- Department of Pathogenic Microorganisms, China Animal Health and Epidemiology Center, Qingdao, China
Chickens contaminated with Campylobacter are a major risk factor for human Campylobacter disease. As a result of the slaughter process, infections should be strictly controlled due to complete exposure of the chickens and the cross-contamination of pathogens. Using @RISK software, quantitative evaluation models of Campylobacter contamination during slaughtering in a large broiler slaughterhouse were constructed. Broiler scalding was set as the starting point of evaluation and four major processes including defeathering, eviscerating, pre-cool rinsing, and splitting-transmission were included. Through the simulation of the constructed model, 90% probability of Campylobacter in 100 g chickens after slaughtering were distributed between 0.3 and 50.2 MPN, which was consistent with simulated actual monitoring data 0–16.6 MPN, indicating that the model shows high credibility. In addition, growth curves of Campylobacter during whole slaughtering showed that contamination significantly increased after defeathering, and increased again after pre-cool rinsing. Using correlation coefficients to analyze the sensitivity of each parameter in the model, it was determined that the concentration of Campylobacter in the pre-cool pond water (correlation coefficient: 0.95) was the most critical risk point of sanitary control in this slaughterhouse. In conclusion, this study is the first to incorporate environmental factors during broiler slaughtering into the risk evaluation of Campylobacter contamination, which provides guidance for the sanitary control and risk management of Campylobacter contamination during broiler slaughtering.
Introduction
The colonization rates of Campylobacter in the intestine, as a symbiotic bacteria in the intestinal tract of broilers, can reach 90%, but broilers show minimal clinical symptoms even when heavily infected (1). Broilers are easily contaminated by Campylobacter viscera when carrying recessive Campylobacter when entering the slaughtering stages due to complete exposure to the environment during slaughtering, leading to a wider cross-contamination and Campylobacter epidemics in chicken. As a result, 55.4% of retail chickens are contaminated by Campylobacter according to the latest surveys by the Food Standards Agency (FSA) in 2019 (2). A survey by the Federal Consumer Protection and Food Safety Agency (BVL) in 2017 found that 51.8% of retail chickens were contaminated by Campylobacter, and that 78.8% of slaughterhouse chickens are contaminated (3). Meanwhile, the contamination rates of Campylobacter in the chicken produced from large slaughterhouses in Eastern China was as high as 36.7% in 2018 (Monitoring data from our lab). It has been reported that 20–30% of Campylobacter cases are related to consumption of chickens contaminated with Campylobacter (4). Controlling Campylobacter during broiler slaughter therefore represents an effective means to prevent the risk of Campylobacter disease in the population.
Quantitative risk evaluation technology, as the optimal model of microbial risk evaluation, can provide important data references for the formulation of risk management policies (5). Combining microbial quantitative risk evaluations with critical control points can effectively reduce the cross-contamination of pathogenic bacteria. In this study, critical risk control points of Campylobacter contamination in a large-scale broiler slaughterhouse (the mainstream mode of broiler slaughtering in China) were analyzed using quantitative evaluation technology to provide guidance for the more targeted control of Campylobacter contamination during the slaughtering process.
Materials and Methods
Campylobacter Contamination Data in a Large Broiler Slaughterhouse
The parameters of broiler slaughter and processing were obtained through on-site investigations or expert consultations during the sampling process. Meanwhile, data on the contamination of Campylobacter in all stages of slaughter were obtained from monitoring data in our laboratory in the “Quality and Safety Risk Assessment Project of Animal and Poultry Products” of the Ministry of Agriculture in 2018. A total of 270 samples of Campylobacter were collected from the four links of scalding, rinsing, pre-cool rinsing, and segmental transmission in a large broiler slaughterhouse in the Shandong Province. As a result, Campylobacter was isolated, cultured and identified. Quantitative and qualitative results of Campylobacter contamination in chickens, viscera and the environment were obtained.
Quantitative Risk Assessment
In this study, risk assessment software @RISK 7 were used to fit the random distribution of data through its distribution fitting functiona. Meanwhile, variables and parameters involved in risk evaluations were expressed by specific values, formulas, or distributions. Data was input in Excel worksheet and models were built using @RISK 7. Monte Carlo simulation was performed using the Latin hypercube sampling method. Random computer extracts and probability distributions were obtained from each time point in simulations of 10,000 iterations.
Construction of Quantitative Risk Assessment Model
A slaughter batch of broiler chickens, with the number of 50,000–150,000 usually, were assessed. Broiler chickens are directly exposed to air and environmental utensils after scalding, so post-scalding was taken as the starting point of the evaluation process. Defeathering, eviscerating, pre-cool rinsing, and splitting-transmission were the four processes that were successively passed downstream.
Defeathering
The contamination of Campylobacter after hot washing was the initial value used for the evaluation model. After hot washing, the majority of bacteria on the chicken surface have been inactivated, and quantitative data cannot be detected directly. The contamination of Campylobacter in samples is converted from qualitative to quantitative data by the formula M = –(2.303/V) × lg (Nneg/Ntotal) (6). Amongst the values, V refers to the volume diluted by 100 cm2 cotton swab sample, Nneg refers to the number of negative samples detected, and Ntotal refers to the total number of samples. The defeathering process of the eviscerating machine can cross-contaminate the remaining Campylobacter to other chickens, and as a consequence, the contamination status of the eviscerating machine can be assessed as a risk contribution factor in the model. The contamination density of the eviscerating machine calculated through the cumulative probability distribution M = Cumulative (Min, Max, {a1, a2, a3…}, {p1, p2, p3…}) Fitting, in which Min is the minimum, Max is the maximum, a is the levels of Campylobacter (1 CFU = 1MPN), and p is the cumulative probability corresponding to the amounts of Campylobacter. Furthermore, it was assumed that half of the Campylobacter in the chicken body contacting the surface can be transferred to the chicken body in each batch of the defeathering machine, with total contamination amounts (N1) on the carcass of broiler chickens after scalding being the sum of Campylobacter remaining after scalding and newly contaminated after defeathering. All parameters are shown in Table 1.
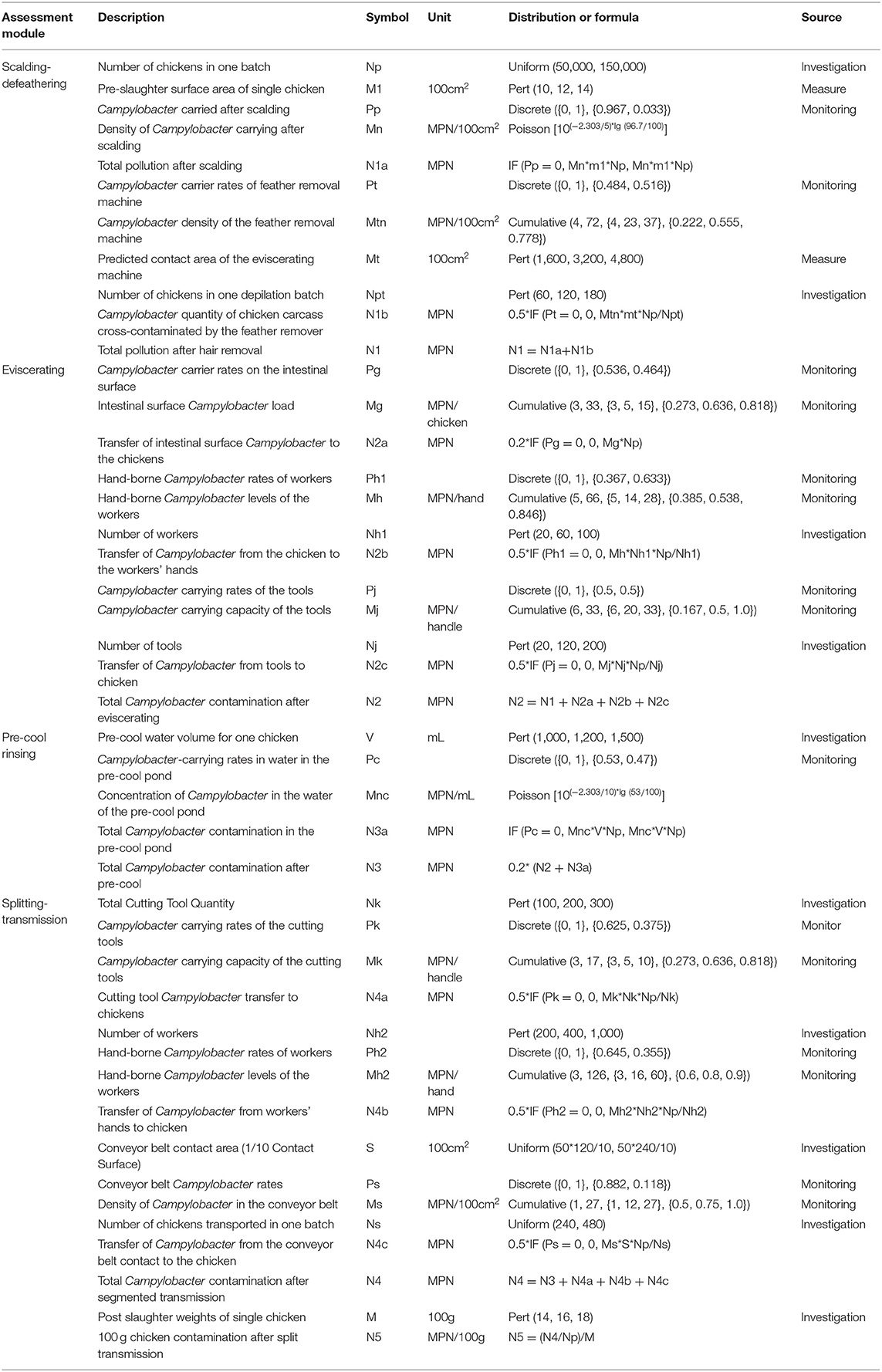
Table 1. Parameter settings of Campylobacter quantitative risk evaluation models for broiler slaughter.
Eviscerating
The intestinal tract of the broilers can carry Campylobacter recessively, and the viscera is easily punctured by the cutter during the eviscerating process. Residual feces in the cloacal orifice can easily contaminate the carcass of other broilers. Therefore, the viscera of chickens, workers' hands and eviscerating tools were considered as risk contributors. The increase of Campylobacter contamination is determined by the bacterial carrying rates, bacterial carrying capacity, the transmissibility of the visceral surface, workers' hands surfaces, and eviscerating tools. Meanwhile, the three risk contributing risk factors can detect the positive quantitative data of Campylobacter, and as a consequence, the bacterial carrying capacity is fitted by the cumulative probability distribution. The carrying rates are fitted by discrete probability distributions p = Discrete ({0, 1}, {Pneg, Ppos}), of which 0 represents negative, 1 represents positive, Pneg is negative rate, and Ppos is positive rate.
Pre-cool Rinsing
Pre-cooling after slaughter can reduce the total contamination of Campylobacter, but increases the chance of cross contamination of Campylobacter when all broiler carcasses are collected in the pre-cool pond. Meanwhile, according to investigations in the broiler slaughterhouse, the volume of pre-cool water corresponding to a broiler is 1–1.5 L. Although the isolation rates of Campylobacter in pre-cool water are high, effective positive quantitative data for direct counting are not obtained. Therefore, the concentration of Campylobacter in the pre-cool pond water was also converted into quantitative data by the formula. The transfer rate of Campylobacter contaminated in the pre-cool pond to the carcass of broilers was calculated by 1/5 (7).
Splitting-Transmission
Splitting-transmission is one of the most complex steps during broiler carcass exposure. Cutting tools, workers' hands and conveyor belts are all included in the evaluation. The number of carcass contact surfaces between the three risk contributing factors and broilers were obtained through on-site investigation. In addition to the positive isolation rates of Campylobacter fitted by discrete probability distributions, the positive quantitative data were fitted by the cumulative probability distribution. The transmissibility of Campylobacter to chickens through the conveyor belt were calculated by 1/2 (7). The total contamination of Campylobacter in one slaughter batch of chickens after splitting-transmission was expressed as N4, and those of Campylobacter in 100 g of chickens was expressed as N5.
Probability Distribution of Actual Contamination of Campylobacter in Chicken Meat
Cotton swab samples directly smeared from 100 cm2 surface of post-slaughtered chickens were collected for qualitative and quantitative detection. Quantitative data are also fitted using the cumulative probability distribution (Table 2). The relationship between the surface area and the weight of the single chickens were converted by the formula m = 2* (0.67*M*100+536) (8), in which M was the weight of single chickens.
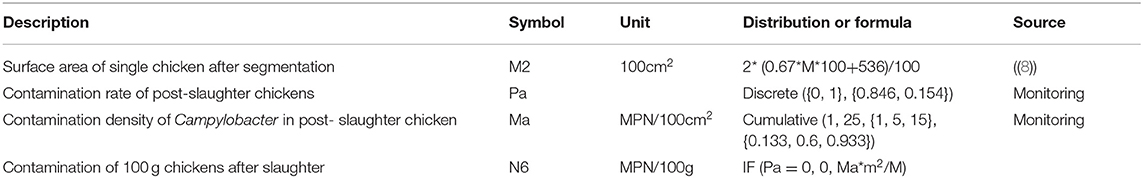
Table 2. Fitting parameters of the probabilistic distribution of Campylobacter contamination in chickens.
Results and Analysis
Quantitative Evaluation Models to Simulate the Contamination Probability Distribution of Campylobacter During Broiler Chickens Slaughtering
The fitting data are shown in Figure 1A. The total contamination of Campylobacter in one batch broiler chickens after scalding was taken as the initial value, although the 90% probability was 0 MPN, the maximum value reached to 3.84 × 106 MPN. Through quantitative risk assessment models of Campylobacter contamination in slaughter chickens, after defeathering, eviscerating, pre-cool rinsing, and splitting-transmission, 90% of the total Campylobacter contamination in broiler chickens was distributed between 0.4 × 106 and 87.2 × 108 MPN (Figure 1B), with an average of 3.06 × 107 MPN. As a result, the slaughter process significantly increased the risk of Campylobacter contamination in chickens.
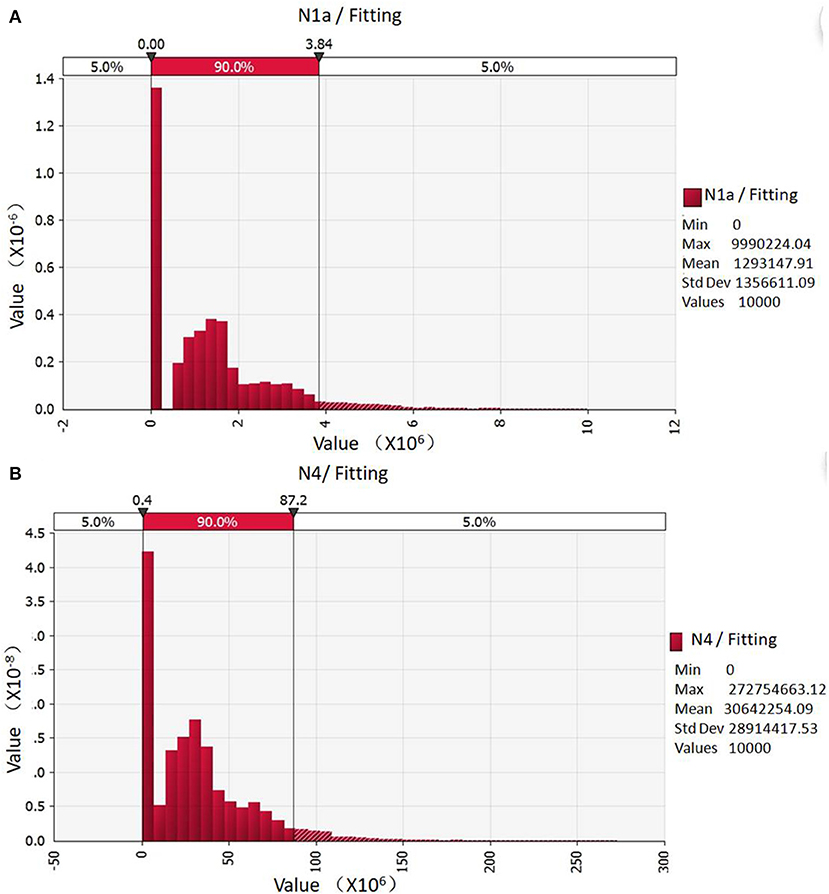
Figure 1. Probabilistic distribution of Campylobacter contamination in one patch of slaughtered broilers. (A) Initial total contamination of Campylobacter; (B) The total contamination of Campylobacter after segmentation simulated by evaluation Model.
Practical Verification of the Output
Using the simulation of the quantitative evaluation model of Campylobacter during the slaughtering of broiler, up to 90% of the contamination of Campylobacter in 100 g of chicken was found to distribute between 0.3 and 50.2 MPN, with an average of 19.19 MPN (Figure 2A). In addition, after fitting the actual monitoring data of the chickens after slaughtering, it was found that the 90% probability distribution of Campylobacter contamination in 100 g of chicken was 0–16.6 MPN (Figure 2B). These data were consistent with the contamination levels simulated by the model, and its average value of 2.19 MPN, was also between 0.3 and 50.2 MPN, which indicating that the quantitative evaluation model showed good reliability.

Figure 2. Distribution of Campylobacter contamination in 100 g of chicken after slaughtering. (A) Output results of the model simulation; (B) Fitting results of the actual monitoring data.
Changes of Campylobacter in Chickens During Slaughtering
The total amounts of Campylobacter contamination in the broilers during defeathering, eviscerating, pre-cool rinsing, and splitting-transmission were further simulated through the quantitative evaluation model. According to the average MPN values obtained, the growth curve of Campylobacter carried by the broilers during slaughtering was constructed (Figure 3). Meanwhile, it was found that the contamination of Campylobacter significantly increased after defeathering, from 1.2 × 106 to 15.3 × 106 MPN, and then miner increased after eviscerating. The contamination of Campylobacter significantly increased after pre-cool rinsing again from 16.2 × 106 to 27.6 × 106 MPN, but then unchanged on the whole after splitting-transmission. It seems that the defeathering and pre-cool rinsing of broiler chickens in the slaughter house were the main risk contributors of Campylobacter.
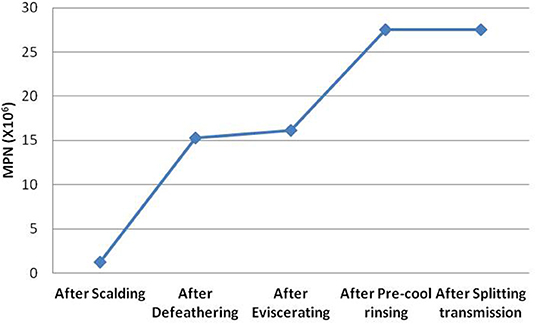
Figure 3. Changes of Campylobacter in broiler chickens during slaughtering. The average MPN of Campylobacter in the broiler chickens after defeathering, eviscerating, pre-cool rinsing and splitting-transmission was simulated, and the growth curves of Campylobacter contamination during slaughtering were constructed.
Analysis of Critical Risk Control Points in Broiler Slaughtering Process
The correlation between the contamination of Campylobacter in segmented chickens and the risk factors in the slaughtering process were discussed through sensitivity analysis of the parameters in the quantitative evaluation model. The risk contribution of each factor to the contamination of Campylobacter in the terminal chicken products were then determined. The results in Figure 4 show that the concentration carried in the water of pre-cool pond was the most critical risk point for Campylobacter contamination in the terminal chicken products (correlation coefficient value: 0.95). In addition, the carrier rate of Campylobacter on the defeathering machine was also contributed some risk to Campylobacter contamination in the terminal chickens (correlation coefficient value: 0.14).
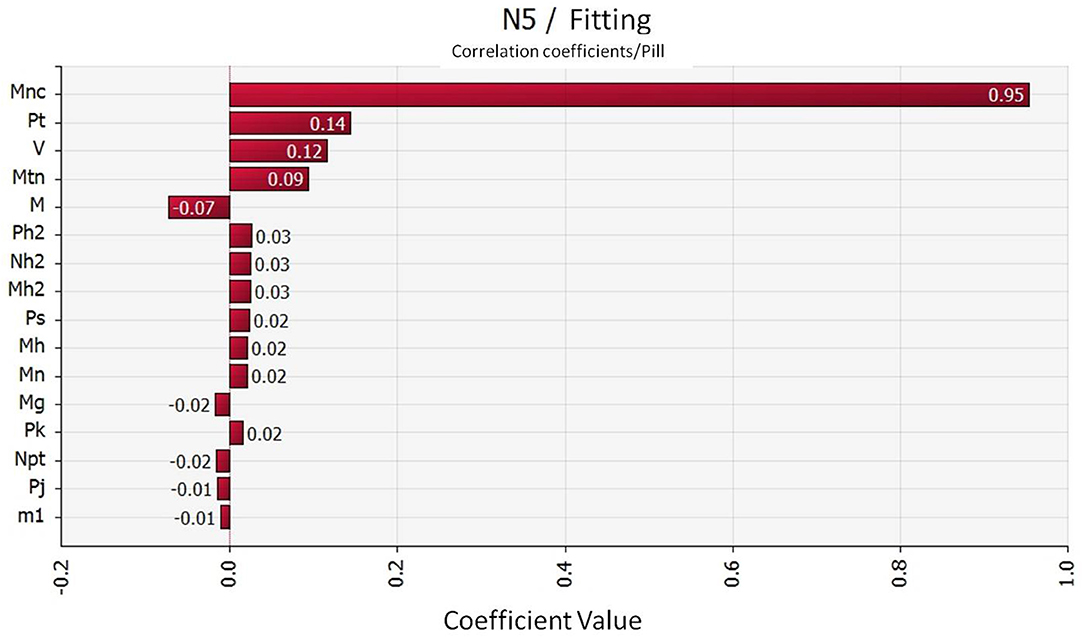
Figure 4. Coorelation coefficient of the Campylobacter hazard factors during slaughtering. Mnc: Campylobacter carrying concentration in the water of the pre-cool pond; Pt: Campylobacter carrying rate of defeathering machine; V: Volume of the pre-cool pond water; Mtn: Campylobacter carrying capacity of defeathering machine.
Uncertainty Analysis of the Quantitative Evaluation Model
The quantitative evaluation model of Campylobacter contamination during the slaughtering links of broilers constructed in the study had several uncertainties. An example is the uncertainty of the process and the model. This study assumed that Campylobacter does not proliferate during the slaughter process, despite its known ability to proliferate at the temperature before pre-cool rinsing. A further limitation is the uncertainty of quantitative data. Some parameters that failed to detect quantitative data were directly converted from the qualitative data, but remained unable to replace real quantitative data obtained from a large number of actual samples. The final uncertainty is from the empirical speculations. The majority of data originated from actual investigations and monitoring, but few were obtained through relevant experience speculation, such as the transfer rates of bacteria from tools and workers to the chickens. Here we assumed that half of the bacteria can be transmitted by contact, without the consideration of other factors.
Discussion
Microbial quantitative evaluations hold high application values for the controlled measurement of risk probabilities and severities. Such predictions can prevent the exposure risk and hazard of pathogenic bacteria in food. The Monte-Carlo method simulates experimental results with a probability model, which better reflects the real risk situation (9). In this study, the quantitative evaluation method was used to study the contamination status of Campylobacter in each link of a large-scale broiler slaughterhouse, and the critical risk control points of the whole slaughtering process were identified to guide the supervision and control of microorganisms in the slaughterhouse more effectively.
China is a big country in chicken production and consumption. Current international data suggest that Campylobacter infections have become more serious (2, 3, 10) and represent a major challenge to food safety (11). Western developed countries have performed a systematic risk assessment on Campylobacter in chicken (12–14) and highlighted that the contamination rates of Campylobacter are proportional to the population incidence. Studies in China assessing the risk of Campylobacter from farms to dining tables in chicken have estimated the incidence of Campylobacter disease in the current contaminated population. In this study, 90% of Campylobacter contamination in chicken was estimated at between 0.4 × 106 and 87.2 × 108 MPN per 100 g, which was consistent with the actual monitoring data. Although this initial contamination is not sufficiently high to cause disease, Campylobacter is a living organism, if it exponentially proliferate under the hotter temperature in summer, it only takes a few hours to reach the pathogenic dose of 400–500 CFU (15).
Previous study (16) highlighted Campylobacter contamination in eviscerating link was the critical risk control point during the slaughtering process, however, the risk of the slaughtering environment to carcass cross-contamination was not analyzed. Chickens are completely exposed to the environment during slaughtering and can be directly cross-contaminated by pathogenic bacteria in the environment. There are differences in the sanitary management in different slaughterhouses, which determine the contamination status of pathogens in the environment and final products is also different. This study selected a large broiler slaughterhouse in the Shandong Province to evaluate the contamination status of Campylobacter in the whole slaughterhouse process, particularly in the exposed environment, and thus to lock the key risk points for environmental sanitation control. It was found that the Campylobacter concentration carried in the pre-cool pond water had the greatest impact on the contamination risk of the terminal chicken products, followed by the bacterial carrying rate on the eviscerating machine. In theory, pre-cooling rinsing can reduce bacterial contamination, but unqualified hygiene can indeed make the pre-cooling pool as “a large dye tank” of bacteria, in which potential cross-contamination to each chicken is extremely serious. Obviously, the hygiene control of pre-cooling in the slaughterhouse we are studying should be specially strengthened.
The control of critical risk points is important during the slaughtering process of broilers. In May 2013, the Food Safety Administration of the United States (FSIS) issued HACCP (Hazard Analysis and Critical Control Point) system certification guidelines (17) and defined the critical risk point control measures for broiler slaughter. In September of the same year, the FSIS added Campylobacter control measures (18) on the basis of Salmonella in broilers. China also developed the corresponding HACCP in livestock and poultry slaughtering (19), but the implementation is the autonomous behavior of the slaughtering enterprise, and is currently not supervised, even some broiler slaughterhouses have not been certified and standardized for implementation. This study analyses and clarifies the critical control points of Campylobacter contamination in broiler slaughterhouses. The slaughtering enterprise can therefore take measures to strengthen the hygienic control of pre-cool pond water effectively, and to ensure the terminal chicken products with better quality and safety.
In conclusion, the quantitative evaluation model of Campylobacter contamination including environmental samples during broiler slaughtering was constructed for the first time in this study. Changes in Campylobacter contamination in chicken meat during slaughtering the process were identified based on the model simulation data, which highlighted that Campylobacter concentration carried in the pre-cool rinsing water was the critical risk control point. Finally, the model provided a theoretical basis for the sanitary control and risk management of Campylobacter contamination in broiler slaughterhouses.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
The animal study was reviewed and approved by Animal Ethics Committee of China Animal Health and Epidemiology Center.
Author Contributions
GZ: model construction and manuscript drafting. XH and NL: Campylobacter isolation in samples. YL and LW: Campylobacter identification and data analysis. JZ and YG: sample and data collection from slaughter house. JuaW, ZQ, and JL: manuscript revision. JunW: research overall guidance.
Funding
This work was funded by the National Key Research and Development Program of China (2018YFD0500505) and the National Special Project for Risk Assessment of Quality & Safety of Agricultural Products (GJFP201800703).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Prof. Ming Zou in Qingdao Agriculture University for contacting the slaughterhouse.
References
1. Hutchison ML, Taylor MJ, Tchòrzewska MA, Ford G, Madden RH, Knowles TG. Modelling-based identification of factors influencing campylobacters in chicken broiler houses and on carcasses sampled after processing and chilling. J. Appl. Microbiol. (2017) 122:1389–401. doi: 10.1111/jam.13434
2. Whitworth J. Rise in UK Chickens Positive for top Levels of Campylobacter. Food Safety News (2019). Available online at: https://www.foodsafetynews.com/2019/06/rise-in-uk-chickens-positive-for-top-levels-of-Campylobacter/ (accessed May16, 2019).
3. Diet and Food. Every other chicken in the trade with infected diarrhoea pathogens (2019). Available online at: https://dailytophealth.com/diet-food/every-other-chicken-in-the-trade-with-infected-diarrhoea-pathogens/
4. BIOHAZ. Scientific opinion on campylobacterin broiler meat production: control options and performance objectives and/or targets at different stages of the food chain. EFSA J. (2011) 9:2105. doi: 10.2903/j.efsa.2011.2105
5. Zhao G, Wang Y, Wang J. Research status and problem analysis of quantitative risk evaluation of pathogenic microorganisms in livestock and poultry products. Agri Product Qual Saf. (2016) 6:41–6. doi: 10.3969/j.issn.1674-8255.2016.06.010
6. Jarvis B. Sampling for microbiological analysis. In: Lund BM, Baird-Parker TC, Gould GW, editors. The Microbiological Safety and Quality of Food, Vol. II. Aspen Publishers, Inc. (2000). p. 1727–28.
7. Zhao G, Liu N, Zhao J, Li Y, Wang J, Qu Z, et al. Quantitative risk evaluation of Salmonella contamination in broiler slaughtering and processing. China Animal Quarantine. (2018) 35:26–31. doi: 10.3969/j.issn.1005-944X.2018.04.007
8. Thomas NL. Observations of the relationship between the surface area and weight of eviscerated carcases of chickens, ducks and turkeys. Int J Food Sci Technol. (2007) 13:81–6. doi: 10.1111/j.1365-2621.1978.tb00780.x
9. Gong C, Wang Z, Wu H, Dong F, Sun Y. Quantitative evaluation of dietary exposure risk of Vibrio parahaemolyticus in seafood of Yantai sea area. J Food Safe Qual Inspect. (2015) 6:3485–90.
10. Zhu H, Ping Y, An Y, Zhou L, Wang F, Yan G, et al. Study on Salmonella and Campylobacter contamination in broiler farming and processing. Chin. Poult. (2018) 40:80–4. doi: 10.16372/j.issn.1004-6364.2018.04.018
11. Guo P, Guo D. The next challenge for the safety of food and drink Chang. pigs and poultry in animal husbandry abroad. CampylobacterVac. (2009) 29:60–1.
12. Crotta M, Georgiev M, Guitian J. Quantitative risk assessment of Campylobacter in broiler chickens - Assessing interventions to reduce the level of contamination at the end of the rearing period. Food Control. (2017) 75:29–39. doi: 10.1016/j.foodcont.2016.12.024
13. DanishAgricultureFoodCouncil. A Quantitative Microbiological Risk Assessment of Campylobacterin the Broiler Meat Chain. EFSA Supporting Publications (2011).
14. WHO. Risk Evaluation of Campylobacterin Broiler Chickens. (2009). Available online at: https://www.who.int/foodsafety/publications/micro/MRA11_En.pdf
15. Zhai W, Jiao Y, Jin L, Jiao X. Current situation of Campylobacter contamination in poultry meat and research progress of prevention and control measures. Adv Anim Med. (2010) 31:180–4. doi: 10.3969/j.issn.1007-5038.2010.z1.046
16. Huang J, Zang X, Zhai W, Guan C, Lei T, Jiao X. Campylobacterspp. in chicken-slaughtering operations: a risk assessment of human campylobacteriosis in East China. Food Control. (2018) 86:249–56. doi: 10.1016/j.foodcont.2017.11.026
17. FSIS. FSIS. Compliance Guideline HACCP Systems Validation (2013). Available online at: https://www.fsis.usda.gov/wps/wcm/connect/a70bb780-e1ff-4a35-9a9a-3fb40c8fe584/HACCP_Systems_Validation.pdf,?MOD=AJPERES.
18. FSIS. Salmonella, and CampylobacterVerification Program for Raw Meat and Poultry Products. (2013). Available online at: https://www.fsis.usda.gov/wps/wcm/connect/ebf83112-4c3b-4650-8396-24cc8d38bf6c/10250.1.pdf?MOD=AJPERES.
Keywords: quantitative risk evaluation, broiler, slaughtering process, Campylobacter, critical control point
Citation: Zhao G, Huang X, Zhao J, Liu N, Li Y, Wang L, Gao Y, Wang J, Qu Z, Liu J and Wang J (2020) Risk Prevention and Control Points Through Quantitative Evaluation of Campylobacter in a Large Broiler Slaughterhouse. Front. Vet. Sci. 7:172. doi: 10.3389/fvets.2020.00172
Received: 04 December 2019; Accepted: 12 March 2020;
Published: 22 April 2020.
Edited by:
Guillermo Tellez, University of Arkansas, United StatesReviewed by:
Roberto Senas Cuesta, University of Arkansas, United StatesCalderon Apodaca Norma, National Autonomous University of Mexico, Mexico
Copyright © 2020 Zhao, Huang, Zhao, Liu, Li, Wang, Gao, Wang, Qu, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ge Zhao, cathyge@163.com; Junwei Wang, yffs2000@sina.com
 Ge Zhao*
Ge Zhao* Xiumei Huang
Xiumei Huang Na Liu
Na Liu Lin Wang
Lin Wang Yubin Gao
Yubin Gao Juan Wang
Juan Wang Junhui Liu
Junhui Liu