries, based on the works conducted in China (37, 41) and France (42). The effectiveness of chloroquine on COVID-19 infection has been reported in vitro (32). A clinical study conducted in more than 10 hospitals in Wuhan (China) also demonstrated the efficacy and safety of chloroquine phosphate in the treatment of COVID-19 associated with pneumonia (41). This antiviral property of chloroquine was already reported 17 years ago (43). Indeed, Savarino et al. (44) demonstrated that chloroquine has antiviral potential by increasing endosomal pH required for virus/cell fusion, and interfering with the glycosylation of cellular receptors of SARS-CoV. The in vitro antiviral activity of chloroquine has been also reported on other viruses such as Middle East Respiratory Syndrome Coronavirus (MERS-CoV), HIV, Ebola, Hendra, and Nipah (43–46).
Antiviral drugs such as favipiravir (an anti-influenza agent), remdesivir (an anti-ebola drug), lopinavir and ritonavi (commonly used against HIV) and pegylated interferon with ribavirin (used in the treatment of HBV and HCV) were urgently administered to patients with COVID-19 (32, 33, 47, 48). In a recent study (without a control group) conducted on 53 COVID-19 patients under remdesivir therapy, a clinical improvement was observed in 36 (68%) patients (49), suggesting the beneficial potential of this protease inhibition in the treatment of COVID-19. Oseltamivir also improved the clinical symptoms of patients with severe COVID19 in 35.8% (393/1099) (39), 89.9% (124/138) (9), and 92.7% (38/41) (38) patients. Although an in vitro study demonstrated the inhibitory potential of lopinavir and ritonavir against SARSCoV (50), no clinical improvement was observed in 199 patients with severe COVID-19 treated with these drugs (51). Clinical studies to identify effective antiviral drugs for the treatment of COVID-19 are underway.
The use of serum or convalescent plasma from patients recovered from COVID-19 represents an effective treatment strategy because the treated patients from the viral infection produced a specific antibody in response to SARS-CoV-2 (52). Although the use of corticosteroids has been reported as an effective treatment of COVID-19 (41), their use is controversial. 30 clinical trials have been initiated to date (as of September 24, 2020) to evaluate the safety and efficacy of dexamethasone against SARS-CoV-2 infection1. Dexamethasone has been proven to significantly reduce the mortality risk in COVID-19 patients on ventilation and oxygen by up to 35 and 20%, respectively. Based on positive results, dexamethasone has been authorized by the UK government for the treatment of critically ill COVID-19 patients (53). However, the comorbidity factors (especially diabetes) should be considered before treatment because some researchers reported that dexamethasone increases the risk of COVID-19 progression to severe disease, especially in patients with diabetes (54, 55).
In China, in a total of 701 patients with severe COVID-19 treated with traditional Chinese medicine based on Qingfei paidu decoction, a clinical improvement was observed in 449 patients while 212 cases were stable (56). An important study carried out on Vero E6 cells by Runfeng et al. (57) demonstrated that Lianhuaqingwen (a traditional Chinese medicine formula) may represent a novel strategy to control COVID-19 because it significantly inhibits the SARS-COV-2 replication, affects virus morphology and exerts anti-inflammatory activity in vitro. Other Chinese plants like Astragulus membranaceus and Pelargonium sidoides commonly used to treat viral respiratory infections (58, 59) may also be useful against COVID-19.
The anti-mouse coronaviral activity (a surrogate of SARS-CoV) of some Indian plants including Indigofera tinctoria, Vitex trifolia, Gymnema sylvestre, Abutilon indicum, Leucas aspera, Cassia alata, Sphaeranthus indicus, Clerodendruminerme gaertn, and Evolvulus alsinoides have been reported (60). These Indian plants may represent a potential therapy of COVID-19. More experimental and clinical studies are highly needed.
In Cameroon, many plants such as Aloe vera, Angelica gigas, Panax ginseng, Scutellaria baicalensis, Trichilia dregeana, Detarium microcarpum, Phragmanthera capitate, and Phyllanthus muellerianus have been reported to exhibit antiviral and immunomodulatory properties through stimulation of cytokines, activation of lymphocytes and improvement of macrophage actions (61, 62). These plants may represent a possible source of COVID-19 therapy.
In Madagascar, a polyherbal formulation called COVID Organics (CVO) composed of Artemisia afra and other Madagascan plants has been recommended by the President of the Republic for the prevention and treatment of COVID-19. The consumption of CVO by the Madagascan population may justify the low prevalence of COVID-19 in the country (8,162 positive cases, 69 deaths, and 4,662 recovered as of 22 July 2020). However, experimental and clinical studies are underway by international researchers to confirm the effectiveness of CVO for the treatment of COVID-19. Researchers at Germany's Max Planck Institute of Colloids and Interfaces in Potsdam are among a group of scientists from Germany and Denmark in collaboration with the US company ArtemiLife to conduct a project on the effects of Artemisia annua on COVID-19 (63). On the other hand, Eucalyptus essential oil found in many countries is reported to improve the innate cell-mediated immune response that can be used as an immunoregulatory agent against infectious diseases (63, 64).
The immunomodulatory potential of some nutrients has been proposed as a possible strategy to prevent and/or treat SARS-CoV-2. For instance, since vitamin C (ascorbic acid) has been used for decades in the treatment of influenza (a coronavirus) (65), it may also be effective against SARS-CoV-2, but clinical studies are needed. Vitamin D intake may reduce the risk of COVID-19 infection and related deaths. Indeed, evidence has shown that vitamin D decreases the risk of COVID-19 outbreak in winter, which is a time when 25-hydroxyvitamin D (25(OH)D) level is low (66). Vegetables rich in vitamin A like carrots, spinach, and sweet potato may also be helpful against COVID-19 infection. Vitamin A comprises a group of fat-soluble compounds (including retinol, retinoic acid, and β-carotene) known to lower the susceptibility to infections (67). For example, isotretinoin (a derivative of vitamin A) mediates the down-regulation of angiotensin-converting enzyme 2 (ACE2), which is a crucial host cellular protein required for the entry of SARS-COV-2 in the body (68). Rice bran, wheat bran, Lawsonia alba (hina), Echinacea purpurea (eastern purple coneflower), Plumbago zeylanica (Ceylon leadwort), and Cissampelos pareira Linn (velvetleaf) also exhibit immunomodulatory properties by stimulating phagocytosis (63, 64). Using these immunomodulatory vitamins and foods could improve the immune system and protect the body against COVID-19. However, experimental and clinical studies are needed to confirm their effectiveness.
Although there is sufficient information about the direct transmission of the new type of beta coronavirus agent, the information about the indirect transmission is not clear. Although there is no evidence that the disease agent is contaminated with food in the studies conducted up to this time, it is known that there is a possibility of contamination from the virus-contaminated surfaces. Based on this information, an idea can be made about how the disease can be transmitted from food contaminated by the disease. In the statement published by the FDA (US Food and Drug Administration) in February 2020, there was no evidence that COVID-19 could be contaminated with consumed food and nutrient packaging, but it has been stated that it is always important for general hygiene and sanitation to be thoroughly disinfected the hands and surfaces when handling food, cooking, and serving. Raw meat products should not be kept together with other foods, and food should be cooked at the right temperature2.
According to the report published by the WHO, there is no information that the SARS-CoV-2 virus has been transmitted by foods, but according to the previous coronavirus outbreaks (SARS-CoV and MERS-CoV), it has been reported that there are some suspicions about the coronavirus can be found in animal foods that have not been heated treatment (20). This shows that animal products at processing plants, slaughterhouses, and butchers can play an important role in spreading the disease agent. According to another study, the emergence and spread of the disease factor in a sea market, where animal products are sold reveal that hygienic measures are very important (69). Moreover, a study reported that viruses that can be transmitted through the respiratory tract remain viable for up to 48 h in fruits and vegetables stored in the cold (70). Technological processes such as heating and cooling applied to prevent microbial growth in foods can be applied to block the reproduction of bacteria. However, if the contaminated agent is a virus, the aim is not to prevent development, but to destroy the virus completely (71).
It has been reported that coronaviruses remain stable in cold environments (71). It is also known that coronaviruses maintain their viability on different surfaces for 3–4 days at appropriate humidity and temperature parameters, and are sensitive to heat treatment at 70°C. Therefore, it has been reported by the WHO that meat, milk, and other animal products should not be consumed raw and contact with other foods should be avoided to prevent cross-contamination (72). Moreover, China's Disease Control and Prevention Center have determined that there is COVID-19 nucleic acid in 33 of 585 environmental samples collected in Wuhan province, China, where the disease originated (73).
The COVID-19 outbreak has raised concerns about potential medical supply issues, including both pharmaceuticals and medical products such as personal protective equipment (e.g., gloves, masks, gowns) and surgical drapes (74). When working in the food industries, basic hygiene measures should always be implemented. The U.S. Centers for Disease Control and Prevention (CDC) recommends frequent disinfection of food and human contact surfaces at production sites. It states that the disinfection of these surfaces should be done like routine cleaning, no additional disinfectants are required. During the new type of beta coronavirus pandemic, it recommends that employees working in food businesses stay home if they show symptoms of the disease and not go out and go to work before the disease symptoms disappear completely (75). To prevent contamination of animal products with disease agents and the spread of the disease with food, the disinfection of the hands should be ensured during animal product processing, and the products should be consumed after being washed thoroughly or disinfected with an appropriate antiseptic. Animal products should not be consumed raw and they should be cooked at least at 70°C for a sufficient period. The devices should be cleaned and disinfected frequently. It is also recommended that the employees working in the animal products processing facilities should be clean during the production, stay at least 2 meters away from each other and disinfect their hands and clothing after leaving the production facility (76). Hence, biosecurity and biosafety should be improved in order to prevent the spread of this pathogen.
A small number of animals worldwide have been reported to be infected with SARS-CoV-2, mostly after close contact with people infected with COVID-19. In March 2020, two dogs (in Hong Kong) and two domestic cats (in Italy and Hong Kong) belonging to SARS-CoV-2 infected persons and exhibiting COVD-19 symptoms were tested positive for SARS-CoV-2 (77–80).
On March 27, 2020, a four-year-old tiger at the Bronx Zoo in New York City developed a dry cough and some wheezing. On April 2, 2020, the samples were collected from the tiger and she was tested positive for SARS-CoV-2 (81). By April 3, three additional tigers and 3 lions in the same Zoo exhibited cough and a loss of appetite (important symptoms of SARS-CoV-2), but were not tested. These animals were immediately isolated. As of April 6, 2020, the presumably infected animals were stable and recovered, although they had not been tested (81).
Presently (as of September 24, 2020), the United States Department of Agriculture (USDA) has reported numerous cases of SARS-CoV-2 in cats, dogs, lions, tigers, and minks3.
Although routine testing of animals for SARS-CoV-2 is not recommended, adequate measures (testing, isolation, and treatments) must be implemented immediately in the case of suspected cases (animals or companion animals), using a One Health approach between local, state, and/or federal public health and animal health officials, as recommended by the Centers for Disease Control and Prevention, US Department of Agriculture4.
Adequate surveillance is needed to detect infected animals and workers at an early stage for efficient response to prevent the spread of disease. The WHO in cooperation with the Food and Agriculture Organization of the United Nations and OIE-World Organization for Animal Health encouraged collaboration, networking, and technical consultation for jointly analyzing epidemiological and human-animal interfaces and promptly sharing and distributing public health information (82).
The severity of the effects of the COVID-19 pandemic on agriculture, food, and therefore feed sector varies by country (83). Many factors, such as the measures taken by countries against the COVID-19 pandemic, the power of the measures taken, the economic structure of the countries, and the level of awareness of the society in terms of the epidemic, have been effective. Failure to import and export the feeds, which are the raw materials of livestock raising, and the continuity of animal production harm the narrowing of the market of animal products and the cost of the products. The mixed feed industry is an intermediate industry branch, but it takes its raw materials mostly from crop production and gives its final product to animal production. The effects of the COVID-19 pandemic on the mixed feed industry are seen as the problems experienced in the supply of raw materials and the supply of mixed feed demand (83).
For instance, in Turkey, the price of maize, barley, cracked wheat, and wheat decreased from January to June 2020 (Figures 2A,B). During the same period, the price of full-fat soybean, soybean meal, canola meal, sunflower meal, cottonseed meal, corn gluten feed, wheat bran, rasmol, molasses, and dried distillers grain with soluble (DDGS) was also unstable (Figures 2C–F). For instance, the price of DDGS, which was almost stable at the beginning of the pandemic (from January to February 2020) increased from February to April, after which it began to decrease and reach the baseline value in June 2020 (Figures 2E,F), indicating a balance between production and consumption. In addition, the price of molasses has continued to increase to date, while the prices of wheat bran and rasmol continue to decrease (Figures 2E,F), indicating high demand for molasses and low production, as well as low demand for wheat bran and rasmol, which reduced sales and lowered prices5.
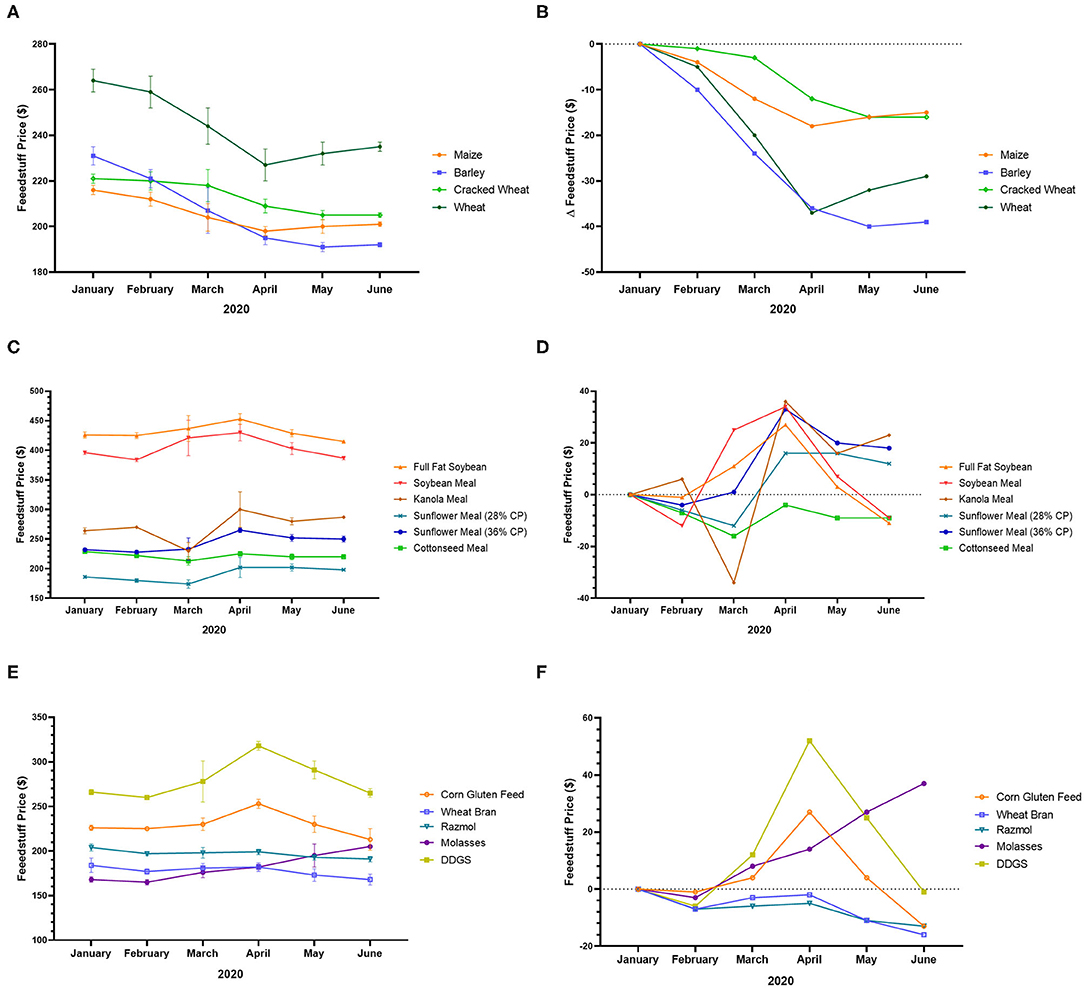
Figure 2. Feedstuff price variation in Turkey during the COVID-19 pandemic. (A) Price of maize, barley, cracked wheat, and wheat from January to June 2020. (B) Variation of the price of maize, barley, cracked wheat, and wheat from January to June 2020. (C) Price of full-fat soybean, soybean meal, canola meal, sunflower meal, and cottonseed meal from January to June 2020. (D) Variation of the price of full-fat soybean, soybean meal, canola meal, sunflower meal, and cottonseed meal from January to June 2020. (E) Price of corn gluten feed, wheat bran, rasmol, molasses, and DDGS from January to June 2020. (F) Variation of the price of corn gluten feed, wheat bran, rasmol, molasses, and DDGS from January to June 2020 (83).
In the UK, the COVID-19 crisis increased the price of feedstuff. Indeed, the price of wheat, barley, oats, soybean, soybean meal, rape meal, sunflower meal, corn gluten feed, and molasses increased from January to May 2020, with a peak generally observed in March-April (except wheat, oats, and sunflower meal) (Figures 3A–F). The peak prices of certain feedstuffs such as wheat, oats, and sunflower meal have not yet been observed (Figures 3A–D). The rising price of feedstuff in the UK may be due to low production and high demand6.
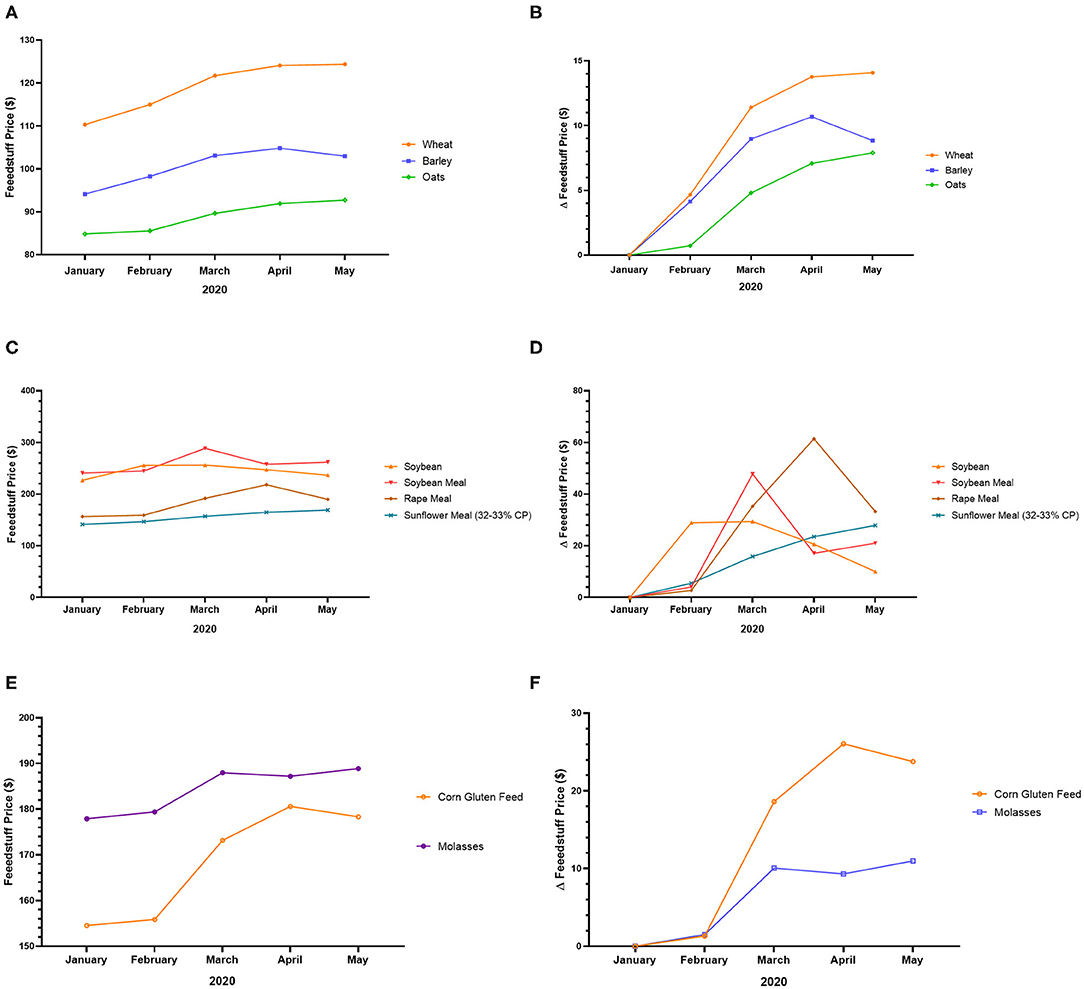
Figure 3. Feedstuff price variation in the UK during the COVID-19 pandemic. (A) Price of wheat, barley, and oats from January to May 2020. (B) Variation of the price of wheat, barley, and oats from January to May 2020. (C) Price of soybean, soybean meal, rape meal, and sunflower meal from January to May 2020. (D) Variation of the price of soybean, soybean meal, rape meal, and sunflower meal from January to May 2020. (E) Price of corn gluten feed and molasses from January to May 2020. (F) Variation of the price of corn gluten feed and molasses from January to May 20205.
Rations cannot be prepared as desired due to the inability to import feed. The basic principle taken into account when preparing the ration is to give animals the carbohydrates, protein, fat, vitamins, and minerals they need to obtain maximum efficiency with minimum cost. Productivity per animal is likely to decrease due to the lack of basic nutrients in animal feed. According to a study with 1,008 participants in Wuhan Province of China during the beginning of the disease, it has been reported that people's interest in game meat has decreased and the demand for organically produced animal products in farms has increased (84).
During the COVID-19 pandemic, the transportation of animal feeds and food, including animal production products, encountered many obstacles. For instance, in China, milk transport was disrupted by tight road traffic controls, leading to milk dumping (85). Moreover, import and export difficulties by maritime transport, linked to several factors such as lack of manpower in Chinese ports, minimum time at sea imposed by certain ports on ships from China seriously disturbed the transport of animal products between China and neighboring countries. In the Philippines, the risk of a shortage of meat caused by the delay in vehicles transporting raw materials for processing has been avoided by stopping movement restriction. Within the countries of the European Union, 35% of beef is exported between member countries. Export bans have caused prices to plummet in Poland, with domestic consumption representing only 15% of production. In Latin America, especially in Argentina and Uruguay, restrictions on the transportation of animal products and the export of meat have lowered the incomes of farmers and ranchers, plunging many into unemployment. In West Africa, the restriction of animal feeds import due to the COVID-19 crisis decreased poultry and pig productions (86, 87).
The impact of the COVID-19 pandemic on animal disease prevention and control is observed around the world. Some examples are summarized in Table 1.
The closure of markets during the COVID-19 crisis created an imbalance between production and consumption, leading to lower demand, lower sales, and falling prices. For instance, American pig prices dropped by about 27 percent in just over a week7. The movement restriction implemented in many countries, the reduction in the number of workers employed in animal production facilities (due to the quarantine of infected or suspected workers) negatively affect the production capacity and contribute to the economic failure. Elleby et al. (83) perform a scenario-based analysis on the IMF economic growth forecasts for 2020 and 2021 using a global multi-commodity agricultural market model and concluded that a decline in economic growth will decrease international meat prices by 7–18% in 2020 and dairy products by 4–7% compared to a business as usual situation.
As reported by Hafez et al. (94), the strategic future after the COVID-19 outbreak should be mainly focused on disease control. For instance, in the poultry industry, eradication, elimination, and/or control of foodborne and zoonotic pathogens present a major challenge. Also, the countries should synchronize their legislation linked to the market, animal nutrition, and the drugs and vaccines licensing for veterinary practice, particularly after the COVID-19 pandemic (94).
The impact of the COVID-19 crisis on animal production is serious. The maintenance of animal production activities should be seen among the priority sectors and the decisions of the governments should be in this direction. Some recommendations and precautionary measures are given below:
- To maintain animal production and prevent possible adversities, it is necessary to ensure the sustainability of ordinary processes such as feed shipment and supply of feed raw materials by applying the necessary disinfection processes (Table 2). It is also important to respect physical distancing and hygiene practices recommended by the WHO (17) to avoid human-to-human transmission.
- The animal health professionals should be informed (from trusted sources) of the evolution of the COVID-19 pandemic in particular in their locality and of the clinical and therapeutic progress of the disease to implement new preventive methods and biosafety measures if necessary.
- Anyone with COVID-19 symptoms (confirmed or suspected cases) including asymptomatic or recovering patients should avoid close contact with animals until cleared by medical providers.
- Prevent animal diseases by maintaining good animal husbandry and production practices.
- Workers, especially veterinarians, should have a contingency plan, such as keeping an inventory of chemicals and equipment and implementing the latest laws and regulations regarding online veterinary consultation during the COVID-19 crisis.
- Animal health professionals should collaborate with pharmaceutical industries, communication channels (reliable social media, radio, and television), and staff in charge of the prevention and treatment of COVID-19 to organize meetings and seminars, preferably webinars, to raise awareness in animal production with important recommendations that can stabilize the activity.
- The countries should maintain open borders for imports and exports to allow trade while respecting all COVID-19 preventives measures.
- The government should provide financial means or loans to animal production centers to increase the supply of animal feed and pay the wages of workers.
- The government should promote alternative sales channels of animal products such as e-commerce instead of public markets to avoid physical contact between people to prevent disease transmission and boost the economy.
- Develop an alternative and effective strategy to increase production with fewer workers, for example by adjusting working arrangements if an employee is absent due to COVID19 or in quarantine and by adjusting the sick leave policy of workers.
- To ensure the optimal functioning of the animal products supply chain, governments can promote collective marketing to maintain demand, and coordinate with suppliers to buy products and redistribute them through adequate channels (for example organizations such as UNICEF, UNHCR, etc.).
The increasing prevalence of COVID-19 infection worldwide is a serious international public health issue as it affects all aspects of life. Although some countries are reopening, the number of confirmed COVID-19 cases is still increasing day by day and the second phase of contamination is observed in many countries. Some researchers have proposed the use of some vitamins (vitamin A, C, D, and E) and foods to boost the immune system and prevent COVID-19 infection, but a specific effective treatment or vaccine against COVID-19 is not yet available. It is imperative to follow the preventive methods recommended by the WHO to limit the spread of this disease. Recent data reported the possibility of transmission from infected patients to animals, so adequate surveillance is also needed to detect infected animals and workers at an early stage for efficient response. Although there is no evidence of disease transmission through the foodstuff or its packaging, it is important to reinforce the current hygiene rules and to make them more attentive to eliminate concerns in consumers' approach to the consumption of foodstuffs. To meet the demand for animal products and boost the economy, it is essential to resolve access to animal feed and other raw materials on a state basis.
PBDD, VK, CO, and KS participated in the study conception, implementation and data analysis, and drafted the manuscript. KS supervised this study from the conception to manuscript review and validation. All co-authors read and approved the final manuscript.
This work was supported in part by the Turkish Academy of Sciences (KS).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with one of the authors KS.
The authors thank the Turkish Academy of Sciences (KS) for partially supporting.
1. ^https://clinicaltrials.gov/ct2/results?cond=covid-19&term=dexamethasone&cntry=&state=&city=&dist= (accessed September 23, 2020).
2. ^https://www.mpg.de/14663263/artemisia-annua-corona-virus (accessed June 24, 2020).
3. ^https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/sa_one_health/sars-cov-2-animals-us (accessed September 23, 2020).
4. ^https://www.cdc.gov/coronavirus/2019-ncov/animals/animal-testing.html (accessed September 23, 2020).
5. ^http://yem.org.tr (accessed July 23, 2020).
6. ^www.gov.uk/government/statistical-data-sets/animal-feed-prices (accessed July 23, 2020).
7. ^https://www.pigprogress.net/World-of-Pigs1/Articles/2020/4/How-are-pig-producers-aroundthe-world-affected-by-Covid-19-568258E/ (accessed July 8, 2020).
2. Antonelli G, Pistello M. Virology: a scientific discipline facing new challenges. Clin Microbiol Infect. (2019) 25:133–5. doi: 10.1016/j.cmi.2018.12.003
3. Corman VM, Muth D, Niemeyer D, Drosten C. Hosts and sources of endemic human coronaviruses. Adv Virus Res. (2018) 100:163–88. doi: 10.1016/bs.aivir.2018.01.001
4. Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. (2019) 17:181–92. doi: 10.1038/s41579-018-0118-9
5. Lu G, Wang Q, Gao GF. Bat-to-human: spike features determining “host jump” of coronaviruses SARS-CoV, MERS-CoV, and beyond. Trends Microbiol. (2015) 23:468–78. doi: 10.1016/j.tim.2015.06.003
6. Woolhouse M, Scott F, Hudson Z, Howey R, Chase-Topping M. Human viruses: discovery and emergence. Philos Trans R Soc Lond B Biol Sci. (2012) 367:2864–71. doi: 10.1098/rstb.2011.0354
7. Lai CC, Liu YH, Wang CY, Wang YH, Hsueh SC, Yen MY, et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARSCoV-2): facts and myths. J Microbiol Immunol Infect. (2020) 53:404–12. doi: 10.1016/j.jmii.2020.02.012
8. Sun J, He WT, Wang L, Lai A, Ji X, Zhai X, et al. COVID-19: epidemiology, evolution, and cross-disciplinary perspectives. Trends Mol Med. (2020) 26:483–95. doi: 10.1016/j.molmed.2020.02.008
9. Chen J. Pathogenicity and Transmissibility of 2019-nCoV—A quick overview and comparison with other emerging viruses. Microbes Infect. (2020) 22:69–71. doi: 10.1016/j.micinf.2020.01.004
10. Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. (2020) 24:91–8. doi: 10.1016/j.jare.2020.03.005
11. Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. (2020) 172:577–82. doi: 10.7326/M200504
12. Ran J, Zhao S, Zhuang Z, Chong MK, Cai Y, Cao P, et al. Quantifying the improvement in confirmation efficiency of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) during the early phase of outbreak in Hong Kong in 2020. Int J Infect Dis. (2020) 96:284–7. doi: 10.1016/j.ijid.2020.05.015
13. Hemida MG, Ba-Abduallah MM. The SARS-CoV-2 outbreak from a one health perspective. One Health. (2020) 10:100127. doi: 10.1016/j.onehlt.2020.100127
14. Cyranoski D. Did Pangolins Spread the China Coronavirus to People? (2020). Available online at: https://www.nature.com/articles/d41586-020-00364-2
15. Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. (2020) 395:809–15. doi: 10.1016/S0140-6736(20)30360-3
16. Poudel PB, Poudel MR, Gautam A, Phuyal S, Tiwari CK, Bashyal N, et al. COVID-19 and its global impact on food and agriculture. J Biol Todays World. (2020) 9:221. doi: 10.35248/2322-3308.20.09.221
17. Schmidhuber J, Pound J, Qiao B. COVID-19: Channels of Transmission to Food and Agriculture. FAO (2020).
18. Food and Agriculture Organization (FAO). Coronavirus Disease 2019 (COVID-19). Addressing the Impacts of COVID-19 in Food Crises. (2020). Available online at: http://www.fao.org/3/ca8497en/ca8497en.pdf (accessed July 8, 2020).
19. Bulut C, Kato Y. Epidemiology of COVID-19. Turk J Med Sci. (2020) 50:563–70. doi: 10.3906/sag-2004-172
20. WHO (World Health Organization). Coronavirus Disease 2019 (COVID-19) Situation Report −32. (2020). Available online at: https://www.who.int/docs/defaultsource/coronaviruse/situation-reports/20200221-sitrep-32-covid19.pdf?sfvrsn=4802d089_2
21. Hansen S, Baptiste KE, Fjeldborg J, Horohov DW. A Review of the equine age-related changes in the immune system: comparisons Between human and equine aging, with focus on lung-specific immune-Aging. Ageing Res Rev. (2015) 20:11–23. doi: 10.1016/j.arr.2014.12.002
22. Akha AAS. Aging and the immune system: an overview. J Immunol Methods. (2018) 463:21–6. doi: 10.1016/j.jim.2018.08.005
23. Bagatini MD, Cardoso AM, Reschke CR, Carvalho FB. Immune system and chronic diseases 2018. J Immunol Res. (2018) 2018:8653572. doi: 10.1155/2018/8653572
24. Singhal TA. Review of coronavirus disease-2019 (COVID-19). Indian J Pediatr. (2020) 87:281–6. doi: 10.1007/s12098-020-03263-6
25. de Lucena TMC, da Silva Santos AF, de Lima BR, de Albuquerque Borborema ME, de Azevêdo Silva J. Mechanism of inflammatory response in associated comorbidities in COVID-19. J.Diabetes Metab Syndr. (2020) 14:597–600. doi: 10.1016/j.dsx.2020.05.025
26. Wang T, Du Z, Zhu F, Cao Z, An Y, Gao Y, et al. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet. (2020) 395:e52. doi: 10.1016/S0140-6736(20)30558-4
27. Zhai P, Ding Y, Wu X, Long J, Zhong Y, Li Y. The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents. (2020) 55:105955. doi: 10.1016/j.ijantimicag.2020.105955
28. WHO. Save Lives: Clean Your Hands. [online]. Geneva (2020). Available online at: https://www.who.int/infection-prevention/campaigns/clean-hands/en/
29. Harapan H, Itoh N, Yufika A, Winardi W, Keam S, Te H, et al. Coronavirus disease 2019 (COVID-19): a literature review. J Infect Public Health. (2020) 13:667–73. doi: 10.1016/j.jiph.2020.03.019
30. Patel A JD. Initial public health response and interim clinical guidance for the 2019 novel coronavirus outbreak—United States, December 31, 2019–February 4, 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:140–6. doi: 10.15585/mmwr.mm6905e1
31. Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DKW, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. (2020) 25:2000045. doi: 10.2807/1560-7917
32. Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. (2020) 395:470–3. doi: 10.1016/S0140-6736(20)30185-9
33. Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. (2020) 30:269271. doi: 10.1038/s41422-020-0282-0
34. Lu R, Wu X, Wan Z, Li Y, Zuo L, et al. Development of a novel reverse transcription loop-mediated isothermal amplification method for rapid detection of SARS-CoV-2. Virol Sin. (2020) 35:344–7. doi: 10.1007/s12250-020-00218-1
35. Broughton JP, Deng X, Yu G, Fasching CL, Servellita V, Singh J, et al. CRISPR–Cas12- based detection of SARS-CoV-2. Nat Biotech. (2020) 38:870–4. doi: 10.1038/s41587-020-0513-4
36. Loeffelholz MJ, Tang YW. Laboratory diagnosis of emerging human coronavirus infections — the state of the art. Emerg Microbes Infect. (2020) 9:747–56. doi: 10.1080/22221751.2020.1745095
37. Tang JW, Tambyah PA, Hui DSC. Emergence of a novel coronavirus causing respiratory illness from Wuhan, China. J Infect. (2020) 80:350–71. doi: 10.1016/j.jinf.2020.01.014
38. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. (2020) 395:507–13. doi: 10.1016/S0140-6736(20)30211-7
39. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of 2019 novel coronavirus infection in China. N Engl J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
40. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
41. Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. (2020) 368:m606. doi: 10.1136/bmj.m606
42. Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. (2020) 14:72–3. doi: 10.5582/bst.2020.01047
43. Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and Azithromycin as a treatment of COVID-19: results of an openlabel non-randomized clinical trial. Int J Antimicrob Agents. (2020) 56:105949. doi: 10.1016/j.ijantimicag.2020.105949
44. Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect Dis. (2003) 3:722–7. doi: 10.1016/s1473-3099(03)00806-5
45. Keyaerts E, Vijgen L, Maes P, Neyts J, Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. (2004) 323:264–8. doi: 10.1016/j.bbrc.2004.08.085
46. Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. (2005) 2:69. doi: 10.1186/1743-422X-2-69
47. Kono M, Tatsumi K, Imai AM, Saito K, Kuriyama T, Shirasawa H. Inhibition of human coronavirus 229E infection in human epithelial lung cells (L132) by chloroquine: involvement of p38 MAPK and ERK. Antiviral Res. (2008) 77:150–2. doi: 10.1016/j.antiviral.2007.10.011
48. Chan KS, Lai ST, Chu CM, Tsui E, Tam CY, Wong MM, et al. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med J. (2003) 9:399–406.
49. Ahn DG, Shin HJ, Kim MH, Lee S, Kim HS, Myoung J, et al. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19). J Microbiol Biotechnol. (2020) 30:313–24. doi: 10.4014/jmb.2003.03011
50. Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. (2020) 382:2327–36. doi: 10.1056/NEJMoa2007016
51. Chu CM, Cheng VCC, Hung IFN, Wong MML, Chan KH, Chan KS, et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. (2004) 59:252–6. doi: 10.1136/thorax.2003.012658
52. Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. The feasibility of convalescent plasma therapy in severe COVID-19 patients: a pilot study. medRxiv [Preprint]. (2020). doi: 10.1101/2020.03.16.20036145
53. Ledford H. Coronavirus breakthrough: dexamethasone is first drug shown to save lives. Nature. (2020) 582:469. doi: 10.1038/d41586-020-01824-5
54. Alessi J, de Oliveira GB, Schaan BD, Telo GH. Dexamethasone in the era of COVID-19: friend or foe? An essay on the effects of dexamethasone and the potential risks of its inadvertent use in patients with diabetes. Diabetol Metab Syndr. (2020)12:80. doi: 10.1186/s13098-020-00583-7
55. Li Q, Li W, Jin Y, Xu W, Huang C, Li L, et al. Efficacy evaluation of early, low-dose, short-term corticosteroids in adults hospitalized with non-severe COVID-19 Pneumonia: A retrospective cohort study. Infect Dis Ther. (2020) 2:1–14. doi: 10.1007/s40121-020-00332-3
56. Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. (2020) 382:1787–99. doi: 10.1056/NEJMoa2001282
57. Runfeng L, Yunlong H, Jicheng H, Weiqi P, Qinhai M, Yongxia S, et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol Res. (2020) 156:104761. doi: 10.1016/j.phrs.2020.104761
58. Kolodziej H. Antimicrobial, antiviral and immunomodulatory activity studies of pelargonium sidoides (EPsfi7630) in the context of health promotion. Pharmaceuticals. (2011) 4:1295–314. doi: 10.3390/ph4101295
59. Luo H, Tang QL, Shang YX, Liang SB, Yang M, Robinson N, et al. Can Chinese medicine be used for prevention of corona virus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chin J Integr Med. (2020) 26:243–50. doi: 10.1007/s11655-020-3192-6
60. Ren JL, Zhang AH, Wang XJ. Traditional Chinese medicine for COVID-19 treatment. Pharmacol Res. (2020) 155:104743. doi: 10.1016/j.phrs.2020.104743
61. Tan BKH, Vanitha J. Immunomodulatory and antimicrobial effects of some traditional Chinese medicinal herbs: a review. Curr Med Chem. (2004) 11:1423–30. doi: 10.2174/0929867043365161
62. Galani BR, Sahuc ME, Njayou FN, Deloison G, Mkounga P, Feudjou WF, et al. Plant extracts from Cameroonian medicinal plants strongly inhibit hepatitis C virus infection in vitro. Front Microbiol. (2015) 6:488. doi: 10.3389/fmicb.2015.00488
63. Serafino A, Vallebona PS, Andreola F, Zonfrillo M, Mercuri L, Federici M, et al. Stimulatory effect of eucalyptus essential oil on innate cell-mediated immune response. BMC Immunol. (2008) 9:17. doi: 10.1186/1471-2172-9-17
64. Sadlon AE, Lamson DW. Immune-modifying and antimicrobial effects of eucalyptus oil and simple inhalation devices. Alternative Med Rev. (2010) 15:33–47.
65. Saul AW. Nutritional treatment of coronavirus. Orthomol Med News Ser. (2020). Available online at: https://drlauda.at/images/pdf/omns/Nutritional_Treatment_of_Coronavirus.pdf
66. Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, et al. Evidence that vitamin d supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. (2020) 12:988. doi: 10.3390/nu12040988
67. Huang Z, Liu Y, Qi G, Brand D, Zheng S. Role of Vitamin A in the immune system. J Clin Med. (2018) 7:258. doi: 10.3390/jcm7090258
68. Sinha S, Cheng K, Schäffer AA, Aldape K, Schiff E, Ruppin E. In vitro and in vivo identification of clinically approved drugs that modify ACE2 expression. Mol Syst Biol. (2020) 16:e9628. doi: 10.15252/msb.20209628
69. FDA (U.S. Food Drup Administration). Coronavirus Disease 2019 (COVID19) the Food Supply Chain. (2020). Available online at: https://www.fda.gov/food/foodsafety-during-emergencies/food-safety-and-coronavirus-disease-2019-covid-19
70. Jalava K. First respiratory transmitted food borne outbreak? Int J Hyg Environ Health. (2020) 226:113490. doi: 10.1016/j.ijheh.2020.113490
71. WHO (World Health Organization) & FAO (Food and Agriculture Organization of the United Nations). Viruses in Food: Scientific Advice to Support Risk Management Activities: Meeting Report. (2008). Available online at: https://apps.who.int/iris/handle/10665/44030
72. Yepiz-Gomez MS, Gerba CP, Bright KR. Survival of respiratory viruses on fresh produce. Food Environ Virol. (2013) 5:150–6. doi: 10.1007/s12560-013-9114-4
73. Bosch A, Gkogka E, Le Guyader FS, Loisy-Hamon F, Lee A, van Lieshout L, et al. Foodborne viruses: detection, risk assessment, and control options in food processing. Int J Food Microbiol. (2018) 285:110–28. doi: 10.1016/j.ijfoodmicro.2018.06.001
74. Krebs, Christopher C. Memorandum on Identification of Essential Critical Infrastructure Workers During Covid-19 Response. U.S. Department of Homeland Security, Cybersecurity and Infrastructure Security Agency (CISA) (2020).
75. El Zowalaty ME, Järhult JD. From SARS to COVID-19: a previously unknown SARSCoV-2 virus of pandemic potential infecting humans –Call for a One Health approach. One Health. (2020) 9:100124. doi: 10.1016/j.onehlt.2020.100124
76. FDA (U.S. Food and Drug Administration). Food Safety and the Coronavirus Disease 2019 (COVID-19). (2020). Available online at: https://www.fda.gov/food/food-safetyduring-emergencies/food-safety-and-coronavirus-disease-2019-covid-19
77. PRO/AH/EDR > COVID-19 Update (30): China (Hong Kong), Dog, Suspected, Serology Pending. (2020). Available online at: http://promedmail.org/post/20200306.7057595
78. PRO/AH/EDR > COVID-19 Update (45): China (Hong Kong) Animal, Dog, 2nd Case PCR Positive. (2020). Available online at: http://promedmail.org/post/20200319.7112693
79. PRO/AH/EDR > COVID-19 Update (58): Belgium, Cat, Clinical Case, Request for Information. (2020). Available online at: http://promedmail.org/post/20200326.7151215
80. D. PRO/AH/EDR > COVID-19 Update (70): China (Hong Kong) Cat, Pets and Stock. (2020). Available online at: http://promedmail.org/post/20200326.7173286
81. James A. A Tiger at Bronx Zoo tests positive for COVID-19. ABC News. Available online at: https://wpde.com/news/coronavirus/a-tiger-at-bronx-zoo-tests-positive-for-covid-19 (accessed April 5, 2020).
82. World Health Organization. Implementation of the IHR at the Human-Animal-Health Interface. Geneva: WHO (2015).
83. Elleby C, Domínguez IP, Adenauer M, Genovese G. Impacts of the COVID-19 pandemic on the global agricultural markets. Environ Resour Econ. (2020) 76:1067–79. doi: 10.1007/s10640-020-00473-6
84. FDA (U.S. Food and Drup Administration). Guidance for Food Businesses on Coronavirus (COVID-19). Available online at: https://www.gov.uk/government/publications/covid-19-guidance-for-foodbusinesses/guidance-for-food-businesses-on-coronavirus-covid-19
85. Xie X, Huang L, Li JJ, Zhu H. Generational differences in perceptions of food health/risk and attitudes toward organic food and game meat: the Case of the COVID-19 Crisis in China. Int J Environ Res Public Health. (2020) 17:3148. doi: 10.3390/ijerph17093148
86. FAO. Mitigating the Impacts of COVID-19 on the Livestock Sector. Rome (2020). Available online at: https://doi.org/10.4060/ca8799en.
87. FAO. African swine fever unprecedented global threat: a challenge to food security, wildlife management and conservation. In: Emergency Prevention System for Animal Health (EMPRES-AH). Rome (2020). Available online at http://www.fao.org/ag/againfo/programmes/en/empres/FAO_ASF_call_for_action.html
88. American Veterinary Medical Association. COVID-19: Drug and medical supply impacts. AVMA. (2020). Available online at: https://www.avma.org/resources-tools/animalhealth-and-welfare/covid-19/covid-19-drug-medical-supply-impacts
89. Slabodkin G. FDA chief warns of supply 'pressure' on reagents for coronavirus tests. MEDTECHDIVE. (2020). Available online at: https://www.medtechdive.com/news/fda-chief-warns-ofsupply-pressure-on-reagents-forcoronavirus-tests/573999/.
90. Herper M, Branswell H. Shortage of crucial chemicals creates new obstacle to U.S. coronavirus testing. STAT. (2020). Available online at: https://www.statnews.com/2020/03/10/shortage-crucialchemicals-us-coronavirus-testing/
91. Vimalanathan S, Ignacimuthu S, Hudson J. Medicinal plants of Tamil Nadu (Southern India) are a rich source of antiviral activities. Pharm. Biol. (2009) 47:422–9. doi: 10.1080/13880200902800196
92. WHO. The Safe Hand Challenge [video]. (2020). Available online at: https://youtu.be/y7e8nM0JAz0
93. Phelps M. COVID-19: African swine fever response challenge. Queensland Country Life [online] (2020). Available online at: https://www.queenslandcountrylife.com.au/story/6715906/covid-19-createsafricanswine-fever-response-challenges/
94. Hafez MH, Attia YA. Challenges to the poultry industry: Current perspectives and strategic future after the COVID-19 outbreak. Front Vet Sci. (2020) 7:516. doi: 10.3389/fvets.2020.00516
95. Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. (2020) 104:246–51. doi: 10.1016/j.jhin.2020.01.022
Keywords: COVID-19, infection, pandemic, animal production, economy
Citation: Defo Deeh PB, Kayri V, Orhan C and Sahin K (2020) Status of Novel Coronavirus Disease 2019 (COVID-19) and Animal Production. Front. Vet. Sci. 7:586919. doi: 10.3389/fvets.2020.586919
Received: 24 July 2020; Accepted: 30 September 2020;
Published: 05 November 2020.
Edited by:
Youssef A. Attia, King Abdulaziz University, Saudi ArabiaReviewed by:
Hafez Mohamed Hafez, Freie Universität Berlin, GermanyCopyright © 2020 Defo Deeh, Kayri, Orhan and Sahin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kazim Sahin, bnNhaGlua21AeWFob28uY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.