- 1Royal (Dick) School of Veterinary Studies and Roslin Institute, University of Edinburgh, Edinburgh, United Kingdom
- 2Moredun Research Institute, Edinburgh, United Kingdom
- 3Global Health Institute, Federal Institute of Technology in Lausanne, Lausanne, Switzerland
- 4Institute of Novel and Emerging Infectious Diseases, Friedrich-Loeffler-Institut, Federal Research Institute for Animal Health, Greifswald-Insel Riems, Germany
- 5Département Homme et Environment, Centre d'Ecologie et des Sciences de la Conservation, Muséum National d'Histoire Naturelle, Paris, France
- 6Agence Française pour la Biodiversité, Centre d'expertise et de Données sur la Nature, Muséum National d'Histoire Naturelle, Paris, France
- 7Dipartimento di Medicina Veterinaria, Università degli Studi di Milano, Milan, Italy
- 8Dipartimento di Scienze Teoriche ed Applicate, Università degli Studi dell'Insubria, Varese, Italy
- 9School of Natural Sciences, Bangor University, Bangor, United Kingdom
- 10Laboratorio Interdisciplinario de Investigación Dermatológica, Servicio de Dermatología, Hospital Universitario, Universidad Autonoma de Nuevo León, Monterrey, Mexico
- 11Melbourne Veterinary School, Faculty of Veterinary and Agricultural Sciences, University of Melbourne, Melbourne, VIC, Australia
- 12Institut Pasteur de Paris, Paris, France
Eurasian red squirrels (Sciurus vulgaris) in the British Isles are the most recently discovered animal reservoir for the leprosy bacteria Mycobacterium leprae and Mycobacterium lepromatosis. Initial data suggest that prevalence of leprosy infection is variable and often low in different squirrel populations. Nothing is known about the presence of leprosy bacilli in other wild squirrel species despite two others (Siberian chipmunk [Tamias sibiricus], and Thirteen-lined ground squirrel [Ictidomys tridecemlineatus]) having been reported to be susceptible to experimental infection with M. leprae. Rats, a food-source in some countries where human leprosy occurs, have been suggested as potential reservoirs for leprosy bacilli, but no evidence supporting this hypothesis is currently available. We screened 301 squirrel samples covering four species [96 Eurasian red squirrels, 67 Eastern gray squirrels (Sciurus carolinensis), 35 Siberian chipmunks, and 103 Pallas's squirrels (Callosciurus erythraeus)] from Europe and 72 Mexican white-throated woodrats (Neotoma albigula) for the presence of M. leprae and M. lepromatosis using validated PCR protocols. No DNA from leprosy bacilli was detected in any of the samples tested. Given our sample-size, the pathogen should have been detected if the prevalence and/or bacillary load in the populations investigated were similar to those found for British red squirrels.
Introduction
Leprosy is one of the oldest known diseases in humans and can be caused by Mycobacterium leprae and Mycobacterium lepromatosis (1, 2). While treatable with antibiotics, >200,000 new human cases are still recorded every year. Transmission is thought to occur during close and frequent contact with infected people, but the exact mechanism remains unknown (3).
Humans were thought to be the only host species susceptible to leprosy bacilli until cases of naturally occurring infection with M. leprae were identified in the 1970s in nine-banded armadillos (Dasypus novemcinctus) in the southern United States of America and Mexico(4, 5). M. leprae has been reported repeatedly and in multiple locations in the Americas in nine-banded armadillos. To a lesser extent, leprosy has been reported in wild chimpanzees (Pan troglodytes) and sooty mangabey monkeys (Cercocebus atys) in Africa and several primate species have been infected experimentally with leprosy bacilli (6–10). In the Southeastern United States of America, identical strains of M. leprae were detected in nine-banded armadillos and humans (11) but the frequency or route of transmission cannot be determined from the data currently available. It has been suggested that prolonged close contact to infected animals and ingestion of their flesh and blood may be risk factors for human infection (12).
More recently, both leprosy bacilli were identified in seemingly healthy as well as diseased Eurasian red squirrels (Sciurus vulgaris) in the British Isles where autochthonous human cases have not occurred for centuries (13, 14). The M. leprae strain identified in the Eurasian red squirrel population on Brownsea Island was found to be closely related to strains from human leprosy cases in medieval England while the M. lepromatosis strains isolated from British squirrels diverged from Mexican human strains about 27,000 years ago (13).
Moreover, within the family Sciuridae two other species have been infected experimentally with leprosy bacilli; Siberian chipmunks (Tamias sibiricus) and Thirteen-lined ground squirrels (Ictidomys tridecemlineatus) (15, 16), but there are no publications about natural infection or disease in wild individuals from either species.
No natural leprosy infection of other rodent species within or outside the British Isles has been described (17). In 2015, during the genome sequencing of M. lepromatosis, two human cases of M. lepromatosis infection in central America were linked to the possible consumption of meat from rata de campo or “field rat” (18). Rodent meat, as well as armadillo meat, has been reported to be eaten by patients suffering from Hansen's disease in Mexico. However, no studies have been conducted yet to detect leprosy bacilli in wild rodents captured for consumption in this country.
It is possible that rodents may be infected with leprosy bacilli in locations other than those previously reported. Our research therefore aimed to assess the presence of M. leprae and M. lepromatosis in several rodent species in Mexico, continental Europe and the British Isles.
Materials and Methods
Study Population and Sample Types
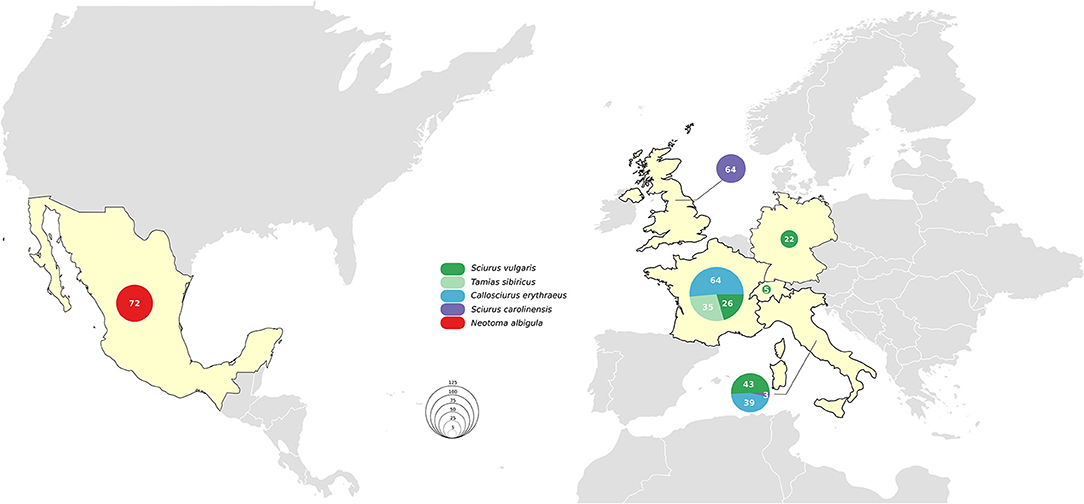
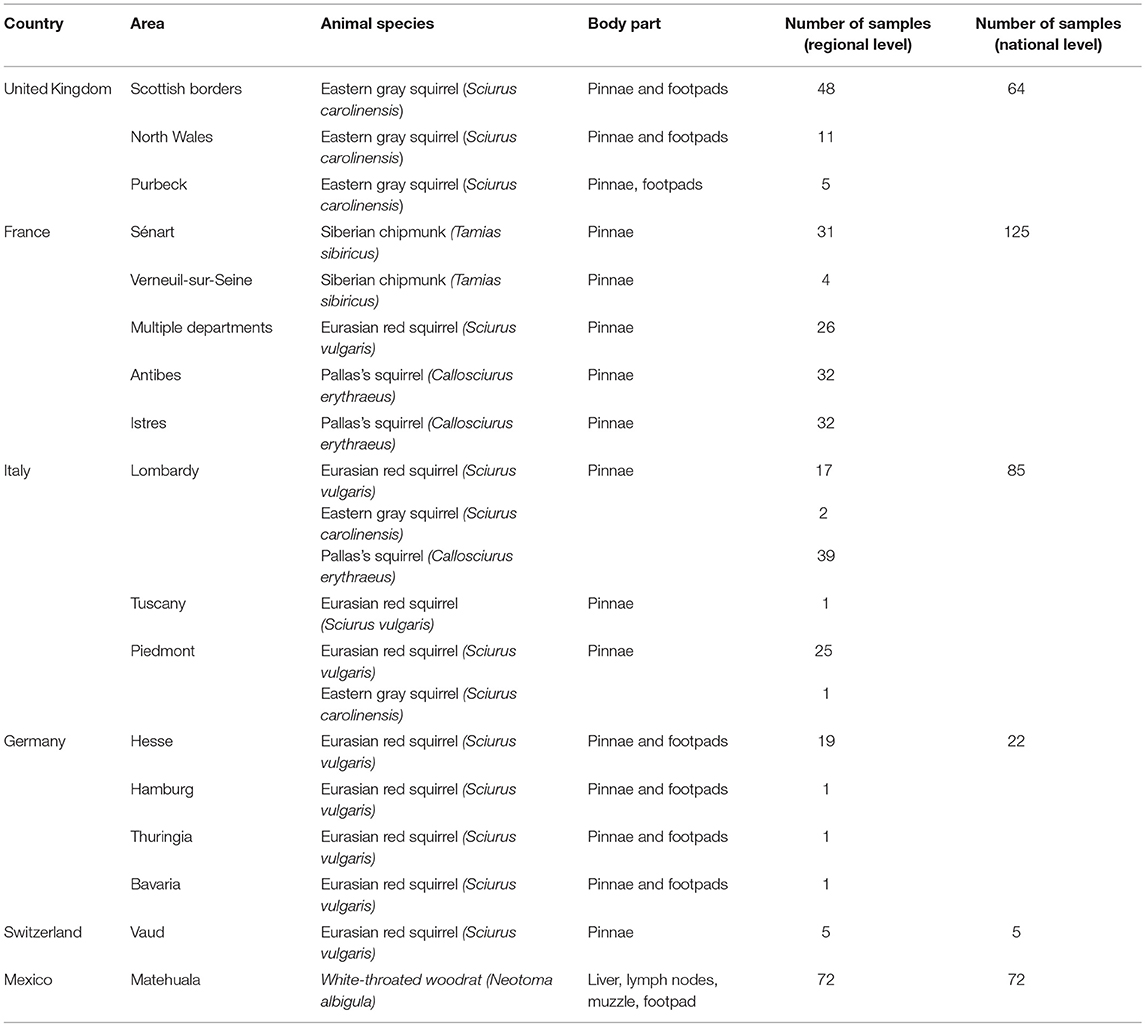
The six countries of origin and five different species included in this study are detailed in Figure 1. Animal carcasses were collected as part of surveillance efforts and were from animals that had been found dead, died at wildlife rescue centers, or had been humanely culled as part of invasive species control efforts. In Mexico, where human M. lepromatosis infections are reported (19), white-throated woodrats (Neotoma albigula) from the Matehuala region were bought at Monterrey meat market. Sample types included pinnae, footpads, liver, and lymph nodes (Table S1). When available, the pinnae samples were favored since it has been reported to be the most effective site for molecular screening (13). Samples were preserved in 70% ethanol for DNA extraction or in 10% formalin for histopathology.
Histopathology
A 2–3 mm wide longitudinal section presenting the full length of the pinna was prepared. The trimmed tissues were submitted for Ziehl-Neelsen-staining according to the protocol described by Avanzi et al. (13). The entire sections were screened for acid-fast bacilli on a light microscope (magnification: 20× to 40×).
DNA Extraction and PCR Screening for the Presence of Leprosy Bacilli
Italian, German, British, and Mexican Samples
DNA was extracted using Qiagen DNeasy Blood and Tissue kit (Italian, German and British samples) or the Qiamp UCP Pathogen kit (Mexican samples) as previously described (13). PCR amplifications were performed in a 50 μl reaction volume containing GoTaq® G2 Flexi DNA Polymerase (1.25 Units/reaction, Promega) or Accustart PCR II SuperMix (Quanta Bioscience), 200 nM of primer pairs to amplify specific fragments of M. lepromatosis (LPM244) and M. leprae (RLEP 7 and 8) (13) and 3 μl of DNA extract. Sterile water was used as a negative PCR control and DNA extracts of M. leprae and M. lepromatosis from clinically diseased red squirrels as positive controls.
Swiss and French Squirrel Samples
Total DNA was extracted from 10 to 100 mg of pinna tissue using the Extracta kit (Quanta Bioscience). PCR amplification was performed using the RLEP and LPM244 primer sets as described above. In addition, a primer set targeting the mitochondrial 12S ribosomal RNA gene of the Eurasian red squirrel genome was used to assess the performance of the Extracta DNA extraction method and to exclude the possibility of PCR inhibitors (13). For the other squirrel species, inhibitory effects were excluded by spiking pure M. leprae DNA (1 pg from strain Thai-53, BEI resources) into each sample followed by RLEP PCR.
Statistics
Using WinPepi (v. 11.35; http://www.brixtonhealth.com/pepi4windows.html) DESCRIBE, we calculated the sample size necessary to find at least one case with 95% confidence if leprosy occurred at a prevalence of 21% in a population based on the estimated values currently available for clinically healthy squirrels in the British Isles (12). The necessary sample size based on this calculation is 19 individuals per population.
Results
No M. leprae or M. lepromatosis DNA was detected in any samples from the 103 Pallas's squirrels, 96 Eurasian red squirrels, 67 Eastern gray squirrels, 35 Siberian chipmunks, and 72 white-throated woodrats analyzed by conventional PCR (Table 1, Figure 1, and Table S1). When Ziehl-Neelsen staining of selected tissues was performed, no acid-fast bacilli were observed (Table S1).
Discussion
The recent discovery of Eurasian red squirrels infected with M. leprae or M. lepromatosis in the British Isles suggested that this rodent could be a reservoir throughout its range and that leprosy bacilli may have more hosts than was previously thought (13, 20–22). This is the first study to investigate the presence of leprosy bacilli in wild populations of squirrels outside the British Isles and in white-throated woodrats sold for consumption in markets in Mexico. It also expands the investigation into the presence of leprosy bacilli in Eastern gray squirrels in Great Britain. In this study, no animals representing five rodent species were found positive for leprosy bacilli DNA. It is therefore possible that only Eurasian red squirrels in the British Isles are a natural host for leprosy bacilli, which is consistent with the fact that to date no clinical signs of leprosy have been reported in wild populations of any squirrel species outside the British Isles. We calculated that a random sample size of 19 individuals should enable detection of at least one infected animal with 95% confidence in a continuous population where leprosy infection occurred at a rate of 21%, based on the estimated rate currently available for Eurasian red squirrels in the British Isles (13). Thus, our sample sizes should have been sufficient to detect at least one infected animal in the populations tested. However, we acknowledge that the prevalence of leprosy infection in Eurasian red squirrels varies widely depending on the geographical area in the British Isles (1–100%) suggesting possible local effects (13, 22). Our negative result could be due to the limited size of animal populations screened or to the limited geographical areas covered by our sampling. Testing larger numbers of squirrels throughout Europe would be necessary to state confidently that leprosy bacilli are absent. Additionally, while very specific tests for the detection of leprosy symptoms and bacilli exist, the sensitivity can be poor, especially for paucibacillary cases characterized by a low number of bacilli associated with few or no clinical lesions (23). Similar to humans, red squirrels display paucibacillary forms, which are difficult to detect even using the most sensitive PCR techniques (13). Serology is less reliable (13) and obtaining suitable blood samples from carcasses is problematic. PCR with tissue samples has so far been the most sensitive and specific method to identify leprosy bacilli in red squirrels (13, 20, 22). Nevertheless, only a small portion of tissue was screened for each animal with a preference for the pinnae, previously reported to be the most productive site for molecular screening (13). Investigation is ongoing to establish the best site and localization for effective detection of leprosy bacilli. Pending these investigations, false negative results cannot be excluded especially for paucibacillary cases. Moreover, our sample set included juvenile and sub-adult individuals, which may also limit the chance of detecting leprosy if disease progression is as slow as in the other known hosts (24). Currently, nothing is known about the timescale of leprosy bacilli infection and disease development in squirrels. It is, therefore, important to intensify research on leprosy progression in squirrels and to expand our detection toolkit to inform and enable future surveillance efforts.
Great Britain, Germany, Switzerland and France have been free of autochthonous human leprosy cases for decades or even centuries (14, 25–27), while autochthonous cases have been noted in more recent times in Italy (28). There are no obvious reasons why continental Eurasian red squirrels are less likely to have had contact with leprosy bacilli from a human source in the past than British squirrels, especially in countries where autochthonous human cases, albeit rare, still appear. For all non-indigenous squirrel species, the time of their introduction to Europe and the existence of leprosy in their native range may be informative. Gray squirrels, Pallas's squirrels, and Siberian chipmunks were introduced at different locations in Europe between 1870's and 1980's, long after human leprosy was eliminated from most European countries (29–32). When looking at their countries of origin, the Asian range of Pallas's squirrels does overlap with regions in which human leprosy is still endemic (33) and when the Siberian chipmunks were first introduced to Europe, human cases still existed in Korea for example (34). The natural range of Eastern gray squirrels does not overlap with areas where leprosy was endemic in humans. However, Eastern gray squirrels have replaced Eurasian red squirrels in large parts of the British Isles and currently share the same habitat in some regions where possible transmission or infection could occur as it has been shown for other pathogens (35, 36). However, we found no evidence of this. The white-throated woodrat samples came from an area in Mexico where autochthonous human leprosy cases occur and these animals are consumed as a food-source. Again, no evidence of infection was found.
Immunogenetics, which plays an important role in human and nine banded armadillo leprosy (37, 38), may also influence the susceptibility of squirrels to leprosy bacilli. Evidence exists for marked differences in disease resistance for different squirrel species (35, 39). Leprosy-specific evidence for this, in the form of polymorphisms in the key innate immunity toll-like receptor gene (TLR1), was recently published (13). Indeed, certain TLR1 polymorphisms were found more frequently in healthy red squirrels infected with leprosy bacilli compared to diseased animals implying that they may prevent the development of clinical disease. Further investigation of the functional genomics of different squirrel populations is required to understand why some Eurasian red squirrels are susceptible to leprosy bacilli while others are not, and whether higher genetic diversity on the European continent (39) prevents infection of Eurasian red squirrels here.
This is the first study to investigate the presence of leprosy bacilli in wild populations of squirrels outside the British Isles and in Mexican white-throated woodrats. The lack of detection of leprosy bacilli or clinical signs of leprosy was unexpected given the prevalence of leprosy in red squirrel populations in the British Isles. It shows that further targeted surveillance efforts and increased knowledge of the genetic determinants of leprosy resistance in animal hosts are necessary to fully understand the disease dynamics of leprosy in wildlife populations.
Author Contributions
A-KS, CA, SC, J-LC, LV-C, AM, KS, PL, and JD-P conceived and designed the study. RU, J-LC, NF, CR, MM, BP, A-KS, WE-F, PL, CS, and JO-C collected samples. A-KS, CA, JM, PB, WE-F, and JO-C extracted DNA. A-KS, CA, JM, KS, PB, WE-F, and JO-C conducted molecular analysis. A-KS and JD-P conducted histopathological analysis. A-KS, CA, SC, KS, AM, PL, and JD-P analyzed and interpreted the data. A-KS, KS, CA, SC, and AM wrote the first draft of the manuscript. RU, CS, and PL wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
Financial support for this study came from the University of Edinburgh Principal's Career Development Scholarship, the Scottish Government Rural and Environment Science and Analytical Services Division, the Fondation Raoul Follereau, the Swiss National Science Foundation grant IZRJZ3_164174 and EU LIFE14 NAT/UK/000467–SciuriousLIFE. The funders had no role in study design, data collection, and interpretation, or the decision to submit the work for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We gratefully acknowledge the provision of gray squirrel samples from the British Isles by Chris Fairgrieve, Ian Kerr, and Douglas Ryder, as well as the assistance of Charles Dutton, Morgan Charbonnier and Marion Jourdan in providing the necessary contact. We thank Korinna Seybold, Sonja Jonas, Sabine Gallenberger, and Tanja Schäfer for supplying us with red squirrel samples from Germany; Dörte Kaufmann for coordinating dissection and shipment of samples from Germany; Luc Wauters for assisting with sample collection in Italy; Jacques Renaud and Laurent Cavallini for collecting carcasses in Switzerland. We thank the staff of the Easter Bush Pathology and Histopathology for their support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2019.00008/full#supplementary-material
Table S1. Overall dataset information and results.
References
2. Han XY, Seo YH, Sizer KC, Schoberle T, May GS, Spencer JS, et al. A new Mycobacterium species causing diffuse lepromatous leprosy. Am J Clin Pathol. (2008) 130:856–64. doi: 10.1309/AJCPP72FJZZRRVMM
3. Goulart IMB, Bernardes Souza DO, Marques CR, Pimenta VL, Gonçalves MA, Goulart LR. Risk and protective factors for leprosy development determined by epidemiological surveillance of household contacts. Clin Vaccine Immunol. (2008) 15:101–5. doi: 10.1128/CVI.00372-07
5. Walsh GP, Meyers WM, Binford CH. Naturally acquired leprosy in the nine- banded armadillo: a decade of experience 1975-1985. J Leukoc Biol. (1986) 40:645–56.
6. Donham KJ, Leininger JR. Spontaneous leprosy-like disease in a chimpanzee. J Infect Dis. (1977) 136:132–6.
7. Leininger JR, Donham KJ, Meyers WM. Leprosy in a chimpanzee. Postmortem lesions. Int J Lepr Mycobact Dis Off Organ Int Lepr Assoc. (1980) 48:414–21.
8. Gormus BJ, Wolf RH, Baskin GB, Ohkawa S, Gerone PJ, Walsh GP, et al. A second sooty mangabey monkey with naturally acquired leprosy: first reported possible monkey-to-monkey transmission. Int J Lepr Mycobact Dis Off Organ Int Lepr Assoc. (1988) 56:61–5.
9. Honap TP, Pfister L, Housman G, Mills S, Tarara P, Suzuki K, et al. Mycobacterium leprae genomes from naturally infected nonhuman primates. PLoS Negl Trop Dis. (2018) 12:e0006190. doi: 10.1371/journal.pntd.0006190
10. Meyers WM, Gormus BJ, Walsh GP, Baskin GB, Hubbard GB. Naturally acquired and experimental leprosy in nonhuman primates. Am J Trop Med Hyg. (1991) 44:24–7.
11. Truman RW, Singh P, Sharma R, Busso P, Rougemont J, Paniz-Mondolfi A, et al. Probable zoonotic leprosy in the Southern United States. N Engl J Med. (2011) 364:1626–33. doi: 10.1056/NEJMoa1010536
12. Clark BM, Murray CK, Horvath LL, Deye GA, Rasnake MS, Longfield RN. Case-control study of armadillo contact and Hansen's disease. Am J Trop Med Hyg. (2008) 78:962–7. doi: 10.4269/ajtmh.2008.78.962
13. Avanzi C, Benjak A, Stevenson K, Simpson VR, Busso P, Mcluckie J, et al. Red squirrels in the British Isles are infected with leprosy bacilli. Science (2016) 354:744–8. doi: 10.1126/science.aah3783
14. Fulton N, Anderson LF, Watson JM, Abubakar I. Leprosy in England and wales 1953-2012: surveillance and challenges in low incidence countries. BMJ Open (2016) 6:e010608. doi: 10.1136/bmjopen-2015-010608
15. Lew J, Yang YT, Pyun WS. Experimental infection of the Korean chipmunk (Tamias Sibiricus asiaticus, Gmelin) with M. leprae. Int J Lepr Other Mycobact Dis. (1974) 42:193–202.
16. Galetti G, Cavicchi G, Ussia G. Replication of Mycobacterium leprae in hibernating ground squirrels (Citellus tridecemlineatus). Acta Leprol. (1982) 88:23–31.
17. Rojas-Espinosa O, Lovik M. Mycobacterium leprae and Mycobacterium lepraemurium infections in domestic and wild animals. Rev Sci Tech. (2001) 20:219–51. doi: 10.20506/rst.20.1.1271
18. Singh P, Benjak A, Schuenemann VJ, Herbig A, Avanzi C, Busso P, et al. Insight into the evolution and origin of leprosy bacilli from the genome sequence of Mycobacterium lepromatosis. Proc Natl Acad Sci USA. (2015) 112:4459–64. doi: 10.1073/pnas.1421504112
19. Vera-Cabrera L, Escalante-Fuentes W, Ocampo-Garza SS, Ocampo-Candiani J, Molina-Torres CA, Avanzi C, et al. Mycobacterium lepromatosis infections in Nuevo León, Mexico. J Clin Microbiol. (2015) 53:1945–6. doi: 10.1128/JCM.03667-14
20. Meredith A, del-Pozo J, Smith S, Milne E, Stevenson K, McLuckie J. Leprosy in red squirrels in Scotland. Vet Rec. (2014) 175:285–6. doi: 10.1136/vr.g5680
21. Simpson V, Hargreaves J, Butler H, Blackett T, Stevenson K, McLuckie J. Leprosy in red squirrels on the Isle of Wight and Brownsea Island. Vet Rec. (2015) 177:206–7. doi: 10.1136/vr.h4491
22. Butler HM, Stevenson K, McLuckie J, Simpson V. Further evidence of leprosy in Isle of Wight red squirrels. Vet Rec. (2017) 180:407. doi: 10.1136/vr.j1920
23. Lastória JC, de Abreu MAMM. Leprosy: review of the epidemiological, clinical, and etiopathogenic aspects - Part 1. An Bras Dermatol. (2014) 89:205–18. doi: 10.1590/abd1806-4841.20142450
24. Scollard DM, Adams LB, Gillis TP, Krahenbuhl JL, Truman RW, Williams DL. The continuing challenges of leprosy. Clin Microbiol Rev. (2006) 19:338–81. doi: 10.1128/CMR.19.2.338-381.2006
25. Reibel F, Chauffour A, Brossier F, Jarlier V, Cambau E, Aubry A. New insights into the geographic distribution of Mycobacterium leprae SNP genotypes determined for isolates from leprosy cases diagnosed in metropolitan France and French territories. PLoS Negl Trop Dis. (2015) 9:e0004141. doi: 10.1371/journal.pntd.0004141
27. Gilsdorf A. Infektionsepidemiologisches Jahrbuch meldepflichtiger Krankheiten für 2016. Berlin (2017) 143–144. doi: 10.17886/rkipubl-2017-002
28. Cusini M, Marzano AV, Barabino G, Benardon S. A case of autochthonous leprosy in an elderly Italian patient leaving in Milan province with peculiar clinical presentation. J Am Acad Dermatol. (2017) 76:AB3. doi: 10.1016/j.jaad.2017.04.030
29. Bertolino S, Mazzoglio PJ, Vaiana M, Currado I. Activity budget and foraging behavior of introduced Callosciurus finlaysonii (Rodentia, Sciuridae) in Italy. J Mammal. (2004) 85:254–9. doi: 10.1644/BPR-009
30. Chapuis J-L, Obolenskaya E, Pisanu B, Lissovsky A. Datasheet onTamias Sibiricus. Wallingford, UK: CABI, Invasive Species Compendium (2011). Available online at: http://www.cabi.org/isc/?compid=5&dsid=62788&loadmodule=datasheet&page=481&site=144 (Accessed Jan 22, 2019).
31. Martinoli A, Bertolino S, Preatoni DG, Balduzzi A, Marsan A, Genovesi P, et al. Headcount 2010: the multiplication of the grey squirrel introduced in italy. Hystrix (2010) 21:127–36. doi: 10.4404/Hystrix-21.2-4463
32. Signorile AL, Reuman DC, Lurz PWW, Bertolino S, Carbone C, Wang J. Using DNA profiling to investigate human-mediated translocations of an invasive species. Biol Conserv. (2016) 195:97–105. doi: 10.1016/j.biocon.2015.12.026
33. Lurz PWW, Hayssen V, Geissler K, Bertolino S. Callosciurus erythraeus (Rodentia: Sciuridae). Mamm Species (2013) 902:60–74. doi: 10.1644/902.1
34. Lee J, Kim J-P, Nishikiori N, Fine PEM. The decline of leprosy in the Republic of Korea; patterns and trends 1977–2013. Lepr Rev. (2015) 86:316–27.
35. Chantrey J, Dale TD, Read JM, White S, Whitfield F, Jones D, et al. European red squirrel population dynamics driven by squirrelpox at a gray squirrel invasion interface. Ecol Evol. (2014) 4:3788–99. doi: 10.1002/ece3.1216
36. Romeo C, Ferrari N, Lanfranchi P, Saino N, Santicchia F, Martinoli A, et al. Biodiversity threats from outside to inside: effects of alien grey squirrel (Sciurus carolinensis) on helminth community of native red squirrel (Sciurus vulgaris). Parasitol Res. (2015) 114:2621–8. doi: 10.1007/s00436-015-4466-3
37. Adams LB, Pena MT, Sharma R, Hagge DA, Schurr E, Truman RW. Insights from animal models on the immunogenetics of leprosy - A review. Mem Inst Oswaldo Cruz. (2012) 107:197–208. doi: 10.1590/S0074-02762012000900028
38. Reibel F, Cambau E, Aubry A. Update on the epidemiology, diagnosis, and treatment of leprosy. Med Mal Infect. (2015) 45:383–93. doi: 10.1016/j.medmal.2015.09.002
Keywords: leprosy, squirrels, white-throated woodrats, PCR, Mycobacterium leprae, Mycobacterium lepromatosis
Citation: Schilling A-K, Avanzi C, Ulrich RG, Busso P, Pisanu B, Ferrari N, Romeo C, Mazzamuto MV, McLuckie J, Shuttleworth CM, Del-Pozo J, Lurz PWW, Escalante-Fuentes WG, Ocampo-Candiani J, Vera-Cabrera L, Stevenson K, Chapuis J-L, Meredith AL and Cole ST (2019) British Red Squirrels Remain the Only Known Wild Rodent Host for Leprosy Bacilli. Front. Vet. Sci. 6:8. doi: 10.3389/fvets.2019.00008
Received: 05 November 2018; Accepted: 14 January 2019;
Published: 01 February 2019.
Edited by:
Dirk Werling, Royal Veterinary College (RVC), United KingdomReviewed by:
Richard W. Truman, Louisiana State University, United StatesKevin Bown, University of Salford, United Kingdom
Carolina Perez-Heydrich, Meredith College, United States
Copyright © 2019 Schilling, Avanzi, Ulrich, Busso, Pisanu, Ferrari, Romeo, Mazzamuto, McLuckie, Shuttleworth, Del-Pozo, Lurz, Escalante-Fuentes, Ocampo-Candiani, Vera-Cabrera, Stevenson, Chapuis, Meredith and Cole. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna L. Meredith, YW5uYS5tZXJlZGl0aEB1bmltZWxiLmVkdS5hdQ==
Stewart T. Cole, c3Rld2FydC5jb2xlQHBhc3RldXIuZnI=
 Anna-Katarina Schilling1,2
Anna-Katarina Schilling1,2 Rainer G. Ulrich
Rainer G. Ulrich Maria Vittoria Mazzamuto
Maria Vittoria Mazzamuto Craig M. Shuttleworth
Craig M. Shuttleworth Stewart T. Cole
Stewart T. Cole