- Department of Clinical Sciences, College of Veterinary Medicine, Cornell University, Ithaca, NY, United States
Background: Platelet rich plasma (PRP) is used extensively in equine regenerative medicine. Differences in preparation protocols give rise to significant variability in the cellular composition of PRP making it very difficult to establish a standard of care in the field. This study aimed to optimize the preparation protocol for leukocyte-reduced PRP (P-PRP).
Methods: Blood (100 mL) was collected from horses (n = 5) and divided into 2 purple top EDTA tubes and 6 (15 mL) double syringesa with a final concentration of 10% acid citrate dextrose anticoagulant. Six double syringesa were collected from each horse; PRP samples were prepared in duplicate and centrifuged at 1,100 rpm (188 × g), 1,300 rpm (263 × g), or 1,500 rpm (350 × g). Duplicates were subjected to +/– braking at the end of centrifugation. The total volume of PRP generated was measured and divided into thirds. Each third (top, middle, and bottom) were drawn off separately using the inner (6 mL syringe) and placed in purple top EDTA tubes. Automated complete blood counts were performed on all peripheral whole blood and PRP samples.
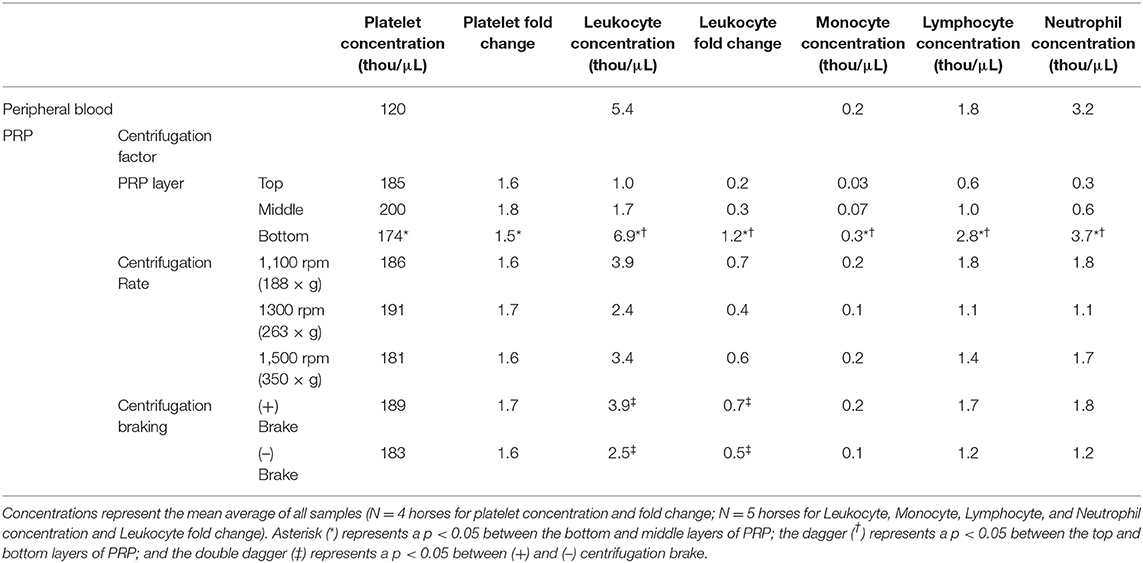
Results: The concentration of leukocytes was higher in the bottom layer of PRP compared to the top and middle layers (p < 0.0001). The concentration of platelets was slightly lower in the bottom layer of PRP than the middle layer (p = 0.02). Centrifugation braking increased the leukocyte concentration in the top (p = 0.03) and middle layers of PRP (p = 0.001). Centrifugation rate had no effect on the cellular composition of PRP (p = 0.1–0.6).
Conclusions: Because layer of plasma affected both platelet and leukocyte concentrations in PRP, the most important modification for the current single spin, double syringe, plasma based PRP preparation protocols is to exclude the bottom 1/3 layer of PRP.
Introduction
Musculoskeletal injuries are common in equine athletes and, due to the poor intrinsic healing capabilities of cartilage and tendons, present a significant clinical challenge (1, 2). PRP has been used extensively by equine practitioners and specialists to promote healing by delivering anabolic growth factors to damaged tissue (3–6). In human medicine, there is considerably more robust evidence for the application of PRP in musculoskeletal injury with several level one studies supporting the use of PRP for tendinopathies and osteoarthritis (7–16). There is however, remaining debate regarding the optimal PRP preparation. Specifically, controversy exists regarding the effects of leukocytes within PRP. Some studies suggest a beneficial effect including increased growth factor and cytokine release as well as increased antibacterial and immunologic resistance (17). Other studies however suggest a negative effect including increased inflammatory cytokines and catabolic mediators release leading to the degradation of tendon, ligament, muscle, and cartilage (18, 19). Recent evidence suggests that minimizing leukocyte concentration in PRP is more important than maximizing platelet concentration for decreasing inflammation and enhancing matrix gene synthesis (18–23). Broadly, plasma-based centrifugation methods produce leukocyte-reduced PRP (P-PRP) in contrast to buffy coat based methods which concentrate both platelets and leukocytes (L-PRP) (24, 25).
Many techniques exist for the preparation of P-PRP in either a liquid or solid fibrin form (18, 26–29). For veterinary applications, liquid injectable preparations of PRP are most commonly used. Preparation protocols for P-PRP differ by centrifugation rate (188–350 × g), centrifugation time (4–6 min), and braking at the end of centrifugation (26). Each of these factors can affect the platelet and leukocyte composition of PRP and therefore the clinical application of PRP for therapeutic application.
Most commercially available PRP systems were designed for use with human blood, posing a challenge for equine practitioners due to the lower average hematocrit in horses (33%) compared to humans (45%) (30, 31). Using the double syringe systema as an example, the lower equine hematocrit leads to generation of an excessive volume of PRP (~9 mL of PRP), which is greater than the maximum 6 mLs the double syringe systema is designed to produce. When the top 6 mLs of PRP are withdrawn, the 3 mLs of PRP closest to the packed red blood cell layer remain. Conventional wisdom would suggest that these remaining 3 mLs of PRP would contain more platelets than the upper 6 mLs simply due to platelet sedimentation during centrifugation, but the platelet and leukocyte concentrations have not been measured in the various levels of ACP. The objective of this study was to assess how centrifugation factors including; centrifugation rate, centrifugation braking, and layer of plasma affected the distribution of platelet and leukocyte concentration in PRP. This information will help establish a more standardized protocol for single-spin P-PRP methods.
Materials and Methods
Study Overview
Platelet rich plasma (PRP) was generated from 5 healthy mature horses (3 geldings and 2 mares) of various breeds (2 Thoroughbreds, 2 Warmbloods, and 1 Quarter Horse) with a mean age of 8 years (range: 4–21 years). Three different centrifugation rates, each with and without a brake were used to generate PRP. The top, middle, and lowest 1/3 of each PRP preparation were analyzed for platelet and leukocyte concentration (Figure 1). This study was approved by the Institutional Animal Care and Use Committee of Cornell University.
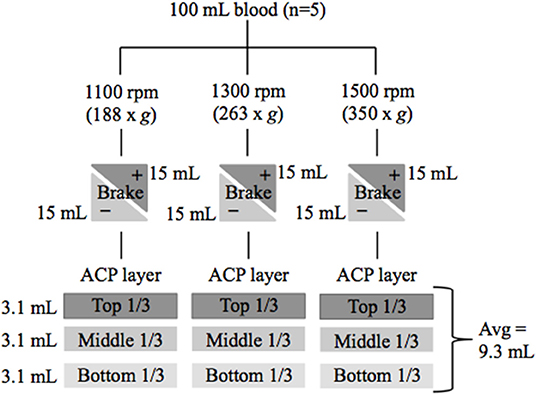
Figure 1. Flow chart of study design. Blood was collected from 5 healthy horses. Platelet rich plasma (PRP) was generated using 6 centrifugation techniques with variations in centrifugation rate and braking. PRP was divided into three layers and CBCs were performed to determine cellular composition. rpm, revolutions per minute; g, g force; (+) brake was applied or (–) brake was not applied at end of centrifugation.
Platelet Rich Plasma
Blood (100 mL) was collected and divided into 2 purple top EDTA tubes and 6 (15 mL) double syringesa containing acid citrate dextrose (ACD) anticoagulant to a final concentration of 10%. To test the effect of centrifugation rate on the distribution of leukocytes and platelets throughout the plasma, PRP was prepared in duplicate with centrifugation at 1,100 (188 × g), 1,300 (263 × g), or 1,500 rpm (350 × g), each for 5 min using a Hettich Rotofix 32 centrifuge. One of the duplicate syringes at each centrifugation rate was subjected to braking (25 s deceleration using the rotor brake) at the end of centrifugation and the other was not (9 min deceleration using gravity/friction).
After centrifugation, the total volume of PRP generated was measured and recorded. For each double syringea, the total PRP volume was divided into thirds (ex. total volume 9 = 3 mL per third). The PRP was extracted and placed into purple top tubes one layer at a time (ex. 3 mL) from the 15 mL double syringea using the inner (6 mL) syringe per manufacturer's instructions. These samples were labeled as top layer (farthest from RBCs), middle layer, and bottom layer (closest to the RBCs). Complete blood counts (CBCs) were performed on both the peripheral whole blood and PRP samples by Cornell University Clinical Pathology using a Bayer ADVIA 2120 automated analyzer.
Statistical Methods
For measures of PRP composition (platelets, total leukocytes, segmented neutrophils, lymphocytes, and monocytes), a mixed-effect model was fitted to the data. Horse was treated as a random effect and centrifugation rate (188, 263, or 350 × g), brake (+/–), and layer (top, middle, or bottom 1/3) were treated as fixed effects, with interaction terms for centrifugation rate*brake, brake*layer, and layer*centrifugation rate. Platelet and leukocyte fold change was calculated by dividing the concentration of cells in PRP by the concentration of cells in peripheral whole blood. PRP layer was evaluated by comparing the top, middle and bottom layers (~3 mL aliquots). Centrifugation rate and braking were evaluated by comparing the average [(top + middle + bottom layers)/3] of each sample vs. samples of varying centrifugation rates and/or braking status. Statistical analysis was performed using a statistical software program with p < 0.05 considered significant. Effect size was calculated for significant findings.
Results
A total of 95 CBCs were performed on 30 PRP and 5 peripheral whole blood samples. The average amount of PRP produced was 9.25 mL (range 7.0–10.0 mL) per double syringea. Data from one horse was excluded from the statistical analysis of platelet fold change due to platelet clumping in the whole blood sample (flagged in automated CBC report and confirmed on blood smear) making a fold change calculation inaccurate. However, for leukocyte concentration, leukocyte fold change, and platelet concentration data from all 5 horses were used.
Platelet Concentration
Platelet concentration was affected by the layer of PRP, but not by centrifugation rate or braking. Platelet concentration varied between the 3 layers of PRP (Table 1), with the bottom layer of PRP having a slightly lower platelet concentration (p = 0.02) and lower platelet fold change (p = 0.04) than the middle layer. There was no difference in platelet concentration between the top and bottom layers of PRP.
Variations in centrifugation rate and braking at the end of centrifugation did not affect platelet concentration in PRP. There was little variation in platelet concentration (p = 0.60) or platelet fold change (p = 0.40) between PRP generated at 188, 263, 350 × g. The greatest difference in platelet concentration was between the 263 and 350 × g groups with the 350 × g group containing an average of 2% more platelets than the 263 × g group. Centrifugation braking had no effect on platelet concentration (p = 0.40) or platelet fold change (p = 0.40). Platelet concentration was increased by <2% when braking was used at the end of centrifugation.
Leukocyte Concentration
In direct contrast to the increased platelet concentration in the middle layer of PRP, leukocyte concentration was increased in the bottom layer. Both the total leukocyte concentration (p < 0.0001) and leukocyte fold change (p < 0.0001) were increased in the bottom layer of PRP compared to the middle and top layers (Table 1). The majority (72%) of leukocytes measured in the PRP were in the bottom layer. The bottom layer of PRP was leukocyte concentrated with a leukocyte fold change of 1.2, whereas the top and middle thirds were leukocyte reduced with a 0.2- and 0.3-fold change, respectively. All three leukocyte subtypes; monocytes (p < 0.0001), lymphocytes (p < 0.0001), and neutrophils (p < 0.0001), were increased in the bottom layer of PRP (Table 1). Monocyte concentration was 5.1 and 12.7 times higher in the bottom layer of PRP than in the middle and top layers, respectively. Lymphocyte concentration was 2.7 and 4.5 times higher in the bottom layer of PRP than in the middle and top layers, respectively, and neutrophil concentration was 6.6 and 10.7 times higher in the bottom layer of PRP than in the middle and top layers, respectively.
Variations in centrifugation rate did not affect leukocyte concentration (p = 0.10) or leukocyte fold change (p = 0.09) in ACP (Table 1). Leukocyte concentration was 1.4 and 1.6 times higher in PRP generated at 350 and 188 × g than at 263 × g, but this difference was not significant (p = 0.10).
Braking at the end of centrifugation increased leukocyte concentration (p = 0.04) and leukocyte fold change (p = 0.04) in PRP (Table 1). Braking increased the leukocyte concentration by ~1,500 (leukocytes/μL) in each layer of PRP leading to a 3.9, 4.6, and 1.1 times increase in leukocyte concentration in the top, middle, and bottom layers of PRP, respectively. Leukocyte concentration increased significantly in the top (p = 0.03) and middle (p = 0.001) layers of PRP when the brake was applied, however the increase was not significant in the bottom layer (p = 0.8). It is important to note that although braking increased leukocyte concentration in PRP, the top and middle layers remained leuko-reduced (lower concentration compared to starting blood sample) regardless of braking.
Discussion
The data from this study suggests that the top 2/3 of PRP produced using the double syringe, single-spin plasma-based method have the optimal platelet: leukocyte ratio and platelet dose for treatment of tendonitis and osteoarthritis. A centrifugation rate between 188 and 350 × g can be used to consistently produce P-PRP using the single spin plasma-based method. Centrifugation braking will increase leukocyte concentration throughout the PRP layer, however the top 2/3 will remain leuko-reduced.
Despite the fact that plasma-based systems concentrate platelets in the plasma portion of the sample, many practitioners believe that the layer closest to the buffy coat contains the highest concentration of platelets and many try to include this layer or even a small portion of the buffy coat into their sample to maximize platelet retrieval. The finding from this study contradict this line of thinking. Hematologic evaluation revealed significant variations in the cellular composition of PRP when divided into three layers. Platelets in the top and middle layers of PRP were increased 1.6 and 1.8-fold over whole blood whereas platelets in the bottom layer were only increased by 1.4-fold. This represented a significant difference in platelet fold change suggesting that the majority of concentrated platelets were in the top 2/3 of PRP (away from the buffy coat).
In regenerative medicine, the “more is better” concept has dominated when considering total platelet dose. Recent investigations have shown that improvement in tissue healing is achieved with low to moderate platelet doses and the amount of improvement in tissue healing plateaus with increasing platelet doses. Multiple level one studies have reported successful outcomes using 0.9–9.0 × 109 platelets/dose (7, 8, 12). In the present study, an average platelet dose of 1.4 × 109 was produced in just the top 2/3 of PRP. This dose is well within the range necessary to improve tissue healing; therefore, it would be acceptable to exclude the bottom 1/3 layer of PRP.
Centrifugation is a key factor in PRP preparation as it separates blood components based on their density gradients. Platelets are the lightest, followed by leukocytes, and then RBCs, which are heaviest (32). Therefore, relative centrifugal force (RCF) must be appropriate to obtain good separation of platelets from other blood cells. In this study, a range of low centrifugation rates and a short duration of centrifugation time resulted in the reliable and consistent production of P-PRP. We compared a range of slow centrifugation rates, 188, 263, and 350 × g, which are standard for the single spin plasma-based method protocol. Our results showed that there was no difference in the cellular composition of PRP between these groups. Previous studies reported a 1.2–2.5-fold increase in platelet concentration and a 0.06–0.2-fold decrease in leukocyte concentration using the standard (350 × g, 5 min) ACP protocol (33–35). PRP generated in our study had an average of 1.5-fold increase in platelet concentration (with no significant difference between centrifugation rate groups). This is in line with previously reported values. The PRP produced in our study also had an average of 0.6-fold decrease in leukocyte concentration. This does not correlate with the results of previous studies, however our study included PRP from the bottom 1/3 layer of plasma (closest to the buffy coat) which had a 1.2-fold increase in leukocyte concentration vs. the middle and top layers which had a 0.2 and 0.3 fold decrease in leukocyte concentration, respectively. Therefore, the composition of PRP generated during our study is in line with previously reported values when the bottom 1/3 layer is excluded. We concluded that a slow centrifugation rate, between 188 and 350 × g, can be used to consistently produce P-PRP using the single spin plasma-based method.
Braking at the end of centrifugation was also evaluated in this study. We found that the use of a rotor brake (which reduces centrifugation time by about 9 min) had no impact on platelet concentration in PRP but caused a slight increase in leukocyte concentration in the top and middle layers of PRP. This is consistent with the results of previous studies which showed that rapid deceleration induced turbulence and mixing of WBCs into the PRP (32). However, the amount of leukocyte contamination in the top and middle layers of PRP after braking was minimal—with the end product continuing to be significantly leuko-reduced at 0.2- and 0.3-fold decrease in leukocyte concentration in the top and middle layers, respectively. Therefore, the use of a rotor brake at the end of centrifugation does not significantly alter the cellular composition of P-PRP.
The results of this study suggest that differences in the cellular composition of PRP due to various centrifugation and preparation factors should be considered when determining the optimal P-PRP protocol. Layer represented the most significant factor influencing the composition of PRP. When seeking to produce P-PRP, the top and middle layers have the best cellular composition with a platelet concentration in the range suggested for improved tissue healing and an 80% reduction in leukocyte concentration.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
This study was approved by the Institutional Animal Care and Use Committee of Cornell University.
Author Contributions
AR and MG executed the study. All authors contributed equally to study design, data review, and manuscript preparation.
Funding
The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of Interest
LF is a consultant to Arthrex, Inc. whom donated the ACP syringes for the study.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Ms. Paula Sharp for assistance in preparation of the manuscript.
References
1. Yang G, Rothrauff BB, Tuan RS. Tendon and ligament regeneration and repair: clinical relevance and developmental paradigm. Birth Defects Res Part C Embryo Today Rev. (2013) 99:203–22. doi: 10.1002/bdrc.21041
2. Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. (1982) 64:460–6. doi: 10.2106/00004623-198264030-00022
3. Bembo F, Eraud J, Philandrianos C, Bertrand B, Silvestre A, Veran J, et al. Combined use of platelet rich plasma micro-fat in sport and race horses with degenerative joint disease: preliminary clinical study in eight horses. Muscles Ligaments Tendons J. (2016) 6:198–204. doi: 10.11138/mltj/2016.6.2.198
4. Geburek F, Gaus M, van Schie HTM, Rohn K, Stadler PM. Effect of intralesional platelet-rich plasma (PRP) treatment on clinical and ultrasonographic parameters in equine naturally occurring superficial digital flexor tendinopathies - a randomized prospective controlled clinical trial. BMC Vet Res. (2016) 12:191. doi: 10.1186/s12917-016-0826-1
5. Garrett KS, Bramlage LR, Spike-Pierce DL, Cohen ND. Injection of platelet- and leukocyte-rich plasma at the junction of the proximal sesamoid bone and the suspensory ligament branch for treatment of yearling Thoroughbreds with proximal sesamoid bone inflammation and associated suspensory ligament branch de. J Am Vet Med Assoc. (2013) 243:120–25. doi: 10.2460/javma.243.1.120
6. Bosch G, van Schie HTM, de Groot MW, Cadby JA, van de Lest CHA, Barneveld A, et al. Effects of platelet-rich plasma on the quality of repair of mechanically induced core lesions in equine superficial digital flexor tendons: a placebo-controlled experimental study. J Orthop Res. (2010) 28:211–7. doi: 10.1002/jor.20980
7. Görmeli G, Görmeli CA, Ataoglu B, Çolak C, Aslantürk O, Ertem K. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. (2017) 25:958–65. doi: 10.1007/s00167-015-3705-6
8. Thanasas C, Papadimitriou G, Charalambidis C, Paraskevopoulos I, Papanikolaou A. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. Am J Sports Med. (2011) 39:2130–4. doi: 10.1177/0363546511417113
9. Gosens T, Peerbooms JC, van Laar W, den Oudsten BL. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis. Am J Sports Med. (2011) 39:1200–8. doi: 10.1177/0363546510397173
10. deVos RJ, Weir A, van Schie HT, Bierma-Zeinstra SM, Verhaar JA, Weinans H, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. (2010) 303:144–9. doi: 10.1001/jama.2009.1986
11. Castricini R, Longo UG, de Benedetto M, Panfoli N, Pirani P, Zini R, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair. Am J Sports Med. (2011) 39:258–65. doi: 10.1177/0363546510390780
12. Chen X, Jones IA, Park C, Vangsness CT. The efficacy of platelet-rich plasma on tendon and ligament healing: a systematic review and meta-analysis with bias assessment. Am J Sports Med. (2018) 46:2020–32. doi: 10.1177/0363546517743746
13. Peerbooms JC, Sluimer J, Bruijn DJ, Gosens T. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. (2010) 38:255–62. doi: 10.1177/0363546509355445
14. Cervellin M, de Girolamo L, Bait C, Denti M, Volpi P. Autologous platelet-rich plasma gel to reduce donor-site morbidity after patellar tendon graft harvesting for anterior cruciate ligament reconstruction: a randomized, controlled clinical study. Knee Surg Sport Traumatol Arthrosc. (2012) 20:114–20. doi: 10.1007/s00167-011-1570-5
15. Cerza F, Carnì S, Carcangiu A, Di Vavo I, Schiavilla V, Pecora A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. (2012) 40:2822–7. doi: 10.1177/0363546512461902
16. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis. Am J Sports Med. (2013) 41:356–64. doi: 10.1177/0363546512471299
17. Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. (2011) 39:266–71. doi: 10.1177/0363546510387517
18. Anitua E, Zalduendo M, Troya M, Padilla S, Orive G. Leukocyte inclusion within a platelet rich plasma-derived ribrin scaffold stimulates a more pro-inflammatory environment and alters fibrin properties. PLoS ONE. (2015) 10:e0121713. doi: 10.1371/journal.pone.0121713
19. Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. (2011) 39:2135–40. doi: 10.1177/0363546511417792
20. Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med. (2016) 44:792–800. doi: 10.1177/0363546515580787
21. Boswell SG, Schnabel LV, Mohammed HO, Sundman EA, Minas T, Fortier LA. Increasing platelet concentrations in leukocyte-reduced platelet-rich plasma decrease collagen gene synthesis in tendons. Am J Sports Med. (2014) 42:42–9. doi: 10.1177/0363546513507566
22. McCarrel TM, Minas T, Fortier LA. Optimization of leukocyte concentration in platelet-rich plasma for the treatment of tendinopathy. J Bone Joint Surg Am. (2012) 94:e143. doi: 10.2106/JBJS.L.00019
23. Zhang L, Chen S, Chang P, Bao N, Yang C, Ti Y, et al. Harmful effects of leukocyte-rich platelet-rich plasma on rabbit tendon stem cells in vitro. Am J Sports Med. (2016) 44:1941–51. doi: 10.1177/0363546516644718
24. Dohan Ehrenfest DM, Bielecki T, Mishra A, Borzini P, Inchingolo F, Sammartino G, et al. In search of a consensus terminology in the field of platelet concentrates for surgical use: platelet-rich plasma (PRP), platelet-rich fibrin (PRF), fibrin gel polymerization and leukocytes. Curr Pharm Biotechnol. (2012) 13:1131–7. doi: 10.2174/138920112800624328
25. Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. (2009) 27:158–67. doi: 10.1016/j.tibtech.2008.11.009
26. Oudelaar BW, Peerbooms JC, Huis In't Veld R, Vochteloo AJH. Concentrations of blood components in commercial platelet-rich plasma separation systems: a review of the literature. Am J Sports Med. (2018) 47:479–87. doi: 10.1177/0363546517746112
27. Trindade-Suedam IK, Leite FR, de Morais JA, Leite ER, Marcantonio EJ, Leite AA. Avoiding leukocyte contamination and early platelet activation in platelet-rich plasma. J Oral Implantol. (2007) 33:334–49. doi: 10.1563/1548-1336(2007)33[334:ALCAEP]2.0.CO;2
28. Russell RP, Apostolakos J, Hirose T, Cote MP, Mazzocca AD. Variability of platelet-rich plasma preparations. Sports Med Arthrosc. (2013) 21:186–90. doi: 10.1097/JSA.0000000000000007
29. Kushida S, Kakudo N, Morimoto N, Hara T, Ogawa T, Mitsui T, et al. Platelet and growth factor concentrations in activated platelet-rich plasma: a comparison of seven commercial separation systems. J Artif Organs. (2014) 17:186–92. doi: 10.1007/s10047-014-0761-5
30. Nostell K, Funkquist P, Nyman G, Essén-Gustavsson B, Connysson M, Muhonen S, et al. The physiological responses to simulated race tests on a track and on a treadmill in standardbred trotters. Equine Vet J Suppl. (2006) 38:123–7. doi: 10.1111/j.2042-3306.2006.tb05527.x
31. Brun JF, Varlet-Marie E, Raynaud de Mauverger E. Hematocrit and hematocrit viscosity ratio during exercise in athletes: even closer to predicted optimal values? Clin Hemorheol Microcirc. (2017) 64:777–87. doi: 10.3233/CH-168012
32. Marx G, Blankenfeld A, Gorodetsky R, BarShany S. Reducing white cells in platelet units. Transfusion. (1991) 31:743–7. doi: 10.1046/j.1537-2995.1991.31892023501.x
33. Hauschild G, Geburek F, Gosheger G, Eveslage M, Serrano D, Streitbürger A, et al. Short term storage stability at room temperature of two different platelet-rich plasma preparations from equine donors and potential impact on growth factor concentrations. BMC Vet Res. (2017) 13:7. doi: 10.1186/s12917-016-0920-4
34. Oh JH, Kim W, Park KU, Roh YH. Comparison of the cellular composition and cytokine-release kinetics of various platelet-rich plasma preparations. Am J Sports Med. (2015) 43:3062–70. doi: 10.1177/0363546515608481
Keywords: platelet rich plasma, PRP, equine, ACP, leukocyte reduced, P-PRP
Citation: Radtke AV, Goodale MB and Fortier LA (2020) Platelet and Leukocyte Concentration in Equine Autologous Conditioned Plasma Are Inversely Distributed by Layer and Are Not Affected by Centrifugation Rate. Front. Vet. Sci. 7:173. doi: 10.3389/fvets.2020.00173
Received: 22 January 2020; Accepted: 13 March 2020;
Published: 12 May 2020.
Edited by:
Fausto Cremonesi, University of Milan, ItalyReviewed by:
Kyla Ortved, University of Pennsylvania, United StatesMarco Patruno, University of Padova, Italy
Copyright © 2020 Radtke, Goodale and Fortier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lisa A. Fortier, bGFmNCYjeDAwMDQwO2Nvcm5lbGwuZWR1
 Alexandra V. Radtke
Alexandra V. Radtke Margaret B. Goodale
Margaret B. Goodale Lisa A. Fortier
Lisa A. Fortier