
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 18 August 2020
Sec. Animal Nutrition and Metabolism
Volume 7 - 2020 | https://doi.org/10.3389/fvets.2020.00338
 Salem R. Alyileili1*
Salem R. Alyileili1* Khaled A. El-Tarabily2,3,4*
Khaled A. El-Tarabily2,3,4* Ibrahim E. H. Belal1
Ibrahim E. H. Belal1 Wissam H. Ibrahim5
Wissam H. Ibrahim5 Mohsin Sulaiman1
Mohsin Sulaiman1 Ahmed S. Hussein1
Ahmed S. Hussein1The long-term use of antimicrobials as growth promoters in poultry feed leads to antimicrobial resistance in pathogens. Thus, alternatives to antibiotics are essential for reasons associated with both safety and cost-effectiveness. Underutilized plant sources need to be developed to replace antibiotics in broiler feed. Several feed resources have been introduced so far, but they have yet to be applied widely. Date pits are a major by-product of the date industry (6–8%) and have the potential antioxidant to replace antibiotics. In this study, fresh date pits were degraded using the mold Trichoderma reesei under solid-state degradation (SSD), resulting in degraded date pits (DDP). A total of 180 Brazilian “Cobb 500” broiler chicks were divided into six feed treatments in triplicate groups. The treatments were corn-soy basal diet (positive control; C+), corn-soy + 20% oxytetracycline at 0.05% (negative control; C–), corn-soy + 10% DDP, corn-soy + 0.2% mannan-oligosaccharides (MOS), corn-soy + 0.1% mannose, and corn-soy + 0.2% mannose. The antioxidant and biochemical effects of DDP, MOS, and mannose were determined in the blood serum, liver, and intestine of broilers at age 21 and 42 days. The results indicated that the contents of antioxidants such as flavonoids and phenolics, as well as the MOS content in DDP, were increased by the degradation process. Additionally, mannose, glucose, arabinose, rhamnose, and glucuronic acid were significantly increased in DDP after degradation. The activity of antioxidant enzymes (GPx—glutathione peroxidase, catalase, and SOD—superoxide dismutase) in the serum, liver, and intestine of broilers fed with diets containing 10% DDP and 0.2% MOS was increased significantly compared to the control group. Malondialdehyde activity was decreased, whereas the mean corpuscular hemoglobin level and the iron content were significantly upregulated in the broilers fed with 10% DDP, 0.1% mannose, and 0.2% MOS diets compared with the control. Thus, DDP can be used to improve the antioxidant status and has a prebiotic-like effect in broiler chicken performance.
Practical applications of agricultural wastes in poultry nutrition play an important role in replacing expensive ingredients and/or additives in their diet particularly after COVID-19 outbreaks in which an expected shortage of feed resources for animal nutrition may have occurred (1–3). Poultry are a valuable source of animal protein of high quality, and nutrition of poultry plays an essential role in controlling the balance of pro-oxidants and antioxidants and thus product quality and shelf life after harvest (4).
The synthesis of reactive oxygen species (ROS) affects the antioxidant system and pro-oxidants, leading to damage, which results in oxidative stress (5). Oxidative stress disrupts redox signaling, and thus measuring the redox ratio is an effective method for studying oxidative stress. Broilers are vulnerable to different types of stressors, such as the stress caused by heat, which induces the generation of ROS. Currently, consideration is being given to new methods of protection against ROS using antioxidant enzymes.
Antioxidant enzymes are present in all organisms, and these enzymes help prevent cell membrane damage, the inactivation of enzymes, and alteration of nucleic acids. The major enzymes that make up the primary defenses are glutathione peroxidase (GPx), catalase (CAT), and superoxide dismutase (SOD). SOD catalyzes the dismutation of superoxide radicals to H2O2 and oxygen, while CAT catalyzes the breakdown of H2O2 to H2O and molecular oxygen. GPx is a selenium-based enzyme, which deactivates peroxides using the peptide glutathione (GSH) as its cosubstrate (6). Catalase and peroxidases are enzymatic ROS scavengers that decrease the concentration of H2O2, which acts as a source of active radical species. ROS are deemed as critical oxygen mediators and crucial messengers that promote cell division (7). The lipid peroxidation end product malondialdehyde (MDA) in tissues acts as a biomarker for radical-prompted deterioration and peroxidation of endogenous lipid.
The catalytic activity of enzymes in erythrocytes and the liver is most commonly monitored for the diagnosis of blood and organ diseases. Erythrocytes are rich in hemoglobin with an efficient system of defense against free radicals, and they contain antioxidant enzymes with a high level of glutathione (8). The liver performs primary detoxification functions and has a central metabolic role in the organism (9). Antioxidant enzymes neutralize the formation and harmful effects of reactive oxygen metabolites (10).
Date palm (Phoenix dactylifera L.) is one of the major fruit crops in most of the Arabian countries. Solid-state degradation (SSD) of date pits with exogenous microbial enzymes like xylanases enhances the production of simpler forms of carbohydrate molecules from fibers present in date pits (11). A considerable investigation is currently concentrated on the cellulolytic filamentous fungus, Trichoderma reesei. When subjected to degradation, enzymes secreted by T. reesei catalyze the degeneration of these substrates to simple sugars and enhance the degradation of plant cell walls. Degradation of date pits improves the chemical constituents and yields bioactive substances of added value for animal nutrition (1–3), and SSD is one of the reasonable methods to prepare degraded date pits (DDP) by using T. reesei.
Microbially degraded feeds and enzymes can be better utilized by animals, which improve the growth performance and protection in stress condition and maintain productive and reproductive performance (12). The advantage of using enzymatically degraded prebiotics like DDP in animal feed promotes the beneficial role of non-digestible sugars for increasing the immune system of animals through the antioxidant system. This property of a natural growth promoter from date pits is a milestone to animal feed research. Thus, the goal of the current study was to investigate the impacts of DDP, mannan-oligosaccharides (MOS), and mannose on the blood serum, liver, and intestine antioxidant and the biochemical responses of broiler chickens.
The scientific committee of the Department of Integrative Agriculture, College of Food and Agriculture, United Arab Emirates University, United Arab Emirates (UAE) approved the present work under experimental protocol 313072. The committee recommended animal welfare and minimum stress during the experimental work.
Fresh pits of dates (P. dactylifera L.), Khalas variety, were acquired from the Al Sad date processing factory in Al-Ain, UAE. Each seed was ~2–2.5 cm long and ~6–8 mm thick. A medium-sized mill (Skiold A/S, Kjeldgaardsvej 3, Saeby 9300, Denmark) was used to finely grind the date pits to about 1 mm in diameter.
T. reesei used in the current study was cultivated as described by Hussein et al. (13). Four lyophilized T. reesei ampoules were purchased from Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (DSMZ), Braunschweig, Germany. A subsample from the rehydrated T. reesei culture was transferred to potato dextrose broth (PDB) (Lab M Limited, Lancashire, UK) amended with 250 μg ml−1 chloramphenicol (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) and 100 μg ml−1 streptomycin sulfate (Sigma-Aldrich). A rotary shaker (Model G76, New Brunswick Scientific, Edison, NJ, USA) was used for the incubation of the flasks at 250 rpm at 25 ± 2°C in the dark for 7 days. The flasks were assessed visually daily to monitor the growth of fungus. The fungus was kept on PDA plates and stored at 4°C.
T. reesei DDP was produced using an SSD inside an incubator as described by Hussein et al. (13). Chemical composition of DDP and non-degraded date pits (NDDP) (n = 3 per treatment) was studied and reported by Alyileili et al. (14). Diets were formulated (Table 1) to be isonitrogenous and isocaloric. The calculated nutrient composition of the diets was based on the feedstuff profiles reported by Hashim et al. (15). All ingredients were ground and mixed in a commercial mixer (Hobart mixer, HL1400, USA) for 20 min.
A total of 180 Brazilian “Cobb 500” broiler chicks were divided into six dietary treatments. The treatments were T1—corn-soy basal diet (Positive control; C+), T2—corn-soy basal diet + 20% oxytetracycline at 0.05% (Negative control; C–), T3—corn-soy basal diet + 10% (DDP), T4—corn-soy basal diet + 0.2% (MOS), T5—corn-soy basal diet + 0.1% mannose, and T6—corn-soy basal diet + 0.2% mannose. DDP was given simultaneously with oxytetracycline to study the antibacterial effect of DDP in broiler intestine. Chickens were housed as 10 chicks per cage (50 × 45 × 45 cm) in an environmentally controlled house.
The experiment lasted for 42 days from 1 to 42 days of age. Birds within each treatment were designated to three replicate groups (n = 10/group). Feed and water were given on an ad libitum basis. The light-dark cycle was 23:1 daily from the 4 days of the experiment. Vaccination and medical care were carried out under the supervision of veterinaries.
Chicken liver and intestinal and blood samples (serum and plasma) were collected in two tubes with or without heparin from two randomly selected broilers at 42 days of age from each treatment replicate (n = 6 samples). Blood samples were centrifuged at 1,716 g for 15 min to separate the plasma and serum, which were kept at 20°C until analysis.
Oligosaccharides were extracted from DDP and NDDP using the method of Huang et al. (16). Extracted sample was purified, and monosaccharide composition was determined by the method of Jahromi et al. (17). In DDP and NDDP (n = 3 per treatment), the ability to scavenge 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radicals was assayed according to a previously described procedure (18). The 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical scavenging was measured using another previously described procedure (19). The ferric reducing activity of the date pits extract was assessed based on the method reported by Benzie and Strain (20).
Malondialdehyde (MDA) was measured in liver and intestinal tissue and in blood serum using the thiobarbituric acid assay method as described by Ohkawa et al. (21). Catalase activity was assayed in liver and intestinal tissue and blood serum as described by Maehly (22). SOD activity was measured in liver and intestinal tissue and blood serum as described by Kakkar et al. (23). The activity of GPx was evaluated in the liver as described by Lawrence and Burk (24) with modifications described by Agergaard and Jensen (25). Alanine aminotransferase (ALT), aspartate aminotransferase (AST), and glutamate-pyruvate transaminase were evaluated in serum using the procedure described by Rietman and Frankel (26). Gamma glutamyl transferase (GGT) activity was measured using a GGT colorimetric assay kit from Sigma-Aldrich. The total calcium, iron, phosphorus, and copper in plasma were quantified by inductively coupled atomic emission spectrometry (ICP-OES), as described by Vanhoe et al. (27). Blood serum urea was measured using the diacetyl monoxime method (28). Blood serum uric acid was estimated using the modified colorimetric technique (29). Total protein, cholesterol, blood plasma glucose, creatinine, triglycerides, and HDL-cholesterol were quantified using commercial kits (Unichem Elite, United Diagnostics Industry, Dammam, KSA) and based on the methods used by Piotrowska et al. (30).
The hemocytometer method using the Natt-Herrick solution was used for the determination of white blood cell (WBC), red blood cell (RBC), and hemoglobin (Hb) count, and hematocrit values were measured using the microhematocrit and cyanate-hemoglobin method (31). The mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) were measured as described by Pampori and Iqbal (32).
Data were subjected to ANOVA (one-way procedure) using a general linear model (GLM), and mean comparisons were done using the Student Newman Keuls test to compare significant differences among means for all analyses (Version 20.0, SPSS Inc., Chicago, IL, USA). The differences among means were considered significant at p ≤ 0.05.
The statistical model was as follows:
Model:
where
Xij = Any observation
u = Overall mean
Ti = Treatments (i = 1, 2…and 4)
eij = Experimental error
The number of samples used in the statistical analyses was six per treatment as two samples per replicate considering the sample as the experimental unit. This was done to improve the precision of analyses of variance. Before analyses of variance, all percentage data were transformed to their analogous arcsine.
The phenolic content in NDDP was 3.2 g GAE/100 g DW, and the flavonoid content was 2.28 g RE/100 g DW. After degradation with T. reesei, the flavonoid and phenolic contents were significantly increased to 11.68 g RE/100 g DW and 14.23 g GAE/100 g DW, respectively. These increases amounted to 11.03 and 9.6 g, respectively (Table 2).
These findings indicated that, after degradation with T. reesei, the DPPH radical scavenging ability was 78% in DDP compared to 59% in NDDP. This represents an increase of 19%. The ABTS radical scavenging activity of NDDP was 6.23 mmol TE/100 g DW, while it was significantly higher at 13.28 mmol TE/100 g DW in DDP. Additionally, the ferric reducing antioxidant power (FRAP) assay showed that the degradation process with T. reesei enhanced the ferric reducing antioxidant power of NDDP from 24.56 mmol TE/100 g DW to 36.23 mmol TE/100 g DW. Metal chelating activity was also increased in DDP to about 17.25 μmol EE/g DW. Table 3 shows the MOS content and its monosaccharide composition in NDDP. Degradation by T. reesei significantly increased the MOS content in date pits. The mannose content of MOS from DDP was 19.97%. After degradation, the contents of glucose, arabinose, rhamnose, and glucuronic acid were significantly increased in MOS.
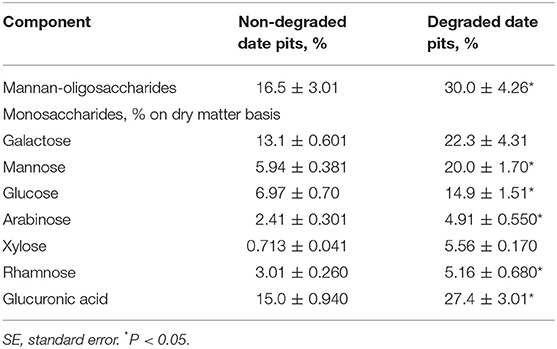
Table 3. Monosaccharides contents in mannan-oligosaccharides of non-degraded and degraded date pits (Means ± SE; n = 3).
The activity of the enzymatic antioxidants SOD, CAT, and GPx in serum was found to be significantly higher in broilers fed with the 10% DDP diet and the 0.2% MOS diet as compared to those fed with the corn-soy diet. The activity of the antioxidant enzymes in broilers fed with the antibiotic and mannose diets increased but was not significantly higher than that in broilers fed with the 10% DDP and 0.2% MOS diets (Table 4). The level of MDA was significantly lower in broilers fed with the 10% DDP diet and 0.2% MOS diet compared with those fed with the positive and negative control diets or the mannose diet.
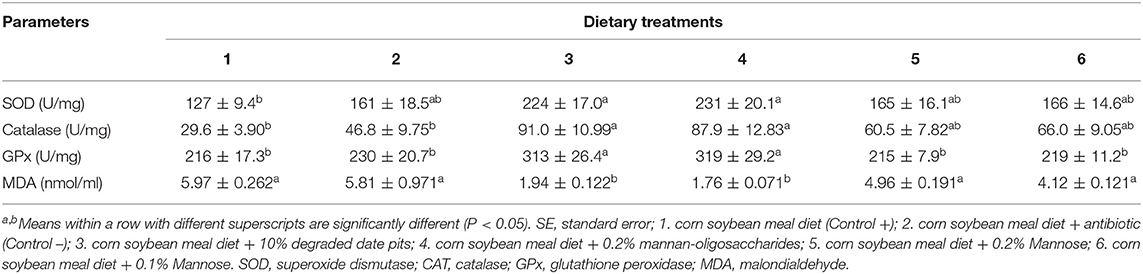
Table 4. Effect of different dietary treatments on activity of SOD, CAT, GPx, and MDA in serum (Means ± SE; n = 6).
The activity of SOD and CAT in the liver was significantly higher in broilers fed with the 10% DDP diet and 0.2% MOS diet compared with those fed with the positive control diet (Table 5). The activity of SOD was similar in mannose and negative control fed-broilers and did not differ significantly from the other groups. The results showed that CAT activity was intermediate in the broilers fed with the positive control diet and did not differ significantly from that in the other groups. The GPx activity was similar in broilers fed with the 0.1 and 0.2% mannose diets and was comparable to that in broilers fed with the 10% DDP and 0.2% MOS diets. The GPx activity in the liver was significantly higher in broilers fed with the 10% DDP diet and 0.2% MOS diet compared with those fed with the positive control diet or 0.2% mannose. The liver glutathione S-transferase (GST) activity was significantly higher in broilers fed with the 10% DDP and 0.2% MOS diets when compared with those fed with the positive control diet. Additionally, the GST activity of broilers fed with the 0.1% mannose and negative control diets was significantly higher than in those fed with the positive control diet, but lower than in those fed with the DDP and MOS diets (Table 5). The liver MDA content was significantly lower in broilers fed with the 10% DDP and 0.2% MOS diets when compared with those fed with the positive control diet. The MDA content in broilers fed with the mannose and positive and negative control diet was similar and significantly higher than that in those fed with other test diets.
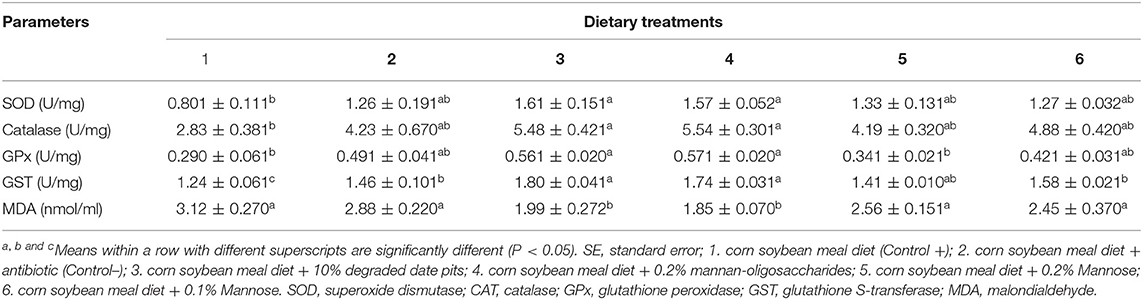
Table 5. Effect of different dietary treatments on activity of SOD, CAT, GPx, GST, and MDA content in liver (Means ± SE; n = 6).
The intestinal SOD, CAT, GPx, and GST activity was significantly higher in broilers fed with the 10% DDP diet and 0.2% MOS diet in both the proximal and distal portions compared with broilers fed with the positive control diet. The SOD activity of broilers fed with the mannose diet in the proximal and distal portions of the intestine was lower when compared with those fed with the 10% DDP and 0.2% MOS diets. In broilers fed with the mannose diets, the activity of CAT and GPx in the proximal and distal portions of the intestine was similar to that in broilers fed with positive and negative control diets. The activity of GST in the proximal portion of the intestine of broilers fed with the mannose diet was low and comparable to that in broilers fed with positive and negative control diets. The MDA content in the proximal and distal portions of the intestine was significantly lower in broilers fed with the 10% DDP and 0.2% MOS diets compared with those fed with the positive control diet. The MDA content in both portions of the intestine in broilers fed with the mannose diet was comparable with that in broilers fed with positive and negative control diets (Table 6).
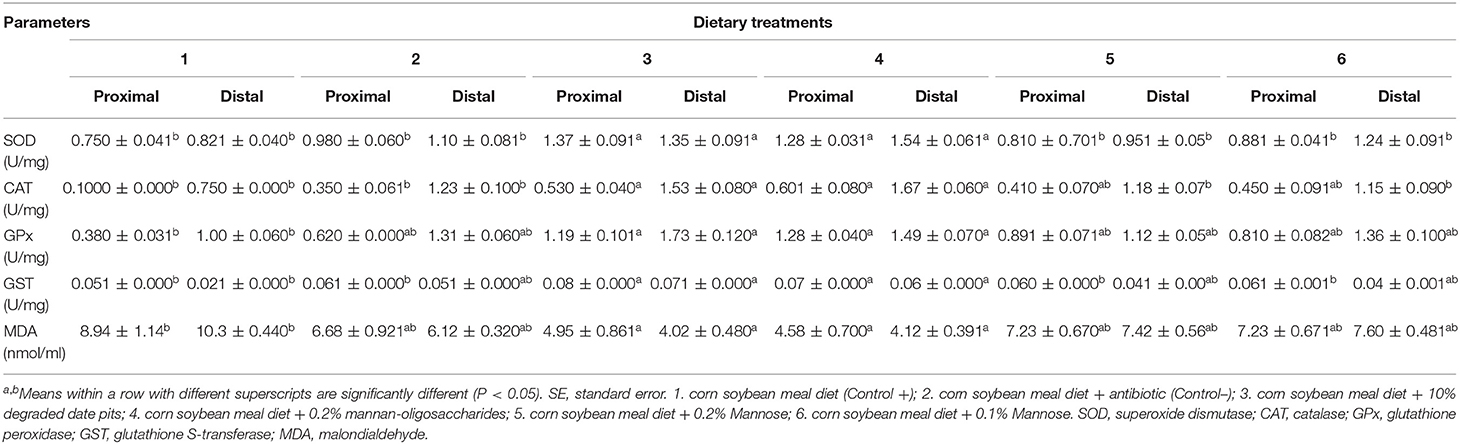
Table 6. Effect of different dietary treatments on activity of SOD, CAT, GPx, GST, and MDA content in intestine (Means ± SE; n = 6).
The supplementation of 10% DDP, 0.2% MOS, and 0.1% or 0.2% mannose in broiler diets had no influence on ALT, AST, GGT, plasma glucose, calcium, creatinine, copper, phosphorus, or uric acid. Triglycerides, total cholesterol, LDL cholesterol, and HDL cholesterol were reduced in broilers fed with the 10% DDP and 0.2% MOS diets, but not significantly compared with the other treatments. The iron content was significantly higher in broilers fed with 10% DDP, 0.2% mannose, and 0.2% MOS when compared with broilers fed with the positive and negative control diets (Table 7).
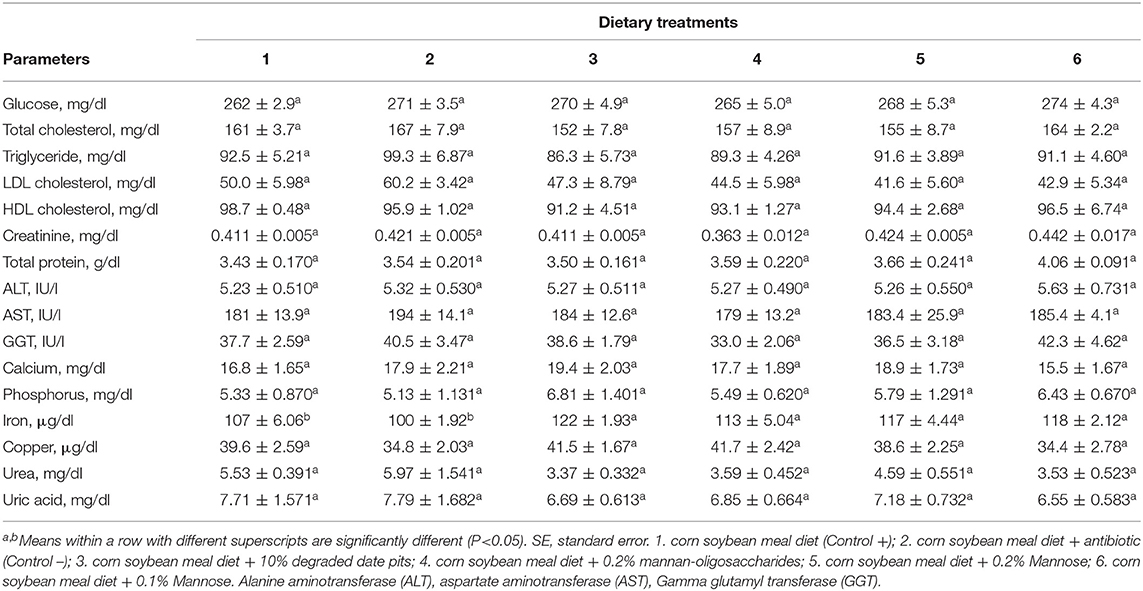
Table 7. Effect of different dietary treatments on blood biochemical parameters of broilers (Means ± SE; n = 6).
The supplementation of 10% DDP, 0.2% MOS, and 0.1% or 0.2% mannose in broiler diets had no significant effect on WBC, RBC, Hb, HCT, MCV, or MCHC. The MCH was significantly upregulated in broilers fed with the 10% DDP, 0.1% mannose, and 0.2% MOS diets. The Hb level, HCT, and MCHC were higher in broilers fed with the 10% DDP and 0.2% MOS diets but did not differ significantly from that in the other groups (Table 8).
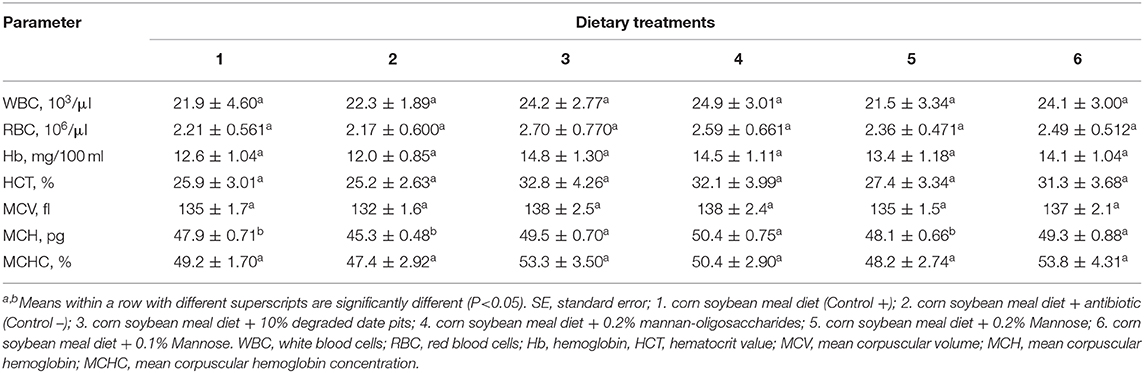
Table 8. Effect of different dietary treatments on blood and serum hematological parameters of broilers (Means ± SE; n = 6).
The antioxidant properties of date pits degraded with T. reesei along with MOS content were considerably increased compared to NDDP. In addition, mannose, glucose, arabinose, rhamnose, and glucuronic acid were significantly increased in the MOS of DDP compared with NDDP. The present results indicate that degradation caused structural modification of date pits. Compared with NDDP, the total phenolic contents in DDP were significantly higher. Phenolic compounds have been used to exhibit a scavenging outcome against free radicals (33).
The nutrient content of food can be enhanced by microbial degradation through the biosynthesis of proteins, vitamins, and essential amino acids, which results in enhanced protein quality and fiber digestibility (34). Microbial degradation removes antinutritional factors and alters the bioavailability of micronutrients (35). During SSD, the catalytic action of β-glucosidase enhances phytochemical constituents such as flavonoids and phenolic compounds through the release of phenolic isoflavone aglycones, and the formation of reductases increases the antioxidant properties of legume seeds (36). The microorganisms involved in SSD cleave the phenolic and flavonoid linkages, which free the compounds to act as antioxidants, thereby improving antioxidant activity (37). The phenolic and flavonoid content of date pits significantly increased following degradation. DDP also showed significant antioxidant activity, as demonstrated by the results of the DPPH, ABTS, and FRAP assays. The increase in bioactive substances in DDP after treatment by T. reesei is of added value to agro-byproducts in view of improving the nutritive value as feedstuffs and extended their utilization as a growth promoter. Furthermore, after COVID-19 outbreaks, shortage in global feed resources is apparent due to strong competition between animals and humans for grains and cereals; thus, improving the utilization of locally available resources is urgent in the present circumstances (1–3).
The current study showed that the activity of CAT, SOD, and GPx in serum was significantly increased in broilers fed with the 10% DDP diet. This probably indicates that DDP enhanced the syntheses of antioxidant enzymes. Broilers are capable of adapting to oxidative stress by encouraging the synthesis of antioxidant enzymes. The antioxidant systems in the body have antioxidant enzymes such as SOD and GPx, which act to protect the body from oxidative stress. The dismutation of superoxide ions to hydrogen peroxide by SOD is usually the primary defense. SOD is widespread in oxygen metabolizing cells and protects aerobic cells against the harmful effects of superoxide radicals and other ROS (38).
The antioxidant enzymes SOD and GPx are essential elements of the first level of antioxidant defense in the cell because they form the main protective system against oxidative damage (39). The serum levels of GSH significantly increased (P < 0.05) in the DDP treatment, especially by the 21st day of the experiment (40). The methanolic extract of P. dactylifera pits is known as an antioxidant exporter of β-carotene and phenolic compounds (41). This antioxidant action was linked with the phenolic contents (42).
The liver controls numerous essential mechanisms in the body, being the major organ for detoxification of different substances. The pathogenesis of liver defects covers different cell types in the liver through cell death and regeneration mechanisms. Ferket et al. (43) studied the advantages of the addition of MOS to poultry feed and reported that MOS enhances liver morphology and functioning. Sarangi et al. (44) studied the effect of dietary supplementation of prebiotics, probiotics, and symbiotics on liver histomorphology in broilers and reported the beneficial effects of MOS. The inclusion of prebiotics and peppermint extract in broiler diet enhanced the performance, enzymatic activity, and histological aspects of the liver during the experimental period (45).
Dietary supplementation of 10% DDP and 0.2% MOS enhanced the activity of antioxidant enzymes in the liver. The scavenger functions of these hepatic antioxidative enzymes protect the liver from oxidative deterioration by inducing the release of SOD and GPx from prebiotics such as 10% DDP and 0.2% MOS. The MDA content was significantly (P < 0.05) reduced in broilers fed with the 10% DDP and 0.2% MOS diets. Binding free radicals to chelating transition metal ions and hydrogen atoms that act as a catalyst for free radical generation could be the major mechanism in the antiperoxidative capability of such prebiotics (46). Dvorska and Surai (47) reported that dietary supplementation of MOS decreased the MDA level in quail liver. It was also reported that carbohydrates and carbohydrate-containing molecules can be used as antioxidants that scavenge ROS (48). Several trials reported that MOS has a protective impact on heat-stressed birds (49) and enhanced the antioxidant function of egg yolk and antioxidant enzymes in the liver (50) when fed to laying hens. Oskoueian et al. (51) reported that an ethanolic extract of palm kernel cake (PKC) has high levels of fatty acids and many bioactive compounds with significant antioxidant activity.
Dietary treatment with 10% DDP and 0.2% MOS upregulated the antioxidant enzyme levels in the intestine of broilers. It was found (52) that MOS from Saccharomyces cerevisiae has antioxidative action in vitro. This indicates that MOS can prevent the gut not only by removing undesirable bacteria but also through improving antioxidant status. Prebiotics like β-glucans have antioxidant action and enhance antioxidant enzyme levels in the intestine of broilers (53). The activity of antioxidant enzymes depends on dietary antioxidants. Oxidative damage increases when the antioxidant/oxidant balance changes in a negative manner due to increasing oxidative stress (54).
The increase in GPx activity in broilers fed with the 10% DDP and 0.2% MOS diets suggests greater protection from oxidative stress. The present results further substantiate the positive effect of 10% DDP and 0.2% MOS on GPx activity in chicken serum, intestine, and liver. GPx is active fundamentally in the cytoplasm of the cells and only about 10% of its activity takes place in the mitochondria (55). The endogenous MDA level reflects lipid peroxidation. The MDA level decreased in broilers fed with the 10% DDP and 0.2% MOS diets, and this was a direct reflection of decreased peroxidation and an enhanced protective effect.
Dietary inclusion of 10% DDP and 0.2% MOS in broiler diets did not affect blood biochemical parameters. The measurement of AST, ALT, and GGT activities helps determine any functional liver damage in broilers before clinical symptoms develop (56). Any abnormality in the increase in serum levels of AST and ALT can reveal hepatocellular damage; thus, normal levels of AST were observed in groups on 10% DDP and 0.2% MOS. Al-Bowait and Al-Sultan (57) showed that date pits had no significant impact on the blood glucose level in broilers. Consistent with our observations, Masoudi et al. (58) demonstrated that replacement of corn by 30% date pits in broiler feed had no significant impact on blood cholesterol, triacylglycerol, HDL, and LDL. The inclusion of date waste at 150 g/kg in isocaloric, isonitrogenous broiler diets from 1 to 42 days of age did not affect growth performance or blood cholesterol (59). Toghyani et al. (60) reported that broilers supplemented with β-glucan prebiotics or corn-soy diet have normal levels of serum biochemical parameters. The MOS from yeast autolysate in the diet lowered serum total cholesterol and triglycerides but had no effect on total protein and uric acid (61). The present study also supports the above findings. Moreover, yeast culture supplementation with MOS had no influence on serum parameters, but increased serum uric acid (62).
The present study showed no considerable changes in the blood hematological parameters in all treatments despite a significant increase in MCH in broilers fed with the 10% DDP, 0.1% mannose, and 0.2% MOS diets. This is consistent with Cetin et al. (63), who found that the addition of prebiotic MOS to the feed significantly (P < 0.05) increased the erythrocyte count, hemoglobin concentration, and hematocrit rates in turkeys. The elevation in MCH concentration observed herein could be attributed to the increase in the RBC count.
In conclusion, T. ressi degradation of date pits increased the antioxidant properties of DDP by increasing the availability of their phenolic compounds. That was reflected in the liver and intestinal enzymes of broilers. Hence, DDP could be fed at 10% to broilers to enhance antioxidant balance, improve product quality, and extend the use of DDP as a feedstuff and growth promoter in animal nutrition. This is of vital importance under COVID-19 circumstances due to the global shortage in feed supply and the unseen future for the availability of feed resources for animal nutrition and the expected damages in agriculture sector.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The scientific committee of the Department of Integrative Agriculture, College of Food and Agriculture, United Arab Emirates University approved the present experiment.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to express their gratitude to the Department of Integrative Agriculture, College of Food and Agriculture, United Arab Emirates University for their financial (Grant number 313072), technical, and valuable help throughout the study.
1. Azizi M, Seidavi AR, Rangni M, Laudadio V, Tufarelli V. Practical applications of agricultural wastes in poultry feeding in Mediterranean and Middle East regions. part 1: citrus, grape, pomegranate and apple wastes. World Poultry Sci J. (2018) 74:489–98. doi: 10.1017/S0043933918000478
2. Seidavi AR, Azizi M, Ragni M, Laudadio V, Tufarelli V. Practical applications of agricultural wastes in poultry feeding in Mediterranean and Middle East regions. part 2: tomato, olive, date, sunflower wastes. World Poultry Sci J. (2018) 74:443–52. doi: 10.1017/S004393391800051X
3. Kadhim MJ, Al-Shammari KI, Ulsagheer MK, Rmul MR. Effect of addition date molasses or/and ascorbic acid with/without feeding method in some productive performance of broiler chickens Ross 308. J Phys Conf Ser. (2019) 1294:92012. doi: 10.1088/1742-6596/1294/9/092012
4. Cowey CB. The role of nutritional factors in the prevention of peroxidative damage to tissues. Fish Physiol Biochem. (1986) 2:171–8. doi: 10.1007/BF02264085
5. Sies H. Role of reactive oxygen species in biological processes. Klinische Wochenschrift. (1991) 69:965–8. doi: 10.1007/BF01645140
6. Halliwell B. Oxidative stress and neurodegeneration, where are we now? J Neurochem. (2006) 97:1634–58. doi: 10.1111/j.1471-4159.2006.03907.x
7. Buetler TM, Krauskopf A, Ruegg UT. Role of superoxide as a signaling molecule. Physiology. (2004) 19:120–3. doi: 10.1152/nips.01514.2003
8. Kostadinovic S, Jovanov D, Mirhosseini H. Comparative investigation of cold pressed essential oils from peel of different mandarin varieties. IIOAB J. (2011) 3:7–14.
9. Samson G, Claude Morisette J, Popovic R. Copper quenching of the variable fluorescence in Dunaliella tertiolecta. New evidence for a copper inhibition effect on PSII photochemistry. Photochem Photobiol. (1998) 48:329–32. doi: 10.1111/j.1751-1097.1988.tb02829.x
10. Cotgreave IA, Moldeus P, Orrenius S. Host biochemical defense mechanisms against prooxidants. Ann Rev Pharmacol Toxicol. (1988) 28:189–212. doi: 10.1146/annurev.pa.28.040188.001201
11. Foreman PK, Brown D, Dankmeyer L, Dean R, Diener S, Dunn-Coleman NS et al. Transcriptional regulation of biomass-degrading enzymes in the filamentous fungus Trichoderma reesei. J Biol Chem. (2003) 278:31988–97. doi: 10.1074/jbc.M304750200
12. Rozan P, Villaum C, Bau HM, Schwertz A, Nicolas JP, Mejean L. Detoxication of rapeseed meal by Rhizopus oligosporus sp-T3: a first step towards rapeseed protein concentrate. Int J Food Sci Tech. (1996) 31:85–90. doi: 10.1111/j.1365-2621.1996.17-315.x
13. Hussein AS, Belal IEH, Alyalyali SRA, El-Tarabily KA. Date Pit Composition for the Treatment of Animals. US. Patent No. 9,682,116. Washington, DC: Patent and Trademark Office (2015).
14. Alyileili SR, Hussein AS, Ibrahim WH, El-Tarabily KA. Phytochemical composition and antioxidant activity of Trichoderma reesei degraded date (Phoenix dactylifera L.) pits. Curr Bioact Compd. (2020) 16:528–36. doi: 10.2174/1573407215666190207093046
15. Hashim IB, Hussein AS, Afifi HS. Quality of breast and thigh meats when broilers are fed rations containing graded levels of sugar syrup. Poult Sci. (2013) 92:2195–200. doi: 10.3382/ps.2012-02940
16. Huang G, Yang Q, Wang ZB. Extraction and deproteinization of mannan oligosaccharides. Z Naturforsch C. (2010) 65:387–90. doi: 10.1515/znc-2010-5-611
17. Jahromi MF, Liang JB, Abdullah N, Goh YM, Ebrahimi R, Shokryazdan P. Extraction and characterization of oligosaccharides from palm kernel cake as prebiotic. Bioresources. (2016) 11:674–95. doi: 10.15376/biores.11.1.674-695
18. Karagozler AA, Erdag B, Emek YC, Uygun DA. Antioxidant activity and proline content of leaf extracts from Dorystoechas hastata. Food Chem. (2008) 111:400–7. doi: 10.1016/j.foodchem.2008.03.089
19. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. (1999) 26:1231–7. doi: 10.1016/S0891-5849(98)00315-3
20. Benzie IF, Strain JJ. Ferric reducing/antioxidant power assay, direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. (1999) 299:15–27. doi: 10.1016/S0076-6879(99)99005-5
21. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. (1979) 95:351–8. doi: 10.1016/0003-2697(79)90738-3
22. Maehly A. The assay of catalases and peroxidases. In: Glick D, editor. Methods of Biochemical Analysis. New York, NY: Interscience Publishers, Inc. (1954). p. 357–424.
23. Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. (1984) 21:130–2.
24. Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. (1976) 71:952–8. doi: 10.1016/0006-291X(76)90747-6
25. Agergaard N, Jensen PT. Procedure for blood glutathione peroxidase determination in cattle and swine (selenium status, methodology, storage). Acta Vet Scand. (1982) 23:515–27.
26. Rietman S, Frankel SA. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminase. Am J Clin Pathol. (1957) 28:56–63. doi: 10.1093/ajcp/28.1.56
27. Vanhoe H, Vandecasteele C, Versieck J, Dams R. Determination of iron, cobalt, copper, zinc, rubidium, molybdenum, and cesium in human serum by inductively coupled plasma mass spectrometry. Anal Chem. (1989) 61:1851–7. doi: 10.1021/ac00192a014
28. Friedman HS. Modification of determination of urea by diacetyl monoxime method. Anal Chem. (1953) 25:662–4. doi: 10.1021/ac60076a040
29. Buchanan MJ, Isdale IC, Rose BS. Serum uric acid estimation, chemical and enzymatic methods compared. Ann Rheum Dis. (1965) 24:285. doi: 10.1136/ard.24.3.285-8
30. Piotrowska A, Burlikowska K, Szymeczko R. Changes in blood chemistry in broiler chickens during the fattening period. Folia Biol. (2011) 59:183–7. doi: 10.3409/fb59_3-4.183-7
31. Kececi T, Oguz H, Kurtoglu V, Demet O. Effects of polyvinylpolypyrrolidone, synthetic zeolite and bentonite on serum biochemical and hematological characters of broiler chickens during aflatoxicosis. Br Poult Sci. (1998) 39:452–8. doi: 10.1080/00071669889051
32. Pampori ZA, Iqbal S. Haematology, serum chemistry and electrocardiographic evaluation in native chicken of Kashmir. Int J Poult Sci. (2007) 6:578–82. doi: 10.3923/ijps.2007.578.582
33. Shahidi F, Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices, antioxidant activity and health effects–A review. J Funct Foods. (2015) 18:820–97. doi: 10.1016/j.jff.2015.06.018
34. Parr AJ, Bolwell GP. Phenols in the plant and in man. The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. J Sci Food Agric. (2000) 80:985–1012. doi: 10.1002/(SICI)1097-0010(20000515)80:7<985::AID-JSFA572>3.0.CO;2-7
35. Oboh G, Puntel RL, Rocha JBT. Hot pepper Capsicum annuum, tepin and Capsicum chinese, habanero, prevents Fe2+-induced lipid peroxidation in brain–in vitro. Food Chem. (2007) 102:178–85. doi: 10.1016/j.foodchem.2006.05.048
36. Oboh G, Ademiluyi AO, Faloye YM. Effect of combination on the antioxidant and inhibitory properties of tropical pepper varieties against α-amylase and α-glucosidase activities in vitro. J Med Food. (2011) 14:1152–8. doi: 10.1089/jmf.2010.0194
37. Moktan B, Saha J, Sarkar PK. Antioxidant activities of soybean as affected by Bacillus-fermentation to kinema. Food Res Int. (2008) 41:586–93. doi: 10.1016/j.foodres.2008.04.003
39. Surai PF, Speake BK, Sparks NHC. Carotenoids in avian nutrition and embryonic development. 1. Absorption, availability and levels in plasma and egg yolk. J Poult Sci. (2001) 38:1–27. doi: 10.2141/jpsa.38.1
40. El-Far AH, Shaheen HM, Abdel-Daim MM, Al Jaouni SK, Mousa SA. Date palm Phoenix dactylifera, protection and remedy food. J Nutraceuticals Food Sci. (2016) 1:9.
41. Shalaby EA, Shanab SM. Antioxidant compounds, assays of determination and mode of action. Afr J Pharm Pharmacol. (2013) 7:528–39. doi: 10.5897/AJPP2013.3474
42. Ardekani MRS, Khanavi M, Hajimahmoodi M, Jahangiri M, Hadjiakhoondi A. Comparison of antioxidant activity and total phenol contents of some date seed varieties from Iran. Iran J Pharm Res. (2010) 9:141–6.
43. Ferket PR, Parks CW, Grimes JL. Benefits of dietary antibiotic and mannan-oligosaccharide supplementation for poultry. In: Multi-State Poultry Meeting. Raleigh, NC: Department of Poultry Science North Carolina State University (2002).
44. Sarangi NR, Babu LK, Kumar A, Pradhan CR, Pati PK, Mishra JP. Effect of dietary supplementation of prebiotic, probiotic, and synbiotic on growth performance and carcass characteristics of broiler chickens. Vet World. (2016) 9:313–9. doi: 10.14202/vetworld.2016.313-319
45. Ahmed AMH, El-Sanhoury MHS, Mostafa MM. Effect of peppermint extracts inclusion in broiler chick diet on chick performance, plasma constituents, carcass traits and some microbial populations, enzymatic activity and histological aspects of small intestine. Asian J Anim Vet Adv. (2016) 11:441–51. doi: 10.3923/ajava.2016.441.451
46. Rageb SM, Abd-Allah EA, Abou Khalil NS, Abdel-Maksoud FM, Mahmoud UT. Effects of mannan-oligosaccharide and β-glucan prebiotic on the brain oxidant/antioxidant balance in broilers under natural Egyptian summer conditions. Egypt Acad J Biol Sci. (2018) 10:35–46. doi: 10.21608/eajbsz.2018.13428
47. Dvorska JE, Surai PF. Effects of T-2 toxin, zeolite and Mycosorb on antioxidant systems of growing quail. Asian Austral J Anim Sci. (2001) 14:1752–7. doi: 10.5713/ajas.2001.1752
48. Stoyanova S, Geuns J, Hideg E, Van Den Ende W. The food additives inulin and stevioside counteract oxidative stress. Int J Food Sci Nutr. (2011) 62:207–14. doi: 10.3109/09637486.2010.523416
49. Sohail MU, Hume ME, Byrd JA, Nisbet DJ, Ijaz A, Sohail A, et al. Effect of supplementation of prebiotic mannan-oligosaccharides and probiotic mixture on growth performance of broilers subjected to chronic heat stress. Poult Sci. (2012) 91:2235–40. doi: 10.3382/ps.2012-02182
50. Bozkurt M, Küçükyilmaz K, Catli AU, Çinar M, Bintaş E, Çöven F. Performance, egg quality, and immune response of laying hens fed diets supplemented with mannan-oligosaccharide or an essential oil mixture under moderate and hot environmental conditions. Poult Sci. (2012) 91:1379–86. doi: 10.3382/ps.2011-02023
51. Oskoueian E, Abdullah N, Idrus Z, Ebrahimi M, Goh YM, Shakeri M, et al. Palm kernel cake extract exerts hepatoprotective activity in heat-induced oxidative stress in chicken hepatocytes. BMC Complement Altern Med. (2014) 14:368. doi: 10.1186/1472-6882-14-368
52. Krizkova L, Durackova Z, Sandula J, Sasinkova V, Krajcovic J. Antioxidative and antimutagenic activity of yeast cell wall mannans in vitro. Mutat Res. (2001) 497:213–22. doi: 10.1016/S1383-5718(01)00257-1
53. Kogani G, Pajtinka M, Babincova M, Miadokova E, Rauko P, Slamenova D, et al. Yeast cell wall polysaccharides as antioxidants and antimutagens: Can they fight cancer? Neoplasma. (2008) 55:387–93.
54. Ibrahim W, Lee US, Yen HC, Clair DKS, Chow CK. Antioxidant and oxidative status in tissues of manganese superoxide dismutase transgenic mice. Free Radic Biol Med. (2000) 28:397–402. doi: 10.1016/S0891-5849(99)00253-1
55. Halliwell B. Establishing the significance and optimal intake of dietary antioxidants, the biomarker concept. Nutr Rev. (1999) 57:104–13. doi: 10.1111/j.1753-4887.1999.tb06933.x
56. Kraljevic P, Simpraga M, Vilic M. Aminotransferase activity in chicken blood plasma after application of a lethal activity of 32P. Acta Vet. (2008) 58:203–10. doi: 10.2298/AVB0803203K
57. Al-Bowait M, Al-Sultan SI. Aspects of the serum biochemistry, carcass quality and organoleptic characteristics of broilers fed alkali-treated date pits. Int J Poult Sci. (2006) 5:284–8. doi: 10.3923/ijps.2006.284.288
58. Masoudi A, Chaji M, Bojarpour M, Mirzadeh KH. Effects of different levels of date pits on performance, carcass characteristics and blood parameters of broiler chickens. J Appl Anim Res. (2011) 39:399–405. doi: 10.1080/09712119.2011.621790
59. Attia YA, Al-Harthi MA. Effect of supplementation of date waste to broiler diets on performance, nutrient digestibility, carcass characteristics and physiological parameters. Europ Poult Sci. (2015) 79:1–10.
60. Toghyani M, Toghyani M, Gheisari A, Ghalamkari G, Mohammadrezaei M. Growth performance, serum biochemistry and blood hematology of broiler chicks fed different levels of black seed (Nigella sativa), and peppermint (Mentha piperita). Livest Sci. (2010) 129:173–8. doi: 10.1016/j.livsci.2010.01.021
61. Yalçin S, Yalçin S, Cakin K, Eltan O, Dagaşan L. Effects of dietary yeast autolysate Saccharomyces cerevisiae, on performance, egg traits, egg cholesterol content, egg yolk fatty acid composition and humoral immune response of laying hens. J Sci Food Agric. (2010) 90:1695–701. doi: 10.1002/jsfa.4004
62. Yalçin S, Cabuk M, Bruggeman V, Babacanoglu E, Buyse J, Decuypere E, Siegel PB. Acclimation to heat during incubation, 3. Body weight, cloacal temperatures, and blood acid-base balance in broilers exposed to daily high temperatures. Poult Sci. (2008) 87:2671–7. doi: 10.3382/ps.2008-00164
Keywords: antioxidant indices, blood biochemistry, broiler chickens, degraded date pits, mannan-oligosaccharides, mannose, poultry nutrition
Citation: Alyileili SR, El-Tarabily KA, Belal IEH, Ibrahim WH, Sulaiman M and Hussein AS (2020) Effect of Trichoderma reesei Degraded Date Pits on Antioxidant Enzyme Activities and Biochemical Responses of Broiler Chickens. Front. Vet. Sci. 7:338. doi: 10.3389/fvets.2020.00338
Received: 22 April 2020; Accepted: 15 May 2020;
Published: 18 August 2020.
Edited by:
Youssef A. Attia, King Abdulaziz University, Saudi ArabiaReviewed by:
Alireza Seidavi, Islamic Azad University, Rasht Branch, IranCopyright © 2020 Alyileili, El-Tarabily, Belal, Ibrahim, Sulaiman and Hussein. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Salem R. Alyileili, salem_alyileili@uaeu.ac.ae; Khaled A. El-Tarabily, ktarabily@uaeu.ac.ae
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.