
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 17 July 2020
Sec. Veterinary Infectious Diseases
Volume 7 - 2020 | https://doi.org/10.3389/fvets.2020.00363
This article is part of the Research Topic The Epidemiology, Diagnosis and Prevention of Infectious Diseases in Livestock View all 46 articles
The Gram-negative bacteria of the genus Chlamydia cause a wide range of diseases in humans and animals. The seroprevalence of Chlamydia in domestic black-boned sheep and goats in China is unknown. In this survey, a total of 481 serum samples were collected randomly from domestic black-boned sheep and goats from three counties in Yunnan province, southwest China, from July to August 2017. The sera were examined by an indirect hemagglutination assay (IHA). Antibodies to Chlamydia were detected in 100/481 [20.79%, 95% confidence interval (CI), 17.16–24.42] serum samples (IHA titer ≥1:64). The Chlamydia seroprevalence ranged from 12.21% (95% CI, 7.81–16.61) to 30.89% (95% CI, 22.72–39.06) across different regions in Yunnan province, and the differences were statistically significant (P < 0.01). The seroprevalence in male domestic black-boned sheep and goats (28.64%; 95% CI, 22.36–34.92) was significantly higher than that in the females (15.25%; 95% CI, 11.05–19.45) (P < 0.01). However, there was no statistically significant difference in Chlamydia seroprevalence in domestic black-boned sheep and goats between ages and species (P > 0.05). To our knowledge, this is the first report of Chlamydia seroprevalence in domestic black-boned sheep and goats in Yunnan Province, southwest China. These data provide baseline information for future implementation of measures to control Chlamydia infection in these animals.
Chlamydia, an obligate intracellular Gram-negative pathogen, is responsible for a broad spectrum of diseases in animals and humans (1, 2). Chlamydia grows and reproduces in the respiratory, urogenital, and gastrointestinal tracts (2). Two species of the genus Chlamydia, namely Chlamydia abortus and Chlamydia pecorum, can cause serious infections in sheep and goats (1). Chlamydia is a leading cause of abortion in sheep and goats, which caused significant economic losses to livestock industry (3–6). Additionally, as a zoonotic pathogen, humans can be infected via exposure to Chlamydia infected animals (7).
Chlamydia infection is prevalent in sheep and goats all over the world, especially in sheep-rearing areas, such as in Northern Europe and North America (8, 9). In China, Chlamydia infection in sheep has been reported in many provinces, such as Qinghai, Shandong, and Hubei (10). However, data about Chlamydia infection in domestic black-boned sheep and goats have been limited. Domestic black-boned sheep and goats have dark tissue compared to ordinary sheep and goats, which has been attributed to the presence of excessive melanin in domestic black-boned sheep and goats (11).
Domestic black-boned sheep and goats are indigenous animals to the Lanping County of Yunnan Province, China (11–13). Because of their unique characteristics of these breeds, black-boned sheep and goats have a strong adaptability, and they have been introduced into other provinces of China, such as Shandong, Henan, and Hebei (14). Therefore, in this study, we examined the seroprevalence and risk factors of Chlamydia infection in domestic black-boned sheep and goats in Yunnan Province, southwest China. Our results provide baseline data for future control strategies of Chlamydia infection in domestic black-boned sheep and goats in China.
This study was approved by the Animal Administration and Ethics Committee of Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences (approval no.: LVRIAEC-2017-06). Domestic black-boned sheep and goats, from which the blood samples were collected, were handled humanely in accordance with the requirements of the Animal Ethics Procedures and Guidelines of the People's Republic of China.
The survey was conducted in Shilin County, Lanping County, and Yongsheng County in Yunnan Province, southwest China (Figure 1). Yunnan Province is the major producing region of domestic black-boned sheep and goats in China. In the present study, the sampling sites are all large-scale farms, which implement a free-range breeding mode for 5–8 h in daytime. The annual temperature difference in Yunnan Province is small, but the daily temperature difference is large.
Figure 1. The map of China showing the geographical regions in Yunnan province, where domestic black-boned sheep and goats were sampled. LP, Lanping County; SL, Shilin County; YS, Yongsheng County.
Between July and August 2017, a total of 481 blood samples were collected randomly from domestic black-boned sheep and goats from four intensive farms (n = 6,100), two of which were from Lanping county (n = 213), followed by Yongsheng county (n = 145) and Shilin county (n = 123), Yunnan province, southwest China. A standardized questionnaire was used to collect information about the region, gender, age, and species of each animal. Blood samples were transported to the laboratory, kept at room temperature for 2 h, and centrifuged at 3,000 g for 10 min, and the supernatants, which represent the serum samples, were collected and stored at −20°C until further analysis.
A commercially available indirect hemagglutination assay (IHA) kit (Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences) was used to determine the level of Chlamydia antibodies in the serum of domestic black-boned sheep and goats. As a mature technology for detecting Chlamydia antibodies, the sensitivity and specificity of the IHA kit used in this study have been verified by the Ministry of Agriculture of China (NY/T 562-2002), which were 100% and 95%, respectively (15). The serological analysis was carried out according to the manufacturer's recommendations as previously described (16–19). Briefly, serum samples were added to 96-well V-bottomed polystyrene plates, which were diluted fourfold serially from 1:4 to 1:1,024. The detection antigen was added to each well, and the plate was then shaken slightly for 2 min followed by incubation at 37°C for 2 h. The samples were considered positive for Chlamydia antibodies when the agglutinated erythrocytes were formed in wells at dilutions of 1:64 or higher. Samples that had agglutination results between 1:4 and 1:64 were considered “suspect” and were retested.
Differences in the seroprevalence of Chlamydia among domestic black-boned sheep and goats of different regions, genders, ages, and species were analyzed by a χ2 test using the SPSS software (release 23.0 standard version; SPSS Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant. Odds ratios (ORs) with 95% confidence intervals (CIs) were also determined.
In the present study, 100 of the examined 481 serum samples of domestic black-boned sheep and goats (20.79%; 95% CI, 17.16–24.42) were seropositive for Chlamydia by IHA test at the cutoff titer of 1:64. The 100 positive samples included 26 samples (of 213) from Lanping Country (12.21%; 95% CI, 7.81–16.61), 36 (of 145) from Yongsheng Country (24.83%; 95% CI, 17.80–31.86), and 38 (of 123) samples from Shilin Country (30.89%; 95% CI, 22.72–39.06). The differences in Chlamydia seroprevalence between these regions were statistically significant (χ2 = 18.59, df = 2, P < 0.01; Table 1). As shown in Table 1, the investigation revealed that the seroprevalence in female and male animals was 15.25% (43/282; 95% CI, 11.05–19.45) and 28.64% (57/199; 95% CI, 22.36–34.92), respectively. The difference in Chlamydia seroprevalence was statistically significant between genders (χ2 = 12.71, df = 1, P < 0.01) of domestic black-boned sheep and goats. Seropositive black-boned sheep and goats were found in all four age groups and varied from 16.41% (21/128; 95% CI, 10.00–22.83) to 25.40% (48/189; 95% CI, 19.19–31.61). In terms of species, the seroprevalence was 22.76% (71/312; 95% CI, 18.11–27.41) in black-boned sheep and 17.16% (29/169; 95% CI, 11.48–22.84) in black-boned goats. There was no statistically significant difference in Chlamydia seroprevalence observed between age groups (χ2 = 4.63, df = 3, P > 0.05) and species (χ2 = 2.09, df = 1, P > 0.05) in domestic black-boned sheep and goats (Table 1). The antibody titers were diverse in domestic black-boned sheep and goats of different regions, genders, ages, and species, with the most frequent titers being 1:64 (87.00%), followed by 1:256 (10.00%) and 1:1,024 (3.00%; Table 1).
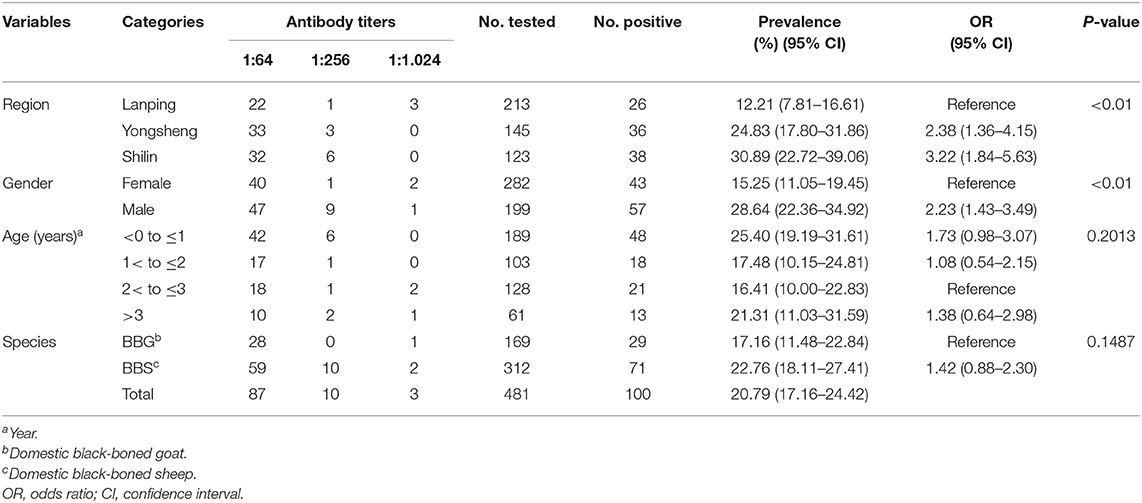
Table 1. Seroprevalence and risk factors for Chlamydia in domestic black-boned sheep and goats in Yunnan Province, southwest China, determined by indirect hemagglutination (IHA) test.
In this study, the seroprevalence of Chlamydia in domestic black-boned sheep and goats in Yunnan province was 20.79%, which was higher than the 8.45% reported in goats in Hunan Province, China (20), but was lower than that reported in sheep in Xinjiang Province (40.13%) in China (10). Chlamydia seroprevalence has been reported in sheep and goats worldwide. For example, 10.60% seroprevalence has been reported in sheep in India (21), and 33% seroprevalence has been reported in Spain (22). The different seroprevalences in different counties in our study is probably attributed to the differences in sanitation, husbandry practices, and animal welfare. In addition, other reasons for the variations of prevalence may include different ecological and geographical factors including temperature, rainfall, altitude, or level of vegetation. Furthermore, differences in the serological methods and cutoff titers used may be other factors that influence the seroprevalence of Chlamydia in different regions.
The overall Chlamydia seroprevalence in domestic black-boned sheep and goats in Shilin County was 30.89%, which was higher than the seroprevalence in Yongsheng County (24.83%) and in Lanping County (12.21%). There was significant difference in Chlamydia seroprevalence in domestic black-boned sheep and goats of different regions (P < 0.01). This result is consistent with a previous study that reported an 18.65% Chlamydia seroprevalence in Tibetan sheep in Gansu province (15). Chlamydia is significantly resistant under dry, cold (5–10°C), and dark conditions (23). Yunnan Province has a generally mild climate as diverse as its terrain. Shilin Country has an average annual temperature of 15°C and a mean annual rainfall of 1,010 mm. The warm temperature and appropriate precipitation in Shilin Country are favorable for the survival of Chlamydia. Therefore, the differences in Chlamydia seroprevalence in domestic black-boned sheep and goats across different regions are probably attributed to the variable climatic conditions in Yunnan Province.
Statistically, the Chlamydia seroprevalence in male (28.64%) domestic black-boned sheep and goats was significantly higher than in the females (15.25%). Statistical analysis showed a significant difference between genders (P < 0.01). Gender-related differences in Chlamydia seroprevalence were related to variations in immune response or antibody persistence between males and females (24). The result was different from a previous study, which reported no effect of the gender on the prevalence of Chlamydia infection in sheep (17).
The seroprevalence of Chlamydia varied across the different age groups of domestic black-boned sheep and goats. The highest seroprevalence was 25.40% in black-boned sheep and goats of the 0 < years ≤ 1 age group, and the lowest prevalence was 16.41% in the 2 < years ≤ 3 age group. But the differences were not statistically significant among different age groups (P > 0.05), which disagree with the study of Qin et al. (15), which reported positive association of Chlamydia seroprevalence with the ages of Tibetan sheep in Gansu Province. The higher seroprevalence in domestic black-boned sheep and goats of the 0 < years ≤ 1 age group may be due to the low levels of antibodies, which makes them more susceptible to infection. The different prevalence in different age groups indicates the possibility of horizontal transmission in investigated black-boned sheep and goat herds (25).
The seroprevalence of Chlamydia in domestic black-boned sheep (22.76%) was slightly higher than that in domestic black-boned goats (17.16%), which may be related to the different susceptibility of goats and sheep to Chlamydia. Statistical analysis suggested that species may not be a crucial factor for Chlamydia infection in black-boned sheep and goats. The difference in Chlamydia seroprevalence in domestic black-boned sheep and goats may be caused by the sample bias, where more domestic black-boned sheep samples were examined than black-boned goats.
There are some limitations to the present investigation. The serum samples of black-boned sheep and goats examined in this study were collected from July to August 2017, a relatively short sampling time; thus, the reported Chlamydia seroprevalence may not fully reflect the true situation of long-term infection of Chlamydia in domestic black-boned sheep and goats. Given that domestic black-boned sheep and goats have been introduced into other provinces of China (14), further research should investigate Chlamydia seroprevalence in domestic black-boned sheep and goats in these provinces, which will provide global baseline data for the prevention of Chlamydia infection in black-boned sheep and goats in China.
The present study revealed that Chlamydia seroprevalence (20.79%) is relatively high in domestic black-boned sheep and goats in Yunnan Province, southwest China. This study also demonstrated that region and gender are the main risk factors for Chlamydia seroprevalence between domestic black-boned sheep and goats. To our knowledge, the present survey is the first to document the seroprevalence of Chlamydia infection in domestic black-boned sheep and goats in China, which provided baseline data for future prevention and control of Chlamydia in domestic black-boned sheep and goats.
All datasets generated for this study are included in the article.
The animal study was reviewed and approved by The Animal Ethics and Administration Committee of Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences.
X-QZ and F-CZ conceived and designed the experiments. L-XS performed the experiments, analyzed the data, and wrote the paper. ZL, J-FY, and F-CZ participated in the collection of serum samples. Q-LL and X-HH participated in the implementation of the study. X-QZ and F-CZ critically revised the manuscript. All authors have read and approved the final version of the manuscript. All authors contributed to the preparation of the manuscript.
This project support was provided, in part, by the Agricultural Science and Technology Innovation Program (ASTIP) (Grant No. CAAS-ASTIP-2016-LVRI-03) and the Promotion Project of ESI Disciplines of Yunnan Agricultural University (Grant No. 2019YNAUESIMS03).
Frontiers Media Ltd., remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank Associate Professor Hany M. Elsheikha from the Faculty of Medicine and Health Sciences, University of Nottingham, UK, for improving the English of the manuscript.
1. Longbottom D, Coulter LJ. Animal chlamydioses and zoonotic implications. J Comp Pathol. (2003) 128:217–44. doi: 10.1053/jcpa.2002.0629
2. Rohde G, Straube E, Essig A, Reinhold P, Sachse K. Chlamydial zoonoses. Dtsch Arztebl Int. (2010) 107:174–80. doi: 10.3238/arztebl.2010.0174
3. Wang FI, Shieh H, Liao YK. Prevalence of Chlamydophila abortus infection in domesticated ruminants in Taiwan. J Vet Med Sci. (2001) 63:1215–20. doi: 10.1292/jvms.63.1215
4. Szeredi L, Bacsadi A. Detection of Chlamydophila (Chlamydia) abortus and Toxoplasma gondii in smears from cases of ovine and caprine abortion by the streptavidin-biotin method. J Comp Pathol. (2002) 127:257–63. doi: 10.1053/jcpa.2002.0591
5. Sharma SP, Baipoledi EK, Nyange JFC, Tlagae L. Isolation of Toxoplasma gondii from goats with history of reproductive disorders and the prevalence of Toxoplasma and Chlamydial antibodies. Onderstepoort J Vet Res. (2003) 70:65–8. doi: 10.1046/j.1365-2915.2003.00404.x
6. Masala G, Porcu R, Sanna G, Tanda A, Tola S. Role of Chlamydophila abortus in ovine and caprine abortion in Sardinia, Italy. Vet Res Commun. (2005) 29:117–23. doi: 10.1007/s11259-005-0842-2
7. Schautteet K, Vanrompay D. Chlamydiaceae infections in pig. Vet Res. (2011) 42:29. doi: 10.1186/1297-9716-42-29
8. Seth-Smith HMB, Busó LS, Livingstone M, Sait M, Harris SR, Aitchison KD, et al. European Chlamydia abortus livestock isolate genomes reveal unusual stability and limited diversity, reflected in geographical signatures. BMC Genomics. (2017) 18:344. doi: 10.1186/s12864-017-3657-y
9. Campos-Hernández E, Vázquez-Chagoyán JC, Salem AZM, Saltijeral-Oaxaca JA, Escalante-Ochoa C, López-Heydeck SM, et al. Prevalence and molecular identification of Chlamydia abortus in commercial dairy goat farms in a hot region in Mexico. Trop Anim Health Prod. (2014) 46:919–24. doi: 10.1007/s11250-014-0585-6
10. Zhou JZ, Li ZC, Lou ZZ, Fei YY. Prevalence, diagnosis, and vaccination situation of animal chlamydiosis in China. Front Vet Sci. (2018) 5:88. doi: 10.3389/fvets.2018.00088
11. Deng WD, Yang SL, Huo YQ, Gou X, Shi XW, Mao HM. Physiological and genetic characteristics of black-boned sheep (Ovis aries). Anim Genet. (2006) 37:586–8. doi: 10.1111/j.1365-2052.2006.01530.x
12. Deng WD, Shu W, Yang SL, Shi XW, Mao HM. Pigmentation in Black-boned sheep (Ovis aries): association with polymorphism of the MC1R gene. Mol Biol Rep. (2009) 36:431–6. doi: 10.1007/s11033-007-9197-9
13. Deng WD, Xi DM, Gou X, Yang SL, Shi XW, et al. Pigmentation in Black-boned sheep (Ovis aries): association with polymorphism of the Tryosinase gene. Mol Biol Rep. (2008) 35:379–85. doi: 10.1007/s11033-007-9097-z
14. Chen D, Wang SS, Zou Y, Li Z, Xie SC, Shi LQ, et al. Prevalence and multi-locus genotypes of Enterocytozoon bieneusi in black-boned sheep and goats in Yunnan Province, southwestern China. Infect Genet Evol. (2018) 65:385–91. doi: 10.1016/j.meegid.2018.08.022
15. Qin SY, Yin MY, Cong W, Zhou DH, Zhang XX, Zhao Q, et al. Seroprevalence and risk factors of Chlamydia abortus infection in Tibetan sheep in Gansu Province, northwest China. Sci World J. (2014) 2:1–6. doi: 10.1155/2014/193464
16. Cong W, Huang SY, Zhang XY, Zhou DH, Xu MJ, Zhao Q, et al. Seroprevalence of Chlamydia psittaci infection in market-sold adult chickens, ducks and pigeons in north-western China. J Med Microbiol. (2013) 62:1211–4. doi: 10.1099/jmm.0.059287-0
17. Huang SY, Wu SM, Xu MJ, Zhou DH, Danba C, Gong G, et al. First record of Chlamydia abortus seroprevalence in Tibetan sheep in Tibet, China. Small Rumin Res. (2013) 112:243–5. doi: 10.1016/j.smallrumres.2012.12.012
18. Wu SM, Huang SY, Xu MJ, Zhou DH, Song HQ, Zhu XQ. Chlamydia felis exposure in companion dogs and cats in Lanzhou, China: a public health concern. BMC Vet Res. (2013) 9:104. doi: 10.1186/1746-6148-9-104
19. Zhang NZ, Zhou DH, Shi XC, Nisbet AJ, Huang SY, Ciren D, et al. First report of Chlamydiaceae seroprevalence in Tibetan pigs in Tibet, China. Vector Borne Zoonotic Dis. (2013) 13:196–9. doi: 10.1089/vbz.2012.1208
20. Hu SF, Li F, Zheng WB, Liu GH. Seroprevalence and risk factors of Chlamydia abortus infection in goats in Hunan province, subtropical China. Vector Borne Zoonotic Dis. (2018) 18:500–3. doi: 10.1089/vbz.2017.2183
21. Chahota R, Gupta S, Bhardwaj B, Malik P, Verma S, Sharma AM. Seroprevalence studies on animal chlamydiosis amongst ruminants in five states of India. Vet World. (2015) 8:72–5. doi: 10.14202/vetworld.2015.72-75
22. Tejedor-Junco MT, González-Martín M, Corbera JA, Santana Á, Hernández CN, Gutiérrez C. Preliminary evidence of the seroprevalence and risk factors associated with Chlamydia abortus infection in goats on the Canary Islands, Spain. Trop Anim Health Prod. (2019) 51:257–60. doi: 10.1007/s11250-018-1654-z
23. Reinhold P, Sachse K, Kaltenboeck B. Chlamydiaceae in cattle: commensals, trigger organisms, or pathogens? Vet J. (2011) 189:257–67. doi: 10.1016/j.tvjl.2010.09.003
24. Zhang NZ, Zhang XX, Zhou DH, Huang SY, Tian WP, Yang YC, et al. Seroprevalence and genotype of Chlamydia in pet parrots in China. Epidemiol Infect. (2015) 143:55–61. doi: 10.1017/S0950268814000363
Keywords: Chlamydia, domestic black-boned sheep and goats, indirect hemagglutination assay, seroprevalence, China
Citation: Sun L-X, Liang Q-L, Hu X-H, Li Z, Yang J-F, Zou F-C and Zhu X-Q (2020) First Report of Chlamydia Seroprevalence and Risk Factors in Domestic Black-Boned Sheep and Goats in China. Front. Vet. Sci. 7:363. doi: 10.3389/fvets.2020.00363
Received: 05 January 2020; Accepted: 26 May 2020;
Published: 17 July 2020.
Edited by:
Anuwat Wiratsudakul, Mahidol University, ThailandReviewed by:
Si-Yang Huang, Yangzhou University, ChinaCopyright © 2020 Sun, Liang, Hu, Li, Yang, Zou and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng-Cai Zou, emZjMTIwN0B2aXAuMTYzLmNvbQ==; Xing-Quan Zhu, eGluZ3F1YW56aHUxQGhvdG1haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.