- 1Bacterial Disease Division, Animal and Plant Quarantine Agency, Gimcheon-si, South Korea
- 2Foot and Mouth Disease Division, Animal and Plant Quarantine Agency, Gimcheon-si, South Korea
- 3Gangwondo Livestock & Veterinary Service, Chuncheon-si, South Korea
- 4Chungcheongbukdo Livestock & Veterinary Service, Chungju-si, South Korea
- 5College of Veterinary Medicine, Kyungpook National University, Daegu, South Korea
Bovine tuberculosis is a chronic disease impacting both public health and the livestock industry. The interferon (IFN)-γ assay has been introduced as an ancillary test for diagnosing bovine tuberculosis to overcome limitations of the skin test. The objective of this study was to assess the IFN-γ assay in terms of diagnostics and as a nationwide surveillance program in South Korea. From 2012 to 2013, cattle (n = 120) with bovine tuberculosis and cattle (n = 426) from bovine tuberculosis free herds were subjected to the IFN-γ assay to evaluate the sensitivity and specificity of the assay, respectively, depending on various cut-offs (0–3.5). When optical density of the cut-off was 0.1, the sensitivity and specificity were found to be 81.7% (74.7–88.6) and 99.5% (98.9–100.0), respectively. After introducing the IFN-γ assay as part of the national control program, the IFN-γ assay and single caudal fold skin test data were collected from 47 regional veterinary services to compare the results of these two tests. Overall, the agreement between the IFN-γ assay and the single caudal fold skin test (n = 492,068) was 98.2%, and Cohen's kappa value for the two methods was 0.47. Serial and parallel use of the IFN-γ assay and skin test for the bovine tuberculosis control program were compared using samples (n = 91) from cattle confirmed as bovine tuberculosis positive in laboratories from 2014 to 2016. Parallel screening for bTB showed much higher sensitivity (86/91, 94.5%) than the following screening approaches: serial (47.2%, 43/91), single screening using CFT (63.7%, 58/91), or the IFN-γ assay (78.0%, 71/91). These results indicate that the IFN-γ assay and single caudal fold skin test are complementary to each other; therefore, parallel use of these two tests is considered a useful approach to reduce the prevalence of bovine tuberculosis in South Korea.
Introduction
Bovine tuberculosis (bTB), caused primarily by Mycobacterium bovis (M. bovis), is a chronic disease impacting both public health and the livestock industry. Worldwide, eradication programs for bTB are based on detection of infected animals and their removal from the herd (test and slaughter) (1–3). In South Korea, although this strategy has been used for over half a century, bTB continues to be endemic. Diagnostic tests for bTB are mainly based on the cell-mediated immune (CMI) response, and the tuberculin skin test is a widely used standard screening method worldwide (2). However, the tuberculin skin test has some limitations, such as subjectivity in interpretation of results, requirement of a skilled technician, and false-positive reactions caused by sensitization of other mycobacteria or by local inflammation (4). The interferon gamma (IFN-γ) assay, used as an ancillary test for bTB diagnosis, has improved the sensitivity of bTB testing (5). This assay is advantageous over the tuberculin skin test because it enables early detection of M. bovis infections in animals without revisits, and this prevents variation in observation when assessing skin reactions (6). The IFN-γ assay has been approved for use in a number of national programs for bTB control in many other countries including European Union, USA, New Zealand, and Australia (7, 8).
The number of dairy and beef cattle in the South Korea have been maintained around 400,000 and 2,700,700 for several years (9). A different surveillance program for bTB has been used for beef vs. dairy cattle in South Korea (10). All dairy cattle over 12 months old should be tested annually by means of the caudal fold test (CFT) using purified protein derivative of M. bovis (PPD-B) (CAVAC, South Korea). In contrast, there was no regular testing for beef cattle except for slaughter house surveillance. A pilot monitoring project using the IFN-γ assay for beef cattle was undertaken from 2014 to November 2016. Approximately 300,000 beef cattle a year, raised at farms with a history of bTB outbreaks, were tested using a commercial IFN-γ kit, TB feron ELISA (Bionote, South Korea). Since November 2016, pre-movement test prior to farm to farm trade has been adopted as a mandatory policy for beef cattle; however, this is not the case for dairy cattle due to their regular testing. Since then, the IFN-γ assay has been adopted as the primary test along with the skin test to diagnose bTB. Both types of cattle farms with ongoing outbreaks should be tested every 2 months by parallel testing (CFT and IFN-γ assay) concurrently until all cattle are identified as not reactive in two successive tests. During this period, cattle movement from outbreak herds is strictly restricted except for movement to slaughter houses. The two primary tests for bTB diagnosis should only be carried out by state veterinarians in South Korea. The objective of this study was to assess the efficacy of the IFN-γ assay in terms of diagnostics and as a large-scale surveillance program in South Korea.
Materials and Methods
Use of the IFN-γ Assay
Samples
Heparinized blood and tissue samples, including lung, and cervicothoracic lymph nodes, were collected from 2012 to 2013. All cattle were examined for visible lesions by veterinarians, and tissue samples sent to the laboratory for bacteriological and pathological examination to identify infection status. Samples for determining the specificity (Sp) of the assay were collected at a slaughter house located in an OIE certified bTB free region in South Korea. In total, 426 cattle were identified as being bTB negative by laboratory tests, and results from the IFN-γ assay of these cattle were used to determine the cut-off as a true negative. Samples for determining sensitivity (Se) of the assay were collected from cattle diagnosed as positive by the CFT from herds in regions with bTB outbreaks. In total, 120 cattle identified as M. bovis infected from tissues and/or identified by polymerase chain reaction (PCR) after detection of typical bTB lesions on gross/microscopic examination were considered as truly bTB-positive (11, 12).
IFN-γ Assay
Whole blood samples were collected in sodium-heparinized tubes from the jugular vein of cattle and transferred to the laboratory at room temperature (18–25°C) on the day of collection. All blood samples were stimulated within 10 h after collection. In the laboratory, blood samples were divided into three aliquots of 1.5 ml each in 24 well plates. Commercial PPD-B, PPD-A (purified protein derivative of M. avium) (CZ veterinaria, Spain), and PBS (phosphate buffered saline) as a nil antigen control were used for stimulation of immune response in whole blood samples. One hundred microliters of PPD-B (0.3 μg/ml), PPD-A (0.3 μg/ml), or PBS were added to and mixed in each well and incubated at 37°C in a CO2 incubator for 24 h. After incubation, 24 well plates were centrifuged for 20 min at 500 × g, and plasma from the upper layer was harvested. Plasma samples were tested in duplicate with a sandwich enzyme immunoassay using the TB feron ELISA (Bionote, South Korea) per the manufacturer's instructions. Optical density (O.D) of each well was measured at 450 nm. The mean O.D of each sample was calculated and used to define the cut-off values. The O.D of a sample stimulated with PPD-B minus the O.D of a sample stimulated with PPD-A (O.DPPD−B-O.DPPD−A), and O.D of a sample stimulated with PPD-B minus the O.D of nil antigen treated sample (O.DPPD−B-O.DPBS) values were used as cut-off criteria.
Statistical Analysis
The Receiver Operating Characteristic (ROC) curve, indicating the Se and Sp of the assay depending on each cut-off value, was determined using STATA 15 (StataCorp, USA). Se and Sp were plotted for various cut-off values (0–3.5) to construct the ROC curve. Area under the curve (AUC) for the ROC curve was calculated to define the diagnostic utility of the IFN-γ assay examined in this study. AUC analysis indicated that 0.5 < AUC ≤ 0.7 represents low accuracy, 0.7 < AUC ≤ 0.9 moderate accuracy, and 0.9 < AUC ≤ 1.0 represents high accuracy (13).
Bacteriological Culture and Identification Using Polymerase Chain Reaction
Five to ten grams of all tissue samples collected at postmortem examination were transferred into sterile stainless-steel containers, cut into small pieces using sterile scissors, and then liquefied in 10 ml of PBS using a homogenizer (Tokken, Chiba, Japan). Homogenized tissues were transferred to centrifuge tubes and an equal volume of 10% oxalic acid was added. Samples were then incubated at room temperature for 10 min to decontaminate tissue samples. Tissue solutions were filtered using 70 & 40 μm cell strainers step by step (Thermo Fisher Scientific, MA, USA) and filtered samples were centrifuged at 3,000 × g for 10 min. The supernatant was decanted and the pellet was re-suspended in 2 ml PBS and vortexed strongly. Finally, tissue suspensions were inoculated into Mycobacteria growth indicator tubes (MGIT) (Becton, Dickinson (BD) and company, NJ, USA), 7H11 slant agar (BD), and Lowenstein Jensen slant agar (BD), and incubated at 37°C for 4 weeks.
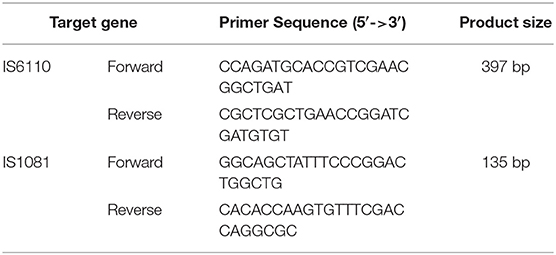
DNA was extracted from suspected bTB colonies for identification by polymerase chain reaction (PCR) using the DNeasy Blood and Tissue kit (Qiagen, Germany) according to the manufacturer's instructions. Primers were designed targeting the insertion elements (IS), IS1081 and IS6110, which specifically exist within the genome of Mycobacterium bovis (7, 14, 15). Multiplex PCR was performed for IS1081 and IS6110 in a reaction mixture (20 μl) (Table 1) containing 10 pmol of each primer, 14 μl AccuPower hotstart PCR premix (Bioneer, South Korea), and 2 μl DNA template. PCR conditions were as follows: 94°C for 5 min, followed by 35 cycles of 94°C for 30 s, 65°C for 30 s min, 72°C for 1 min, with a final extension at 72°C for 5 min. PCR products were analyzed using agarose gel electrophoresis with a 1.5% agarose gel. When one or two PCR bands corresponding to the two sets of primers were observed, they were considered to be M. bovis DNA (see Figure S1).
Introduction and Application of the IFN-γ Assay as Part of the bTB Control Program in South Korea
Agreement Between the IFN-γ Assay and CFT
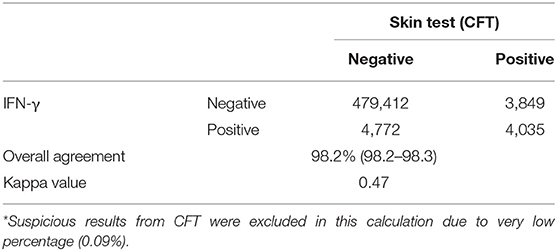
From 2014 to 2017, a total of 1,678,291 and 1,716,179 cattle were tested by 47 regional veterinary services in South Korea using the IFN-γ assay and CFT, respectively. Of these, 8,157 and 8,949 cattle were diagnosed as positive by the two tests, respectively. Of the total tested cattle, results from 492,068 cattle that were tested concurrently using these two methods were analyzed for agreement using Cohen's Kappa value (16).
Evaluation of Parallel Use of the IFN-γ Assay and the CFT in Herds With Ongoing bTB Outbreaks
From 2014 to 2016, 116 cattle from herds with bTB tested concurrently using both the CFT and IFN-γ assay were used to verify that parallel use of the two tests can effectively eliminate reactors from herds. Results from these two screening tests, gross/microscopic examination, acid fast staining, bacteriological culture, and direct PCR from tissue samples were compared. The cut-off for the IFN-γ assay was >O.D 0.1 (450 nm) for O.DPPD−B-O.DPPD−A and O.DPPD−B-O.DPBS. Gross/microscopic examinations were conducted by a veterinary pathologist to detect typical lesions such as tubercle, granuloma with central caseation necrosis, and acid-fast staining. Additionally, culture & tissue direct PCR was performed according to the method mentioned in section Bacteriological culture and identification using polymerase chain reaction. Residual homogenized tissues, after bacterial culture, were pretreated as follows for direct PCR: incubation at 37°C for 30 min after the addition of 180 μl of lysozyme (20 μl/ml, Sigma-Aldrich, USA), followed by incubation at 56°C for 60 min after the addition of 25 μl of proteinase K (Sigma-Aldrich, USA). DNA was extracted from the lysed tissue samples using the NucleoSpin® Soil kit (Macherey Nagel, Germany) according to the manufacturer's instructions.
Results
Optimal Test Cut-Off for the IFN-γ Assay
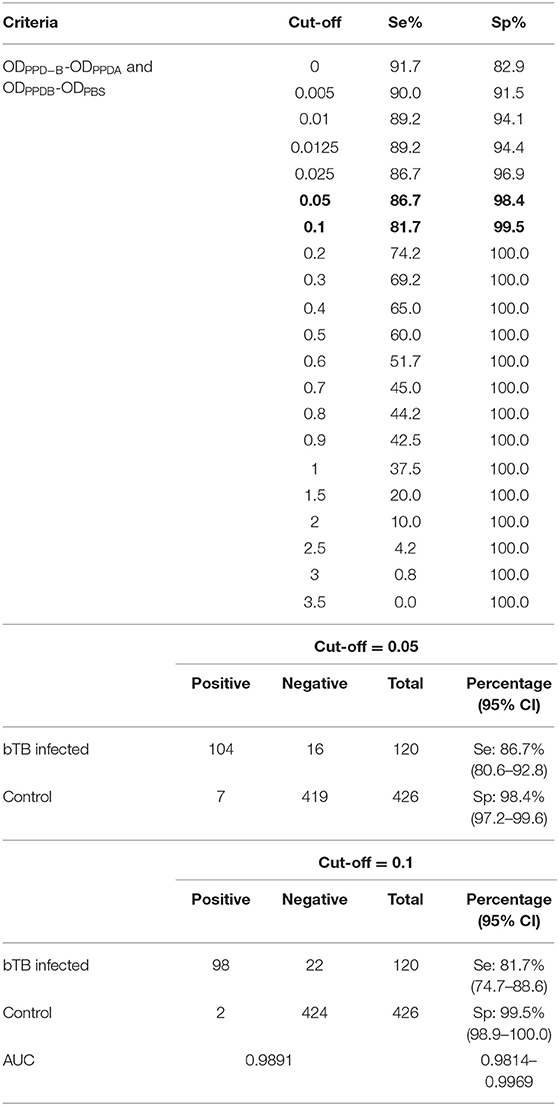
Se (n = 120) and Sp (n = 426) of the IFN-γ assay were calculated based on cut-off values ranging from 0 to 3.5. When cut-off O.D for the IFN-γ assay was 0.1, Se and Sp were 81.7% (95% CI, 74.7–88.6) and 99.5% (95% CI, 98.9–100.0), respectively (Table 2). When cut-off O.D was 0.05, the assay showed higher sensitivity (86.7, 95% CI, 80.6–92.8); however, Sp was found to be lower (98.4, 95% CI, 97.2–99.6) than when O.D was 0.1. The AUC of this ROC curve was 0.9891.
Agreement Between the IFN-γ Assay and CFT, and Evaluation of Parallel Use of the Two Tests
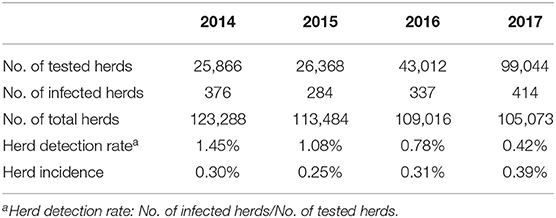
Agreement between results of the IFN-γ assay and CFT for 492,068 cattle tested from 2014 to 2017 is shown in Table 3. The overall agreement of the two tests was markedly high (98.2%); however, Cohen's kappa value for the two methods was 0.47, indicating moderate agreement. Since the introduction of the IFN-γ test as one of the elements of the control program, the rate of detection of bTB from 2014 to 2017 in South Korea has decreased continuously from 1.45 to 0.42%, but the herd incidence has remained at around 0.3% (Table 4). The regional detection rate for most regions has decreased from 2014 to 2017, similar to nationwide figures; however, regional herd incidence was found to fluctuate during the same period except on Jeju Island and in Gyeongnam province where the number of bTB outbreaks increased profoundly in 2017 (Figure 1).
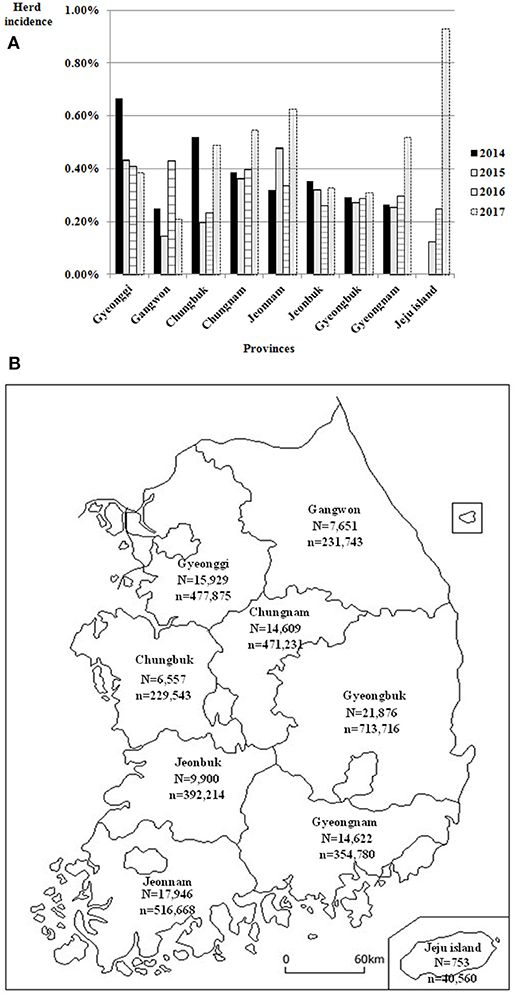
Figure 1. (A) Regional herd incidence in nine provinces of South Korea from 2014 to 2017. (B) Location of these nine provinces of South Korea and number of cattle herds and heads in each province in 2017. N, No. of cattle herds; n, No. of cattle heads.
After the introduction of the IFN-γ assay as part of the national control program from 2014 to 2016, 91 out of a total of 116 samples collected from herds with ongoing bTB outbreak were confirmed as bTB positive by M. bovis culture and/or typical bTB lesions. Positive samples were most frequently found using the IFN-γ assay (71/91, 78.0%), followed by IS1081 PCR (76.9%), gross/microscopic examination (70.3%), CFT (63.7%), M. bovis isolation (58.2%), IS6110 PCR (51.6%), and acid-fast staining (29.7%). Differences in detection efficiency for bTB depending on the application of the two tests are shown in Table 5. Parallel screening for bTB showed much higher Se (86/91, 94.5%) than the following screening approaches: serial (47.2%, 43/91), single screening using CFT (63.7%, 58/91), or the IFN-γ assay (78.0%, 71/91). When true infection was strictly defined using M. bovis cultures (n = 53), normally used as a gold standard test to evaluate diagnostic tools, gross/ microscopic examination showed the highest Se (46/53, 86.8%), followed by the IFN-γ assay (44/53, 83.0%), IS1081 PCR (36/53, 67.9%), IS6110 PCR (30/53, 56.6%), CFT (29/53, 54.7%), and acid-fast staining (24/53, 45.3%).
Discussion
The IFN-γ assay is designed to measure the CMI, the dominant immunological response to tuberculosis in cattle and is mainly used as an ancillary test (2). In this study, we attempted to assess the efficacy of the IFN-γ assay as a diagnostic tool and large-scale surveillance program for detection of bTB in South Korea.
Generally, the calculation formula for the IFN-γ assay is based on the difference between the titers of IFN-γ that exist in each sensitized plasma sample using three kinds of stimulating materials, including bovine PPD, avian PPD, and PBS (O.DPPD−B-O.DPPD−A, O.DPPD−B-O.DPBS, O.DPPD−B > O.DPPD−A or O.DPPD−B-O.DPPD−A / O.Dpositivecontrol-O.Dnegativecontrol). This approach has been used depending on the epidemiological context in various countries or regions (6, 8, 17). This calculation using PPDs accounts for sensitization by non-tuberculous organisms such as M. avium and IFN-γ in blood released for other reasons (6). Additionally, defined mycobacterial antigens, including ESAT-6 and CFP-10, are also being used to increase Sp of results in regions with low bTB prevalence or officially bTB free (OTF) countries (17, 18). The selected cut-off value is one important factor in the application of the IFN-γ test. The cut-off value for this study is estimated at O.D 0.1 for Se 81.7% (74.7–88.6) and Sp of 99.5% (98.9–100.0), whereas the cut-off value at O.D 0.05 provides Se of 86.7% (80.6–92.8) and Sp of 98.5% (97.2–99.6). As the cut-off value changes, Se and Sp of the IFN-γ assay will vary in opposing directions; thus, the analysis criteria may require adaptation depending on the region's infection status and the test application conditions (8, 19). Previous studies have reported the range of Se for the IFN-γ assay to be between 73.0 and 100.0% and showed that Sp ranged from 85.0 to 99.6% (6, 17, 20). These results were likely influenced by several factors, such as cattle population, cut-off values, the source of PPD tuberculin, and the animals defined as the “gold standard” (6). The AUC of ROC indicated that this IFN-γ assay has good power of discrimination to be used for diagnosis of bTB. Although bTB has been endemic in South Korea since first reported in 1913, recently, herd incidence for bTB in South Korea has not been very high (about 0.3%) (10). In terms of Sp, a 1% difference implies that there would be over 10,000 false positive cattle from one million cattle tests per annum. Consequently ~100 million dollars would be required for compensation and slaughter of uninfected cattle diagnosed as false positives. Therefore, 0.1 as a cut-off value obtained using the formula devised using PPDs is regarded as adequate to control bTB in South Korea. Since the introduction of the bTB eradication program, bTB testing by private veterinarians has not been allowed in South Korea. Moreover, the number of state veterinarians has been insufficient to perform the CFT as a pre-movement test or as a regular interval test for all cattle. Consequently, the CFT has only been used for parallel testing in outbreak herds and in regular interval testing for dairy cattle, which have a population one-seventh the size of beef cattle. The IFN-γ assay was introduced to reinforce the control policy of bTB as a screening test for beef cattle, which were monitored only by slaughterhouse surveillance, in spite of their larger population size relative to dairy cattle. However, many studies on the IFN-γ assay have reported that it is appropriate as an ancillary test in herds exposed to bTB due to its higher Se and lower Sp than the skin test (6, 8). Hence, long term monitoring of the IFN-γ assay as a primary test should be conducted, although Sp was very high in South Korea from our results.
Our results showed high agreement (98.2%) on identification of most cattle as bTB negative using both the skin test and IFN-γ assay; however, the results had a relatively low Cohen's kappa value (0.47) caused by diagnosis of some cattle as bTB positive based on only one kind of test (CFT-positive and IFN-γ assay-negative or CFT-negative and IFN-γ assay-positive (21, 22). Several other studies also reported agreement between the two tests at various levels. In a study conducted in Brazil, kappa values were 0.54–0.66 depending on time points when the IFN-γ assay (Bovigam, criteria for the IFN-γ assay not provided) was performed. Agreement increased to 0.649–0.713 when data from cattle affected by M. avium complex were withdrawn (23). A study performed in Ireland in 2015 showed relatively higher agreement between the single intradermal comparative tuberculin test and IFN-γ assay (Bovigam, cut-off for the INF-γ assay = 0.05) (20). Contrastingly, a study from dairy cattle in southern Chile showed a kappa value of ~0.3, indicating fair agreement in efficacy between the two tests when the cut off for the IFN-γ assay (Bovigam) was 0.05 or 0.1 (24). Most instances of low agreement resulted from false negatives obtained due to the diagnostic methods used. Potential causes of identification of false negatives using the skin test are as follows: difficulty of intra-cutaneous injection of the PPD (too superficial or too deep), taking observations too early after injection (reading the result of the skin test 72 h after injection is recommended), error in recording the thickness of skin, problems arising from the tuberculin used (expired or inadequately stored or manufacturing errors), desensitization, and reduction of immune response due to co-infection with Fasciola hepatica (6, 25, 26). Two different PPD-B preparations, manufactured by different companies, were used for skin test (CAVAC) and IFN-γ assay (CZ veterinaria) in South Korea. These two companies don't provide the potency of PPD-B as international unit (IU) but as protein concentration (CAVAC-0.2 mg/0.1 ml and CZ veterinaria-0.3 μg/ml). Therefore, the potency of the two PPD-B preparations may be not equivalent, although each stimulating agent is standardized through comparison with reference material. This difference of potency of PPD-B may also be one of the causes of the low agreement between the two tests. But analysis for potency of two commercial PPD-B preparations is not included in this study. Other than the false negative results mentioned above, it is probable that the IFN-γ assay could detect the immune response for bTB earlier than the skin test (6, 20, 21). In contrast, one of the potential causes of false negative diagnosis using the IFN-γ assay might be incorrect blood status, showing an overall reduction in titer by degradation of T lymphocyte activity, which is associated with the time required for transport or temperature of storage. Several studies reported that delays in the processing of blood and improper temperatures, which might be encountered in field situations or upon delivery, might impact IFN-γ production (27, 28). Some field veterinarians in South Korea prefer the caudal vein over the jugular vein to collect blood due to the relatively easier restraint of cattle for caudal vein blood collections. However, the smaller diameter of the caudal vein vs. that of the jugular vein might cause blood cell damage and micro-clotting, which could lead to the capture of lymphocytes, lowering IFN-γ production (29). Co-infection or pre-sensitization with other organisms, such as M. avium subsp. paratuberculosis could also impact the result showing O.DPPD−B-O.DPBS higher than the cut-off value; however, O.DPPD−B-O.DPPD−A lower than the cut-off value. Some studies reported that Se of the IFN-γ assay was reduced in the group of cattle co-infected with bTB and Johne's disease (30, 31). In South Korea, there is no regular surveillance program for paratuberculosis. However, some studies reported that the sero-prevalence of paratuberculosis in South Korean cattle ranged from 3.3 to 7.1% (32). Therefore, it is possible that the diagnosis of bTB by the IFN-γ assay is likely to be influenced by co-infection or pre-sensitization, although additional investigations are needed to confirm this. Similar to other studies, our data, obtained from large-scale surveillance, also support the results of the skin test, and found that the IFN-γ assay could detect slightly different sub-populations of bTB infected cattle due to the abovementioned reasons, even though both tests use the host's CMI response (6, 33).
After the introduction of the IFN-γ assay in South Korea, the herd detection rate for bTB continuously decreased because the number of tested herds markedly increased as compared to the period before this assay was introduced. However, herd incidence increased slightly from 2015 to 2017. This was speculated to be due to the fact that the IFN-γ assay could detect more reactor cattle recently in the regions of South Korea where there was no field surveillance for beef cattle, except slaughterhouse surveillance until 2013. Regional differences in herd incidence were not significant from 2014 to 2017 because cattle trades between regions are common in South Korea. Moreover, there was no obvious correlation between regional incidence and size of cattle herds or heads. On account of frequent inter-regional movement and unclear regional differences in herd incidence for bTB, differential regional application of detailed parts of the control program, such as testing interval and frequency, which are operational in other countries, have not yet been considered in South Korea (34–36).
Many studies have stated that parallel testing using a combination of two different tests could improve Se of assays and accelerate the resolution of a TB outbreak (6, 8, 12, 37). In this study, when the two kinds of tests were used as single, serial, or in parallel for reactors identified using several laboratory tests, the parallel screening showed the highest Se (86/91, 94.5%). Thus, parallel screening using the IFN-γ assay and skin test based on CMI could be a suitable strategy to eliminate reactors from bTB herds, as already reported in previous studies.
In South Korea, although the control program based on the CFT and meat inspection has been conducted over the last 50 years since the first bTB detection in 1913, bTB is still regarded as an endemic disease, which greatly affects cattle husbandry. The IFN-γ assay was introduced to overcome some disadvantages of the skin test and is currently used as an ancillary test to complement the skin test in the EU, USA, New Zealand, and Australia (4, 20, 33). Unlike most countries that adapted the IFN-γ assay as a supplementary test, in South Korea, it is included in the bTB surveillance program as a primary test equivalent to the skin test. This is the first study to describe the nationwide surveillance of bTB using the IFN-γ assay in South Korea. Our study revealed a change of bTB incidence after the introduction of the IFN-γ assay and its agreement with the skin test as a diagnostic method. Furthermore, our study showed that parallel use of the IFN-γ assay and skin test for bTB outbreak herds may serve as an efficient strategy in field conditions in South Korea.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
We confirmed that there is no requirement to obtain the approval for research on animals slaughtered due to infectious diseases from IACUC (Institutional Animal Care and Use Committee) in South Korea.
Author Contributions
Y-HJ performed the statistical analyses. Y-HJ and JK drafted the manuscript. Y-HJ, YL, S-SY, and JK designed the study and coordinated the work. CP and SK collected clinical samples and conducted gross and microscopic examinations. Y-HJ, SR, TK, MJ, and YS participated in the laboratory experiments, and generation and collection of the data, and collaborated in the interpretation of the results. All authors critically revised the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by the Animal and Plant Quarantine Agency, Ministry of Agriculture, Food and Rural Affairs, South Korea (project B-1541778-2012-13-02 & project M-1543081-2017-17-01).
Conflict of Interest
Y-HJ was employed by the Animal and Plant Quarantine Agency (government-affiliated), and CP and SK were employed by the Regional Government Institute (veterinary service).
The remaining authors, including CP and SK, declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Kang, Dr. Byeon and the other state veterinarians from 46 regional veterinary services in South Korea for technical support for sampling and data collection for the CFT and IFN-γ assay.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2020.00222/full#supplementary-material
Figure S1. PCR result for identification of M. bovis. Line M: DNA molecular weight marker (100 bp); Line 1~2: M. bovis isolates; Line 3: M. intracellulare ATCC 13950, Line 4: M. avium subsp. hominissuis 104, Line 5: M. avium subsp. paratuberculosis ATCC 19698; Line -: negative control, Line +: M. bovis AN5.
References
1. Casal C, Bezos J, Diez-Guerrier A, Alvarez J, Romero B, de Juan L, et al. Evaluation of two cocktails containing ESAT-6, CFP-10 and Rv-3615c in the intradermal test and the interferon-gamma assay for diagnosis of bovine tuberculosis. Prev Vet Med. (2012) 105:149–54. doi: 10.1016/j.prevetmed.2012.02.007
2. Schiller I, Vordermeier HM, Waters WR, Whelan AO, Coad M, Gormley E, et al. Bovine tuberculosis: effect of the tuberculin skin test on in vitro interferon gamma responses. Vet Immunol Immunopathol. (2010) 136:1–11. doi: 10.1016/j.vetimm.2010.02.007
3. Kuria JKN. Disease Caused by bacteria in cattle: tuberculosis. Intechopen. (2019). doi: 10.5772/intechopen.82051
4. Abdellrazeq GS, Elnaggar MM, Osman HS, Davis WC, Singh M. Prevalence of bovine tuberculosis in Egyptian cattle and the standardization of the interferon-gamma assay as an ancillary test. Transbound Emerg Dis. (2016) 63:497–507. doi: 10.1111/tbed.12291
5. Wood PR, Corner LA, Rothel JS, Baldock C, Jones SL, Cousins DB, et al. Field comparison of the interferon-gamma assay and the intradermal tuberculin test for the diagnosis of bovine tuberculosis. Aust Vet J. (1991) 68:286–90. doi: 10.1111/j.1751-0813.1991.tb03254.x
6. de la Rua-Domenech R, Goodchild AT, Vordermeier HM, Hewinson RG, Christiansen KH, Clifton-Hadley RS. Ante mortem diagnosis of tuberculosis in cattle: a review of the tuberculin tests, γ-interferon assay and other ancillary diagnostic techniques. Res Vet Sci. (2006) 81:190–210. doi: 10.1016/j.rvsc.2005.11.005
7. OIE. Bovine tuberculosis. In: Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, Chapter 3.4.6. Paris. (2018). p. 1058–74.
8. EFSA. Scientific opinion on the use of a gamma interferon test for the diagnosis of bovine tuberculosis. EFSA J. (2012) 10:2975. doi: 10.2903/j.efsa.2012.2975
9. KOSIS. Number of Farms in Households and Animals in Heads by Type. Korean statistical information service (South Korea). (2017) Available online at: http://kosis.kr/eng/statisticsList/statisticsListIndex.do?menuId=M_01_01&vwcd=MT_ETITLE&parmTabId=M_01_01&statId=1962008&themaId=#F12.4 (accessed August 17, 2019).
10. Ku BK, Jeon B-Y, Kim JM, Jang Y-B, Lee H, Choi JY, et al. Investigation of bovine tuberculosis outbreaks by using a trace-back system and molecular typing in Korean Hanwoo beef cattle. J Vet Sci. (2018) 19:45–50. doi: 10.4142/jvs.2018.19.1.45
11. Michelet L, de Cruz K, Karoui C, Tambosco J, Moyen JL, Henault S, et al. Second line molecular diagnosis for bovine tuberculosis to improve diagnostic schemes. PLoS ONE. (2018) 13:e0207614. doi: 10.1371/journal.pone.0207614
12. Keck N, Boschiroli M-L, Smyej F, Vogler V, Moyen J-L, Desvaux S. Successful application of the gamma-interferon assay in a bovine tuberculosis eradication program: the french bullfighting herd experience. Front Vet Sci. (2018) 5:27. doi: 10.3389/fvets.2018.00027
13. Swets J. Measuring the accuracy of diagnostic systems. Science. (1988) 240:1285–93. doi: 10.1126/science.3287615
14. Khosravi AD, Alami A, Meghdadi H, Hosseini AA. Identification of Mycobacterium tuberculosis in clinical specimens of patients suspected of having extrapulmonary tuberculosis by application of nested PCR on five different genes. Front Cell Infect Microbiol. (2017) 7:3. doi: 10.3389/fcimb.2017.00003
15. Collins DM, Erasmuson SK, Stephens DM, Yates GF, De Lisle GW. DNA fingerprinting of Mycobacterium bovis strains by restriction fragment analysis and hybridization with insertion elements IS1081 and IS6110. J clin Microbiol. (1993) 31:1143–7. doi: 10.1128/JCM.31.5.1143-1147.1993
16. Petrie A, Watson P. Statistics for Veterinary and Animal Science, 3rd Edn. Hoboken, NJ: Wiley Blackwell (2013). p. 408.
17. Schiller I, Oesch B, Vordermeier HM, Palmer MV, Harris BN, Orloski KA, et al. Bovine tuberculosis: a review of current and emerging diagnostic techniques in view of their relevance for disease control and eradication. Transbound Emerg Dis. (2010) 57:205–20. doi: 10.1111/j.1865-1682.2010.01148.x
18. Strain SAJ, McNair J, McDowell SWJ. A review of the international application of the interferon-gamma Test. Agri-Food and Biosciences Institute (UK). (2012). Available online at: http://www.daera-ni.gov.uk/sites/default/files/publications/dard/review-of-international-application-of-the-interferon-gamma-test.pdf (accessed 10 September, 2019).
19. Faye S, Moyen J-L, Gares H, Benet J-J, Garin-Bastuji B, Boschiroli M-L. Determination of decisional cut-off values for the optimal diagnosis of bovine tuberculosis with a modified IFNγ assay (Bovigam®) in a low prevalence area in France. Vet Microbiol. (2011) 151:60–7. doi: 10.1016/j.vetmic.2011.02.026
20. Clegg TA, Good M, Doyle M, Duignan A, More SJ, Gormley E. The performance of the interferon gamma assay when used as a diagnostic or quality assurance test in Mycobacterium bovis infected herds. Prev Vet Med. (2017) 140:116–21. doi: 10.1016/j.prevetmed.2017.03.007
21. Neill SD, Cassidy J, Hanna J, Mackie DP, Pollock JM, Clements A, et al. Detection of Mycobacterium bovis infection in skin test-negative cattle with an assay for bovine interferon-gamma. Vet. Rec. (1994) 135:134–5. doi: 10.1136/vr.135.6.134
22. Pollock JM, Welsh MD, McNair J. Immune responses in bovine tuberculosis: towards new strategies for the diagnosis and control of disease. Vet Immunol Immunopathol. (2005) 108:37–43. doi: 10.1016/j.vetimm.2005.08.012
23. Lopes LB, Alves TM, Stynen APR, Mota PMPC, Leite RC, Lage AP. Parameter estimation and use of gamma interferon assay for the diagnosis of bovine tuberculosis in Brazil. Pesqui Vet Bras. (2012) 32:279–83. doi: 10.1590/S0100-736X2012000400001
24. Raffo E, Monti G, Salgado M. Comparison of common tests performance for Mycobacterium bovis infection diagnosis in low prevalence dairy cattle herds of southern Chile. Austral J Vet Sci. (2018) 50:51–4. doi: 10.4067/S0719-81322018000100109
25. Claridge J, Diggle P, McCann CM, Mulcahy G, Flynn R, McNair J, et al. Fasciola hepatica is associated with the failure to detect bovine tuberculosis in dairy cattle. Nat Commun. (2012) 3:853. doi: 10.1038/ncomms1840
27. Waters WR, Nonnecke BJ, Olsen SC, Palmer MV. Effects of pre-culture holding time and temperature on interferon-gamma responses in whole blood cultures from Mycobacterium bovis-infected cattle. Vet Microbiol. (2007) 119:277–82. doi: 10.1016/j.vetmic.2006.08.014
28. Gormley E, Doyle MB, McGill K, Costello E, Good M, Collins JD. The effect of the tuberculin test and the consequences of a delay in blood culture on the sensitivity of a gamma-interferon assay for the detection of Mycobacterium bovis infection in cattle. Vet Immunol Immunopathol. (2004) 102:413–20. doi: 10.1016/j.vetimm.2004.08.002
29. Pucken V-B, Knubben-Schweizer G, Döpfer D, Groll A, Hafner-Marx A, Hörmansdorfer S, et al. Evaluating diagnostic tests for bovine tuberculosis in the southern part of Germany: a latent class analysis. PLoS ONE. (2017) 12:e0179847. doi: 10.1371/journal.pone.0179847
30. Álvarez J, de Juan L, Bezos J, Romero B, Sáez JL, Marqués S, et al. Effect of paratuberculosis on the diagnosis of bovine tuberculosis in a cattle herd with a mixed infection using interferon-gamma detection assay. Vet Microbiol. (2009) 135:389–93. doi: 10.1016/j.vetmic.2008.09.060
31. Aranaz A, De Juan L, Bezos J, Alvarez J, Romero B, Lozano F, et al. Assessment of diagnostic tools for eradication of bovine tuberculosis in cattle co-infected with Mycobacterium bovis and M. avium subsp. paratuberculosis. Vet Res. (2006) 37:593–606. doi: 10.1051/vetres:2006021
32. Yoo HS, Shin SJ. Recent research on bovine paratuberculosis in South Korea. Vet Immunol Immunopathol. (2012) 148:23–8. doi: 10.1016/j.vetimm.2012.06.005
33. Veterinary Handbook for Herd Management in the bovine TB eradication programme. Department of Agriculture, Fisheries and Food (Ireland). (2010). Available online at: http://www.bovinetb.info/docs/veterinary-handbook-for-herd-management-in-the-bovine-tb-eradication-programme.pdf (accessed 19 February, 2020).
34. Bovine TB: get your cattle tested in England. Animal and Plant Health Agency (UK). (2015). Available online at: http://www.gov.uk/guidance/bovine-tb-getting-your-cattle-tested-in-england (accessed 28 December, 2019).
35. Bovine TB eradication Uniforms and Rules (UMR). United states Department of Agriculture (USA). (2005). Available online at: https://www.aphis.usda.gov/animal__health/animal__diseases/tuberculosis/downloads/tb-umr.pdf (accessed 28 December, 2019).
36. Napp S, Ciaravino G, Pérez de Val B, Casal J, Saéz JL, Alba A. Evaluation of the effectiveness of the surveillance system for tuberculosis in cattle in Spain. Prev Vet Med. (2019) 173:104805. doi: 10.1016/j.prevetmed.2019.104805
Keywords: bovine tuberculosis, Mycobacterium bovis, IFN-γ, CFT, cattle
Citation: Jang Y-H, Kim T, Jeong MK, Seo YJ, Ryoo S, Park CH, Kang S, Lee YJ, Yoon S-S and Kim JM (2020) Introduction and Application of the Interferon-γ Assay in the National Bovine Tuberculosis Control Program in South Korea. Front. Vet. Sci. 7:222. doi: 10.3389/fvets.2020.00222
Received: 05 November 2019; Accepted: 01 April 2020;
Published: 28 April 2020.
Edited by:
Armanda Bastos, University of Pretoria, South AfricaReviewed by:
Eduard Otto Roos, Pirbright Institute, United KingdomAnthony Duignan, Department of Agriculture Food and the Marine, Ireland
Copyright © 2020 Jang, Kim, Jeong, Seo, Ryoo, Park, Kang, Lee, Yoon and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jae Myung Kim, dmV0a2ltam04OCYjeDAwMDQwO2dtYWlsLmNvbQ==
 Yun-Ho Jang
Yun-Ho Jang Tae-woon Kim1
Tae-woon Kim1