
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 07 January 2021
Sec. Veterinary Infectious Diseases
Volume 7 - 2020 | https://doi.org/10.3389/fvets.2020.539444
This article is part of the Research Topic The Belt and Road of Animal Diseases View all 12 articles
 Zhiguo Liu1
Zhiguo Liu1 Chengling Wang1
Chengling Wang1 Kongjiao Wei1
Kongjiao Wei1 Zhongzhi Zhao2
Zhongzhi Zhao2 Miao Wang3
Miao Wang3 Dan Li1
Dan Li1 Heng Wang4
Heng Wang4 Qiang Wei5*
Qiang Wei5* Zhenjun Li1*
Zhenjun Li1*In this study, MLVA (multiple-locus variable-number tandem repeat analysis) genotype data of Brucella strains from 11 countries along the Silk Road were downloaded from the MLVAbank. MLVA data of strains were applied to the constructed Minimum Spanning Tree to explore the species/biovars distribution, geographic origins, and genetic relationships of the strains analyzed. Moreover, whole-genome sequencing–single-nucleotide polymorphism (WGS-SNP) phylogenetic analysis of the genome of Brucella melitensis strains from GenBank was performed to discriminate the relatedness of strains further and investigate the transmission pattern of B. melitensis brucellosis. A total of 1,503 Brucella strains were analyzed in this study: 431 Brucella abortus strains (29.8%), 1,009 B. melitensis strains (65.7%), and 63 Brucella suis strains (4.5%). B. melitensis biovar 3 was the dominant species and was shown to be widespread in all of the examined regions, suggesting that the prevention and surveillance of the B. melitensis population are a main challenge in these countries. A wide host spectrum was observed for this Brucella population; many animal reservoirs are a potential reason for the continuous brucellosis circulation in these countries. Although the B. abortus strains from the examined regions had common geographic origins, only a few shared genotypes were observed in different countries. These data revealed that the majority of B. abortus strains were spreading within the national borders. However, the B. melitensis strains from Italy originated from a Western Mediterranean lineage; strains from the other 10 countries originated from Eastern Mediterranean lineage, and this lineage was shared by strains from three to nine different countries, suggesting that the introduction and reintroduction of the disease in the 10 countries might have occurred in the past. Furthermore, the most shared MLVA-16 genotypes were formed in the B. melitensis strains from China, Kazakhstan, and Turkey, suggesting that the introduction and trade in sheep and goats have occurred frequently in these countries. WGS-SNP analysis showed that the B. melitensis in this study originated from the Malta (Italy) region. According to their territorial affiliation between four clade strains from these countries in genotype B, the absence of a clear differentiation suggests that strains continuously expand and spread in countries along with Silk Road. Active exchange and trade of animals (sheep and goats) among these countries are reasonable explanations. B. suis strains from different nations showed unique geographic origins and epidemiological characteristics. Therefore, there is an urgent need for the control of transfer and trade of infected sheep (goats) in countries along the Silk Road, namely, the strengthening of the entry–exit quarantine of sheep and goats and improvements in the diagnosis of animal brucellosis.
Brucellosis is a zoonotic infectious disease caused by members of the genus Brucellae, circulating in more than 170 countries, especially in the Mediterranean, Asia, and Central and South America (1). The disease is primarily contracted through direct or indirect contact with infected animals and their contaminants, although the consumption of contaminated animal products can also cause infection. Low fever, sweating, fatigue, and joint pain are the most common clinical manifestations of patients (2). Chronic brucellosis is challenging to cure, which affects the quality of life of patients and causes great family and socioeconomic burdens (3). In females, abortion and stillbirth are the main manifestations, whereas orchitis and sterility are common in males (4). Therefore, brucellosis not only has a significant impact on the husbandry and health of the human population, but is also closely related to many fields such as public safety, food hygiene, and foreign trade (5).
Although human brucellosis (HB) is one of the most common bacterial zoonotic diseases, its occurrence greatly differs between geographic areas. The highest annual incidences of HB per million of the population are observed in Syria and Mongolia (1); for instance, 39,838 human cases were reported in Syria in 2007. Mongolia has a high incidence of brucellosis in humans and animals due to livestock husbandry; livestock seroprevalence ranged from 0.5 to 1.8% in 2011 (6). Moreover, a total of 684,380 HB cases were reported in mainland China during 1950–2018, and the incidence of HB peaked in 2014 (4.32/100,000) (7). Kazakhstan is a hyperendemic area, considering the very high incidence rates in the human population and farm animals (1). From 1999 to 2016, 38,557 new cases of HB were recorded, with annual counts of cases ranging from 1,443 (2014) to 3,596 (2004) (8). In Pakistan, the high age-wise prevalence was recorded as 32.25% (98/304) by enzyme-linked immunosorbent assay (ELISA) (P < 0.05) in 21- to 30-year-old females (9). In India, the seroprevalence of brucellosis among cattle and goats was estimated to be 1.1 and 11.2%, respectively (10). Brucellosis is still reported from all types of farms and domestic livestock in Russia; between 1999 and 2009, the number of infected sites increased, ranging from 54 in 2001 to 112 in 2009 (11). The incidence of HB in Turkey is 26 per 100,000 (12), and 18,264 new brucellosis cases were reported in 2004 (13); moreover, pediatric brucellosis may constitute 20% to 30% of all brucellosis cases in the world (14). Previous research reported that 22 (5.1%) tested by Rose Bengal Test (RBT) and 58 (13.5%) tested using the blocking ELISA assay were positive for Brucella in wild boar hunted in the Campania region of Italy during 2016–2017 (15). Each year a relatively small number of cases are reported in Germany, but within the last 2 years, an increase of cases was observed (16). Many comprehensive brucellosis controls have been developed in the majority of countries, including testing-slaughter, restricted infected animal transfer, and vaccination of livestock. However, brucellosis is still an endemic zoonosis in these nations (17).
The Silk Road Economic Belt has had a profound effect on economic prosperity, cultural diversity, foreign trades, and many other characteristics of nations along the road; therefore, it might also provide opportunities for the spread of zoonotic diseases (18). Indeed, zoonotic diseases such as plague and anthrax are associated with commercial activities related to the Silk Road (19, 20), especially in countries along the line of the road. Indeed, these countries have high amounts of husbandry, and brucellosis circulates between humans and animals in Italy, Germany, Turkey, Iran, and Kazakhstan (21–25). Accordingly, there is the potential for transmission of brucellosis during processing of animals and trade of animal products. Currently, MLVA-16 genotyping of Brucella strains can be used for trace-back and trace-forward investigations, as well as the identification of the spreading route (26), and MLVA-11 is often used to trace back the geographic origin of strains (27). Moreover, whole-genome sequencing–single-nucleotide polymorphism (WGS-SNP) is likely to be a suitable tool for trace-back analysis of Brucella melitensis, as it is capable of suggesting the potential geographic origin of a given strain (16). Therefore, this study was conducted to better understand the epidemiological characteristics of brucellosis in countries along the Silk Road, provide valuable information for strengthening trade cooperation, and launch a corresponding strategy of prevention and control of brucellosis.
Data pertaining to MLVA-16 of 1,503 Brucella from 11 related countries including China, Kazakhstan, Turkey, Germany, Italy, Iran, Israel, Lebanon, Mongolia, Syria, and India were obtained from the international MLVAbank database (http://microbesgenotyping.i2bc.paris-saclay.fr/databases) (2016–2018·V1.4.0), including the strain number (Named by the database), species/biovars, host types, isolated region, Panel 1 genotype, MLVA-11 genotype, and the number of tandem repeats at MLVA-16 loci. Moreover, strains from Kyrgyzstan and Tajikistan were not found in the international MLVAbank database.
A total of 1,503 Brucella strains were collected from samples of patients and animals from 11 different countries. According to standard bacteriology approaches, all of the strains were isolated and identified (28). Conventional identification methods were used to assign the strains to biotypes. All of the strains were gram-negative, agglutinated with polyvalent brucellosis serum, had oxidase and catalase activity, synthesized urease, and were capable of growing in atmospheric conditions. Subsequently, BCSP-31 polymerase chain reaction (PCR) (29), AMOS-PCR (30), and/or Brucella ladder PCR (31) were applied to verify the results from the biotyping assays.
A fresh single Brucella strain clone was picked up from a solid agar medium surface and then heat-inactivated at 80°C for 20 min. Subsequently, DNA was isolated using a phenol/chloroform extraction method and/or the QIAamp DNA Mini Kit (Qiagen, United States) according to the manufacturer's instructions. The MLVA-16 assay was performed as previously described (32, 33). Briefly, 16 loci were divided into three panels: Panel 1 (also called MLVA-8), Panel 2A, and Panel 2B. The combination of panels MLVA-8 and 2A was called MLVA-11, whereas the combination of all three panels (16 loci) was designated MLVA-16. The MLVA-11 panel allows for tracing the geographic origin of the strains analyzed, whereas the Panel 2B loci are highly discriminatory, and their combination with MLVA-11 was used for molecular epidemiology investigation. PCR was used to determine the number of repeats from each sample, and its products were purified and directly sequenced using an ABI Prism Big Dye Terminator. Size analysis of VNTR repeats was performed using GeneMapper 4.1 (Applied Biosystems).
A total of 1,503 Brucella strains were downloaded from the MLVAbank database, including 394 in China, 359 in Italy, 338 in Kazakhstan, 234 in Turkey, 58 in Germany, 37 in India, 28 in Lebanon, 20 in Syria, 14 in Israel, 12 in Mongolia, and 9 in Iran (Supplementary Table 1). The downloaded data were processed according to the species/biovars and genotypes of all Brucella strains using Excel 2016 software (Microsoft Corporation, Redmond, WA, USA). At the same time, the species/biovars and distribution regions of the strains were analyzed to investigate the epidemiological correlation of Brucella in the above countries. Subsequently, the Minimum Spanning Tree (MST) of Brucella abortus strains, B. melitensis strains, and Brucella suis strains were constructed using the BioNumerics 7.6 software based on the MLVA-11 data, and the genotype distribution and geographical origin of the strains were analyzed. The BioNumerics 7.6 software (Applied Maths, Sint-Martens-Latem, Belgium) based on unweighted pair group method using arithmetic averages was applied to construct the MST of B. abortus strains, B. melitensis strains, and B. suis strains based on MLVA-16 data and to explore their genetic relatedness. Moreover, the WGS-SNP phylogenetic analysis (34) was performed in 39 B. melitensis from 14 countries along the Silk Road (Supplementary Table 2) to explore further the genetic relatedness of strains and the transmission pattern of brucellosis in these nations.
Among the 1,503 strains of Brucella, 431 (28.7%) were B. abortus, 1,009 (67.1%) were B. melitensis, and 63 (4.2%) were B. suis (Table 1). B. abortus strains included four biovars: 63 in biovar 1, 188 in biovar 3, 79 in biovar 6, four in biovar 7, one in rough strain, and another 98 strains have not been identified (Figure 1A). Among the B. melitensis strains, 99 strains in biovar 1, 50 strains in biovar 2, 532 strains in biovar 3, five strains in rough, and nine strains were atypical, and 313 strains of unknown biovars were observed (Figure 1B). Among the 63 B. suis strains, 18 were in biovar 1, 44 in biovar 2, and one in biovar 3 (Figure 1C). All three Brucella species were observed in China, Germany, and Italy (Table 1). Both B. abortus and B. melitensis were circulating in the remaining seven countries. Five different biotypes were found in B. melitensis species; five biotypes were observed in B. abortus species, whereas three biotypes were recorded in B. suis species. B. melitensis was the dominant population in all countries examined, and B. abortus biovar 3 and B. melitensis biovar 3 were the dominant species in countries along the Silk Road (Table 1). B. suis biovar 1 was found exclusively in Chinese strains, and B. suis biovar 2 was observed in Germany and Italy (Table 1).
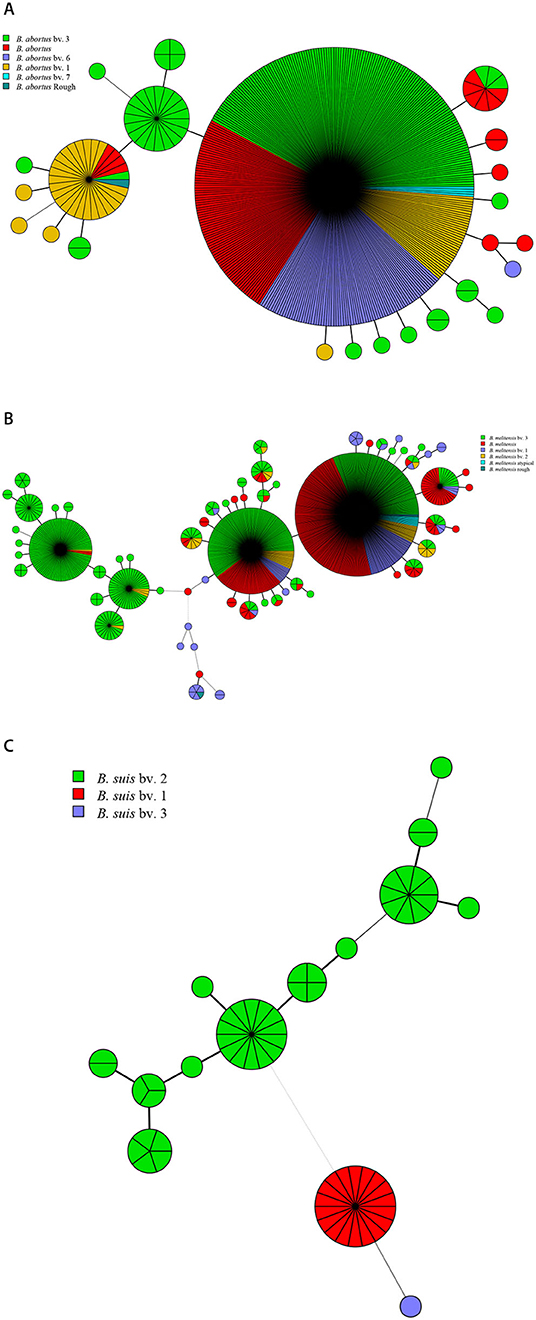
Figure 1. Species/biovars distributed characteristics of B. abortus (A), B. melitensis (B), and B. suis (C). Color coding according to species/biovar; circle size depicts the number of strains.
In this study, a total of 13 kinds of hosts were observed in all of the countries analyzed, including humans, cattle, sheep, camels, buffalo, goats, bharals, dogs, deer, swine, boars, hares, and wild swine, of which 583 strains were found in humans, 448 in cattle, and 363 in sheep (Table 2). The hosts of B. abortus strains included cattle, sheep, camels, buffalo, and humans, of which 92.0% of strains were collected from cattle (Figure 2A). The hosts of B. melitensis strains included goats, sheep, cattle, deer, bharals, camels, humans, and dogs. Remarkably, approximately 56.8% (573/1,009) of strains were from humans, and this host was distributed widely in all of the countries; moreover, 35.3% (353/1,009) of the strains were obtained from sheep (Figure 2B, Table 2). Deer, humans, sheep, swine, boars, hares, and wild swine were common hosts from isolated B. suis strains, and 55.6% (35/63) of the strains from Germany and Italy were found in wild swine (Figure 2C). However, humans, cattle, and sheep were the main hosts of the B. suis strains in China (Table 2). The most extensive hosts of Brucella in this study were observed in China, followed by Italy, Kazakhstan, and India (Table 2).
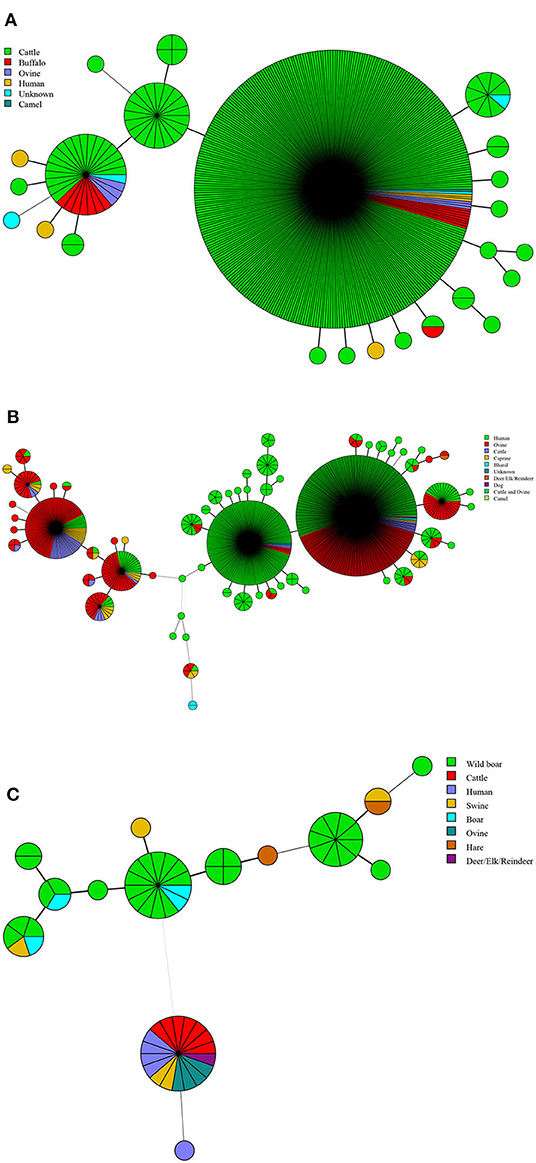
Figure 2. Host lineages profile of B. abortus (A), B. melitensis (B), and B. suis strains (C). Color-coding according to hosts types; circle size depicts the number of strains.
There were 24 MLVA-11 genotypes among B. abortus strains (72, 82, 201, and 70 were the most common genotypes). Of these, genotype 72 was overwhelming, accounting for 80.5% (347/431) (Figure 3A). Genotype 72 was shared by strains from Kazakhstan, China, Italy, Mongolia, and Turkey. Genotype 210 was shared by Italian and Chinese strains, whereas genotype 82 was shared by strains from Kazakhstan, German, Italy, China, and India (Figure 3B). MST analysis of B. abortus strains revealed that some MLVA-16 shared genotypes were found in strains from Kazakhstan, Italy, and China. Moreover, in Kazakhstan and China, Kazakhstan and Italy, and Kazakhstan, Italy, and China, a limited number of strains consisted of shared genotypes. In contrast, no shared genotype was found in other countries (Figure 3C).
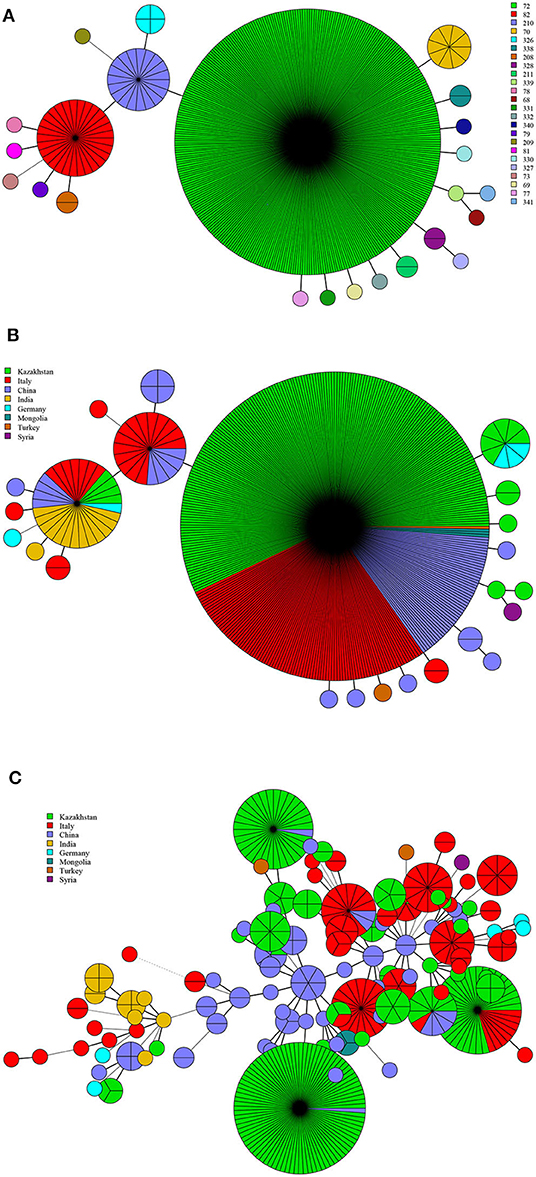
Figure 3. MLVA-11 genotype (A), geographic origin (B), and genetic relatedness characteristics (C) of B. abortus strains. (Color-coding according to MLVA-11 genotypes (A,B) and MLVA-16 genotypes (countries, C); circle size depicts the number of strains).
There were 62 MLVA-11 genotypes in 1,009 strains of B. melitensis, of which 116, 125, 87, 96, 111, and 91 were the most common genotypes (Figure 4A). Among these, genotype 116 was overwhelmingly dominant, accounting for 40.9% (413/1,009), whereas genotypes 125, 87, 96, 111, and 91 accounted for 20.0% (202/1,009), 10.6% (107/1,009), 4.6% (45/1,009), 3.8% (38/1,009), and 2.2% (22/1,009), respectively. Based on the geographic origin, B. melitensis genotypes 116, 125, and 111 belong to an Eastern Mediterranean lineage, whereas genotypes 87, 96, 91, and 162 belong to the Western Mediterranean lineage. Genotypes 116, 125, and 111 were shared by strains from 10 countries, except Italy (Figure 4B). Genotype 116 was shared by strains from China, Germany, India, Iran, Kazakhstan, Lebanon, Mongolia, Syria, and Turkey, whereas type 125 was shared by strains from nine countries, namely, China, Germany, Iran, Israel, Italy, Kazakhstan, Lebanon, Syria, and Turkey. Otherwise, genotype 111 was observed in China, Kazakhstan, and Turkey. However, genotypes 87, 96, 91, and 162 were found almost exclusively in the Italian strains, and only two strains from Germany were represented in genotype 96 (Figure 4B). MST analysis based on MLVA-16 data of B. melitensis strains indicated that 1,009 strains of B. melitensis were clustered into two groups (A and B). The strains in Italy have formed an independent group A, whereas the other 10 countries' strains were clustered into group B (Figure 4C). Multiple shared genotypes in group B were observed in different countries, including China, Kazakhstan, and Turkey; China, Turkey, and Syria; China, Kazakhstan, and Mongolia; China and India; Turkey, Lebanon, and Syria; Turkey and Lebanon; Turkey and Syria; Turkey and India; and Turkey and Iran. The most shared genotypes were observed among the Chinese and Kazakhstan strains (Figure 4C).
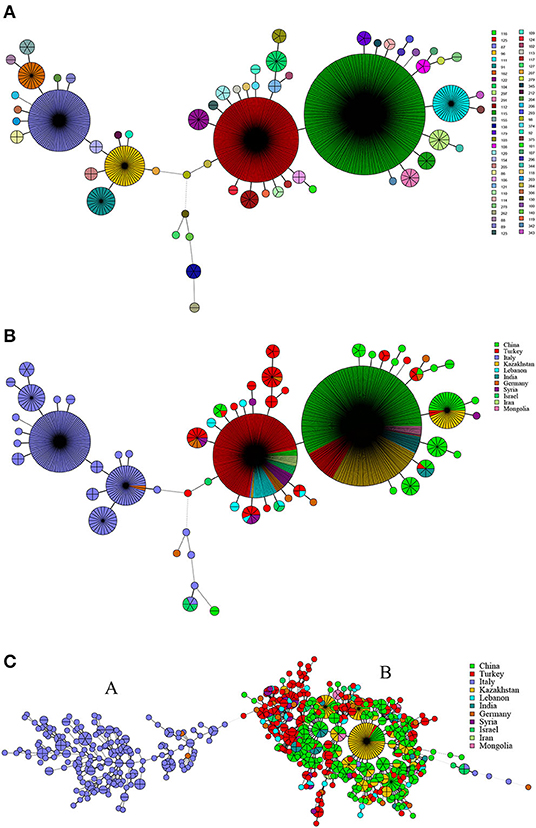
Figure 4. MLVA-11 genotype (A), geographic origin (B), and genetic relatedness characteristics (C) of B. melitensis strains. (Color-coding according to MLVA-11 genotypes (A,B) and MLVA-16 genotypes (countries, C); circle size depicts the number of strains).
Phylogenetic analysis based on WGS-SNPs revealed the geographic clustering profile of B. melitensis strains from these countries; 39 B. melitensis strains were divided into two groups (A and B). Group A included the strains from the Western Mediterranean Region (Italy and Malta) and located at the base of the tree. Group B included the strains from regions of Asia, covering a broad geographic area that could be subdivided into four clades: (I) Middle East (Iran, Iraq, Turkey, Syria), China, and Bulgaria; (II) Turkey, Russia, China, Georgia, and India; (III) China-Russia; (IV) Turkmenistan, Afghanistan, Iran, and Pakistan (Figure 5).
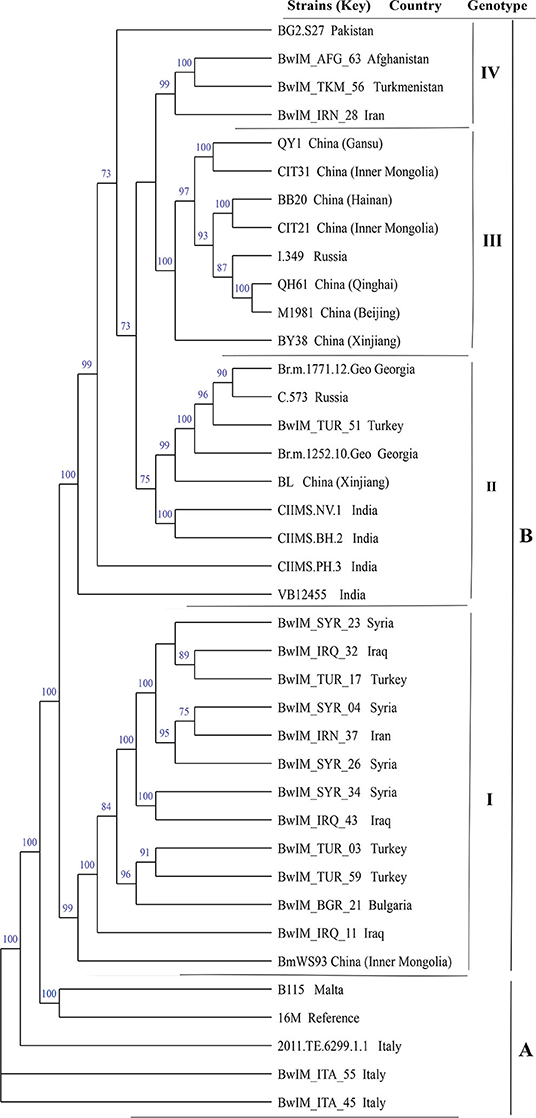
Figure 5. Whole-genome sequencing–single-nucleotide polymorphism phylogenetic analysis of 39 B. melitensis strains from 14 countries along the Silk Road.
There were 14 MLVA-11 genotypes in 63 strains of B. suis, and MLVA-11 genotypes 33, 57, and 44 were predominant, accounting for 28.6% (18/63), 22.2% (14/63), and 7.0% (9/63), respectively; the remaining 11 genotypes contained one to five strains from the respective country (Figure 6A). Genotypes 33, 31, 57, and 44 were unique to Chinese, India, German, and Italian strains, respectively (Figure 6B). MST analysis based on MLVA-16 data revealed that 63 strains of B. suis from Germany, Italy, China, and India formed three independent branches (A–C). The majority of strains from Germany clustered in branch A, and all Italy strains and a few German strains clustered in branch B. Strains from China and India (n = 1) were grouped in branch C. No shared MLVA-16 genotypes were found in strains from different countries (Figure 6C).
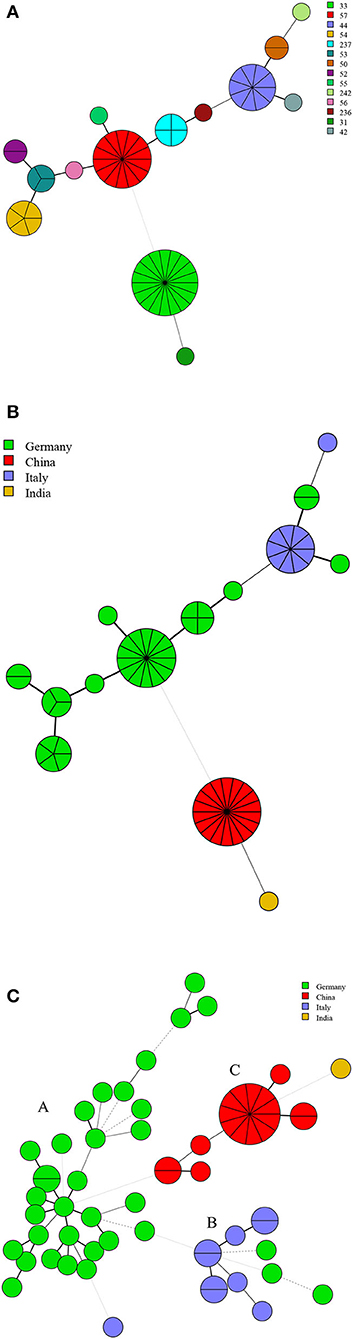
Figure 6. MLVA-11 genotype (A), geographic origin (B), and genetic relatedness characteristics (C) of B. suis strains. (Color-coding according to MLVA-11 genotypes (A,B) and MLVA-16 genotypes (countries, C); circle size depicts the number of strains).
Brucellosis is prevalent in Italy, Germany, Turkey, Iran, Kazakhstan, China, and other countries, along with the Silk Road. Moreover, a considerable number of Brucella strains have been isolated, providing a basis for investigation of the genetic correlation characteristics among strains. In this study, B. abortus, B. melitensis strains, and B. suis strains accounted for 28.7% (431/1,503), 67.1% (1,009/1,503), and 4.2% (63/1,503) of the total, respectively. Moreover, B. melitensis biovar 3 accounted for 52.9% (534/1,009) of the total. The distribution characteristic of pathogenic bacteria population and Brucella biovars was similar to the previous study; B. melitensis is the most important zoonotic agent, followed by B. abortus and B. suis (35, 36). This correlates with the fact that the worldwide control of bovine brucellosis (due to B. abortus) has been achieved to a greater extent than the control of sheep and goat brucellosis (due to B. melitensis), these latter species being the most important domestic animals in many developing countries (17). B. melitensis strains were distributed widely in all of the countries examined; 56.8% (573/1,009) strains were obtained from humans, and 35.3% (353/1,009) were from sheep. These data indicated that B. melitensis strains are the predominant pathogen responsible for human and animal brucellosis in these countries. These findings suggest that identical or similar epidemiological characteristics of brucellosis might be found in countries along the Silk Road (37). Globally, despite the remarkable results achieved by the majority of industrialized countries, where bovine brucellosis has been eradicated or controlled, small ruminant brucellosis remains a problem in some of these countries (38). Our current study confirmed that B. melitensis is a latent “travel bacterium” that continuously spreads and expands from Northern China to Southern China (39). From 1970 to 2015, B. melitensis has been the pathogen isolated most frequently in human cases, accounting for more than 99% of Brucella spp. isolated from humans in Italy (40). Similarly, the species that causes the most frequent brucellosis and produces the most severe disease picture is B. melitensis in Turkey (13). Although 28.7% of total strains were of B. abortus, most of them were obtained from cattle, and its pathogenicity to humans was significantly lower than B. melitensis strains (17). Moreover, B. suis biovar 2 strains in this study were from a wild animal; B. suis biovar 1 and biovar 3 from humans and B. suis strains were observed in limited countries; fewer strains were isolated from humans, and the B. suis biovar 2 is not an essential pathogen for humans in contrast to B. suis biovars 1, 3, and 4 (17). Therefore, to solve the brucellosis problem in countries along the Silk Road, we should focus more on the B. melitensis strains than B. abortus and B. suis populations, and it is urgent to strengthen the detection and management of the infected sheep and goats in these countries.
In the present study, B. abortus strains and B. melitensis strains had a wide host spectrum, including many livestock and wild animals. Some shared genotypes were observed in different hosts among each species, indicating that these wild animals might become a source for reintroducing Brucella strains in domestic ruminants and humans (41). B. abortus and B. melitensis are the most regularly transmitted species between wild and domestic ungulates. They are most frequently associated with the conflicting needs of wildlife and agriculture and the risk of human disease (42). This confirmed the widespread existence and natural epidemic origin of brucellosis in countries along the Silk Road (43, 44), which poses severe challenges to the prevention and surveillance of brucellosis in these countries.
Based on the MLVA-11 analysis of B. abortus strains, genotypes 72, 201, 82, and 70 were more common; each of them was shared by strains from two to five countries, especially 72 being the predominant genotype and shared by strains from Kazakhstan, China, Italy, Mongolia, and Turkey. These findings demonstrate that the B. abortus strains from these countries have high homogeneity and common geographic origins (45, 46). MST analysis of B. abortus strains revealed that there were bovine brucellosis outbreaks in Kazakhstan, Italy, and China, but the number of cases of cross-infection among countries was limited (47). This was not only closely related to the reduction in virulence of B. abortus strains, but also restricted by factors such as larger size in cattle, higher transportation costs, and trade policies.
Among the 1,009 B. melitensis population, genotypes 116, 125, and 111 originated from the Eastern Mediterranean lineage, and genotypes 87, 96, and 91 originated from Western Mediterranean lineage. These findings demonstrate that the B. melitensis strains from countries along the Silk Road have two different geographical origins. The strains from Italy originated from a Western Mediterranean lineage, and strains from the other 10 countries originated from an Eastern Mediterranean lineage. Moreover, genotypes 116, 125, and 111 were shared by strains from nine, nine, and three countries, respectively, indicating that the strains from these countries had the same geographical origin. This suggests that the introduction and reintroduction of the disease among the 10 countries might have occurred in the past (48, 49). Based on MST analysis revealed that B. melitensis strains clustered into two groups, with strains in Italy forming a relatively independent group A, whereas strains in the remaining 10 countries clustered into group B. In addition, there were at least 10 shared MLVA-16 genotypes in strains from group B. The most shared genotypes were formed in strains from China, Kazakhstan, and Turkey. It was previously suggested that B. melitensis strains in China, Turkey, and Kazakhstan were epidemiologically correlated, and the prevalence of B. melitensis brucellosis in these countries resulted from a common infectious source (16, 50, 51). The breeding and trade of small ruminants were the main economic source and industrial pillar of the vast agricultural and pastoral areas in Turkey, Kazakhstan, and China, and animal introduction and trade were extremely frequent in these countries. The results were consistent with those of previous studies that showed the majority of B. melitensis strains in Kazakhstan were genetically related to strains transmitted in China and were closely related to the long-term trading partnership between the two countries (52). Therefore, WGS-SNP analysis indicated that B. melitensis were originated from Malta (Italy) regions, strains from group B forming the four subclades, but do not have a clear differentiation according to their territorial affiliation, indicating the frequent penetration of the B. melitensis strains from one country to another (53). Active exchange and trade of animals (sheep and goats) among these countries could promote pathogen dissemination, and there was continuous expansion and spread in countries along with Silk Road. Recently, many infectious diseases have emerged, and most of their pathogens originated from zoonosis; some of the diseases spread worldwide by the international flow of goods, people, and animals (54). We suggest that implementing a larger scale of vaccine coverage of small ruminants would significantly reduce infection in livestock and humans. Moreover, restricting movement in positive sheep and goats among different countries and the regular disinfection of the farm environments could effectively reverse the trend of increasing brucellosis in countries along the Silk Road.
B. suis strains in Germany, China, Italy, and India represent a unique predominated genotype, respectively, suggesting that each of these strains had an exclusive geographic origin. MST analysis revealed that MLVA-16 shared genotypes were not found in different countries' strains, demonstrating that B. suis strains were only prevalent in their respective countries. This was possibly related to environmental adaptability and economic and social factors influencing hosts of B. suis strains. B. suis biovar 2 is largely restricted to continental Europe and is maintained in wild animal (Suidae and hares) populations (40). We suggest that enhanced surveillance regulation for wild animals in Europe is essential for the prevention of strain spill over into the livestock. Moreover, further genome comparison analysis of more B. suis strains from all over the world will increase our understanding of B. suis brucellosis epidemiology.
Our research has some limitations. Most of the data were derived from highly concentrated time and location, rather than from continuous time points and wide coverage areas, so they may only partly reflect the truth situation of brucellosis in the regions examined. Second, data on animals' epidemiology and movements in these countries were not available. A focus on risk analysis or statistical analysis of hosts from the spatiotemporal or species-location perspective is essential.
In this study, the species distribution, host lineage profiles, geographical origin, and genetic relatedness and the epidemiological correlation of Brucella species in countries along the Silk Road were discussed. B. abortus was found to share the same geographic origin, but the transmission area was limited to their respective countries. However, B. melitensis strains were the dominant population in these countries and had two main geographic origins, and strains from the same geographic origin showed extensive gene sharing. B. suis strains from respective countries showed unique geographic origins and epidemiological characteristics. The surveillance and control of B. melitensis strains were identified as major problems among Brucella species in countries along the Silk Road. The control of its main host (sheep and goat) should be strengthened. Effective vaccination limits Brucella infection, restricts shedding, hampers transmission from animal to animal, and diminishes zoonosis risk (55). The corresponding regulations should be strictly implemented when introducing animals to prevent the spread of this species. Moreover, improving scientific research to achieve early diagnosis and implement joint prevention and control is recommended.
All of the data generated or analyzed during this study are included in this published article, and the supplementary information files will be freely available to any scientist wishing to use them for non-commercial purposes upon request via e-mail with the corresponding author.
ZhiL performed strain download, MLVA typing and cluster analysis. CW drafted the manuscript. KW, HW, MW, ZZ and DL conducted data processing. ZheL and ZhiL participated in design of the study and critically reviewed the manuscript and ZheL managed the project. QW revised the manuscript critically. All authors have read and approved the final manuscript.
This study was supported by the National Key R&D Program of China (Grant Numbers 2018ZX10734404, 2018ZX10734401, and 2017ZX10303401), the Nature Science Fund of Inner Mongolia Autonomous Region (Grant Number 2018MS08004), and the National Science and Technology Major Project on Important Infectious Diseases Prevention and Control (Grant Number 2018ZX10734404). The funders played no role in study design, data collection, analysis, decision to publish, or manuscript preparation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank all the staff of MLVAbank for the data collected and standardized during Brucella genotyping. The authors also thank Accdon (www.accdon.com) for linguistic assistance during the preparation of this manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2020.539444/full#supplementary-material
1. Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. (2006) 6:91–9. doi: 10.1016/S1473-3099(06)70382-6
2. Esmaeilnejad-Ganji SM, Esmaeilnejad-Ganji SMR. Osteoarticular manifestations of human brucellosis: a review. World J Orthop. (2019) 10:54–62. doi: 10.5312/wjo.v10.i2.54
3. Ahmed W, Zheng K, Liu ZF. Establishment of chronic infection: brucella's stealth strategy. Front Cell Infect Microbiol. (2016) 6:30. doi: 10.3389/fcimb.2016.00030
4. Mcelwain TF, Thumbi SM. Animal pathogens and their impact on animal health, the economy, food security, food safety and public health. Rev Sci Tech. (2017) 36:423–433. doi: 10.20506/rst.36.2.2663
5. Al Dahouk S, Nöckler K. Implications of laboratory diagnosis on brucellosis therapy. Expert Rev Anti Infect Ther. (2011) 9:833–45. doi: 10.1586/eri.11.55
6. Kang SI, Her M, Erdenebaataar J, Vanaabaatar B, Cho H, Sung SR, et al. Molecular epidemiological investigation of Brucella melitensis circulating in Mongolia by MLVA16. Comp Immunol Microbiol Infect Dis. (2017) 50:16–22. doi: 10.1016/j.cimid.2016.11.003
7. Yang H, Zhang S, Wang T, Zhao C, Zhang X, Hu J, et al. Epidemiological characteristics and spatiotemporal trend analysis of human brucellosis in China, 1950-2018. Int J Environ Res. Public Health. (2020) 17:82. doi: 10.3390/ijerph17072382
8. Shevtsova E, Vergnaud G, Shevtsov A, Shustov A, Berdimuratova K, Mukanov K, et al. Genetic diversity of Brucella melitensis in Kazakhstan in relation to world-wide diversity. Front Microbiol. (2019) 10:1897. doi: 10.3389/fmicb.2019.01897
9. Niaz S, Raqeeb A, Khan A, Nasreen A., mir S, Zhu L, et al Status of human brucellosis in district Malakand, Khyber Pakhtunkhwa, Pakistan. J Infect Public Health. (2020) doi: 10.1016/j.jiph.2019.12.013
10. Behera SK, Das D, Balasubramani K, Chellappan S, Rajaram K, Kumar Mohanta H, et al. P. Seroprevalence and risk factors of brucellosis in livestock in the wildlife and livestock interface area of Similipal Biosphere Reserve, India. Vet World. (2020) 13:465–70. doi: 10.14202/vetworld.2020.465-470
11. Denisov AA, Sclyarov OD, Salmakov KM, Shumilov KV. The Russian experience in brucellosis veterinary public health. Rev Off Int Epizoot. (2013) 32:229–37. doi: 10.20506/rst.32.1.2199
12. Kutlu M, Ergonul O, Sayin-Kutlu S, Guven T, Ustun C, Alp-Cavus S, et al. Risk factors for occupational brucellosis among veterinary personnel in Turkey. Prev Vet Med. (2014) 117:52–8. doi: 10.1016/j.prevetmed.2014.07.010
13. Denk A, Demirdag K, Kalkan A, Ozden M, Cetinkaya B, Kilic SS. In vitro activity of Brucella melitensis isolates to various antimicrobials in Turkey. Infect Dis. (2015) 47:364–9. doi: 10.3109/00365548.2014.988748
14. Gül S, Satilmi,ş OK, Ozturk B, Gökçe MI, Kuscu F. Seroprevalence of brucellosis among children in the Middle Anatolia Region of Turkey. J Health Popul Nutr. (2014) 32:577–9.
15. Montagnaro S, D'ambrosi F, Petruccelli A, Ferrara G, D'alessio N, Iovane V, et al. A serological survey of brucellosis in eurasian wild boar (Sus scrofa) in campania Region, Italy. J Wildl Dis. (2020) 56:424–8. doi: 10.7589/2019-04-095
16. Georgi E, Walter MC, Pfalzgraf MT, Northoff BH, Holdt LM, Scholz HC, et al. Whole genome sequencing of Brucella melitensis isolated from 57 patients in Germany reveals high diversity in strains from Middle East. PLoS ONE. (2017) 12:e0175425. doi: 10.1371/journal.pone.0175425
17. Godfroid J, Cloeckaert A, Liautard JP, Kohler S, Fretin D, Walravens K, et al. From the discovery of the Malta fever's agent to the discovery of a marine mammal reservoir, brucellosis has continuously been a re-emerging zoonosis. Vet. Res. (2005) 36:313–326. doi: 10.1051/vetres:2005003
18. Acharya KP. Brucellosis in Nepal - a potential threat to public health professionals. Curr Health Sci J. (2016) 42:396–407. doi: 10.12865/CHSJ.42.04.10
19. Simonson TS, Okinaka RT, Wang B, Easterday WR, Huynh L, U'ren JM, et al. Bacillus anthracis in China and its relationship to worldwide lineages. BMC Microbiol. (2009) 9:71. doi: 10.1186/1471-2180-9-71
20. Cui Y, Yu C, Yan Y, Li D, Li Y, Jombart T, et al. Historical variations in mutation rate in an epidemic pathogen, Yersinia pestis. Proc Natl Acad Sci USA. (2013) 110:577–582. doi: 10.1073/pnas.1205750110
21. Al Dahouk S, Neubauer H, Hensel A, Schoneberg I, Nockler K, Alpers K, et al. Changing epidemiology of human brucellosis, Germany, 1962-2005. Emerg Infect Dis. (2007) 13:1895–900. doi: 10.3201/eid1312.070527
22. Yumuk Z, O'callaghan D. Brucellosis in Turkey – an overview. Int J Infect Dis. (2012) 16:e228–35. doi: 10.1016/j.ijid.2011.12.011
23. Beauvais W, Coker R, Nurtazina G, Guitian J. Policies and livestock systems driving brucellosis re-emergence in Kazakhstan. Ecohealth. (2017) 14:399–407. doi: 10.1007/s10393-015-1030-7
24. Garofolo G, Di Giannatale E, Platone I, Zilli K, Sacchini L, Abass A, et al. Origins and global context of Brucella abortus in Italy. BMC Microbiol. (2017) 17:28. doi: 10.1186/s12866-017-0939-0
25. Golshani M, Buozari S. A review of brucellosis in Iran: epidemiology, risk factors, diagnosis, control, and prevention. Iran Biomed J. (2017) 21:349–59. doi: 10.18869/acadpub.ibj.21.6.349
26. Wareth G, El-Diasty M, Melzer F, Schmoock G, Moustafa SA, El-Beskawy M, et al. MLVA-16 genotyping of Brucella abortus and Brucella melitensis isolates from different animal species in Egypt: geographical relatedness and the mediterranean lineage. Pathogens. (2020) 9:498. doi: 10.3390/pathogens9060498
27. Liu ZG, Wang LJ, Piao DR, Wang M, Liu RH, Zhao HY, et al. Molecular investigation of the transmission pattern of Brucella suis 3 from inner Mongolia, China. Front Vet Sci. (2018) 5:271. doi: 10.3389/fvets.2018.00271
29. Baily GG, Krahn JB, Drasar BS, Stoker NG. Detection of Brucella melitensis and Brucella abortus by DNA amplification. J Trop Med Hyg. (1992) 95:271.
30. Bricker BJ, Halling SM. Differentiation of Brucella abortus bv. 1, 2, and 4, Brucella melitensis, Brucella ovis, and Brucella suis bv. 1 by PCR. J Clin Microbiol. (1994) 32:2660–6. doi: 10.1128/JCM.32.11.2660-2666.1994
31. Ledwaba MB, Gomo C, Lekota KE, Le Flèche P, Hassim A, Vergnaud G, et al. H. Molecular characterization of Brucella species from Zimbabwe. PLoS Negl Trop Dis. (2019) 13:e0007311. doi: 10.1371/journal.pntd.0007311
32. Le Flèche P, Jacques I, Grayon M, Al Dahouk S, Bouchon P, Denoeud F, et al. Evaluation and selection of tandem repeat loci for a Brucella MLVA typing assay. BMC Microbiol. (2006) 6:9. doi: 10.1186/1471-2180-6-9
33. Liu ZG, Di DD, Wang M, Liu RH, Zhao HY, Piao DR, et al. (2017) MLVA genotyping characteristics of human Brucella melitensis isolated from Ulanqab of inner mongolia China. Front Microbiol. 8:6. doi: 10.3389/fmicb.2017.00006
34. Li Z, Wang XM, Zhu X, Wang M, Cheng H, Li D, et al. Molecular characteristics of brucella isolates collected from humans in Hainan Province, China. Front Microbiol. (2020) 11:452. doi: 10.3389/fmicb.2020.00452
35. Deqiu S, Donglou X, Jiming Y. Epidemiology and control of brucellosis in China. Vet Microbiol. (2002) 90:165–182. doi: 10.1016/S0378-1135(02)00252-3
36. Lim JS, Min KD, Ryu S, Hwang SS, Cho SI. Spatial analysis to assess the relationship between human and bovine brucellosis in South Korea, 2005-2010. Sci Rep. (2019) 9:6657. doi: 10.1038/s41598-019-43043-7
37. Tan KK, Tan YC, Chang LY, Lee KW, Nore SS, Yee WY, et al. Full genome SNP-based phylogenetic analysis reveals the origin and global spread of Brucella melitensis. BMC Genom. (2015) 16:93. doi: 10.1186/s12864-015-1294-x
38. Madut NA, Muwonge A, Nasinyama GW, Muma JB, Godfroid J, Jubara AS, et al. The sero-prevalence of brucellosis in cattle and their herders in Bahr el Ghazal region, South Sudan. PLoS Negl Trop Dis. (2018) 12:e0006456. doi: 10.1371/journal.pntd.0006456
39. Zhu X, Zhao Z, Ma S, Guo Z, Wang M, Li Z, et al. Brucella melitensis, a latent “travel bacterium,” continual spread and expansion from Northern to Southern China and its relationship to worldwide lineages. Emerg Microbes Infect. (2020) 9:1618–27. doi: 10.1080/22221751.2020.1788995
40. De Massis F, Zilli K, Di Donato G, Nuvoloni R, Pelini S, Sacchini L, et al. Distribution of Brucella field strains isolated from livestock, wildlife populations, and humans in Italy from 2007 to 2015. PLoS ONE. (2019) 14:e0213689. doi: 10.1371/journal.pone.0213689
41. Mailles A, Rautureau S, Le Horgne JM, Poignet-Leroux B, D'arnoux C, Dennetière G, et al. Re-emergence of brucellosis in cattle in France and risk for human health. Euro Surveill. (2012) 17:20227.
42. Bengis RG, Leighton FA, Fischer JR, Artois M, Mörner T, Tate CM. The role of wildlife in emerging and re-emerging zoonoses. Rev Sci Tech. (2004) 23:497–511. doi: 10.20506/rst.23.2.1498
43. Freeman BA, Pearson GR, Hines WD. Host-parasite relationships in brucellosis. 3. behavior of avirulent Brucella in tissue culture monocytes. J Infect Dis. (1964) 114:441–9. doi: 10.1093/infdis/114.5.441
44. Vered O, Simon-Tuval T, Yagupsky P, Malul M, Cicurel A, Davidovitch N. The price of a neglected zoonosis: case-control study to estimate healthcare utilization costs of human brucellosis. PLoS ONE. (2015) 10:e0145086. doi: 10.1371/journal.pone.0145086
45. Shevtsova E, Shevtsov A, Mukanov K, Filipenko M, Kamalova D, Sytnik I, et al. Epidemiology of Brucellosis and Genetic Diversity of Brucella abortus in Kazakhstan. PLoS ONE. (2016) 11:e0167496. doi: 10.1371/journal.pone.0167496
46. Sun MJ, Di DD, Li Y, Zhang ZC, Yan H, Tian LL, et al. Genotyping of Brucella melitensis and Brucella abortus strains currently circulating in Xinjiang, China. Infect Genet Evol. (2016) 44:522–529. doi: 10.1016/j.meegid.2016.07.025
47. Ma SY, Liu ZG, Zhu X, Zhao ZZ, Guo ZW, Wang M, et al. Molecular epidemiology of Brucella abortus strains from cattle in Inner Mongolia, China. Prev Vet Med. (2020) 183:105080. doi: 10.1016/j.prevetmed.2020.105080
48. Garofolo G, Sacchini F, Ancora M, Travaglini D, Tittarelli M. Multiple-locus variable-number tandem repeat analysis (MLVA) of Brucella melitensis strains isolated in Italy, Greece and Israel. In: International Research Conference Including the 67th Annual Brucellosis Research Meeting, Berlin (2014).
49. Vergnaud G, Hauck Y, Christiany D, Daoud B, Pourcel C, Jacques I, et al. Genotypic expansion within the population structure of classical brucella species revealed by MLVA16 typing of 1404 brucella isolates from different animal and geographic origins, 1974-2006. Front Microbiol. (2018) 9:1545. doi: 10.3389/fmicb.2018.01545
50. Tappe D, Melzer F, Schmoock G, Elschner M, Lam TT, Abele-Horn M, et al. Isolation of Brucella melitensis biotype 3 from epidural empyema in a Bosnian immigrant in Germany. J Med Microbiol. (2012) 61:1335–7. doi: 10.1099/jmm.0.038612-0
51. Shevtsov A, Ramanculov E, Shevtsova E, Kairzhanova A, Tarlykov P, Filipenko M, et al. Genetic diversity of Brucella abortus and Brucella melitensis in Kazakhstan using MLVA-16. Infect Genet Evol. (2015) 34:173–80. doi: 10.1016/j.meegid.2015.07.008
52. Daugaliyeva Capital A, C., Sultanov A, Usserbayev B, Baramova S, Modesto P, et al. Genotyping of Brucella melitensis and Brucella abortus strains in Kazakhstan using MLVA-15. Infect Genet Evol. (2018) 58:135–44. doi: 10.1016/j.meegid.2017.12.022
53. Pisarenko SV, Kovalev DA, Volynkina AS, Ponomarenko DG, Rusanova DV, Zharinova NV, et al. Global evolution and phylogeography of Brucella melitensis strains. BMC Genomics. (2018) 19:353. doi: 10.1186/s12864-018-4762-2
Keywords: Brucella spp., silk road, species/biovar, host lineages, geographic origin, genetic relatedness
Citation: Liu Z, Wang C, Wei K, Zhao Z, Wang M, Li D, Wang H, Wei Q and Li Z (2021) Investigation of Genetic Relatedness of Brucella Strains in Countries Along the Silk Road. Front. Vet. Sci. 7:539444. doi: 10.3389/fvets.2020.539444
Received: 01 March 2020; Accepted: 17 November 2020;
Published: 07 January 2021.
Edited by:
Shao-Lun Zhai, Guangdong Academy of Agricultural Sciences, ChinaReviewed by:
Jo Stevens, University of Edinburgh, United KingdomCopyright © 2021 Liu, Wang, Wei, Zhao, Wang, Li, Wang, Wei and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Wei, d2VpcWlhbmdAY2hpbmFjZGMuY24=; Zhenjun Li, bGl6aGVuanVuQGljZGMuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.