- 1College of Veterinary Medicine, South China Agricultural University, Guangzhou, China
- 2Guangdong Provincial Key Laboratory of Prevention and Control for Severe Clinical Animal Diseases, Guangzhou, China
- 3Guangdong Provincial Pet Engineering Technology Research Center, Guangzhou, China
- 4Institute of Animal Science and Technology, Zhongkai University of Agriculture and Engineering, Guangzhou, China
The canine influenza virus (CIV) outbreaks have raised concerns as they pose a threat to the health of dogs. The successful construction of a canine influenza (CI) infection model is essential to study the CIV. Here we investigated the pathogenicity of different infectious doses of H3N2 CIV in Beagle dogs. Thirty-seven healthy Beagle dogs were used in the experiment and were infected with 103, 104, 105, and 106 50% egg-infectious doses (EID50). Compared to the dogs in the other three groups, those in the 106 EID50 group presented with obvious clinical symptoms, high virus titer, and typical pathological changes. Considering the ensemble of clinical scores, body temperature, virus shedding, lung lesions, pathological section scores, and visceral virus titers, we determined that 106 EID50 is the minimum infectious dose for the Beagle infection model. The other three infectious doses had almost no clinical symptoms. These results indicate that 106 EID50 is the minimum infectious dose of H3N2 CIV that can cause obvious clinical manifestations and pathological changes associated with CI in Beagle dogs. The theoretical framework developed in this research will guide the establishment of an infection model of CIV for future research.
Introduction
Canine influenza virus (CIV) belongs to the family Orthomyxoviridae and contains eight single-stranded negative-sense RNA segments that encode more than 15 viral proteins (1). In recent years, it has posed a serious threat to the health of dogs and public health security. The avian-origin H3N2 CIV was first discovered in southern China (2), then spread quickly to other parts of Asia (3), and broke out in the United States in 2015 (4). It is worth noting that, H3N2 CIV can recombine with other influenza viruses (IAVs). It was found that natural co-infection with H3N2 CIV and H1N1 pdm09 virus in a single host could result in a wild-type H3N1 virus (5). In addition, in 2015, a novel H3N2 CIV carrying the polymerase acidic (PA) gene from the H9N2 avian influenza virus was isolated in South Korea (6). Therefore, dogs are potential mixing vessels of influenza viruses (7). Owing to the susceptibility of dogs to infection and their close physical contact with humans, the risks for human infection with new influenza strains increases exponentially (8).
Clinical symptoms caused by influenza virus infections vary widely and depend on the route of inoculation and the immune status of the host (9). Different researchers have used different doses of H3N2 CIV to test Beagle dogs for animal infection models. Hong et al. injected Beagles with 2 × 106 50% egg-infectious doses (EID50) of H3N2 CIV to study the clinical symptoms of influenza virus infections (10). Su et al. injected 106 EID50 of H3N2 CIV into Beagle dogs to study proteomics changes triggered by CIV (11). Lyoo et al. inoculated dogs intranasally with 106.5 EID50 of H3N2 CIV to build a model for CI infection (12). However, there is no systematic study on the minimum infectious dose of H3N2 CIV that causes severe clinical symptoms in Beagles.
The successful construction of an infection model is essential for the study of CIV. Jirjis et al. (13) and Deshpande et al. (14) established a scoring system for clinical signs to study H3N8 CIV. Luo used this scoring system for clinical signs of respiratory disease when studying H3N2 CIV (15). We found that the clinical sign scores of Beagles with CI symptoms were >3 in all three studies. Bodewes infected Beagles with 106 TCID50 of H3N2 CIV and found that all dogs with obvious clinical signs had an affected lung area >10% (9). Hence, we believe that referring to this standard clinical scoring method, a model that initiates an infection with a clinical score >3 and an affected lung area >10% can be considered a successful H3N2 CIV infection model. However, the minimum infectious dose of H3N2 CIV to develop CI has not yet been systematically investigated. In this study, we infected Beagles with different doses of H3N2 CIV and evaluated the body temperature, clinical scores, virus titers, area of the affected lung, and histopathology scores with the aim of finding the minimum challenge dose of H3N2 CIV. This study could provide valuable information for constructing the H3N2 CIV infection model for further studies on CIV.
Materials and Methods
Virus
The A/canine/Guangdong/04/2014 (H3N2), referred to as the GD14 strain, was isolated from a pet dog in Guangdong province, China. The virus was propagated in 9-day-old specific-pathogen-free (SPF) embryonated chicken eggs (Wenshi Group Co., Ltd.) at 37°C for 48 h and stored at −80°C. Viral titers were evaluated by EID50/mL and calculated by the Reed-Muench method (15).
Clinical Studies and Virus Challenge
Thirty-seven (10-week-old) Beagle dogs were obtained from the Fuzhou Zhenhe experimental animal Co., Ltd. (China). Prior to the experiment, serum samples were collected from all dogs and tested by hemagglutination inhibition (HI) assays to ensure that the animals had not been exposed to CIV H3N2. Five dogs were used as controls. For intranasal administration, the dogs were divided into 4 groups of 8 and inoculated intranasally with 1 mL of 103, 104, 105, or 106 EID50 of GD14. The control group was inoculated intranasally with the same volume of sterile phosphate-buffered saline. The dogs were housed in the negative pressure room of the Animal Experimental Center of South China Agricultural University. Each dog was housed in a separate cage. They were observed for 1 week and fed regularly before starting the experiment.
After inoculation, the rectal temperature of the dogs was measured at 10:00 every day. Nasal swabs were also collected, during which the clinical symptoms of the dog were monitored. The clinical score of every dog was evaluated using the previously described scoring system (13) (Table 1). Nasal secretions were collected from the left and right nostrils of each animal every day until 10 days after inoculation (0–10 dpi) and diluted with 1 mL of PBS containing 1% Penicillin and streptomycin. The nasal swabs were used for measuring the EID50 using 9–11-day-old embryonated chicken eggs (Wenshi Group Co., Ltd.). Three dogs from the control group and five from each experimental group were euthanized at 5 dpi. Turbinates, tracheas, and lung tissues were collected and the EID50 in each sample was determined. Briefly, 1 mL of sterile PBS was added per g of collected tissue, which was then grinded in a liquid nitrogen homogenizer. The supernatant was collected by centrifugation and inoculated in 9-day-old chicken embryos with different dilution concentrations. After incubation at 37°C for 48 h, the hemagglutination titer was determined, and the EID50 was calculated by the Reed-Muench method. Animal health and behavior were monitored daily in order to implement euthanasia measures (Humane Endpoint) immediately on animals if the following occurred: complete loss of appetite for up to 5 days or poor appetite (<50% of normal amount) for up to 7 days, showing depression and hypothermia (<37°C) without anesthesia or sedation. After the experiment, all remaining Beagles were euthanized (Figure 1).
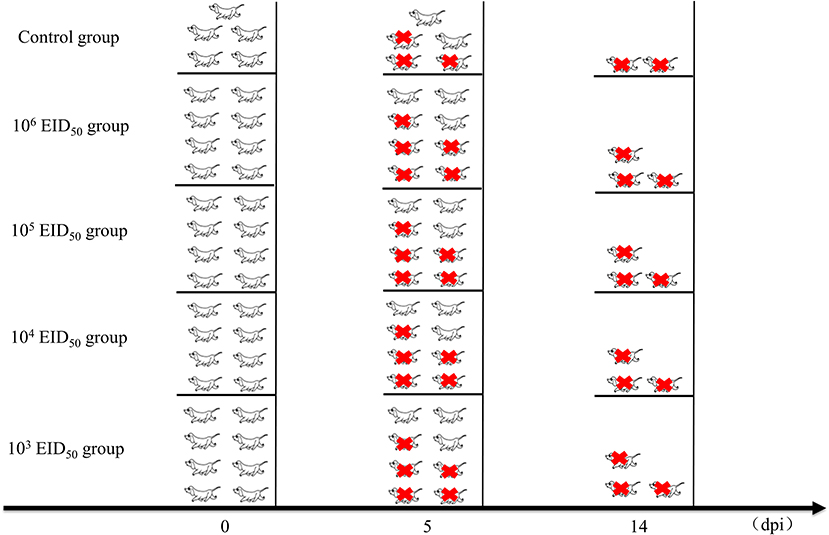
Figure 1. Clinical studies and experimental grouping. Five dogs were used as controls. For intranasal administration, the dogs were divided into 4 groups of 8 and inoculated intranasally with 1 mL of 103, 104, 105, or 106 EID50 of A/canine/Guangdong/04/2014. The control group was inoculated intranasally with the same volume of phosphate-buffered saline. Three dogs from the control group and five from each experimental group were euthanized at 5 dpi. After the experiment, all remaining Beagles were euthanized. The red icons refer to euthanasia.
Serological Testing
Blood samples were collected from the dogs at days 0, 3, 6, 9, 12, and 14 dpi. Approximately 500 μL of serum was collected from each dog and stored at −20°C until use. First, 1 volume of serum was mixed with 3 volumes of receptor destroying enzyme (RDE, Denka Seiken Co., LTD.) and incubated for 18 h at 37°C followed by 30 min at 56°C. The antiserum titer was then determined by the hemagglutination inhibition assay (16). The 1% red blood cells used in this experiment were collected from specific pathogen-free cocks and diluted using sterile PBS.
Histopathological Examination and IHC Analysis
Lung tissues were fixed in 10% formalin for more than 48 h. Samples were washed overnight, dehydrated with alcohol, and embedded in paraffin. Next, the paraffin-embedded tissues were cut into 4–7-μm-thick sections and deposited onto glass slides. These were then mounted and left overnight at 37°C, prior to hematoxylin and eosin (H&E) staining. Antigen distribution in tissue was detected by immunohistochemistry. Briefly, tissue sections were incubated at 4°C for 12 h with a mouse polyclonal antibody against the H3N2 CIV nucleoprotein (primary antibody; preserved by our laboratory). Then, the sections were incubated with a horseradish peroxidase goat anti-mouse IgG (H + L) antibody (secondary antibody; Abbkine, Wuhan, China) at 25°C for 50 min and finally stained with diaminobenzidine. The development of an amber hue indicated positive staining.
Gross Pathology and Histopathology Scoring
For pathology analysis of the lungs, the percentage of the affected lung area was assessed by visual inspection (15, 17). A total of 11 and 12 points were assigned to the right and left anterior lobe, respectively, 25 points (13 for the left and 12 for the right) to the cardiac lobes, 10 points to the accessory lobe, and 20 and 22 points to the left and right caudal lobes, respectively, to reach a total of 100 points (Figure 4A). Each HE section was randomly selected from five fields using an optical microscope with a 10× objective lens to score the size and severity of the inflammatory lesions (9). Dimensions of the inflammation area per field that were ≤10×, >10× and ≤2.5×, and >2.5× of the objective lens were scored as 1, 2, 3, respectively. The severity of inflammation was expressed as follows: 1 for “mild,” 2 for “moderate,” and 3 for “obvious.” The average cumulative value of the size and severity of inflammation was used as the histopathological score for each dog.
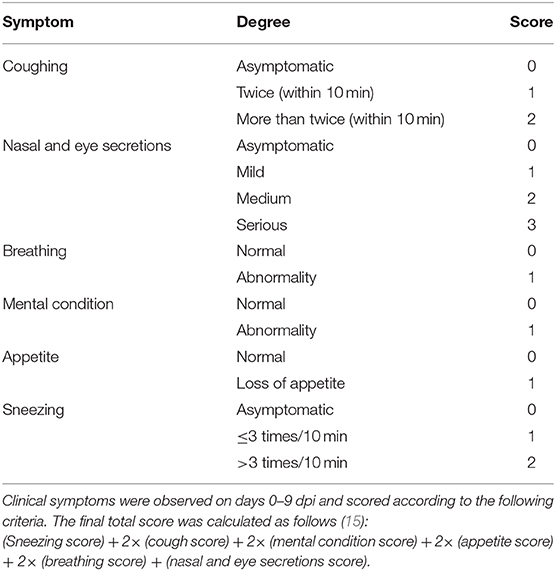
Clinical Symptom Score Criteria
Clinical symptoms were observed on days 0–9 post-infection and scored according to the following criteria with the final total score calculated as follows (15):
(Sneezing score) + 2× (cough score) + 2× (mental condition score) + 2× (appetite score) + 2× (breathing score) + (nasal and eye secretions score). The detailed clinical symptom score criteria are shown in Table 1.
Statistical Analyses
Statistical significance was determined using the conventional Student's t-test results. The differences in means were tested using the one-way ANOVA with post-hoc Tukey's multiple-comparison test (GraphPad Software, Inc., La Jolla, CA). A p < 0.05 was considered significant (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001).
Ethics Approval
All procedures in animal experiments were supervised and inspected by the Experimental Animal Ethics Committee of South China Agricultural University [SYXK (YUE) 2014-0136]. All experimented animals were monitored by university-licensed veterinarians. Ethical approval for this experiment was obtained from the Laboratory Animal Center of South China Agricultural University. Laboratory experiments were conducted under biosafety level 2 conditions, with investigators wearing appropriate personal protective equipment.
Results
Clinical Symptoms
Compared to the control group, the body temperatures of the dogs in the four challenge groups showed varying degrees of fluctuations, and there was fever at 2 and 4 dpi. When the infectious dose was 106 EID50, the dogs' body temperature was higher than the other three challenge groups at all points in time. There was a significant difference when the dose of 105 EID50 was compared at 4, 6, and 8 dpi (p < 0.05). Nevertheless, the other three groups only showed mild body temperature increases (Figure 2A). After infection with a virus titer of 106 EID50, all the dogs showed some respiratory symptoms at 2 dpi, including sneezing, clearing of the nose, as well as coughing on the 3rd day after inoculation; the symptoms continued until day 6. The clinical symptoms in the other three groups were slightly milder. Nevertheless, when the infectious dose was 104 EID50 and 103 EID50, almost no clinical symptoms were observed. When the infectious dose was 106 EID50, the clinical symptoms scores were higher than in the other three groups at 0–8 dpi and were >3 at 4, 5, 6, and 7 dpi. Comparing the infectious dose at 106 EID50 with 105 EID50, the clinical symptom scores of dogs were extremely different at 3, 4, and 5 dpi (p < 0.01). The difference was significant at 2, 6, and 7 dpi (p < 0.05) (Figure 2B). None of the animals met the Humane Endpoint during this study.
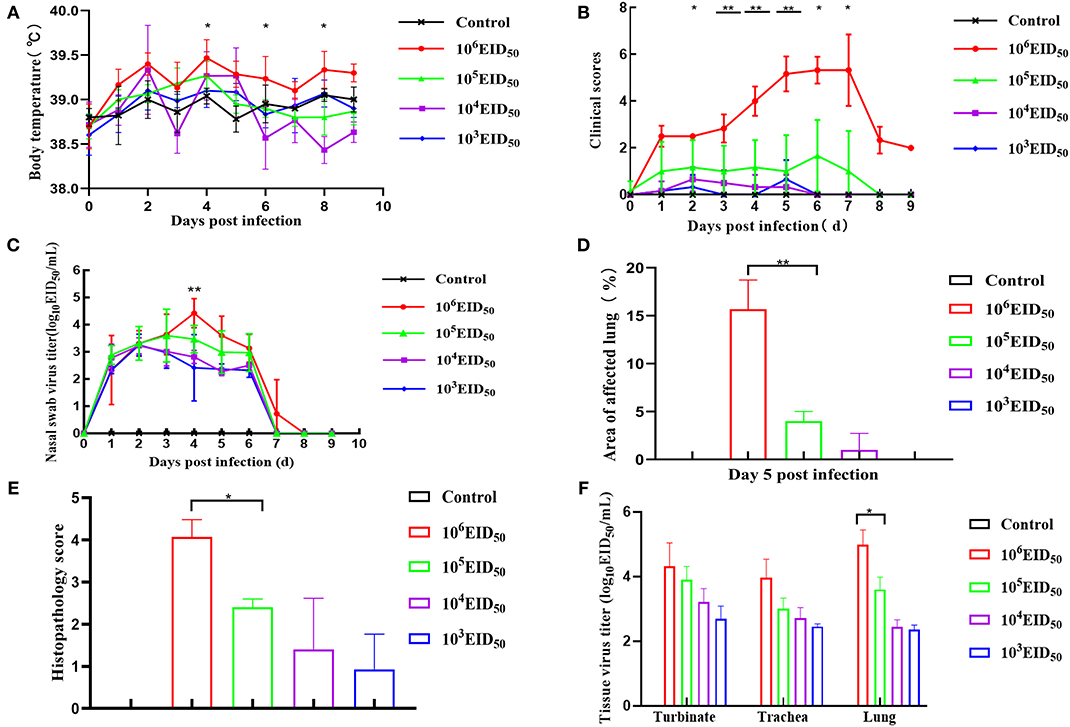
Figure 2. Clinical symptoms. Dogs were inoculated with 106, 105, 104, and 103 EID50 (n = 8) of GD14 virus or PBS (n = 5) as control. Nasal turbinate, tracheal, and lung tissues were collected after euthanizing three dogs in every group at 5 dpi. (A) Body temperature was recorded at days 0–10 dpi; (B) after infection with H3N2 CIV, days 0–10 clinical scores were recorded based on previous criteria (15); (C) nasal swabs were determined by EID50 assay. **P < 0.01 indicates that at 4 dpi, the nasal swab virus titer of the 106 EID50 group was extremely different from that in the 105 EID50 group; (D) lung lesion score; (E) histopathology score. **P < 0.01 indicates that at 5 dpi, the lung lesion scores of 106 EID50 were extremely significantly higher than that of 103 EID50. (F) Tissue virus titer. An EID50 assay was conducted to evaluate the virus titers in the turbinate, tracheal, and lung tissues at 5 dpi (n = 5 per group). Error bars represent the standard errors of the mean; p-values using Student's t-test are indicated.
Virus Titers
The virus was detected within 48 h post-infection, and virus shedding via rhinorrhea lasted for 6–7 days in all the challenged dogs. On the third day after inoculation, the virus titer reached its highest level. The virus titer of the 106 EID50 group was higher than the other three groups at 4 dpi, and the virus shedding in the 106 EID50 group was significantly higher than that in the 105 EID50 group (P < 0.01) (Figure 2C). No virus was detected on the 8th day, showing that virus shedding terminated at 8 dpi in all dogs. As shown in Figure 2F, virus titers were evaluated from turbinates, tracheas, and lungs of infected dogs at 5 dpi. Samples of all three tissues from the 106 EID50 group had the highest virus titer among those from all experimental groups. Nevertheless, tracheas and lungs had higher virus titers than turbinates among the three groups. This suggests that the CIV replicated more easily in the trachea and the lungs. The virus titers in the lungs were significantly different between the 105 EID50 and 106 EID50 groups (P < 0.05).
Seroconversion (HI Titer)
Low antibody levels were detected in canine sera at 9 dpi. At 9, 12, and 14 dpi, the serum antibody levels of the 106 EID50 group were higher than those of the other three experimental groups. The antibody levels at 14 dpi in the two groups, 103 EID50 and 104 EID50, were significantly different from those in the 106 EID50 group (P < 0.0001) (Figure 3).
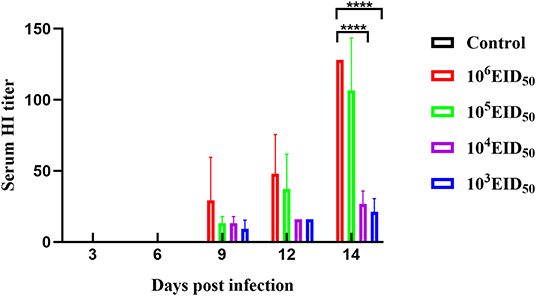
Figure 3. A HI titer was conducted to evaluate the sera specific antibody levels. Error bars represent the standard errors of the mean.
Anatomical Examination
Anatomical examination of the lungs revealed that the 106 EID50 group had obvious lesions, including those in the lung interlobular lobe and the septum intumescentia. The lung surfaces had visible bleeding spots (Figure 4E). However, visible lesions were hardly seen in the lungs of the other three challenge groups (Figures 4B–D). The area of the affected lung of the dogs increased as the challenge dose increased (Figure 2D). When the challenge dose was 106 EID50, the area of the affected lung was >10%, indicating that this challenge dose, not 105, 104, or 103 EID50, could successfully construct the H3N2 CIV infection model.
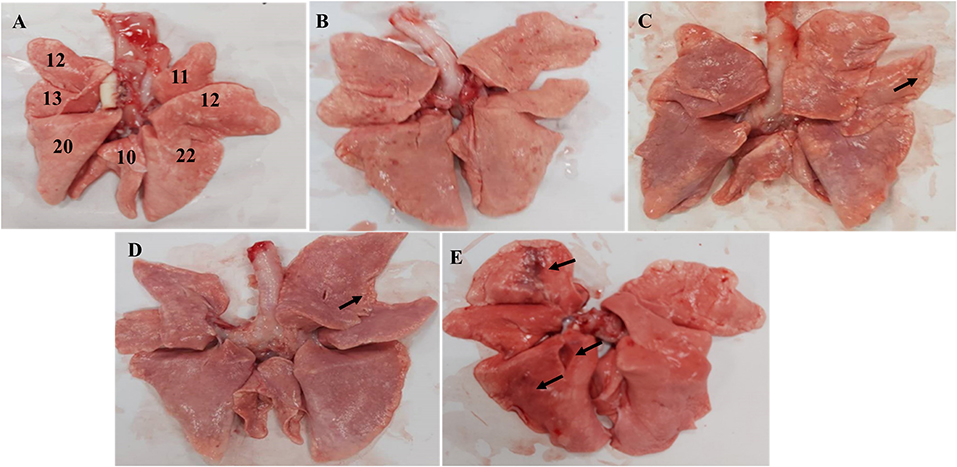
Figure 4. Macroscopic image of the Beagle lung. (A) Normal Beagle lung. Lungs of the (B) 103 EID50; (C) 104 EID50; (D) 105 EID50; and (E) 106 EID50 challenge groups. The arrows refer to tissue damage.
Pathological Changes and IHC Analysis
The histopathology scores in the 106 EID50 group were significantly higher than those in the 105 EID50 group (P < 0.05, as shown in Figure 2E). Lung pathological lesions, including emphysema and pulmonary congestion, pulmonary interstitial hyperplasia, and narrow alveolar cavity, were observed in the 106 EID50 group (Figure 5E) Tissue hyperemia and inflammatory reaction were more serious, with the infiltration of inflammatory cells such as granulocytes, plasma cells, and lymphocytes. When the challenge dose was 105 EID50, only slight alveolar stenosis could be observed in the lungs of the dogs (Figure 5D). However, when the challenge dose was 104 and 103 EID50, there were no obvious pathological changes observed in the lungs (Figures 5B,C), with no differences compared with the control group. IHC analyses were conducted to determine the location of tissue damage and the distribution of the CIV antigen. As shown in Figure 6E, strong staining for CIV antigens was observed in the tracheal epithelial cells, bronchial epithelial cells, and alveolar epithelial cells of the 106 EID50 group, while weak staining for CIV antigens was detected in the bronchial epithelial cells of the other three infection groups (Figures 6B–D).
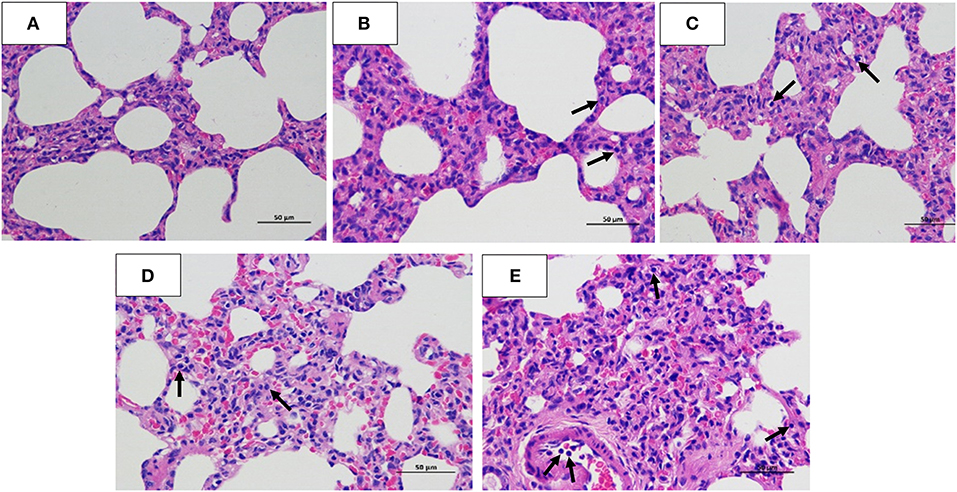
Figure 5. Hematoxylin and eosin staining. Lung tissues were collected for histopathology (hematoxylin and eosin) staining analysis at 5 dpi. (A) Normal Beagle lung histopathology. Pathological lung sections of the (B) 103 EID50; (C) 104 EID50; (D) 105 EID50; and (E) 106 EID50 challenge groups. The arrows refer to the infiltration of inflammatory cells.
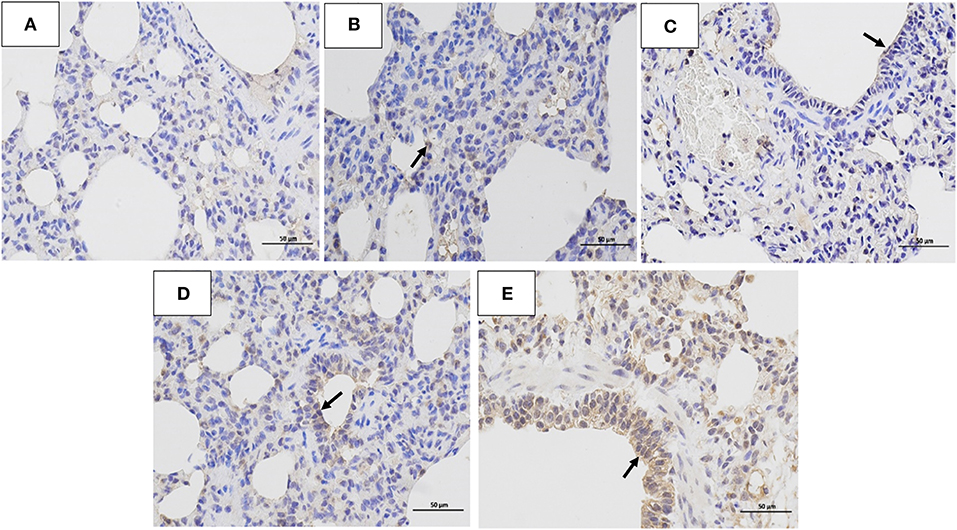
Figure 6. Immunohistochemistry analysis. Immunohistochemistry (IHC) analysis of the lungs of dogs challenged with different doses of H3N2 CIV: (A) control group; (B) 103 EID50 challenge group; (C) 104 EID50 challenge group; (D) 105 EID50 challenge group; and (E) 106 EID50 challenge group. The arrows refer to GD14 antigen expression.
Discussion
Dogs can not only be infected by CIV but also by other IAV subtypes such as H3N8, H3N1, H1N1 (18). As a popular companion, dogs have close contact with humans and are therefore considered as potential intermediate hosts for IAVs (19). The main clinical symptoms of CIV include sneezing, coughing, running nose, tearing of the eyes, fever, conjunctivitis, and anorexia. In the case of CIV infection, histological examination often reveals bronchitis, bronchiolitis, and pneumonia (3, 20). H3N2 CIV is a new respiratory pathogen in dogs (21). Vaccination is the primary measure to prevent and control it (22).
Determining the minimum infectious dose of H3N2 CIV in Beagles is critical to the development of the CIV vaccine. In 2003, Richt et al. established a scoring standard for tissue damage after infection with the H3N2 swine influenza virus (17). Jirjis and Deshpande (13, 14) established the H3N8 CIV-challenged Beagles clinical symptoms and lung affected area scoring criteria. Based on our previous research (11, 23, 24), we developed the scoring criteria for clinical symptoms and lung tissue damage. To determine the minimum infectious dose of H3N2 CIV causing obvious clinical characterization and typical lung damage in Beagles, different doses of the virus were used to infect the Beagles.
The dose of 106 EID50 inoculated intranasally caused obvious clinical symptoms and tissue damage compared to the other three doses. The 106 EID50 group showed clinical symptoms at 2 dpi, which persisted longer than those in the other three groups. At the same time, the clinical symptom score was >3. To evaluate the immune response, HI antibody titers were tested after infection. At 9 dpi, antibodies were detected in all four experimental groups. This indicates that to resist virus invasion, the body induces an immune response against IAVs which often causes secondary bacterial diseases and lung diseases after infection (25). To assess the degree of lung injury caused by different challenge doses, we collected lung tissues from the dogs at 5 dpi. When the dose was 103 EID50, 104 EID50, and 105 EID50, the lung injury was mild, not showing obvious symptoms. Conversely, when the dose was 106 EID50, lung lesions were evident. We determined that the infectious dose of 106 EID50 could cause moderate disease symptoms, with virus shedding for 7–8 days and tissue damage. Although the three groups infected with low doses of CIV did not show severe clinical signs, they could still shed the virus through the nasal cavity for 7 days. Therefore, these asymptomatic infected animals can still transmit CIV. Because we did not test for broad tissue tropism (except for the respiratory tract) and the presence of the virus in rectal swabs, we cannot tell whether CIV can be transmitted through the digestive tract, but it is reported that the virus can be detected in rectal swabs from dogs infected with H3N2 CIV (26), indicating the possibility of fecal-oral transmission of CIV.
Altogether our results show that 106 EID50 is the minimum dose of H3N2 CIV to trigger obvious clinical manifestations in Beagles. When Beagles received an infectious dose of <106 EID50, although the virus could be detected in the respiratory tract and nasal cavity, it usually did not cause noticeable clinical signs. Therefore, animal owners and veterinarians may not detect and treat a CIV infection in time, allowing the virus to circulate and further adapt in dogs. These results can provide valuable information for developing an H3N2 CIV infectious model in Beagles for subsequent vaccine development and prevention and treatment of CI.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
The animal study was reviewed and approved by Experimental Animal Ethics Committee of South China Agricultural University.
Author Contributions
SL: conceptualization, funding acquisition, project administration, and visualization. CF: data curation and formal analysis. XW, SY, JO, LW, JL, GL, XZ, HX, JH, and PW: methodology. JL: software. XW, SY, JO, and JL: validation. YL: writing—original draft and writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key Research and Development Program of China [2016YFD0501004] and The Guangdong Provincial Key Laboratory of Prevention and Control for Severe Clinical Animal Diseases [2017B030314142]; this work was supported in part by the National Natural Science Foundation of China [31672563].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Lee IW, Kim YI, Lim GJ, Kwon HI, Si YJ, Park SJ, et al. Comparison of the virulence and transmissibility of canine H3N2 influenza viruses and characterization of their canine adaptation factors. Emerg Microbes Infect. (2018) 7:17. doi: 10.1038/s41426-017-0013-x
2. Li S, Shi Z, Jiao P, Zhang G, Zhong Z, Tian W, et al. Avian-origin H3N2 canine influenza A viruses in Southern China. Infect Genet Evolut. (2010) 10:1286–8. doi: 10.1016/j.meegid.2010.08.010
3. Song D, Kang B, Lee C, Jung K, Ha G, Kang D, et al. Transmission of avian influenza virus (H3N2) to dogs. Emerg Infect Dis. (2008) 14:741–6. doi: 10.3201/eid1405.071471
4. Pulit-Penaloza JA, Simpson N, Yang H, Creager HM, Jones J, Carney P, et al. Assessment of molecular, antigenic, and pathological features of canine influenza A(H3N2) viruses that emerged in the United States. J Infect Dis. (2017) 216(suppl_4):S499–S507. doi: 10.1093/infdis/jiw620
5. Song D, Moon HJ, An DJ, Jeoung HY, Kim H, Yeom MJ, et al. A novel reassortant canine H3N1 influenza virus between pandemic H1N1 and canine H3N2 influenza viruses in Korea. J General Virol. (2012) 93(Pt 3):551–4. doi: 10.1099/vir.0.037739-0
6. Lee IH, Le TB, Kim HS, Seo SH. Isolation of a novel H3N2 influenza virus containing a gene of H9N2 avian influenza in a dog in South Korea in 2015. Virus Genes. (2016) 52:142–5. doi: 10.1007/s11262-015-1272-z
7. Chen Y, Trovao NS, Wang G, Zhao W, He P, Zhou H, et al. Emergence and evolution of novel reassortant influenza A viruses in canines in Southern China. mBio. (2018) 9:e00909–18. doi: 10.1128/mBio.00909-18
8. Sun H, Blackmon S, Yang G, Waters K, Li T, Tangwangvivat R, et al. Zoonotic risk, pathogenesis, and transmission of avian-origin H3N2 canine influenza virus. J Virol. (2017) 91:e00637–17. doi: 10.1128/JVI.00637-17
9. Bodewes R, Kreijtz JH, van Amerongen G, Hillaire ML, Vogelzang-van Trierum SE, Nieuwkoop NJ, et al. Infection of the upper respiratory tract with seasonal influenza A(H3N2) virus induces protective immunity in ferrets against infection with A(H1N1)pdm09 virus after intranasal, but not intratracheal, inoculation. J Virol. (2013) 87:4293–301. doi: 10.1128/JVI.02536-12
10. Hong M, Kang B, Na W, An D, Moon H, Kim DJ, et al. Prolonged shedding of the canine influenza H3N2 virus in nasal swabs of experimentally immunocompromised dogs. Clin Exp Vaccine Res. (2013) 2:66–8. doi: 10.7774/cevr.2013.2.1.66
11. Su S, Tian J, Hong M, Zhou P, Lu G, Zhu H, et al. Global and quantitative proteomic analysis of dogs infected by avian-like H3N2 canine influenza virus. Front Microbiol. (2015) 6:228. doi: 10.3389/fmicb.2015.00228
12. Lyoo KS, Na W, Yeom M, Jeong DG, Kim CU, Kim JK, et al. Virulence of a novel reassortant canine H3N2 influenza virus in ferret, dog and mouse models. Arch Virol. (2016) 161:1915–23. doi: 10.1007/s00705-016-2868-x
13. Jirjis FF, Deshpande MS, Tubbs AL, Jayappa H, Lakshmanan N, Wasmoen TL. Transmission of canine influenza virus (H3N8) among susceptible dogs. Vet Microbiol. (2010) 144:303–9. doi: 10.1016/j.vetmic.2010.02.029
14. Deshpande M, Abdelmagid O, Tubbs A, Jayappa H, Wasmoen T. Experimental reproduction of canine influenza virus H3N8 infection in young puppies. Vet Ther Res Appl Vet Med. (2009) 10:29–39.
15. Luo J, Lu G, Ye S, Ou J, Fu C, Zhang X, et al. Comparative pathogenesis of H3N2 canine influenza virus in beagle dogs challenged by intranasal and intratracheal inoculation. Virus Res. (2018) 255:147–53. doi: 10.1016/j.virusres.2018.05.023
16. Zhou P, Cao Z, Zeng W, Hao X, Zheng Q, Lin X, et al. PB2 E627K or D701N substitution does not change the virulence of canine influenza virus H3N2 in mice and dogs. Vet Microbiol. (2018) 220:67–72. doi: 10.1016/j.vetmic.2018.05.004
17. Richt JA, Lager KM, Janke BH, Woods RD, Webster RG, Webby RJ. Pathogenic and antigenic properties of phylogenetically distinct reassortant H3N2 swine influenza viruses cocirculating in the United States. J Clin Microbiol. (2003) 41:3198–205. doi: 10.1128/JCM.41.7.3198-3205.2003
18. Nogales A, Chauche C, DeDiego ML, Topham DJ, Parrish CR, Murcia PR, et al. The K186E amino acid substitution in the canine influenza virus H3N8 NS1 protein restores its ability to inhibit host gene expression. J Virol. (2017) 91:e00877–17. doi: 10.1128/JVI.00877-17
19. Sun H, Blackmon S, Yang G, Waters K, Li T, Tangwangvivat R, Erratum for Sun, et al. “Zoonotic risk, pathogenesis, and transmission of avian-origin H3N2 canine influenza virus”. J Virol. (2018) 92:e02227–17. doi: 10.1128/JVI.02227-17
20. Piccirillo A, Pasotto D, Martin AM, Cordioli P. Serological survey for influenza type A viruses in domestic dogs (Canis lupus familiaris) and cats (Felis catus) in north-eastern Italy. Zoonoses Public Health. (2010) 57:239–43. doi: 10.1111/j.1863-2378.2009.01253.x
21. Song D, Lee C, Kang B, Jung K, Oh T, Kim H, et al. Experimental infection of dogs with avian-origin canine influenza A virus (H3N2). Emerg Infect Dis. (2009) 15:56–8. doi: 10.3201/eid1501.080755
22. Cox NJ, Bridges CB. Inactivated and live attenuated influenza vaccines in young children–how do they compare? N Engl J Med. (2007) 356:729–31. doi: 10.1056/NEJMe078003
23. Zhao FR, Su S, Zhou DH, Zhou P, Xu TC, Zhang LQ, et al. Comparative analysis of microRNAs from the lungs and trachea of dogs (Canis familiaris) infected with canine influenza virus. Infect Genet Evolut. (2014) 21:367–74. doi: 10.1016/j.meegid.2013.11.019
24. Zheng Y, Fu X, Wang L, Zhang W, Zhou P, Zhang X, et al. Comparative analysis of MicroRNA expression in dog lungs infected with the H3N2 and H5N1 canine influenza viruses. Microbial Pathog. (2018) 121:252–61. doi: 10.1016/j.micpath.2018.05.015
25. De Luna X, Hartshorn KL. Influenza casts a lung shadow. Am J Pathol. (2017) 187:697. doi: 10.1016/j.ajpath.2017.01.007
Keywords: canine influenza virus, H3N2, beagle, minimum infectious dose, pathogenicity
Citation: Liu Y, Fu C, Lu G, Luo J, Ye S, Ou J, Wang X, Xu H, Huang J, Wu L, Zhang X, Wu P and Li S (2020) Comparison of Pathogenicity of Different Infectious Doses of H3N2 Canine Influenza Virus in Dogs. Front. Vet. Sci. 7:580301. doi: 10.3389/fvets.2020.580301
Received: 05 July 2020; Accepted: 08 September 2020;
Published: 13 November 2020.
Edited by:
Maxim C.-J. Cheeran, University of Minnesota, United StatesReviewed by:
Frederick Joseph Fuller, North Carolina State University, United StatesStefania Lauzi, University of Milan, Italy
Copyright © 2020 Liu, Fu, Lu, Luo, Ye, Ou, Wang, Xu, Huang, Wu, Zhang, Wu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shoujun Li, c2hvdWp1bmxpJiN4MDAwNDA7c2NhdS5lZHUuY24=
†These authors have contributed equally to this work
 Yongbo Liu
Yongbo Liu Cheng Fu
Cheng Fu Gang Lu
Gang Lu Jie Luo1,2,3
Jie Luo1,2,3 Shaotang Ye
Shaotang Ye Shoujun Li
Shoujun Li