
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Vet. Sci. , 13 June 2019
Sec. Veterinary Infectious Diseases
Volume 6 - 2019 | https://doi.org/10.3389/fvets.2019.00188
This article is part of the Research Topic The Animal Microbiome in Health and Disease View all 14 articles
As antibiotics continue to be phased out of livestock production, alternative feed amendments have received increased interest not only from a research standpoint but for commercial application. Most of the emphasis to date has focused on food safety aspects, particularly on lowering the incidence of foodborne pathogens in livestock. Several candidates are currently either being examined or are already being implemented in commercial settings. Among these candidates are chemical compounds such as formaldehyde. Formaldehyde has historically been used to inhibit Salmonella in feeds during feed processing. Currently, there are several commercial products available for this purpose. This review will cover both the historical background, current research, and prospects for further research on the poultry gastrointestinal tract and feeds treated with formaldehyde.
Treatment of animal feeds has always been considered a critical component to food animal management to prevent the formation of mycotoxins and other biological contributors to contamination and feed quality decline during storage (1). There have been numerous research studies and applications for various chemicals to be added to animal and poultry feeds during feed processing, and these have been well-documented in published review articles over the past few decades (1–5). Not surprisingly much of the focus for the application of chemical additives particularly in poultry feeds has been directed toward limiting Salmonella in the feed (2–7). Research studies have ranged from the assessment of feed contamination at the feed mill to bird feeding trials and involved both natural Salmonella contamination and inoculation of a marker Salmonella strain.
In order for a particular chemical feed additive to possess commercial attractiveness to be promoted for routine use in animal and poultry feeds, several criteria essential to meet this demand would have to be considered. Some of these specifically for feed antimicrobials have been outlined previously (3) but would still apply in a general sense. Effectiveness in the presence of a high organic load that is characteristic of a typical mixed feed and/or individual feed ingredient would be a must. The effective dose would have to be safe in the target animal and not result in undesired residues in animal products. The relative cost to be applied to large bulk quantities of feed would also need to be of a commercial scale level of utility as well as ease of application and minimal damage to milling equipment. Governmental regulatory approval both domestically in the United States and internationally for use in animal diets should be in place. The worker safety during application in the feed mill and post-milling, delivery to the farm and use at the farm would have to be established.
In this review a discussion of Salmonella occurrence in feeds will be described in brief, followed by discussion of one of the more prominent and widespread used chemical group of compounds, namely, aldehydes with the primary focus on formaldehyde/formalin in terms of antimicrobial mechanism(s) and efficacy as feed additives in the poultry gastrointestinal tract. Finally, future directions for application and improving efficacy will be discussed.
In general aldehydes are relatively ubiquitous in the environment originating not only as a natural compound but as an intermediary endogenous product in biological metabolism and other processes as well-generation from automobile exhaust gases and indoor environments from sources such as building materials and furniture (8–10). The chemistry and pathways for their formation have been extensively discussed in a review by O'Brien et al. (8) and will not be discussed in detail in the current review. Numerous aldehydes including formaldehyde are detectable in a wide range of foods including fruits, vegetables, meat, cheese, and seafood (8, 11, 12). Aldehydes can be detected in the air, feed, tissue, and feces via personal monitors, spectrophotometric measurement of color reaction between tissue distillate, and chromatographic-sulphuric acid reaction, respectively (13). They can be formed as volatile aldehydes during cooking, particularly from edible oils, auto oxidation of unsaturated fatty acids, odor compounds emanating from rancid high-fat foods, and occurring as products from the storage of beer (12). Aldehydes and ketones are known to increase during milk thermal processing and storage of milk powder, resulting in changes in flavor and milk powder porosity (14). Formaldehyde in foods is released in the stomach and absorbed into the bloodstream where it is metabolized to formic acid by the red blood cells. Formic acid is further metabolized to carbon dioxide and water (15, 16). The metabolic half-life of formaldehyde is 60 to 90 s. This route of metabolism may be similar for other aldehydes. Aldehydes are also an important set of useful compounds for industrial processes such as flavors, fragrances, and pharmaceutical precursors. In addition, efforts have been made to genetically modify microorganisms to accumulate sufficient quantities for commercial purposes (17, 18).
Formaldehyde can serve as a fixative preventing cell autolysis and reacting with proteins, lipids and nucleic acids (19–21). The interaction of formaldehyde with peptides has been characterized by Metz et al. (17) as occurring via formation of either methylol groups, Schiff bases, or methylene bridges. Methylol and Schiff base modifications are considered reversible whereas methylene bridge products are stable and can lead to cross-linking of protein chains (17, 22–24). The type of bond formed between formaldehyde and protein/ amino acids is dependent on the reaction conditions (25).
The reaction of formaldehyde with aqueous solutions of crystalline amino acids (98:2 ratio) at 24°C resulted in the formation of a compound (described as an adduct) exhibited antimicrobial activity against E. coli and Salmonella (26). Only lysine, arginine, histidine and asparagine were reported to form the adduct. The bond between lysine and formaldehyde was found to be reversible and was broken by distillation in a mildly acidic solution suggesting a methylol or Schiff base linkage. This is consistent with the findings of Alexander et al. (25) that reported that methylol derivatives of formaldehyde and amino groups are unstable and dissociate under mildly acidic conditions. Additional research by Barlow (27) and Rude et al. (28) indicate formaldehyde added to fishmeal or corn amended with crystalline lysine under mild reaction conditions (ambient temperature) does not affect availability at the 3 kg/ton level.
The ability of formaldehyde to form methylene bridges and cross-link protein was first utilized to improve the elasticity of wool. Intensive research has been conducted in this area and various reaction conditions utilized. Reaction conditions required to cross-link amino have been found to be dependent on the ratio of formaldehyde to protein, reaction temperature, reaction time and pH (25). Theis and Jacoby (29) first reported that protein could be cross-linked by formaldehyde when a 3:2 ratio of amino acid to formaldehyde was incubated at 60°C for 30 min, but that at a 3:1 ratio of amino acid, the bond was reversible. Other researchers have utilized higher reaction temperatures (up to 100°C), longer reaction times (up to 24 h) and higher formaldehyde to protein ratios to form a cross-linked protein (25, 30). However, the interaction of formaldehyde with proteins may be somewhat more complex and variable compared to isolated peptides. For example, formaldehyde peptide cross-linking has been examined in more detail by Toews et al. (31) who reported that some regions within proteins are more susceptible to formaldehyde cross-linking than other regions of the respective proteins, and the variation in three dimensional structures of proteins dictate relative reactivities to formaldehyde (31).
Regardless of the exact mechanism(s) in which proteins are cross-linked, exposure of proteins to formaldehyde results in decreased water sensitivity, and increased resistance to chemical and enzyme exposure (22). This has been used for several practical applications in biology. Historically, formaldehyde has been used as a tissue fixative for clinical sample preservation that ensures stability for several years (32, 33). The ability to modify proteins has been taken advantage in the process of inactivating bacterial toxins and viruses for generating vaccines (17). Formaldehyde has also been implemented as a means to stabilize and retain intact whole cells, particularly bacteria. This has been used to preserve a consistent set of rumen bacterial cells to serve as an agent for immunization in layer hens to generate egg yolk polyclonal antibodies (34). Fixation of bacterial cells harvested after growth in large scale growth vessels and subsequent addition of thimerosol allowed for extended frozen storage of whole intact cells without the growth of bacterial contaminants until they could be used to immunize hens (35).
Using formaldehyde to stabilize bacterial cells has benefits for other types of studies where retaining intact whole cells may be critical. For example, formalin solutions have also been used to harvest and preserve rumen bacterial cells after continuous culture growth studies for cell dry weight determinations (36–38). Isaacson et al. (36) incorporated formalin fixation as part of the recovery process due to concerns over cell lysis occurring during the centrifugation and washing steps of mixed cultures that could impact the accuracy of dry weight estimates of rumen bacterial populations from continuous cultures. They concluded that the addition of formalin did not alter the dry weight results appreciably to impact the interpretation of the dry cell data. In a more recent study, Baker et al. (39) used formalin solutions to preserve pathogenic Escherichia coli strains for use in flow cytometry detection. In their study, there was a need to standardize an immuno-based flow cytometry analyses with known quantities of particular pathogenic E. coli pure culture isolates to serve as standards before assessing food samples. In this particular study, they demonstrated that formalin preserved sets of E. coli could be spiked into ground beef samples, recovered, quantified by both quantitative polymerase chain reaction and flow cytometry, and demonstrated that the two methods did not statistically differ from each other. They concluded that formalin fixed solutions of pathogenic E. coli could serve as internal standards for calibrating flow cytometry-based assays by providing stable known quantities of E. coli cells.
Given the ability of formaldehyde to interact with macromolecules and serve as a fixative agent for bacterial cells it is not surprising the formaldehyde would be a potential antimicrobial compound. Glutaraldehyde-based chemicals have been used for sterilization in clinical settings such as dental, medical and veterinary surgical facilities (40). Glutaraldehydes have also been employed as disinfectant sprays in broiler and animal housing and livestock transportation vehicles for limiting bacterial and viral contamination (41–45). Formaldehyde fumigation has been used for eggshell surface decontamination, but hazard concerns have motivated research for alternative methods that are as effective as formaldehyde in reducing bacterial loads even though formaldehyde remains one of the more effective antibacterials that are available (46–52). While it has been noted by Carrique-Mas et al. (53) that there are concerns regarding the safety of formaldehyde to humans, in order to reduce occupational exposure, formaldehyde is applied in an enclosed system [mixer/enclosed auger; USDA (54)]. In a recent risk assessment, the European Food Safety Authority indicated that formaldehyde would not be considered a risk to humans when employed as an animal nutrition product, but anyone handling the product should avoid exposure to the respiratory tract, skin, and eyes (3, 55, 56).
Historically only limited microbial data responses mostly based on culture methods have been generated for evaluating aldehyde disinfectants in poultry operations (46–52). Consequently, microbial profiling is confined to which media is used, the respective selective processes, and the segment of the microbial population capable of forming visible colonies. Now that microbiome sequencing has become routine, more comprehensive microbial community profiling has become possible to conduct a comparative assessment of disinfectant treatments on microbial populations such as those that inhabit poultry houses. For example, Jiang et al. (45) compared different disinfectant sprays and reported that glutaraldehyde not only reduced overall airborne bacterial contamination in empty broiler houses but based on 16S rDNA sequencing using an Illumina HiSeq sequencer, decreased the number of detectable phyla by nearly half (from 32 phyla to 17 phyla) compared to the non-disinfected house. Phyla diversity was even more substantially decreased (6 phyla detected) when a disinfectant mixture (aldehyde, alcohol, and quaternary ammonium salt) was used leading the authors to suggest a much broader antibacterial spectrum for the disinfection mixture. In future studies, it would be of interest to conduct metagenomic profiling to determine the frequency of antibacterial resistance genes in these microbial populations that are specific to certain disinfectants being implemented routinely.
Formaldehyde was first utilized in the animal feed sector as a mold inhibitor for the preservation of high moisture corn (57). Formaldehyde has also been used extensively as a feed chemical antimicrobial to reduce Salmonella and improve general bacterial hygiene in feeds [Figure 11, (3, 4, 53)]. In general, potential cell targets of formaldehyde include the spore cores of bacterial spores, the cell walls of bacteria, and the amino groups of fungi (58, 59). The antimicrobial activity of glutaraldehyde and formaldehyde is believed to be elicited primarily by both the formation of a Schiff base product and irreversible cross-linking of proteins, RNA, and DNA in bacteria and of proteins in feeds (3, 4, 26, 53, 58, 59).
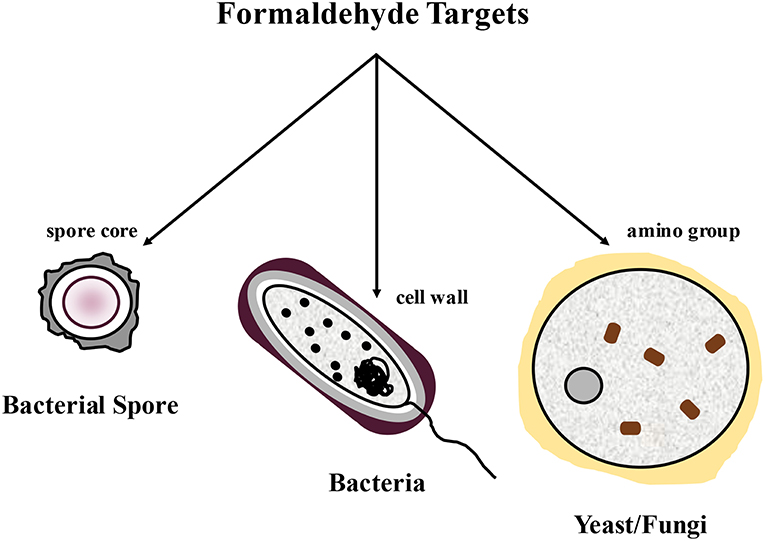
Figure 1. The impact of the use of formaldehyde on bacterial spores, bacteria, and yeast/fungus by targeting key components.
Unlike some of the other feed additive acids that have been used over the years, little bacteriological work has been conducted to determine mechanisms of formaldehyde exposure on Salmonella. Temcharoen and Thilly (60) examined toxic and mutagenic effects of formaldehyde in a mutant Salmonella Typhimurium test strain that lacked either membrane translocation or phosphoribosyl-transferase. The basic concept in using the Salmonella tester strain (his+ revertant of an Ames Salmonella tester strain) is that if a particular compound is mutagenic then the histidine auxotrophic version of the tester strain will revert to a version that no longer requires histidine and can grow on media plates without histidine supplementation (61). Based on their results, Temcharoen and Thilly (60) concluded that formaldehyde was toxic and mutagenic to the S. Typhimurium tester strain and the minimum concentration required to induce mutagenicity was 0.167 mM. They hypothesized that formaldehyde may lead to mutations either by direct interaction with the bacterial cell's DNA, or reacting with amino groups, simple amines, amino acids, nucleic acids, or proteins to form mutagenic product(s).
As of date, there is no clear evidence linking the use of formaldehyde in poultry operations to the expression of resistance factors in Salmonella. For example, when Salmonella isolates exposed to different disinfectants including formaldehyde in Danish broiler houses were characterized by Gradel et al. (62) for minimum inhibitory concentrations (MIC), no clear-cut association could be detected among serovar persistences, tendencies to persist, or use of a particular disinfectant. Likewise, S. Enteritidis isolates from egg-laying flocks where a quaternary ammonium-formaldehyde disinfectant was used also did not exhibit alterations in susceptibility/resistance responses (63). This again proved to be true in Salmonella isolates known to be persistent in a fish feed plant (64) where even though these isolates had been exposed to a commercial organic acid- formaldehyde mixture they were no more resistant to disinfectants than Salmonella isolates from other sources. In feed applications as a chemical antimicrobial additive, formaldehyde is unlikely to directly interact with Salmonella cells in a fashion similar to the pure culture Salmonella incubations conducted by Temcharoen and Thilly (60) as described above. Instead, it is much more likely to chemically interact with the proteins present in feeds upon exposure and potentially affecting bird performance.
Biological contamination of feeds by organisms has always been considered a complex issue with numerous factors influencing levels and types of organisms likely to be present on a particular feed or feed ingredient at any given time or location as previously discussed (1, 3, 65–67). Although few conclusions can be drawn, the microbial composition associated with animal and poultry feeds can be quite diverse (1, 3, 68). Prokaryotes, bacteriophage, fungi, and yeast have all been identified in feeds and in some cases isolated from a wide range of feeds (1–3, 69–72). Detecting particular patterns or critical factors that dictate specific bacterial and/or non-bacterial populations associated with feed remains elusive. Indeed, factors such as environmental conditions during storage and subsequent feeding to animals, storage time, and feed treatments would be expected to contribute to the final composition of a feed or feed ingredient but to what degree and what other factors may be involved remains unknown. As molecular techniques develop, it is conceivable that such methods could be employed to begin comprehensive studies that establish signature populations in the feed that do correlate with certain influential factors and potentially identify which factors are most critical to certain feed processing operations.
Among the bacterial contaminants potentially present in animal and poultry feeds several organisms would also be considered foodborne pathogens that could cause disease in humans. These include Salmonella, Clostridium perfringens, Clostridium botulinum, and Listeria, some of which have been more frequently identified with feed than others (2, 3, 73–77). Of the foodborne pathogens isolated from feeds, foodborne Salmonella serovars have received the most attention particularly with poultry feeds and feed ingredients and remain an issue for all aspects of vertically integrated poultry operations (6, 78–82). Salmonella-contaminated feed certainly has to be considered a potential risk factor for salmonellosis.
Poultry feed has been known to be a source of Salmonella since 1948 (83). In integrated operations, Salmonella control typically begins at the breeder level (84). Snoeyenbos (85) reported that the transmission of Salmonella in breeder eggs occurred with sufficient frequency to require control measures for Salmonella at the breeder and multiplier level. Wilding and Baxter-Jones (86) estimated that colonization of one breeder/multiplier by Salmonella might affect 65 broilers. Shapcott (87) reported that the presence of Salmonella in breeder feed might impact the transmission to broiler chicks. After implementing a rigorous program for the control of Salmonella at the breeder farm, the hatchery and the feed mill, both the broiler and breeder operations were Salmonella negative for >3 years. However, in June of 1980, a single breeder feed tested positive for Salmonella Sofia. Within 1 year, 100% of the flocks tested positive for S. Sofia. Jenson and Rosales (88) reported that 80% of the Salmonella serotypes found in breeder feed might be detected weeks later in breeder birds or their offspring.
The significance of Salmonella in feed and animal produce is less understood. There are many vectors for Salmonella transmission to poultry and animal produce, including breeders, hatchery, farm, feed mill, and the processing plant. Morris et al. (89) first discussed Salmonella in feed and its association with processing plant contamination. Of 12 serotypes of Salmonella isolated from the processing plant, six isolates were also present in feed. Only S. Montevideo isolates displayed a relationship in the frequency of detection between the feed mill and processing plant. Lahellec and Collins (90) reported that 8 of 16 serotypes of Salmonella isolated from the processing plant were found in feed. In a 3 year study of a large integrated broiler operation, McKenzie and Bains (91) observed that Salmonella in broiler carcasses displayed a 100% correlation with Salmonella in feed ingredients and grains. In Europe where Salmonella contamination rates of feed are low (<2%), Davies et al. (92) used a slightly different approach to determine if Salmonella in feed was associated with processing plant contamination. During a 2 year study, samples of dust and residues from feed mills of two large integrated broiler operations were analyzed for Salmonella. Corry et al. (93) compared isolated serotypes from feed to those present at the processing plant and found that 55% of Salmonella isolates from the processing plant originated from the feed. The connection of potential for salmonellosis to feed has been made in other ways as well. As an illustration of this particular point, Bucher et al. (94) characterized Salmonella isolates from chicken nuggets, strips, and pelleted broiler feed and concluded that Salmonella strains isolated from broiler feed were indistinguishable from isolates recovered from packaged raw, frozen chicken nuggets, and strips. Similar observations have been noted in commercial egg operations. Shirota et al. (95, 96) reported that both the frequency and the serotypes of Salmonella in feed were correlated to the frequency and serotypes of Salmonella in eggs (58% of egg isolates was identical to feed isolates). The authors of these studies concluded that Salmonella contamination of carcasses and egg contamination could be significantly reduced by minimizing the incidence of Salmonella in the feed. This would suggest that Salmonella possesses the capability of being transmitted from feed production, broiler growout/egg production, poultry processing and eventually retail establishments.
As a result of the widespread prevalence of Salmonella spp. in the environment and its capacity for survival under relatively harsh conditions such as increases in temperature (97–99) it is not surprising that Salmonella would come in contact with different stages of feed production all the way from cereal grain harvesting to feed milling and in turn lead to cross-contamination in places such as feed mills (3, 6, 78, 80, 81, 100–105). It is clear that better tracking methods will be needed to pinpoint ultimate origins for particular Salmonella spp., but this will be somewhat of a challenge given the high number of serovars that have been identified. Likewise, this makes developing effective control measures difficult due to the complexity of Salmonella occurrence in all phases of feed production and the range of potential Salmonella serovars that could be contaminants.
Given the effectiveness of formaldehyde as a general sanitizer, it is not surprising that there would be interest in applying it as an antimicrobial treatment for poultry feeds. Duncan and Adams (106) examined the use of formaldehyde gas as a potential treatment to fumigate feeds and eliminate Salmonella loads using chick starter, fish meal, and meat and bone meal artificially contaminated with S. Senftenberg as their test model. They initially tested a commercial acid-based blended product containing propionic acid, isopropyl alcohol, and phosphoric acid but found this to be relatively ineffective at reducing S. Senftenberg levels in the various feeds. Following this experiment, they formaldehyde fumigated contaminated feed samples at 37°C and 60% relative humidity in a forced-draft incubator. They concluded that 5 min of formaldehyde fumigation was adequate and that the maximum fumigant penetration was <2.54 cm, but at least 1.91 cm and effective depth was increased to over 5 cm for 500 gm samples if they were continuously mixed.
While formaldehyde fumigation applications were initially tested, formaldehyde liquid solutions that could be incorporated/mixed directly into the feed matrices were examined as potential chemical feed additives to feeds as a means to reduce Salmonella contamination. Moustafa et al. (107) artificially contaminated commercial poultry with S. Typhimurium after the feeds had initially been sterilized via autoclaving. They concluded that a 40 % formaldehyde solution applied at a rate of 10 L/ton resulted in complete reduction of S. Typhimurium within the first hour of treatment while only 94% reduction was achieved with a 5 L/ton rate during this same application time frame. More recently, Sbardella et al. (108) examined the effect of a 3.0 g per kg formaldehyde-propionic acid blend on natural bacterial populations in pig feed and reported reductions in natural populations of the enterobacteria populations. Based on these studies it appeared that formaldehyde solutions could be directly added to feeds and once mixed into the feed were effective in substantially reducing Salmonella contamination.
Studies on the residual activity of formaldehyde treated feed/ingredients to prevent recontamination by Salmonella was first reported by Barlow et al. (27). Fishmeal was treated with a formaldehyde-based product at 2 kg/ton and subsequently challenged with 200–500 cfu/g of S. Senftenberg and the time required to kill Salmonella determined. At 2 kg/ton, 5 to 9 days were required. When fishmeal was treated with 3 kg/ton and challenged with 1,500 to 2,000 cfu/g, all Salmonella was eliminated within the first 24 h. A similar study was conducted by Primm (109) using a mixed culture of Salmonella serotypes and higher challenge rates. At a challenge rate of 3,400 cfu/g, no Salmonella was detected at 3 kg/ton. The 3 kg/ton failed to protect the feed at challenge rates of >34,000 cfu/g.
Commercially, there are several chemical options for treatment of feeds to control Salmonella as described in several reviews published over the years (1–7). From a management perspective it is important to be able to compare various sanitizers to identify either single compounds or combinations that are optimal for the particular conditions they are being applied. Along these lines, studies have been conducted over the years to directly compare feed additive organic acid blends with formaldehyde. In early work Smyser and Snoeyenbos (110) compared 12 different compounds as antimicrobials for Salmonella when these compounds were added to meat and bone meal (MBM). Several acids and non-acid antimicrobials were examined including among others, acetic acid, oleic acid, propionate salts, benzoic, sorbic, methylparaben, formalin at 0.05, 0.1, 0.12, and 0.2 % (w/w) and some commercial blends. A nalidixic acid resistant S. Infantis strain was used as the marker strain to inoculate the samples set at a moisture level in the MBM to support Salmonella growth. Plate enumerations were conducted beginning at 2 to 3 days post-inoculation and subsequently continued for anywhere from 1 to 2 weeks afterwards. All compounds except formalin at levels >0.1 % failed to prevent S. Infantis growth. The authors noted that while initial declines in S. Infantis occurred for many of the additives, the pH of the feed mixtures also became alkaline over time with spoilage ensuing.
Smyser and Snoeyenbos (110) commented that from their previous work that most of these compounds including formalin had minimal effect on Salmonella in MBM when added to the MBM matrix with a much lower moisture content. This would suggest that water activity is an important component for ensuring optimal antimicrobial activity. In a more recent study, Carrique-Mas et al. (53) used a spray application of a Salmonella inocula to a feed matrix to compare the respective efficacies of four different commercial organic acid (various combinations of formic, propionic, and sorbic acids) and formaldehyde-based feed additives in either fishmeal or MBM. The Salmonella inocula (S. Enteritidis, S. Typhimurium, S. Senftenberg, and S. Mbandaka) were sprayed onto the feed matrix accompanied by mixing, subsequently allowed to incubate over time followed by recovery for pre-enrichment. A critical outcome of the research results noted by the authors was that the carryover of the antimicrobials into the recovery media in turn appeared to “mask” and/or reduce the population recovery levels of the inoculated Salmonella and thus led to an overestimation of the antimicrobial effect due to decreased levels of Salmonella surviving in the recovery media. To counter this masking effect, the authors employed antimicrobial neutralizing antagonists such as histidine for formaldehyde or sodium hydroxide for organic acids to the pre-enrichment media to neutralize artifactual antimicrobial decreases resulting from the respective feed additive to add. One of the formaldehyde-based treatments elicited less masking and more efficacy against Salmonella with no differences among the serovars. Clearly, as more feed studies are done, caution will need to be exercised to avoid Salmonella methodology misinterpretations occurring from masking regardless of the antimicrobial used. This will mean that some quantitative methodology validation will need to be conducted to ensure that the results represent the Salmonella populations originally present in the feed matrix after treatment of the feeds. This may not only be a concern for Salmonella but may need to be considered for all non-Salmonella bacterial population enumerations to avoid artificial selection by masking in either the dilutions or the plating media.
Other factors for optimizing feed treatments to control Salmonella may be influential as well. Carrique-Mas et al. (53) pointed out that the timing of when a feed additive is applied could be important as they and others (111) have noted that pretreatment with organic acids and formaldehyde prior to inoculation of Salmonella results in a more rapid decline in bacterial populations suggesting that pretreated feeds may be more resistant to subsequent contamination. This has practical significance as the potential for Salmonella cross contamination during milling is considered a concern. This is illustrated in a study by Jones and Richardson (80) where they detected Salmonella recontamination originating from dust in the feed mill. This led them to conclude that potential cross contamination between areas of the mill operation are possible and must be taken into account as part of control strategy for Salmonella feed contamination. Even if Salmonella levels in feed are initially decreased during milling, risk of exposure to Salmonella remains. For example, Jones (81) concluded that thermal processes such as pelleting could reduce Salmonella levels, but recontamination could occur post-pelleting and suggested that the addition of chemical disinfectants could diminish potential recontamination.
There are strategies that can be utilized to limit recontamination. To this point, Cochrane et al. (112) examined post rendering chemical treatment of rendered feed ingredients by comparing a wide range of feed additives including a medium chain fatty acid (MCFA) blend (caproic, caprylic, and capric acids) with an organic acid blend (lactic, formic, propionic, and benzoic acids), an EO blend (garlic oleoresin, tumaric oleoresin, capsicum oleoresin, rosemary extract, and wild oregano), sodium bisulfate, and a commercial formaldehyde product. They initially treated the rendered protein feed ingredients (feather meal, blood meal, MBM, and poultry by-product meal) with the corresponding feed additive followed by spray inoculation with a S. Typhimurium strain. They observed that the feed ingredient matrix impacted Salmonella persistence as similar populations were recovered from both blood meal and MBM and, in turn, were higher than the populations enumerated from feather meal and poultry by-product meal. Out of all the products examined, they concluded that the MCFA blend and the formaldehyde commercial product were the most effective in preventing S. Typhimurium post processing contamination, but time and feed matrix type were all factors in reducing S. Typhimurium levels.
In summary, formaldehyde is an effective control agent for limiting Salmonella in feeds but when and where to apply it to achieve maximum efficacy needs to be standardized. This can be accomplished by developing a more complete picture of the microbial ecology of feed production (3). Understanding the microbial ecology of the feed mill as well as the feed ingredient and mixed feed matrices could potentially be helpful not only for Salmonella tracking but general microbial contamination that occurs in feed processing. While many of these non-Salmonella organisms are probably not deleterious to animals and/or humans their presence could be indicative of the impact of processing environmental conditions on the feed prior to being fed to the animal.
Application of next-generation sequencing (NGS) technologies would offer a more complete profile of the microbial population and depending on the bioinformatics analysis identify core feed microbial populations that align with certain characteristics including feed type, feed mill location, individual processing steps in the feed mill (such as before and after pelleting). These identified populations could also serve as indicator organism(s) for the likelihood of occurrence of Salmonella. This may be important if Salmonella occurs relatively infrequently in feeds and/or is dramatically reduced after antimicrobial treatments. Therefore, if based on natural contamination, screening of antimicrobials for control of Salmonella would be more difficult and identification of indicator organisms that are more frequent and parallel Salmonella behavior would have utility for routine testing.
Knowing the core feed microbial populations may be helpful not only for establishing effectiveness of antimicrobial treatments such as formaldehyde in the feed matrix but would also enhance understanding of the GIT microbial population response to formaldehyde treated feed as it enters the GIT. In most poultry studies, emphasis has been placed on antibacterial activities in either the feed matrix or the subsequent impact on Salmonella occurrence in the GIT of birds consuming treated feed. As more is becoming understood about the avian microbiome it is becoming possible to establish relationships between diets, dietary components and the specific responses of the avian microbial community. While this relationship has not been explored extensively with formaldehyde treated feeds there is indirect evidence of potential impact on the poultry GIT based on poultry performance and digestibility studies.
Wales et al. (4) concluded that formaldehyde, when applied as an antimicrobial feed additive, has not been generally shown as a cause of adverse responses in animals. However, Ricke (3) suggested given the dynamics of GIT digestion and microbial responses that a more detailed impact of formaldehyde on dietary protein availability for the concentrations of formaldehyde used as a feed antimicrobial treatment may also need to be considered. As more studies are conducted to examine the utility of formaldehyde as a chemical antimicrobial for poultry feed application, more specific nutritional responses such as amino acid and protein availability for digestion and absorption should also be taken into account in the overall determination of optimal concentrations to be used for antimicrobial applications.
Spears et al. (113) evaluated the impact of soybean meal treated with 0, 3, 6 or 9 kg/ton of formaldehyde (37%) on the performance of broiler chicks through 10 days of age. No adverse effects on body weight gain, feed consumption or feed conversion were observed at the 3 kg/ton treatment. At 6 kg/ton, feed intake was adversely affected. Spratt (57) reported no negative effect of high moisture corn diets treated with 2.5 kg/ton of formaldehyde (37%) in broilers (6 wks) or pullets (6 wks) or laying hens (20–33 wks). In more recent trials with broilers (114, 115), white layers (116), and brown layers (117), consumption of feed treated with a formaldehyde-based product (33% formaldehyde) at 2 to 3 kg/ton was not observed to negatively affect performance. The effect of higher levels of formaldehyde in feed has been evaluated in broilers and cockerels (118, 119). At 2.5 and 5 kg/ton of formaldehyde (37% solution), no adverse effects were reported. Feeding poultry 10 kg of formaldehyde/ton depressed feed intake, reduced body weight gain, and caused ulceration of the crop/gastrointestinal tract.
Barlow (27) was the first to examine the digestibility of formaldehyde in fishmeal destined for aquaculture. Fishmeal was treated with 0, 2, 4, or 6 kg/ton and fed to mink (test animal digestibility trials in aquaculture). No negative effects on protein digestibility occurred at levels up to 6 kg per ton. In digestibility trials in broilers, FBP treated feed/soybean meal was not observed to significantly affect protein digestibility broilers when fed at 2 kg/ton in both non-cecectomized broilers (115) and cecectomized broilers (120). Ironically, in both studies, protein digestibility was numerically improved but was not significantly different (P > 0.05). However, in both studies, it was not disclosed if the feed was subjected to pelleting thus the possibility of cross-linking of formaldehyde and amino acids at high reaction temperatures was not addressed. Jones et al. (121) conducted a study in which feed was treated with 3 kg/ton of a formaldehyde-based product and subjected to extreme pelleting conditions (86°C for 5.5 min). Feed was subsequently fed to cecectomized roosters (20 replicates/treatment), and amino acid digestibility determined. Formaldehyde was observed to not impact amino acid digestibility except for arginine (<1% reduction in digestibility).
While poultry performance and digestibility have been determined for birds fed formaldehyde treated feeds very little is known about the GIT microbial responses to these feeds. The lack of general influence on performance and digestibility would suggest that minimal impact occurs on the poultry GIT microbial populations except for the higher levels of formaldehyde when bird performance effects are noted. However, this does not rule out specific GIT microbial population responses in birds fed formaldehyde treated feeds. Historically, it was difficult to discern more subtle GIT microbial responses to differences in diets due to the limitations associated with culture methods for recovery of representative GIT microbial populations, particularly the more strict anaerobic GIT microorganisms. Development of molecular identification approaches such as NGS has made total GIT microbial populations much more accessible. As 16S rDNA sequencing methodologies for poultry microbiomes continue to advance it should be possible to achieve more indepth resolution for specific poultry GIT microbial population responses to formaldehyde treated feeds. Even when differences in overall poultry performance or digestibility responses are not detectable in the presence of formaldehyde treated feed, it is still possible that shifts in GIT could occur in response to changes in individual dietary components such as free amino acids and/or proteins. This response could vary depending on the particular GIT compartment in the bird such as the crop at the beginning of the GIT vs. the ceca at the terminal of the GIT. Not only are the microbial populations distinct in each of these GIT compartments but the lumen environment, pH, and metabolite composition are likely to be different as well (122–124). Application of metabolomics and transcriptomics may further reveal poultry GIT microbial responses even when detectable changes in GIT microbial populations composition do not occur.
While a fairly wide range of chemical, physical and biological agents have been examined and in some cases commercially applied over the years as feed additives, formaldehyde remains one of the more frequently used from a commercial standpoint. It is considered effective as a feed additive, but it also may possess different antimicrobial mechanism(s) against Salmonella and other organisms such as GIT indigenous bacteria. However, its activity in the GIT, once consumed by the bird, may be different as well. It is conceivable since formaldehyde may bind directly to feed proteins that perhaps it is more stable in the GIT and therefore is more likely to reach the lower parts of the tract. It would be interesting to conduct studies on labeled formaldehyde similar to the work done by Hume et al. (125) with labeled propionate to determine whether that is indeed the case.
Formaldehyde can react with several different amino acids including the epsilon-amino group of lysine, the primary amide groups of asparagine and glutamine, the sulphydryl group of cysteine among others (23). This differential reactivity for particular amino acids could account for some of the variation seen in feed protein additive studies and the interaction with the GIT microbial community as proteins become modified with formaldehyde linkages and potentially present unique targets for protein hydrolysis by GIT microorganisms. In conclusion, the introduction of microbiome sequencing and bioinformatic tools should help to sort out some of the microbial ecology complexities associated with formaldehyde treated feeds both in the feed itself as well as once it is consumed by the bird.
All authors significantly contributed to the work of the current review. SR wrote the review with the assistance from DD and KR.
KR is employed by the company Anitox Corporation, 1055 Progress Circle, Lawrence, GA 33043, USA.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author DD would like to knowledge the Graduate College at the University of Arkansas for its support through the Distinguished Academy Fellowship and the continued support from the Cell and Molecular Biology Program and Department of Food Science at the University of Arkansas. Furthermore, the authors would like to acknowledge Anitox for its support in writing this review.
1. Maciorowski KG, Herrera P, Jones FT, Pillai SD, Ricke SC. Effects of poultry and livestock of feed contamination with bacteria and fungi. Animal Feed Sci Technol. (2007) 133:109–36. doi: 10.1016/j.anifeedsci.2006.08.006
2. Ricke SC. Chapter 7. Ensuring the Safety of Poultry Feed. In: Mead GC, editor. Food Safety Control in Poultry Industry. Cambridge: Woodhead Publishing Limited (2005). p. 174–94.
3. Ricke SC. Chapter 8. Feed Hygiene. In: Dewulf J, Van Immerseel F, editors. Biosecurity in Animal Production and Veterinary Medicine. Leuven: ACCO (2017). p. 144–76.
4. Wales AD, Allen VM, Davies RH. Chemical treatment of animal feed and water for the control of Salmonella. Foodborne Path Dis. (2010) 7:1–15. doi: 10.1089/fpd.2009.0373
5. Williams JE. Salmonellas in poultry feeds–A worldwide review. Part III Methods in control and elimination. World Poultry Sci J. (1981) 37:97–105. doi: 10.1079/WPS19810009
6. Maciorowski KG, Jones FT, Pillai SD, Ricke SC. Incidence, sources, and control of food-borne Salmonella spp. in poultry feed. World Poultry Sci J. (2004) 60:446–57. doi: 10.1079/WPS200428
7. Maciorowski KG, Herrera P, Kundinger MM, Ricke SC. Animal feed production and contamination by foodborne Salmonella. J Consumer Prot Food Safe. (2006) 1:197–209. doi: 10.1007/s00003-006-0036-z
8. O'Brien PJ, Siraki AG. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit Revs Toxicol. (2005) 35:609–62. doi: 10.1080/10408440591002183
9. Yang X, Yu X, Lin M, Ge M, Zhao Y, Wang F. Interface effect of mixed phase Pt/ZrO2 catalysts for HCHO oxidation at ambient temperature. J Mater Chem A. (2017) 5:13799–806. doi: 10.1039/C7TA03888G
10. Wang Q, Zhang C, Shi L, Zeng G, Zhang H, Li S, et al. Ultralow Pt catalyst for formaldehyde removal: the determinant of support. Science. (2018) 9:487–501. doi: 10.1016/j.isci.2018.11.011
11. WHO. Formaldehyde. Environmental Programme on Chemical Safety. Geneva: World Health Organization (1989).
12. Osório VM, Cardeal ZL. Analytical methods to assess carbonyl compounds in foods and beverages. J Braz Chem Soc. (2013) 24:1711–8. doi: 10.5935/0103-5053.20130236
13. Scientific Committee for Animal Nutrition. Update of the Opinion of the Scientific Committee for Animal Nutrition on the Use of formaldehyde as a Preserving Agent for Animal Feedingstuffs of 11 June 1999. Brussels: European Commission: Health and Consumer Protection Directorate-General (2002).
14. Li Y, Zhang L, Wang W. Formation of aldehyde and ketone compounds during production and storage of milk powder. Molecules. (2012) 17:9900–11. doi: 10.3390/molecules17089900
15. Koivusalo M. Studies on the metabolism of methanol and formaldehyde in the animal organism. Acta Physiol Scandin. (1956) 39:2–181.
16. Malorny G, Rietbrook N, Scheider M. The oxidation of formaldehyde to formic acid in blood, a contribution to the metabolism of formaldehyde. Naunyn Schmiedebergs Arch Exp Path Pharmak. (1965) 250:419–36.
17. Metz B, Kersten GFA, Hoogerhout P, Brugghei HF, Timmermans HAM, de Jong A, et al. Identification of formaldehyde-induced modifications in proteins. J Biol Chem. (2004) 279:6235–43. doi: 10.1074/jbc.M310752200
18. Kunjapur AM, Prather KLJ. Microbial engineering for aldehyde synthesis. Appl Environ Microbiol. (2015) 81:1892–901. doi: 10.1128/AEM.03319-14
19. French D, Edsall JT. The reactions of formaldehyde with amino acids and proteins. Adv Protein Chem. (1945) 2:277–335. doi: 10.1016/S0065-3233(08)60627-0
20. Hopwood D. Fixatives and fixation: a review. Histochem J. (1969) 1:323–60. doi: 10.1007/BF01003278
21. Kiernan JA. Formaldehyde, formalin, paraformaldehyde and glutaraldehyde: what they are and what they do. Microscopy Today. (2000) 1:8–12. doi: 10.1017/S1551929500057060
22. Walker JF. Formaldehyde, Third Edition. Malabar, FL: Robert A. Krieger Publishing Company (1975).
23. Barry TN. The effectiveness of formaldehyde treatment in protecting dietary protein from rumen microbial degradation. Proc Nutr Soc. (1976) 35:221–9. doi: 10.1079/PNS19760035
24. Dapson RW. Macromolecular changes caused by formalin fixation and antigen retrieval. Biotech Histochem. (2007) 82:133–40. doi: 10.1080/10520290701567916
25. Alexander P, Carter D, Johnson KG. Formation by formaldehyde of a cross-link between lysine and tyrosine residues in wool. Biochem J. (1951) 38:435–41. doi: 10.1042/bj0480435
27. Barlow SM. Use of Salmex (Termin-8) With Fish Meal. Research Report. International Association of Fish Meal Manufacturers (1992). p. 1992–4. Available online at: http://www.iffo.net/system/files/Salmex%20with%20fishmeal%201992-4_1.pdf
28. Rude C, Mellick D, Lamptey A, Bienhoff M. Evaluation of the effects of a formaldehyde based feed additive on free lysine. Poult Sci. (2016) 94(Suppl. 2):135. doi: 10.2527/msasas2016-288
30. Gustavason KH. Note on the reaction of formaldehyde with colllagen. J Bio Chem. (1948) 169:531–6.
31. Toews J, Rogalski JC, Clark TJ, Kast J. Mass spectrometic of formaldehyde-induced peptide modifications under in vivo protein cross-linking conditions. Anal Chim Acta. (2008) 618:168–83. doi: 10.1016/j.aca.2008.04.049
32. Fox CH, Johnson FB, Whiting J, Roller PP. Formaldehyde fixation. J Histochem Cytochem. (1985) 33:845–53. doi: 10.1177/33.8.3894502
33. O'Rourke MB, Padula MP. Analysis of formalin-fixed, paraffin-embedded (FFPE) tissue via proteomic techniques and misconceptions of antigen retrieval. BioTechniq. (2016) 60:229–38. doi: 10.2144/000114414
34. Ricke SC, Schaefer DM, Cook ME, Kang KH. Differentiation of ruminal bacterial species by enzyme-linked immunosorbent assay using egg yolk antibodies from immunized chicken hens. Appl Environ Microbiol. (1988) 54:596–9.
35. Ricke SC, Schaefer DM. Characterization of egg yolk antibodies for detection and quantitation of rumen selenomonads using an enzyme - linked immunosorbent assay. Appl Environ Microbiol. (1990) 56:2795–800.
36. Isaacson HR, Hinds FC, Bryant MP, Owens FN. Efficiency of energy utilization by mixed rumen bacteria in continuous culture. J Dairy Sci. (1975) 58:1645–59. doi: 10.3168/jds.S0022-0302(75)84763-1
37. Schaefer DM, Davis CL, Bryant MP. Ammonia saturation constants for predominant species of rumen bacteria. J Dairy Sci. (1980) 63:1248–63. doi: 10.3168/jds.S0022-0302(80)83076-1
38. Ricke SC, Schaefer DM. Growth and fermentation responses of Selenomonas ruminantium. to limiting and non-limiting concentrations of ammonium chloride. Appl Microbiol Biotechnol. (1996) 46:169–75. doi: 10.1007/s002530050800
39. Baker CA, Park SH, Kim SA, Rubinelli PM, Roto SM, Lee SI, et al. Formalin-fixed cells as an internal standard approach for the detection and quantitative assessment of shiga toxin-producing Escherichia coli. (STEC). Food Control. (2016) 63:76–82. doi: 10.1016/j.foodcont.2015.11.014
40. McLeod V. Sterilizing safely–A closer look at glutaldehyde based chemical agents. Lab Manager. (2018) 13:30–3.
41. Böhm R. Disinfection and hygiene in the veterinary and disinfection of animal houses and transpost vehicles. Int Bioter Biodegrad. (1998) 41:217–24. doi: 10.1016/S0964-8305(98)00030-4
42. Reuter G. Disinfection and hygiene in the field of food of animal origin. Int Biodeter Biodegrad. (1998) 41:209–15. doi: 10.1016/S0964-8305(98)00029-8
43. Battersby T, Walsh D, Whyte P, Bolton D. Evaluating and improving terminal hygiene practices on broiler farms to prevent Campylobacter cross-contamination between flocks. Food Microbiol. (2017) 64:18. doi: 10.1016/j.fm.2016.11.018
44. Castro Burbarelli MF, do Valle Polycarpo G, Deliberali Lelis K, Granghelli CA, Carão de Pinho AC, Ribeiro Almeida Queiroz S, et al. Cleaning and disinfection programs against Campylobacter jejuni for broiler chickens: productive performance, microbiological assessment and characterization. Poultry Sci. (2017) 96:3188–98. doi: 10.3382/ps/pex153
45. Jiang L, Li M, Tang J, Zhao X, Zhang J, Zhu H, et al. Effect of different disinfectants on bacterial aerosol diversity in poultry houses. Front Microbiol. (2018) 9:2113. doi: 10.3389/fmicb.2018.02113
46. Williams JE. Effect of high-level formaldehyde fumigation on bacterial populations on the surface of chicken hatching eggs. Avian Dis. (1970) 14:386–92. doi: 10.2307/1588482
47. Proudfoot FG, Nash DM, Hulan HW. Effects of glutaraldehyde-surfactant solution on the hatchability of hen's eggs. Poultry Sci. (1985) 64:2400–2. doi: 10.3382/ps.0642400
48. Sacco RE, Renner PA, Nestor KE, Saif M, Dearth RN. Effect of hatching egg sanitizers on embryonic survival and hatchability of turkey eggs from different lines and on egg shell bacterial populations. Poultry Sci. (1989) 68:1179–84. doi: 10.3382/ps.0681179
49. Whistler PE, Sheldon BW. Biocidal activity of ozone versus formaldehyde against poultry pathogens inoculated in a prototype setter. Poultry Sci. (1989) 68:1068–73. doi: 10.3382/ps.0681068
50. Scott TA, Swetnam C. Screening sanitizing agents and methods for hatching eggs I. Environmental and user friendliness. J Appl Poultry Res. (1993) 2:1–6. doi: 10.1093/japr/2.1.1
51. Scott TA, Swetnam C. Screening sanitizing agents and methods for hatching eggs II. Effectiveness against microorganisms on the egg shell. J Appl Poultry Res. (1993) 2:7–11. doi: 10.1093/japr/2.1.7
52. Scott TA, Swetnam C, Kinsman R. Screening sanitizing agents and methods for hatching eggs III. Effect of concentration and exposure time embryo viability. J Appl Poultry Res. (1993) 2:12–8. doi: 10.1093/japr/2.1.12
53. Carrique-Mas JJ, Bedford S, Davies RH. Organic acid and formaldehyde treatment of animal feeds to control Salmonella: efficacy and masking during culture. J Applied Microbiol. (2007) 103:88–96. doi: 10.1111/j.1365-2672.2006.03233.x
54. USDA. Risk That Poultry Feed Made With Corn-Potentially Contaminated With Eurasian-North American Lineage H5N2 HPAI Virus From Wild Migratory Birds – Results in Exposure Of Susceptible Commercial Poultry. Document 294.0515 cl. Fort Collins, CO: United States Department of Agriculture, Center for Epidemiology and Animal Health. (2015).
55. EFSA. FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2014. Scientific Opinion on the safety and efficacy of formaldehyde for all animal species based on a dossier submitted by Regal, BV. EFSA J. (2014) 12:3561. doi: 10.2903/j.efsa.2014.3561
56. Arts JH, Rennen MA, de Heer C. Inhaled formaldehyde: evaluation of senosry irritation in relation to carcinogenicity. Regul Toxicol Pharmacol. (2006) 44:144–60. doi: 10.1016/j.yrtph.2005.11.006
57. Spratt CD. Effect of Mold Inhibitor Treated High Moisture Corn on Performance of Poultry. M.Sc.Thesis, University of Guelph (1985).
58. Cloete TE. Resistance mechanisms of bacteria to antimicrobial compounds. Int Biodeterior Biodegr. (2003) 51:277–82. doi: 10.1016/S0964-8305(03)00042-8
59. Russell AD, Furr JR, Maillard JY. Microbial susceptibility and resistance to biocides. ASM News. (1997) 63:481–7.
60. Temcharoen P, Tilly WG. Toxic and mutagenic effects of formaldehyde in Salmonella typhimurium. Mutat Res. (1983) 119:89–93. doi: 10.1016/0165-7992(83)90115-X
61. Ames BN, Durston WE, Yamasaki E, Lee FD. Carcinogens are mutagens: a simple test system combining liver homogenates for activation and bacteria for detection. Proc Natl Acad Sci USA. (1973) 70:2281–5. doi: 10.1073/pnas.70.8.2281
62. Gradel RO, Randall L, Sayers AR, Davies RH. Possible associations between Salmonella persistence in poultry houses and resistance to commonly used disinfectants and a putative role of mar. Vet Microbiol. (2005) 107:127–38. doi: 10.1016/j.vetmic.2005.01.013
63. Davison S, Benson CE, Munro DS, Rankin SC, Ziegler AE, Eckroade RJ. The role of disinfectant resistance of Salmonella enterica serotype Enteritidis in recurring infections in Pennsylvania egg quality assurance program monitored flocks. Avian Dis. (2003) 47:143–8. doi: 10.1637/0005-2086(2003)047[0143:TRODRO]2.0.CO;2
64. Møretrø T, Midtgaard ES, Nesse LL, Langsrud S. Susceptibility of Salmonella isolated from fish feed factories to disinfectants and air-drying at surfaces. Vet Microbiol. (2003) 94:207–17. doi: 10.1016/S0378-1135(03)00105-6
65. Fenlon DR. Wild birds and silage as reservoirs of Listeria in the agricultural environment. J Appl Bacteriol. (1985) 59:537–43. doi: 10.1111/j.1365-2672.1985.tb03357.x
66. Pelhate J. Microbiology of moist grains. In: Multon JL, editor. Preservation and Storage of Grains, Seeds, and Their By-Products. New York, NY: Lavoisier Publishing, Inc. (1988). p. 328–46.
67. Pedersen J. Insects identification, damage, and detection. In: Sauer DB, editor. Storage of Cereal Grains and Their Products, 4th ed. St. Paul, MN: American Association of Cereal Chemists (1992). p. 435–89.
68. Sauer DB, Meronick RA, Christenesen CM. Microflora. In: Sauer DB, editor. Storage of Cereal Grains and Their Products, 4th ed. St. Paul, MN: American Association of Cereal Chemists (1992). p. 313–40.
69. Dänicke S. Prevention and control of mycotoxins in the poultry production chain: a European view. World Poultry Sci J. (2002) 58:451–74. doi: 10.1079/WPS20020033
70. Hesseltine CW, Shotwell OL, Kwolek WF, Lillehof EB, Jackson WK, Bothast RJ. Aflatoxin occurence in 1973 corn at harvest II Mycological studies. Mycologia. (1976) 68:341–53. doi: 10.1080/00275514.1976.12019915
71. Oguz H. A review from experimental trials on detoxification of aflatoxin in poultry feed. Eurasian J Vet Sci. (2011) 27:1–12.
72. Wilson DM, Abramson D. Mycotoxins. In: Sauer DB, editor. Storage of Cereal Grains and Their Products, 4th ed. St. Paul, MN: American Association of Cereal Chemists (1992). p. 341–91.
73. Blank G, Savoie S, Campbell LD. Microbiological decontamination of poultry feed – evaluation of steam conditioners. J Sci Food Agr. (1996) 72:299–305.
74. Greenham LW, Harber C, Lewis E, Scullion FT. Clostridium perfringens in pelleted feed. Vet Rec. (1987) 120:557. doi: 10.1136/vr.120.23.557-a
75. Skovgaard N, Morgen C-A. Detection of Listeria spp. in faeces from animals, in feeds, and in raw foods of animal origin. Int J Food Microbiol. (1988) 6:229–42. doi: 10.1016/0168-1605(88)90015-3
76. Whyte P, McGill K, Collins JD. A survey of the prevalence of Salmonella and other enteric pathogens in a commercial poultry feedmill. J Food Safe. (2003) 23:13–24. doi: 10.1111/j.1745-4565.2003.tb00348.x
77. Lindström M, Heikinheimo A, Lahti P, Korkeala H. Novel insights into the epidemiology of Clostridium perfringens type A food poisoning. Food Microbiol. (2011) 28:192–8. doi: 10.1016/j.fm.2010.03.020
78. Alvarez J, Porwollik S, Laconcha I, Gisakis V, Vivanco AB, Gonzalez I, et al. Detection of Salmonella enterica serovar California strain spreading in Spanish feed mills and genetic chracterization with DNA microarrays. Appl Environ Microbiol. (2003) 69:7531–4. doi: 10.1128/AEM.69.12.7531-7534.2003
79. Jarquin R, Hanning I, Ahn S, Ricke SC. Development of rapid detection and genetic characterization of Salmonella in poultry breeder feeds. Sensors. (2009) 9:5308–23. doi: 10.3390/s90705308
80. Jones FT, Richardson KE. Salmonella in commercially manufactured feeds. Poultry Sci. (2004) 83:384–91. doi: 10.1093/ps/83.3.384
81. Jones FT. A review of practical Salmonella control measures in animal feed. J Appl Poultry Res. (2011) 20:102–13. doi: 10.3382/japr.2010-00281
82. Williams JE. Salmonellas in poultry feeds – A worldwide review. World Poultry Sci J. (1981) 37:6–19. doi: 10.1079/WPS19810002
83. Ellis EM. Salmonella reservoirs in animal and feeds. J Am Oil Chem Soc. (1969) 46:227–9. doi: 10.1007/BF02544802
84. Henken AM, Frankena K, Goelema JO, Graat EAM, Noordhuizen JPTM. Multivariate epidemiological approach to salmonellosis in broiler breeder flocks. Poultry Sci. (1992) 71:838–43. doi: 10.3382/ps.0710838
85. Snoeyenbos GH. An approach to maintaining Salmonella free chickens. Avian Dis. (1971) 15:28–31. doi: 10.2307/1588384
86. Wilding GP, Baxter-Jones C. Egg borne Salmonellas: Is prevention feasible? In: Proceedings of the International Symposium on Salmonella. New Orleans, LA (1984) p. 126–33.
87. Shapcott RC. Practical aspects of Salmonella control: progress report on a programme in a large broiler integration. In: Proceedings of the International Symposium on Salmonella. New Orleans, LA (1984). p. 109–14.
89. Morris GK, McMurray BL, Galton MM, Wells JG. A study of the dissemination of Salmonellosis in a commercial broiler chicken operation. Am J Vet Res. (1969) 30:1413–21.
90. Lahellec C, Collins P. Relationship between serotypes of Salmonella from hatcheries and rearing farms and those from processed poultry carcases. Brit Poult Sci. (1985) 26:179–86. doi: 10.1080/00071668508416802
91. McKenzie A. Bains BS. Dissemination of salmonella serotypes from raw feed ingredients to chicken carcasses. Poult Sci. (1976) 55:957–60. doi: 10.3382/ps.0550957
92. Davies R, Breslin M, Corry JE, Hudson W, Allen VM. Observations on the distribution and control of Salmonella species in two integrated broiler companies. Vet Rec. (2001) 149:227–32. doi: 10.1136/vr.149.8.227
93. Corry JE, Allen VM, Hudson WR, Breslin MF, Davies RH. Sources of Salmonella on broiler carcasses during transportation and processing: modes of contamination and methods of control. J Appl Microbiol. (2002) 92:424–32. doi: 10.1046/j.1365-2672.2002.01543.x
94. Bucher O, Holley RA, Ahmed R, Tabor H, Nadon C, Ng LK, et al. Occurence and characterization of Salmonella from chicken nuggests, strips, and pelleted feed. J Food Prot. (2007) 70:2251–8. doi: 10.4315/0362-028X-70.10.2251
95. Shirota K, Katoh H, Musase T, Ito T, Otsuki K. Monitoring of layer feed and eggs for Salmonella in eastern Japan between 1993 and 1998. J Food Prot. (2001) 64:734–7. doi: 10.4315/0362-028X-64.5.734
96. Shirota K, Katoh H, Murase T, Ito T, Otsuki K. Salmonella contamination in commercial layer feed in Japan. J Vet Med Sci. (2000) 62:789–91. doi: 10.1292/jvms.62.789
97. Jarvis NA, O'Bryan CA, Dawoud TM, Park SH, Kwon YM, Crandall PG, et al. An overview of Salmonella thermal destruction during food processing and preparation. Food Control. (2016) 68:280–90. doi: 10.1016/j.foodcont.2016.04.006
98. Dawoud TM, Davis ML, Park SH, Kim SA, Kwon YM, Jarvis N, et al. Salmonella thermal resistance - Molecular responses. Front Vet Sci. (2017) 4:93. doi: 10.3389/fvets.2017.00093
99. Park SH, Jarquin R, Hanning I, Almeida G, Ricke SC. Detection of Salmonella spp. survival and virulence in poultry feed by targeting the hilA gene. J Appl Microbiol. (2011) 111:426–32. doi: 10.1111/j.1365-2672.2011.05054.x
100. Davies RH, Wales AD. Investigation into Salmonella contamination in poultry feedmills in the United Kingdom. J Appl Microbiol. (2010) 109:1430–40. doi: 10.1111/j.1365-2672.2010.04767.x
101. Ge B, LaFon PC, Carter PJ, McDermott SD, Abbott J, Glenn A, et al. Retrospective analysis of Salmonella, Campylobacter, Escherichia coli, and Enterococcus in animal feed ingredients. Foodborne Path Dis. (2013) 10:684–91. doi: 10.1089/fpd.2012.1470
102. Gong C, Jiang X. Characterizing Salmonella contamination in two rendering processing plants. J Food Prot. (2017) 80:263–70. doi: 10.4315/0362-028X.JFP-16-210
103. Jiang X. Prevalence and characterization of Salmonella in animal meals collected from rendering plants. J Food Prot. (2016) 79:1026–31. doi: 10.4315/0362-028X.JFP-15-537
104. Jones FT, Ricke SC. Researchers propose tentative HACCP plan for feed manufacturers. Feedstuffs. (1994) 66:40–42.
105. Park SY, Woodward CL, Kubena LF, Nisbet DJ, Birkhold SG, Ricke SC. Environmental dissemination of foodborne Salmonella in preharvest poultry production: reservoirs, critical factors and research strategies. Crit Revs Environ Sci Technol. (2008) 38:73–111. doi: 10.1080/10643380701598227
106. Duncan MS, Adams AW. Effects of a chemical additive and of formaldhyde-gas fumigation on Salmonella in poultry feeds. Poultry Sci. (1972) 51:797–802. doi: 10.3382/ps.0510797
107. Moustafa GZ, Zaki MM, Badawy EM. Hygienic control of Salmonella in artificially contaminated feed. Vet Med J. (2002) 50:239–46.
108. Sbardella M, do Prado Perina D, de Andrade C, Longo FA, Miyada VS. Effects of a dietary added formaldehyde-propoinc acid blend on feed enterobacteria counts and on growing pig performance nd fecal formaldehyde excretion. Ciência Rural. (2015) 45:474–9. doi: 10.1590/0103-8478cr20131660
109. Primm ND. Field experiences with the control of salmonellae introduction into turkey flocks via contaminated feeds. In: Proceedings of the 47th Annual Western Poultry Disease Conference. Sacramento, CA (1998). p.27–30.
110. Smyser CF, Snoeyenbos GH. Evaluation of organic acids and other compounds in Salmonella antagonists in meat and bone meal. Poultry Sci. (1979) 58:50–4. doi: 10.3382/ps.0580050
111. Rouse J, Rolow A, Nelson CE. Research note: effect of chemical treatment of poultry feed on survival of Salmonella. Poultry Sci. (1988) 67:1225–8. doi: 10.3382/ps.0671225
112. Cochrane RA, Huss AR, Aldrich GC, Stark CR, Jones CA. Evaluating chemical mitigation of Salmonella Typhimurium ATCC 14028 in animal feed ingredients. J Food Prot. (2016) 79:672–6. doi: 10.4315/0362-028X.JFP-15-320
113. Spears JW, Hatfield EE, Clark JH. Influence of formaldehdye treatment of soybeal mela on the performance of growing steers and protein availability in the chick. J Anim Sci. (1980) 50:750–5. doi: 10.2527/jas1980.504750x
114. Yakhkeshi S, Rahimi S, Naseri KG. The effects of comparison of herbal extracts, antibiotic, probiotic and organic acid on serum lipids, immune response, GIT microbial population, intestinal morphology and performance of broilers. J Med Plants. (2011) 10:80–92.
115. Yakhkeshi S, Shawrang P, Rahimi S. Effect of some acidifiers on gastrointestinal tract characteristics and performance of broiler chicks. Iran J Appl Anim Sci. (2014) 4:143–9.
116. Anderson KE, Richardson KE. Effect of termin-8 compound on the microbiological and physical quality of shell eggs from commercial laying chickens. In: 21st Annual Meeting of the Southern Poultry Science Association (2000).
117. Anderson KE, Sheldon BW, Richardson KE. Effect of Termin-8 Compound on the productivity and environmental microbial populations from brown egg laying chickens. Poult Sci. (2002) 81(Suppl. 1):14.
118. Babar A, Khan M, Ahmad S, Khan A. Toxico-pathological effects of formalin (37% formaldehyde) feeding in broiler chicks. Pakistan Vet J. (2001) 21:13–6.
119. Khan A, Hussain S, Khan M. Effects of formalin feeding or administering into the crops of white leghorn cockerels on hematological and biochemical parameters. Poult Sci. (2006) 85:1513–9. doi: 10.1093/ps/85.9.1513
120. Ferreira NT, Sakomura NK, Longo FA, Vittori J, Dorigam JCP, Lima MB, et al. Effects of soybean meal treatment with Salmex on protein and energy digestability for broilers. Poult Sci. (2014) 93(Suppl. 1):144.
121. Jones MK, Richardson KE, Starkey CW, Dale NM, Davis AJ. Impact of extended heat treatment on the amino acid digestibility and TMEn content of a formaldehyde treated diet. J Appl Poult Res. (2018) 98:1–5. doi: 10.3382/japr/pfy038
122. Rehman HU, Vahjen W, Awad WA, Zentek J. Indigenous bacteria and bacterial metabolic products in the gastrointestinal tract of broiler chickens. Arch Anim Nutr. (2007) 61:319–35. doi: 10.1080/17450390701556817
123. Stanley D, Hughes RJ, Moore RJ. Microbiota of the chicken gastrointestinal tract: influence on health, productivity and disease. App Microbiol Biotechnol. (2014) 98:4301–10. doi: 10.1007/s00253-014-5646-2
124. Oakley BB, Morales CA, Line J, Berrang ME, Meinersmann RJ, Glenn E, et al. The poultry-associated microbiome: network analysis and farm-to-fork characterizations. PLoS ONE. (2013) 8:e57190. doi: 10.1371/journal.pone.0057190
Keywords: formaldehyde, feed, poultry, Salmonella, gastrointestinal tract
Citation: Ricke SC, Richardson K and Dittoe DK (2019) Formaldehydes in Feed and Their Potential Interaction With the Poultry Gastrointestinal Tract Microbial Community–A Review. Front. Vet. Sci. 6:188. doi: 10.3389/fvets.2019.00188
Received: 30 December 2018; Accepted: 28 May 2019;
Published: 13 June 2019.
Edited by:
Timothy J. Johnson, University of Minnesota Twin Cities, United StatesReviewed by:
Lisa Bielke, The Ohio State University, United StatesCopyright © 2019 Ricke, Richardson and Dittoe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Steven C. Ricke, c3JpY2tlQHVhcmsuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.