
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Vet. Sci., 05 November 2020
Sec. Veterinary Epidemiology and Economics
Volume 7 - 2020 | https://doi.org/10.3389/fvets.2020.558481
The “One Health” concept recognizes that human health is connected to animal health and to the ecosystems. Coxiella burnetii–induced human Q fever is one of the most widespread neglected zoonosis. The main animal reservoirs responsible for C. burnetii transmission to humans are domesticated ruminants, primarily goats, sheep, and cattle. Although studies are still too sparse to draw definitive conclusions, the most recent C. burnetii serosurvey studies conducted in herds and farms in Africa, North Africa, Arabian Peninsula, and Asia highlighted that seroprevalence was strikingly higher in dromedary camels (Camelus dromedarius) than in other ruminants. The C. burnetii seroprevalence in camel herds can reach more than 60% in Egypt, Saudi Arabia, and Sudan, and 70 to 80% in Algeria and Chad, respectively. The highest seroprevalence was in female camels with a previous history of abortion. Moreover, C. burnetii infection was reported in ticks of the Hyalomma dromedarii and Hyalomma impeltatum species collected on camels. Even if dromedary camels represent <3% of the domesticated ruminants in the countries of the Mediterranean basin Southern coast, these animals play a major socioeconomic role for millions of people who live in the arid zones of Africa, Middle East, and Asia. In Chad and Somalia, camels account for about 7 and 21% of domesticated ruminants, respectively. To meet the growing consumers demand of camel meat and milk (>5 million tons/year of both raw and pasteurized milk according to the Food and Agriculture Organization) sustained by a rapid increase of population (growth rate: 2.26–3.76 per year in North Africa), dromedary camel breeding tends to increase from the Maghreb to the Arabic countries. Because of possible long-term persistence of C. burnetii in camel hump adipocytes, this pathogen could represent a threat for herds and breeding farms and ultimately for public health. Because this review highlights a hyperendemia of C. burnetii in dromedary camels, a proper screening of herds and breeding farms for C. burnetii is urgently needed in countries where camel breeding is on the rise. Moreover, the risk of C. burnetii transmission from camel to human should be further evaluated.
Q fever is a neglected zoonotic disease caused by bacteria (1, 2). It is generally admitted that clones of Coxiella burnetii, the etiologic agent of Q fever, circulate in wildlife and infects domestic ruminants. Very few bacteria are required to initiate the infection process (3). Usually, humans become infected through the aerosol route during contact with C. burnetii–positive domestic animals or their products (2, 4). Infection of humans concerns first the farmers and other professionals that have contacts with animals (e.g., veterinarians), but epidemics have been reported in other social groups. C. burnetii is a strict intracellular Gram-negative bacterium entering different cell types with progressive variation in the structure of its lipopolysaccharide (LPS): a smooth LPS for the virulent phase I and a rough LPS for the less virulent phase II (5–7). For symptomatic cases, human Q fever usually occurs 2 to 6 weeks after bacterial exposure (8, 9). The symptomatic primo-infection (10–60% of cases), called acute Q fever, is characterized by high fever, headache, myalgia, pneumonia, and hepatitis (2, 10). It usually resolves spontaneously in a few weeks. When Q fever is suspected, confirmation is provided by serological diagnosis based on anti–C. burnetii immunoglobulin (Ig) detection. An IgG anti–phase II Ig titer above 1:200 and an IgM titer above 1:50 are considered significant for the diagnosis of acute Q fever. Sometimes the symptoms do not resolve (about 5% of cases) and settle in a persistent way mainly in the heart valve and vascular wall but also lymph node, and bone (11). Other disorders can be associated with persistent infections, including lung diseases, hepatitis, and B-cell lymphoma (12, 13).
Regarding domestic ruminants, C. burnetii is responsible for epizooties with increased morbidity and mortality in livestock. It has long been considered that sheep, goats, and cattle were the main domestic source of C. burnetii worldwide among ecosystems in which C. burnetii clones circulate. Although C. burnetii has been classified as a notifiable animal disease by the World Organization for Animal Health (14), notifications concern only a subgroup of domestic animal species and ignore the bacteria dynamics in different ecosystems. Among ruminant species, camels are present in the countries of the Southern coast of Mediterranean basin but absent from countries of the Northern coast. The fact that some Southern countries practice intensive camel breeding, that a high percentage of these animals are carriers of anti–C. burnetii Ig, that C. burnetii was found in camels raw milk, and that camel ticks carries the bacteria must make us question our global perception of the mode of C. burnetii transmission in the Southern coast countries of Mediterranean basin. This review compiles data from the literature regarding the countries around the Mediterranean basin and the Arabic peninsula where camel breeding is practiced and highlights that the potential role of camels as a bacterial reservoir in the transmission of C. burnetii to humans should be considered.
As far back as 1950, the third World Health Assembly was aware of the potential danger of Q fever to public health and passed a resolution calling for study of the C. burnetii prevalence worldwide. Since then, numerous epidemiological studies have shown that human Q fever is found almost everywhere on the planet, with only exceptions of the Antarctica continent and New Zealand. Although seroprevalence data are available for most countries, it can be considered that the true incidence of Q fever in humans is largely underestimated because (i) Q fever is a neglected infectious disease; (ii) there is a predominance of asymptomatic forms; (iii) Q fever is rarely a notifiable disease, and there is a lack of mandatory reporting (e.g., Q fever become a notifiable illness in 1977 and 1978 in Australia and the Netherlands, respectively, and a reportable disease in 1999 in the United States and Japan); and (iv) several local reports written in languages other than English remain ignored (Figure 1).
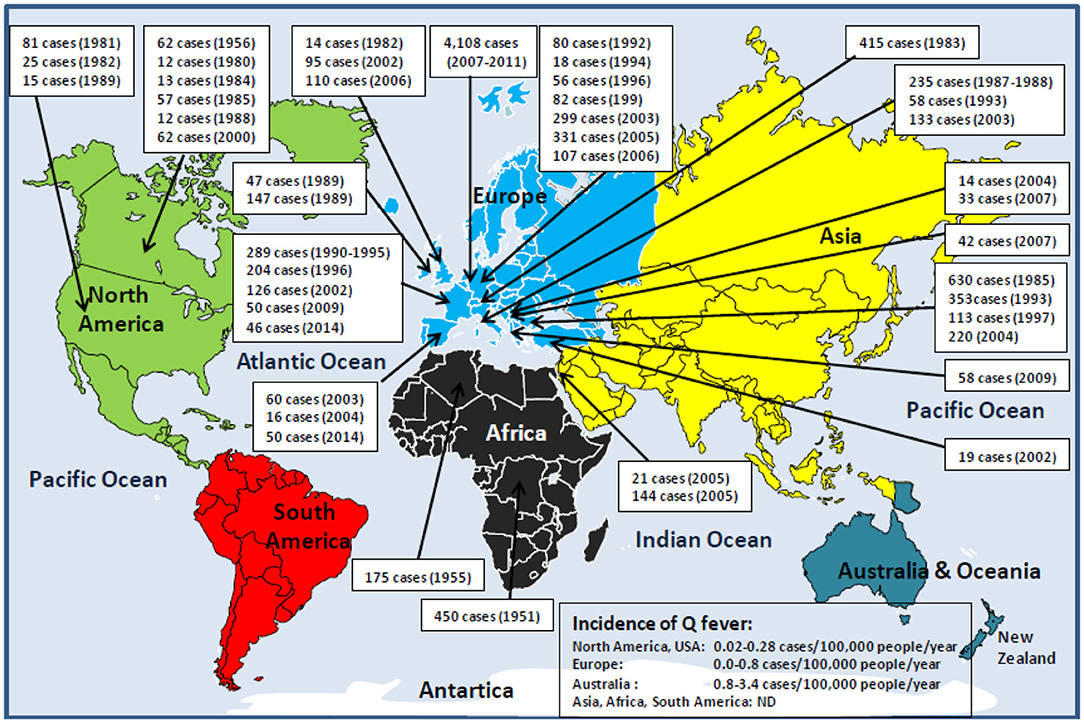
Figure 1. Schematic representation of human Q fever epidemiology around the world. With the exception of Antarctica and New Zealand, Q fever is a global zoonosis present in North America, South America, Europe, Asia, Africa, and Australia/Oceania. The clinical manifestation of Q fever in human is usually an undifferentiated febrile illness. Q fever was described for the first time in humans in 1937 by Burnet, who investigated several cases of Australian abattoir workers suffering from undifferentiated febrile illness (15, 16). During the Second World War (1941–1944), the Q fever disease was reported among German soldiers stationed in the Balkans, Southern Italy, Corsica, in English and American allied troops in Central Italy, and soldiers in Crimea and Ukraine. That is why the disease has had many synonyms: Olympus fever, Crimean fever, flu Balkan flu, Cretan pneumonia, Euboea fever, fever of the 7 days, or Derrick and Burnet's disease (17). The causative agent of the disease first identified by Cox in the United States, and formerly named Rickettsia diasporica, was definitively renamed Coxiella burnetii (18–20). The figure illustrates the history of the major human epidemics of Q fever (outbreaks >10 linked cases) from 1950 (when the Third World Health Assembly passed a resolution calling for study of the prevalence of Q fever throughout the world) to the present day. Although C. burnetii infection has been classified as a notifiable animal disease by the World Organization for Animal Health, OIE (14), the lack of mandatory reporting of human Q fever cases in most countries, the predominance of asymptomatic forms, the clinical polymorphism, and the difficulty of diagnosis are likely to lead to a significant underestimation of the true incidence of the disease in humans. In Europe where the ECDC carries out a regular epidemiological surveillance, only 1,023 of 4,245 Q fever cases confirmed during the 2013–2017 period were reported by the European countries (21). It is impossible to evaluate the number of cases for the Asian and African continents. ND, not determined.
On the North American continent, between 1946 and 1977, a total of 1,169 human Q fever cases were reported in the United States (22–25). Then, from 1978 to 2016, about 200 cases of human Q fever were reported annually, a mean of about 0.25 cases/100,000 inhabitants/year (cases/100 kI/y), with a seropositivity of 3.1% in adult populations rising 22% among the veterinarians (26–29). In Canada, C. burnetii in humans was first reported in 1952 (30, 31). In 1956, an outbreak with 62 human cases was reported in people working in a slaughterhouse, then individual cases in 1960 and 1966, followed by several outbreaks between 1975 and 1989 (32–34). In Central America, Q fever cases were reported in El Salvador and Mexico (35, 36). In the South American continent, during the 2013–2014 outbreak of dengue in Brazil, C. burnetii was identified in 3.3% of patients (37). Q fever cases were also reported in most South American countries (38–41). A very high incidence was also observed in the French Guiana in 2005 (150 cases/100 kI/y) (42).
On the European continent, more than 1,000 human Q fever cases were reported among soldiers in the Balkans in the early 1940s. Large-scale outbreaks were documented over recent decades, and serosurveys suggest a seroprevalence between 2 and 14% of the population (18, 43). The disease is endemic in Germany with 27 to 100 Q fever annual cases (incidence is 0.08–0.14 cases/100 kI/y), and 40 Q fever outbreaks documented (44, 45). In the United Kingdom, from 1975 to 1996, between 67 and 169 Q fever annual cases were reported (incidence of 0.15–0.35 cases/100 kI/y), including eight outbreaks (46–49). In 1983, a large outbreak of 415 human Q fever cases was reported in Switzerland (50). Until 2007, in the Netherlands, 5 to 16 Q fever cases were reported annually (51, 52). In 2007–2010, a large human outbreak with an estimated 44,000 people infected in 3 years was reported, among which were 4,108 cases of Q fever (53–56). In Portugal, the average frequency of Q fever is 0.1 case/100 kI/y, yet it is likely underestimated (57, 58). In the Spanish Canary Islands, a seroprevalence of infection by C. burnetii in humans of 36% was reported during an outbreak of Q fever (59). In France, the seroprevalence for anti–C. burnetii Ig was estimated 5/100 kI/y (60). In Bulgaria, from 1949 to 1993, more than 20 Q fever outbreaks occurred with three major outbreaks between 1982 and 1985, and next in 1993 and 1997 (61–64). In the late 2010s, 139 Q fever cases were reported (incidence of 0.27 cases/100 kI/y) (65). In Slovakia, a seroprevalence of 3% was estimated for the period before 1993 (63). According to OIE, between 1996 and 2001, eight Q fever cases were reported in Hungary, 26 cases in Ukraine, and 138 cases in Yugoslavia. In Russia, an outbreak in Leningrad affected 48 people in 1957 and between 1957 and 1995 up to 11,058 Q fever cases were reported (66–68).
On the Asian continent, 1% of patients hospitalized for infectious endocarditis and 14.6% of patients hospitalized for acute febrile illness/pneumonitis in India were infected by C. burnetii (69, 70). In Iran, 4.2% of patients with febrile illness and 18.1% of butchers and slaughterhouse workers carried anti–C. burnetii Ig (71, 72). In China, Q fever was initially reported in 1950 in a patient with pneumonia, and then in the 1960s, five outbreaks of Q fever occurred in abattoir workers, stockyard men, and troops (73, 74). Between 1989 and 2013, human Q fever cases were reported in people from 15 provinces in China and 4% of patients with infectious endocarditis suffered from Q fever (75, 76). In Japan, serosurveys indicated the presence of anti–C. burnetii Ig in 16.5% of human serum samples collected between 1978 and 1991 (77, 78). Since 1999, 7 to 46 Q fever cases were reported annually (79–82). In the Arabian Peninsula, the presence of C. burnetii in humans was reported in 1968, and a recent serological analysis detected C. burnetii Ig in 35.2% of patients with pyrexia of undetermined cause (83–85). In Qatar, Q fever data are rare, yet a seroprevalence of 2.1% was found in US soldiers deployed in this country (86).
On the Australian/Oceanian continent, since the first description of Q fever in 1937, the disease has continued to be endemic in Australia (87). Between 1977 and 1994, 202 to 860 cases were reported annually (incidence 3.11–4.99 cases/100 kI/y), despite a vaccine is recommended to farmers since 1989 (88–91). New Zealand is considered free from Q fever.
On the African continent, outbreaks of Q fever were reported in the early 1950s, but the disease remained neglected and underestimated (92–95). In Rwanda, an outbreak with 450 Q fever cases and 40 deaths linked to C. burnetii was reported (96). In Western Africa, seroprevalence in human was found to be 5% in rural Western Ivory Coast, 8% among nomads in rural Northern Burkina Faso, and 6–9% of patients hospitalized for pneumonia in Cameroun (97–101). C. burnetii was incriminated in 10% of children with non-malaria febrile illness (NMFI) in Niger, 8% in Gambia, and 17% in Ghana (102–104). Q fever is responsible for 2 to 9% of human hospitalization for NMFI in Middle, Central, and West Africa (105–107). In Eastern Africa, C. burnetii seroprevalence was reported to be 5% in pregnant women (108). A serological testing carried out in Kenya in 2016 indicated that 2.5% of people were seropositive for C. burnetii (109). In South Africa, a recent study reported that 38% of NMFI patients and 61% of workers in contact with camels (farmers, herders, and veterinary) carried anti–C. burnetii Ig (110).
The Mediterranean is bordered by 22 riparian countries (Figure 2) including the following:
• in the North: Spain, France, Monaco principality, Italy, Slovenia, Croatia, Bosnia Herzegovina, Montenegro, Albania, Greece, Turkey, Malta, and Cyprus; and
• in the South: Syria, Lebanon, Israel, Palestine, Egypt, Libya, Tunisia, Algeria, and Morocco.
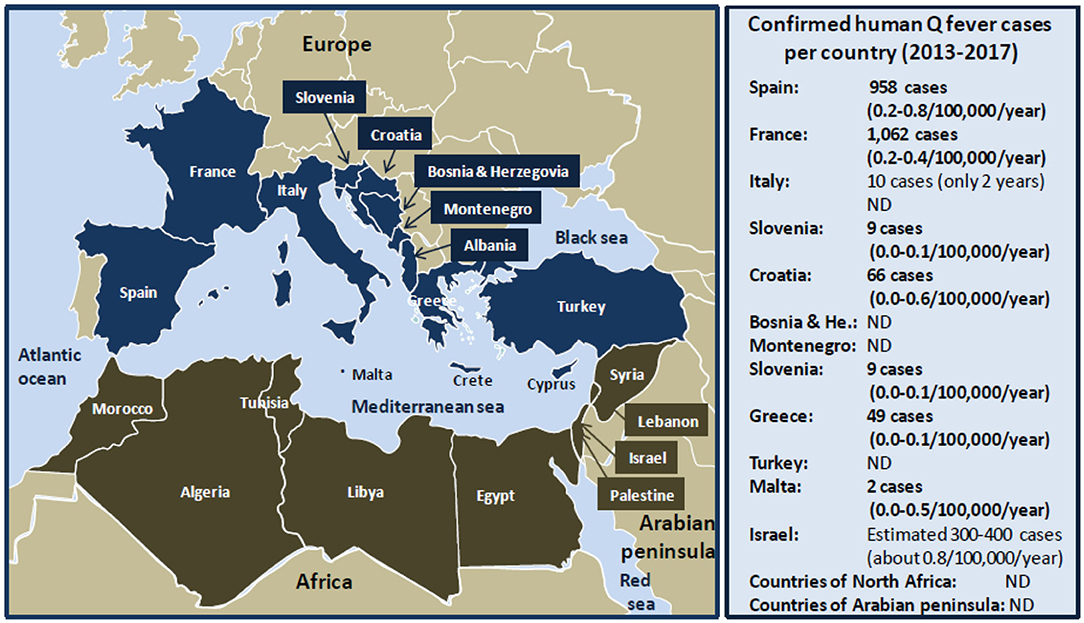
Figure 2. Schematic representation of human Q fever around the Mediterranean. Left panel: map of the Mediterranean basin. The Mediterranean Sea is bordered by 22 riparian countries. The countries of the Northern Mediterranean coast are represented in dark blue, and the countries of the Southern Mediterranean coast are represented in tanned brown. Right panel: confirmed human Q fever cases per country during the period 2013 to 2017 according to the ECDC (21). Q fever surveillance report, 2017. Values between brackets indicate the average number of Q fever cases per 100,000 inhabitants per year over the 5 years period. Regarding Italy, no data were available for the years 2013, 2014, and 2015. The number of cases of Q fever for Israel over the period 2013 to 2017 was extrapolated from the data published by Yarrow and colleagues (111). ND, not determined. Human Q fever occurs mostly in the form of sporadic cases. Sometimes outbreaks of Q fever were reported in humans. The main epidemics of Q fever described during the last 40 years in people living on the Northern coast of the Mediterranean basin are as follows: 2003 (60 cases), 2004 (16 cases), 2014 (50 cases) in Spain; 1992 (40 cases), 1996 (204 cases), 2002 (126 cases), 2009 (50 cases), 2014 (46 cases) in France; 1993 (58 cases), 2003 (133 cases) in Italy; 2007 (33 cases) in Slovenia; 2004 (14 cases) in Croatia; 2007 (42 cases) in Albania; 2009 (58 cases) in Greece; and 2002 (19 cases) in Turkey. Human Q fever outbreaks are poorly documented concerning the countries of the Southern Mediterranean Sea coast. An epidemics of Q fever was described in 1955 in Algeria with 175 cases.
On the Northern Mediterranean coast, Q fever is endemic in countries of the South Europe (Spain, France, Italia). From 1981 to 1998, more than 600 cases of Q fever were reported in Spain, most of which sporadic, except three outbreaks in 1989 (5 cases), 1990 (30 cases), and 1998 (14 cases) (112–116). Between 2000 and 2009, hundreds of Q fever cases were reported, most of which sporadic with an epidemic episode in the Asturias with 60 cases in 2003, and two outbreaks (16 and 22 cases, respectively) in Madrid (117–119). During the 2011–2015 period, 50 human Q fever cases were reported in Vizcaya and among 155 subjects with febrile illness from Galicia, 25% (39/155) were diagnosed with Q fever, and 6 patients died (120, 121). In France, Q fever was first observed in 1948 among slaughterhouse workers in Strasbourg. Between 1949 and 1953, cases were reported in Paris, in the region of Lyon and Northwestern (122). An intrafamily Q fever outbreak was induced by infected pigeons (18). The seroprevalence in humans can go up to 30% in the Alps rural populations (123). In the South of France, 5 to 8% of cases of endocarditis are due to C. burnetii, and a retrospective analysis performed on 22,496 sera showed a seroprevalence of 7.8% (1,754/22,496) with 323 acute Q fever (124, 125). Between 1990 and 1996, three outbreaks (including 289 Q fever cases in Martigues and 204 cases in Briançon) were linked to meet with infected sheep or goat, animal carcasses, and/or consumption of unpasteurized milk (126, 127). In 2002, an outbreak of 126 human Q fever cases possibly contaminated by ovine livestock occurred in Chamonix (128). In 2009, an outbreak of 50 human cases of Q fever was reported in Cholet (129), and in 2014, an outbreak of 46 cases of Q fever occurred after people had visited a sheep farm (130). In Italy, Q fever emerged in the late 1940s with epidemic outbreaks, and then it became endemic with sporadic occurrence (131). However, an epidemic outbreak was reported in 1996 in the Vicenza region with 58 human cases after contact with infected sheep (132). In 2003, an outbreak of 133 human Q fever was reported in Como, the prison being mainly concerned with a prevalence of disease of 10.8% (59/547) in prisoners, 16.5% (37/224) in guards, and 3.2% (33/1,025) in the city residents (133).
In the countries of the North coast of the Adriatic sea (Slovenia, Croatia, Bosnia Herzegovina, Montenegro, Albania), several reports indicated the presence of the pathogen. A group of 33 veterinarians contracted Q fever during a training course in Slovenia in 2007 (134). In Croatia between 1985 and 2002, 155 acute Q fever cases were hospitalized in Split, and the annual mean incidence was 0.20–4,64 cases/100 kI/y (135, 136). In 2004, an outbreak of 14 Q fever cases occurred in Zadar linked to contacts with infected sheep (137). During the 2008–2010 period in Croatia, a C. burnetii seroprevalence study indicated that 27.5% (152/552) of febrile patients with prolonged cough showed anti–C. burnetii Ig, and 5.8% developed acute Q fever (138). In the 2000s, a Q fever outbreak was reported in Albania in a group of 115 Argentinean police officers who were exposed to contaminated dust from infected sheep during a United Nations mission in Prizren in the South Kosovo, among whom 42 showed clinical symptoms of Q fever (139).
Q fever occurred in Greece in 1946 possibly due to consumption of milk from infected ovines (140). A serosurvey performed in Northern Greece in 1990 showed that 4.7% (173/3,686) of patients with atypical pneumonia had anti–C. burnetii Ig. During a 2-year survey on children hospitalized in Athens, acute Q fever was diagnosed in 0.67% (8/1,200) of patients, and Q fever accounted for 2.9% of the cases with prolonged fever (141). In 2009, 58 cases of Q fever were reported in Northern Greece (142). The mean rate of Q fever during 2004 to 2012 was 0.033 cases/100 kI/y. C. burnetii is endemic in the island of Crete. A high seroprevalence (38.7%) of anti–C. burnetii Ig was found in humans living in Crete, and 98 cases of Q fever were reported between 1989 and 1993 (143). In addition, a study over a period of 6 years (1989–1995) confirmed that 4.6% (152/3,300) of patients suspected of infection had anti–C. burnetii Ig (144). More recently, another serosurvey found a seroprevalence of 48.7% (240/493) (145). C. burnetii is also present in the islands of Malta and Cyprus. In Cyprus, a serosurvey study that investigated serum samples from 547 people found that 5.3% contained anti–C. burnetii Ig, whereas a more recent study using a similar number of samples indicated a seroprevalence of 52.7% (146, 147).
Q fever is considered endemic in Turkey. A total of 191 human Q fever cases were documented before 1953, most of them being sporadic (148, 149). In 2002, 46 cases of febrile illness were reported around the Black Sea in Northern Turkey, 19 with confirmed acute Q fever (150). The search for anti–C. burnetii Ig in 83 veterinarians indicated that 7–8% of them had been exposed to C. burnetii. A serosurvey on blood donors in Ankara showed that anti–C. burnetii IgG was detected in 32.3% (194/601) (151). In 2009, an investigation of C. burnetii prevalence in a group of 407 healthy subjects living in North Turkey indicated that 8.1% (33/407) of them showed evidence of contact with C. burnetii and 5.4% (22/407) were symptomatic with 17 acute Q fever and 5 persistent forms (152). Recently, the case of a young woman with Q fever endocarditis was reported (153). A recent seroprevalence study performed in the Erzincan province in the Eastern Turkey showed the presence of anti–C. burnetii Ig in 8.7% (32/368) of people (154).
In the Maghreb countries (Morocco, Algeria, Tunisia, Libya), C. burnetii was found in the early 1950s (94). In Morocco, a seroprevalence study conducted in 1995 reported that anti–C. burnetii Ig was present in 1% (1/300) of sera samples from Casablanca and 18.3% (23/126) of samples from Fez citizens (155). In Algeria, the first detection of human Q fever dates back to 1948 with 172 cases (156, 157). In 1955, an outbreak of Q fever concerned 175 infected soldiers from a French battalion who was quartered in stables recently occupied by horses and sheep (158). In 1960, several Q fever cases were reported in Eastern Algeria (17). A study performed on children younger than 16 years in Hoggar indicated a seroprevalence of anti–C. burnetii Ig of 20% (159). The follow-up of a human cohort of infective endocarditis in Algiers in 2000–2003 found a C. burnetii seroprevalence of 3% (2/61 patients) (160). A C. burnetii seroprevalence of 15.5% (113/729) was reported in humans in the Wilaya of Setif, an agropastoral region (161). In recent years, a limited number of human cases of Q fever were reported in Algeria, and most cases occurred in the Northern part of the country (160, 162). In Tunisia, a study of samples from a cohort of blood donors collected in 1993 in Sousse and its rural surrounding areas reported that 26% (130//500) of subjects had antibodies against C. burnetii (163). Yet most of serosurveys performed in Tunisia between 1990 and 2008 suggest a seroprevalence of C. burnetii between 1 and 3% (164–167). Information is missing regarding the human prevalence of C. burnetii infections in Libya. The serological study of foreigners (Czechoslovak citizens) returning to their country after they had worked in Libya between 1984 and 1988 showed an anti–C. burnetii Ig in 48 people, and about half of them had clinical symptoms of Q fever (168).
In the Mashreq (Egypt, Jordan, Palestine, Lebanon, Syria), a seroprevalence for anti–C. burnetii Ig ranging from 3 to 32%, was reported. An early study reported anti–C. burnetii Ig in 14.3% (11/77) of sera samples from Egypt (169). A C. burnetii seroprevalence of 32% (285/883) was reported in humans living near the Nile River Delta (170). In North Sinai in 2006, anti–C. burnetii Ig was found in 5.3% (8/150) of patients with pyrexia of unknown origin and 3.3% (1/30) of healthy controls (171). Another study found a seroprevalence of 16.3% (15/92) in humans who lived in agricultural districts (172). A more recent (2016–2017) study in El Minya Governorate reported a seroprevalence of anti–C. burnetii IgG of 25.7% (9/35) in farmers (173). Besides a case of Q fever in a Belgian patient who developed the disease after a journey in Syria was reported (174), there is no information available regarding human seroprevalence of anti–C. burnetii Ig in the Palestinian, Lebanese, Jordanian, and Syrian populations.
Q fever is endemic in Israel. Between 1981 and 1990, 758 cases of Q fever were reported. A more recent series of 34 cases of endocarditis allowed estimating the annual incidence of Q fever at 3.5 cases per year, or 0.075 cases/100 kI/y, likely linked to infected ruminants exposure (18, 175). In 2005, two outbreaks of Q fever (21 cases in Haifa and 144 cases in a school in central Israel) were reported (176, 177). A recent retrospective study reported 16 pediatric cases of Q fever (178). Another study investigated a cohort of patients admitted to Tel Aviv, Haifa, Hadera, and Kfar Saba hospitals between 2006 and 2016 and confirmed 38 cases of Q fever on 205 patients (179).
Domestic ruminants are considered the principal reservoirs for Coxiella burnetii and are frequently incriminated as sources of Q fever outbreaks in humans who become infected following inhalation of aerosols containing particles loaded with the bacteria or bacteria that survive in a spore-like state (95, 180, 181). C. burnetii was sometimes found in other domestic animals such as poultry, cats, dogs, rabbits, and pigeons (182–186). Different C. burnetii genotypes circulate in wildlife including clones that are more likely to cross species barrier for infection of livestock and humans (187–190).
Former epidemiological studies performed on cattle showed that when imported into an area of endemic infection, 40% of uninfected cows became C. burnetii infected within 6 months (191). Although the animals can develop metritis and mastitis, in cattle farms the disease usually evolves subclinically (79). The different clinical manifestations of the disease can lead to late gestation abortions, fertility disorders, and premature delivery (192, 193). Up to 109 bacteria per gram can be contained in the placenta from infected ruminants (194–196). C. burnetii shedding is higher in vaginal mucus and feces than milk in the first 3 weeks postabortion or postpartum (197).
On the Australian/Oceanian continent, Q fever is the most commonly reported notifiable zoonotic disease in Australia after food-borne pathogens (198). Australia became the first country to use ruminants' vaccination. In New Zealand, in 1993, a large study conducted on 2,181 cattle and 12,556 sheep concluded that the country was free from coxiellosis. On the North American continent, a serosurvey performed in 1964 revealed that Quebec had the highest seroprevalence of anti–C. burnetii Ig in bovine (39.6%) (199). Sheep and goat occupy only a minor segment of farm activities in Canada, and their seroprevalence was 6.7 and 10.5%, respectively (19). Decades ago in the United States, a seroprevalence of C. burnetii Ig study in farm animals showed the highest seroprevalence among goats (41.6%), followed by sheep (16.5%) and cattle (3.4%) (200). In Asia, a seroprevalence study in Iran indicated that 13.6% (45/330) of sheep had anti–C. burnetii Ig (201). The overall prevalence of anti–C. burnetii Ig in China was 15% (288/1,918) in cattle and 12% (176/1,440) in goats (75). A recent study of seroprevalence in goats from the Hubei province of China reported that 4.75% (55/1,157) of animal had anti–C. burnetii Ig (202). In Europe, the main sources of human infection by C. burnetii were ovine products (203, 204). In 1983, a large outbreak of human Q fever was reported in Switzerland after sheep transhumance, with 38% of the animals being positive for anti–C. burnetii Ig (50). Most of the human epidemics reported in Germany were related to handling of infected sheep products (24 outbreaks), to contact with cattle (6 epidemics) or livestock (4 epidemics), or to work in slaughterhouses (2 epidemics). In 2003, 299 people were infected when a sheep gave birth at a livestock market in Soest (205). The large Q fever epidemics reported in 2007–2010 in the Netherlands were probably associated with the increase in goat farming (e.g., 5,000 in 1985 and up to 375,000 in 2009) (206–208), and a very high number of infected females as suggested by the frequency (20%) of abortions (209). The retrospective investigation of the origin of this C. burnetii outbreak in humans revealed that C. burnetii was also found in dogs and horses, as well as in wild deer (210, 211). In Portugal, the frequency of exposure of ovine herds at C. burnetii seems to be increasing with possible impact on humans (212). On the African continent, a C. burnetii surveys of ovines indicated seroprevalences of 13% in Chad, 24% in Sudan, and 29% in Niger (105, 213, 214).
In the countries from the Northern coast of the Mediterranean basin, cattle, goats, and sheep are considered the major reservoir of C. burnetii related to human infections. Serological studies performed on livestock in Madrid indicated that up to 76.6% of goats and 8.8% of cattle had anti–C. burnetii Ig (215). The investigations in livestock revealed that in Northern Spain, 3% of ovine carried C. burnetii (216). Other investigations reported the highest C. burnetii seroprevalence for sheep (31.5%), followed by goat (22.4%) and cattle (5.6%), respectively (217), and 7.7% (80/1,039) of ticks (mainly Hyalomma rufipes) (218). Surveys carried out on 5,081 cattle abortion cases from four rural regions in France between 1993 and 1996 confirmed C. burnetii infection in 0.5% to 3.8% of cases, while suspected for an additional 2 to 16% of cases (219). Serosurvey of C. burnetii in ruminant in Sicily (Southern Italy) also showed a very high seroprevalence of 73.6% in farm sheep (220). A serosurvey in Slovenia indicated that 46% of cattle, 36% (36/100) of sheep, and 2.4% (17/701) of ticks (mainly Ixodes ricinus) were exposed to C. burnetii, and ticks found positive by polymerase chain reaction (PCR) were most commonly (5.09%) sampled from wild deer (221). A recent serosurvey performed on 1,970 serum samples collected from farm cattle in three regions of Bosnia and Herzegovina indicated that 8.8% of animals were exposed to C. burnetii (222). In Turkey, the prevalence of C. burnetii exposed animals varies widely with species and geographic location (223). In Cyprus, a serosurvey indicated that many farm animals had been in contact with C. burnetii including 48.2% of goats, 24% of bovines, and 18.9% of sheep, with an overall abortion rate in the livestock population of Cyprus at 2 to 5% (147, 224). Among a total of 622 cow abortions in Cyprus in 2008–2009, C. burnetii infection was documented in 57% (29/51) of the tested samples (225). In 2013, in Malta, a 6-month ban was imposed on the transfer of cattle between farms because of an outbreak of C. burnetii infection in nine goats in one farm and two human cases. Altogether, these data (Table 1) strengthen the hypothesis that human Q fever epidemics in the countries of the Northern coast of the Mediterranean basin found their origin in sheep and/or goats mainly.
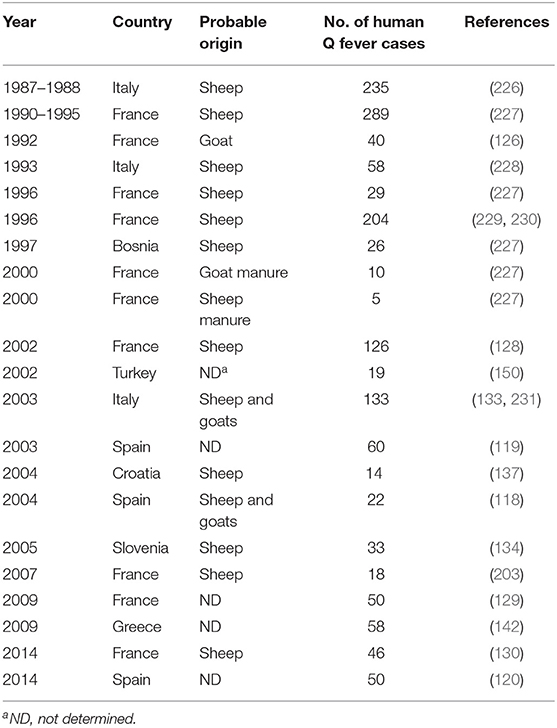
Table 1. History of the main human Q fever epidemics in countries of the Northern coast of the Mediterranean Sea and identification of the zoonotic source of C. burnetii.
In the countries from the Southern coast of the Mediterranean basin, the earliest investigations of C. burnetii in the ecosystem of Morocco indicated the presence of the bacteria in sheep, goat, cattle, camels, gerbil, and ticks (94). A recent serosurvey of cattle in the North-East state of Setif indicated a seroprevalence of 11.36% (77/678) in cows (232). A study indicated that ticks collected on camels (Hyalomma dromedarii) and bulls (Hyalomma excavatum) imported in Egypt from Sudan were infected with C. burnetii (233). Other studies found the presence of C. burnetii in livestock with a seroprevalence of 22.5 to 32.7% in sheep, 16.8 to 28.2% in goat, and 13 to 13.2% in cattle, respectively (171–173, 234). A large survey that included livestock from Western desert, Nile River Valley, and Delta region reported anti–C. burnetii Ig in 19.3% (162/840) of cattle, 8.9% (64/716) of sheep, and 6.8% (21/311) of goats (235). The C. burnetii surveys of cattle indicated seroprevalences of 16 and 10% to 29% in Tunisia and Algeria, respectively (236–239).
Regarding the different human epidemics of Q fever in the countries of the Southern coast of the Mediterranean basin, similar to what has been demonstrated for the countries of the North Mediterranean coast, it was assumed that the source of bacteria came from cows, sheep, and/or goats (124), although it may be partly wrong because of the presence of animal species endemic to these countries.
A listing of camel diseases considered a major threat, which ignored Q fever as “major threat” (although it appears as “notifiable disease”), was drawn by OIE in 2008 and updated in 2010 (240). Compared to other domestic species present on both sides of the Mediterranean, little is known about the pathogens that circulate in camel herds (241–244), probably due to a lack of international concern for camels (the earliest serosurveys were performed by biologists from Northern countries where camel is absent) (Figure 3). In the last decade, several camel diseases with overmortalities that occurred in African countries as well as Saudi Arabia attracted epidemiologists' curiosity. Today, OIE draws particular attention to camelpox and rabies viruses, to parasite-induced trypanosomosis, and to a few bacterial diseases including brucellosis, tuberculosis, paratuberculosis, pasteurellosis, anthrax, and caseous lymphadenitis.
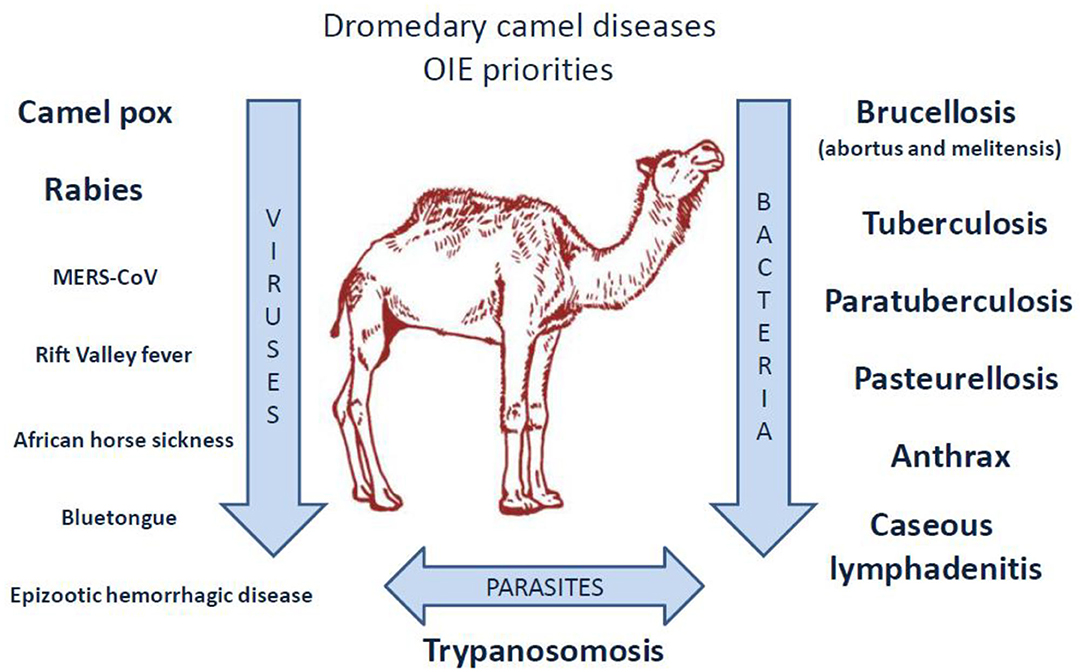
Figure 3. Priority diseases of camelids according to OIE (240). A list of diseases affecting camels and that appear to be priorities for the OIE to improve diagnostic capacity and establish guidelines for trade of camels and camel products was drawn up in 2014 by the OIE ad hoc Group on camel diseases. These experts divided the priority diseases into three groups: (1) significant diseases; (2) diseases for which camelids are potential pathogen carriers; (3) minor or non-significant diseases. Regarding the priority viral diseases (significant diseases), only camelpox and rabies were listed [foot and mouth disease (FMD) that concerns bactrian camels only, also belonged to the list]. The ad hoc Group classified MERS-CoV, Rift valley fever, and orbivirus-induced diseases (BT, AHS, EHD) among the diseases for which camelids are potential pathogen carriers (the bovine viral diarrhea that concerns the New World camelids also belonged to that list). Regarding bacteria, the ad hoc Group classified brucellosis, tuberculosis, paratuberculosis, anthrax, caseous lymphadenitis, and pasteurellosis in the significant diseases category. Trypanosomosis was classified in the significant parasitic diseases of camelids. It should be noted that coxiellosis is not mentioned in the lists of priority camel diseases for the World Organization for Animal Health, OIE. However, C. burnetii has been classified as a notifiable animal disease by this international office (14).
Among the viral diseases affecting camels, camelpox is an economically important disease, notifiable to OIE (245–249). Camelpox is contagious in camel husbandry, and its mortality ranges from 0 to 40% (250, 251). This virus is a risk to the human population (252, 253), yet the disease can be prevented by vaccine and/or antiviral drugs such as cidofovir and ribavirin (254, 255). Rabies in camels is also observed in many countries from Africa, Arabian Peninsula, and Asia (256–262). Infection of camels was found preventable by canine inactivated rabies vaccine (263). Several other viruses able to infect camels are of concern for OIE. These viruses are the Rift valley fever (RFV), the Middle East respiratory syndrome coronavirus (MERS-CoV), the foot and mouth disease virus, the bluetongue virus, the epizootic hemorrhagic disease virus), the African horse sickness virus, and the Alkhurma hemorrhagic fever virus (AHFV) (264–297). In humans, the MERS-CoV and AHFV infections are known to be of high fatality rate (272–275, 298). Camel can also be infected by a number of other viruses (299–302).
Specific attention was drawn by OIE to Trypanosoma parasites (Trypanosoma evansi, Trypanosoma vivax), which can be the cause of abortion in camel herds (303–310). Other parasites and fungi also circulate in camel herds, including Aspergillus fumigatus considered responsible for the death of 40 racing camels in United Arab Emirates (UAE) during an outbreak of bronchopneumonia and gastroenteritis (311–313).
Because of the economic impact of brucellosis in ruminant herds (with losses on meat and milk sales due to abortion), special attention was focused on this disease in camels (314–321). In Saudi Arabia, whole herd vaccination using S19 or Rev1 vaccinal strains was reported to be successful for camel protection (322, 323). Finally, there is a public health concern linked to the risk of transmission to humans (324–327). Dromedary camel infection by Mycobacterium tuberculosis or Mycobacterium bovis was reported in several countries (328–341). Another mycobacterium, Mycobacterium avium subsp. paratuberculosis, is the causative agent of Johne disease that affects camels more severely than other ruminants (342–347). There is also concern by OIE for lung pseudotuberculosis abscesses, a frequent disease of camels, as well as pasteurellosis, anthrax, and plague (348–350). Cases of camel plague/Yersinia pestis were reported in Libya (351, 352), and human cases were described after consumption of meat from infected camels (353, 354). Obviously, camels are susceptible to a wide range of bacterial-induced diseases including mastitis (242, 355, 356), upper respiratory tract diseases (357–360), skin necrosis (361, 362), botulism (363), tetanus (364, 365), and diarrhea (299, 366–368).
Dromedary camels that are almost absent from the Northern countries of Mediterranean basin account for 3% of the domestic ruminant populations in the Southern countries of the Mediterranean basin (Table 2). Although this percentage is relatively low, it became necessary to revisit the epidemiological data and question the possible role of camels as a source of human Q fever. Sixty-five years ago, the presence of C. burnetii in camels was already reported (94). Regarding animal serosurvey, it is hazardous to directly compare the data obtained from one country to another by different laboratories under the format of a meta-analysis because of size of tested population, sample selection bias, and different technical methods of diagnosis. Yet, it remains intriguing that in most studies that included dromedary camels in the panels of ruminants tested for C. burnetii exposure or infection, the highest seroprevalence corresponded to dromedary camels ahead from the other ruminants (Table 3).
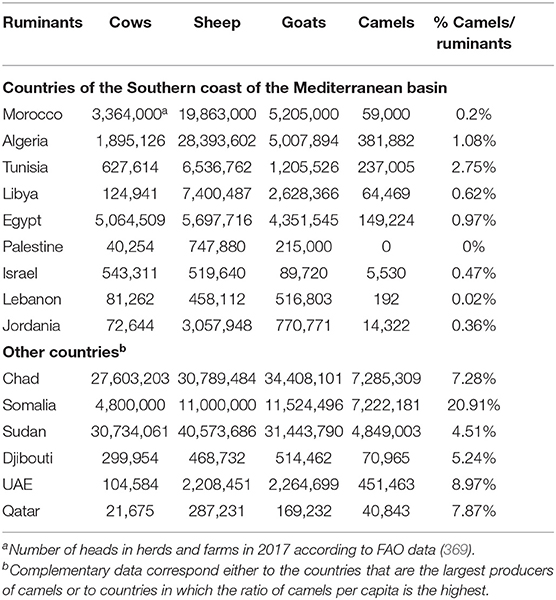
Table 2. National productions of farm ruminants and percentage of dromedary camels with respect to the total number of other domestic ruminants (cows, sheep, and goats).
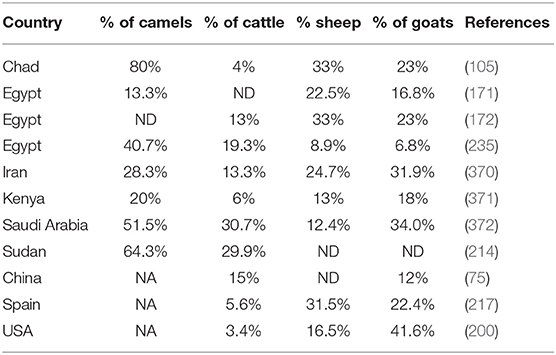
Table 3. The seroprevalence of Coxiella burnetii in Camelus dromedarius camels compared to other ruminants.
An investigation in Egypt that tested 200 camels for C. burnetii reported a seroprevalence of 66% (373). Another serosurvey two decades later reported anti–C. burnetii Ig in 40.7% of dromedary camels (mainly imported from Sudan), followed by cattle (19.3%), sheep (8.9%), and goat (6.8%) (235). In the study by Klemmer et al. the seroprevalence in camels from Aswan governorate bordering Sudan was 67.5%. This corroborates a study in Sudan that reported a seroprevalence of 64.3% (49/76) in camels and 29.9% in cattle (214). A recent study in Egypt reported that 4.5% (5/112) of camel sera were positive for anti–C. burnetii Ig, whereas a standard quantitative PCR found an overall prevalence of 15 to 19% (374). The only study that reported a higher seroprevalence in ovines than camels was performed in North Sinai, with the higher seroprevalence in sheep (22.5%), followed by goat (16.8%) and camels (13.3%), respectively (171). A serosurvey in Chad, highlighted that seroprevalence was the highest in dromedary camels (80%), followed by sheep (33%), goats (23%), and cattle (4%) (105). In Iran, on 167 camels that originated from 11 regions, a mean seroprevalence of 28.7% for C. burnetii (seropositivity ranging from 0 to 63.6%) was observed (375). A more recent study confirmed a seroprevalence of Q fever in camels of 28.3% in Iran, whereas for the other ruminants, the results were 31.9% in goats, 24.7% in sheep, and 13.3% in cattle (370). Studies in Saudi Arabia reported a seroprevalence around 50 to 60% of dromedary camels, with the most recent investigation reporting a seroprevalence of 51.5% in 489 camels from Saudi Arabia, whereas the seroprevalence was 34.0% in goats, 30.7% in cattle, and 12.4% in sheep, respectively (372, 376, 377). A serosurvey performed in Algeria revealed that 71.2% of dromedary camels had circulating C. burnetii Ig (378). A recent study conducted in Kenya confirmed that the highest seroprevalence was in dromedary camels (20%), followed by goats (18%), sheep (13%), and cattle (6%) (379). These results corroborate those from another study that reported a seroprevalence of 18.6% in camels (371).
Many questions remain unanswered regarding the origins of the high prevalence of anti–C. burnetii Ig in dromedary camels (Table 4), the ways by which camels become infected, and their role as putative reservoir in transmission of C. burnetii to other ruminants and/or humans. It was reported that the preferred route of C. burnetii shedding by infected camels is feces (27.6% positive samples by PCR), followed by urine (23.8%) and milk (6.5%) (396) (Figure 4). A study on 534 healthy camels in Tunisia indicated that 44% (235/534) were seropositive to C. burnetii, and it reached 70% in female camels with a previous history of abortion (391). It is also possible that the high prevalence of anti–C. burnetii Ig in camels was related to infections by fleas or ticks during blood-sucking (397, 398). Among ticks, the H. dromedarii that colonize dromedary camels were found infected with C. burnetii (1, 233, 399). At every developmental stage of their life cycle, the H. dromedarii ticks feed only once, and their camel blood meal is sufficient for the molt to occur to the next stage (400). Female ticks deposit 10,000 to 20,000 eggs on the camel host body. Recently, a survey performed on dromedary camels and H. dromedarii ticks in Egypt found that 46% (52/113) of camels (27.1% of dromedary camels in Giza and 67.9% in Cairo) and 5.6% (10/177) of H. dromedarii ticks were positive for C. burnetii (383). In contrast, in the hot and dry regions of Southern Europe, other ticks such as Dermacentor marginatus were considered a possible vector of C. burnetii among ruminants (171, 401–403).
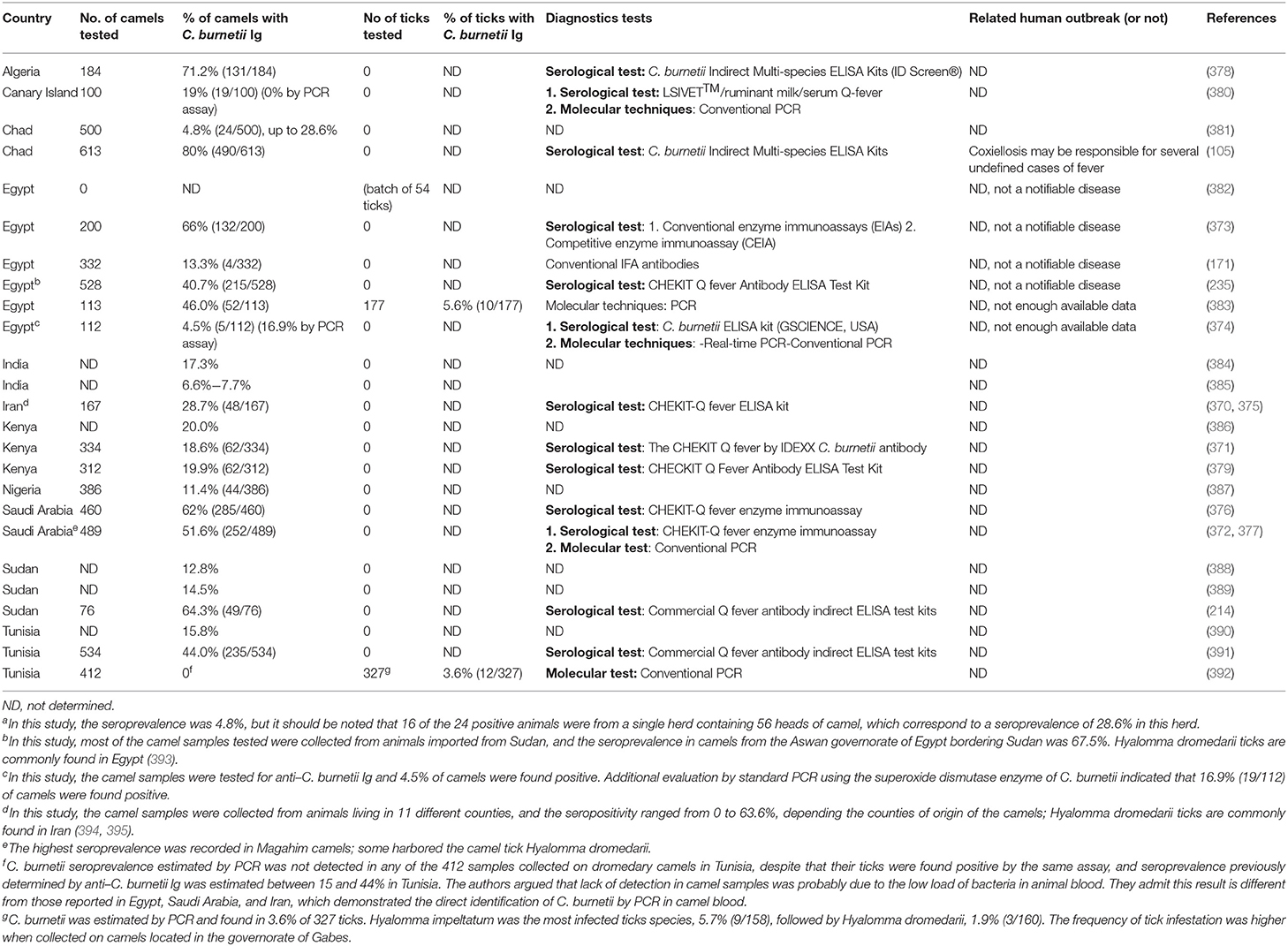
Table 4. The seroprevalence of Coxiella burnetii in Camelus dromedarius camels and Hyalomma dromedarii ticks.
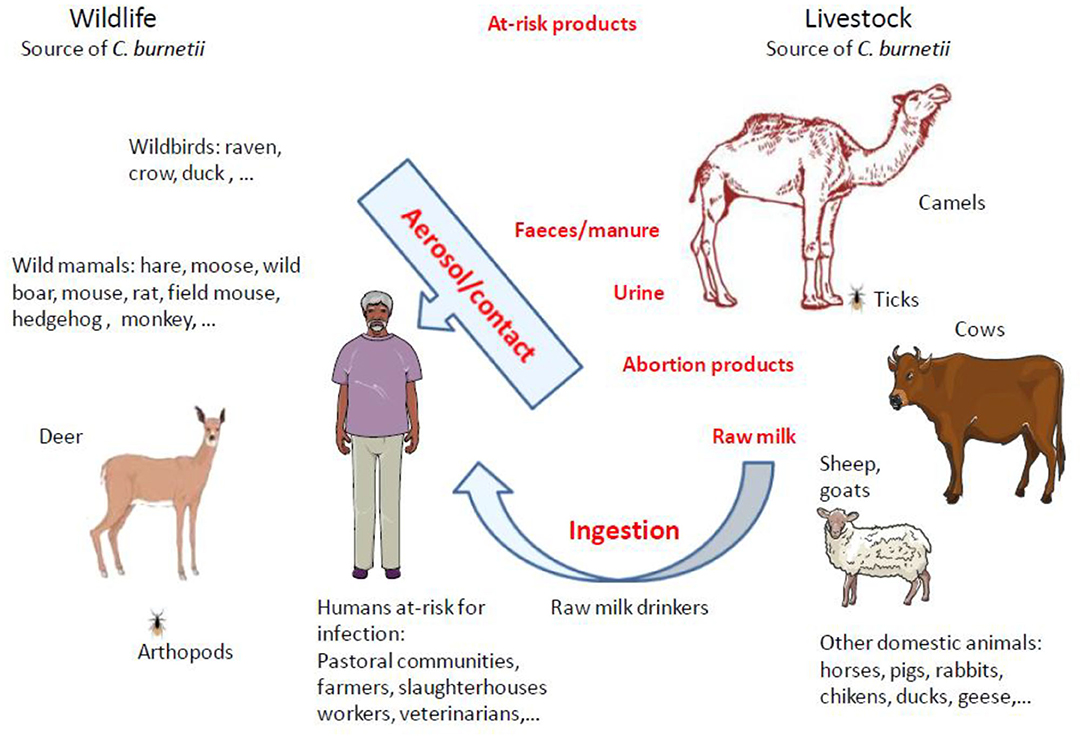
Figure 4. Schematic representation of animal reservoirs diversity and main routes of animal-to-human transmission of Q fever. Up to now, domestic ruminants were considered the principal reservoirs for C. burnetii and were frequently incriminated as sources of Q fever outbreaks in humans. However, in North Africa and Middle East were camels live, it was reported that most of the time the seroprevalence of anti–C. burnetii Ig was much higher in dromedary camels than in other ruminants. C. burnetii shedding is higher in vaginal mucus and feces than in milk. The infection in humans by Coxiella burnetii is mainly by inhalation of aerosols. It was evidenced that C. burnetii can be transmitted during arthropod blood-sucking. Among ticks, the Hyalomma dromedarii that colonize dromedari camels was found infected with C. burnetii. Human populations at risk of C. burnetii infection are pastoral communities, farmers, slaughterhouses workers, tanneries workers, veterinarians, or individuals handling infected livestock, especially animals giving birth. Raw milk drinkers are also at risk. With the increasing demand of milk and camel meat in urban areas, there is a potential threat for millions of people.
It could also be interesting to investigate the role of the camel hump adipocytes in the long-term storage of C. burnetii. In a murine model, it was demonstrated that once C. burnetii has gained the host bloodstream, during the first week of infection it penetrates different organs, and bacteria can be found in spleen, liver, epididymis, prostate, and semen. At 3 weeks, degenerative changes in capillary blood vessels and the surrounding tissues of the adipose envelope of the epididymis are concomitant to the circulation of infected macrophages, and bacteria shed to semen can be transmitted from male to female by sexual intercourse (404). At 4 months postinfection, C. burnetii was detected in abdominal, inguinal, and dorsal adipose tissues, whereas no bacteria were detected in blood, liver, lung, and spleen, and the transfer of adipose tissue from convalescent mice to naive immunodeficient mice resulted in the infection of the recipient host (405). Altogether these results acquired in other models than camels indicate that adipose tissues may be the reservoir in which C. burnetii persists for prolonged periods after the end of clinical symptoms. Although infection by C. burnetii of camel hump adipocytes has not been evaluated so far, the elevated concentration of adipocytes in camel hump could provide C. burnetii with an ideal long-term storage site unique among the ruminants (406). Moreover, when food is scarce, C. burnetii could be released from hump adipocytes during lipolysis. During dehydration and underfeeding periods, camels mobilize their hump adipose tissue accumulated during overfeeding periods to compensate for the deficit (406). In the pastoral communities, the close physical contacts with dromedary camels create the conditions for the transfer to the man zoonotic diseases. A meta-analysis that searched in nine databases, the 929 unique articles regarding C. burnetii epidemiology in Africa concluded that close contact with camels was associated with increased seroprevalence in humans (95).
The large camelids include two domestic species: Camelus bactrianus (the two-humped camel) and Camelus dromedarius (the single-humped camel) (Figure 5). Regarding the bactrian camel, a strain adapted to cold winters that inhabit mainly the mountains of central Asia, historians reported that the camel production was already recommended in the pre-Islamic sacred religious books (412). The dromedary camel, C. dromedarius, nicknamed “desert vessel,” was domesticated in the Arabian Peninsula around the 1st millennium and the second century BC (413–418). It is usually considered that the dromedary camel domestication appears late compared to other ruminants because it took place about 8,000 years after that of sheep and 6,000 years after that of cattle (419, 420). The use of the dromedary camel gradually developed with caravan trade of spices in the Arabian Peninsula and Mediterranean cities markets.
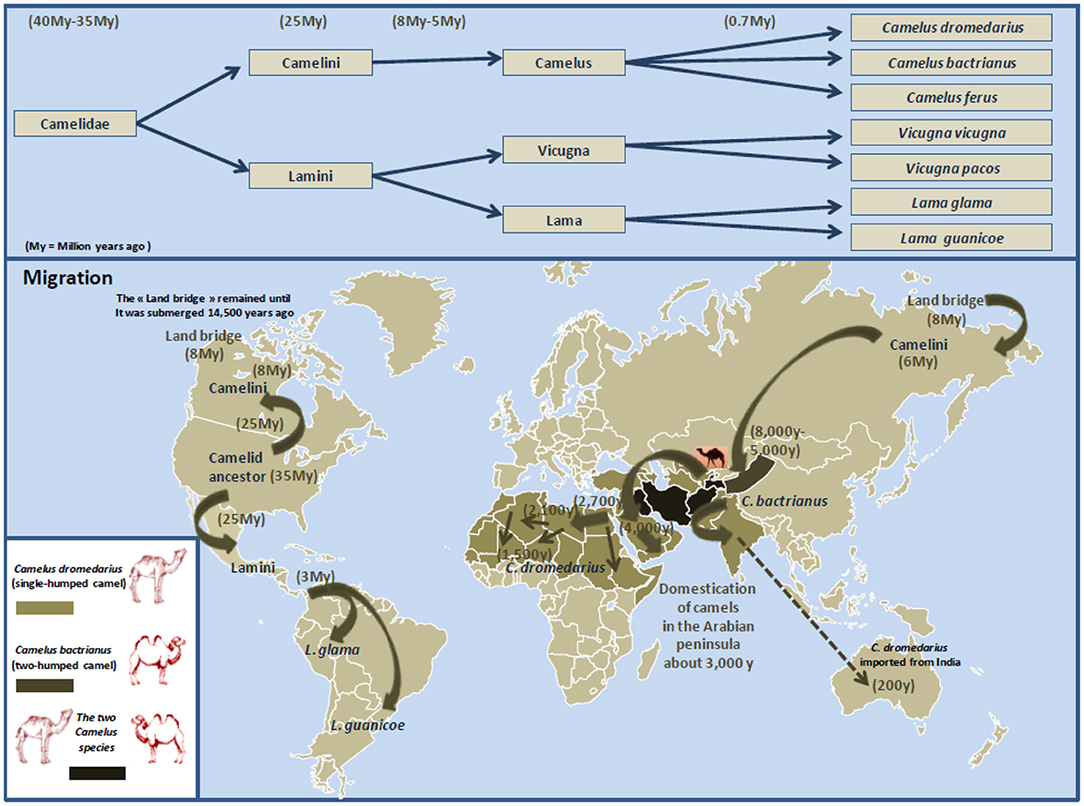
Figure 5. Schematic representation of Camelidae evolution, migration, and domestication. The earliest known Camelidae, named Protylopus and Poebrotherium, appeared roughly 40 million years ago in the North American. During the transition from the Eocene to the Oligocene geological period (about 34 million years ago), the climate in North America is expected to have changed for cooler and drier, and Camelidae began to genetically diverge (407). This was supported by the discovery of the fossil of Paracamelus in Canada in 1913 where this ancestor of Camelus bactrianus and Camelus dromedarius was expected to have inhabited there about 3.5 million years ago when a warmer climate allowed forests to spread near the Arctic Circle (408). About 6 to 8 million years ago, Camelini gradually moved across the land that connected North America to Asia (this land bridge appeared some 8 million years ago, and it remained practicable until it was submerged about 14,500 years ago). According to Wu et al. (409), during the species evolution, they diverged into (i) large Camelidae (Camelini), which lived in North America and next moved westward across the land that connected North America with Asia, then Middle East and North Africa; and (ii) small Camelidae (Lamini), which dispersed South (currently South America). Subsequently about 5 to 8 million years ago, Camelini further evolved into Camelus, which include two species: Camelus bactrianus (the two-humped camel; weight: 600–1,000 kg; size 1.6–1.8 m) and Camelus dromedarius (the single-humped camel; weight: 400–600 kg; size 1.6–2.0 m). Lamini subdivided into two genera: Lama and Vicugna. The earliest evidence for the dromedary domestication is dated about 3,000 years ago near Abu Dhabi on the Arabian Gulf. Northern Arabian tribes began to use dromedary camels as riding animals (410). Dromedary camels were progressively domesticated in North Africa. Gift of camels was a source of camel spread around the Mediterranean. Currently, there are 33 million of domestic Camelus dromedarius living in semiarid and arid regions of Africa and the Middle East, 3 million of domestic Camelus bactrianus that live from the cold steppes of Central Asia to the border of Manchuria in China, and a small population (1,000 camels) of Camelus ferus, the Wild Bactrian, which survives in the Northwest China and the Gobi Desert of Mongolia (Camelus ferus diverged from Camelus bactrianus about 0.7 million years ago) (411). Dromedary camels from India were also introduced in central Australia. Females are only able to conceive from 3 years old and can live up to 30 to 40 years old.
Dromedary camel domestication was crucial for livelihood of pastoral communities in which camels are kept for multiple uses including transport of people (camels can travel several hours per day at a speed of 15–20 Km/h), transport of loads (they can carry between 150 and 250 kg), the maintenance of an agricultural activity around oasis, the control of desertification and rational management of water resources, milk production and consumption, source of meat, and traditional medicine (421–423). Camels feed on herbaceous plants, shrubs, shoots, cacti, and date stones and can spend months in semiarid regions without drinking (424, 425). During millennia, camels were reared according to three breeding systems: sedentary, nomadic, and transhumant. Given the ecological zone in which they live, the last two systems are the most frequent, with a predominance of the transhumant mode (426–428). In most areas, dromedary camels are multipurpose animals with the females used primarily as milk producers and the males for transport or draft. The usual selection criteria of dromedary camels were color, morphometric characteristics, milk production, and endurance. For example, the Guerzni type is a pack camel maintained by nomads; the Marmouri type is a dromedary camel used for riding, whereas the Malhah- and Wadhah-type breeds were selected for high milk production (429, 430).
Economically, dromedary camel exploitation appear problematic because of slow reproductive cycle (13 months of pregnancy) and high mortality of young (431, 432). Reproductive losses in camel herds are due to infertility (uterine infection), pregnancy loss (infectious pathogen–induced abortions), mastitis (female udder infections), and neonatal diseases (433). A large investigation (11,200 camels from different herds) in Ethiopia regarding the major constraints to camel production emphasized widespread diseases, lack of attention to camels, lack of experience and knowledge, inadequate veterinary service, lack of attention by the government, poor infrastructures, and feed shortage. Yet, camel production remains attractive for low-income people, and renewed interest for camel breeding was observed in the Maghreb (e.g., Morocco) because of the increasing food needs for urbanized population. For meat consumption, at 3 years old, the weight of dromedary camels can reach about 500 kg (251). Regarding milk, the production of dromedary camel milk varies within camel breeds (434). The Hoor Somalian breed can produce 8 L of milk per day for 8 to 16 months, whereas the Eydimmo breed can produce 4 L of milk for 6 to 12 months (435). Camel milk is considered the closest to human mother milk, highly nutritious and with high minerals and low sugar and cholesterol (436). More recently, a fourth dromedary camel breeding mode has been developed that is camel breeding farms.
Currently, the population of dromedary camels is ~33 million heads (Figure 6), with highest numbers in Africa and the Middle East. The numbers of dromedary camels from one country to another have been very variable over the last 50 years. In the countries of the Southern shore of the Mediterranean, the population of dromedary camels had drastically declined in Palestine, Syria, Lebanon, and Turkey between the 1960s and 1990s, rising from 89,000 to 10,550, and then returning to growth with 62,000 heads in 2011; most of them (about 54,000) breed in Syria. In 2017, the Syrian livestock of camels was 66,390 heads. For the countries of North Africa (Morocco, Algeria, Tunisia, Libya, Egypt), the total population decreased from 1,031,000 heads in the 1960s to 879,000 in 2011 with 163,000 heads in Morocco; 315,000 in Algeria; 237,000 in Tunisia; 57,000 in Libya; and 107,000 in Egypt (369). The density of dromedary camels per inhabitant has been estimated at one dromedary camel per 45 humans in Tunisia, one per 98 in Libya, one per 119 in Algeria, one per 200 in Morocco, and one per 792 in Egypt, but these values calculated on the global populations of dromedary camels and humans do not reflect the regional discrepancies (438). For example, in Morocco, 58% of dromedary camels are found in the Southern Saharan region and 26% in the East-West band from Ouarzazate to Figuig passing by Rachidia (430). It should be noted that between 2011 and 2017, Tunisia has stabilized its livestock, whereas the population of dromedary camels increased in Algeria and Egypt and decreased in Morocco and Libya. In the country producing the largest livestock in Africa, Somalia kept almost stable livestock with 6,411,000 camels in 1985, 7,000,000 in 2011, and 7,222,081 in 2017, whereas Chad showed a marked increase in livestock with 481,060 in 1985, 1,435,000 in 2011, and 7,285,309 in 2017. The highest density of camels by land area or human population in the Arabian Peninsula is found in UAE and Qatar (439), with 451,463 camel heads and 9.4 million inhabitants (one camel per 21 inhabitants) in UAE, and 40,843 camel heads for 2.6 million inhabitants (one camel per 63 inhabitants) in Qatar in 2017, respectively. In the early 2000s, the relative importance of camels with respect to the total animal biomass was 6.2% in Africa, 0.7% in Asia, and 15.1% in the Arab countries, respectively. Moreover, the total world meat and milk productions from camels were about 376,000 tons/year, and 5,100,000 tons/year (440). In the Arab countries, the animal biomass was mainly composed of cattle (54.8%), followed by sheep (13.6%), camels (10.1%), goats (8.8%), buffalo (8.0%), and equine (4.6%) (440). In countries such as Sudan, Niger, Chad, and Tunisia, camel breeding represents a significant part of the agricultural economics, whereas it is of primary importance in the economy of Somalia, Mauritania, and Djibouti (427). As shown in Table 5, in 2017, the production of camel milk in Somalia was 953,673 tons, whereas it was 26,470 tons for Morocco, Algeria, Tunisia, and Libya altogether.
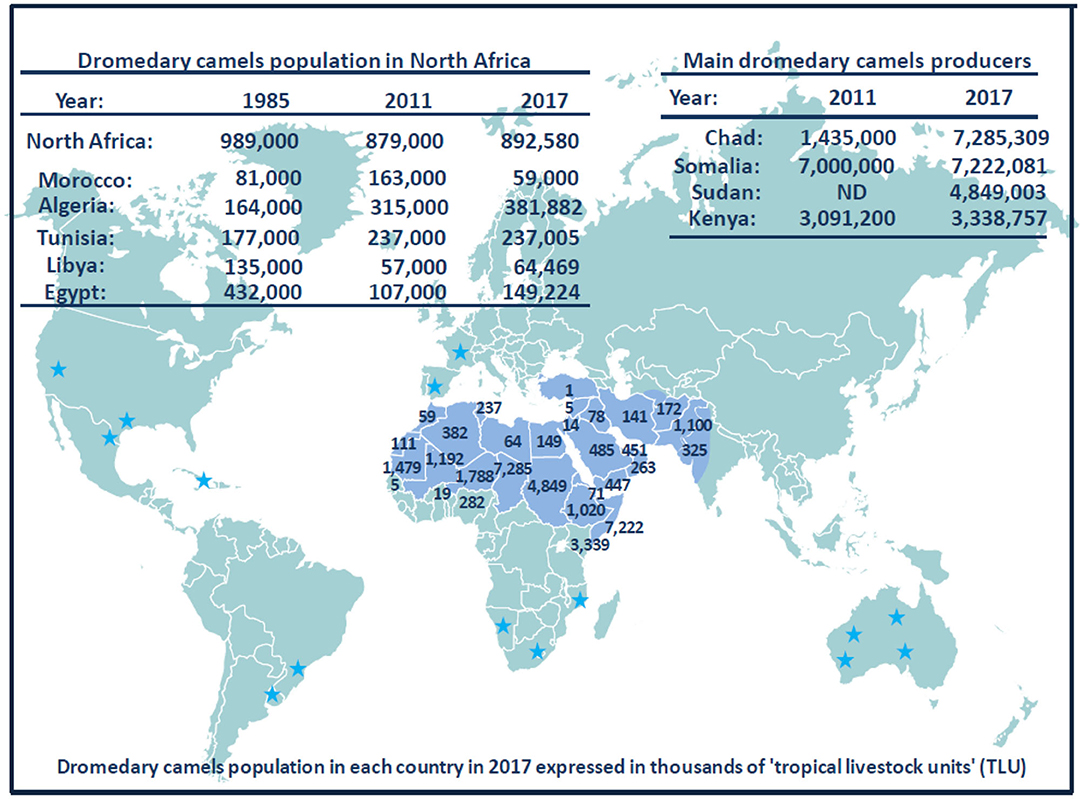
Figure 6. Schematic representation of dromedary camel distribution in the world. The Arabic generic term commonly used for camel is “ibl.” Male camel between 6 and 20 years old are named “jamal” (also the Arabic word for beauty). Currently, there are ~33 million dromedary camels (or humpback camels) worldwide, with highest numbers (77%) in Africa and the Middle East. The geographical location of the dromedary is in the belt of the tropical and subtropical dry zones of Africa, but its presence extends to Western Asia and Northwest India (blue area on the map). Dromedary camels are found in 35 native countries ranging from Senegal to India and from Kenya to Turkey. The number on the map show the population of dromedary camels in each country in 2017 expressed in thousands of “tropical livestock units” (TLUs), according to FAO (369). For example, the largest dromedary camel populations are found in Chad (7,285,309 camel heads) and Somalia (7,222,081 camel heads), followed by Sudan (4,849,003 camel heads) and Kenya (3,338,757 camel heads). Regarding the countries of the Southern coast of the Mediterranean Sea, the current livestock situation is as follows: 59,000 camels for Morocco; 381,882 camels for Algeria; 237,005 camels for Tunisia; 64,469 camels for Libya; 149,224 camels for Egypt; 5,530 camels for Israel; 66,390 camels for Syria; and 14,322 camels for Jordania. A massive dromedary camel (300,000 now-feral dromedary camels) implantation was made in the last century in Australia from camels imported from India (not shown); very specific introductions were also made in the United States, Central America, South Africa, and Europe; they are indicated by blue stars (437). Another member of the Camelidae family, the Camelus bactrianus (or two-humped camels) with a distinguished geographical distribution is present from the cold deserts of Central Asia to the border of Manchuria in China (see Figure 3). Both C. bactrianus and Camelus dromedary species can cohabit in a few places such as western Asia.
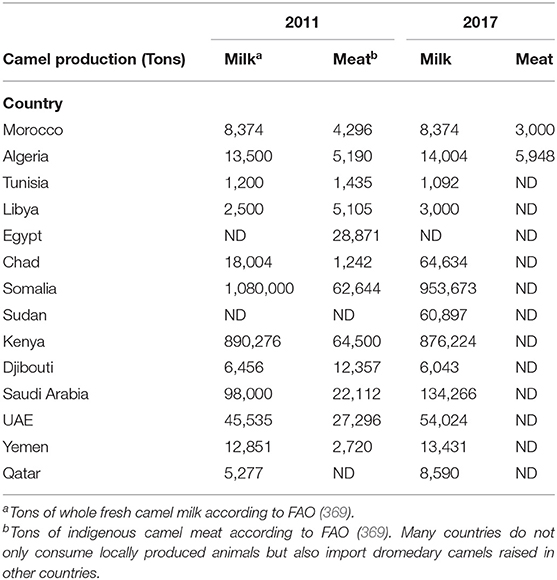
Table 5. Production of whole fresh camel milk and indigenous camel meat in North Africa compared to other countries of Africa and Arabic peninsula.
After a decrease of the dromedary camel populations in several countries, the recovery can be associated with the increasing demand for milk and camel meat that parallel the increase of human population (441). Unlike camel meat production whose market price is lower than that of sheep and cattle meat, milk production remains poorly valued, and its price is higher than that of cow's milk (428). In the case of Tunisia, the camel red meat production increased from 2,150 tons in 1997 to 3,500 tons in 2003 (439). Regarding North Africa (Morocco, Algeria, Tunisia, Libya, Egypt), the dromedary camel production systems are characterized by major differences in the sizes of herds ranging from a few heads in the agropastoral systems to thousands of heads. Some highly intensive farms are currently emerging all over the Arabian Peninsula both for milk production (including pasteurized milk) and meat production (feed-lot farms of young male camels, named hachi) (274). The Maghreb countries and Egypt favor import (from Sudan, Ethiopia, and Chad), rather than breeding of camels. Libya imports camels mainly from Chad and Niger for food. Only Tunisia is self-sufficient. Because of desertification, camels may become an interesting issue to replace cattle as a source of milk and meat in the newly desertified areas of the world. Indeed, adaptation of camels to desert ecosystems has attracted the attention of international organizations including the International Fund for Agricultural Development, the Islamic Development Bank, which together with a funding agency from the French Government have established the Camel Applied Research and Development Network (CARDN) in 1991. These funding agencies have next contracted with the Arab Center for the Studies of Arid Zones and Dry Lands, which started to operate as the executing agency of the consortium since 1996 to develop camel husbandry. CARDN supports laboratories, units for artificial insemination and embryo transfer, and mobile veterinary units. Several Southern countries are at a socioeconomic crossroads, which means choosing to modernize the sector in order to improve the productive performances of dromedary camels. Studies on production and marketing of camels were conducted in Tunisia, Egypt, Sudan, Pakistan, and Mauritania. In 2017, date of the latest statistics available according to the FAO, the population of dromedary camels in North Africa was 891,000 heads in North Africa, with 59,000 heads in Morocco, 382,000 in Algeria, 237,000 in Tunisia, 64,000 in Libya, and 149,000 in Egypt (369).
Besides food, camels play a role in local tradition and economy. The hides and skins sector, long neglected, is improving rapidly. Tunisia and Egypt develop good practices for killing the animals and better quality of tannery treatment. Tunisia has thus created a technical center leather and footwear, which is interested in dromedary product valorization. It is especially in the field of recreation and tourism that the dromedary camels know a continued interest, either to animate meharias in the desert (although this activity has decreased in some countries for security reasons), or as part of scenery of tourist places (e.g., camel rides at the foot of the pyramids in Egypt, at Djerba in Tunisia, or Essaouira in Morocco). The riding of the dromedary camels as saddle animal is regularly practiced in most countries of the Maghreb, particularly among the Saharawis (Morocco) or Tuareg (Algeria, Libya) populations. Dromedary camels are also attracting tourists around races that are very popular in the countries of the Arabian Golf and North Africa (432, 442, 443). Over many years, typical racing dromedary camels (slim, lightweight, high-speed) were selected to confer highest sports performance (Table 6). The racing stables are maintained with great care, selected feeding, and training of animals. In North Africa, racing of dromedary camels is the occasion of festivities like the Douz marathon (Tunisia), the festival of Marrakech (Morocco), or the fantasia of Ouargla (Algeria). In the Arabian Peninsula, camel shows called Mazayin al-Ibl (“best of camels”) are held annually with the 100-camel herds competition day and the camel beauty contest. One of the largest camel shows (about 160,000 camels) is usually held in Um al-Rughaiba (300 km from Riyadh in Saudi Arabia), where thousands of people come to attend the show (459). During thousands of years, Arab Bedouins have bred camels for speed and endurance, whereas camel racing became a professional sport in the UAE only after discovery of oil. Today, camel racing is considered a strategy to reinforce national identity by preserving the ancestral heritage in a modern country (460). Dromedary camels in the UAE are mainly grouped into three breeds (Al-Arabiat; Al-Kazmiat; racing camels); the government imported well-known racing camels from different countries, and they were used for endogenous breeds leading to new racing breeds including Sokan, Hamlol, Msehan, and Al-Thenian (444). Several Omani camels have been selected for racing such as Al-Azkiyah and Al-Bahree famous for short-distance racing or Kudsha and Arjaa famous for long-distance racing. In Saudi Arabia, there are 4 main camel breeds (Al-Majahem; Al-Makater; Lorak; racing camel breeding of Al-Omaniat, Al-Hurah, and Al-Sodaniat) (444). In a past period now over, the UAE and other Gulf states involved child jockeys in camel racing, drawing lawsuits from human rights groups (461). This led to a change in practices and the founding of the Camel Racing Association in 1992 and Camel Racing Federation in 2003. The practice of child jockeys was banned, and since then, camels are spurred on by small robots jockeys. There are several racetracks across the country with spacious and well-kept stadiums for viewers. The Abu Dhabi Authority of Culture and Heritage annually organizes a famous camel international festival in April. Another major racing competition is held in February in the Janadrriyah suburb of Riyadh (459). The King Abdulaziz Camel Festival (28 day celebration) attracts more than 300,000 people, almost 2,000 owners, and 40,000 camels. The winner of a beauty contest can get a prize of several 100 thousands of US dollars. In Saudi Arabia, $57 million are distributed annually in Camel Festivals. Several other international camel festivals are held annually in Oman, Qatar, and Kuwait.
Although racing camels receive a lot of attention at the sanitary level, outbreaks of bronchopneumonia and gastroenteritis sometimes affect racing camels (311, 462). Yet there has been so far no report of C. burnetii outbreak in racing camels. Knowledge about the genetic characteristic and diversity of camels is improving (463–465). The size of camel genome is roughly 2 to 2.4 GB, encoding for more than 20,000 genes (452, 466). Growing interest in racing camels has led to set up research centers (e.g., Camel Research Center at King Faisal University in Hofuf, Saudi Arabia, and Camel reproductive center in Dubai) aimed at improving breeding stock. In 2009, in Dubai, the world's first successful cloning of a C. dromedarius was reported (467). More recently, the same research center reported the first cloning of a C. bactrianus (468).
Q fever is transmitted to humans through inhalation or ingestion of infected animal products such as meat, milk, or cheese. Camel milk is a major component of the diet in many pastoralist societies. When nomads move in search of pasture, they can live for up to a month in the desert on nothing but dromedary camel milk. Daily female camel milk production ranges from 2 to 6 L under desert conditions and up to 20 L under a more favorable environment (469). Most camel milk is drunk fresh, which may be a source of infection if the animal excretes C. burnetii in milk. With increasing urbanization, it has gained a wider market, and commercialization and consumption of camel milk are on the rise (470). Every year, 5.4 million tons of camel milk are produced (369). Although not extensively investigated for camel, the rate of excretion of C. burnetii in milk would be expected to be low, except in the early days after parturition (471).
There is also an increase of camel meat consumption, which is parallel to the urban development (441). For example, there has been a radical change in dromedary camel farming practices in the Arabian Peninsula since the 1960s, with an intensification of the production around cities. The annual camel meat consumption is estimated to be 21,500 tons in Saudi Arabia, a country where 33 million people are living (369). This change in methods of breeding camels might increase the frequency of zoonotic infections from camels to humans. It seems reasonable to assume that the sensitivity of public health surveillance to detect infectious microorganism in camels and to investigate the source of sporadic human cases of infectious diseases is higher in Saudi Arabia and then developing countries of East Africa. However, camel meat consumption is also very high in the Africa countries (e.g., 6,000 tons in Chad, 3,000 tons in Niger) (369). In Djibouti, where a population of about 1 million people is living and with a livestock of 70,965 camels (one camel per 14 humans), 300 tons of camel meat are eaten annually. In several North African countries, there is a long supply circuit of camel meat with several intermediary operators who, for example, carry herds from the El-Obeid region in Sudan to Aswan in Egypt where the dromedary camels are killed and dispatched on markets. The cross-border trade of camels from Sahelian countries to North Africa could represent a sanitary risk since the percent of Sahelian animals exposed to, or infected by, C. burnetii seems to be very high. Disease transmission associated with cross-border transport of dromedary camels was previously documented for RFV and pestis (472, 473).
In the past decade, at-risk populations for C. burnetii infection were limited to pastoral communities, farmers, slaughterhouse and tannery workers, veterinarians, and raw milk drinkers. With the increasing demand of milk and camel meat in urban areas, there is a potential threat for millions of people (474). Because camels suffer from lack of attention in several countries, the control of C. burnetii within livestock is severely hampered. Insufficient serological surveillance and uncontrolled trade of infected animals may therefore have direct consequence on the flock sanitary evolution. Effort should be made to increase awareness of Q fever in public, veterinary health authorities, and decision makers. Human is not the sole species at risk during meeting with infected camel. The bacteria can spread intraspecies in dromedary camel flocks, and interspecies transmission to other cattle remains possible. This could possibly impact the economy of affected countries and also their food reserves.
To fight against C. burnetii transmission (camel-to-humans, camel-to-camel, camel-to-other livestock), global hygiene procedures should be introduced (rational vaccination schemes, antibiotics, disinfectants, hygiene procedure to handle the products of abortion). Appropriated management of the risk requires clear information of camel keepers/owners, regular investigations of the animal sanitary statute by veterinary (regular serological tests would improve surveillance) crosstalk and collaboration between the veterinary and medical sectors, the establishment of health guidelines in all countries concerned, and intergovernmental cooperation between trading countries. Sometimes, very simple procedures may also improve the health status of herds. For example, to control mastitis in camel, it is a good practice to remove ticks, even when the animal is not lactating (475).
Regarding the general hygiene measures, the farmer must keep the premises clean. The professionals must wear a mask and gloves in areas expected to be possibly contaminated, remove afterbirth and birth fluids, and disinfect areas where camels have given birth and material in contact with camels. Immediate reporting of outbreaks is required to quickly start investigations of camel farms, other livestock, and domestic animals. Killing infected animals remains a possible strategy in extreme uncontrolled situations.
In an epidemic case, it should be recommended to pregnant women to avoid participating in farming activities, and vaccination of professionals should be considered. A vaccine against human Q fever was developed using the formalin-inactivated C. burnetii Henzerling strain phase I (Q-Vax®, Commonwealth Serum Laboratories, Parkville, Victoria), but it is distributed only in Australia (88, 90, 476–478). The availability of a human vaccine for at-risk professionals would be of benefit to prevent human outbreaks. In case of febrile illness following contact with ruminants, the diagnosis of human Q fever is established by serology and bacterial identification (8). Molecular techniques have an added value to the diagnostic of acute Q fever and for the clinical follow-up of infected people. Infected people should be treated with doxycycline 100 mg twice daily for at least 2 weeks. In case of gastric intolerance to doxycycline or in the case of meningoencephalitis, fluoroquinolones (ofloxacin 200 mg three times a day or pefloxacin 400 mg twice daily) are preferred (18, 479–481).
In farms, the prevention of C. burnetii shedding by infected animals (sheep, goats) is possible through vaccination of livestock with a phase I vaccine (482). Formaldehyde-inactivated whole C. burnetii made with phase I antigens confers greater protection than those made with phase II antigens (483, 484). To counteract the undesirable effects (induration, abscesses) of formaldehyde inactivation, chloroform–methanol vaccines were proposed (485, 486). A trichloroacetic acid–treated C. burnetii phase I vaccine is used in Slovakia (487). During the 2007–2010 Q fever outbreak in the Netherlands, vaccination of livestock was used to reduce the transmission of C. burnetii to humans. In France, an inactivated C. burnetii phase I vaccine (Coxevac®) was found to efficiently protect goats against abortion. However, vaccination did not clear infection in previously infected goats and cattle (488–490). More recently, a vaccination of red deer with Coxevac® was found to reduce C. burnetii shedding in feces, but not yet in vaginal secretions and milk (491). C. burnetii phase II vaccines protective for small ruminants have been developed in France (Chlamyvax-FQ® from Merial and Abortstop® from Rhône-Poulenc Rorer) (492). In already infected animals, these vaccines seem to only reduce C. burnetii shedding in feces. So far, there are no data available regarding the vaccination of camels against C. burnetii.
Currently, it is of major importance to monitor both the camel herds and camel farms. The search for anti–C. burnetii antibodies must be carried out on the serum in an abortive context. This low-cost method is not the most reliable, with a significant percentage of false-negative results but useful for rapid milk screening. Detection can also be performed on tissue samples using Gimenez staining. The sample must be made as sterile as possible and be quickly transported to the laboratory (within 48 h at 4°C; otherwise, it must be frozen). Because of their higher sensitivity and specificity, immunofluorescence or immunoperoxidase immunodetection assays should be preferred to microscopic identification. The two most reliable methods are enzyme-linked immunosorbent assay and PCR. PCR diagnosis using specific oligonucleotide probes is probably the fastest, most sensitive, and feasible method. Yet, it is still too expensive to use it routinely in monitoring of camel herds. A less expensive strategy could consist in testing pool of samples from 10 animals and return to individual tests only in case of a positive result. Finally, the culture of the bacteria on agar followed by C. burnetii identification by matrix-assisted laser desorption/ionization–time-of-flight or genomic sequencing is rarely used in veterinary medicine, but it brings irrefutable proof of the infection and can allow the comparison of strains isolated in camels, humans, and the ecosystem to identify the reservoir of bacteria.
The attitude to be adopted in case of contamination of a camel herd by C. burnetii did not drastically differ from those already applied to the treatment of other ruminants (Figure 7). In veterinary medicine, oxytetracyclines (OTC) were reported to be effective in decreasing the number of abortions in ruminants without preventing bacterial shedding and transmission (187, 493–495). Veterinarians recommended that cow with metritis or abortion be isolated and treated by parenteral antibiotic injections and intrauterine injections of OTC (8 mg OTC/kg/day for 30 days). The female must then be vaccinated and may later be inseminated (493). Yet, OTC treatments do not guarantee the elimination of bacteria from the milk of infected female (496). In France, when C. burnetii is detected in a herd of ruminants, sale of milk and transformation into cheese of milk from aborted female are strictly forbidden. Milk of the remainder of the flock can be sold after pasteurization (72°C during 15 min) (497). A similar preventive strategy is used in small ruminants (498). Because OTC at a dose of 25 mg/kg administered intramuscularly every 2 days for 30 days was found effective in eliminating bacterial shedding in camels infected by Brucella melitensis (322), it suggests that a similar treatment could be used for the treatment of C. burnetii infection, although such therapy has not yet been evaluated in camels infected with C. burnetii.
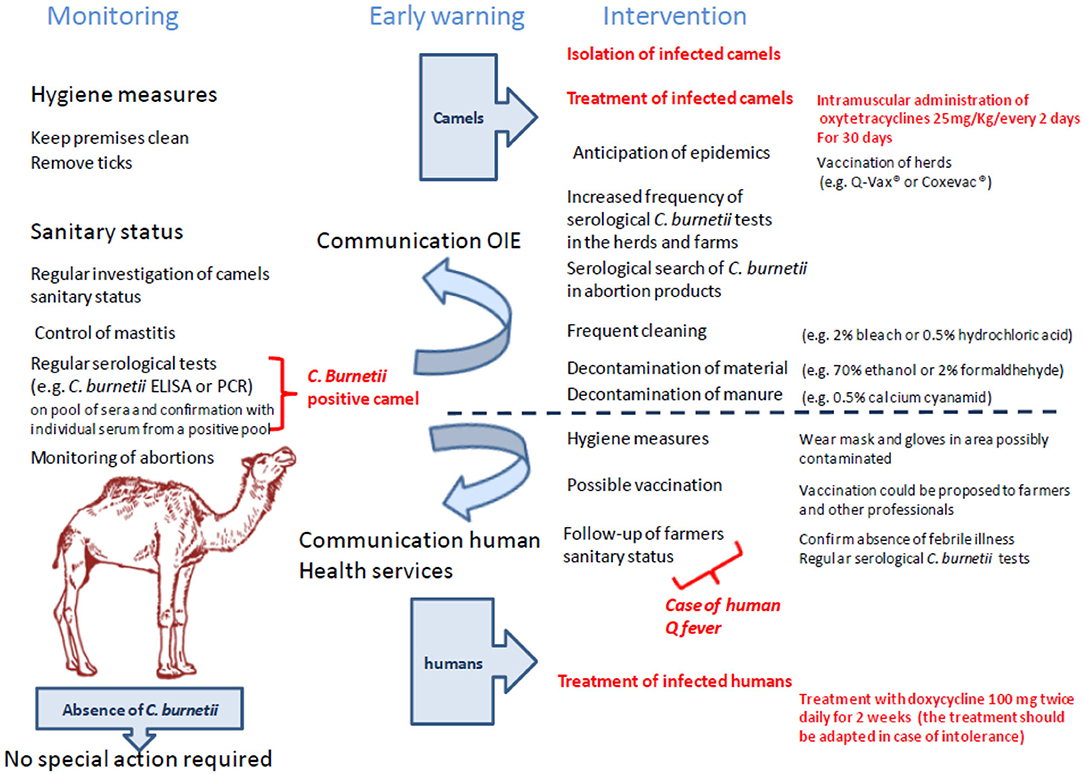
Figure 7. Suggested flowchart to monitor farms against the risk of C. burnetii infection. This logigram takes into account the surveillance, the alert, and the measures to be taken in case of presence of C. burnetii–infected animals in a herd. With regard to the specific actions to be implemented, they relate to both general safety measures and specific measures for sick camels or herds with sick animals and farmers.
To design an effective strategy of control and prevention, it is necessary to remember that C. burnetii is able to survive in the external environment for a long period under a pseudosporulated form. Survival was estimated 1 h at 60°C in milk; 5 months in the soil, 6 months in the dried blood, 24 months in tick waste, and 1 month in the dried milk and meat (494, 499–501), suggesting that the bacteria can be transmitted within camel herds, as well as to other livestock and humans. In farms, the prevention of transmission is not an easy challenge because C. burnetii is also resistant to conventional disinfectants such as 0.5% formalin, 1% phenol, and 0.5% hypochlorite (495); C. burnetii is nevertheless destroyed by 0.5% hydrochloric acid, chlorinated lime at 2%, 1% formalin, 5% hydrogen peroxide, and 2% bleach (502, 503). In the Netherlands, spread of manure (feces) from infected herds is forbidden for at least 3 months after suspicion of infection (52). Decontamination of feces from infected animals is possible by adding 0.5% of calcium cyanamide to contaminated dung (504). Decontamination of surface and materials can be performed using 2% formaldehyde, 5% hydrogen peroxide, 70% ethanol, or 5% chloroform (439).
The Mediterranean population has experienced a growth rate of 20% between 1970 and 2019. In 2016, the number of inhabitants living in the 22 riparian countries of the Mediterranean basin was estimated 502 million people (7% of the world population). According to World Bank, the population of this region will reach 524 million inhabitants by 2025. North Africa, with a population of almost 200 million people (about 36 million inhabitants in Morocco, 43 million in Algeria, 12 million in Tunisia, 6.5 million in Libya, 97.5 million in Egypt) in 2019, represents about 40% of the population of all the Mediterranean basin, and this is where the population growth is fastest. During the period 1965–2000, the urban population growth rate increased by 3.76, 2.96, 2.82, and 2.26 per year in Libya, Algeria, Morocco, and Tunisia, respectively. Improvement of standards of living combined with a high population growth rate and rapid urbanization has caused a massive increase in demand of livestock products that native breeds could not satisfy. This situation resulted in massive food importation and intensification of livestock production systems. So far, the food of the inhabitants of North Africa was based on farms quite similar to those found in the Northern regions of the Mediterranean basin, especially dairy cattle and poultry. Until recently, little was invested in dromedary camel production development. Nowadays, dromedary camel breeding becomes an issue to face the growing food demand of North Africa.
C. burnetii was first reported in Africa in the late 1940s (157, 505). Until recently, C. burnetii serosurvey focused mainly on goats, sheep, and cattle, probably because in Australia, America, and Europe, where C. burnetii outbreaks in herds have produced significant economic losses and epidemics in humans, there are hardly any camels. Yet, in the North Africa and Arabic peninsula, dromedary camels could become the most important reservoir of C. burnetii in ruminants. This hypothesis is supported by the fact that in the past decade the camel seroprevalence was always the highest among all ruminants. In this area of the world, close contacts with dromedary camels were associated with increased C. burnetii seropositivity in humans (95). For the moment, dromedary camels represent <3% of livestock in countries where camels live. However, if governments decide a sharp rise in dromedary camel production, it will become urgent to better control the sanitary status of these animals and to implement breeding methods to protect herds from epizooties and humans from C. burnetii zoonoses.
As shown in Table 4, when dromedary camel seroprevalences for anti–C. burnetii Ig are compared over time, it appears that there are many more seropositive animals today than in the past. This could indicate either a strong progression in dromedary camel seroprevalences to C. burnetii in recent decades or that a recent increase in the number of serological test performed or the improvement of the diagnosis methods has artificially suggested a progression while the seroprevalence in camels has always been very high. Even if a change in the frequency of serosurvey or in diagnosis methods has allowed a better evaluation of C. burnetii occurrence in herds, it cannot introduce an experimental bias accounting for the highest seroprevalence for C. burnetii found in camels among ruminants. An important information that is generally absent from the publications on the subject concerns the period of collection of samples for which it is not known whether they have been taken during an outbreak or not. Likewise, it remains difficult to estimate the accuracy of the serological tests in camels, and it should be borne in mind that antigenic cross-reactions between C. burnetii and other bacteria are possible and have been reported for Legionella pneumophila (506). This could introduce a bias into these studies without, however, influencing the relative percentages of seroprevalence within the different species studied in the same series. The serosurveys by Schelling and colleagues in Chad, Hussein and colleagues in Sudan, and Klemmer and colleagues in Egypt are representative of this disturbing finding. In Chad, the anti–C. burnetii Ig seroprevalence was 80% in camels, 33% in sheep, 23% goats, and 4% in cattle; in Sudan, the seroprevalence was 64.3% in camels and 29.9% in cattle; in Egypt, the seroprevalence was 40.7% in camels, 19.3% in cattle, 8.9% in sheep, and 6.8% in goat. This intriguing high rate of C. burnetii infection in camel herds compared to other ruminants could be attributed to a number of factors, yet none was demonstrated so far to account for the high seroprevalence in camels. Because camels are known to show a high rate of ticks infestation (>99%) (507), and because ticks were found to carry C. burnetii and to transmit the pathogen (401, 402), it has been hypothesized that ticks widespread in the Sahara and North Africa could be vectors of C. burnetii among camels (378). It could fit with the observation that the highest rate of C. burnetii infection in camel herds was in old animals that have had more chance to be bitten by ticks in their life. However, this does not easily agree with the observation that female had higher seroprevalence than male (378). It is also difficult to determine whether these high seroprevalences are related to spreads of the pathogen in local herds or to increased transmission through contacts with animals imported for commercial purposes. Whatever the reasons, the dromedary camel could be the first source of C. burnetii among ruminants in countries of the Southern coast of the Mediterranean basin.
The incidence of abortion due to C. burnetii in animal herds in African ruminants remains to be better documented. In Morocco, Chlamydia abortus and C. burnetii were found associated with abortions of small ruminants, causing a significant economic loss (508, 509). A survey in Rabat reported that sheep with history of abortions were more likely to be seropositive for C. burnetii than those with normal births, 33% vs. 15%, respectively (510). More recently, a survey of abortion highlighted an average abortion of 12.1% in ewes, and the serological analysis of sheep and goat indicated that 57% were seropositive for C. burnetii, their seroprevalence being 91% for Chlamydia, 74% for Toxoplasma, 43% for Brucella, and 22% for Leptospira, respectively (510). To our knowledge, C. burnetii–induced abortion in dromedary camels was not evaluated so far.
The measures to be held for prevention, treatment, and control of zoonoses are usually available to humans and livestock in high-income countries but less present in low-income settings, which are the most vulnerable (511). Although dromedary camels can be suspected to play a major role in the spreading of C. burnetii to humans, their serosurvey remains rare. In addition, it is not known whether the camels found seropositive for C. burnetii still produce infectious bacteria or if the seroprevalence is simply the immunological signature of an old infection in animal that has gotten rid of the bacteria. Yet, the high prevalence of Q fever in camels, coupled with the widespread habit of consuming raw camel milk, underscores a possible role of camels in Q fever transmission to humans. If human Q fever cases have been documented in connection with consumption of raw milk from camels infected with C. burnetii, there are so far no reports of a human outbreak traced to camels. This does not mean that it does not exist especially in a context where it has not been systematically sought. Further research to evaluate the role of camels in C. burnetii transmission to humans should be assessed keeping in mind that infection with C. burnetii in humans is most often asymptomatic. Moreover, there is also a risk for people who come into close animal contact during mass gatherings like the annual Hajj pilgrimage to Saudi Arabia where more than 10 million Muslims from around 184 Islamic countries meet together. The overall correlation of camel numbers and human Q fever remains to be further explored. International cooperation and intersectorial governance are required for the control of C. burnetii zoonosis. Finally, these observations must raise physicians' awareness to the importance of notifying Q fever so that the real incidence may be found.
CD wrote the paper. All authors contributed to conceive the paper.
This work was supported by the French Government under the Investissements d'avenir' (Investments for the Future) program managed by the Agence Nationale de la Recherche (reference: Méditerranée Infection 10-IAHU-03). IO was supported by a grant from the Caisse Nationale de Sécurité Sociale de Djibouti (Social security, Djibouti). The images are available under a Creative Commons CCBY 3.0 license.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Benoît Desnues for helpful discussion.
2. Eldin C, Mélenotte C, Mediannikov O, Ghigo E, Million M, Edouard S, et al. From Q fever to Coxiella burnetii infection: a paradigm change. Clin Microbiol Rev. (2017) 30:115–90. doi: 10.1128/CMR.00045-16
3. La Scola B, Lepidi H, Raoult D. Pathologic changes during acute Q fever: influence of the route of infection and inoculum size in infected guinea pigs. Infect Immun. (1997) 65:2443–7. doi: 10.1128/IAI.65.6.2443-2447.1997
5. Vishwanath S, Hackstadt T. Lipopolysaccharide phase variation determines the complement-mediated serum susceptibility of Coxiella burnetii. Infect Immun. (1988) 56:40–4. doi: 10.1128/IAI.56.1.40-44.1988
6. Beare PA, Samuel JE, Howe D, Virtaneva K, Porcella SF, Heinzen RA. Genetic diversity of the Q fever agent, Coxiella burnetii, assessed by microarray-based whole-genome comparisons. J Bacteriol. (2006) 188:2309–24. doi: 10.1128/JB.188.7.2309-2324.2006
7. Narasaki CT, Toman R. Lipopolysaccharide of Coxiella burnetii. Adv Exp Med Biol. (2012) 984:65–90. doi: 10.1007/978-94-007-4315-1_4
9. van Schaik EJ, Chen C, Mertens K, Weber MM, Samuel JE. Molecular pathogenesis of the obligate intracellular bacterium Coxiella burnetii. Nat Rev Microbiol. (2013) 11:561–73. doi: 10.1038/nrmicro3049
10. Porter SR, Czaplicki G, Mainil J, Guatteo R, Saegerman C. Q fever: current state of knowledge and perspectives of research of a neglected zoonosis. Int J Microbiol. (2011) 2011:1–22. doi: 10.1155/2011/248418
11. Tattevin P, Arvieux C, Dupont M, Guggenbuhl P, Lemeur A, Michelet, et al. Granulomatous lymphadenitis as a manifestation of Q Fever. Emerg Infect Dis. (2003) 9:137–8. doi: 10.3201/eid0901.020211
12. Melenotte C, Million M, Audoly G, Gorse A, Dutronc H, Roland G, et al. B-cell non-Hodgkin lymphoma linked to Coxiella burnetii. Blood. (2016) 127:113–21. doi: 10.1182/blood-2015-04-639617
13. Melenotte C, Protopopescu C, Million M, Edouard S, Carrieri MP, Eldin C, et al. Clinical features and complications of Coxiella burnetii infections from the French National Reference Center for Q fever. JAMA Netw Open. (2018) 1:e181580. doi: 10.1001/jamanetworkopen.2018.1580
14. OIE. Q fever: Chapter 2·1·12. In: Manual of Standard Diagnostic Tests and Vaccines for Terrestrial Animals. 7th ed. Paris: World Organization for Animal Health (2012). p. 1–13.
15. Burnet FM, Freeman M. Experimental studies on the virus of “Q” fever. Rev Infect Dis. (1983) 5:800–8. doi: 10.1093/clinids/5.4.800
16. Derrick EH. “Q” fever, a new fever entity: clinical features, diagnosis and laboratory investigation. Res Infect Dis. (1983) 5:790–800. doi: 10.1093/clinids/5.4.790
17. Joubert L, Fontaine M, Bartoli M, Garrigue G. Sheep Q fever zoonosis risk: a professional, rural, and military concern. “La fièvre Q ovine, zoonose d'actualité de type professionnel, rural et militaire” [in French]. Rev Med Vet. (1976) 3:361–81.
20. Wentworth BB. Historical review of the literature on Q fever. Bacteriol Rev. (1955) 19:129–49. doi: 10.1128/MMBR.19.3.129-149.1955
21. ECDC. European Centre for Disease Prevention and Control. Q Fever Surveillance Report. Annual Epidemiological Report for 2017 (2019).
22. Topping NH, Shepard CC, Irons JV. Q fever in the United States. I. Epidemiologic studies of an outbreak among stock handlers and slaughter house workers. JAMA. (1947) 133:813–21. doi: 10.1001/jama.1947.02880120001001
23. Shepard CC. An outbreak of Q fever in Chicago packing house. Am J Hyg. (1947) 46:185–92. doi: 10.1093/oxfordjournals.aje.a119162
24. D'Angelo JL, Baker EF, Schlosser W. Q fever in the United States, 1948–1977. J Infect Dis. (1979) 139:613–5. doi: 10.1093/infdis/139.5.613
25. Willenberg P, Ruppanner R, Behymer DE, Haghighi S, Kaneko JJ, Franti CE. Environmental exposure to Coxiella burnetii: a sero-epidemiologic survey among domestic animals. Am J Epidemiol. (1980) 111:437–43. doi: 10.1093/oxfordjournals.aje.a112919
26. Pinsky RL, Fishbein DB, Greene CR, Gensheimer KF. An outbreak of cat-associated Q fever in the United States. J Infect Dis. (1991) 164:202–4. doi: 10.1093/infdis/164.1.202
27. Anderson AD, Kruszon-Moran D, Loftis AD, McQuillan G, Nicholson WL, Priestley RA, et al. Seroprevalence of Q fever in the United States, 2003–2004. Am J Trop Med Hyg. (2009) 81:691–4. doi: 10.4269/ajtmh.2009.09-0168
28. Whitney EA, Massung RF, Candee AJ, Ailes EC, Myers LM, Patterson NE, et al. Seroepidemiologic and occupational risk survey for Coxiella burnetii antibodies among US veterinarians. Clin Infect Dis. (2009) 48:550–7. doi: 10.1086/596705
29. Biggs HM, Turabelidze G, Todd SR, Slifka KJ, Drexler NA, Pratt D, et al. Q fever outbreak on a large United States Goat and cattle dairy: a one health investigation. In: 63rd Annual Meeting. New Orleans, LA (2014).
30. Pavilanis V, Lepine P, Morisset N. The presence of Q fever complement fixing antibodies in sera of inhabitants of the Province of Quebec. Can Med Assoc J. (1952) 66:333–4.
31. Marc-Aurèle J, Gregoire F, Comeau M. Clinical report on Q fever: first case in Canada. Can Med Assoc J. (1956) 75:931–3.
35. Kovacova E, Sixl W, Stunzner D, Urvolgyi J, Kazar J. Serological examination of human and animal sera from six countries of three continents for the presence of rickettsial antibodies. Eur J Epidemiol. (1996) 12:85–9. doi: 10.1007/BF00144434
36. Araujo-Melendez J, Sifuentes-Osomio J, Bobadilla-Del-Valle JM, Aquilar-Cruz A, Torres-Angeles O, Ramirez-Gonzalez JL, et al. What do we know about Q fever in Mexico? Rev Inves Clin. (2012) 64:541–5. Available online at: https://www.medigraphic.com/pdfs/revinvcli/nn-2012/nn126g.pdf
37. Mares-guia MAMM, Rozental T, Guterres A, dos Santos Ferreira M, De Gasperis Botticini R, Terra AKC, et al. Molecular identification of Q fever in patients with a suspected diagnosis of dengue in Brazil in 2013-2014. Am J Trop Med Hyg. (2016) 94:1090–4. doi: 10.4269/ajtmh.15-0575
38. Brandão H, Ribeiro do Valle LA, Christovão DA. Investigacão sobre a febre Q em São Paulo. 1. Estudo sorológico em operários de um frigorífico [in Portuguese]. Arq Fac Hig Saúde Publ Univ São Paulo. (1953) 7:127–34.
39. Cicuttin GL, Deqiuseppe JI, Mamianetti A, Corin MV, Linares MC, De Salvo MN, et al. Serological evidence of Rickettsia and Coxiella burnetii in humans of Buenos Aires, Argentina. Comp Immunol Microbiol Infect Dis. (2015) 43:57–60. doi: 10.1016/j.cimid.2015.10.007
40. Epelboin L, Nacher M, Mahamat A, Pommier de Santi V, Berlioz-Arthaud A, Eldin C, et al. Q fever in French Guiana: tip of the iceberg or epidemiological exception? PLoS Negl Trop Dis. (2016) 10:e0004598. doi: 10.1371/journal.pntd.0004598
41. Damasceno IAM, Guerra RC. Coxiella burnetii and Q fever in Brazil: a public health issue [in Portuguese]. Cien Saude Colet. (2018) 23:4231–9. doi: 10.1590/1413-812320182312.27772016
42. Eldin C, Mahamat A, Demar M, Abboud P, Djossou F, Raoult D. Q fever in French Guiana. Am J Trop Med Hyg. (2014) 91:771–6. doi: 10.4269/ajtmh.14-0282
43. Forland F, De Carvalho Gomes H, Nokleby H, Escriva A, Coulombier D, Giesecke J, et al. Applicability of evidence-based practice in public health: risk assessment on Q fever under an ongoing outbreak. Euro Surveill. (2012) 17:20060.
44. Lyytikainen O, Ziese T, Schwartlander B, Matzdorff P, Kuhnhen C, Jager C, et al. An outbreak of sheep-associated Q fever in a rural community in Germany. Eur J Epidemiol. (1998) 14:193–9. doi: 10.1023/A:1007452503863
45. Hellenbrand W, Breuer T, Peterson L. Changing epidemiology of Q fever in Germany, 1947-1999. Emerg Infect Dis. (2001) 7:789–96. doi: 10.3201/eid0705.010504
46. Winner SJ, Eglin RP, Moore VI, Mayon-White RT. An outbreak of Q fever affecting postal workers in Oxfordshire. J Infect. (1987) 14:255–61. doi: 10.1016/S0163-4453(87)93560-2
47. Smith DL, Ayres JG, Blair I, Burge PS, Carpenter MJ, Caul EO, et al. A large Q fever outbreak in the West Midlands: clinical aspects. Respir Med. (1993) 87:509–16. doi: 10.1016/0954-6111(93)90006-L
48. Thomas DR, Salmon RL, Smith RMM, Caul EO, Treweek L, Kench SM, et al. Epidemiology of Q fever in the UK. In: Kazar J, Toman R, editors. Rickettsiae and Rickettsial Diseases. Bratislava: Slovak Academy of Sciences (1996) p. 512–7.
49. Rahaman R, Milazzo A, Marshall H, Bi P. Is a one health approach utilized for Q fever control? a comprehensive literature review. Int J Environ Res Public Health. (2019) 16:730. doi: 10.3390/ijerph16050730
50. Dupuis G, Petite J, Peter O, Vouilloz M. An important outbreak of human Q fever in a Swiss Alpine valley. Int J Epidemiol. (1987) 16:282–7. doi: 10.1093/ije/16.2.282
51. Georgiev M, Afonso A, Neubauer H, Needham H, Thiery R, Rodolakis A, et al. Q fever in humans and farm animals in four European countries, 1982 to 2010. Euro Surveill. (2013) 18:20407.
52. Schimmer B, Morroy G, Dijkstra F, Schneeberger PM, Weers-Pothoff G, Timen A, et al. Large ongoing Q fever outbreak in the south of the Netherlands, 2008. Euro Surveill. (2008) 13:2. doi: 10.2807/ese.13.37.18976-en
53. van Loenhout JA, Paget WJ, Vercoulen JH, Wijkmans CJ, Hautvast JL, van der Velden K. Assessing the longterm health impact of Q-fever in the Netherlands: a prospective cohort study started in 2007 on the largest documented Q-fever outbreak to date. BMC Infect Dis. (2012) 12:280. doi: 10.1186/1471-2334-12-280
54. van der Hoek W, Schneeberger PM, Oomen T, Wegdam-Blans MC, Dijkstra F, Notermans DW, et al. Shifting priorities in the aftermath of a Q fever epidemic in 2007 to 2009 in the Netherlands: from acute to chronic infection. Euro Surveill. (2012) 17:20059.
55. Van Leuken JPG, Swart AN, Branddsma J, Terink W, Van de Kassteele J, Droogers P, et al. Human Q fever incidence is associated to spatiotemporal environmental conditions. One Health. (2016) 2:77–87. doi: 10.1016/j.onehlt.2016.03.004
56. van Roeden SE, Wever PC, Kampschreur LM, Gruteke P, van der Hoek W, Hoepelman AIM, et al. Chronic Q fever-related complications and mortality: data from a nationwide cohort. Clin Microbiol Infect. (2019) 25:1390–8. doi: 10.1016/j.cmi.2018.11.023
57. Fonseca F, Pinto MR, Azevedo JF, Lacerda MT. Q fever in Portugal. “Febre Q em Portugal” [in Portuguese]. Clín Contemp. (1949) 3:1159–64.
58. Palmela C, Badura R, Valadas E. Acute Q fever in Portugal. Epidemiological and clinical features of 32 hospitalized patients. Germs. (2012) 2:43–59. doi: 10.11599/germs.2012.1013
59. Bolanos M, Santana OE, Angel-Moreno A, Perez-Arellano JL, Liminana JM, Serra-Majem L, et al. Seroprevalence of infection by Coxiella burnetii in Canary Islands (Spain). Eur J Epidemiol. (2003) 18:259–62. doi: 10.1023/A:1023342624475
60. Frankel D, Richet H, Renvoise A, Raoult D. Q fever in France, 1985-2009. Emerg Infect Dis. (2011) 17:350–6. doi: 10.3201/eid1703.100882
61. Mitov A. Diagnosis of two cases of Q fever in southern Bulgaria [in Bulgarian]. Bulgarskaja Klin. (1949) 8:610–23.
62. Novkirishki V, Bojadzhian CH, Kijanovska E. Epidemiologic studies of Q fever outbreak in the region of the Knyezha town. Infektologija. (1994) 31:16–9.
63. Serbezov V, Kazar J, Novkirishki V, Gatcheva N, Kovacova E, Voynova V. Q fever in Bulgaria and Slovakia. Emerg Infect Dis. (1999) 5:388–94. doi: 10.3201/eid0503.990309
64. Tilburg JJHC. Molecular investigation of the Q fever epidemic in Netherlands, the largest outbreak caused by Coxiella burnetii ever reported. Thesis. Nijmegen: Radboud University (2013).
65. Genova-Kalou P, Vladimirova N, Stoitsova S, Krumova S, Kurchatova A, Kantardjiev T. Q fever in Bulgaria: laboratory and epidemiological findings on human cases and outbreaks, 2011 to 2017. Euro Surveill. (2019) 24:1900119. doi: 10.2807/1560-7917.ES.2019.24.37.1900119
66. Tokarevich NK, Vasilyeva LD. Q-fever in Leningrad. In: Nikitin MY, editors. Zoonoses Proceedings of the Institut Pasteur. St. Petersburg: Institut Pasteur of Leningrad (1959). p. 7–19.
67. Rudakov NV. Ecologico-epidemiological observations of Q fever in Russia. In: Kazar J, Toman R, editors. Rickettsiae and Rickettsial Diseases. Bratislava: Slovak Academy of Sciences (1996). p. 524–7.
68. Freylikhman O, Kiselev A, Kazakov S, Sergushichev A, Panferova Y, Tokarevich N, et al. Draft genome sequence of Coxiella burnetii historical strain Leningrad-2, isolated from blood of a patient with acute Q fever in Saint Petersburg, Russia. Genome Announc. (2018) 6:e01464-17. doi: 10.1128/genomeA.01464-17
69. Balakrishnan N, Menon T, Fournier PE, Raoult D. Bartonella quintana and Coxiella burnetii as causes of endocarditis. India Emerg Infect Dis. (2008) 14:1168–9. doi: 10.3201/eid1407.071374
70. Pradeep J, Kumar S, Stephen S, Kamboj DV, Gunasekaran D, Hanifah M. Detection of acute Q fever human cases by indirect immunofluorescence and real-time polymerase chain reaction in a tertiary care hospital in Puducherry. Indian J Med Res. (2018) 148:449–52. doi: 10.4103/ijmr.IJMR_692_17
71. Esmaeili S, Naddaf SR, Pourhossein B, Hashemi Shahraki A, Bagheri Amiri F, Gouya MM, et al. Seroprevalence of brucellosis, leptospirosis, and Q fever among butchers and slaughterhouse workers in South-Eastern Iran. PLoS ONE. (2016) 11:e0144953. doi: 10.1371/journal.pone.0144953
72. Esmaeili S, Mohabati Mobarez A, Khalili M, Mostafavi E, Moradnejad P. Genetic evidence of Coxiella burnetii infection in acute febrile illnesses in Iran. PLoS Negl Trop Dis. (2019) 13:e0007181. doi: 10.1371/journal.pntd.0007181
74. Yu S. Coxiella burnetii in China. In: Williams JC, Thompson HA, editors. Q Fever: The Biology of Coxiella burnetii. Boca Raton, FL; Ann Arbor, MI; Boston, MA; London: CRC Press (1991). p. 327–39.
75. El-Mahallawy HS, Lu G, Kelly P, Xu D, Li Y, Fan W, et al. Q fever in China: a systematic review, 1989-2013. Epidemiol Infect. (2015) 143:673–81. doi: 10.1017/S0950268814002593
76. Han X, Hsu J, Miao Q, Zhou BT, Fan HW, Xiong XL, et al. Retrospective examination of q fever endocarditis: an underdiagnosed disease in the Mainland of China. Chin Med J. (2017) 130:64–70. doi: 10.4103/0366-6999.196566
77. Oda H, Yoshiie K. Isolation of a Coxiella burnetii strain that has low virulence for mice from a patient with acute Q fever. Microbiol Immunol. (1989) 33:969–73. doi: 10.1111/j.1348-0421.1989.tb00984.x
78. Htwe KK, Yoshida T, Hayashi S, Miyake T, Amano KI, Morita C, et al. Prevalence of antibodies to Coxiella burnetii in Japan. J Clin Microbiol. (1993) 31:722–3. doi: 10.1128/JCM.31.3.722-723.1993
79. To H, Htwe KK, Kako N, Kim HJ, Yamaguchi T, Fukushi H, et al. Prevalence of Coxiella burnetii infection in dairy cattle with reproductive disorders. J Vet Med Sci. (1998) 60:859–61. doi: 10.1292/jvms.60.859
80. Nagaoka H, Akiyama M, Sugieda M, Nishio T, Akahane S, Hattori H, et al. Isolation of Coxiella burnetii from children with influenza-like symptoms in Japan. Microbiol Immunol. (1996) 40:147–51. doi: 10.1111/j.1348-0421.1996.tb03330.x
81. Yuasa Y, Yoshiie K, Takasaki T, Yoshida H, Oda H. Retrospective survey of chronic Q fever in Japan by using PCR to detect Coxiella burnetii DNA in paraffin-embedded clinical samples. J Clin Microbiol. (1996) 34:824–7. doi: 10.1128/JCM.34.4.824-827.1996
82. Porter SR, Czaplicki G, Mainil J, Horii Y, Misawa N, Saegerman C. Q fever in Japan: an update review. Vet Microbiol. (2011) 149:298–306. doi: 10.1016/j.vetmic.2010.11.017
83. Lippi M, Sebastiani A, el-Mutabakani H. Detection of serum antibodies against Reoviruses, Adenoviruses and Coxiella burneti in a group of inhabitants of Riyad (Saudi Arabia) [in Italian]. Arch Ital Sci Med Trop Parasitol. (1968) 49:129–36.
84. Al-Hajjar S, Hussain Qadri SM, al-Sabban E, Jäger C. Coxiella burnetii endocarditis in a child. Pediatr Infect Dis J. (1997) 16:911–3. doi: 10.1097/00006454-199709000-00020
85. Almogren A, Shakoor Z, Hasanato R, Adam MH. Q fever: a neglected zoonosis in Saudi Arabia. Ann Saudi Med. (2013) 33:464–8. doi: 10.5144/0256-4947.2013.464
86. Royal J, Riddle MS, Mohareb E, Monteville MR, Porter CK, Faix DJ. Seroepidemiologic survey for Coxiella burnetii among US military personnel deployed to Southwest and Central Asia in (2005). Am J Trop Med Hyg. (2013) 89:991–5. doi: 10.4269/ajtmh.12-0174
87. Spelman DW. Q fever: a study of 111 consecutive cases. Med J Aust. (1982) 1:547–53. doi: 10.5694/j.1326-5377.1982.tb124169.x
88. Garner MG, Longbottom HM, Cannon RM, Plant AJ. A review of Q fever in Australia 1991–1994. Aust N Z Publ Health. (1997) 21:722–30. doi: 10.1111/j.1467-842X.1997.tb01787.x
89. Bond KA, Vincent G, Wilks CR, Franklin L, Sutton B, Stenos J, et al. One health approach to controlling a Q fever outbreak on an Australian goat farm. Epidemiol Infect. (2016) 144:1129–41. doi: 10.1017/S0950268815002368
90. Sellens E, Norris JM, Dhand NK, Heller J, Hayes L, Gidding HF, et al. Willingness of veterinarians in Australia to recommend Q fever vaccination in veterinary personnel: implications for workplace health and safety compliance. PLoS ONE. (2018) 13:e0198421. doi: 10.1371/journal.pone.0198421
91. Australian Government Department of Health. National Notifiable Diseases Surveillance System. (2019). Available online at: http://www9.health.gov.au/cda/source/cda-index.cfm
92. Giroud P, Jadin J. Essais d'isolement de souches de fièvre Q au Ruanda-Urundi du lait de vache, du cerveau de chèvres et de tiques d'animaux domestiques et sauvages. Bull Soc Pathol Exot. (1950) 435:672–3.
93. Giroud P. Rickettsial diseases in Equatorial Africa. “Les rickettsioses en Afrique Equatoriale” [in French]. Bull World Health Org. (1951) 4:535–46.
95. Vanderburg S, Rubach MP, Halliday JEB, Cleaveland S, Reddy EA, et al. Epidemiology of Coxiella burnetii Infection in Africa: a one health systematic review. PLoS Negl Trop Dis. (2014) 8:e2787. doi: 10.1371/journal.pntd.0002787
96. Jadin J. Rickettsial diseases in the Belgian Congo and Ruanda Urundi. “Les rickettsioses du Congo Belge et Ruanda Urundi” [in French] [Ph.D. thesis]. Louvain: Louvain University (1951).
97. Gidel R, Lefevre M, Athawet B. Investigation on the epidemiology of rickettsiosis in a rural section of the Ivory Coast. Med Trop. (1966) 26:649–61.
98. Gidel R, Athawet B. Serological survey of human brucellosis and rickettsial diseases in a group of a nomad population in the sahelian regions of Upper Volta. Ann Soc Belg Med Trop. (1975) 55:77–83.
99. Koulla-Shiro S, Kuaban C, Belec L. Acute community-acquired bacterial pneumonia in human immunodeficiency virus (HIV) infected and non-HIVinfected adult patients in Cameroon: aetiology and outcome. Tuber Lung Dis. (1996) 77:47–51. doi: 10.1016/S0962-8479(96)90075-1
100. Koulla-Shiro S, Kuaban C, Belec L. Microbial etiology of acute community-acquired pneumonia in adult hospitalized patients in Yaounde-Cameroon. Clin Microbiol Infect. (1997) 3:180–6. doi: 10.1111/j.1469-0691.1997.tb00595.x
101. Ki-Zerbo GA, Tall F, Nagalo K, Ledru E, Durand G, Patey O, et al. Rickettsiosis and Q fever in pyretic patients hospitalized at the Bobo- Dioulasso Hospital (Burkina Faso). Med Mal Infect. (2000) 30:270–4. doi: 10.1016/S0399-077X(00)89140-4
102. Julvez J, Michault A, Kerdelhue C. Serological study of rickettsioses in Niamey, Niger. Med Trop. (1997) 57:153–6.
103. Kobbe R, Kramme S, Kreuels B, Adjei S, Kreuzberg C, Panning M, et al. Q fever in young children, Ghana. Emerg Infect Dis. (2008) 14:344–6. doi: 10.3201/eid1402.070971
104. van der Hoek W, Sarge-Njie R, Herremans T, Chisnall T, Okebe J, et al. Short communication: prevalence of antibodies against Coxiella burnetii (Q fever) in children in The Gambia, West Africa. Trop Med Int Health. (2013) 18:850–3. doi: 10.1111/tmi.12116
105. Schelling E, Diguimbaye C, Daoud S, Nicolet J, Boerlin P, et al. Brucellosis and Q-fever seroprevalences of nomadic pastoralists and their livestock in Chad. Prev Vet Med. (2003) 61:279–93. doi: 10.1016/j.prevetmed.2003.08.004
106. Prabhu M, Nicholson WL, Roche AJ, Kersh GJ, Fitzpatrick KA, et al. Q fever, spotted fever group, and typhus group rickettsioses among hospitalized febrile patients in northern Tanzania. Clin Infect Dis. (2011) 53:e8–15. doi: 10.1093/cid/cir411
107. Crump JA, Morrissey AB, Nicholson WL, Massung RF, Stoddard RA, et al. Etiology of severe non-malaria febrile illness in northern Tanzania: a prospective cohort study. PLoS Negl Trop Dis. (2013) 7:e2324. doi: 10.1371/journal.pntd.0002324
108. Anstey NM, Tissot Dupont H, Hahn CG, Mwaikambo ED, McDonald MI, et al. Seroepidemiology of Rickettsia typhi, spotted fever group rickettsiae, and Coxiella burnetti infection in pregnant women from urban Tanzania. Am J Tro Med Hyg. (1997) 57:187–9. doi: 10.4269/ajtmh.1997.57.187
109. Wardrop NA, Thomas LF, Cook EAJ, de Glanville WA, Atkinson PM, Wamae CN, et al. The sero-epidemiology of Coxiella burnetii in humans and cattle, Western Kenya: evidence from a cross-sectional study. PLoS Negl Tropic Dis. (2016) 10:e0005032. doi: 10.1371/journal.pntd.0005032
110. Simpson GJG; Quan V, Frean J, Knobel DL; Rossouw J, Weyer J, Marcotty T, Godfroid J, et al. Prevalence of selected zoonotic diseases and risk factors at a human-wildlife-livestock interface in Mpumalanga province, South Africa. Vector Borne Zoonotic Dis. (2018) 18:303–10. doi: 10.1089/vbz.2017.2158
112. Montejo Baranda M, Corral Carranceja J, Aguirre Errasti C. Q fever in the Basque country: 1981–1984. Rev Infect Dis. (1985) 7:700–1. doi: 10.1093/clinids/7.5.700
113. Tellez A, Sainz C, Echevarria E, De Carlos S, Fernandez MV, Leon P, et al. Q fever in Spain: acute and chronic cases 1981–1985. Rev Infec Dis. (1988) 10:198–202. doi: 10.1093/clinids/10.1.198
114. Rotaeche del Campo R, Anta Unanue JL. Q fever. A familial outbreak of 5 cases. Aten Primaria. (1990) 7:211–2.
115. Martinez Eizaguirre JM, Pérez Rizo M, Olivella Pedregal A, Garcia Ventura S, Cancio Fanlo M, BasabeZapirain M. Q fever: epidemic outbreak of the pure febrile form. Aten Primaria. (1992) 9:425–8.
116. Pascual-Velasco F, Montes M, Marimon JM, Cilla G. High seroprevalence of Coxiella burnetii infection in Eastern Cantabria (Spain). Int J Epidemiol. (1998) 27:142–5. doi: 10.1093/ije/27.1.142
117. Nebreda T, Contreras E, JesuÂs Merino F, Dodero E, Campos A. Outbreak of Q fever and seroprevalence in a rural population from Soria Province. Enferm Infecc Microbiol Clin. (2001) 19:57–60. doi: 10.1016/S0213-005X(01)72561-X
118. de los Ríos-Martín R, Sanz-Moreno JC, Martín-Martínez F, Tébar-Betegón MA, Cortes-Garcia M, Escudero-Nieto R. Q fever outbreak in an urban area associated with school farm visit. “Brote de fiebre Q en un área urbana asociado a la visita a una granja-escuela” [in Spanish]. Med Clin. (2006) 126:573–5. doi: 10.1157/13087697
119. Garcia-Clemente M, Seco-Garcia AJ, Gutiérrez-Rodriguez M, Romero-Alvarez P, Fernandez-Bustamante J, Rodriguez-Pérez M. Outbreak of Coxiella burnetii pneumonia. Enferm Infecc Microbiol Clin. (2007) 25:184–6. doi: 10.1157/13099370
120. Alonso E, Lopez-Etxaniz I, Hurtado A, Liendo P, Urbaneja F, Aspiritxaga I, et al. Q fever outbreak among workers at a waste-sorting plant. PLoS ONE. (2015) 10:e0138817. doi: 10.1371/journal.pone.0138817
121. Alende-Castro V, MacõÂa-RodrõÂguez C, Novo-Veleiro I, GarcõÂa-FernaÂndez X, Treviño-Castellano M, RodrõÂguez-FernaÂndez S, et al. Q fever in Spain: description of a new series, and systematic review. PLoS Negl Trop Dis. (2018) 12:e0006338. doi: 10.1371/journal.pntd.0006338
122. Marchal JP. Q fever zoonosis: 55 cases among French soldiers in federal Germany. “La fièvre Q zoonose sur une endémie de 55 cas chez des soldats français en Allemagne fédérale” [in French] [Ph. D. thesis]. Université Claude Bernard, Lyon, France (1975).
123. Bru JP, Stahl JP, Gaillat J, Favier A, Micoud M. Epidemiological survey of Q fever in rural commune. “Enquete épidémiologique de la fièvre Q dans une commune rurale” [in French]. Lyon Med. (1983) 249:459–61.
124. Tissot Dupont H, Raoult D, Brouqui P, Janbon F, Peyramond D, Weiller PJ, et al. Epidemiologic features and clinical presentation of acute Q fever in hospitalized patients–323 French cases. Am J Med. (1992) 93:427–34. doi: 10.1016/0002-9343(92)90173-9
125. Dupont HT, Thirion X, Raoult D. Q fever serology: cutoff determination for microimmunofluorescence. Clin Diagn Lab Immunol. (1994) 1:189–96. doi: 10.1128/CDLI.1.2.189-196.1994
126. Fishbein DB, Raoult D. A cluster of Coxiella burnetii infections associated with exposure to vaccinated goats and their unpasteurized dairy products. Am J Trop Med Hyg. (1992) 47:35–40. doi: 10.4269/ajtmh.1992.47.35
127. Tissot Dupont H, Torres S, Nezri M. Raoult D. A hyperendemic focus of Q fever related to sheep and wind. Am J Epidemiol. (1999) 150:67–74. doi: 10.1093/oxfordjournals.aje.a009920
128. INVS. Q Fever Epidemic in the Chamonix Valley, Haute-Savoie, June-September 2002. “Epidémie de Fièvre Q dans la Vallée de Chamonix, Haute-Savoie, Juin-Spetembre 2002” [in French]. Inst National Veille Sanitaire (2005) Available online at: https://www.santepubliquefrance.fr/ (accessed October 15, 2020).
129. INVS. Q Fever Epidemic in a Meat Industry, Maine-et-Loire, February 2009. “Epidémie de Fièvre Q Dans une Usine de Traitement de Viande, Maine-et-Loire, Février 2009”. Inst National Veille Sanitaire (2010). Available online at: http://opac.invs.sante.fr/ (accessed 15 October, 2020).
130. Santé Publique France. Q Fever Epidemic Linked to the Visit of a Breeding Farm, Vaucluse-Drôme. “Epidémie de Fièvre Q liée à la Visite d'une Ferme D'élevage, Vaucluse-Drôme. Mai-Juin 2014” [in French]. Santé Publique France (2016). Available online at: https://www.santepubliquefrance.fr/regions/provence-alpes-cote-d-azur-et-corse/documents/rapport-synthese/2016/epidemie-de-fievre-q-liee-a-la-visite-d-une-ferme-d-elevage-vaucluse-drome.-mai-juin-2014
131. Tringali G, Mansueto S. Epidemiology of Q fever in Italy and the other Mediterranean countries. Zentralbl Bakteriol Mikrobiol Hyg. (1987) 267:20–5. doi: 10.1016/S0176-6724(87)80181-5
132. Selvaggi TM, Rezza G, Scagnelli M, Rigoli R, Rassu M, De Lalla F, et al. Investigation of a Q-fever outbreak in Northern Italy. Eur J Epidemiol. (1996) 12:403–8.
133. Santoro D, Giura R, Colombo MC, Antonelli P, Gramegna M, Gandola O, et al. Q fever in Como, Northern Italy. Emerg Infect Dis. (2004) 10:159–60. doi: 10.3201/eid1001.030467
134. Grilc E, Socan M, Koren N, Ucakar V, Avsic T, Pogacnik M, et al. Outbreak of Q fever among a group of high school students in Slovenia, March-April 2007. Euro Surveill. (2007) 12:3237. doi: 10.2807/esw.12.29.03237-en
135. Luksic B, Punda-Polic V, Ivic I, Bradaric I, Bradaric N. Clinical and epidemiological features of hospitalized acute Q fever cases from Split-Dalmatia county (Croatia), 1985-2002. Med Sci Monit. (2006) 12:CR126-131.
136. Racic I, Spicic S, Galov A, Duvnjak S, Zdelar-Tuk M, Vujnovic A, et al. Identification of Coxiella burnetii genotypes in Croatia using multi-locus VNRT analysis. Vet Microbiol. (2014) 173:340–7. doi: 10.1016/j.vetmic.2014.08.016
137. Medic A, Dzelalija B, Punda Polic V, Gjenero Margan I, Turkovic B, Gilic V. Q fever epidemic among employees in a factory in the suburb of Zadar, Croatia. Croat Med J. (2005) 46:315–9.
138. Vilibic-Cavlek T, Kucinar J, Ljubin-Sternak S, Kolaric B, Kaic B, Lazaric-Stefanovic L, et al. Prevalence of Coxiella burnetii antibodies among febrile patients in Croatia. Vector Borne Zoonotic Dis. (2012) 12:293–6. doi: 10.1089/vbz.2011.0681
139. Faas AA, Engeler A, Zimmermann A, Zöller L. Outbreak of query fever among Argentinean special police unit officers during a United Nations Mission in Prizren, South Kosovo. Mil Med. (2007) 172:1103–6. doi: 10.7205/MILMED.172.10.1103
140. Caminopetros JP. The Q fever in Greece. Milk, the source of infection for humans and animals [in French]. Ann Parasit Hum Comp. (1948) 23:107–18. doi: 10.1051/parasite/1948231107
141. Maltezou HC, Constantopoulou I, Kallergi C, Vlahou V, Georgakopoulos D, Kafetzis DA, et al. Q fever in children in Greece. Am J Trop Med Hyg. (2004) 70:540–4. doi: 10.4269/ajtmh.2004.70.540
142. Pape M, Mandraveli K, Arvanitidou-Vagiona M, Nikolaidis P, Alexiou-Daniel S. Q fever in northern Greece: epidemiological and clinical data from 58 acute and chronic cases. Clin Microbiol Infect. (2009) 15:150–1. doi: 10.1111/j.1469-0691.2008.02163.x
143. Tselentis Y, Gikas A, Kofteridis D, Kyriakakis E, Lydatakis N, Bouros D, et al. Q fever in the Greek island of Crete: epidemiologic, clinical, and therapeutic data from 98 cases. Clin Infect Dis. (1995) 20:1311–6. doi: 10.1093/clinids/20.5.1311
144. Spyridaki I, Gikas A, Kofterdis D, Psaroulaki A, Tselentis Y. Q fever in the Greek island of crete: detection, isolation, and molecular identification of eight strains of Coxiella burnetii from clinical samples. J Clin Microbiol. (1998) 36:2063–7. doi: 10.1128/JCM.36.7.2063-2067.1998
145. Vrankis I, Kokkini S, ChoChlakis D, Sandalakis V, Pasparaki E, Minadakis G, et al. Serological survey of Q fever in Crete, southern Greece. Comp Immunol Microbiol Infect Dis. (2012) 35:123–5. doi: 10.1016/j.cimid.2011.11.006
146. Kelly H. Q fever in Cyprus: report of cases and a survey of United Nations personnel. Int. J Epidemiol. (1974) 3:47. doi: 10.1093/ije/3.1.47
147. Psaroulaki A, Hadjichristodoulou C, Loukaides F, Soteriades E, Konstantinidis A, Papastergiou P, et al. Epidemiological study of Q fever in humans, ruminant animals, and ticks in Cyprus using a geographical information system. Eur J Clin Microbiol Infect Dis. (2006) 25:576–86. doi: 10.1007/s10096-006-0170-7
150. Gozalan A, Esen B, Rolain JM, Akin L, Raoult D. Is Q fever an emerging infection in Turkey? East Mediterr Health J. (2005) 11:384–91.
151. Killic S, Yilmaz GR, Komiya T, Kurtoglu Y, Karakoc EA. Prevalence of Coxiella burnetii antibodies in blood donors in Ankara, Central Anatolia, Turkey. New Microbiol. (2008) 31:527–34.
152. Gozalan A, Rolain JM, Ertek M, Angelakis E, Coplu N, Basbulut EA, et al. Seroprevalence of Q fever in a district located in the west Black Sea region of Turkey. Eur J Clin Microbiol Infect Dis. (2010) 29:465–9. doi: 10.1007/s10096-010-0885-3
153. Yavuz SS, Ozbek E, Basaran S, Celebi B, Yilmaz E, Basaran M, et al. The first case of chronic Q fever endocarditis and aortis from Turkey: a 5-year infection before diagnosis with drain in sternum. Anatol J Cardiol. (2016) 16:814–6. doi: 10.14744/AnatolJCardiol.2016.7329
154. Clikman A, Aydin M, Gulhan B, Karakecili F, Ozcicek A, Arif Kesik O, et al. The seroprevalence of Coxiella burnetii in Erzincan, Turkey: identification of the risk factors and their relationship with geographical features. J Vector Borne Dis. (2017) 54:157–63.
155. Meskini M, Beati L, Benslimane A, Raoult D. Seroépidemiology of rickettsial infections in Morocco. Eur J Epidemiol. (1995) 11:655–60. doi: 10.1007/BF01720299
156. Blanc G, Bruneau J, Martin LA, Maurice A. New data on Moroccan Q fever. “Quelques donées nouvelles sur le virus de la fièvre Q marocaine” [in French]. CR Acad Sci Paris. (1948) 226:607–8.
157. Pierrou M, Mimoune G, Vastel G. A major outbreak of Q fever (175 cases) in Algeria. “Une importante épidémie de fièvre Q (175 cas) 345 observée à Batna (Algérie)” [in French]. Presse Med. (1956) 64:471–3.
158. Spicer AJ. Military significance of Q fever: a review. J Royal Soc Med. (1978) 71:762–7. doi: 10.1177/014107687807101011
159. Dumas N. Rickettsioses et chlamydioses au Hoggar (République Algérienne): sondage épidémiologique [in French]. Bull Soc Pathol Exot. (1984) 77:278–83.
160. Benslimani A, Fenollar F, Lepidi H, Raoult D. Bacterial zoonoses and infective endocarditis, Algeria. Emerg Infect Dis. (2005) 11:216–24. doi: 10.3201/eid1102.040668
161. Lacheheb A, Raoult D. Seroprevalence of Q-fever in Algeria. Clin Microbiol Infect. (2009) 15:167–8. doi: 10.1111/j.1469-0691.2008.02211.x
162. Angelakis E, Raoult D. Q fever. Vet Microbiol. (2010) 140:297–309. doi: 10.1016/j.vetmic.2009.07.016
163. Omezzine-Letaief A, Yacoub S, Tissot-Dupont H, Le Cam C, Ghachem L, Letaief J, et al. Seroépidemiological survey of rickettsial infectious among blood donors in central Tunisia. Trans R. Soc Trop Med Hyg. (1995) 89:266–8. doi: 10.1016/0035-9203(95)90531-6
164. Omezzine-Letaief A, Dupont HT, Bahri F, Ernez M, Raoult D, et al. Seroepidemiologic study among 300 febrile patients in a infectious disease hospital ward. Med Mal Infect. (1997) 27:663–6. doi: 10.1016/S0399-077X(97)80221-1
165. Omezzine-Letaief A, Alaoui FZ, Bahri F, Mahdhaoui A, Boughzela E, et al. Infectious endocarditis with negative blood cultures. Arch Mal Coeur Vaiss. (2004) 97:120–4.
166. Kaabia N, Rolain JM, Khalifa M, Ben Jazia E, Bahri F, et al. Serologic study of rickettsioses among acute febrile patients in central Tunisia. Ann NY Acad Sci. (2006) 1078:176–9. doi: 10.1196/annals.1374.126
167. Znazen A, Trabelsi I, Maaloul I, Gargouri S, Maazoun Y, et al. Investigation of blood culture negative endocarditis in a tertiary care centre in Tunisia. Int J Antimicrob Agents. (2009) 33:S9. doi: 10.1016/S0924-8579(09)70047-0
168. Lukacovicova Z, Zboncakova L, Bazilkova M, Brezina R, Kazar J. Clinical and serologic study of Czecho-Slovak citizens working in developing countries from the aspect of risk of infection with Coxiella burnetii. Bratisl Lek Listy. (1993) 94:415–8.
170. Corwin A, Habib M, Watts D, Darwish M, Olson J, et al. Community based prevalence profile of arboviral, rickettsial, and Hantaan-like viral antibody in the Nile River Delta of Egypt. Am J Trop Me Hyg. (1993) 48:776–83. doi: 10.4269/ajtmh.1993.48.776
171. Mazyad SA, Hafez AO. Q fever (Coxiella burnetii) among man and farm animals in North Sinai, Egypt. J Egypt Soc Parasitol. (2007) 37:135–42.
172. Nahed HG, Khaled AAM. Seroprevalence of Coxiella burnetii antibodies among farm animals and human contacts in Egypt. J Am Sci. (2012) 8:619–21.
173. Abushahba MFN, Abdelbaset AE, Rawy MS, Ahmed SO. Cross-sectional study for determining the prevalence of Q fever in small ruminants and humans at El Minya Governorate, Egypt. BMC Res Notes. (2017) 10:538. doi: 10.1186/s13104-017-2868-2
174. Bottieau E, De Raeve H, Colebunders R, van den Ende J, Vervoort T, van Marck E. Q fever after a journey in Syria/ diagnosis suggested by bone marrow biopsy. Acta Clin Belg. (2000) 55:30–3. doi: 10.1080/17843286.2000.11754269
175. Siegman-Igra Y, Kaufman O, Keysary A, Rzotkiewicz S, Shalit I. Q fever endocarditis in Israel and a worldwide review. Scand J Infect Dis. (1997) 29:41–9. doi: 10.3109/00365549709008663
176. Oren I, Kraoz Z, Hadani Y, Kassis I, Zaltzman-Bershadsky N, Finkelstein R. An outbreak of Q fever in an urban area in Israel. Eur J Clin Microbiol Infect Dis. (2005) 24:338–41. doi: 10.1007/s10096-005-1324-8
177. Amitai Z, Bromberg M, Bernstein M, Raveh D, Keysary A, David D, et al. A large Q fever outbreak in an urban school in central Israel. Clin Infect Dis. (2010) 50:1433–8. doi: 10.1086/652442
178. Sachs N, Atiya-Nasagi Y, Beth-Din A, Levy I, Ben-Shimol S, Tasher D, et al. Chronic Q fever infections in Israeli children: a 25-year nationwide study. Pediatr Infect Dis J. (2018) 37:212–7. doi: 10.1097/INF.0000000000001790
179. Reisfeld S, Hasadia Mhamed S, Stein M, Chowers M. Epidemiological, clinical and laboratory characteristics of acute Q fever in an endemic area in Israel, 2006-2016. Epidemiol Infect. (2019) 147:e131. doi: 10.1017/S0950268818003576
180. McCaul TF. The development cycle of Coxiella burnetii. In: Williams JC, Thompson HA, editors. Q fever: The Biology of Coxiella burnetii. Boca Raton, FL: CRC Press, Inc. (1991). p. 223–58.
181. Alvarez J, Perez A, Mardones FO, Pérez-Sancho M, García-Seco T, Pagés E, et al. Epidemiological factors associated with the exposure of cattle to Coxiella burnetii in the Madrid region of Spain. Vet J. (2012) 194:102–7. doi: 10.1016/j.tvjl.2012.02.022
182. Durand MP, Durand JL. Q fever: human and animal epidemiology and prophylaxis. “Fièvre Q. Epidémiologie et prophylaxie humaine et animale”. Bull Mens Soc Vet Prat France. (1993) 77:269–97.
183. Khaled H, Sidi-Boumedine K, Merdja S, Dufour P, Dahmani A, Thiéry R, et al. Serological and molecular evidence of Q fever among small ruminant flocks in Algeria. Comp Immunol Microbiol Infect Dis. (2016) 47:19–25. doi: 10.1016/j.cimid.2016.05.002
184. Candela MG, Caballol A, Atance PM. Wide exposure to Coxiella burnetii in ruminant and feline species living in a natural environment: zoonoses in a human livestock-wildlife interface. Epidemiol Infect. (2017) 145:478–81. doi: 10.1017/S0950268816002454
185. Selim A, Ali AF, Moustafa SM, Ramadan E. Molecular and serological data supporting the role of Q fever in abortions of sheep and goats in northern Egypt. Microb Pathog. (2018) 125:272–5. doi: 10.1016/j.micpath.2018.09.034
186. Tokarevich NK, Panferova YA, Freylikhman OA, Blinova OV, Medvedev SG, Mironov SV, et al. Coxiella burnetii in ticks and wild birds. Ticks Tick Borne Dis. (2019) 10:377–85. doi: 10.1016/j.ttbdis.2018.11.020
187. Astobiza I, Barandika JF, Hurtado A, Juste RA, Garcia-Perez AL. Kinetics of Coxiella burnetii excretion in a commercial dairy sheep flock after treatment with oxytetracycline. Vet J. (2010) 184:172–5. doi: 10.1016/j.tvjl.2009.01.017
188. Prigent M, Rousset E, Yang E, Thiéry R, Sidi-Boumedine K. Validation study for using lab-on-chip technology for Coxiella burnetii multi-locus-VNTR analysis (MLVA) typing: application for studying genotypic diversity of strains from domestic ruminants in France. Microbes Infect. (2015) 17:782–8. doi: 10.1016/j.micinf.2015.09.026
189. Ceglie L, Guerrini E, Rampazzo E, Barberio A, Tilburg JJ, Hagen F, et al. Molecular characterization by MLVA of Coxiella burnetii strains infecting dairy cows and goats of north eastern Italy. Microbes Infect. (2015) 17:776–81. doi: 10.1016/j.micinf.2015.09.029
190. Gonzalez-Barrio D, Hagen F, Tilburg JJHC, Ruiz-Fons F. Coxiella burnetii genotypes in Iberian wildlife. Microb Ecol. (2016) 72:890–7. doi: 10.1007/s00248-016-0786-9
191. Huebner RJ, Bell JA. Q fever studies in southern California: summary of current results and a discussion of possible control measures. JAMA. (1951) 145:301–5. doi: 10.1001/jama.1951.02920230025005
192. Tissot-Dupont H, Raoult D. Q fever. Infect Dis Clin North Am. (2008) 22:505–14. doi: 10.1016/j.idc.2008.03.002
193. Angelakis E, Mediannikov O, Socolovschi C, Mouffok N, Bassene H, Tall A, et al. Coxiella burnetii-positive PCR in febrile patients in rural and urban Africa. Int J Infect Dis. (2014) 25:107–10. doi: 10.1016/j.ijid.2014.05.029
194. Luoto L, Huebner RJ. Q fever studies in Southern California: IX. Isolation of Q fever organisms from parturient placentas of naturally infected dairy cows. Publ Health Rep. (1950) 65:541–4. doi: 10.2307/4587315
195. Abdel-Moein KA, Hamza DA. The burden of Coxiella burnetii among aborted dairy animals in Egypt and its public health implications. Acta Trop. (2017) 166:92–5. doi: 10.1016/j.actatropica.2016.11.011
196. Rahal M, Tahir D, Eldin C, Bitam I, Raoult D, Parola P. Genotyping of Coxiella burnetii detected in placental tissues from aborted dairy cattle in the north of Algeria. Comp Immunol Microbiol Infect Dis. (2018) 57:50–4. doi: 10.1016/j.cimid.2018.06.001
197. Joulié A, Laroucau K, Bailly X, Pringent M, Gasqui P, Lepetitcolin E, et al. Circulation of Coxiella burnetii in a naturally infected flock of dairy sheep: shedding dynamics, environmental contamination, and genotype diversity. Appl Environ Microbiol. (2015) 81:7253–60. doi: 10.1128/AEM.02180-15
198. Safe Work Australia. Occupational Disease Indicators Report 2014. Canberra, ACT: Safe Work Australia (2014).
199. McKiel JA. Q fever in Canada. Can Med Assoc J. (1965) 91:573–7. doi: 10.1001/archderm.1965.01600120005001
200. McQuiston JH, Childs JE. Q fever in humans and animals in the United States. Vector Borne Zoonot Dis. (2002) 2:179–91 doi: 10.1089/15303660260613747
201. Kayedi MH, Mokhayeri H, Birjandi M, Chegeni-Sharafi A, Esmaeili S, Mostafavi E. Seroepideomiological study of Q fever in Lorestan province, Western Iran 2014. Iranian J Microbiol. (2017) 9:213–8.
202. Li K, Luo H, Shahzad M. Epidemiology of Q fever in goats in Hubei province of China. Trop Anim Health Prod. (2018) 50:1395–8. doi: 10.1007/s11250-018-1561-3
203. EFSA Panel on Animal Health and Welfare (AHAW). Scientific Opinion on Q fever. EFSA J. (2010) 8:1595. doi: 10.2903/j.efsa.2010.1595
204. Boarbi S, Fretin D, Mori M. Coxiella burnetii, agent de la fièvre Q. Can J Microbiol. (2016) 62:102–22. doi: 10.1139/cjm-2015-0551
206. Karagiannis I, Schimmer B, Van Lier A, Timen A, Schneeberger P, Van Rotterdam B, et al. Investigation of a Q fever outbreak in a rural area of the Netherlands. Epidemiol. Infect. (2009) 137:1283–94. doi: 10.1017/S0950268808001908
207. Delsing CE, Kullberg BJ, Bleeker-Rovers CP. Q fever in the Netherlands from 2007 to 2010. Neth J Med. (2010) 68:382–7.
208. Hackert VH, van der Hoek W, Dukers-Muijrers N, de Bruin A, Al Dahouk S, Neubauer H, et al. Q fever: single-point source outbreak with high attack rates and massive numbers of undetected infections across an entire region. Clin Infect Dis. (2012) 55:1591–9. doi: 10.1093/cid/cis734
209. van den Brom R, Roest HI, de Bruin A, Dercksen D, Santman-Berends I, van der Hoek W, et al. A probably minor role for land-applied goat manure in the transmission of Coxiella burnetii to humans in the 2007-2010 Dutch Q fever outbreak. PLoS ONE. (2015) 10:e0121355. doi: 10.1371/journal.pone.0121355
210. Rijks JM, Roest HI, van Tulden PW, Kik MJ, Grone A. Coxiella burnetii onfection in roe deer during Q fever epidemic, the Netherlands. Emerg Infect Dis. (2011) 17:2369–71. doi: 10.3201/eid1712.110580
211. Roest HI, van Solt CB, Tilburg JJ, Klaassen CH, Hovius EK, Roest FT, et al. Search for possible additional reservoirs for human Q fever, the Netherlands. Emerg Infect Dis. (2013)19:834–5. doi: 10.3201/eid1905.121489
212. Cruz R, Esteves F, Vasconcelos-Nobrega C, Santos C, Feirreira AS, Mega C, et al. Outbreaks of abortions by Coxiella burnetii in small ruminant flocks and a longitudinal serological approach on archived bulk tank milk suggest Q fever emergence in Central Portugal. Transbound Emerg Dis. (2018) 65:972–5. doi: 10.1111/tbed.12913
213. Haumesser JB, Poutrel B. Rickettsiosis in the Niger. Epidemiological investigation carried out in the Maradi area. Rev Elev Med Vet Pays Trop. (1973) 26:293–8. doi: 10.19182/remvt.7837
214. Hussien MO, Enan KA, Alfaki SH, Gafar RA, Taha KM, El Hussein RM. Seroprevalence of Coxiella burnetii in dairy cattle and camel in Sudan. Int J Infect. (2017) 4:e42945. doi: 10.5812/iji.42945
215. Téllez A, Martin A, Anda P, de la Fuente L, Benitez P, Garcia C, et al. Study of C. burnetii human and animal seroprevalence in a rural population in Madrid community. Eur J Epidemiol. (1989) 5:444–6. doi: 10.1007/BF00140138
216. Opporto B, Barandika JF, Hurtado A, Aduriz G, Moreno B, Garcia-Perez AL. Incidence of ovine abortion by Coxiella burnetii in Nothern Spain. Ann N Y Acad Sci. (2006) 1078:498–501. doi: 10.1196/annals.1374.095
217. Carballedo AD, Olmeda AS, Díez de Tejada Martín P, Jado I, Díez A, Blanco J, et al. Seroprevalence of Coxiella burnetii in ruminants from central Spain. Abstr P074. In: 5th Int Meet Rickettsiae and Rickettsial Dis. Marseille (2008) p. 60–61.
218. Toledo A, Jado I, Olmeda AS, Casado-Nistal MA, Gil H, Escudero R, et al. Detection of Coxiella burnetii in ticks collected from Central Spain. Vector-Borne Zoonot Dis. (2008) 9:465–8. doi: 10.1089/vbz.2008.0070
219. Berger E. A study of infectious pathogens (other than Brucella)-induced sheep abortion. “Contribution à l'étude des avortements d'origine infectieuse non brucellique chez les ovins” [Ph.D. thesis]. Ecole Vétérinaire Maison, Alfort, France (1999).
220. Villari S, Galluzzo P, Arnone M, Alfano M, Geraci F, Chiarenza G. Seroprevalence of Coxiella burnetii infection (Q fever) in sheep farms located in Sicily (Southern Italy) and related risk factors. Small Rumin Res. (2018) 164:82–6 doi: 10.1016/j.smallrumres.2018.05.006
221. Knap N, Zele D, Glinsek-Biskup U, Avsic-Zupanc T, Vengust G. The prevalence of Coxiella burnetii in ticks and animals in Slovenia. BMC Vet Res. (2019) 15:368. doi: 10.1186/s12917-019-2130-3
222. Cekani M, Papa A, Kota M, Velo E, Berxholi K. Report of a serological study of Coxiella burnetii in domestic animals in Albania. Vet J. (2008) 175:276–8. doi: 10.1016/j.tvjl.2007.01.005
223. Kilic S, Pasa S, Babur C, Ozlem MB. Investigation of Coxiella burnetii antibodies in sheep in Aydin region, Turkey. Rev Med Vet. (2005) 156:336–40.
224. Polydorou K. Q fever in Cyprus: a short review. Br Vet J. (1981) 137:470. doi: 10.1016/S0007-1935(17)31584-1
225. Cantas H, Muwonge A, Sareyyupoglu B, Yardimci H, Skerve E. Q fever abortions in ruminants and associated on-farm risk factors in northern Cyprus. BMC Vet Res. (2011) 7:13. doi: 10.1186/1746-6148-7-13
226. Boschini A, Di Perri G, Legnani D, Fabbri P, Ballarini P, Zucconi R, et al. Consecutive epidemics of Q fever in a residential facility for drug abusers: impact on persons with human immunodeficiency virus infection. Clin Infect Dis. (1999) 28:866–72. doi: 10.1086/515192
227. Arricau-Bouvery N, Rodolakis A. Is Q fever an emerging or re-emerging zoonosis? Vet Res. (2005) 36:327–49. doi: 10.1051/vetres:2005010
228. Selvaggi TM, Rezza G, Scagnelli M, Rigoli R, Rassu M, De Lalla, et al. Investigation of a Q-fever outbreak in northern Italy. Eur J Epidemiol. (1996) 12:403–8.
229. Armengaud A, Kessalis N, Desenclos JC, Maillot E, Brousse P, Brouqui P, et al. An urban epidemic of Q fever, Briançon, France. “Une épidémie urbaine de fièvre Q, Briançon, France, mars-juin 1996” [in French]. Eurosurveillance. (1997). 2:12–3. doi: 10.2807/esm.02.02.00137-en
230. Carrieri MP, Tissot-Dupont H, Rey D, Brousse P, Renard H, Obadia Y, et al. Investigation of a slaughterhouse-related outbreak of Q fever in the French Alps. Eur J Clin Microbiol Infect Dis. (2002) 21:17–21. doi: 10.1007/s10096-001-0645-5
231. Starnini G, Caccamo F, Farchi F, Babudieri S, Brunetti B, Rezza G. An outbreak of Q fever in a prison in Italy. Epidemiol Infect. (2005) 133:377–80. doi: 10.1017/S0950268804003383
232. Menadi S, Mura A, Santucciu C, Ghalmi F, Hafsi F, Massala G. Seroprevalence and risk factors of Coxiella burnetii infection in cattle in northeast Algeria. Trop Anim Hlth Prod. (2019) 52:935–42. doi: 10.1007/s11250-019-02083-x
234. Gwida M, El-Ashker M, El-Diasty M, Engelhardt C, Khan L. Q fever in cattle in some Egyptian governorates: a preliminary study. BMC Res Notes. (2014) 7:881. doi: 10.1186/1756-0500-7-881
235. Klemmer J, Njeru J, Emam A, El-Sayed A, Moawad AA, Henning K, et al. Q fever in Egypt: epidemiological survey of Coxiella burnetii specific antibodies in cattle, buffaloes, sheep, goats and camels. PLoS ONE. (2018) 13:e0192188 doi: 10.1371/journal.pone.0192188
236. Dechicha A, Gharbi S, Kebbal S, Chatagnon G, Tainturier D, Ouzrout R, et al. Serological survey of etiological agents associated with abortion in two Algerian dairy cattle breeding farms. J Vet Med Anim Health. (2010) 2:1–5. Available online at: https://academicjournals.org/journal/JVMAH/article-full-text-pdf/07DEC7D1282.pdf
237. Abdelhadi FZ, Abdelhadi SA, Niar A, Benallou B, Meliani S, Smail NL, et al. Abortions in cattle on the level of Tiaret area (Algeria). Global Vet. (2015) 14:638–45. Available online at: https://www.idosi.org/gv/gv14(5)15/2.pdf
238. Elandalousi RB, Ghram A, Maaroufi A, Mnif W. Seroprevalence of zoonotic abortive diseases in ruminants in northern Tunisia. “Séroprévalence des maladies abortives zoonotiques chez les ruminants au nord de la Tunisie” [in French]. Research fr. (2015) 2:1419. Available online at: http://www.research-journal.net/fr/Seroprevalence-of-abortive-zoonotic-diseases-in-ruminants-in-northern-Tunisia.html
239. Agag S, Kaidi R, Khelef D. Seroprevalence of Q fever in cows in Bejaia area (Algeria). Rev Elev Med Vet Pays Trop. (2016) 69:155–9. doi: 10.19182/remvt.31200
240. El Harrak M. OIE AHG on Diseases of Camelids Priority Diseases of Camelids. (2014). Available online at: https://www.oie.int/eng/refcentre2014/pdf/Presentations/S6_Parallel_Sessions/PS_Camelids_1_DrElharrak_2014.pdf
241. McGrane JJ, Higgins AJ. Infectious diseases of the camel: viruses, bacteria and fungi. Br Vet J. (1985) 141:529–47. doi: 10.1016/0007-1935(85)90049-1
244. Zhu S, Zimmerman D, Deem SL. A review of zoonotic pathogens of dromedary camels. Ecohealth. (2019) 16:356–77. doi: 10.1007/s10393-019-01413-7
245. Borisovich YF, Skalinskii EL. Camel pox virus [in Russian]. In: Sjurin VN, editor. Guidance on Veterinary Virology. Moscow (1966) p. 632–3.
246. Higgins AJ, Silvey RE, Abdelgafir AE, Kitching RP. The epidemiology and control of an outbreak of camel pox in Bahrain. In: Allen WR, Higgins AJ, Maybew IG, Snow DH, Wade JF, editors. Proceedings of the First International Camel Conference. Dubai; Newmarket: R & W Publications (1992). p. 101–4.
247. Khalafalla AI, Mohamed MEH. Clinical and epizootiological features of camelpox in eastern Sudan. J Camel Pract Res. (1996) 3:99–102.
248. Kinne J, Cooper JE, Wernery U. Pathological studies on camelpox lesions of the respiratory system in the United Arab Emirates(UAE). J Comp Pathol. (1998) 18:257–66. doi: 10.1016/S0021-9975(07)80002-8
249. Erster O, Melamed S, Paran N, Weiss S, Khinich Y, Gelman B, et al. First diagnosied case of camelpox in Israel. Virus. (2018) 10:E78. doi: 10.3390/v10020078
250. Wernery U, Zacharia R. Experimental camelpox infection in vaccinated and unvaccinated dromedaries. Zenteralblatt Veterinarmed. (1999) 46:131–5. doi: 10.1111/j.0931-1793.1999.00250.x
251. Megersa B. An Epidemiological Study of Major Camel Diseases in the Borana Lowland, Southern Ethiopia. DCG Report n°58, September 2010. Oslo: Drylands Coordination Group (2010). Available online at: http://www.drylands-group.org
252. Coetzer JAW. Poxviridae. In: Coetzer JAW, Tustin RC, editors. 2nd ed. Infect Disease Livestock. Southern Africa: Oxford University Press (2004). p. 1265–67.
253. Balamurugan V, Venkatesan G, Bhanuprakash V, Singh RK. Camelpox, an emerging orthopox viral disease. Indian J Virol. (2013) 24:295–305. doi: 10.1007/s13337-013-0145-0
254. Hafez SMA, Al-Sukayran A, Dela Cruz D, Mazloum KS, Al-Bokmy AM, Al-Mukayel A, et al. Development of a live cell culture camel pox vaccine. Vaccine. (1992) 10:533–9. doi: 10.1016/0264-410X(92)90353-L
255. Smee DF, Sidwell RW, Kefauver D, Bray M, Huggins JW. Characterization of wild type and cidofovir – resistant strains of camelpox, cowpox, monkeypox, and vaccinia viruses. Antimicrob Ag Chemother. (2002) 46:1329–35. doi: 10.1128/AAC.46.5.1329-1335.2002
256. Afzal M, Sakir M, Majid Hussain M. Corynebacterium pseudotuberculosis infection and lymphadenitis (taloa or mala) in the camel. Trop Anim Health Prod. (1996) 28:158–62. doi: 10.1007/BF03030838
257. Bloch N, Diallo IA. Probable outbreak of rabies in a group of camels in Niger. Vet Microbiol. (1995) 46:281–3. doi: 10.1016/0378-1135(95)00092-O
258. Wernery U, Kaaden OR. Infectious Diseases of Camelids. 2nd ed. Berlin: Blackwell Wissenschafts Verlag (2002). p. 110.
259. Peck EF. In: Dalling T, Robertson A, Boddie GE, Spruell JS, editors. International Encyclopaedia of Veterinary Medicine. 1st ed. Edinburgh; London: W Green and Son Ltd. (1966). p. 577.
260. Ata FA, Tageldin MH, Al Sumry HS, Al–Ismaily SI. Rabies in the sultanate of Oman. Vet Rec. (1993) 131:68–9. doi: 10.1136/vr.132.3.68
261. Al-Duraib AM. Rabies in camels at Qassim region of central Saudi Arabia. J Camel Pract Res. (2007) 1:101–3.
262. Memish ZA, Assiri AM, Gautret P. Rabies in Saudi Arabia: a need for epidemiological data. Int J Infect Dis. (2015) 34:99–101. doi: 10.1016/j.ijid.2015.03.016
263. Liu Y, Zhang H-P, Zhang S-F, Wang J-X, Zhou H-N, Zhang F, et al. Rabies outbreaks and vaccination in domestic camels and cattle in Northwest China. PLoS Negl Trop Dis. (2016) 10:e0004890. doi: 10.1371/journal.pntd.0004890
264. Imam IZE, Karamany RE, Darwish MA. Epidemic of rift valley fever in Egypt. Isolation of Rvf virus from animals. J Egypt Publ Health Assoc. (1978) 21:265–9.
265. Eisa M. Rift Valley Fever. Technical Reports, Series 1. Paris: Office Int Epizootics (OIE) (1981). p. 2–13.
266. Britch SC, Binepal YS, Ruder MG, Kariithi HM, Linthicum KJ, Anyamba A, et al. Rift Valley fever risk map model and seroprevalence in selected wild ungulates and camels from Kenya. PLoS ONE. (2013) 8:e66626. doi: 10.1371/journal.pone.0066626
267. Abdallah MMM, Adam IA, Abdalla TM, Abdelaziz SA, Ahmed ME, Aradaib IE. A survey of rift valley fever and associated risk factors among the one-humed camel (Camelus dromedaries) in Sudan. Irish Vet J. (2016) 69:6. doi: 10.1186/s13620-016-0065-6
268. Scott GR, Coakley W, Roach RW, Cowdy NR. Rift valley fever in camels. J Pathol Bacteriol. (1963) 86:229–31. doi: 10.1002/path.1700860131
269. Meegan JM, Hoogstraal H, Mousa MI. An epizootic of Rift Valley Fever in Egypt in 1977. Vet Rec. (1977) 105:124–5. doi: 10.1136/vr.105.6.124
270. Shoemaker T, Boulianne C, Vincent MJ, Pezzanite L, Al-Qahtani MM, Al-Mazrou Y, et al. Genetic analysis of viruses associated with emergence of Rift Valley fever in Saudi Arabia and Yemen, 2000–2001. Emerg Infect Dis. (2002) 8:1415–20. doi: 10.3201/eid0812.020195
271. Ould El Mamy AB, Ould Baba M, Barry Y, Isselmou K, Dia ML, Hampate B, et al. Unexpected rift valley fever outbreak, Northern Maurtitania. Emerg Infect Dis. (2011) 17:1894–6. doi: 10.3201/eid1710.110397
272. Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus ADME, Fouchier RAM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. New Engl J Med. (2012) 367:1814–20. doi: 10.1056/NEJMoa1211721
273. Azhar EI, El-Kafrawy SA, Farraj SA, Hassan AM, Al-Saeed MS, Hashem AM, et al. Evidence for camel-to-human transmission of MERS coronavirus. N Engl J Med. (2014) 370:2499–505. doi: 10.1056/NEJMoa1401505
274. Hemida MG, Elmoslemany A, Al-Hizab F, Alnaeem A, Almathen F, Faye B, et al. Dromedary camels and the transmission of Middle East Respiratory Syndrome Coronavirus (MERS-CoV). Transbound Emerg Dis. (2017) 64:344–53. doi: 10.1111/tbed.12401
275. Afelt A, Devaux C, Serra-Cobo J, Frutos R. Chapter 8: Bats. In: Mikkola H, editor. Bats, Bat-Borne Viruses, and Environment. London: IntechOpen (2018). p. 113–32. doi: 10.5772/intechopen.74377
276. Omrani AS, Al-Tawfiq JA, Memish ZA. Middle East respiratory syndrome coronavirus (MERS-CoV): animal to human interaction. Pathog Glob Health. (2015) 109:355–62. doi: 10.1080/20477724.2015.1122852
277. Sabir JSM, Lam TTY, Ahmed MMM, Li L, Shen Y, Abo-Aba SEM, et al. Co-circulation of three camel coronavirus species and recombination of MERS-CoVs in Saudi Arabia. Science. (2016) 351:81–4. doi: 10.1126/science.aac8608
278. Perera RA, Wang P, Gomaa MR, El-Shesheny R, Kandeil A, Bagato O, et al. Seroepidemiology for MERS coronavirus using microneutralisation and pseudoparticle virus neutralisation assays reveal a high prevalence of antibody in dromedary camels in Egypt, June (2013). Euro Surveill. (2013) 18:20574. doi: 10.2807/1560-7917.ES2013.18.36.20574
279. Reusken CB, Haagmans BL, Muller MA, Gutierrez C, Godeke GJ, Meyer B, et al. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative serological study. Lancet Infect Dis. (2013) 13:859–66. doi: 10.1016/S1473-3099(13)70164-6
280. Reusken CB, Farag EA, Haagmans BL, Mohran KA, Godeke GJ, Raj VS, et al. Occupational exposure to dromedaries and risk for MERS-CoV infection, Qatar, 2013–2014. Emerg Infect Dis. (2015) 21:1422–5. doi: 10.3201/eid2108.150481
281. Hemida MG, Chu DK, Poon LL, Perera RA, Alhammadi MA, Ng HY, et al. MERS coronavirus in dromedary camel herd, Saudi Arabia. Emerg Infect Dis. (2014) 20:1231–4. doi: 10.3201/eid2007.140571
282. Alexandersen S, Kobinger GP, Soule G, Wernery U. Middle East respiratory syndrome coronavirus antibody reactors among camels in Dubai, United Arab Emirates, in (2005). Transbound Emerg Dis. (2014) 61:105–8. doi: 10.1111/tbed.12212
283. Haagmans BL, van den Brand JMA, Raj VS, Volz A, Wohlsein P, Smits SL, et al. An orthopoxvirus-based vaccine reduces virus excretion after MERS-CoV infection in dromedary camels. Science. (2016) 351:77–81. doi: 10.1126/science.aad1283
284. Wernery U, Kaaden OR. Foot-and-Mouth disease in camelids: a review. Vet J. (2004) 168:134–42. doi: 10.1016/j.tvjl.2003.10.005
285. Yousef MR, Mazloum KS, Al-Nakhli HM. Serological evidence of natural exposure of camels (Camelus dromedaries) to foot and mouth disease virus. Vet World. (2012) 5:197–200. doi: 10.5455/vetworld.2012.197-200
286. Hedger RS, Barnett TR, Gray DF. Some virus diseases of domestic animals in the Sultanate of Oman. Trop Anim Health Prod. (1980) 12:107–14. doi: 10.1007/BF02242618
287. Richard D. Manuel des Maladies du Dromadaire: Project de Development de L'Elevage dans le Niger Centre-Est. Maisons-Alfort: IEMVT (1986). p. 118.
288. Moussa AA, Daoud A, Omar A, Metwally MA, El-Nimr M, McVicar JW. Isolation of foot and mouth disease virus from camels with ulcerative disease syndromes. J Egypt Vet Med Assoc. (1987) 47:219–9.
289. Frederiksen T, Borch J, Christensen J, Tjørnehøj K, Wernery U, Alexandersen S. Comparison of FMDV Type O, A and Asia 1 Antibody Levels After Vaccination of Cattle, Sheep, Dromedary Camels and Horses. Report of the Session of the Research Group of the Standing Technical Committee of the European Commission for the Control of Foot-and-Mouth Disease. Paphos (2006). p. 243–7.
290. Wernery U, Kinne J. Foot and mouth disease and similar virus infections in camelids: a review. Rev Sci Tech. (2012) 31:907–18. doi: 10.20506/rst.31.3.2160
291. Abu Elzen EME. Bluetongue in camels: a serological survey of the one- humped camel (Camelus dromedarius) in the Sudan. Rev Elev Med Vet Pays Trop. (1985) 38:438–42.
292. Chandel BS, Chauhan HC, Kher HN. Comparison of the standard AGID test and competitive ELISA for detecting bluetongue virus antibodies in camels in Gujarat, India. Trop Anim Health Prod. (2003) 35:99–104. doi: 10.1023/A:1022896117122
293. Madani H, Casal J, Alba A, Allepuz A, Cêtre-Sossah C, Hafsi L, et al. Animal diseases caused by orbiviruses, Algeria. Emerg Inf Dis. (2011) 17:2325–7. doi: 10.3201/eid1712.110928
294. Touil N, Cherkaoui Z, Lmrabih Z, Loutfi C, Harif B, El Harrak M. Emerging viral diseases in dromedary camels in Southern Morocco. Transboundary Emerg Dis. (2012) 59:177–82. doi: 10.1111/j.1865-1682.2011.01282.x
295. Baba SS, Olaleye OD, Ayanbadejo OA. Haemaglutination-inhibition antibodies against African horse sickness virus in domestic animals in Nigeria. Vet Res. (1993) 24:483–7.
296. Awad FI, Amin MM, Salama SA, Khide S. The role played by Hyalomma dromedarii in the transmission of African horse sicknessin Egypt. Bull Anim Health Prod Afr. (1981) 29:337–40.
297. Charrel RN, Fagbo S, Moureau G, Alqahtani MH, Temmam S, de Lamballerie X. Alkhurma hemorrhagic fever virus in Ornithodoros savignyi ticks. Emerg Infect Dis. (2007) 13:153–5. doi: 10.3201/eid1301.061094
298. Madani TA. Alkhumra virus infection, a new viral hemorrhagic fever in Saudi Arabia. J Infect. (2005) 51:91–7. doi: 10.1016/j.jinf.2004.11.012
299. Al-Ruwaili MA, Khalil OM, Selim SA. Viral and bacterial infections associated with camel (Camelus dromedarius) calf diarrhea in North Province, Saudi Arabia. Saudi J Biol Sci. (2012) 19:35–41. doi: 10.1016/j.sjbs.2011.10.001
300. El-Fakharany EM, El-Baky NA, Linjawi MH, Aljaddawi AA, Saleem TH, Nassar AY, et al. Influence of camel milk on the hepatitis C virus burden of infected patients. Exp Therap Med. (2017) 13:1313–20. doi: 10.3892/etm.2017.4159
301. Alghamdi A, Hassan AM, TolahAM Alamari SS, Alzahrani AA, Alsaaidi GA, et al. Molecular evidence of influenza A virus circulation in African dromedary camels imported to Saudi Arabia, 2017-2018. Open Forum Infect Dis. (2018) 6:ofz370. doi: 10.1093/ofid/ofz370
302. Saidi R, Bessas A, Bitam I, Ergün Y, Ataseven VS. Bovine herpesvirus-1 (BHV-1), bovine leukemia virus (BLV), and bovine viral diarrhea virus (BVDV) infection in Algerian dromedary camels (Camelus dromedarius). Trop Anim Hlth Prod. (2018) 50:561–4. doi: 10.1007/s11250-017-1469-3
303. Salim B, de Meeûs T, Bakheit MA, Kamau J, Nakamura I, Sugimoto C. Population genetics of Trypanosoma evansi from camel in the Sudan. PLoS Negl Trop Dis. (2011) 5:e1196. doi: 10.1371/journal.pntd.0001196
304. Bennoune O, Adili N, Amri K, Bennecib L, Ayachi A. Trypanosomiasis of camels (Camelus dromedarius) in Algeria: first report. Vet Res Forum. (2013) 4:273–5.
305. Khosravi A, Hakimi Parizi M, Bamorovat M, Borhani Zarandi M, Ali Mohammadi M. Prevalence of Trypanosoma evansi in camels using molecular and parasitological methods in the southeast of Iran 2011. J Parasit Dis. (2015) 39:422–5. doi: 10.1007/s12639-013-0355-9
306. El Wathig M, Faye B, Thevenon S, Ravel S, Bossard G. Epidemiological surveys of camel trypanosomosis in Al-jouf, Saudi Arabia based on PCR and ELISA. Emirates J Food Agric. (2016) 28:212–6. doi: 10.9755/ejfa.2015-09-759
307. Aregawi WG, Agga GE, Abdi RD, Büsher P. Systematic review and meta-analysis on the global distribution, host range, and prevalence of Trypanosoma evansi. Parasit Vectors. (2019) 12:67. doi: 10.1186/s13071-019-3311-4
308. Mossaad E, Salim B, Suganuma K, Musinguzi P, Hassan M, Elamin E, et al. Trypanosoma vivax is the second leading cause of camel trypanosomosis in Sudan after Trypanosoma evansi. Parasit Vectors. (2017) 10:176. doi: 10.1186/s13071-017-2117-5
309. Zakian A, Nouri M, Safaei P, Mohammad-Sadegh M, Kahroba H, Mokhber-Dezfouli MR, et al. An acute outbreak of natural Trypanosoma evansi infection in camel (Camelus dromedarius) herds in the southwestern Iran. Comp Clin Pathol. (2017) 26:51–9. doi: 10.1007/s00580-016-2345-7
310. Barghash SM, Hafez AA, Darwish AM, El-Naga TRA. Molecular detection of pathogens in ticks infesting camels in Matrouh Governorate, Egypt. J Bacteriol Parasitol. (2016) 7:259. doi: 10.4172/2155-9597.1000269
311. El-Khouly AB, Gadir FA, Cluer DD, Manefield GW. Aspergillosis in camels affected with a specific respiratory and enteric syndrome. Austral Vet J. (1992) 69:182–6. doi: 10.1111/j.1751-0813.1992.tb07515.x
312. El-Naga Barghash J. Blood parasites in camels (Camelus dromedarius) in Northern West coast of Egypt. J Bacteriol Parasitol. (2016) 7:1. doi: 10.1016/j.parepi.2016.07.002
313. Riley J, Garner MM, Kiupel M, Hammond EE. Disseminated toxoplasmosis in a captive adult dromedary camel (Camelus dromedarius). J Zoo Wildl Med. (2017) 48:937–40. doi: 10.1638/2016-0057.1
314. Abbas B, Agab H. Review of camel brucellosis. Prev Vet Med. (2002) 55:47–56. doi: 10.1016/S0167-5877(02)00055-7
315. Hamdy ME, Amin AS. Detection of Brucella species in milk of infected cattle, sheep, goat and camels by PCR. Vet J. (2002) 163:299–305. doi: 10.1053/tvjl.2001.0681
316. Sprague LD, Al-Dahouk S, Neubauer H. A review on camel brucellosis: a zoonosis sustained by ignorance and indifference. Pathog Glob Health. (2012) 106:144–9. doi: 10.1179/2047773212Y.0000000020
317. Gwida M, El-Gohary A, Melzer F, Khan I, Rösler U, Neubauer H. Brucellosis in camels. Res Vet Sci. (2012) 92:351–5. doi: 10.1016/j.rvsc.2011.05.002
318. Ramadan RO, Hatim ME, Abdin-Bey MR. Isolation of Brucella melitensis from carpal hygroma in camel. J Camel Pract Res. (1998) 5:239–41.
319. Al-Khalaf S, El-Khaladi A. Brucellosis of camels in Kuwait. Comp Immunol Microbiol Infect Dis. (1989) 12:1–4. doi: 10.1016/0147-9571(89)90002-7
320. Gameel SE, Mohamed SO, Mustafa AA, Azwai SM. Prevalence of camel brucellosis in Libya. Trop Anim Health Prod. (1993) 25:91–3. doi: 10.1007/BF02236513
321. Bayasgalan C, Chultemdorj T, Roth F, Zinsstag J, Hattendorf J, Badmaa B, et al. Risk factors of brucellosis seropositivity in bactrian camels of Mongolia. BMC Vet Res. (2018) 14:342. doi: 10.1186/s12917-018-1664-0
322. Radwan AI, Bekairi SI, Mukayel AA, al-Bokmy AM, Prasad PV, Azar FN, et al. Control of Brucella melitensis infection in a large camel herd in Saudi Arabia using antibiotherapy and vaccination with rev.1 vaccine. Rev Sci Tech. (1995) 14:719–32. doi: 10.20506/rst.14.3.860
323. Agab H, Angus RD, Abbas B, Mamoun IE. Serologic response of camels (Camelus dromedarius) to Br. abortus S19 vaccine. J Camel Pract Res. (1995) 2:93–5.
324. Pappas G, Papadimitriou P, Akritidis N, Chistou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. (2006) 6:91–9. doi: 10.1016/S1473-3099(06)70382-6
325. Skalsky K, Yahav D, Bishara J, Pitlik S, Leibovici L, Paul M. Treatment of human brucellosis: systematic review and meta-analysis of randomised controlled trials. Brit Med J. (2008) 336:701–4. doi: 10.1136/bmj.39497.500903.25
326. Hasanjani Roush MR, Ebrahimpour S. Human brucellosis: an overview. Caspian J Intern Med. (2015) 6:46–7.
327. Garcell HG, Garcia EG, Pueyo PV, Martin IR, Arias AV, Alfonso Serrano RN. Outbreaks of brucellosis related to the consumption of unpasteurized camel milk. J Infect Publ Health. (2016) 9:523–7. doi: 10.1016/j.jiph.2015.12.006
328. Chartier F, Chartier C, Thovel MF, Crespeau F. A new case of Mycobacterium bovis pulmonary tuberculosis in the dromedary (Camelus dromedarius) in Mauritania. Rev Elev Med Vet Pays Trop. (1991) 44:43–7.
329. Refai M. Bacterial and mycotic diseases of camels in Egypt. In: Proceedings of the First International Camel Conference. Dubai: Newmarket Press (1992).
330. Kinne J, Johnson B, Jahans KL, Smith NH, Ul-Haq A, Wernery U. Camel tuberculosis-a case report. Trop Anim Health Prod. (2006) 38:38. doi: 10.1007/s11250-006-4366-8
331. Mamo G, Bayleyegn G, Sisay Tessema T, Legesse M, Medhin G, et al. Pathology of camel tuberculosis and molecular characterization of its causative agents in pastoral regions of Ethiopia. PLoS ONE. (2011) 6:e15862. doi: 10.1371/journal.pone.0015862
332. Legesse M, Mamo G, Ameni G, Medhin G, Bjune G, Abebe F. Community-based prevalence of undiagnosed mycobacterial disease in the Afar region, north-east Ethiopia. Int J Mycobacteriol. (2013) 2:94–102. doi: 10.1016/j.ijmyco.2013.04.001
333. Beyi AF, Gezahegne KZ, Mussa A, Ameni G, Ali MS. Prevalence of bovine tuberculosis in dromedary camels and awareness of pastoralists about its zoonotic importance in eastern Ethiopia. J Vet Med Anim Health. (2014) 6:109–15. doi: 10.5897/JVMAH2014.0284
334. Higgins A. The camel in health and disease. Br Vet J. (1984) 140:482–4. doi: 10.1016/0007-1935(84)90044-7
336. Mamo G, Kassaye A, Sanni M, Ameni G. A cross sectional study of camel tuberculosis in Ethiopia. Bull Anim Health Prod Afr. (2009) 57:13–20. doi: 10.4314/bahpa.v57i1.44047
337. Elnaker YF, Diab MS, Ibrahim NA, El-Gedawy A, Zaki RS, Radwan A. Seroprevalence and molecular characterization of Mycobacterium bovis infection in camels (Camelus dromedarius) in the Delta region, Egypt. Vet World. (2019) 12:1180–7. doi: 10.14202/vetworld.2019.1180-1187
338. Gumi B, Schelling E, Berg S, Firdessa R, Erenso G, Mekonnen W, et al. Zoonotic transmission of tuberculosis between pastoralists and their livestock in south-east Ethiopia. EcoHealth. (2012) 9:139–49. doi: 10.1007/s10393-012-0754-x
339. Devaux CA, Mediannikov O, Medkour H, Raoult D. Infectious disease risk acroos the growing human-non human primate interface: a review of the evidence. Front Pub Health. (2019) 7:305. doi: 10.3389/fpubh.2019.00305
342. Buchnev KN, Tulepbaev SZ, Sansyzbaev AR. Infectious diseases of camels in the USSR. Rev Sci Tech. (1987) 6:487–95. doi: 10.20506/rst.6.2.297
343. Radwan A, El Magwary S, Hawari A, Al Bikairi SJ, Aziz S, Rebelza RM. Paratuberculosis enteritis (Johne's disease) in camels in Saudi Arabia. J Saudi Soc Biol Sci. (1991) 1:57–66.
>344. Salem MA, El-Deeb WM, Zaghawa AA, Housawi FM, Alluwaimi AM. Investigation of mycobacterium paratuberculosis in Arabia n dromedary camels (Camelus dromedarius). Vet World. (2019) 12:218–23. doi: 10.14202/vetworld.2019.218-223
345. Gluecks IV, Bethe A, Younan M, Ewers C. Molecular study on Pasteurella multocida and Mannheimia granulomatis from Kenyan camels (Camelus dromedarius). BMC Vet Res. (2017) 13:265. doi: 10.1186/s12917-017-1189-y
346. Hassan AKM, Mustafa AA. Isolation of Pasteurella multocida type B from an outbreak of haemorrhagic septicemia in camels in Sudan. Rev Elev Med Vet Pays Trop. (1985) 38:31–3.
347. Momin RR, Petkar DK, Jaiswal TN, Jhala VM. An outbreak of pasteurolosis in camels. Indian Vet J. (1987) 64:896–7.
348. Abubakr MI, Nayel MN, Fadlalla ME. Corynebacterium abscesses in camels in Bahrain. J Camel Prac Res. (1999) 6:107–9.
349. Barakat AA, Sayour E, Fayed AA. Investigation of an outbreak of anthrax in camels in the western desert. J Egypt Vet Med Assoc. (1976) 36:183–6.
350. Dawood Hawari A. Corynebacterium pseudotuberculosis infection (Caseous lymphadenitis) in camels (Camelus dromedarius) in Jordan. Am J Anim Vet Sci. (2008) 3:68–72. doi: 10.3844/ajavsp.2008.68.72
351. Christie AB, Chen TH, Elberg SS. Plague in camels and goats: their role in human epidemics. J Infect Dis. (1980) 141:724–6. doi: 10.1093/infdis/141.6.724
352. Barbieri R, Drancourt M, Raoult D. Plague, camels, and lice. Proc Natl Acad Sci USA. (2019) 116:7620–1. doi: 10.1073/pnas.1901145116
353. Arbaji A, Kharabsheh S, Al-Azab S, Al-Kayed M, Amr ZS, Abu Baker M, et al. A 12-case outbreak of pharyngeal plague following the consumption of camel meat, in north-eastern Jordan. Ann Trop Med Parasitol. (2005) 99:789–93. doi: 10.1179/136485905X65161
354. Bin Saeed AA, Al-Hamdan NA, Fontaine RE. Plague from eating raw camel liver. Emerg Infect Dis. (2005) 11:1456–7. doi: 10.3201/eid1109.050081
355. Younan M, Ali Z, Bornestein S, Muller W. Application of the California mastitis test in intramammary Streptococcus agalctiae and Staphylococcus aureus infections of camels (Camelus dromedarius) in Kenya. Prev Vet Med. (2001) 51:307–16. doi: 10.1016/S0167-5877(01)00228-8
356. Guliye AY, van Creveld C, Yagil R. Detection of subclinical mastitis in dromedary camels (Camelus dromedarius) using somatic cell counts and the Nacetyl- beta-glucosaminidase test. Trop Anim Health Prod. (2002) 34:95–104.
357. Shigidi MTA. Aerobic microflora of the respiratory tract of camels. Sudan J Vet Sci Anim Husbandry. (1973) 14:19–4.
358. Chauhan RS, Gupta SC, Satiya KC, Kulshreshitha RC, Kaushik RK. Bacterial flora of upper respiratory tract in apparently healthy camels. Indian J Anim Sci. (1987) 57:424–6.
359. Al-Rawashdeh OF, Al-Ani FK, Sharif LA, Al-Qudah KM, Al-Hami Y, Frank N. A survey of camel (Camelus dromedarius) diseases in Jordan. J Zoo Wildlife Med. (2000) 31:335–8. doi: 10.1638/1042-7260(2000)031[0335:ASOCCD]2.0.CO;2
360. Bekele T. Studies on the respiratory disease 'Sonbobe' in camels in the eastern lowlands of Ethiopia. Trop Anim Health Prod. (1999) 31:333–45.
361. Yagoub SO, Mohamed GE. Incidence, clinical observation and etiology of contagious skin necrosis in camels (Camelus dromedarius) in the Sudan. J Camel Pract Res. (1996) 3:95–8.
362. Tejedor MT, Martin JL, Lupiola P, Gutierrez C. Caseous lymphadenitis caused by Corynebactrium ulcerans in the dromedary camel. Canad Vet J. (2000) 41:126–7.
363. Provost A, Haas P, Dembelle M. First case of animal botulism (type c) in Chad: Intoxication of camels by well water. “Premiers cas au Tchad du botulisme animal (type c): intoxication des dromadaires par l'eau d'un puit.” Rev Elev Med Vet Pays Trop. (1975) 28:9–12. doi: 10.19182/remvt.8070
365. Chamoiseau G, Bah SO, Fall SMOA. A case of pulmonary tuberculosis in a dromedary. “Un cas de tuberculose pulmonaire chez un dromadaire” [in French]. Rev Elev Med Vet Pays Trop. (1985) 38:28–30.
366. Cheyne IA, Pegram RG, Cartwright CF. An outbreak of salmonellosis in camels in the North-East of the Somali democratic republic. Trop Anim Health Prod. (1977) 9:238–40. doi: 10.1007/BF02240346
367. Pegram RG. Camel salmonellosis in the horn of Africa. In: Allen WR, Higgins AJ, Maybew IG, Snow DH, Wade JF, editors. Proceedings of the 1st International Camel Conference. Dubai: Newmarket, UK: R & W Publications (1992). p. 402.
368. Bengoumi M, Berrada J, Rochdi M, Hidane K, De Lafarge F, Faye B. Physiopathology of diarrhea in the camel calf in Morocco. Clinical signs and metabolic disturbances “Physiopathologie des diarrhees du chameleon au Maroc. signes cliniques et perturbations metaboliques” [in French]. Rev Elev MedVet Pays Trop. (1998) 51:277–81.
369. FAOSTAT (2019). Available online at: http://www.fao.org/faostat/fr/#data (accessed October 7, 2020).
370. Mohabbati Mobarez A, Bagheri Amiri F, Esmaeili S. Seroprevalence of Q fever among human and animal in Iran: a systematic review and meta-analysis. PLoS Negl Trop Dis. (2017) 11:e0005521. doi: 10.1371/journal.pntd.0005521
371. Browne AS, Deem SL, Fèvre EM, Kinnaird M, Muloi DM, Wang CA, et al. Serosurvey of Coxiella burnetii (Q fever) in Dromedary Camels (Camelus dromedarius) in Laikipia County, Kenya. Zoonoses Publ Health. (2017) 64:543–9. doi: 10.1111/zph.12337
372. Jarelnabi AA, Alshaikh MA, Bakhiet AO, Omer SA, Aljumaah RS, Harkiss GD, et al. Seroprevalence of Q fever in farm animals in Saudi Arabia. Biomed Res. (2018) 29:895–900. doi: 10.4066/biomedicalresearch.29-17-770
373. Soliman A, Boulos A, Botros M, Watts D. Evaluation of a competitive enzyme immunoassay for detection of Coxiella burnetii antibody in animal sera. J Clin Microbiol. (1992) 30:1595–7. doi: 10.1128/JCM.30.6.1595-1597.1992
374. Abdullah HHAM, Hussein HA, Abd Ei-Razik KA, Barakat AMA, Soliman YA. Q fever: a neglected disease of camels in Giza and Cairo Provinces Egypt. Vet World. (2019) 12:1945–50. doi: 10.14202/vetworld.2019.1945-1950
375. Janati Pirouz H, Mohammadi G, Mehrzad J, Azizzadeh M, Nazem Shirazi MH. Seroepidemiology of Q fever in one-humped camel population in northeast Iran. Trop Anim Hlth Prod. (2015) 47:1293–8. doi: 10.1007/s11250-015-0862-z
376. Hussein MF, Alshaikh M, Gad El-Rab MO, Aljumaah RS, Gar El Nabi AR, Abdel Bagi AM. Serological prevalence of Q fever and chlamydiosis in camels in Saudi Arabia. J Anim Vet Adv. (2008) 7:685–8. Available online at: https://medwelljournals.com/abstract/?doi=javaa.2008.685.688
377. Hussein MF, Alshaikh MA, Al-Jumaah RS, GarelNabi A, Al-Khalifa I, Mohammed OB. The Arabian camel (Camelus dromedarius) as a major reservoir of Q fever in Saudi Arabia. Comp Clin Pathol. (2014) 215:887–92 doi: 10.1007/s00580-014-2002-y
378. Benaissa MH, Ansel S, Mohamed-Cherif A, Benfodil K, Khelef D, Youngs CR, et al. Seroprevalence and risk factors for Coxiella burnetii, the causative agent of Q fever in the dromedary camel (Camelus dromedarius) population in Algeria. Onderstepoort J Vet Res. (2017) 84:a1461. doi: 10.4102/ojvr.v84i1.1461
379. Larson PS, Espira L, Grabow C, Wang CA, Muloi D, Browne AS, et al. The sero-epidemiology of Coxiella burnetii (Q fever) across livestock species and herding contexts in Laikipia County, Kenya. Zoonoses Publ Health. (2019) 66:316–24. doi: 10.1111/zph.12567
380. Mentaberre G, Gutiérrez C, Rodriguez NF, Joseph S, Gonzalez-Barrio D, Cabezon O, et al. A transversal study on antibodies against selected pathogens in dromedary camels in the Canary Islands, Spain. Vet Microbiol. (2013) 167:468–73. doi: 10.1016/j.vetmic.2013.07.029
381. Maurice Y, Bares JF, Baille M. Serological survey on rickettsial diseases in dromedaries in Chad “Enquête sérologique sur les rickettsioses chez le dromadaire au Tchad” [in French]. Rev Elev Med Vet Pays trop. (1967) 20:543–50. doi: 10.19182/remvt.7454
383. Abdullah HHAM, El-Shanawany EE, Abdel-Shafy S, Abou-Zina HAA, Abdel-Rahman EH. Molecular and immunological characterization of Hyalomma dromedarii and Hyalomma excavatum (Acari: Ixodidae) vectors of Q fever in camels. Vet World. (2018) 11:1109–19. doi: 10.14202/vetworld.2018.1109-1119
384. Kulshreshtha RC, Arora RG, Kalra DS. Sero-prevalence of Q fever in camels, buffaloes and pigs. Indian J Med Res. (1974) 62:1314–6.
385. Mathur KN, Bhargava SC. Seroprevalence of Q fever and brucellosis in camels of Iorbeer and Bikaner Raiasthan State, India. Indian J Med Res. (1979) 70:391–3.
386. Brown RD. Serological testing of Q fever in domestic animals in Kenya. “La mise en évidence, par tests sérologiques, de la fièvre Q chez les animaux domestiques au Kenya” [in French]. Bull Epiz Dis Afr. (1956) 4:115–9.
387. Addo PB. A serological survey for evidence of Q fever in camels in Nigeria. Br Vet J. (1980) 136:519–21. doi: 10.1016/S0007-1935(17)32198-X
388. Harbi MSMA, Awad El Karim MH. Serological investigation into Q fever in Sudanese camels (Camelus dromedarius). Bull Epizoot Dis Afr. (1972) 20:15–7.
389. Abbas B, Yassin TTM, Elzubir AEA. Survey for certain zoonotic diseases in camels in the Sudan. Rev Elev Med Vet Pays Trop. (1987) 40:231–3.
390. Burgemeister R, Leyk W, Goessler R. Investigations on the occurrence of parasites and of bacterial and virus infections in Southern Tunisia dromedaries. “Untersuchungen ûber Vorkommen von Parasitosen, bakteriellen und viralen Infektions-krankheiten bei Dromedaren in Sûdtunesien” [in German]. Dtsch Tierärztl Wschr. (1975) 82:352–4.
391. Selmi R, Mamlouk A, Ben Yahia H, Abdelaali H, Ben Said M, Sellami K, et al. Coxiella burnetii in Tusian dromedary camels (Camelus dromedarius): seroprevalence, associated risk factors and seasonal dynamics. Acta Trop. (2018) 188:234–9. doi: 10.1016/j.actatropica.2018.09.008
392. Selmi R, Ben Said M, Mamlouk A, Ben Yahia H, Messadi L. Molecular detection and genetic characterization of the potentially pathogenic Coxiella burnetii and the endosymbiotic Candidatus Midichloria mitochondrii in ticks infesting camels (Camelus dromedarius) from Tunisia. Microb Pathogenesis. (2019) 136:103655. doi: 10.1016/j.micpath.2019.103655
393. Abdullah HHAM, El-Molla A, Salib FA, Allam NAT, Ghazy AA, Abdel-Shafy S. Morphology and molecular identification of the brown dog tick Rhipicephalus sanguineus and the camel tick Hyalomma dromedarii (Acari: Ixodidae), vector of Rickettsioses in Egypt. Vet World. (2016) 9:1087–101. doi: 10.14202/vetworld.2016.1087-1101
394. Salim abadi Y, Telmadarraiy Z, Vatandoost H, Chinikar S, Oshaghi MA, Moradi M, et al. Hard ticks on domestic ruminants and their seasonal population dynamics in Yazd province, Iran. Iran J Arthropod Borne Dis. (2010) 4:66–71.
395. Ganjali M, Dabirzadeh M, Sargolzaie M. Species diversity and distribution of ticks (Acari: Ixodidae) in Zabol county, Eastern Iran. J Arthropod Borne Dis. (2014) 8:219–23.
396. Mohammed OB, Jarelnabi AA, Aljumaah RS, Alshaikh MA, Bakhiet AO, Omer SA, et al. Coxiella burnetii, the causative agent of Q fever in Saudi Arabia: molecular detection from camel and other domestic livestock. Asian Pacific J Trop Med. (2014):715–9. doi: 10.1016/S1995-7645(14)60122-X
397. Loftis AD, Reeves WK, Szumlas DE, Abbassy MM, Helmy IM, Moriarity JR, et al. Surveillance of Egyptian fleas for agents of public health significance: Anaplasma, Bartonella, Coxiella, Ehrlichia, Rickettsia, and Yersinia pestis. Am J Trop Med Hyg. (2006) 75:41–8. doi: 10.4269/ajtmh.2006.75.41
398. Leulmi H, Aouadi A, Bitam I, Bessas A, Benakhla A, Raoult D, et al. Detection of Bartonella tamiae, Coxiella burnetii and rickettsiae in arthropods and tissues from wild and domestic animals in northeastern Algeria. Parasit Vectors. (2016) 9:27. doi: 10.1186/s13071-016-1316-9
400. Balashov YS. Interaction between blood-sucking arthropods and their hosts, and its influence on vector potential. Ann Rev Entomol. (1984) 29:137–56. doi: 10.1146/annurev.en.29.010184.001033
401. Mediannikov O, Fenollar F, Socolovschi C, Diatta G, Bassene H, Molez JF, et al. Coxiella burnetii in humans and ticks in rural Senegal. PLoS Negl Trop Dis. (2010) 4:e654 doi: 10.1371/journal.pntd.0000654
402. Aouadi A, Leulmi H, Boucheikhchoukh M, Benakhla A, Raoult D, Parola P. Molecular evidence of tick-borne hemoprotozoan-parasites (Theileria ovis and Babesia ovis) and bacteria in ticks and blood from small ruminants in Northern Algeria. Comp Immunol Microbiol Infect Dis. (2017) 50:34–9. doi: 10.1016/j.cimid.2016.11.008
403. Bolanos-Rivero M, Carranza-Rodriguez C, Rodriguez NF, Gutierrez C, Pérez-Arellano JL. Detection of Coxiella burnetii DNA in peridomestic and wild animals and ticks in an endemic region (Canary Islands, Spain). Vector Borne Zoon Dis. (2017) 17:630–4. doi: 10.1089/vbz.2017.2120
404. Kruszewka D, Tylewska-Wierzbanowska SK. Coxiella burnetii penetration into the reproductive system of male mice, promoting sexual transmission of infection. Inf Immun. (1993) 61:4188–95. doi: 10.1128/IAI.61.10.4188-4195.1993
405. Bechah Y, Verneau J, Ben Amara A, Barry AO, Lépolard C, Achard V, et al. Persistence of Coxiella burnetii, the agent of Q fever, in murine adipose tissue. PLoS ONE. (2014) 9:e97503. doi: 10.1371/journal.pone.0097503
406. Bengoumi M, Faulconnier Y, Tabarani A, Sghiri A, Faye B, Chilliard Y. Effect of feeding level on body weight, hump size, lipid content and adipocyte volume in the dromedary camel. Anim Res. (2005) 54:383–93. doi: 10.1051/animres:2005029
407. Reed CA. The origin of the domestic animals of Africa. Science. (1972) 176:656–7. doi: 10.1126/science.176.4035.656
409. Wu H, Guang X, Al-Fageeh MB, Cao J, Pan S, Zhou H, et al. Camelid genomes reveal evolution and adaptation to desert environments. Nat Commun. (2014) 5:5188. doi: 10.1038/ncomms6188
411. Ji R, Cui P, Ding F, Geng J, Gao H, Zhang H, et al. Monophyletic origin of domestic bactrian camel (Camelus bactrianus) and its evolutionary relationship with the extant wild camel (Camelus bactrianus ferus). Anim Genet. (2009) 40:377–82. doi: 10.1111/j.1365-2052.2008.01848.x
412. Moqaddam E, Namaz-Zadeh KP. An Introduction to Various Breeds of Camel in Iran. Mazraeh: Anal Educ Magazine (1998) 11:73–8.
414. Jianlin H, Quau J, Men Z, Zhang Y, Wang W. Three unique restriction fragment length polymorphisms of EcoR I, Pvu II and Sca I digested mitochondrial DNA of wild Bactrian camel (Camelus bactrianus ferus) in China. J Animal Sci. (1999) 77:2315–6. doi: 10.2527/1999.7782315x
415. Dupuy C. The appearance and expansion of Camelus dromedarius in northern Africa (Nile Valley, Maghreb, Sahara, Sahel). “L'apparition et l'expansion du Camelus dromedarius dans le nord de l'Afrique (Vallée du Nil, Maghreb, Sahara, Sahel)”. Le Saharien. (2009) 190:7–10.
416. Trinks A, Burger P, Beneke N, Burger J. Simulations of populations ancestry of the two-humped camel (Camelus bactrianus). In: Knoll E, Burger P, editors. Camels in Asia and North Africa,” Interdisciplinary Perspectives on Their Significance in Past and Present. Vienna: Academy of Science Press (2012). p. 79–86.
417. Almathen F, Charruau P, Mohandesan E, Mwacharo JM, OrozcoterWengel P, Pitt D, et al. Ancient and modern DNA reveal dynamics of domestication and cross-continental dispersal of the dromedary. Proc Natl Acad Sci USA. (2016) 113:6707–12. doi: 10.1073/pnas.1519508113
418. Khanna ND. Camels in India from photo-historic to the present time. Ind J Anim Sci. (1990) 60:1093–101.
419. Zeder MA, Hesse B. The initial domestication of goats (Capra hircus) in the Zagros mountains 10,000 years ago. Science. (2000) 287:2254–7. doi: 10.1126/science.287.5461.2254
420. Bradley DG, MacHugh DE, Cunningham P, Loftus RT. Mitochondrial DNA diversity and the origins of African and European cattle. Proc Natl Acad Sci USA. (1996) 93:5131–5. doi: 10.1073/pnas.93.10.5131
421. Stiles N. The dromedary against desert progression. “Le dromadaire contre l'avancée du désert”. [in French]. La Recherche. (1988) 201:948–52.
422. Konuspayeva G, Loiseau G, Faye B. The health added value of raw and fermented camel milk: the experience of Kazakhstan. La plus-value ≪ santé ≫ du lait de chamelle cru et fermenté: l'expérience du Kazakhstan [in French]. Renc Rech Rumin. (2004) 11:47–50.
423. Faye B. Combating desertification: the added value of the camel farming. Ann Arid Zones. (2011) 50:1–11.
424. Yagil R. The desert camel. Comparative Physiological Adaptation. Comparative Animal Nutrition. Basel: Karger (1985)
425. Peyre de Fabregues B. The dromedary in its natural environment. “Le dromadaire dans son milieu naturel”. [in French]. Rev Elev Méd Vét Pays Trop. (1989) 42:127–32.
426. Ben Aissa R. Le dromadaire en Algérie [in French]. In: Tisserand JL, editor. Séminaire sur la Digestion, la Nutrition et L'alimentation du Dromadaire. Zaragoza: CIHEAM, Options Méditerranées (1989) p. 19–28.
427. Sghaier M. Camel production systems in Africa. In: Cardellino R, Rosati A, Mosconi C, editors. FAO-ICAR Seminar on Camelids: Current Status of Genetic Resources, Recording and Production Systems in African, Asian and Americans Camelids. Sousse: ICAR Tech Series (2004). p. 19–30.
428. Bourbouze A. Livestock production in the steppes of northern Africa: a rereading of the Pastoral Society in Maghreb. “Systèmes d'élevage et production animale dans les steppes du nord de l'Afrique: une relecture de la société pastorale du Maghreb” [in French]. Sci Chang Planétaires/Sécheresse. (2006) 17:31–9.
429. Djemali M. Camel resources in North Africa. In: Cardellino R, Rosati A, Mosconi C, editors. FAO-ICAR Seminar on Camelids: Current Status of Genetic Resources, Recording and Production Systems in African, Asian and Americans Camelids. Sousse: ICAR Tech Series (2004). p. 51–8.
430. Guerouali A, Acharbane R. Camel genetic resources in Morocco. In: Cardellino R, Rosati A, Mosconi C, editors. FAO-ICAR Seminar on Camelids: Current Status of Genetic Resources, Recording and Production Systems in African, Asian and Americans Camelids. Sousse: ICAR Tech Series (2004). p. 61–72.
432. Zarrouk A, Souilem O, Beckers JF. News about female dromedary (Camelus dromedarius) breeding. “Actualités sur la reproduction chez la femelle dromadaire (Camelus dromedarius)”. [in French]. Rev Elev Méd Vét Pays Trop. (2003) 56:95–102. doi: 10.19182/remvt.9882
433. Tibary A, Fite C, Anouassi A, Sqhiri A. Infectious causes of reproductive loss in camelids. Theriogenology. (2006) 66:633–47. doi: 10.1016/j.theriogenology.2006.04.008
434. Ismail MD, Al Mutairi SE. Milk production potential of dairy camels in northern Saudi Arabia. In: Syomposia on Dromedary Camels as Milk Producing Livestock. Nouakchott (1998).
435. Faye B. Dairy production potential of camels. In: Cardellino R, Rosati A, Mosconi C, editors. FAO-ICAR Seminar on Camelids: Current Status of Genetic Resources, Recording and Production Systems in African, Asian and Americans Camelids. Sousse: ICAR Tech Series (2004) 11:93–104.
436. Zibaee S, Hosseini SM, Yousefi M, Taqhipour A, Kiani MA, Noras MR. Nutritional and therapeutic characteristics of camel milk in children: a systematic review. Electron Physician. (2015) 7:1523–8. doi: 10.19082/1523
437. Wilson RT. The one-humped camel in the word. Options Méditerranéennes -Série Séminaires. (1989) 2:15–7.
438. Faye B, Jaouad M, Bhrawi K, Senoussi A, Bengoumi M. Camel breeding in North Africa: current situation and perspectives/camel farming in North Africa. “Elevage camelin en Afrique du Nord: état des lieux et perspectives/camel farming in North Africa”. [in French] Rev Elev Med Vet Pays Trop. (2014) 67:213–21. doi: 10.19182/remvt.20563
439. Gossner C, Danielson N, Gervelmeyer A, Berthe F, Faye B, Kaasik Aaslav K, et al. Human-dromedary camel interactions and the risk of acquiring zoonotic middle east respiratory syndrome coronavirus infection. Zoonoses Publ Health. (2016) 63:1–9. doi: 10.1111/zph.12171
440. Wardeh MF, Dawa M. Camels and dromedaries: general perpectives. In: Cardellino R, Rosati A, Mosconi C, editors. FAO-ICAR Seminar on Camelids: Current Status of Genetic Resources, Recording and Production Systems in African, Asian and Americans Camelids. Sousse: ICAR Tech Series (2004). p. 1–9.
441. Ahmad S, Yaqoob N, Hashmi N, Ahmad S, Zaman MA, Tariq M. Economic importance of camel: a unique alternative under crisis. Pak Vet J. (2010) 30:191–7.
443. Seboussi R, Faye B, Alhadrami G. Factor of variation of selenium copper and zinc and enzymatic biomarker of muscle injury in the serum of Camelus dromedarius in UAE. “Facteurs de variation de quelques éléments trace (sélénium, cuivre, zinc) et d'enzymes témoins de la souffrance musculaire dans le sérum du dromadaire (Camelus dromedarius) aux Emirats arabes unis” [in French]. Rev Elev Méd Vét Pays Trop. (2004) 57:87–94. doi: 10.19182/remvt.9911
444. Kadim IT, Mahgoub O. Camelid genetic resources. a report on three Arabian Gulf countries. In: Cardellino R, Rosati A, Mosconi C, editors. FAO-ICAR Seminar on Camelids: Current Status of Genetic Resources, Recording and Production Systems in African, Asian and Americans Camelids. Sousse: ICAR Tech Series (2004). p. 81–92.
445. FAO. Characterization and Value Addition to Local Breeds and their Products in the Near East and North Africa – Regional Workshop, Rabat, Morocco, 19-21 November 2012. Rome: Animal Production and Health Report No. 3 (2014).
446. Ali Fouad E, Abu Elnaga ASM, Kandil MM. Antibacterial efficacy of Moringa oleifera leaf extractagainst pyogenic bacteria isolated from a dromedary camel (Camelus dromedarius) abscess. Vet World. (2019) 12:802–8. doi: 10.14202/vetworld.2019.802-808
448. Bakory MA. Preliminary genetic study of Libyan camels using DNA based PAPD and SSR markers. Anim Sci J. (2012) 3:6–10.
449. Aissa B. Dromedary in Algeria. “Le dromadaire en Algérie” [in French]. Opt Méditerr. (1989) 2:19–28.
450. Amine CY, Samir GSB, Nasreddine M, Nacera TA, Nadhira SM. Study of camelina biodiversity in southwestern of algeria. J Life Sci. (2013) 7:416.
451. Cherifi YA, Gaouar SBS, Guastamacchia R, El-Bahrawy KA, Abushady AMA, Sharaf AA, et al. Weak genetic structure in northern African dromedary camels reflects their unique evolutionary history. PLoS ONE. (2017) 12:e0168672. doi: 10.1371/journal.pone.0168672
452. Ali A, Baby B, Vijayan R. From desert to medicine: a review of camel genomics and therapeutic products. Front Genet. (2019) 10:17. doi: 10.3389/fgene.2019.00017
453. Mukasa-Mugerwa E. The Camel (Camelus dromedarius): a Bibliographical Review. Nairobi: ILRI. (1981).
454. Ramadan S, Inoue-Murayama M. Advances in camel genomics and their applications: a review. J Anim Genet. (2017) 45:49–58. doi: 10.5924/abgri.45.49
455. Abdallah HR, Faye B. Phenotypic classification of Saudi Arabian camel (Camelus dromedarius) by their body measurements. Emir J Food Agric. (2012) 24:272–80.
456. Massad JA. Colonial Effects: The Making of National Identity in Jordan. New York, NY: Columbia University Press (2012).
457. Abdelrahman MM, Aljumaa RS, Ayadi M. Selenium and iodine status of two camel breeds (Camelus dromedaries) raised under semi intensive system in Saudi Arabia. Ital J Anim Sci. (2013) 12:e14. doi: 10.4081/ijas.2013.e14
458. Al-Atiyat RM, Suliman G, AlSuhaibani E, El-Waziry A, Al-Owaimer A, Basmaeil S. The differentiation of camel breeds based on meat measurements using discriminant analysis. Trop Anim Health Prod. (2016) 48:871–8. doi: 10.1007/s11250-015-0990-5
459. Harrigan P. At Mazayin al-Ibl, spectators admire a prizewinning herd. Saudi Aramco World. (2008) 59:48–57.
460. Khalaf S. Poetics and politics of newly invented traditions in the Gulf: camel racing in the United Arab Emirates. Ethnology. (2000) 39:243–61. doi: 10.2307/3774109
461. Asghar SM, Farhat S, Naiz S. Camel jockeys of Rahimyar Khan. In: Coleridge A, Qadri G, editors. Findings of a Participatory Research on Life and Situation of Child Camel Jockeys. Peshawar: Save the Children Sweden (2005).
462. Moore JE, McCalmont M, Xu J, Nation G, Tinson AH, Crothers L, et al. Prevalence of faecal pathogens in calves of racing camels (Camelus dromedarius) in the United Arab Emirates. Trop Anim Hlth Prod. (2002) 34:283–7. doi: 10.1023/A:1015626601014
463. Chuluunbat B, Charruau P, Silbermayr K, Khorloojav T, Burger PA. Genetic diversity and population structure of Mongolian domestic Bactrian camels (Camelus bactrianus). Anim Genet. (2014) 45:550–8. doi: 10.1111/age.12158
464. Ming L, Yi L, Guo FC, Siriguleng S, Jirimutu J. Molecular phylogeny of the Bactrian camel based on mitochondrial cytochrome b gene sequences. Genet Mol Res. (2016) 15:1–8. doi: 10.4238/gmr.15038983
465. Othman OE, Abd El-Kader, HAM Alam SS, Abd El-Aziem SH. Cytochrome b conservation between six camel breeds reared in Egypt. J Genet Eng Biotechnol. (2017) 15:1–6. doi: 10.1016/j.jgeb.2017.04.006
466. Jirimutu Wang Z, Ding G, Chen G, Sun Y, Sun Z, Zhang H, et al. Genome sequences of wild and domestic bactrian camels. Nat Commun. (2012) 3:1202. doi: 10.1038/ncomms2192
467. Wani NA, Wernery U, Hassan FA, Wernery R, Skidmore JA. Production of the first cloned camel by somatic cell nuclear transfer. Biol Reprod. (2010) 82:373–9. doi: 10.1095/biolreprod.109.081083
468. Wani NA, Vettical BS, Hong SB. First cloned Bactrian camel (Camelus bactrianus) calf produced by interspecies somatic cell nuclear transfer: a step towards preserving the critically endangered wild Bactrian camels. PLoS ONE. (2017) 12:e0177800. doi: 10.1371/journal.pone.0177800
469. Richard D, Gérard D. Camel milk production in Ethiopia. “La production laitière des dromadaires Dankali (Ethiopie)”. [in French]. Rev Elev Med Vét Pays Trop. (1989) 42:97–103.
470. Farah Z, Mollet M, Younan M, Dahir R. Camel dairy in Somalia: limiting factors and developmental potential. Livestock Sci. (2007) 110:187–91. doi: 10.1016/j.livsci.2006.12.010
471. Rousset E, Russo P, Pepin M, Raoult D. Epidémiologie de la fièvre Q animale. Situation en France [in French]. Méd Mal Infect. (2001) 31:233–46. doi: 10.1016/S0399-077X(01)80064-0
472. Faye B. Surveillance and control procedures for camel diseases. In: Regional Workshop Surveillance and Control of Camels and Wildlife Diseases in the Middle East. Sanaa (2003).
473. Pinauldt G. Epizootics and geographic distribution of lifestok trade in the horn of Africa. “Epizooties et géographie du commerce du bétail dans la Corne de l'Afrique. La guerre des quarantaines dans la région nord-Somali”. Echogeo. (2009) 8. doi: 10.4000/echogeo.11021
474. Perry BD, Grace D, Sones K. Current drivers and future directions of global livestock disease dynamics. Proc Natl Acad Sci USA. (2011) 110:20871–7. doi: 10.1073/pnas.1012953108
475. Amenu K, Szonyi B, Grace D, et al. Important knowledge gaps among pastoralists on causes and treatment of udder health problems in livestock in Southern Ethiopia: results of qualitative investigation. BMC Vet Res. (2017) 13:303. doi: 10.1186/s12917-017-1222-1
476. Ackland JR, Worswick DA, Marmion BP. Vaccine prophylaxis of Q fever—a follow-up study of the efficacy of Q-Vax (CSL) 1985–1990. Med J Austral. (1994) 160:704–8. doi: 10.5694/j.1326-5377.1994.tb125909.x
477. Gefenaite G, Munster JM, van Houdt R, Hak E. Effectiveness of the Q fever vaccine: a metaanalysis. Vaccine. (2011) 29:395–8. doi: 10.1016/j.vaccine.2010.11.008
478. Sellens E, Norris JM, Dhand NK, Heller J, Hayes L, Gidding HF, et al. Q fever knowledge, attitudes and vaccination status of Australia's veterinary workforce in 2014. PLoS ONE. (2016) 11:e0146819. doi: 10.1371/journal.pone.0146819
479. Gikas A, Spyridaki I, Scoulica E, Psaroulaki A, Tselentis Y. In vitro susceptibility of Coxiella burnetii to linezolid in comparison with its susceptibilities to quinolones, doxycycline, and clarithromycin. Antimicrob Agents Chemother. (2001) 45:3276–8. doi: 10.1128/AAC.45.11.3276-3278.2001
481. Melenotte C, Million M, Raoult D. New insights in Coxiella burnetii infection: diagnostic and therapeutic update. Expert Rev Anti Infect Ther. (2019) 6:1–12. doi: 10.1080/14787210.2020.1699055
482. van den Brom R, van Engelen E, Roest HI, van der Hoek W, Vellema P. Coxiella burnetii infections in sheep or goats; an opinionated review. Vet Microbiol. (2015) 181:119–29. doi: 10.1016/j.vetmic.2015.07.011
484. Acha PN, Szyfres B. Q fever. In: Zoonoses and Communicable Diseases Common to Man and Animals, Vol 2: Chlamydioses, Rickettsioses, and Viroses. 3rd ed. Washington, DC: The Pan American Health Organization (2003). p. 16–27.
485. Schmeer N, Miller HP, Langel J, Krauss H, Frost JW, Wieda J. Q fever vaccines for animals. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg. (1987) 267:79–88. doi: 10.1016/S0176-6724(87)80191-8
486. Lang GH, Prescott JF, Williams JC. Serological response in sheep vaccinated against Coxiella burnetii (Q fever). Can Vet J. (1994) 35:373–4.
487. Kazar J, Schramek S, Lisak V, Brezina R. Antigenicity of Chloroform-methanol-treated Coxiella burnetii preparations. Acta Virol. (1987) 31:158–67.
488. Guatteo R, Seegers H, Joly A, Beaudeau F. Prevention of Coxiella burnetii shedding in infected dairy herds using a phase I C. burnetii inactivated vaccine. Vaccine. (2008) 26:4320–8. doi: 10.1016/j.vaccine.2008.06.023
489. Rousset E, Durand B, Champion JL, Prigent M, Dufour P, Forfait C, et al. Efficiency of a phase 1 vaccine for the reduction of vaginal Coxiella burnetii shedding in a clinically affected goat herd. Clin Microbiol Infect. (2009) 15:188–9. doi: 10.1111/j.1469-0691.2008.02220.x
490. Hogerwerf L, van den Brom R, Roest HIJ, Bouma A, Vellema P, Pieterse M, et al. Reduction of Coxiella burnetii prevalence by vaccination of goats and sheep, the Netherlands. Emerg Infect Dis. (2011) 17:379–86. doi: 10.3201/eid1703.101157
491. Gonzalez-Barrio D, Ortiz JA, Ruiz-Fons F. Estimating the efficacy of commercial phase I inactivated vaccine in decreasing the prevalence of Coxiella burnetii infection and shedding in red derr (Cervus elaphus). Front Vet Sci. (2017) 4:208. doi: 10.3389/fvets.2017.00208
492. Arricau-Bouvery N, Souriau A, Bodier C, Dufour P, Rousset E, Rodolakis A. Effect of vaccination with phase I and phase II Coxiella burnetii vaccines in pregnant goats. Vaccine. (2005) 23:4392–402. doi: 10.1016/j.vaccine.2005.04.010
493. Coche B. Bovine Q fever in France. “La fièvre Q bovine en France. Aspects pratiques et importance de la sérologie” [in French]. Point Vet. (1981) 12:95–100.
494. Rodolakis A. Chlamydia and Q fever as abortion agents and their implication in zoonoses. “Chlamydiose et fièvre Q: agents d'avortements et zoonoses ?” [in French]. Point Vet. (1994) 26:845–50.
495. Rousset E, Eon L, Russo P, Pepin M, Aubert M. La fièvre Q: épidémiologie d'une zoonose [in French]. Bull Group Tech Vét. (2002) 17:9–15.
496. Szymanska-Czerwinska M, Niemczuk K. Evaluation of the effectiveness of Q fever treatment with oxytetracycline. Bull Vet Inst Pulawy. (2012) 56:513–7. doi: 10.2478/v10213-012-0090-5
497. Cerf O, Condron R Coxiella burnetii and milk pasteurization: an early application of the precautionary principle? Epidemiol Infect. (2006) 134:946–51. doi: 10.1017/S0950268806005978
498. Astobiza I, Barandika JF, Juste RA, Hurtado A, Garcia-Perez AL. Evaluation of the efficacy of oxytetracycline tretment followed by vaccination against Q fever in a highly infected sheep flock. Vet J. (2013) 196:81–5. doi: 10.1016/j.tvjl.2012.07.028
499. Kersh GJ, Fitzpatrick KA, Self JS, et al. Presence and persistence of coxiella burnetii in the environments of goat farms associated with a Q fever outbreak. Appl Environ Microbiol. (2013) 79:1697–703. doi: 10.1128/AEM.03472-12
500. Moffat MAJ. Zoonotic implications of Q fever and Chlamydial infections in animals and man: part1-Q fever. Ir Vet J. (1990) 43:115–7.
501. Woldehiwet Z, Aitken ID. Coxiellosis (Q fever). In: Woldehiwet Z, Ristic M, editors. Rickettsial and Chlamydial Diseases of Domestic Animals. Oxford: Pergamon Press (1993). p. 131–51.
502. Orfila J. Chapitre 50: Rickettsiales [in French]. In: Le Minor L, Véron M, editors. Bactériologie Médicale. 2 éd. Paris: Flammarion Médecine-Sciences (1989). p. 1069–71.
503. Behymer D, Riemann P. Coxiella burnetii infection (Q fever). J Am Vet Assoc. (1989) 194:164–767.
504. Arricau-Bouvery N, Souriau A, Moutoussamy A, Ladenise K, Rodolakis A. Study of Coxiella burnetii excretion in an experimental goat model and decontamination of dung with calcium Cyanamid. Rencontres autour des Recherches sur les Ruminants. (2001) 8:153–6.
505. Blanc GM, Maurica A. Présence du virus de la ‘Q fever' dans le Maroc méridional [in French]. Bull Acad Natl Med. (1947) 131:138–43.
506. Edouard S, Million M, Casalta JP, Collart F, Amphoux B, Raoult D. Low antibodies titer and serological cross-reaction between Coxiella burnetii and Legionella pneumophila challenge the diagnosis of mediastinitis, an emerging Q fever clinical entity. Infection. (2017) 45:911–5. doi: 10.1007/s15010-017-1048-6
507. Bouhous A, Aissi M, Harhoura KH. Etude des Ixodidae chez le dromadaire dans le sud algérien, région d'Adrar [Study of Ixodidae on camels in Southwest Algeria, Adrar region] [in French]. Annal Méd Vét. (2008) 152:52–8.
508. El Idrissi AH, Manyari A, Benkirane A. Fréquence des avortements infectieux des ovins au Maroc (Région de Zaer et du Moyen Atlas) [in French]. Actes Inst Agron Vét. (1995) 15:11–4.
509. El Jay S, Bouslikhane M, El Idrissi AH. Epidemiological monitoring of small ruminant abortions in pastoral areas of Morocco. “Suivi épidémiologique des avortements de petits ruminants dans les zones pastorales du Maroc.” Actes Inst Agron Vet. (2003) 23:95–100.
510. Benkirane A, Essamkaoui S, El Idrissi A, Lucchese L, Natale A. A sero-survey of major infectious causes of abortion in small ruminants in Morocco. Vet Italian. (2015) 51:25–30. doi: 10.12834/VetIt.389.1814.1
Keywords: Coxiella burnetii, dromedary camel (Camelus dromedarius), zoonoses awareness, epidemiology, human—animal coexistence
Citation: Devaux CA, Osman IO, Million M and Raoult D (2020) Coxiella burnetii in Dromedary Camels (Camelus dromedarius): A Possible Threat for Humans and Livestock in North Africa and the Near and Middle East? Front. Vet. Sci. 7:558481. doi: 10.3389/fvets.2020.558481
Received: 16 July 2020; Accepted: 28 September 2020;
Published: 05 November 2020.
Edited by:
Julio Alvarez, VISAVET Health Surveillance Centre (UCM), SpainReviewed by:
Wendy Beauvais, Cornell University, United StatesCopyright © 2020 Devaux, Osman, Million and Raoult. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christian A. Devaux, Y2hyaXN0aWFuLmRldmF1eEBtZWRpdGVycmFuZWUtaW5mZWN0aW9uLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.