
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Vet. Sci., 02 September 2020
Sec. Veterinary Infectious Diseases
Volume 7 - 2020 | https://doi.org/10.3389/fvets.2020.00526
This article is part of the Research TopicThe Epidemiology, Diagnosis and Prevention of Infectious Diseases in LivestockView all 46 articles
 Qudrat Ullah1,2,3†
Qudrat Ullah1,2,3† Tariq Jamil1,4*†
Tariq Jamil1,4*† Falk Melzer1
Falk Melzer1 Muhammad Saqib5*
Muhammad Saqib5* Muhammad Hammad Hussain6
Muhammad Hammad Hussain6 Muhammad Aamir Aslam7
Muhammad Aamir Aslam7 Huma Jamil3
Huma Jamil3 Muhammad Amjad Iqbal8
Muhammad Amjad Iqbal8 Usman Tahir9
Usman Tahir9 Shakeeb Ullah2
Shakeeb Ullah2 Zafar Iqbal Qureshi3
Zafar Iqbal Qureshi3 Stefan Schwarz4
Stefan Schwarz4 Heinrich Neubauer1
Heinrich Neubauer1Brucellosis is reportedly endemic in ruminants in Pakistan. Both Brucella abortus and B. melitensis infections have been decumented in domestic animals and humans in the country. This study aimed to identify the burden of anti-Brucella antibodies in small ruminants as well as associated potential risk factors with its occurrence at nine institutional livestock farms in Punjab, Pakistan. The sera collected from equal number of sheep and goats (500 from each species) were screened by indirect-ELISA for anti-smooth-Brucella antibodies followed by a serial detection by real-time PCR. Overall, 5.1% (51/1000) seropositivity was registered corresponding to 5% (25/500) prevalence in goats and 5.2% (26/500) in sheep. Brucella-DNA could not be detected in any of the tested sera by real-time PCR. Multiple logistic regression model indicated that farm location (OR 34.05), >4 years of age (OR 2.88), with history of reproductive disorders (OR 2.69), and with BCS of ≤ 3 (OR 12.37) were more likely to test positive for brucellosis at these farms. A routine screening, stringent biosecurity, and quarantine measures are warranted for monitoring and eradication of the infection. Similarly, isolation and molecular investigation of the etiologic agent(s) are needed to understand the relationship of epidemiology and out-breaks of brucellosis in the country.
Brucellosis is a bacterial zoonosis with worldwide distribution, which is caused by bacteria of the genus Brucella. This genus comprises; B. melitensis, B. abortus, B. suis, B. canis, B. ovis, and B. neotome (classical Brucella species), B. ceti and B. pinipedialis from marine mammals, B. microti from voles, B. inopinata from human females, B. papionis from baboons and recently B. vulpis from red foxes (1–6). Based upon host preference; B. abortus predominantly infects bovines, B. melitensis small ruminants, B. canis dogs, B. suis pigs, and B. ovis rams, however, infection in non-prefered hosts is transmissible (7–9). In developing countries, a higher prevalence rate is observed where it causes abortion and retention of fetal membranes (10). The infection may stay undiagnosed due to its asymptomatic form and the infected animals may conceive subsequently, but remain carriers for their life. The infection is of economic importance, especially in developing countries (11). Direct or indirect contact with infected animals and consumption of contaminated raw milk and products are the main routes of transmission, respectively, in animals and humans (12). Brucellosis is an established occupational health hazard (13–16). Diagnosis remains a challenge and is based primarily on serology [e.g., Rose Bengal Test (RBT) and Milk Ring Test (MRT)]. Molecular detection of Brucella-DNA (e.g., PCR) in clinical/biological samples, is coupled with serology to identify the etiology precisely where necessary. The bacterial isolation is a gold standard for the diagnosis, but requires specific growth conditions. Moreover, owing to fastidious nature of the organism (B. abortus for one), the turn-around-time for the samples is beyond a week. Vaccination is recommended but practiced mostly in elite herds in developing countries including in Pakistan (17). Treatment of brucellosis in ruminants is also not very popular in the country hence, test and slaughter/culling policy remains a sole solution for eradication of the infection in farm animals.
Pakistan is an agriculture-based country in south-Asia, where livestock plays a vital role in the national economy. The total livestock population in the country is 142.8 millions, where small ruminants (sheep and goat) share 80.27 million heads (18). In the past, brucellosis has been reported in both large and small ruminants in Punjab, Pakistan (19–23). This study was aimed to ascertain the current status of brucellosis in small ruminants at institutional livestock farms located in Punjab. Additionally, we determined the risk factors associated with the occurrence of the disease.
A total of 1,000 sera (500 each from sheep and goats) were collected from nine different institutional livestock farms maintained under the Livestock and Dairy Development Department (L&DD), Government of Punjab, Lahore, Pakistan (Figure 1) (24). The sample size was calculated for an estimated disease prevalence of 50% at a 95% confidence interval, and 5% desired absolute precision (Table 1) (25). A minimum of 384 samples from each species were required by this method. The sample size was further inflated to accommodate for the potential losses during the transportation. The final sample size was proportionally allocated to each farm according to the population of the animals at each farm. Available identification record was used at each farm, to randomly select animals by using a random number generator and to collect the animal level data. Individual animals were restrained and blood was collected in a 9 mL vacutainer tube without anticoagulant through the jugular vein. No animals were harmed during this process. The animals had no prior history of brucellosis vaccination.
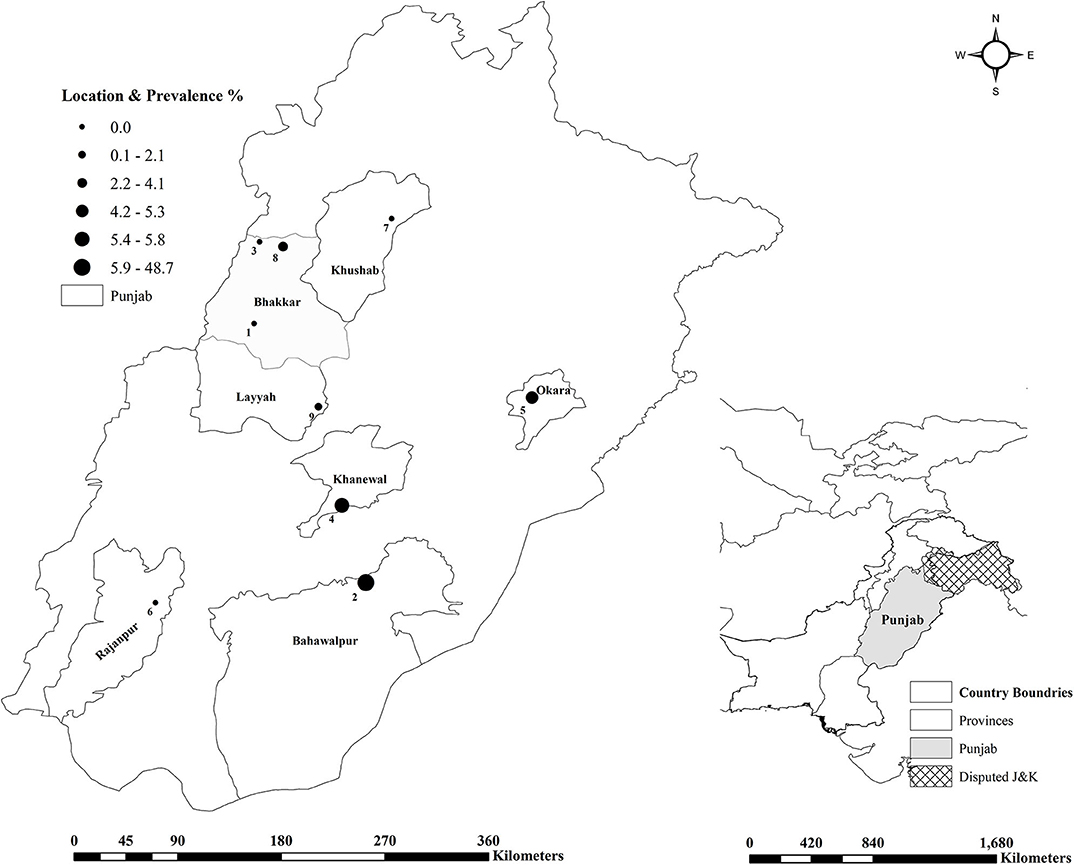
Figure 1. Frontiers Media SA remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Gegraphical representation of the small ruminant farms tested for brucellosis in Punjab, Pakistan.
Sera were screened by ID Screen® Brucellosis Serum Indirect Multi-species (IDVet, Grabels, France), an indirect-ELISA for detection of anti-smooth-lipopolysaccharide (LPS) (B. abortus, B. melitensis, and B. suis). The samples were tested at the National Reference Laboratory (NRL) for brucellosis, Friedrich-Loeffler-Institut (FLI), Jena, Germany as per manufacturer's recommendations. DNA was extracted from sera by using the High Pure Template Kit (Roche, Rotkreuz, Switzerland) and molecular detection was serially done by real-time PCR as described by Probert et al. (26). The DNA extraction was run along with E. coli controls. The real-time PCR was run along with B. abortus (ATCC 23448) and B. melitensis (ATCC 23456) as positive controls. In tandem with positive controls, nuclease-free water was run as negative control (NTC).
Brucellosis prevalence at species level was calculated by dividing the number of positive animals (numerator) by the total number of animals sampled (denominator). The statistical analysis was performed in two parts. In the first part, univariate and multivariate analysis were conducted to determine the association of the risk factors with the seroprevalence. The univariate analysis was conducted for farm related and animal level variables. Seroprevalence of brucellosis was considered as an outcome or dependent variable while biological plausible variables [e.g., farm location, species, sex, age/parity status, breed, history of reproductive disorders, and body condition score (BCS)] were considered as explanatory or independent variables. A p ≤ 0.05 was considered as a level of significance. A backward stepwise approach was used for the binary logistic regression analysis (27). Nagelkerke R2 (NR2) and Hosmer and Lemeshow test (HLT) were used to assess the model-fitness. The statistical analysis was conducted using the IBM SPSS Statistics (IBM Corporation, Armonk, New York, USA).
The second part of statistical analysis was performed using R software and each of the variable was tested one by one alone in a mixed effect model approach with “farm” variable as random factor and using “lmer” function from lme4 package, and logistic binary model function (28). The results of these models showed that five variables were significantly associated with seroprevalence of brucellosis, i.e., species, age, parity status, reproductive disorders, and body condition score (see Table 4). To check if any of these variables showing significance association were confounded, all the five variables were tested in one single model and stepwise backward regression was performed (i.e., least significant variables were taken out in the next model). After running the model, collinearity and confounding behavior was tested by determining variance inflation factor using “vif” function from “car” package. Those variables were taken out of the model which showed high p-value and high variance inflation factor. In the next model if the p-value and variance inflation factor of the other remaining variables changed by a factor of 20%, then the taken-out variable was considered to be confounded with other variables. The maps were generated by using ArcGIS version 10.5.1 (ESRI, Redlands, CA, USA).
Anti-Brucella antibodies were detected in 51 (5.1%, CI 3.8–6.7) samples from sheep and goats. The farm-herd based and univariate analysis showed the seroprevalence almost identical in goats (5.2%) and sheep (5.0%), p = 0.886 (Tables 1, 2). Seropositive animals were detected at the five of nine sampled farms, and the prevalence varied from 2.1% (Farm 9) to 48.7% (Farm 2), p < 0.001. In goats, the highest seroprevalence was recorded in the small ruminants at Farm 5 (15.9%) and the lowest at the Farm 9 (2.9%), p = 0.001. In sheep, the seropositivity ranged from 2.5% (Farm 4) to 48.7% (Farm 2), p < 0.001 (Figure 1). None of the samples contained Brucella DNA as confirmed by negative real-time PCR results.
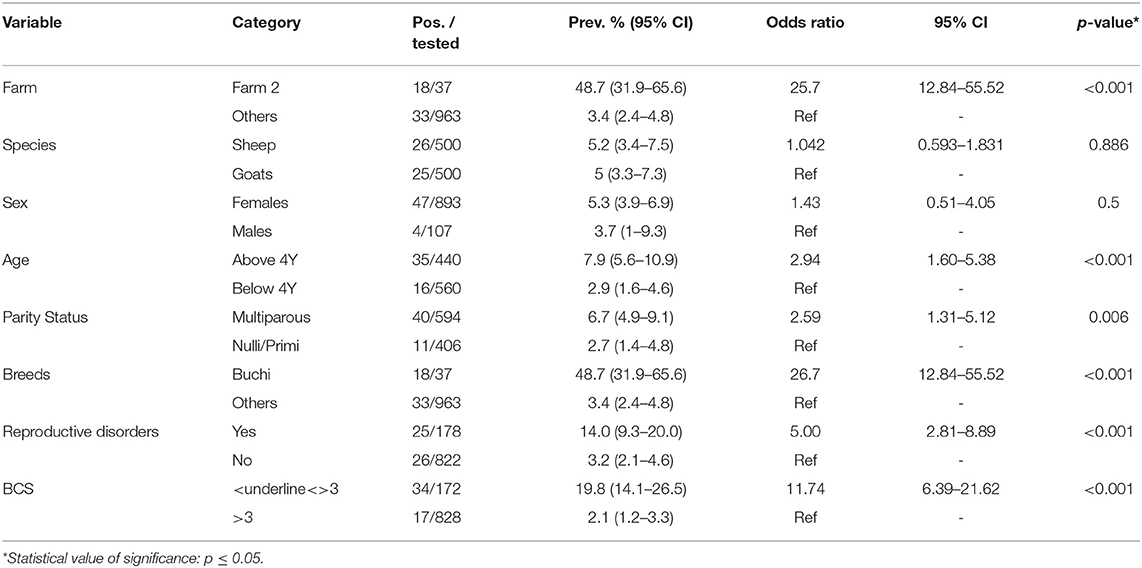
Table 2. Univariable analysis of the seroprevalence of brucellosis in small ruminants sampled from nine institutional livestock farms of Punjab, Pakistan.
The univariable analysis indicated that sheep at Farm 2 were significantly (p < 0.001) more likely to test positive for anti-Brucella antibodies (OR 25.7, CI 12.84–55.52). In females, the seropositivity (5.3%) and odds for testing positive (OR 1.43, 0.51–4.05) were higher as compared to males (3.7%), p = 0.5. The small ruminants; above 4 years of age (7.9%, OR 2.94 CI 1.60–5.38), of multiparous status (6.7%, OR 2.59 CI 1.31–5.12), belonging to Buchi breed (48.7%, OR 26.7 CI 12.84–55.52), with history of reproductive disorders (13.6%, OR 3.19 CI 1.29–7.95) and having BCS ≤ 3 (19.8%, OR 11.74 CI 6.39–21.62) were found significantly (p < 0.05) more likely to test seropositive (Table 2).
The multivariable analysis indicated that small ruminants; kept at Farm 2 (OR 34.05 CI 13.47–86.10), above 4 years of age (OR 2.88 CI 1.39–5.94), with history of reproductive disorders (OR 2.69 CI 1.33–5.42), and BCS ≤ 3 (OR 12.37 CI 5.98–25.57) were significantly (p < 0.01) more likely to test positive for anti-Brucella antibodies (Table 3). The values of Nagelkerke R2 (0.407) and Hosmer and Lemeshow test (Ci-square value; χ2 = 3.092, p = 0.543) indicated that it was a reasonable model to predict seroprevalence of brucellosis at the sampled farms.
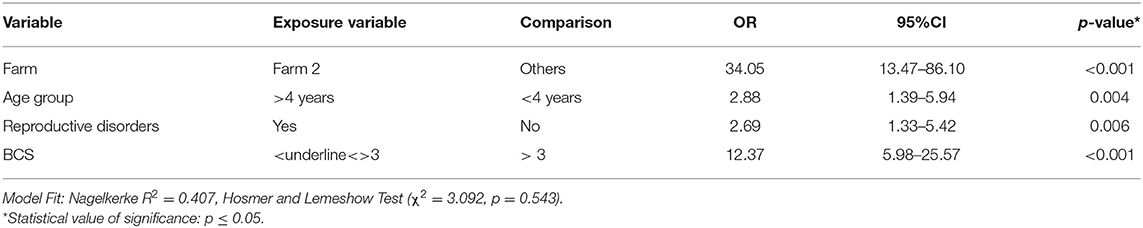
Table 3. Multivariable analysis of the seroprevalence of brucellosis in small ruminants sampled from nine institutional livestock farms of Punjab, Pakistan.
In the second part of statistical analysis, using mixed effects model approach while testing each variable one by one in each model, the following were significant, i.e., species, age, parity status, reproductive disorders, and body condition score while sex and breed were non-significant (Table 4). Using backward regression analysis, testing all these five significant variables together, species and body condition score were found significant while age, parity status, and reproductive disorders were non-significant, with age showing least significant p-value (0.82) and high vif value (3.50) (Table 5). Variable “age” was taken out in the next model, and species, parity status, and body condition score were significant while reproductive disorders was non-significant (0.33) in this model and all variables showed lower vif values. Variable “reproductive disorders” was taken out in the next model, and all the remaining three variables (i.e., species, parity status, and body condition score) were significant and displayed low vif values. Low vif values in the last model pointed out that all the three variables were not confounded (Table 5).
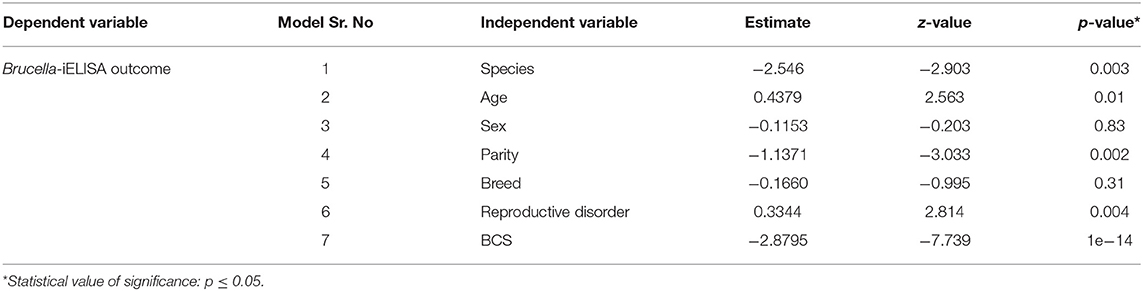
Table 4. Each independent variable was tested separately in Mixed effect logistic regression model with farm as random factor.
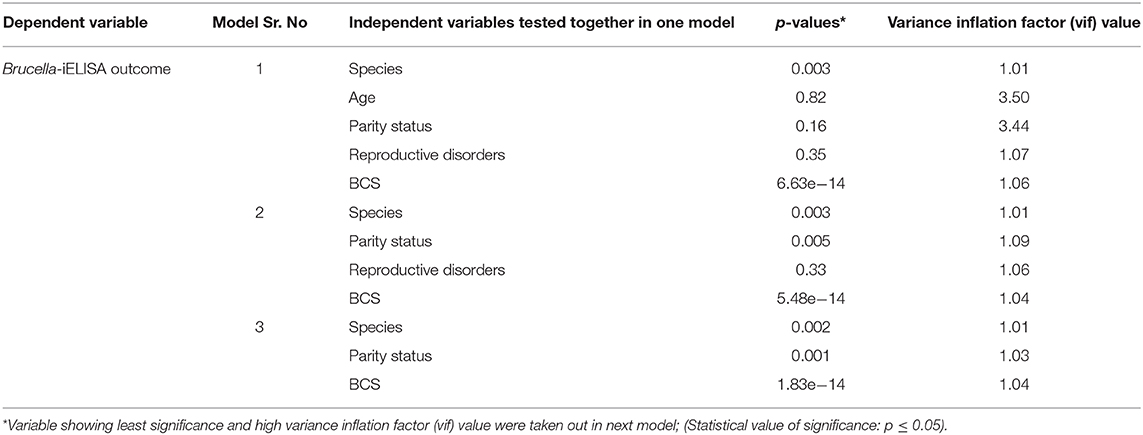
Table 5. Stepwise backward regression models with starting model containing five independent variables and farm as random factor*.
Brucellosis remains an endemic infection in livestock in Pakistan (17, 29). Serology is a preferred and handy choice for diagnosis of brucellosis. ELISA is a sensitive test and is useful for diagnostic screening on larger scale but is unable to differentiate precisely between vaccinated and infected animals (30, 31). Molecular biological tests e.g., PCR, focus on the presence of DNA in the sample and are potentially able to differentiate the vaccine and field strains of Brucella (32). Real-time PCR can even detect and differentiate at lower amounts of DNA in a clinical sample when compared to conventional PCR. However, it requires the presence of bacterial DNA in the sample, which may not be present at every time during and after an infection and might be affected by laboratory procedures (33). Hence, a proper validation process is needed for every test. We used indirect-ELISA as a single screening test and real-time PCR for confirmation of the etiology.
Among variables, the odds for testing positive varied significantly depending upon the farm location and were significantly higher in the animals kept at Farm 2 (Tables 1, 3). These findings are supported by previous reports (20, 22, 34). This could be related to the environmental factors including herd management system at these farms. Furthermore, small ruminants had a close contact with bovines at Farms (2, 5, 6, 7, and 8), where brucellosis was reported previously (21, 23, 35). Moreover, common grazing and watering areas, use of brucellosis positive males for breeding and introduction of new animals without testing could be the factors responsible for brucellosis incidence at these locations (36, 37).
Age (>4 years) and parity status (multiparous) were found significantly associated (p < 0.05) with higher odds as compared to younger (<4 years) and null/primiparous (≤ 1 parturited) animals, respectively. Furthermore, age was also found significantly associated (p < 0.05) with seroprevalence (OR 2.88) by multivariate analysis (Table 3). A similar trend was reported in both sheep and goats with significant association (21, 38), non-significant association (22), and without determination of association (39, 40). This may be ascribed to increased frequency of contact with other animals with respect to age, higher coital chances, and sexual maturity as compared to younger animals (12, 41).
Reproductive disorders showed significant association (OR 2.69, p = 0.006) with brucellosis in the current study (Tables 2, 3). It is understandable as late abortion and retention of fetal membranes are characteristic signs of brucellosis. These findings are supported by similar results reported previously by others investigators (19, 34, 42). However, a non-significant association (p > 0.05) in sheep has also been documented (22). Furthermore, animals having BCS ≤ 3, were more likely to test positive (OR 12.37, p < 0.001) in our study which is concordance with findings of Ethiopian workers (43). A possible reason could be the higher susceptibility of animals already infected with brucellosis to other infections or the loss in BCS caused by the brucellosis itself.
In conclusion, we found anti-Brucella antibodies in sheep and goats at these livestock farms in Punjab, Pakistan. Farm location, age, and species of the animals, history of reproductive disorders and BCS were found to play a significant role for brucellosis seropositivity in these animals. Although vaccination is recommended and treatment is possible for brucellosis, they are not considered safe for human health, hence regular screening and culling of the reactor animals remain the only choice to monitor and eradicate brucellosis. Introduction of the new stock at these farms should be carried out only after screening and quarantine. Furthermore, farm workers should be advised to adopt protection measures as a routine. Abortion at these farms should not go unnoticed and must be investigated to confirm its cause to adopt recommended control measures. If abortions occur, disinfection of the area should be ensured along with strict biosecurity measures to restrict chances of dissemination of infection through the dogs, cats, other domestic animals, visitors, and farm equipment/supply movement. Standardization and validation of the diagnostic tests are required based on the local conditions. Isolation and molecular investigations of the etiological agents might be helpful for future understanding of the epidemiology of the infection and the relationship of the outbreaks.
All datasets generated for this study are included in the article/supplementary material.
Blood and serum samples were collected as per bio-safety, ethical, and animal welfare guidelines defined by Research Board of the University of Agriculture, Faisalabad, Pakistan (letter No. 3253/ORIC, dated: 25.11.2015). Permission was granted by the Livestock and Dairy Development Department (LNDD), Government of Punjab, Pakistan to collect samples at the farms (vide letter No. SO (I&C)/L&DD/2-6/2016, dated: 15.02.2016).
QU and TJ: Conceptualization. FM and MS: methodology. MH and MA: formal analysis. TJ and QU: investigation. UT, MI, and QU: data curation. TJ: writing-original draft preparation. SU, ZQ, HJ, SS, and HN: writing-review and editing. All authors contributed to the article and approved the submitted version.
Authors would like to thank German Federal Foreign Office for financially supporting the study under the project German Biosecurity Program. Funding agencies had no role in designing, performing, and publication of the results.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Authors are thankful to Higher Education Commission (HEC)-Pakistan to support the stay of QU under the project International Research Support Program (IRSP) and TJ under the project 90% Overseas Scholarship Scheme at Friedrich-Loeffler-Institut (FLI), Jena, Germany. Authors would also like to thank the Livestock and Dairy Development (L&DD), Government of Punjab, Pakistan for their cooperation in the provision of samples. The agencies had no role in designing, performing, and publication of the results.
1. Garrity GM, Bell JA, Lilburn TG. Taxonomic Outline of the Prokaryotes. Bergey's Manual of Systematic Bacteriology. New York, NY: Springer-Verlag (2004). p. 55.
2. Foster G, Osterman BS, Godfroid J, Jacques I, Cloeckaert A. Brucella ceti sp nov and Brucella pinnipedialis sp nov for Brucella strains with cetaceans and seals as their preferred hosts. Int J Syst Evol Micr. (2007) 57:2688–93. doi: 10.1099/ijs.0.65269-0
3. Scholz HC, Hubalek Z, Sedlacek I, Vergnaud G, Tomaso H, Al Dahouk S, et al. Brucella microti sp. nov., isolated from the common vole Microtus arvalis. Int J Syst Evol Micr. (2008) 58:375–82. doi: 10.1099/ijs.0.65356-0
4. Scholz HC, Nöckler K, Göllner C, Bahn P, Vergnaud G, Tomaso H, et al. Brucella inopinata sp. nov., isolated from a breast implant infection. Int J Syst Evol Micr. (2010) 60:801–8. doi: 10.1099/ijs.0.011148-0
5. Whatmore AM, Davison N, Cloeckaert A, Al Dahouk S, Zygmunt MS, Brew SD, et al. Brucella papionis sp. nova, isolated from baboons (Papio spp.). Int J Syst Evol Micr. (2014) 64:4120–8. doi: 10.1099/ijs.0.065482-0
6. Scholz HC, Revilla-Fernandez S, Dahouk SA, Hammerl JA, Zygmunt MS, Cloeckaert A, et al. Brucella vulpis sp nov., isolated from mandibular lymph nodes of red foxes (Vulpes vulpes). Int J Syst Evol Micr. (2016) 66:2090–8. doi: 10.1099/ijsem.0.000998
7. Saleem MZ, Akhtar R, Aslam A, Rashid MI, Chaudhry ZI, Manzoor MA, et al. Evidence of Brucella abortus in non-preferred caprine and ovine hosts by real-time PCR assay. Pak J Zool. (2019) 51:1187–9. doi: 10.17582/journal.pjz/2019.51.3.sc3
8. Jamil T, Melzer F, Khan I, Iqbal M, Saqib M, Hussain MH, et al. Serological and molecular investigation of Brucella species in dogs in Pakistan. Pathogens. (2019) 8:294. doi: 10.3390/pathogens8040294
9. Saeed U, Ali S, Khan TM, El-Adawy H, Melzer F, Khan AU, et al. Seroepidemiology and the molecular detection of animal brucellosis in Punjab Pakistan. Microorganisms. (2019) 7:449. doi: 10.3390/microorganisms7100449
11. McDermott J, Grace D, Zinsstag J. Economics of brucellosis impact and control in low-income countries. Rev Sci Tech. (2013) 32:249–61. doi: 10.20506/rst.32.1.2197
12. Gul S, Khan A. Epidemiology and epizootology of brucellosis: a review. Pak Vet J. (2007) 27:145–51.
13. Ali S, Ali Q, Neubauer H, Melzer F, Elschner M, Khan I, et al. Seroprevalence and risk factors associated with brucellosis as a professional hazard in Pakistan. Foodborne Pathog Dis. (2013) 10:500–5. doi: 10.1089/fpd.2012.1360
14. Asif M, Waheed U, Farooq M, Ali T, Khan QM. Frequency of brucellosis in high risk human groups in Pakistan detected through Polymerase Chain Reaction and its comparison with conventional slide agglutination test. Int J Agric Biol. (2014) 16:986–90.
15. Mukhtar F. Brucellosis in a high risk occupational group: seroprevalence and analysis of risk factors. J Pak Med Assoc. (2010) 60:1031.
16. Mukhtar F, Kokab F. Brucella serology in abattoir workers. J Ayub Med Coll Abbottabad. (2008) 20:57–61.
17. Nawaz G, Malik MN, Mushtaq MH, Ahmad FM, Shah AA, Iqbal F, et al. Surveillance of brucellosis in livestock in rural communities of Punjab, Pakistan. Pak J Agric Res. (2016) 29:392–8.
18. Anonymous. Economic Survey of Pakistan: Chapter: Livestock Population of Pakistan. Pakistan Bureue of Statistics (2006).
19. Arshad M, Munir M, Iqbal K, Abbas R, Rasool M, Khalil N. Sero-prevalence of brucellosis in goats from public and private livestock farms in Pakistan. Online J Vet Res. (2011) 15:297–304.
20. Gul ST, Khan A, Ahmad M, Rizvi F, Shahzad A, Hussain I. Epidemiology of brucellosis at different livestock farms in the Punjab Pakistan. Pak Vet J. (2015) 35:309–14.
21. Gul ST, Khan A, Rizvi F, Hussain I. Sero-prevalence of brucellosis in food animals in the Punjab Pakistan. Pak Vet J. (2014) 34:454–8.
22. Iqbal Z, Jamil H, Qureshi ZI, Saqib M, Lodhi LA, Waqas MS, et al. Seroprevalence of ovine brucellosis by modified Rose Bengal test and ELISA in southern Punjab Pakistan. Pak Vet J. (2013) 33:455–7.
23. Jamil T, Melzer F, Saqib M, Shahzad A, Khan KK, Hammad HM, et al. Serological and molecular detection of bovine brucellosis at institutional livestock farms in Punjab, Pakistan. Int J Environ Res Public Health. (2020) 7:1412. doi: 10.3390/ijerph17041412
24. Ullah Q, El-Adawy H, Jamil T, Jamil H, Qureshi ZI, Saqib M, et al. Serological and molecular Investigation of Coxiella burnetii in small ruminants and ticks in Punjab, Pakistan. Int J Environ Res Public Health. (2019) 16:4271. doi: 10.3390/ijerph16214271
26. Probert WS, Schrader KN, Khuong NY, Bystrom SL, Graves MH. Real-time multiplex PCR assay for detection of Brucella spp. B. abortus and B. melitensis. J Clin Microbiol. (2004) 42:1290–3. doi: 10.1128/JCM.42.3.1290-1293.2004
27. Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. (2008) 3:17. doi: 10.1186/1751-0473-3-17
28. The R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing (2018).
29. Farooq U, Fatima Z, Afzal M, Anwar Z, Jahangir M. Sero-prevalence of brucellosis in bovines at farms under different management conditions. British J Dairy Sci. (2011) 2:35–9.
31. Uzal FA, Carrasco AE, Echaide S, Nielsen K, Robles CA. Evaluation of an indirect ELISA for the diagnosis of bovine brucellosis. J Vet Diagn Invest. (1995) 7:473–5. doi: 10.1177/104063879500700408
32. López-Goñi D, García-Yoldi CM, Marín MJ, de Miguel PM, Muñoz JM, Blasco I, et al. Evaluation of a Multiplex PCR Assay (Bruce-ladder) for molecular typing of all Brucella species, including the vaccine strains. J Clin Microbiol. (2008) 46:3484. doi: 10.1128/JCM.00837-08
33. Jamil T, Melzer F, Njeru J, El-Adawy H, Neubauer H, Wareth G. Brucella abortus: current research and future trends. Curr Clin Microbiol Rep. (2017) 4:1. doi: 10.1007/s40588-017-0052-z
34. Naeem KN, Kamran J, Ullah A. Seroprevalence and risk factors associated with Crimean-Congo haemorrhagic fever and brucellosis in people and livestock in Baluchistan and Khyber Pakhtunkhwa Provinces Pakistan. In: Proceedings of South Asia Regional One Health Symposium (Paro) (2013). p. 50–1.
35. Nasir AA, Parveen Z, Shah MA, Rashid M. Seroprevalence of brucellosis in animals at government and private livestock farms in Punjab. Pak Vet J. (2004) 24:144–6.
36. Ullah S, Jamil T, Mushtaq M, Saleem M. Prevalence of brucellosis among camels in district Muzaffargarh Pakistan. J Infec Mol Biol. (2015) 3:52–6. doi: 10.14737/journal.jimb/2015/3.2.52.56
37. Cárdenas L, Peña M, Melo O, Casal J. Risk factors for new bovine brucellosis infections in Colombian herds. BMC Vet Res. (2019) 15:81. doi: 10.1186/s12917-019-1825-9
38. Ali S, Akbar A, Shafee M, Tahira B, Muhammed A, Ullah N. Sero-epidemiological study of brucellosis in small ruminants and associated human beings in district Quetta Balochistan. Pure Appl Microbiol. (2017) 6:797–804. doi: 10.19045/bspab.2017.60084
39. Ghani M, Siraj M, Zeb A, Naeem M. Sero-epidemiological study of brucellosis among goats and sheep at Pshawar district. Asian-Australas J Anim Sci. (1995) 8:489–94. doi: 10.5713/ajas.1995.489
40. Hussain MA, Nazir S, Rajput IR, Khan IZ, Rehman M-u-, Hayat S. Study on seroprevalence of brucellosis in caprine in Kohat (Khyber Pakhtoon Khwa). Lasbela Uni J Sci Tech. (2014) 3:14–20.
41. Abubakar M, Mansoor M, Arshed MJ. Bovine brucellosis: old and new concepts with Pakistan perspective. Pak Vet J. (2012) 32:147–55.
42. Khan AQ, Haleem SK, Shafiq M, Khan NA, ur Rahman S. Seropositivity of brucellosis in human and livestock in Tribal-Kurram agency of Pakistan indicates cross circulation. Wetchasan Sattawaphaet Thai J Vet Med. (2017) 47:349–55.
Keywords: sheep, goats, brucellosis, risk factors, Pakistan
Citation: Ullah Q, Jamil T, Melzer F, Saqib M, Hussain MH, Aslam MA, Jamil H, Iqbal MA, Tahir U, Ullah S, Qureshi ZI, Schwarz S and Neubauer H (2020) Epidemiology and Associated Risk Factors for Brucellosis in Small Ruminants Kept at Institutional Livestock Farms in Punjab, Pakistan. Front. Vet. Sci. 7:526. doi: 10.3389/fvets.2020.00526
Received: 13 January 2020; Accepted: 08 July 2020;
Published: 02 September 2020.
Edited by:
Anuwat Wiratsudakul, Mahidol University, ThailandReviewed by:
Sidharath Dev Thakur, Chaudhary Sarwan Kumar Himachal Pradesh Krishi Vishvavidyalaya, IndiaCopyright © 2020 Ullah, Jamil, Melzer, Saqib, Hussain, Aslam, Jamil, Iqbal, Tahir, Ullah, Qureshi, Schwarz and Neubauer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tariq Jamil, dGFyaXEuamFtaWxAZmxpLmRl; Muhammad Saqib, ZHJzYXFpYl92ZXRAaG90bWFpbC5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.