- 1Central Research Laboratory, Siberian State Medical University, Tomsk, Russia
- 2Department of General Practice and Polyclinic Therapy, Siberian State Medical University, Tomsk, Russia
- 3Department of Microbiology, Immunology and Tropical Medicine, Research Center for Neglected Diseases of Poverty, School of Medicine & Health Sciences, George Washington University, Washington, DC, United States
Aims: There is a general, inverse relationship between helminth infection and allergic diseases including bronchial asthma (BA). Proteins and other mediators released from parasitic worms exert cogent downmodulation of atopic and other allergic reactivity. We investigated the immune activities of an immortalized murine dendritic cell (mDC) line (JAWSII) and of primary human dendritic cells (hDCs) collected from study participants with and without BA after Opisthorchis felineus hemozoin (OfHz) treatment.
Methods and Results: in vitro, expression of lymphocyte-activating factors—T helper 1 (Th1) induction and anti-inflammatory cytokines including tumor necrosis factor alpha (TNF-α), interleukin-1beta (IL-1β), IL-10, and IL-12β–increased significantly in mDCs pulsed with OfHz. In parallel, primary dendritic cells (hDC) from cases clinically diagnosed with BA along with healthy controls were exposed ex vivo to OfHz in combination with lipopolysaccharide (LPS). Whereas no significant change in the cellular maturation markers, CD83, CD86, and CD40, was apparent in BA vs. healthy hDC, pulsing hDC from BA with OfHz with LPS induced significant increases in expression of IL-10 and IL-12β, although not of TNF-α or tumor growth factor-beta (TGF-β).
Conclusions: Liver fluke hemozoin OfHz stimulated production of Th1 inducer and anti-inflammatory cytokines IL-10 and IL-12β from BA-hDC pulsed with OfHz, an outcome that enhances our understanding of the mechanisms whereby opisthorchiasis contributes to protection against the atopic disease in liver fluke infection-endemic regions.
Introduction
Helminth parasites establish chronic infections, characterized by modulation of both the innate and adaptive host immune response. A generalized, negative relationship between helminth infection and immune-related disorders is apparent for several disorders and helminth parasite species (1–3). Excretory–secretory (ES) products released from eukaryotic parasites are potent modulators of the immune response,which is central to the survival of these pathogens and for the maintenance of a chronic infection. There is active, sustained investigation of the mechanisms of the immunomodulation induced by helminths and the characterization of parasite-derived products with immunomodulation properties for treatment and prevention of the autoimmune-related diseases (4, 5). Indeed, investigation of the activities and properties of ES products has provided insights into the mechanisms of the host immune response modulation by parasites, which may be exploited for therapeutic intervention for allergic, autoimmune, metabolic, and inflammatory diseases (6–9).
Hemozoin (Hz) is an ES product with immunomodulatory properties known in a variety of eukaryotic parasites including Plasmodium falciparum (10), schistosomes (11–14), and liver flukes (15, 16). Hz is an inert, dark brown crystalline polymer of ferriprotoporphyrin IX produced by blood-feeding parasites. The synthesis of Hz by the parasite results in the detoxification of heme from digested blood. The effects of Hz purified from parasites [native Hz (nHz)] and of Hz synthesized (sHz) from hemin-synthetic Hz (β-hematin) have been investigated in vitro and in vivo. In vitro, dendritic cell (DC) and other cells such as monocytes phagocytize nHz (17). ES products of many helminths suppress DC maturation and modulate DC response to lipopolysaccharide (LPS) and other stimuli. Similar responses have been described following the incubation of DC with nHz purified from P. falciparum (Pf Hz). Exposure to Pf Hz impairs the maturation of immature DC (18). Pf Hz inhibits the loss of podosomes by DC and inhibits the upregulation of CD83 after stimulation by LPS, a potent activator of DC maturation (19). The addition of Pf Hz or sHz decreases production of LPS/IFN-promoted interleukin (IL)-12p70 [T helper 1 (Th1) responses development] and increases immune homeostasis cytokines including IL-10 and tumor necrosis factor alpha (TNF-α) from peripheral blood mononuclear cells (PBMCs) and CD14+ antigen-presenting cells (20).
Because the exposition and subsequent increased acceptance of the hygiene hypothesis, many diseases have been linked to exposure during childhood to microbial and eukaryotic pathogens, including helminth parasites (21–23). Asthma is a chronic inflammatory disease of the lung airways especially of the bronchi and bronchioles. Numerous factors contribute to asthma including environmental insults and allergens such as dust mites, pollen, and airborne pollutants from motor vehicles, along with a susceptible genetic phenotype. A rapid increase of incidence of asthma is evident in developed countries but is generally unapparent in less developed countries, especially in regions endemic for parasites. According to the hygiene hypothesis, the appearance of allergic diseases is inversely related to the decrease in prevalence of parasite infection, which can modulate the immune response and establish a host–parasite relationship and immunological milieu that accommodates both the helminth and its host (24–27). This provides a new area in which to search for intervention for the treatment and prevention of asthma.
Infection with the food-borne trematode Opisthorchis felineus is a highly prevalent liver fluke infection in Western Siberia, Russia (28, 29). Inverse relationships between O. felineus infection and allergy in a liver fluke endemic region have been reported (30). Epidemiological investigation has indicated that opisthorchiasis has a negative association with skin prick test reactivity (31). Infection with O. felineus diminishes the risk of atopic bronchial asthma (BA) associated with the SOCS5 gene polymorphism (32). The treatment with filarial cystatin of human PBMCs from patients who are sensitive to timothy grass pollen caused a Th1 polarization, pointing to a potential therapy for asthmatics (33). Infection with Schistosoma japonicum and Heligmosomoides polygyrus can downregulate allergic airway inflammation (34, 35). However, given that intact infectious agents are likely to be pathogenic, parasite extracts such as O. felineus Hz (OfHz) might offer a safer biotherapeutic. Accordingly, here, we addressed here whether OfHz modulates DC immune function in patients during BA, a Th2 immune response-associated disease.
Materials and Methods
Hz Extraction and Purification
To extract Hz from Opisthorchis felineus, we used a slightly modified protocol based on methods reports for studies on host immunomodulation to Hz from Schistosoma mansoni (14) and Plasmodium falciparum (18). The purification of OfHz involved the removal of host or parasite products adsorbed on the surface of OfHz, which leads to variable and undetermined residue by decontamination of lipids and proteins. Briefly, O. felineus mature worms were collected from O. felineus metacercariae-infected hamster (50 metacercariae/hamster, Supplement 1). Pooled mature worms were washed three times with 1 × phosphate-buffered saline (PBS), and then 7 mL of packed parasites was final resuspended in PBS with 10 mL total volume. Worms were sonicated on ice until homogenized; the lysate was subjected to centrifugation at 1,000 × g for 60 s at room temperature. The upper aqueous contents of the supernatant were transferred to a new tube, and the pellet containing the Hz was collected after centrifugation at 8,000 × g for 20 min. Pelleted Hz was resuspended in PBS and then subjected to precipitation in chloroform, methanol, and water (36). Pelleted Hz was resuspended in 2 mL of PBS, briefly sonicated on ice, and exposed to 1% proteinase K at 37°C for 18 h. Subsequently, the Hz was re-pelleted at 10,000 × g for 20 min and washed sequentially three times in PBS containing 2% sodium dodecyl sulfate (SDS), three times in 0.1 M of NaHCO3 (pH 9.1) containing 2.5% SDS, and five times in distilled water. The contamination of Hz samples was assessed for proteins by silver-stained SDS–polyacrylamide gel electrophoresis (PAGE) and for nucleic acids by use of ethidium bromide-stained agarose gels. The absence of O. felineus egg contamination was confirmed by light microscopy.
Levels of endotoxin level were determined using an endpoint chromogenic Limulus amebocyte lysate assay (Lonza AG, Visp, Switzerland,). A standard curve was established using a range of concentrations of hematin diluted in 100 mM of NaOH, 2% SDS, and 3 mM of EDTA, for which absorbance at 401 nm was measured using a spectrophotometer. To determine the concentration of OfHz, the absorbance at 401 nm of aliquots of OfHz dissolved in 100 mM of NaOH, 2% SDS, and 3 mM of EDTA was compared with the standard curve.
DC Stimulation—Exposure of JAWSII Cells to OfHz
The semi-adherent JAWSII cell line, a granulocyte-macrophage colony-stimulating factor (GM-CSF)-dependent dendritic (DC) line established from bone marrow cells of p53-knockout C57Bl/6 mouse, was purchased from the American Type Culture Collection (CRL-1194; ATCC, Manassas, VA). JAWSII cells were maintained in complete Roswell Park Memorial Institute medium (RPMI) 1640 culture with 4 mM of L-glutamine, HEPES (Thermo Fisher Scientific) consisting of 10% (v/v) fetal bovine serum (FBS), 5 ng/mL of GM-CSF, 10 U/mL of penicillin and 100 μg/mL of streptomycin, 0.5 mM of 2-ME, and 1 mM of sodium pyruvate, in 5% CO2 in air at 37°C. Cultures were maintained by transferring nonadherent cells to a centrifuge tube and treating attached cells with 0.25% trypsin−0.03% EDTA (Gibco) at 37°C for 5 min, followed by pooling the two populations of cells together and dispensing into new flasks and/or for downstream analysis. To stimulate JAWSII cells, 300,000 cells/mL were seeded into wells of 6-well plates for 24 h before exposure to liver fluke Hz, OfHz. The cells were divided into three groups: control (no treatment), mock (1 × PBS treatment), and OfHz-treated (100 nm of OfHz in 1 × PBS). JAWSII cells were pulsed with OfHz for 48 h and harvested as above. The cells were washed three times with 1 × PBS before proceeding to RNA extraction.
RNA Extraction and Quantitative Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) of Anti-inflammatory and Inflammatory Cytokine
Total RNA was extracted from JAWSII and OfHz-treated JAWSII cells using the RNAzol RT reagent (Molecular Research Center, Inc., Cincinnati, OH), which removes contaminated DNA (37), and its concentration and purity were determined using the NanoDrop ND-1000 spectrophotometer (OD260/280, ~2.0). RNAs were reverse transcribed into cDNA using iScript Reverse Transcript (Bio-Rad). First-strand cDNA was performed RT-qPCR using SsoAdvanced Universal SYBR Green Supermix (Bio-Rad) and run in triplicate using the iQ5 Real time PCR thermal cycler (Bio-Rad); thermal cycling was as follows: initial denaturation at 95°C for 30 s, 40 amplification cycles each consisting of denaturation at 95°C for 15 s, annealing at 60°C for 30 s, and final heating at 60–95°C to obtain the melting curve. Samples were run in triplicates after which the output was analyzed using the iQ5 software (Bio-Rad). The delta-delta Ct method with normalization to mouse GAPDH expression (38) was used to calculate the relative expression of the IL-1β, IL-10, IL-12b, and TNF-α genes. The data were expressed as differential fold change compared with the control group, with fold changes reported as mean and 95% confidence interval (CI) of difference. The statistical significance of cytokine transcript induction from OfHz-treated groups were compared with control groups using two-way analysis of variance (ANOVA), followed by Dunnett's multiple comparison test (Prism software).
Monocyte-Derived DCs
Purification of human PBMCs was performed using a two-step gradient centrifugation (39). Briefly, peripheral blood was diluted with Hank's balanced salt solution (HBSS) (1:1), loaded on a Ficoll–Hypaque gradient (Sigma, Moscow, Russia), and centrifuged for 30 min at 600 × g at room temperature. PBMCs were collected, washed twice in HBSS (pH 7.4), resuspended in serum-free RPMI, and mixed with 1.5 × volume of isotonic Percoll solution (IPS) (Percoll:PBS, 9:1 v/v, p = 1.123 g/mL). Thereafter, PBMCs were overlaid carefully with Percoll–RPMI solution 1 (IPS:RPMI, p = 1.064 g/mL) and Percoll–RPMI solution 2 (IPS:RPMI, p = 1.032 g/mL). Monocytes were collected from the RPMI–Percoll interface after centrifugation at 2,000 × g for 50 min at 20°C. The purity of monocytes was 79–87% as determined by flow cytometric quantification of CD14+-positive cells. Monocytes were seeded into 24-well plates at 0.5–1 × 106 cells/well and cultured for 96 h in complete RPMI 1640 medium supplemented with 10% heat-inactivated FBS (HyClone, Thermo Fisher Scientific,), 50 μM of β-mercaptoethanol, 110 mg/L of 2 mM of L-glutamine, 1 × penicillin–streptomycin (PanEco, Russia) in the presence of GM-CSF (100 ng/mL), and IL-4 (50 ng/mL) (Sigma) in 5% CO2 in air at 37°C. Maturation of DC was induced by pulsing with LPS; immature DCs were incubated with LPS (100 ng/mL) and LPS plus OfHz (15 μg/mL). DCs were harvested 36 h later and supernatants collected for immunoassays; aliquots of supernatants were stored at −80°C for cytokine analysis.
Immunoassays for DC Markers and Secreted Cytokines
The levels of the co-stimulatory and maturation markers CD40, CD83, and CD86 on human DCs (40) were analyzed by flow cytometry using antibodies from BD Biosciences, San Diego, CA. Cells (104) were monitored using the Accuri C6 flow cytometer (Becton Dickinson, Franklin Lakes, NJ), and the data analyzed using the Cell Quest software (BD Biosciences). Levels of IL-10, IL-12β, TNF-α, and tumor growth factor-beta (TGF-β) were measured by enzyme-linked immunosorbent assay (ELISA) (eBioscience, Thermo Fischer Scientific).
Study Participants Diagnosed With BA
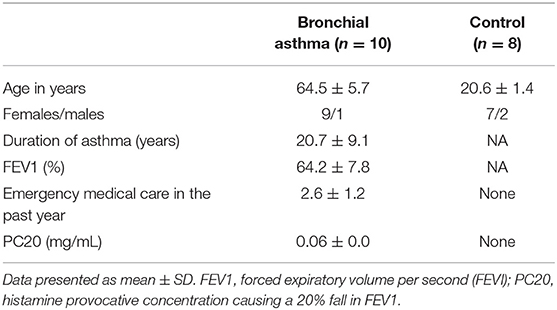
The Ethics Committee of the Siberian State Medical University, Russia, approved the study, with approval number 4815. All participants provided written consent. Blood from 10 cases with severe BA and nine healthy volunteers (personnel at the Siberian State Medical University, Russia) was examined in this study. The elderly asthma patients (>58 years old), eight females and one male, were diagnosed as BA (Table 1) according to the Global Initiative for Asthma criteria (41), using a standardized questionnaire and results of physical and laboratory examinations for more than 20 years. Non-atopic and non-smoking individuals without a family history of asthma/allergy were included as healthy controls. All of the BA cases and the healthy controls were negative for O. felineus infection, by stool examination, at the time of blood collection. Table 1 outlines the epidemiological characteristics and other records of the participants.
RNA Extraction From Human DCs and Differential Cytokine Gene Expression
Total RNAs were isolated from non-stimulated human DCs and from DCs stimulated with Hz using the TRIzol (Invitrogen, USA) extraction method followed by purification on columns (Qiagen, UK). Total RNA was reverse transcribed into cDNA following the protocol for RevertAid First-Strand cDNA Synthesis Kit (Thermo Fisher). The cDNAs were amplified using PCR with gene-specific primers designed by using CLC Main Workbench 7.0 (CLC Bio, Aarhus, Austria). Each PCR was carried out in duplicate with optimized primer concentrations using qPCRmix-HS SYBR (Evrogen, Russia) in a thermal cycler CFX-96 (Bio-Rad), with the following thermal cycling conditions: one cycle of 10 min denaturation at 95°C; 34–37 cycles of 1 min at 94°C, 1 min at 53–57°C, and 1 min at 72°C; and a final extension step at 72°C for 10 min. A dissociation curve was included in each run to ensure specificity of amplification. Beta-actin (ACTB) was used as the reference gene. The relative expression of genes was calculated using delta-delta Ct method; data were expressed as fold change compared with the untreated group (38).
Statistics
Data analysis was accomplished with the assistance of the Prism 6, GraphPad software. Cell culture data were analyzed by two-way ANOVA with Dunnett's multiple comparison test. When the variables did not show normal distribution, they were compared using the Mann–Whitney U-test (two-tailed). The Wilcoxon matched pairs test was applied to dependent samples (P ≤ 0.05 were considered to be statistically significant.
Results
Chemokine Transcript Profiles in JAWSII After OfHz Stimulation
The transcript levels encoding IL-1β, IL-10, IL-12β, and TNF-α were analyzed after JAWSII cells were pulsed with 100 nm of OfHz in PBS for 48 h. Whereas, differences in expression of IL-1β in OfHz-treated and control groups (mock and no treatment) were not seen, there were statistically significant increases in transcription of IL-10, IL-12β, and TNF-α by 6.4-, 151-, and 3.6-fold, respectively (Figure 1).
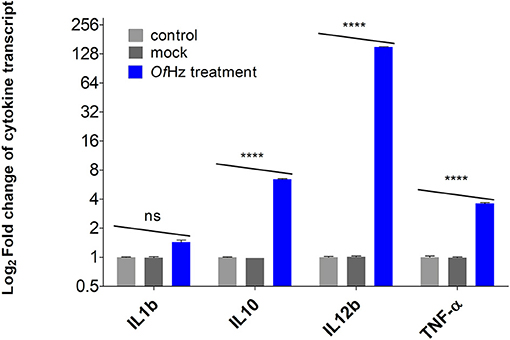
Figure 1. Log2-transformed difference fold changes in cytokine mRNA levels [OfHz treated (blue color bar)/JAWSII control cells (gray color bars)] after GAPDH normalization. Error bars represent mean and 95% CI of difference (n = 3). OfHz, Opisthorchis felineus hemozoin. ****P ≤ 0.001.
Maturation of DCs Induced by LPS Was Unaffected by OfHz
The effect of OfHz on maturation of DCs in response to LPS was investigated by analysis of hallmark markers of the mature DC phenotype (40). Monocyte-derived DCs from participants with BA and controls were stimulated with LPS alone and in combination with OfHz. CD83, CD40, and CD86 expression on DCs were measured. Expression of CD83, CD40, and CD86 was unaffected by OfHz in DCs activated by LPS (Figure 2, Table S1).
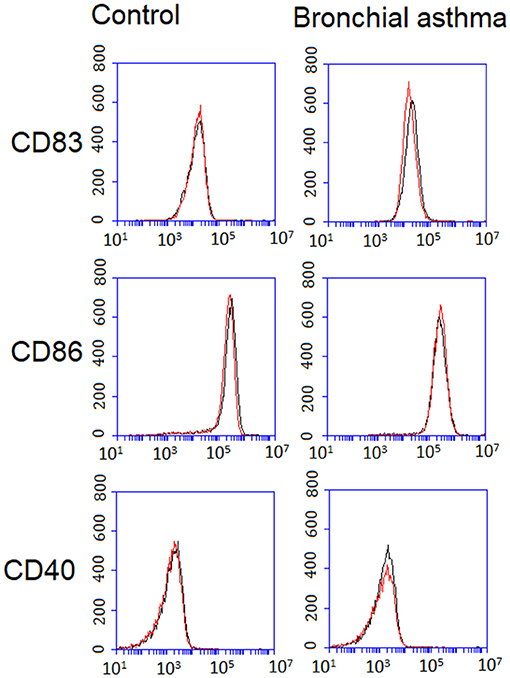
Figure 2. Expression of CD83, CD86, and CD40 on DCs from control and bronchial asthma groups. Mature DC phenotype was analyzed after LPS along stimulation (Hz–) (black line) and stimulation with LPS and OfHz combination (Hz+) (red line). Expression of CD83, CD40, and CD86 was unaffected by OfHz in DCs activated by LPS. DCs, dendritic cells; Hz, hemozoin; LPS, lipopolysaccharide.
Change of DCs Cytokines Expression and Secretion Related to OfHz Treatment
DCs from BA cases exposed in vitro to OfHz exhibited significant upregulation of expression of IL10 and IL12b. In addition, we measured the levels of IL-10, TGF-β, TNF-α, and IL-12β by ELISA in culture supernatants. Concentrations of IL-10 and IL-12β were increased in supernatants of the DC from the BA cases treated with OfHz in comparison with non-OfHz-treated DCs from BA cases. Thus, the IL-10 and IL-12b gene expression and ELISA data were in concordance. By contrast, treatment of DC donated by healthy controls with OfHz failed to affect cytokine gene expression and secretion (Figure 3).
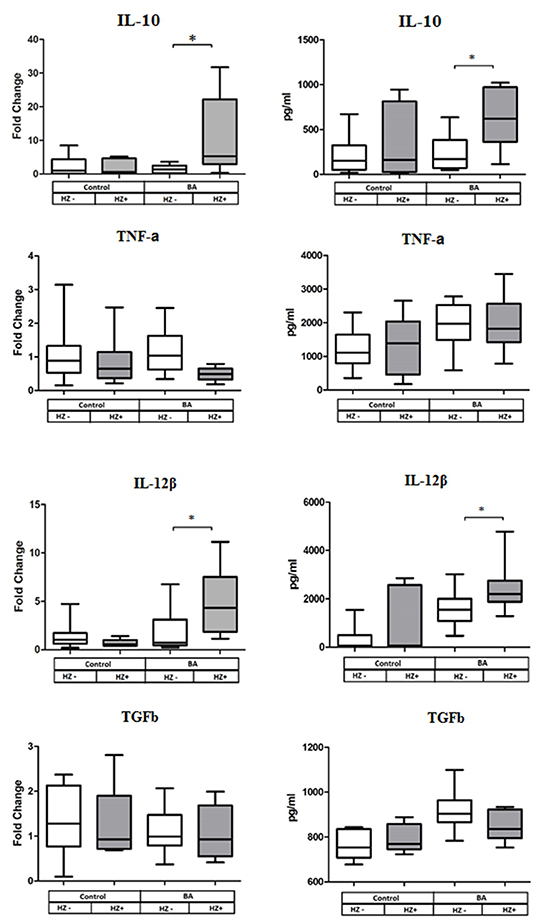
Figure 3. Cytokine gene expression (left panel of the figure) and cytokine secretion (right panel) by human DC after LPS along stimulation (Hz–) and stimulation with LPS and OfHz combination (Hz+). Data are presented as medians (Q1–Q3); the Wilcoxon matched pairs test was applied for dependent samples. BA, bronchial asthma group; DCs, dendritic cells; LPS, lipopolysaccharide; Hz, hemozoin; OfHz, Opisthorchis felineus hemozoin. *P ≤ 0.05.
Discussion
Synthetic Hz has been investigated as an adjuvant in the anti-allergen vaccines. Atopic dermatitis that develops in beagles is similar to that seen in humans and is associated with elevated titers of IgE antibodies against the house dust mite allergen, Derf2. Immunization of dogs with Derf2 together with alum and/or sHZ, with subsequent sensitization by the allergen, provoked significantly elevated levels of IgG2, but not IgG1, antibodies in the sHz-treated group that resembled a Th1-like immune response (42). Treatment of PBMCs from healthy, malaria-free donors with sHz induced increases in IL-12p40 and IL-10 transcripts at 24 h of exposure with further stabilization of the expression levels of the cytokines relative to control conditions (43). Much less in known about properties of Hz isolated from blood-feeding helminths.
This is the first report to investigate the effect of liver fluke Hz on immature mouse DC cytokine expression level and immunomodulation activity on human DC maturation and cytokine expression and secretion. Specifically, the study investigated cytokine responses in an immature DC, JAWSII cells, following OfHz stimulation to estimate OfHz immunomodulation activity. Second, using leads from the responses of these JAWSII monocytes, we investigated the ability of stimulation with OfHz to affect LPS-induced maturation of human DCs and the consequent cytokine responses. Two groups of participants were included in the study to isolate monocytes for DCs generation: healthy volunteers and BA adults. Initially, exposure to OfHz induced expression of IL-12β, IL-10, and TNF-α in JAWSII. Immune-activating and immune-suppressive effects have been reported for Hz in experiments in vitro.
DCs play critical roles in determining T-cell differentiation in the context of allergen exposure (23). Marked differences were evident between asthma and healthy control groups in the expression and release of cytokines by DCs in response to co-incubation with OfHz and LPS. OfHz potentiated the upregulation by LPS of cytokines IL-10 and IL-12β by human DC but only in the asthma group. There was no clear trend in IL-12β production by DCs in the asthma-free group in response to OfHz. In general, we observed the variable individual responses in cytokine production by DCs after OfHz exposure among the healthy control participants. Cell immunophenotypic characteristics can influence diverse responses to stimulation by Hz. Fibronectin incubated at physiologic concentrations with fibronectin-free Hz binds with high affinity to Hz. The addition of fibronectin-containing Hz to adherent monocytes induces rapid stimulation of reactive oxygen species production and increase of TNF and monocyte chemotactic protein 1 by human monocytes. These responses arise from the interaction of fibronectin with fibronectin-receptors TLR4 and integrin CD11b/CD18 (44). TLR4 plays a key role in the allergic inflammation and severity of asthma (45).We hypothesize that the prevalence of the fibronectin receptors, notably TLR4, on the DC surface impairs the effects of stimulation by OfHz.
Mature DCs secrete IL-10, IL-12, and proinflammatory cytokines, and these cytokines are known to participate in T-cell differentiation. OfHz stimulation induced IL-12b, IL-10 expression by immature DC and potentiated LPS-induced expression and production of IL-10 and IL-12β by human DCs in the BA asthma group. Asthma is a Th2-associated inflammatory disease with increased levels of IgE and Th2 cytokines. IL-12 as Th1-promoting cytokine has potential roles in the antagonism of Th2 cytokine responses and IgE synthesis that prevents the progress of airway inflammation in asthma (46). IL-10 has immunosuppressive properties, and induction of IL-10 by helminths is considered as one of the possible mechanisms of parasite survival in the anti-inflammatory environment (47). The production of Th1-promoting cytokine IL-12β and anti-inflammatory cytokine IL-10 by asthma DCs upon OfHz stimulation can suggest a role for Hz released during infection with Opisthorchis felineus in the Th1/Th2 balance regulation.
To extend the findings related to OfHz-activated cytokine expression in DC and to determine whether OfHz may be immunomodulatory, maturation markers, cytokine expression, and secretion by human DCs were investigated. OfHz did not appear to affect LPS-induced upregulation of key markers associated with a DC mature phenotype, including CD83, CD86, and CD40. It is noteworthy that exposure with crude O. felineus extract leads to the downregulation of costimulatory molecules CD83 and CD86 in LPS-induced DCs, generated from monocytes of asthma patients (48). Accordingly, OfHz may be considered as a mediator that plays a key role in the O. felineus-derived immune response. Further investigation of the local liver immune cell response to secretion and accumulation of OfHz in bile ducts might provide insights into the chronic inflammation and other hepatic morbidity due to opisthorchiasis. Moreover, investigation of OfHz in asthma model in vivo can be expected to increase our understanding of the potential benefits of treatment with OfHz for the treatment of asthma.
In conclusion, our data demonstrate that OfHz induces expression of Th1 cytokines in immature mouse DC and that OfHz potentiates LPS-induced expression and production of IL-10 and IL-12β by DC in asthma patients. These results complement earlier findings that demonstrated the regulation of the host immune response by the helminth ES products, which play a protective role against allergy and allergic diseases (49), including findings in regions endemic for O. felineus, and that demonstrated the inverse relationship between opisthorchiasis and allergic diseases (31).
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by The Ethics Committee of the Siberian State Medical University, Russia. The patients/participants provided their written informed consent to participate in this study. The animal study was reviewed and approved by The Ethics Committee of the Siberian State Medical University, Russia.
Author Contributions
IS conceived and planned the experiments, analyzed the data, contributed to the interpretation of the results, and wrote the manuscript with input from all authors. WI performed the experiments, analyzed the data, and wrote the manuscript with input from all authors. YD, KN, WI, and VI performed the experiments. VI were involved in planning and supervised the work. EK and NK contributed to patients sample collection. AP and VM provided critical feedback and helped shape the research. PB supervised the project and wrote the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
IS thanks the Fulbright Visiting Scholar program for support and acknowledges support of grant number 18-75-00036 from Russian Science Foundation (IS). We gratefully acknowledge the support of colleagues and collaborations in the Tomsk OPIsthorchiasis Consortium, TOPIC, www.topic-global.org. We also acknowledge support of award R01CA164719 (WI, VM and PB) from the National Cancer Institute (NCI) and P50AI098639 (PB) from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH). The contents are solely the responsibility of the authors and do not necessarily represent the official views of these sponsors.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2019.00332/full#supplementary-material
References
1. Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. (2003) 3:733–44. doi: 10.1038/nri1183
2. Maizels RM, McSorley HJ. Regulation of the host immune system by helminth parasites. J Allergy Clin Immunol. (2016) 138:666–75. doi: 10.1016/j.jaci.2016.07.007
3. Heylen M, Ruyssers NE, Gielis EM, Vanhomwegen E, Pelckmans PA, Moreels TG, et al. Of worms, mice and man: an overview of experimental and clinical helminth-based therapy for inflammatory bowel disease. Pharmacol Ther. (2014) 143:153–67. doi: 10.1016/j.pharmthera.2014.02.011
4. Broadhurst MJ, Leung JM, Kashyap V, McCune JM, Mahadevan U, McKerrow JH, et al. IL-22+ CD4+ T cells are associated with therapeutic trichuris trichiura infection in an ulcerative colitis patient. Sci Transl Med. (2010) 2:60ra88. doi: 10.1126/scitranslmed.3001500
5. Pineda MA, Al-Riyami L, Harnett W, Harnett MM. Lessons from helminth infections: ES-62 highlights new interventional approaches in rheumatoid arthritis. Clin Exp Immunol. (2014) 177:13–23. doi: 10.1111/cei.12252
6. Shepherd C, Navarro S, Wangchuk P, Wilson D, Daly NL, Loukas A. Identifying the immunomodulatory components of helminths. Parasite Immunol. (2015) 37:293–303. doi: 10.1111/pim.12192
7. Alvarado R, O'Brien B, Tanaka A, Dalton JP, Donnelly S. A parasitic helminth-derived peptide that targets the macrophage lysosome is a novel therapeutic option for autoimmune disease. Immunobiology. (2015) 220:262–9. doi: 10.1016/j.imbio.2014.11.008
8. Doonan J, Lumb FE, Pineda MA, Tarafdar A, Crowe J, Khan AM, et al. Protection against arthritis by the parasitic worm product ES-62, and its drug-like small molecule analogues, is associated with inhibition of osteoclastogenesis. Front Immunol. (2018) 9:1016. doi: 10.3389/fimmu.2018.01016
9. Dastpeyman M, Bansal PS, Wilson D, Sotillo J, Brindley PJ, Loukas A, et al. Structural variants of a liver fluke derived granulin peptide potently stimulate wound healing. J Med Chem. (2018) 61:8746–53. doi: 10.1021/acs.jmedchem.8b00898
10. Goldie P, Roth EF, Oppenheim J, Vanderberg JP. Biochemical characterization of Plasmodium falciparum hemozoin. Am J Trop Med Hyg. (1990) 43:584–96. doi: 10.4269/ajtmh.1990.43.584
11. Oliveira MF, Kycia SW, Gomez A, Kosar AJ, Bohle DS, Hempelmann E, et al. Structural and morphological characterization of hemozoin produced by Schistosoma mansoni and Rhodnius prolixus. FEBS Lett. (2005) 579:6010–6. doi: 10.1016/j.febslet.2005.09.035
12. Jiang Y, Xue X, Chen X, Zhuang W, Sun J, Shen L, et al. Hemozoin from Schistosoma japonicum does not affect murine myeloid dendritic cell function. Parasitol Res. (2010) 106:653–9. doi: 10.1007/s00436-009-1717-1
13. Xiao SH, Sun J. Schistosoma hemozoin and its possible roles. Int J Parasitol. (2017) 47:171–83. doi: 10.1016/j.ijpara.2016.10.005
14. Truscott M, Evans DA, Gunn M, Hoffmann KF. Schistosoma mansoni hemozoin modulates alternative activation of macrophages via specific suppression of Retnla expression and secretion. Infect Immun. (2013) 81:133–42. doi: 10.1128/IAI.00701-12
15. Pershina AG, Saltykova IV, Ivanov VV, Perina EA, Demin AM, Shevelev OB, et al. Hemozoin “knobs” in Opisthorchis felineus infected liver. Parasites Vectors. (2015) 8:459. doi: 10.1186/s13071-015-1061-5
16. Lvova M, Zhukova M, Kiseleva E, Mayboroda O, Hensbergen P, Kizilova E, et al. Hemozoin is a product of heme detoxification in the gut of the most medically important species of the family Opisthorchiidae. Int J Parasitol. (2016) 46:147–56. doi: 10.1016/j.ijpara.2015.12.003
17. Olliaro P, Lombardi L, Frigerio S, Basilico N, Taramelli D, Monti D. Phagocytosis of hemozoin (native and synthetic malaria pigment), and Plasmodium falciparum intraerythrocyte-stage parasites by human and mouse phagocytes. Ultrastruct Pathol. (2000) 24:9–13. doi: 10.1080/019131200281264
18. Skorokhod OA, Alessio M, Mordmüller B, Arese P, Schwarzer E. Hemozoin (malarial pigment) inhibits differentiation and maturation of human monocyte-derived dendritic cells: a peroxisome proliferator-activated receptor-gamma-mediated effect. J Immunol. (2004) 173:4066–74. doi: 10.4049/jimmunol.173.6.4066
19. Bujila I, Schwarzer E, Skorokhod O, Weidner JM, Troye-Blomberg M, Östlund Farrants AK. Malaria-derived hemozoin exerts early modulatory effects on the phenotype and maturation of human dendritic cells. Cell Microbiol. (2016) 18:413–23. doi: 10.1111/cmi.12521
20. Keller CC, Yamo O, Ouma C, Ong'echa JM, Ounah D, Hittner JB, et al. Acquisition of hemozoin by monocytes down-regulates interleukin-12 p40 (IL-12p40) transcripts and circulating IL-12p70 through an IL-10-dependent mechanism: in vivo and in vitro findings in severe malarial anemia. Infect Immun. (2006) 74:5249–60. doi: 10.1128/IAI.00843-06
21. Wills-Karp M, Santeliz J, Karp CL. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat Rev Immunol. (2001) 1:69–75. doi: 10.1038/35095579
22. Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. (2002) 296:490–4. doi: 10.1126/science.296.5567.490
23. Rook GA. Review series on helminths, immune modulation and the hygiene hypothesis: the broader implications of the hygiene hypothesis. Immunology. (2009) 126:3–11. doi: 10.1111/j.1365-2567.2008.03007.x
24. Wu Z, Wang L, Tang Y, Sun X. Parasite-derived proteins for the treatment of allergies and autoimmune diseases. Front Microbiol. (2017) 8:2164. doi: 10.3389/fmicb.2017.02164
25. Hamid F, Versteeg SA, Wiria AE, Wammes LJ, Wahyuni S, Supali T, et al. Molecular diagnostics and lack of clinical allergy in helminth-endemic areas in Indonesia. J Allergy Clin Immunol. (2017) 140:1196–9.e6. doi: 10.1016/j.jaci.2017.04.040
26. Pacífico LG, Marinho FA, Fonseca CT, Barsante MM, Pinho V, Sales-Junior PA, et al. Schistosoma mansoni antigens modulate experimental allergic asthma in a murine model: a major role for CD4+ CD25+ Foxp3+ T cells independent of interleukin-10. Infect Immun. (2009) 77:98–107. doi: 10.1128/IAI.00783-07
27. Dittrich AM, Erbacher A, Specht S, Diesner F, Krokowski M, Avagyan A, et al. Helminth infection with Litomosoides sigmodontis induces regulatory T cells and inhibits allergic sensitization, airway inflammation, and hyperreactivity in a murine asthma model. J Immunol. (2008) 180:1792–9. doi: 10.4049/jimmunol.180.3.1792
28. Ogorodova LM, Fedorova OS, Sripa B, Mordvinov VA, Katokhin AV, Keiser J, et al. Opisthorchiasis: an overlooked danger. PLoS Negl Trop Dis. (2015) 9:e0003563. doi: 10.1371/journal.pntd.0003563
29. Fedorova OS, Kovshirina YV, Kovshirina AE, Fedotova MM, Deev IA, Petrovskiy FI, et al. Opisthorchis felineus infection and cholangiocarcinoma in the Russian Federation: a review of medical statistics. Parasitol Int. (2017) 66:365–71. doi: 10.1016/j.parint.2016.07.010
30. Ogorodova LM, Freidin MB, Sazonov AE, Fedorova OS, Gerbek IE, Cherevko NA, et al. A pilot screening of prevalence of atopic states and opisthorchosis and their relationship in people of Tomsk Oblast. Parasitol Res. (2007) 101:1165–8. doi: 10.1007/s00436-007-0588-6
31. Fedorova OS, Janse JJ, Ogorodova LM, Fedotova MM, Achterberg RA, Verweij JJ, et al. Opisthorchis felineus negatively associates with skin test reactivity in Russia-EuroPrevall-International Cooperation study. Allergy. (2017) 72:1096–104. doi: 10.1111/all.13120
32. Saltykova IV, Ogorodova LM, Bragina EY, Puzyrev VP, Freidin MB. Opisthorchis felineus liver fluke invasion is an environmental factor modifying genetic risk of atopic bronchial asthma. Acta Trop. (2014) 139:53–6. doi: 10.1016/j.actatropica.2014.07.004
33. Daniłowicz-Luebert E, Steinfelder S, Kühl AA, Drozdenko G, Lucius R, Worm M, et al. A nematode immunomodulator suppresses grass pollen-specific allergic responses by controlling excessive Th2 inflammation. Int J Parasitol. (2013) 43:201–10. doi: 10.1016/j.ijpara.2012.10.014
34. Kitagaki K, Businga TR, Racila D, Elliott DE, Weinstock JV, Kline JN. Intestinal helminths protect in a murine model of asthma. J Immunol. (2006) 177:1628–35. doi: 10.4049/jimmunol.177.3.1628
35. Qiu S, Fan X, Yang Y, Dong P, Zhou W, Xu Y, et al. Schistosoma japonicum infection downregulates house dust mite-induced allergic airway inflammation in mice. Fan G-C, editor. PLoS ONE. (2017) 12:e0179565. doi: 10.1371/journal.pone.0179565
36. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. (1959) 37:911–7. doi: 10.1139/y59-099
37. Chomczynski P, Wilfinger W, Kennedy A, Rymaszewski M, Mackey K. RNAzol® RT: a new single-step method for isolation of RNA. Nat Methods. (2010) 7:4–5. doi: 10.1038/nmeth.f.315
38. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(–delta delta C(T)) Method. Methods. (2001) 25:402–8. doi: 10.1006/meth.2001.1262
39. Yuryeva K, Saltykova I, Ogorodova L, Kirillova N, Kulikov E, Korotkaya E, et al. Expression of adenosine receptors in monocytes from patients with bronchial asthma. Biochem Biophys Res Commun. (2015) 464:1314–20. doi: 10.1016/j.bbrc.2015.07.141
40. Blüml S, Kirchberger S, Bochkov VN, Krönke G, Stuhlmeier K, Majdic O, et al. Oxidized phospholipids negatively regulate dendritic cell maturation induced by TLRs and CD40. J Immunol. (2005) 175:501–8. doi: 10.4049/jimmunol.175.1.501
41. Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald JM, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. (2008) 31:143–78. doi: 10.1183/09031936.00138707
42. Coban C, Yagi M, Ohata K, Igari Y, Tsukui T, Horii T, et al. The malarial metabolite hemozoin and its potential use as a vaccine adjuvant. Allergol Int. (2010) 59:115–24. doi: 10.2332/allergolint.10-RAI-0194
43. Ong'echa JM, Remo AM, Kristoff J, Hittner JB, Were T, Ouma C, et al. Increased circulating interleukin (IL)-23 in children with malarial anemia: in vivo and in vitro relationship with co-regulatory cytokines IL-12 and IL-10. Clin Immunol. (2008) 126:211–21. doi: 10.1016/j.clim.2007.08.007
44. Barrera V, Skorokhod OA, Baci D, Gremo G, Arese P, Schwarzer E. Host fibrinogen stably bound to hemozoin rapidly activates monocytes via TLR-4 and CD11b/CD18-integrin: a new paradigm of hemozoin action. Blood. (2011) 117:5674–82. doi: 10.1182/blood-2010-10-312413
45. Li M, Wang ZN, Yang LF, Yan Y, Cai LM, Li YT, et al. TLR4 antagonist suppresses airway remodeling in asthma by inhibiting the T-helper 2 response. Exp Ther Med. (2017) 14:2911–6. doi: 10.3892/etm.2017.4898
46. Mobley JL, Chin JE, Richards IM. Cytokine networks in allergic lung inflammation: an opportunity for drug intervention. Expert Opin Investig Drugs. (1997) 6:1–6. doi: 10.1517/13543784.6.1.1
47. Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. (2008) 180:5771–7. doi: 10.4049/jimmunol.180.9.5771
48. Kremer E, Ogorodova LM, Kirillova NA, Khvorilova KV, Perevozchikova TV, Fait EA. Immunophenotypic characteristic of dendritic cells in bronchial asthma in conditions of extract Opisthorchis felineus in vitro. Vestn Ross Akad Med Nauk. (2013) 5:66–70. doi: 10.15690/vramn.v68i5.665
Keywords: Th1, immunoregulation, cytokine, asthma, dendritic cell, hemozoin, Opisthorchis
Citation: Saltykova IV, Ittiprasert W, Nevskaya KV, Dorofeeva YB, Kirillova NA, Kulikov ES, Ivanov VV, Mann VH, Pershina AG and Brindley PJ (2019) Hemozoin From the Liver Fluke, Opisthorchis felineus, Modulates Dendritic Cell Responses in Bronchial Asthma Patients. Front. Vet. Sci. 6:332. doi: 10.3389/fvets.2019.00332
Received: 17 July 2019; Accepted: 16 September 2019;
Published: 16 October 2019.
Edited by:
Yadong Zheng, State Key Laboratory of Veterinary Etiological Biology, Lanzhou Institute of Veterinary Research (CAAS), ChinaReviewed by:
Xianyong Liu, China Agricultural University (CAU), ChinaSi-Yang Huang, Yangzhou University, China
Copyright © 2019 Saltykova, Ittiprasert, Nevskaya, Dorofeeva, Kirillova, Kulikov, Ivanov, Mann, Pershina and Brindley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Irina V. Saltykova, aXJhLnNhbHRpa292YUBtYWlsLnJ1; Paul J. Brindley, cGJyaW5kbGV5QGd3dS5lZHU=
 Irina V. Saltykova
Irina V. Saltykova Wannaporn Ittiprasert
Wannaporn Ittiprasert Kseniya V. Nevskaya
Kseniya V. Nevskaya Yulia B. Dorofeeva
Yulia B. Dorofeeva Natalia A. Kirillova2
Natalia A. Kirillova2 Evgeniy S. Kulikov
Evgeniy S. Kulikov