
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Vet. Sci., 18 January 2019
Sec. Veterinary Emergency and Critical Care Medicine
Volume 5 - 2018 | https://doi.org/10.3389/fvets.2018.00336
After a safety review of hydroxyethyl starch (HES) solutions in 2013, restrictions on the use of HES were introduced in the European Union (EU) to reduce the risk of kidney injury and death in certain patient populations. Similar restrictions were introduced by the Food and Drug Administration in the United States and other countries. In October 2017, a second safety review of HES solutions was triggered by the European pharmacovigilance authorities based on a request by the Swedish Medical Products Agency to completely suspend HES. After several meetings and repeated evaluations, the recommendation to ban HES was ultimately not endorsed by the responsible committee; however, there was a vote for more restricted access to the drug and rigorous monitoring of policy adherence. This review delineates developments in the European pharmacovigilance risk assessment of HES solutions between 2013 and 2018. In addition, the divergent experts' opinions and the controversy surrounding this official assessment are described. As the new decisions might influence the availability of HES products for veterinary patients, potential alternatives to HES solutions, such as albumin solutions and gelatin, are briefly discussed.
First introduced in the 1960s, hydroxyethyl starch (HES) rapidly became the most commonly used synthetic colloid in human intensive care units (ICUs) throughout the world, with over 60 products registered in Europe and four in the United States (US) by the year 2010 (1–3). The first veterinary reviews advocating the use of HES date from the 1980s and, until recently, veterinarians have been widely using these products to treat anesthesia-induced hypotension and hypovolemia non-responsive to crystalloids (4, 5). Additionally, HES has been used to increase intravascular colloid osmotic pressure in hypoalbuminemic animals by administering it as a low-dose constant rate infusion over several days (6–8). However, an increasing number of potential side effects of HES administration in both humans and animals have since come to light. These include tissue accumulation, acute kidney injury (AKI), coagulopathies and bleeding tendencies, anaphylactoid reactions, and pruritus (the latter only described in humans) (9–11). The nephrotoxic effects of HES have been proposed to be secondary to renal tissue uptake and intracellular storage based on studies in small populations of patients (12, 13). As HES molecules cannot be degraded once they leave the blood, HES leads to vacuolization, swelling, and subsequent cellular dysfunction in the kidney (12, 13). HES-induced coagulopathies and bleeding are suggested to be the result of direct effects on hemostasis through impaired fibrinogen/fibrin polymerization and platelet dysfunction leading to a weaker clot (14, 15).
Awareness of these side effects has resulted in a succession of changes to the warnings, restrictions, and contraindications on HES product packaging (Table 2) and prompted lively debates in the intensive care community about the potential risks and theoretical benefits of HES administration. This review traces back the history and summarizes the current state of regulations on HES use in human medicine. Its purpose is to inform the reader on the origins of the controversy surrounding HES use. Furthermore, a brief overview on HES-related veterinary literature as well as alternatives to HES is presented to help the reader understand the impact (or lack thereof) of the potential loss of access to HES for veterinary patients.
In 2008, the Efficacy of Volume Substitution and Insulin Therapy in Severe Sepsis (VISEP) trial, a multicenter human randomized controlled trial (RCT) conducted in 537 septic patients in Germany, found higher rates of AKI and need for renal-replacement therapy (RRT) associated with the use of 10% HES 200/0.5 than with Ringer's lactate (16). In this study, a number of patients received higher than recommended dosages of the hyperoncotic HES 200/0.5 (>22 ml/kg per day); however, the increased risk to need RRT was also seen in patients treated with HES 200/0.5 at the recommended daily doses (16). This trial was followed by three other large human multicenter RCTs in critically ill or septic patients in 2012 (17–19). The Scandinavian Starch for Severe Sepsis/Septic Shock (6S trial) involving 798 septic patients, found an increased requirement for RRT and higher mortality at 90 days in patients receiving 6% HES 130/0.42 compared to Ringer's acetate (17). The Crystalloid vs. Hydroxyethyl Starch Trial (CHEST trial) involving 7,000 critically ill patients in Australia and New Zealand, also found a higher requirement for RRT but no difference in 90-day mortality in patients receiving 6% HES 130/0.4 compared to saline (18). Finally, the Assessment of Hemodynamic Efficacy and Safety of 6% Hydroxyethylstarch 130/0.4 vs. 0.9% NaCl Fluid Replacement in Patients with Severe Sepsis (Crystalloids Morbidity Associated with severe Sepsis, CRYSTMAS) trial involving 196 septic patients in France and Germany, found no difference in adverse events, including AKI and mortality, between patients receiving 6% HES 130/0.4 compared to saline (19).
Simultaneously with the aforementioned trials, between 2009 and 2013, one of the largest scandals of scientific misconduct in history was unraveled (20, 21). This involved retractions of over 90 HES-related scientific articles of the (back then) prominent German anesthetist and prolific defender of HES, Joachim Boldt, for data fabrication and lack of ethics approval (22, 23). Data from studies by Boldt and coworkers were included in systematic reviews and used to form clinical guidelines worldwide (24, 25). In particular, re-evaluation of one meta-analysis that originally reported no association between HES administration and all-cause mortality, revealed a significantly increased risk of mortality and AKI when 7 studies from Boldt were subsequently excluded from the analysis (26). This elucidates the fact that the fraudulent data from Boldt's many years of research, which mostly favored HES, might have previously tipped the “meta-analytical scales” and masked the potential harm of HES products for many years (21).
Following, VISEP (16), 6S (17), CHEST (18), and CRYSTMAS (19), both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) initiated a safety review of HES products. Both agencies reviewed data from RCTs, meta-analyses and observational studies, with a particular emphasis on 6S (17) (FDA and EMA), CRYSTMAS (19) (FDA only), and CHEST (18) and VISEP (16) (EMA only) (3). The EMA review was started in November 2012 by the Pharmacovigilance Risk Assessment Committee (PRAC) as a referral procedure (Article 31 of Directive 2001/83/EC) and was initiated at the request of the German medicines agency (Data Sheet 1) (27, 28) (Table 1). The PRAC is a section of the EMA composed of experts from each of the member states of the EU as well as members representing patient organizations responsible for all aspects of risk management of medicines (43). In June 2013, the PRAC issued a recommendation to suspend marketing authorization for all HES products as it concluded that the risks of HES outweighed its benefits (29) (Table 1). In the interim, HES was banned by the British Medicines and Healthcare Products Regulatory Agency, and a drug alert was issued in the United Kingdom calling for the return of all HES products to the manufacturers (44). Simultaneously, the United Kingdom triggered an urgent Union procedure (Article 107i of Directive 2001/83/EC) which was performed by PRAC as well (31). Such procedure is automatically triggered when a member state considers suspending marketing authorization or prohibiting supply of a medical product (45) (Table 1).

Table 1. Timeline for regulatory key events in 2013 and 2018 for hydroxyethyl starch (HES) restrictions by the European Medicines Agency's (EMA) Pharmacovigilance Risk Assessment Committee (PRAC).
Numerous commentaries in scientific journals were exchanged between experts with different opinions regarding the risks and benefits of HES, and the quality of the aforementioned landmark trials. Concerns raised by HES proponents (and Marketing Authorization Holders [MAHs]) with regard to the VISEP (16), 6S (17), and CHEST (18) trials included that patients were entered into studies several hours after admission to ICU (up to 24 h after) and were possibly hemodynamically stable at randomization, wherefore HES was no longer indicated. Also, a significant number of patients who were randomized to receive HES were in renal failure. Namely, renal failure without RRT was not an absolute contraindication for HES according to the steering committee and scientific advisors of the 6S (17) trial, despite that renal failure was already a contraindication for HES [stated e.g., by the American FDA (46)] before 6S was initiated (47). Some patients randomized to the crystalloid group had received initial treatment with colloids, and there was a lack of specific criteria for starting RRT. Finally, there was no justification for extrapolating findings in critically ill patients to the entire patient population (47–50). HES opponents disagreed with these critiques against VISEP (16), 6S (17), and CHEST (18) and argued that data were misread and misinterpreted by the critics (51).
In March 2013, another large human RCT was published: The Colloids vs. Crystalloids for the Resuscitation of the Critically Ill (CRISTAL) trial randomized 2,857 critically ill patients in 57 ICUs in France, Belgium, North Africa, and Canada, requiring fluid resuscitation for acute hypovolemia with either colloids (gelatins, dextrans, HES, or albumin) or crystalloids (isotonic saline, hypertonic saline, or any other buffered solution) (52). Even though the trial was open-label, the outcome assessment was blinded, and patients received HES within the maximum dose limit. The study found no significant difference in the 28-day mortality between subjects receiving colloids and those receiving crystalloids, but a significant reduction in mortality at 90 days in the colloid group, as well as more vasopressor-free and more ventilator-free days by day 28 (52). Subgroup analysis confirmed a significantly reduced 90-day mortality in patients treated with HES when compared with patients treated with 0.9% saline. In contrast to VISEP (16), 6S (17), and CHEST (18) the authors of CRISTAL (52) recruited patients newly admitted to the ICU as soon as resuscitation was required. Nevertheless, the trial had limitations, such as long duration of the trial (9 years), open-label design, only 70% of patients in the colloid group received HES, overlap between treatments with the different colloids, some patients received more than one type of colloid, and a high proportion of patients in both groups received colloids prior to ICU colloid administration (52).
Following requests for re-examination by MAHs, including Fresenius Kabi and B. Braun Melsungen AG (located in Germany), a second PRAC committee, composed of a different expert group, re-evaluated the evidence (30) (Table 1). The re-examination focused on the benefit-risk ratio of HES in the treatment of hypovolemic shock in surgery and trauma patients (53). Consequently, two reviews were running in parallel by different expert committees of the PRAC: The re-examination under Article 31 of Directive 2001/83/EC [requested by the MAHs) (30)] and the additional review under Article 107i of Directive 2001/83/EC [triggered by the United Kingdom in June 2013 (31)]. Both reviews were finalized in October 2013 but came to different conclusions. The PRAC review under Article 31 maintained its recommendation (from June 2013) for suspension of marketing authorization for HES (53). However, a re-examination only looks at the evidence provided for the original procedure and therefore, this committee did not include new data (53). In contrast, the PRAC review under Article 107i included new data, which were not available or not considered in the referral under Article 31 and did not recommend an absolute HES suspension (54). Factors that led to a different assessment of that review were short-term hemodynamic improvement (55), volume-sparing effect (56), prevention and limitation of edema formation (57), significantly lower estimated blood loss (58), and reduction in red blood cell transfusions (59) in surgical and trauma patients receiving HES (54). Further, studies provided some evidence that the risks of AKI (60, 61) and death (52, 62) in these patients may be lower than in critically ill and septic patients (54). The PRAC's final recommendation on the use of HES solutions was based on the new evidence considered in the Article 107i procedure and the decision was published in October 2013 on the EMA website (32) (Table 1). Notably, in the online regulatory texts provided by the EMA, no difference is made regarding different HES preparations (e.g., second generation HES 200/0.5 vs. third generation HES 130/0.4 or 0.42, respectively). The EMA recommended that HES should no longer be used in critically ill patients or those with sepsis or burn injuries (Table 2), but it could still be administered to patients with hypovolemia due to acute blood loss if treatment with crystalloids was inadequate. In these patients, HES should be only given for initial volume resuscitation with a maximum dose of 30 ml/kg and kidney function should be monitored for 90 days (although the type of monitoring was not specified) (32). Only Annex III “Amendments to relevant sections of the summary of product characteristics and package leaflet” specifically mentioned the HES preparations (HES 130/0.4 and HES 130/0.42, respectively) and stated that for other HES products (e.g., HES 200/0.5) the maximum daily dose should be recalculated accordingly (63).
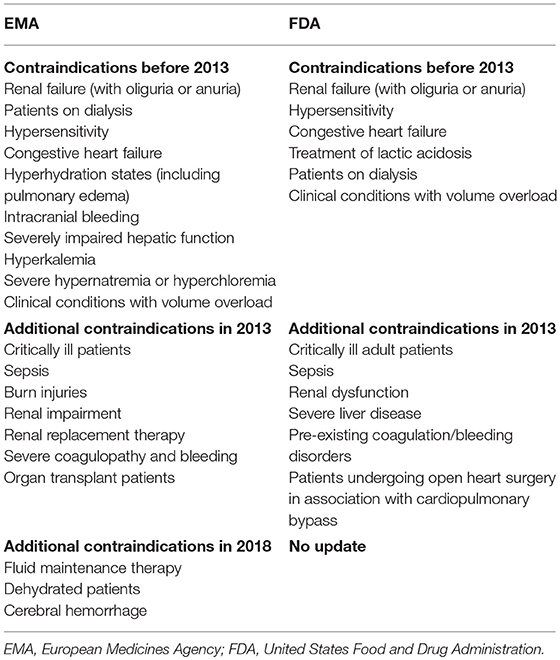
Table 2. Contraindications for hydroxyethyl starch products before and after 2013 in the European Union (EMA) and United States (FDA).
This decision was not unanimously supported, as 14 of the 33 PRAC members voted against the revision (54). Nevertheless, the decision was endorsed by the Coordination Group for Mutual Recognition and Decentralized Procedures—Human (CMDh, responsible for examining questions relating to marketing authorization of human medicines in the EU, composed of one representative per EU Member State) (33), and then by the European Commission, the governing body of the EU, for legal binding in the EU (34) (Table 1). As a condition from the PRAC, the MAHs were asked to conduct drug utilization studies in several member states to evaluate the effectiveness of the risk minimization measures taken. The goal of these drug utilization studies was to characterize prescribing practices during typical clinical use in representative groups of prescribers to verify adherence to the updated product information. As further conditions, changes to the product information, information to the healthcare professionals and patients, and RCTs conducted by the MAHs in order to demonstrate the efficacy and safety of HES in the perioperative and trauma populations were requested (54).
For the FDA review, an expert workshop was set up to review HES safety (64). This led to new safety information added to HES product labeling in November 2013 in the form of a black box warning. It stated not to use HES in critically ill adult patients, and patients with sepsis, severe liver disease, pre-existing coagulopathy, and in patients undergoing open heart surgery in association with cardiopulmonary bypass (Data Sheet 2) (65) (Table 2).
The revised EMA decision was criticized, as it was (for the most part) based on the same studies as the ban had been based on, but with different conclusions (66). HES opponents argued that the risks of HES outweighed the benefits, that there was insufficient evidence that colloid resuscitation improved outcome in surgical and trauma patients, and that a number of other, safer alternative intravenous fluids existed (67, 68). In a 2014 open letter to the Executive Director of the EMA, the authors (overall 70 intensive care researchers) asked, “what assumptions or clinical data would indicate that the same pathological mechanisms [tissue storage with subsequent organ injury, and coagulopathy] do not apply in patients with hypovolemia from blood loss” and argued that the known side effects should be considered to be potential risks in all patient groups. Further, the clinical trials recommended by the PRAC to prove the safety of HES would expose surgical and trauma patients to known risks of harm [e.g., risk of AKI and bleeding] without a proven benefit (67). At the same time, CHEST (18) investigators were heavily criticized by HES defenders due to changes in their methods, statistical analysis, and data after publication in 2012 as well as refusal to share their raw data for independent reanalysis (69, 70). A reanalysis of the CHEST (18) trial was ultimately published in 2016 (confirming the conclusion of the original article), but only two of the eight authors of this reanalysis were from an independent institution. All other listed authors came from the institution which conducted the original study, including three authors who were part of the original 2012 publication, one of which was the prior study's principal investigator (71).
Based on the 2013 PRAC recommendation for the MAHs to conduct human RCTs, Fresenius Kabi and B. Braun Melsungen AG, together with the European Society of Anesthesiology, launched two RCTs in 2017 (72, 73). The Safety and Efficacy of a 6% Hydroxyethyl Starch Solution vs. an Electrolyte Solution in Trauma Patients (TETHYS) trial will include up to 350 patients with blunt or penetrating trauma suffering from an estimated blood loss of ≥ 500 ml and subsequent hypovolemia, who are undergoing surgery within 24 h. The primary endpoint is 90-day mortality and 90-day renal failure (defined as biomarker increase as defined by AKIN stage 2 or RIFLE injury stage or need for RRT at any time during the first 3 months) (73). The Safety and Efficacy of 6% Hydroxyethyl Starch Solution vs. an Electrolyte Solution in Patients Undergoing Elective Abdominal Surgery (PHOENICS) trial will include up to 2,280 patients undergoing elective abdominal surgery with an expected blood loss of ≥ 500 ml and subsequent hypovolemia. Primary outcome is the difference in glomerular filtration rate between the two treatment groups (72).
The regulatory changes and controversy regarding HES led to a worldwide decrease in synthetic colloid use in human ICUs between 2007 and 2014, with considerable geographic variations and an increase in the use of human albumin instead (74).
In February 2017, the US-based Public Citizen Foundation sent a petition to the FDA requesting the immediate removal of all HES products from the market in the US (75). The FDA has yet to undertake official action. The Public Citizen Foundation also joined other experts in signing an open letter to the EMA urging the executive director to reconsider the 2013 PRAC decision and ban the use of HES products for all patients in Europe (Data Sheet 4) (76). In addition, in October 2017, the Swedish Medical Products Agency requested a review of HES products and considered its suspension due to concerns regarding non-compliance to restrictions in their use (Data Sheet 3) (77). Two survey-based drug utilization studies conducted in 2016 and 2017, involving 11 European countries, showed non-adherence to the 2013 PRAC restrictions of up to 77% (77). They showed that HES solutions have continued to be used in high-risk populations (up to 34% of patients) (Data Sheet 5) (78, 79).
In October 2017, the EMA started a new review of HES under Article 107i of Directive 2001/83/EC (35) (Table 1). Upon request from the PRAC, the EMA convened an ad-hoc expert group meeting in December 2017 [minutes of this meeting are not publicly available (80)]. In January 2018, the PRAC recommended removing all HES products from the European market (36) (Table 1). The recommendation was endorsed by the CMDh (37) and forwarded to the European Commission (Table 1). Several experts worldwide supported this decision by publishing open letters and comments in scientific journals (81, 82). A group of British, Australian, Danish, and German scientists [some of them 6S (17) or CHEST (18) trial investigators] even appealed to the World Health Organization (WHO), demanding a worldwide ban of HES (82). In contrast, a group of European scientists [some of them CRISTAL (52) trial investigators] responded that the recommendation to suspend HES was “not scientifically grounded and is potentially hazardous to patients” (83). In addition, experts who were part of the EMA ad-hoc expert group from December 2017 complained that part of their recommendation not to suspend HES was left out in the official PRAC recommendation on the EMA website (80, 84). Criticism regarding the PRAC recommendation also came from a group of 19 European anesthesia societies in the form of an open letter to the European Commission (85). In this letter, they urged the European Commission to denounce the suspension of HES products. They criticized the validity of the survey-based drug utilization studies that showed non-compliance of PRAC recommendations, because respondents could only select dehydration or overhydration as a reason for administering HES (no check box for hypovolemia was provided). Moreover, it was unclear as to whether or not some septic patients had received HES before the development of sepsis. It was also criticized that suspension of HES would lead to unmet clinical needs for colloids in specific situations, such as plasmapheresis, pediatric cardiac surgery, and prevention of hypotension in patients undergoing cesarean section with spinal anesthesia. They claimed that if HES were to be suspended, there would be no alternative colloid, as dextran, gelatin, or albumin are not superior to HES. Finally, the experts also strongly recommended that HES should not be suspended before the results of the PHOENICS (72) and TETHYS (73) trials become available (84). Not surprisingly, European HES solution manufacturers also claimed that HES should remain available on the market, stating that the off-label use of a product by clinicians is not a sufficient argument to withdraw it from the market (86, 87). Accordingly, the new PRAC recommendation and CMDh position were referred back to the EMA by the European Commission in April 2018, as the concern regarding unmet medical needs (e.g., no safe alternative to HES solutions) and the feasibility and effectiveness of risk minimization measures (e.g., changes to the product information, direct health care professional communication) had not been adequately addressed (38) (Table 1).
In May 2018, after re-assessing data on these specific aspects, the PRAC confirmed its previous recommendation that HES solutions should be suspended and sent it again to the CMDh for consideration (39) (Table 1). For the re-assessment, the PRAC reviewed “all newly available data since the previous referral procedures, including results from drug utilization studies, clinical studies, meta-analyses of clinical studies, post-marketing experience, EudraVigilance data (adverse reactions reporting system), literature reviews, responses submitted by MAHs as well as stakeholders' submissions (e.g., different European anesthesia societies), and views expressed by experts during an ad-hoc experts meeting” (40). In regard to the efficacy of HES, the PRAC concluded that although a volume-sparing effect in patients undergoing surgery was demonstrated in some studies, it remained uncertain to what extent this leads to improved postoperative outcomes (88–90). In regard to safety in septic, critically ill, and surgical patients, the quality of new studies were deemed to be insufficient to change the existing restriction (40). In fact, since 2013, some post-hoc analyses of the 6S (17) trial have been published, evaluating AKI in the first 5 days (results in favor of crystalloids) (91), the risk of bleeding and death (results in favor of crystalloids) (92), cytokine concentrations (results reveal no difference between crystalloids and HES) (93), and endothelial damage (results in favor of HES) (94). However, there have been no large RCTs performed, comparable with the previous landmark trials VISEP (16), 6S (17), and CHEST (18). In summary, the PRAC concluded that the volume-sparing effect of HES in patients with hypovolemia is only modest, the key results of the two drug utilization studies were reliable, and that non-adherence to the revised product information was high. Accordingly, the PRAC stated that the benefits of HES solutions did not outweigh the risks, its continued use raised important public health concerns, and current risk minimization measures (e.g., information on the package insert) were inadequate (40).
This time, the CMDh disagreed with the conclusions of the PRAC and decided that HES products should not be withdrawn from the market in June 2018 (41) (Table 1). The rationale for this dissenting decision was the potential unmet medical needs in some of the EU member states, in which HES alternatives may be of limited availability or very expensive. In addition, measures to minimize risk were to be implemented, including contraindications (Table 2), limitations on supply to accredited hospitals, training of healthcare professionals, and additional packaging warnings (42). Like the 2013 sanctions, the PRAC recommended that HES use should be limited to initial volume resuscitation with a dose not exceeding 30 ml/kg over a period of administration not exceeding 24 h, and that kidney function should be monitored for at least 90 days thereafter (Data sheet 3) (42).
At the time of writing this review and to the authors' knowledge, no new regulations beyond the 2013/2014 restrictions have been published from non-EU countries, such as Canada, US, Australia, New Zealand, and Switzerland.
Synthetic colloids are widely used in veterinary medicine with HES being the most frequently used according to a recent international internet-based survey in small animals from 2016 (4) and a previous Veterinary Information Network-based survey from 2013 (95). According to the survey from 2016 (4), HES was selected as the most frequently used synthetic colloid by 84%, gelatin by 4.3%, and dextran by 2.7% of the survey participants. Several review articles critically highlighting the expected effects (e.g., volume effect, increase in colloid-osmotic pressure, plugging endothelial leaks), limitations, and side effects of synthetic colloids, and HES in particular, were recently published (10, 96–99). However, no official guidelines from veterinary experts on the use of HES have thus far been provided. The gaps in species-specific HES-related evidence in veterinary medicine has led to extrapolation of previous indications from human medicine with some exceptions. Notably, many veterinarians seem to use HES as a constant rate infusion for colloid osmotic support, which is uncommon in human medicine (4, 8). Not surprisingly, the changes in human recommendations impacted veterinary medicine. Reduction of frequency, dose, and length of administration of HES products was noted in the 2016 survey (4), with 71% of participants having changed their use of HES due to safety concerns. Of these, AKI and coagulopathy were most often considered contraindications for HES (4). Participants who completely stopped using HES, replaced it with isotonic crystalloids in 85%, plasma in 63%, hypertonic saline in 57%, albumin in 28%, and other/unspecified in 3% of respondents. About half of these participants reported using vasopressors more frequently (4).
The evidence regarding the risk of HES-induced AKI in dogs and cats remains controversial. Four retrospective studies found no increased risk of AKI after 6% HES 130/0.4 (tetrastarch) administration in dogs and cats (100–103), and one retrospective study found evidence for an increased risk of AKI and mortality in dogs receiving 10% HES 200/0.5 (pentastarch) (104). Reasons for these discrepant findings may lie in different types of HES products used (older-generation pentastarch in the study that found increased incidence of AKI vs. newer generation tetrastarch in the other four studies), definitions of AKI, co-morbidities, severity of illness scores and dose regimens. Prospective RCTs evaluating HES-induced AKI in critically ill and/or anesthetized dogs or cats are still lacking. Only one recent study in a small population of dogs undergoing emergency abdominal surgery reported increased levels of urinary neutrophil gelatinase-associated lipocalin (NGAL) after 6% tetrastarch administration compared to the use of an isotonic crystalloid after surgery (105). Notably, this study was not designed to compare AKI incidence after HES vs. crystalloid use, fluid administration was not randomized, and therefore results should be interpreted with caution. In a prospective crossover study in healthy, anesthetized dogs with acute controlled hemorrhage, resuscitation with 20 ml/kg 6% tetrastarch did not reveal evidence of AKI (assessed by urine and plasma NGAL and creatinine) for up to 72 h after its administration (106). Likewise, a recent abstract presented a controlled hemorrhagic shock model in anesthetized dogs treated with a bolus of either 6% tetrastarch, 4% succinylated gelatine, fresh whole blood, or an isotonic crystalloid, HES did not lead to a greater incidence of AKI (assessed by different renal biomarkers, e.g., urinary NGAL, and renal histopathology) compared to the other resuscitations fluids (107). Furthermore, a 72-h infusion of 6% tetrastarch at 50 ml/kg per 24 h did not significantly impact renal function (assessed by urinary NGAL and renal histopathology) in healthy dogs as shown in a recent study presented in an abstract (108). The small study populations and the studies' experimental nature, not representative of the typical cohort of critically ill patients, needs to be taken in consideration before clinical decisions can be based on these results. Furthermore, scientific abstracts often present only partial information and are not submitted to the full scrutiny of a peer-reviewed process reviewing the entire dataset, methods, and limitations of the respective study.
A fair amount of in vitro and in vivo studies evaluating the effects of 6% tetrastarch on hemostasis exists in dogs (8, 109–116), but only one in vitro study in cats (117). In dogs administered 6% tetrastarch, a dose-dependent impairment of platelet function and changes in viscoelastic coagulation testing (rotational thromboelastometry [ROTEM] or thromboelastography) in healthy dogs (109, 110, 113, 114), dogs with controlled hemorrhagic shock (112, 116), and dogs with sepsis was found (111). However, no difference in platelet function was found between 20 ml/kg of 6% tetrastarch and a 3- to 4-fold volume of 0.9% NaCl in healthy dogs (in vitro) (114) or in dogs with controlled hemorrhagic shock (in vivo) (112). These findings suggest that 6% tetrastarch does not cause platelet dysfunction beyond the effects of hemodilution alone. In a similar experimental model in dogs with controlled hemorrhagic shock, dogs received 20 ml/kg of 6% tetrastarch, 4% succinylated gelatine, fresh whole blood, or 80 ml/kg of an isotonic crystalloid solution. Plasmatic coagulation testing and ROTEM showed evidence of mild hypocoagulability beyond hemodilution after HES and gelatin administration, with gelatin administration leading to impaired platelet function and HES administration causing hypocoagulable ROTEM and plasma coagulation assays (116). In dogs with naturally-occurring hemorrhagic shock due to spontaneous hemoperitoneum receiving boluses of either 10 ml/kg of 6% tetrastarch or 30 ml/kg of isotonic crystalloid, an exacerbation of the pre-existing coagulopathy was found after both solutions in plasma coagulation and ROTEM assays, with more pronounced effects on ROTEM after HES (118). Therefore, HES should be avoided or used with caution in dogs at risk for hemorrhage or with pre-existing coagulopathy.
The impact HES restrictions will have on veterinary practice in Europe and elsewhere is uncertain and alternative products will have to be considered. Potential available replacements for HES in people [that have been said to be safer but just as effective, as stated e.g., by a regulatory citizen petition (75)], are gelatin solutions (Europe), dextran (US), albumin solutions, and isotonic crystalloids. A detailed discussion of the benefits and risks of each of these products is beyond the scope of this review. However, significantly less evidence exists in the small animal literature about the efficacy and safety of gelatin and dextran compared to HES.
Gelatin solutions are derived from the degradation of bovine collagen with subsequent chemical modifications to increase solubility (119). Due to their smaller molecular weight compared to HES, gelatins are rapidly excreted by the kidneys and provide a shorter and less pronounced intravascular volume effect (10). Similar to HES, gelatin solutions were introduced into clinical practice in the 1960s before current information requirements for licensing and extensive safety studies were mandatory (119). A meta-analysis in humans concluded that the safety and efficacy of gelatins cannot be assessed based on available evidence despite 60 years of use (120). Gelatin solutions increase the risk of anaphylaxis and may further be harmful by increasing mortality through renal failure and bleeding due to extravascular uptake and coagulation impairment, respectively (121). In a recent prospective study in people with severe sepsis, AKI occurred in 70% of patients receiving HES and in 68% of patients receiving gelatin vs. 47% patients receiving crystalloids. Moreover, in the same study, fluid resuscitation with only crystalloids was equally effective (122). Gelatin has been investigated much less in dogs or cats than HES. Only a few and mostly experimental studies (not related to the risk of AKI) on the use of gelatins in dogs and cats have been published (123–129). In a recent abstract, a greater incidence of kidney injury after the administration of a 4% gelatin solution compared to the administration of 6% tetrastarch, fresh whole blood, or an isotonic crystalloid in a controlled hemorrhagic shock model in anesthetized dogs was reported (107). In this study, a variety of urinary biomarkers as well as renal histopathology were evaluated, and dogs given gelatin had significantly increased NGAL concentrations (with up to a 23-fold increase) within 3 h and increased cystatin C levels, compared to other treatments. Tubular injury scores assessed by histopathology were comparable across treatments, while microvesiculation (intracellular storage of colloid molecules) was significantly higher in the gelatin group (107). Notably, gelatin was withdrawn from the US market in 1978 due to safety concerns over increased blood viscosity and blood coagulation (130). In other countries (e.g., Germany), it is licensed and despite evidence of AKI after intravenous gelatin administration in people (131), it is not labeled to be contraindicated in kidney disease. Indeed, there is no clear dose or maximum daily limit, and the recommendation on the package insert states that the “maximum daily dose is determined by the degree of hemodilution” (Data Sheet 6) (132, 133).
Dextran, a macromolecular polysaccharide, has been withdrawn from the market in a number of European countries (e.g., Germany) due to its adverse effects, such as anaphylactic reactions, osmotic kidney failure with hypertonic dextran preparations, and impaired coagulation (2). By contrast, dextran is licensed in the US, Russia, China, some eastern European countries, and Scandinavia (e.g., Sweden, Norway) (2). In dogs, dextran and hypertonic dextran have been studied in septic shock secondary to pyometra (134), hemorrhagic shock (135, 136), and gastric dilatation volvulus (137, 138). In addition, investigations of its effects on hemostasis are available (139, 140). Evaluation of the effects of oxypolygelatin and dextran 70 on hemostatic variables in healthy dogs showed that both dextran and oxypolygelatin interfered with hemostatic variables (e.g., plasma coagulation assays, platelet numbers, factor VIII coagulant activity, von Willebrand factor antigen concentration, and platelet function and buccal mucosal bleeding time), but dextran's effect was more profound and prolonged when compared to oxypolygelatin (140).
Canine albumin (e.g., lyophilized canine albumin) manufactured in the US, is currently shipped only to Hong Kong, Taiwan, Singapore, and Canada. Consequently, other countries are forced to use human serum albumin products if they wish to administer albumin to their canine or feline patients. No feline albumin products are currently commercially available. Clear evidence of anaphylactic reactions and life-threatening complications after administration of human serum albumin were reported in dogs (141, 142), although large retrospective studies have not demonstrated a high complication rate with human serum albumin solutions use in critically ill dogs and cats (143, 144).
Despite two PRAC expert committees (in 2013 and 2018) recommending suspension of the use of HES and after several reviews and intense discussions, the European Commission decided against a total suspension of HES in July of 2018. Instead of a suspension, additional restrictions have been implemented, allowing its continued use as long as access is controlled and warnings on the packaging inserts are clearly stated. Most likely, this is not the final episode of the HES “saga” as large human RCTs are currently ongoing. In spite of, or maybe because of the HES-controversy, HES is currently the most studied synthetic colloid in human and veterinary medicine. The complicated process of these safety reviews illustrates the difficulties in decision making regarding such a wide-spread drug, particularly when multiple countries with different claims are involved. In spite of the seeming transparency of regulatory authorities in publicly disclosing data and regulatory documents, the exact methods used in their risk assessment procedures remain convoluted and unclear. This was probably one of the main reasons for a remarkable “flood” of commentaries and editorial letters from different experts with strongly held opinions, further fueling the controversy. This situation also reflects the challenges in designing and implementing clinical trials and appropriately interpreting large amounts of data. In such instances, where substantial controversy exists in a field, it is critical that original data from pivotal trials (such as colloid 6S and CHEST) be made available for independent (re-) analysis (145). Therefore, the reluctance of the authors to share original data further exacerbated the debate (69). Examining the available literature leaves the authors of this review with the impression that HES safety and efficacy has become a matter of opinion rather than evidence. Despite efforts to apply evidence-based medicine, grounded guidelines for HES-related clinical decision making are lacking. If and how the 2018 changes may impact the availability and use of HES in veterinary medicine remains unclear. Moreover, if clinicians seek alternatives to HES, the paucity of evidence for their safety and efficacy in veterinary medicine should also be carefully considered.
K-NA and IY performed the literature search and wrote the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2018.00336/full#supplementary-material
6S, Scandinavian Starch for Severe Sepsis/Septic Shock; AKI, acute kidney injury; CHEST, Crystalloid vs. Hydroxyethyl Starch Trial; CHRYSTMAS, Crystalloids Morbidity Associated with severe Sepsis; CMDh, Coordination Group for Mutual Recognition and Decentralized Procedures—Human; EMA, European Medicines Agency; EU, European Union; FDA, Food and Drug Administration; HES, hydroxyethyl starch; ICU, intensive care unit; MAH, marketing authorization holders; NGAL, neutrophil gelatinase-associated lipocalin; PRAC, Pharmacovigilance Risk Assessment Committee; RCT, randomized clinical trial; RRT, renal-replacement therapy; US, United States; VISEP, Volume Substitution and Insulin Therapy in Severe Sepsis.
1. Finfer S, Liu B, Taylor C, Bellomo R, Billot L, Cook D, et al. Resuscitation fluid use in critically ill adults: an international cross-sectional study in 391 intensive care units. Crit Care (2010) 14:R185. doi: 10.1186/cc9293
2. Schortgen F, Deye N, Brochard L, Group CS. Preferred plasma volume expanders for critically ill patients: results of an international survey. Intensive Care Med. (2004) 30:2222–9. doi: 10.1007/s00134-004-2415-1
3. Wiedermann CJ, Eisendle K. Comparison of hydroxyethyl starch regulatory summaries from the Food and Drug Administration and the European Medicines Agency. J Pharm Policy Pract. (2017) 10:12. doi: 10.1186/s40545-016-0090-6
4. Yozova ID, Howard J, Sigrist NE, Adamik KN. Current trends in volume replacement therapy and the use of synthetic colloids in small animals-an internet-based survey 2016. Front Vet Sci. (2017) 4:140. doi: 10.3389/fvets.2017.00140
6. Hughes D, Boag A. Fluid therapy with macromolecular plasma volume expanders. In: Fluid, Electrolyte, and Acid-Base Disorders in Small Animal Practice. DiBartola SP, editor. St. Louis, MO: Elsevier Saunders (2012). p. 647–64.
7. Rudloff E, Kirby R. Colloid fluid therapy, In: Kirk's Current Veterinary Therapy XV. Bonagura J, Twedt D, editors. St. Louis, MO: Elsevier Saunders (2014). p. 8–14.
8. Botto A, Bruno B, Maurella C, Riondato F, Tarducci A, Mengozzi G, et al. Thromboelastometric assessment of hemostasis following hydroxyethyl starch (130/0.4) administration as a constant rate infusion in hypoalbuminemic dogs. BMC Vet Res. (2018) 14:33. doi: 10.1186/s12917-018-1357-8
9. Westphal M, James MF, Kozek-Langenecker S, Stocker R, Guidet B, Van Aken H. Hydroxyethyl starches: different products–different effects. Anesthesiology (2009) 111:187–202. doi: 10.1097/ALN.0b013e3181a7ec82
10. Adamik KN, Yozova ID, Regenscheit N. Controversies in the use of hydroxyethyl starch solution in small animal emergency and critical care. J Vet Emerg Crit Care (2015) 25:20–47. doi: 10.1111/vec.12283
11. Wiedermann CJ, Joannidis M. Accumulation of hydroxyethyl starch in human and animal tissues: a systematic review. Intensive Care Med. (2014) 40:160–70. doi: 10.1007/s00134-013-3156-9
12. Auwerda JJ, Leebeek FW, Wilson JH, van Diggelen OP, Lam KH, Sonneveld P. Acquired lysosomal storage caused by frequent plasmapheresis procedures with hydroxyethyl starch. Transfusion (2006) 46:1705–11. doi: 10.1111/j.1537-2995.2006.00962.x
13. Dickenmann M, Oettl T, Mihatsch MJ. Osmotic nephrosis: acute kidney injury with accumulation of proximal tubular lysosomes due to administration of exogenous solutes. Am J Kidney Dis. (2008) 51:491–503. doi: 10.1053/j.ajkd.2007.10.044
14. Franz A, Braunlich P, Gamsjager T, Felfernig M, Gustorff B, Kozek-Langenecker SA. The effects of hydroxyethyl starches of varying molecular weights on platelet function. Anesth Analg. (2001) 92:1402–7. doi: 10.1097/00000539-200106000-00008
15. Kozek-Langenecker SA. Effects of hydroxyethyl starch solutions on hemostasis. Anesthesiology (2005) 103:654–60. doi: 10.1097/00000542-200509000-00031
16. Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. (2008) 358:125–39. doi: 10.1056/NEJMoa070716
17. Perner A, Haase N, Guttormsen AB, Tenhunen J, Klemenzson G, Aneman A, et al. Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. N Engl J Med. (2012) 367:124–34. doi: 10.1056/NEJMoa1204242
18. Myburgh JA, Finfer S, Bellomo R, Billot L, Cass A, Gattas D, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. (2012) 367:1901–11. doi: 10.1056/NEJMoa1209759
19. Guidet B, Martinet O, Boulain T, Philippart F, Poussel JF, Maizel J, et al. Assessment of hemodynamic efficacy and safety of 6% hydroxyethylstarch 130/0.4 vs. 0.9% NaCl fluid replacement in patients with severe sepsis:The CRYSTMAS study. Crit Care (2012) 16:R94. doi: 10.1186/cc11358
20. Shafer SL. Notice of retraction. Anesth Analg. (2010) 111:1567. doi: 10.1213/ANE.0b013e3182040b99
22. Farrugia A. The Boldt affair: correcting a collective failure. Anesth Analg. (2012) 115:207. doi: 10.1213/ANE.0b013e318256faf2
23. Editors-in-Chief statement regarding published clinical trials conducted without IRB approval by Joachim Boldt. Minerva Anestesiol. (2011) 77:562–3.
24. Perel P, Roberts I Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. (2007) 2007:CD000567. doi: 10.1002/14651858.CD000567.pub3
25. Roberts I, Alderson P, Bunn F, Chinnock P, Ker K, Schierhout G. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. (2004) 2004:CD000567. doi: 10.1002/14651858.CD000567.pub2
26. Zarychanski R, Abou-Setta AM, Turgeon AF, Houston BL, McIntyre L, Marshall JC, et al. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis. JAMA (2013) 309:678–88. doi: 10.1001/jama.2013.430
27. European Medicines Agency. 03/03/2015: Questions & Answers on Article 31 Pharmacovigilance Referral Procedures. Available online at: https://www.ema.europa.eu/documents/regulatory-procedural-guideline/questions-answers-article-31-pharmacovigilance-referral_en.pdf
28. European Medicines Agency. 11/29/2012: Review of Hydroxyethyl-Starch-Containing Solutions for Infusion Started. Available online at: https://www.ema.europa.eu/documents/referral/hydroxyethyl-starch-article-31-referral-review-started_en.pdf
29. European Medicines Agency. 06/14/2013: PRAC Recommends Suspending Marketing Authorisations for Infusion Solutions Containing Hydroxyethyl-Starch. Available online at: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Solutions_for_infusion_containing_hydroxyethyl_starch/Recommendation_provided_by_Pharmacovigilance_Risk_Assessment_Committee/WC500144448.pdf
30. European Medicines Agency. 07/12/2013: Recommendation to Suspend Marketing Authorisations for Hydroxyethyl-Starch Solutions to be Re-examined Under Article 31 of Directive 2001/83/EC. Available online at: https://www.ema.europa.eu/documents/referral/recommendation-suspend-marketing-authorisations-hydroxyethyl-starch-solutions-be-re-examined_en.pdf
31. European Medicines Agency. 07/12/2013: New Review of Hydroxyethyl Starch-Containing Solutions for Infusion Started Under Article 107i of Directive 2001/83/EC (urgent Union procedure). Available online at: https://www.ema.europa.eu/documents/referral/hydroxyethyl-starch-article-107i-procedure-review-started_en.pdf
32. European Medicines Agency. 10/11/2013: PRAC Confirms that Hydroxyethyl-Starch Solutions (HES) Should No Longer be Used in Patients With Sepsis or Burn Injuries or in Critically Ill Patients. Available online at: http://www.ema.europa.eu/docs/en_gb/document_library/press_release/2013/10/wc500151964.pdf
33. European Medicines Agency. 10/25/2013: Hydroxyethyl-Starch Solutions (HES) Should No Longer be Used in Patients With Sepsis or Burn Injuries or in Critically Ill Patients – CMDh Endorses PRAC Recommendations. Available online at: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Hydroxyethyl_starch-containing_medicines_107/Position_provided_by_CMDh/WC500153118.pdf
34. European Medicines Agency. 03/06/2014: European Commission final decision: Hydroxyethyl-starch Solutions (HES) No Longer to be Used in Patients With Sepsis or Burn Injuries or in Critically Ill Patients. HES will be available in restricted patient populations Available online at: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Solutions_for_infusion_containing_hydroxyethyl_starch/European_Commission_final_decision/WC500162361.pdf
35. European Medicines Agency. 10/27/2017: EMA Starts New Review of Hydroxyethyl-Starch Containing Medicines. Available online at: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2017/10/WC500237822.pdf
36. European Medicines Agency. 01/12/2018: PRAC Recommends Suspending Hydroxyethyl-Starch Solutions for Infusion From the Market. Available online at: https://www.ema.europa.eu/documents/referral/hydroxyethyl-starch-article-107i-referral-summary-prac-recommendation_en.pdf
37. European Medicines Agency. 01/26/2018: Hydroxyethyl-Starch Solutions for Infusion to be Suspended – CMDh Endorses PRAC Recommendation. Available from: https://www.ema.europa.eu/documents/referral/hydroxyethyl-starch-article-107i-referral-cmdh-endorses-prac-recommendation_en.pdf (Accessed March 11, 2018).
38. European Commission Directorate-General for Health and Food Safety. Meeting of the Standing Committee On Medical Products For Human Use Held in Brussels on 9 April 2018 (2018). Available online at: http://ec.europa.eu/transparency/regcomitology/index.cfm?do=Search.getPDF&ds_id=56176&version=1&AttLang=en&db_number=1&docType=SUMMARY_RECORD
39. European Medicines Agency. 05/10/2018: Meeting Highlights From the Pharmacovigilance Risk Assessment Committee (PRAC) 14-17 May 2018. Available online at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2018/05/news_detail_002954.jsp&mid=WC0b01ac058004d5c1
40. European Medicines Agency. 05/17/2018: Assessment Report Referral Under Article 107i of Directive 2001/83/EC Resulting From Pharmacovigilance Data. Available online at: http://www.ema.europa.eu/docs/en_GB/documet_library/Referrals_document/Hydroxyethyl_starch_107i/European_Commission_final_decision/WC500252551.pdf
41. European Medicines Agency. 06/29/2018: Hydroxyethyl Starch Solutions: CMDh Introduces New Measures to Protect Patients. Available online at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2018/06/news_detail_002982.jsp&mid=WC0b01ac058004d5c1
42. European Medicines Agency. 07/27/2018: Scientific Conclusions and CMDh's Detailed Explanation on the Scientific Grounds for Differences With the PRAC Recommendation. Available online at: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Hydroxyethyl_starch_107i/European_Commission_final_decision/WC500252550.pdf
43. European Medicines Agency. What Is a European Safety Referral? Available online at: http://www.ema.europa.eu/docs/en_GB/document_library/Presentation/2017/03/WC500222615.pdf
44. Medicines & Healthcare products Regulatory Agency United Kingdom. Drug Alert Class 2 Medicines Recall. Action Within 48 Hours Pharmacy, Clinic and Wholesaler Level Recall 2013. Available online at: http://webarchive.nationalarchives.gov.uk/20160125032611/https://assets.digital.cabinet-office.gov.uk/media/5485ab7ced915d4c0d00022f/con287026.pdf
45. European Medicines Agency. 03/03/2015: Questions & Answers on Urgent Union Procedures (Article 107i of Directive 2001/83/EC) Available online at: https://www.ema.europa.eu/documents/regulatory-procedural-guideline/questions-answers-urgent-union-procedures-article-107i-directive-2001/83/ec_en.pdf
46. Press Release of the Food US, and Drug Administration (FDA). December 27:2007: FDA Approves Voluven to Treat Serious Blood Volume Loss following Surgery. December 27:2007: FDA Approves Voluven to Treat Serious Blood Volume Loss following Surgery. Available online at: https://wayback.archive-it.org/7993/20170112002645/http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucm109048.htm
47. Chappell D, Jacob M. Twisting and ignoring facts on hydroxyethyl starch is not very helpful. Scand J Trauma Resusc Emerg Med. (2013) 21:85. doi: 10.1186/1757-7241-21-85
48. Chappell D, Jacob M. Protocols, physiology, and trials of hydroxyethyl starch. N Engl J Med. (2012) 367:1266; author reply 1267. doi: 10.1056/NEJMc1209905
49. Chappell D, Jacob M. Hydroxyethyl starch - the importance of being earnest. Scand J Trauma Resusc Emerg Med. (2013) 21:61. doi: 10.1186/1757-7241-21-61
50. Vincent JL, Kellum JA, Shaw A, Mythen MG. Should hydroxyethyl starch solutions be totally banned? Crit Care (2013) 17:193. doi: 10.1186/cc13027
51. Haase N, Muller R, Perner A. Debate on HES safety is important, but must be based on facts. Scand J Trauma Resusc Emerg Med. (2013) 21:66. doi: 10.1186/1757-7241-21-66
52. Annane D, Siami S, Jaber S, Martin C, Elatrous S, Declere AD, et al. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA (2013) 310:1809–17. doi: 10.1001/jama.2013.280502
53. European Medicines Agency. 11/11/2013: Assessment Report for Solutions for Infusion Containing Hydroxyethyl Starch. Procedure under Article 31 of Directive 2001/83/EC Available online at: https://www.ema.europa.eu/documents/referral/hydroxyethyl-starch-article-31-referral-prac-assessment-report_en.pdf
54. European Medicines Agency. 11/11/2013: Assessment Report for Solutions for Infusion Containing Hydroxyethyl Starch. Procedure under Article 107i of Directive 2001/83/EC. Available online at: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Hydroxyethyl_starch-containing_medicines_107/Recommendation_provided_by_Pharmacovigilance_Risk_Assessment_Committee/WC500154254.pdf
55. Madi-Jebara S, Ghosn A, Sleilaty G, Richa F, Cherfane A, Haddad F, et al. Prevention of hypotension after spinal anesthesia for cesarean section: 6% hydroxyethyl starch 130/0.4 (Voluven) versus lactated Ringer's solution. J Med Liban. (2008) 56:203–7.
56. Hartog CS, Kohl M, Reinhart K. A systematic review of third-generation hydroxyethyl starch (HES 130/0.4) in resuscitation: safety not adequately addressed. Anesth Analg. (2011) 112:635–45. doi: 10.1213/ANE.0b013e31820ad607
57. Vincent JL. Issues in contemporary fluid management. Crit Care (2000) 4(Suppl. 2):S1–2. doi: 10.1186/cc964
58. Martin G, Bennett-Guerrero E, Wakeling H, Mythen MG, el-Moalem H, Robertson K, et al. A prospective, randomized comparison of thromboelastographic coagulation profile in patients receiving lactated Ringer's solution, 6% hetastarch in a balanced-saline vehicle, or 6% hetastarch in saline during major surgery. J Cardiothorac Vasc Anesth. (2002) 16:441–6. doi: 10.1053/jcan.2002.125146
59. Hamaji A, Hajjar L, Caiero M, Almeida J, Nakamura RE, Osawa EA, et al. Volume replacement therapy during hip arthroplasty using hydroxyethyl starch (130/0.4) compared to lactated Ringer decreases allogeneic blood transfusion and postoperative infection. Braz J Anesthesiol. (2013) 63:27–35. doi: 10.1016/j.bjane.2012.03.002
60. Endo A, Uchino S, Iwai K, Saito K, Sanui M, Takinami M, et al. Intraoperative hydroxyethyl starch 70/0.5 is not related to acute kidney injury in surgical patients: retrospective cohort study. Anesth Analg. (2012) 115:1309–14. doi: 10.1213/ANE.0b013e31826ba8d7
61. Martin C, Jacob M, Vicaut E, Guidet B, Van Aken H, Kurz A. Effect of waxy maize-derived hydroxyethyl starch 130/0.4 on renal function in surgical patients. Anesthesiology (2013) 118:387–94. doi: 10.1097/ALN.0b013e31827e5569
62. Wiedermann CJ, Joannidis M. Mortality after hydroxyethyl starch 130/0.4 infusion: an updated meta-analysis of randomized trials. Swiss Med Wkly. (2012) 142:w13656. doi: 10.4414/smw.2012.13656
63. European Medicines Agency. 11/11/2013: Annex III Amendments to Relevant Sections of the Summary of Product Characteristics and Package Leaflet Under Article 107i. Available online at: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Hydroxyethyl_starch-containing_medicines_107/Position_provided_by_CMDh/WC500153120.pdf
64. Food and Drug Administration. July 17:2012: Risks and Benefits of Hydroxyethyl Starch Solutions; Notice of Public Workshop (2012). Available online at: https://www.gpo.gov/fdsys/pkg/FR-2012-07-25/pdf/2012-18110.pdf
65. Food and Drug Administration. FDA Safety Communication: Boxed Warning on Increased Mortality and Severe Renal Injury, and Additional Warning on Risk of Bleeding, for Use of Hydroxyethyl Starch Solutions in Some Settings (2013). Available online at: http://wayback.archive-it.org/7993/20170112095648/http://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/ucm358271.htm
66. Bion J, Bellomo R, Myburgh J, Perner A, Reinhart K, Finfer S. Hydroxyethyl starch: putting patient safety first. Intensive Care Med. (2014) 40:256–9. doi: 10.1007/s00134-013-3167-6
67. Bellomo R, Bion J, Finfer S, Myburgh J, Perner A, Reinhart K, et al. Open letter to the Executive Director of the European Medicines Agency concerning the licensing of hydroxyethyl starch solutions for fluid resuscitation. Br J Anaesth. (2014) 112:595–600. doi: 10.1093/bja/aeu025
68. Hartog CS, Natanson C, Sun J, Klein HG, Reinhart K. Concerns over use of hydroxyethyl starch solutions. BMJ (2014) 349:g5981. doi: 10.1136/bmj.g5981
69. Doshi P. Data too important to share: do those who control the data control the message? BMJ (2016) 352:i1027. doi: 10.1136/bmj.i1027
70. Doshi P. Update: New England Journal of Medicine publishes correction to 2012 CHEST trial of hydroxyethyl starch versus colloids. BMJ (2016) 352:i1571. doi: 10.1136/bmj.i1571
71. Doshi P. “Independent” reanalysis of landmark starch solutions trial was published by original authors. BMJ (2017) 358:j3552. doi: 10.1136/bmj.j3552
72. ClinicalTrials.gov. Safety and Efficacy of 6% Hydroxyethyl Starch (HES) Solution Versus an Electrolyte Solution in Patients Undergoing Elective Abdominal Surgery (PHOENICS) (2017). Available online at: https://clinicaltrials.gov/ct2/show/NCT03278548
73. ClinicalTrials.gov. Safety and Efficacy of a 6% Hydroxyethyl Starch (HES) Solution Versus an Electrolyte Solution in Trauma Patients (TETHYS) (2017). Available online at: https://clinicaltrials.gov/ct2/show/NCT03338218
74. Hammond NE, Taylor C, Finfer S, Machado FR, An Y, Billot L, et al. Patterns of intravenous fluid resuscitation use in adult intensive care patients between 2007 and 2014: an international cross-sectional study. PLoS ONE (2017) 12:e0176292. doi: 10.1371/journal.pone.0176292
75. Public Citizen. Citizen Petition From Public Citizen: Immediately Require the Removal From the Market of HES IV Solutions (2017). Available online at: https://www.regulations.gov/document?D=FDA-2017-P-0867-0001
76. Public, Citizen,. 08/02/2017: Dangerous IV Solutions Must Be Banned Immediately, Public Citizen Tells FDA. Available online at: https://www.citizen.org/media/press-releases/dangerous-iv-solutions-must-be-banned-immediately-public-citizen-tells-fda
77. Gårdmark, M. Notification to the PRAC/EMA Secretariat of a Referral Under Article 107i of Directive 2001/83/EC (2017). Available online at: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Hydroxyethyl_starch_107i/Procedure_started/WC500237819.pdf
78. European Network of Centres for Pharmacoepidemiology and Pharmacovigilance. Retrospective Drug Utilisation Study to Investigate the Routine Use of Hydroxyethyl Starch (HES)- Containing Infusion Solutions in Hospital (2017). Available online at: http://www.encepp.eu/encepp/viewResource.htm?id=24380
79. European Network of Centres for Pharmacoepidemiology and Pharmacovigilance. Retrospective Drug Utilisation Study to Investigate the Routine Use of Hydroxyethyl Starch (HES)-Containing Infusion Solutions of B. Braun Melsungen AG in Hospitals (2017). Available online at: http://www.encepp.eu/encepp/viewResource.htm?id=22703
80. Doshi P. EMA recommendation on hydroxyethyl starch solutions obscured controversy. BMJ (2018) 360:k1287. doi: 10.1136/bmj.k1287
81. Laake JH, Tonnessen TI, Chew MS, Lipcsey M, Hjelmqvist H, Wilkman E, et al. The SSAI fully supports the suspension of hydroxyethyl-starch solutions commissioned by the European Medicines Agency. Acta Anaesthesiol Scand. (2018) 62, 874–5. doi: 10.1111/aas.13120
82. Roberts I, Shakur H, Bellomo R, Bion J, Finfer S, Hunt B, et al. Hydroxyethyl starch solutions and patient harm. Lancet (2018) 391:736. doi: 10.1016/S0140-6736(18)30255-1
83. Annane D, Fuchs-Buder T, Zoellner C, Kaukonen M, Scheeren TWL. EMA recommendation to suspend HES is hazardous. Lancet (2018) 391:736–8. doi: 10.1016/S0140-6736(18)30254-X
84. Priebe HJ. Should hydroxyethyl starch be banned? Lancet (2018) 392:117–8. doi: 10.1016/S0140-6736(18)31172-3
85. Deutsche Gesellschaft für Anästhesiologie und lntensivmedizin e.V.” (DGAI). Open letter to the European Commission: Marketing Authorization of Colloid Solutions Containing Hydroxyethyl Starch (HES) (2018). Available online at: https://www.dgai.de/aktuelles-2/794-hes-open-letter-ec.html
86. Fresenius Kabi AG? (Germany). 01/29/2018: HES Solutions Should Remain on the Market. Available online at: https://www.fresenius-kabi.com/news/hes-solutions-should-remain-on-the-market
B. Braun AG (Germany). 01/26/2018: HES Should be Kept on the Market. Available online at: https://www.bbraun.com/en/company/news-room/news/2018/1st-quarter-2018/b–braun–hes-should-be-kept-on-the-market.html
88. Patel J, Prajapati M, Solanki A, Pandya H. Comparison of albumin, hydroxyethyl starch and ringer lactate solution as priming fluid for cardiopulmonary bypass in paediatric cardiac surgery. J Clin Diagn Res. (2016) 10:UC01–4. doi: 10.7860/JCDR/2016/18465.7918
89. Schramko A, Suojaranta-Ylinen R, Niemi T, Pesonen E, Kuitunen A, Raivio P, et al. The use of balanced HES 130/0.42 during complex cardiac surgery; effect on blood coagulation and fluid balance: a randomized controlled trial. Perfusion (2015) 30:224–32. doi: 10.1177/0267659114540022
90. Yates DR, Davies SJ, Milner HE, Wilson RJ. Crystalloid or colloid for goal-directed fluid therapy in colorectal surgery. Br J Anaesth. (2014) 112:281–9. doi: 10.1093/bja/aet307
91. Muller RB, Haase N, Lange T, Wetterslev J, Perner A. Acute kidney injury with hydroxyethyl starch 130/0.42 in severe sepsis. Acta Anaesthesiol Scand. (2015) 59:329–36. doi: 10.1111/aas.12453
92. Haase N, Wetterslev J, Winkel P, Perner A. Bleeding and risk of death with hydroxyethyl starch in severe sepsis: post hoc analyses of a randomized clinical trial. Intensive Care Med. (2013) 39:2126–34. doi: 10.1007/s00134-013-3111-9
93. Anthon CT, Muller RB, Haase N, Hjortrup PB, Moller K, Lange T, et al. Effects of hydroxyethyl starch 130/0.42 vs. Ringer's acetate on cytokine levels in severe sepsis. Acta Anaesthesiol Scand. (2017) 61:904–13. doi: 10.1111/aas.12929
94. Muller RB, Ostrowski SR, Haase N, Wetterslev J, Perner A, Johansson PI. Markers of endothelial damage and coagulation impairment in patients with severe sepsis resuscitated with hydroxyethyl starch 130/0.42 vs Ringer acetate. J Crit Care (2016) 32:16–20. doi: 10.1016/j.jcrc.2015.11.025
95. Hopper K, Garcia Rojas A, Barter L. An online survey of small animal veterinarians regarding current fluid therapy practices in dogs and cats. J Am Vet Med Assoc. (2018) 252:553–9. doi: 10.2460/javma.252.5.553
96. Brooks A, Thomovsky E, Johnson P. Natural and synthetic colloids in veterinary medicine. Top Companion Anim Med. (2016) 31:54–60. doi: 10.1053/j.tcam.2016.08.003
97. Cazzolli D, Prittie J. The crystalloid-colloid debate: Consequences of resuscitation fluid selection in veterinary critical care. J Vet Emerg Crit Care (2015) 25:6–19. doi: 10.1111/vec.12281
98. Glover PA, Rudloff E, Kirby R. Hydroxyethyl starch: a review of pharmacokinetics, pharmacodynamics, current products, and potential clinical risks, benefits, and use. J Vet Emerg Crit Care (2014) 24:642–61. doi: 10.1111/vec.12208
99. Wong C, Koenig A. The colloid controversy: are colloids bad and what are the options? Vet Clin North Am Small Anim Pract. (2017) 47:411–21. doi: 10.1016/j.cvsm.2016.09.008
100. Sigrist NE, Kalin N, Dreyfus A. Changes in serum creatinine concentration and Acute Kidney Injury (AKI) grade in dogs treated with hydroxyethyl starch 130/0.4 from 2013 to 2015. J Vet Intern Med. (2017) 31:434–41. doi: 10.1111/jvim.14645
101. Sigrist NE, Kalin N, Dreyfus A., Effects of hydroxyethyl starch 130/0.4 on serum creatinine concentration and development of acute kidney injury in nonazotemic cats. J Vet Intern Med. (2017) 31:1749–56. doi: 10.1111/jvim.14813
102. Yozova ID, Howard J, Adamik KN. Retrospective evaluation of the effects of administration of tetrastarch (hydroxyethyl starch 130/0.4) on plasma creatinine concentration in dogs (2010-2013): 201 dogs. J Vet Emerg Crit Care (2016) 26:568–77. doi: 10.1111/vec.12483
103. Yozova ID, Howard J, Adamik KN. Effect of tetrastarch (hydroxyethyl starch 130/0.4) on plasma creatinine concentration in cats: a retrospective analysis (2010-2015). J Feline Med Surg. (2017) 19:1073–9. doi: 10.1177/1098612X16676160
104. Hayes G, Benedicenti L, Mathews K. Retrospective cohort study on the incidence of acute kidney injury and death following hydroxyethyl starch (HES 10% 250/0.5/5:1) administration in dogs (2007-2010). J Vet Emerg Crit Care (2016) 26:35–40. doi: 10.1111/vec.12412
105. Cortellini S, Pelligand L, Syme H, Chang YM, Adamantos S. Neutrophil gelatinase-associated lipocalin in dogs with sepsis undergoing emergency laparotomy: a prospective case-control study. J Vet Intern Med. (2015) 29:1595–602. doi: 10.1111/jvim.13638
106. Diniz MS, Teixeira-Neto FJ, Celeita-Rodriguez N, Girotto CH, Fonseca MW, Oliveira-Garcia AC, et al. Effects of 6% tetrastarch and lactated ringer's solution on extravascular lung water and markers of acute renal injury in hemorrhaged, isoflurane-anesthetized healthy dogs. J Vet Intern Med. (2018) 32:712–21. doi: 10.1111/jvim.14853
107. Boyd C, Claus M, Raisis A, Cianciolo R, Sharp C, Nabity M, et al. Kidney injury in dogs with hemorrhagic shock treated with hydroxyethyl starch 130/0.4 OR 4% succinylated gelatine (abstract). J Vet Emerg Crit Care (2016) 26:S1–37. doi: 10.1111/vec.12516
108. Wong C, Koenig A, Schmiedt C, Brainard B, Bazzle L, Brown S, et al. Effect of a 72-hour infusion OF 6% hydroxyethyl starch 130/0.4 on canine renal function (abstract). J Vet Emerg Crit Care (2016) 26:S1–37. doi: 10.1111/vec.12516
109. Classen J, Adamik KN, Weber K, Rubenbauer S, Hartmann K. In vitro effect of hydroxyethyl starch 130/0.42 on canine platelet function. Am J Vet Res. (2012) 73:1908–12. doi: 10.2460/ajvr.73.12.1908
110. Falco S, Bruno B, Maurella C, Bellino C, D'Angelo A, Gianella P, et al. In vitro evaluation of canine hemostasis following dilution with hydroxyethyl starch (130/0.4) via thromboelastometry. J Vet Emerg Crit Care (2012) 22:640–5. doi: 10.1111/j.1476-4431.2012.00816.x
111. Gauthier V, Holowaychuk MK, Kerr CL, Bersenas AM, Wood RD. Effect of synthetic colloid administration on coagulation in healthy dogs and dogs with systemic inflammation. J Vet Intern Med. (2015) 29:276–85. doi: 10.1111/jvim.12492
112. McBride D, Hosgood G, Raisis A, Smart L. Platelet closure time in anesthetized Greyhounds with hemorrhagic shock treated with hydroxyethyl starch 130/0.4 or 0.9% sodium chloride infusions. J Vet Emerg Crit Care (2016) 26:509–15. doi: 10.1111/vec.12468
113. Reuteler A, Axiak-Flammer S, Howard J, Adamik KN. Comparison of the effects of a balanced crystalloid-based and a saline-based tetrastarch solution on canine whole blood coagulation and platelet function. J Vet Emerg Crit Care (2017) 27:23–34. doi: 10.1111/vec.12556
114. Wurlod VA, Howard J, Francey T, Schweighauser A, Adamik KN. Comparison of the in vitro effects of saline, hypertonic hydroxyethyl starch, hypertonic saline, and two forms of hydroxyethyl starch on whole blood coagulation and platelet function in dogs. J Vet Emerg Crit Care (2015) 25:474–87. doi: 10.1111/vec.12320
115. Griego-Valles M, Buriko Y, Prittie JE, Fox PR. An in vitro comparison of the effects of voluven (6% hydroxyethyl starch 130/0.4) and hespan (6% hydroxyethyl starch 670/0.75) on measures of blood coagulation in canine blood. J Vet Emerg Crit Care (2017) 27:44–51. doi: 10.1111/vec.12541
116. Boyd C, Claus M, Raisis A, Hosgood G, Sharp C, Smart L. Hypocoagulability and platelet dysfunction are exacerbated by synthetic colloids in a canine hemorrhagic shock model. Front. Vet. Sci. 5:279. doi: 10.3389/fvets.2018.00279
117. Albrecht NA, Howard J, Kovacevic A, Adamik KN. In vitro effects of 6 % hydroxyethyl starch 130/0.42 solution on feline whole blood coagulation measured by rotational thromboelastometry. BMC Vet Res. (2016) 12:155. doi: 10.1186/s12917-016-0767-8
118. Iannucci C, Howard J, Adamik K. Evaluation of coagulation impairment ini dogs with spontaneus hemoperitoneum treated with hydroxyethyl starch pr polyinonic crystalloid (abstract). J Vet Emerg Crit Care (2017) 27:S1–29. doi: 10.1111/vec.12758
119. Ertmer C, Rehberg S, Van Aken H, Westphal M. Relevance of non-albumin colloids in intensive care medicine. Best Pract Res Clin Anaesthesiol. (2009) 23:193–212. doi: 10.1016/j.bpa.2008.11.001
120. Thomas-Rueddel DO, Vlasakov V, Reinhart K, Jaeschke R, Rueddel H, Hutagalung R, et al. Safety of gelatin for volume resuscitation–a systematic review and meta-analysis. Intensive Care Med. (2012) 38:1134–42. doi: 10.1007/s00134-012-2560-x
121. Moeller C, Fleischmann C, Thomas-Rueddel D, Vlasakov V, Rochwerg B, Theurer P, et al. How safe is gelatin? A systematic review and meta-analysis of gelatin-containing plasma expanders vs crystalloids and albumin. J Crit Care (2016) 35:75–83. doi: 10.1016/j.jcrc.2016.04.011
122. Bayer O, Reinhart K, Kohl M, Kabisch B, Marshall J, Sakr Y, et al. Effects of fluid resuscitation with synthetic colloids or crystalloids alone on shock reversal, fluid balance, and patient outcomes in patients with severe sepsis: a prospective sequential analysis. Crit Care Med. (2012) 40:2543–51. doi: 10.1097/CCM.0b013e318258fee7
123. Holbeck S, Grande PO. Effects on capillary fluid permeability and fluid exchange of albumin, dextran, gelatin, and hydroxyethyl starch in cat skeletal muscle. Crit Care Med. (2000) 28:1089–95. doi: 10.1097/00003246-200004000-00030
124. Tait AR, Larson LO Resuscitation fluids for the treatment of hemorrhagic shock in dogs: effects on myocardial blood flow and oxygen transport. Crit Care Med. (1991) 19:1561–5. doi: 10.1097/00003246-199112000-00020
125. Lutz H. Animal experiments on the effect of colloidal plasma substitutes in hemorrhagic shock in the dog. Bibl Haematol. (1969) 33:232–47. doi: 10.1159/000384844
126. Michell AR. Fluid therapy: some specific applications to medical conditions in small animals. Vet Rec. (1979) 104:572–5. doi: 10.1136/vr.104.25.572
127. Wells BT. Use of a gelatin solution in hypovolaemia and toxaemia in small animals. Vet Rec. (1980) 107:85–7. doi: 10.1136/vr.107.4.85
129. Parkins WM, Koop CE, Riegel C, Vars HM, Lockwood JS. Gelatin as a plasma substitute with particular reference to experimental hemorrhage and burn shock. Ann Surg. (1943) 118:193–214. doi: 10.1097/00000658-194308000-00004
130. Food and Drug Administration. List of Drug Products That Have Been Withdrawn or Removed From the Market for Reasons of Safety or Effectiveness. 63 FR 54082. (1998). Available online at: https://www.gpo.gov/fdsys/pkg/FR-1998-10-08/pdf/98-26923.pdf
131. Albrecht FW, Glas M, Rensing H, Kindgen-Milles D, Volk T, Mathes AM. A change of colloid from hydroxyethyl starch to gelatin does not reduce rate of renal failure or mortality in surgical critical care patients: Results of a retrospective cohort study. J Crit Care (2016) 36:160–5. doi: 10.1016/j.jcrc.2016.07.005
132. Rote Liste Service GmbH Fachinfo Service. Fachinformation: Gelafundin 4% Infusionslösung, BBraun (2015). Available online at: https://www.bbraun.de/content/dam/catalog/bbraun/bbraunProductCatalog/S/AEM2015/de-de/b0/gelafundin-4-infusionsloesung.pdf.bb-.00443836/gelafundin-4-infusionsloesung.pdf
133. iMedi.co.uk. Gelaspan Solution For Infusion, B. Braun Melsungen AG (2015) Available online at: https://imedi.co.uk/gelaspan-solution-for-infusion/summary.
134. Fantoni DT, Auler Junior JO, Futema F, Cortopassi SR, Migliati ER, Faustino M, et al. Intravenous administration of hypertonic sodium chloride solution with dextran or isotonic sodium chloride solution for treatment of septic shock secondary to pyometra in dogs. J Am Vet Med Assoc. (1999) 215:1283–7.
135. Schertel ER, Allen DA, Muir WW, Hansen BD. Evaluation of a hypertonic sodium chloride/dextran solution for treatment of traumatic shock in dogs. J Am Vet Med Assoc. (1996) 208:366–70.
136. Tobias TA, Schertel ER, Schmall LM, Wilbur N, Muir WW. Comparative effects of 7.5% NaCl in 6% Dextran 70 and 0.9% NaCl on cardiorespiratory parameters after cardiac output-controlled resuscitation from canine hemorrhagic shock. Circ Shock (1993) 39:139–46.
137. Allen DA, Schertel ER, Muir WW III Valentine AK. Hypertonic saline/dextran resuscitation of dogs with experimentally induced gastric dilatation-volvulus shock. Am J Vet Res. (1991) 52:92–6.
138. Schertel ER, Allen DA, Muir WW, Brourman JD, DeHoff WD. Evaluation of a hypertonic saline-dextran solution for treatment of dogs with shock induced by gastric dilatation-volvulus. J Am Vet Med Assoc. (1997) 210:226–30.
139. Concannon KT, Haskins SC, Feldman BF. Hemostatic defects associated with two infusion rates of dextran 70 in dogs. Am J Vet Res. (1992) 53:1369–75.
140. Glowaski MM, Moon-Massat PF, Erb HN, Barr SC. Effects of oxypolygelatin and dextran 70 on hemostatic variables in dogs. Vet Anaesth Analg. (2003) 30:202–10. doi: 10.1046/j.1467-2995.2003.00111.x
141. Cohn LA, Kerl ME, Lenox CE, Livingston RS, Dodam JR. Response of healthy dogs to infusions of human serum albumin. Am J Vet Res. (2007) 68:657–63. doi: 10.2460/ajvr.68.6.657
142. Francis AH, Martin LG, Haldorson GJ, Lahmers KK, Luther TY, Alperin DC, et al. Adverse reactions suggestive of type III hypersensitivity in six healthy dogs given human albumin. J Am Vet Med Assoc. (2007) 230:873–9. doi: 10.2460/javma.230.6.873
143. Mathews K, Barry M. The use of 25% human serum albumin: outcome and efficacy in raising serum albumin and systemic blood pressure in critically ill dogs and cats J Vet Emerg Crit Care (2005) 15:110–8. doi: 10.1111/j.1476-4431.2005.00141.x
144. Vigano F, Perissinotto L, Bosco VR. Administration of 5% human serum albumin in critically ill small animal patients with hypoalbuminemia: 418 dogs and 170 cats (1994-2008). J Vet Emerg Crit Care (2010) 20:237–43. doi: 10.1111/j.1476-4431.2010.00526.x
Keywords: gelatin, dextran, plasma expanders, pharmacovigilance, synthetic colloids, fluid therapy, European Medicines Agency, Food and Drug Administration
Citation: Adamik K-N and Yozova ID (2019) Starch Wars—New Episodes of the Saga. Changes in Regulations on Hydroxyethyl Starch in the European Union. Front. Vet. Sci. 5:336. doi: 10.3389/fvets.2018.00336
Received: 30 May 2018; Accepted: 14 December 2018;
Published: 18 January 2019.
Edited by:
Julie Menard, Cornell University, United StatesReviewed by:
Liz Guieu, University of Tennessee, Knoxville, United StatesCopyright © 2019 Adamik and Yozova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katja-Nicole Adamik, a2F0amEuYWRhbWlrQHZldHN1aXNzZS51bmliZS5jaA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.