- Department of Infectious Diseases and Public Health, Jockey Club College of Veterinary Medicine and Life Sciences, City University of Hong Kong, Hong Kong, Hong Kong
Infectious causes of reproductive failure in cattle are important in Australia and New Zealand, where strict biosecurity protocols are in place to prevent the introduction and spread of new diseases. Neospora caninum ranks highly as an important cause of reproductive wastage along with fungal and bacterial infections. Brucella, a leading cause of abortion elsewhere in the world, is foreign, following successful programs to control and eradicate the disease. Leptospirosis in cattle is largely controlled by vaccination, while Campylobacter and Tritrichomonas infections occur at low rates. In both countries, Bovine Viral Diarrhea virus (BVDV) infection rates as the second most economically important disease of cattle and one that also has an effect on reproduction. Effective disease control strategies require rapid diagnoses at diagnostic laboratories. To facilitate this process, this review will discuss the infectious causes of reproductive losses present in both countries, their clinical presentation and an effective pathway to a diagnosis.
Introduction
Infectious organisms that often cause only mild and unapparent disease, such as Neospora caninum, Listeria monocytogenes, Bluetongue virus (BTV), and Bovine Viral Diarrhea virus (BVDV), can cause reproductive failures when transmitted vertically from dam to offspring. Down regulation of the maternal immune system during pregnancy is necessary to prevent allogeneic rejection of the embryo. Meanwhile, the fetal immune system only starts to develop during the second trimester. Immunosuppression of the dam, coupled with the developing status of the fetal immune system, affords pathogens the opportunity to infect and grow unchecked. The mechanism of transplacental transmission of many abortifacients has not been completely defined, although there is evidence to suggest that placental macrophages may contribute to transmission of bacteria and fungi (1). Insights have also been provided by studies in mice with malaria induced abortion (2) and Campylobacter infection in sheep (3).
The response of the fetus to infection depends on the stage of gestation when infection first occurs. In the first trimester, when the fetus has no effective immune system, infectious agents kill fetal cells directly (4). If infection occurs at this stage, the calf may be born immunotolerant, as in the case of BVDV. As the fetus develops, the immune system response becomes more complete. From 98 days gestation, for example, the fetus is capable of mounting an IgG immune response against N. caninum (5). Within a few more days, developed bovine fetal lymphocytes are capable of mitogenic stimulation and the production of IL-2 (5). Once the immune system has matured, infection may be repelled, or, conversely, the products of inflammation may negatively affect (6) or kill the fetus (7).
In Australia and New Zealand, abortion and reproductive failure in cattle are significant limitations to cattle productivity. In the dairy industry in Australia, fetal loss accounts for between 2–3% (8) and 7% (9) of total pregnancies. Losses in New Zealand are reported to be similar, at around 6% (10). Losses in the beef industries are harder to quantify because of rangeland rearing and less supervision of animals, still, estimates of fetal losses are thought to be about half of those in the dairy industries (8). Both countries have deregulated cattle farming industries where the responsibility for diagnosing reproductive disease is the responsibility of the farmer. In cases of suspected notifiable exotic disease, government authorities will take responsibility for diagnosis and control (New Zealand1, Australia2).
The geographical isolation of these island states has allowed them to successfully implement and maintain high biosecurity protocols, maintaining freedom from Foot and Mouth disease and Bovine Spongiform Encephalopathy (BSE). It has also enabled both countries to successfully eradicate bovine brucellosis (Brucella abortus) (11, 12). This disease formerly had a worldwide distribution but has now been eradicated from many developed countries; although it still occurs in the USA and certain parts of Europe. Successful eradication in both countries was achieved using a combination of vaccination and compulsory test and slaughter programs. Biosecurity measures such as tail tagging and movement restrictions were also critical in the control of B. abortus transmission. Information on brucellosis has been extensively reviewed (13). Contagious bovine pleuropneumonia (CBPP) (Mycoplasma mycoides) has also been successfully eradicated in Australia (14) and New Zealand (15), while bovine tuberculosis has been eradicated from Australia (16, 17), but not from New Zealand (18). Bovine ephemeral fever virus, Q-fever (Coxiella burnetii) and Bluetongue virus are present within Australia (19, 20) but are absent from New Zealand3 (21, 22).
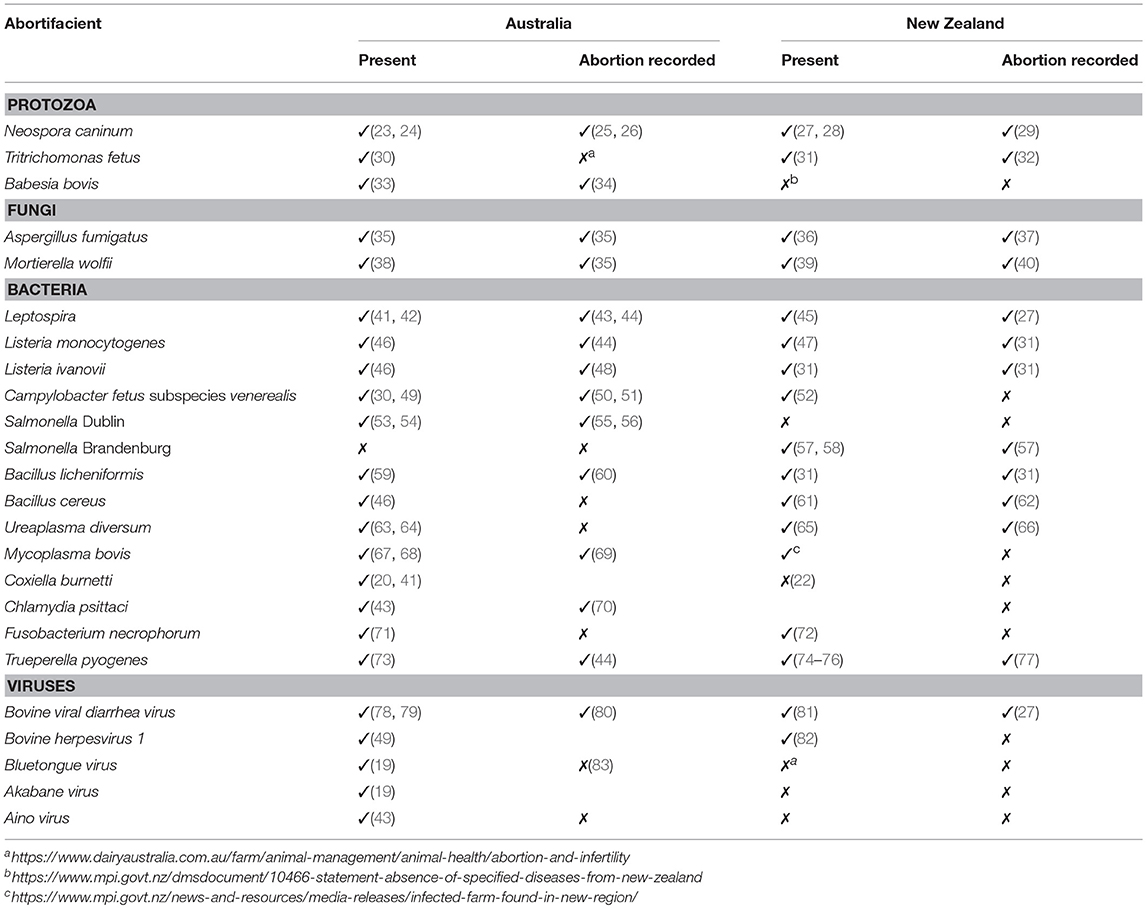
There are many infectious causes of bovine abortion in Australia and New Zealand. While some of these diseases are responsible for significant reproductive failures in other parts of the world, in Australia and New Zealand their clinical presentation is less severe or, occasionally, entirely benign. Table 1 summarizes these abortifacients, and their abortion record in Australia and New Zealand. Where possible, a reference has been given for the most recent evidence of disease presence, and for documented cases of abortion. The most important abortifacients in both countries, in terms of both prevalence and economic impact, are N. caninum, BVDV, and Leptospira. N. caninum infections were estimated to cost the cattle industries in both countries approximately US$ 135 million annually in 2012 (8). Seroprevalence of BVDV is high in both countries (84), and it rates as the second most economically important disease of the cattle industries (85). Multiple Leptospira serovars infect the countries cattle populations (86, 87). In addition to their impact on cattle reproduction, their presence poses a public health risk due to their zoonotic potential.
This review focuses on the protozoa, fungi, bacteria and viruses responsible for reproductive failures of cattle in Australia and New Zealand. Discussed below are the important and interesting features of these pathogens, organized in order of importance within their phyla. The clinical presentation at necropsy is described, and the approaches required for diagnostic laboratories to make a successful definitive diagnosis of abortion etiology are outlined. Diseases that are not prevalent in these two countries are not discussed.
Abortifacients in Australia and New Zealand
Protozoa
Neospora Caninum
Since its recognition as an abortifacient in cattle in the late 1980s (88), N. caninum has quickly become the most frequently diagnosed cause of fetal loss in cattle in Australia and New Zealand (89). Until 1990 in New Zealand, a diagnosis in a case of bovine abortion was only reached 5–16% of the time (90, 91). However, once N. caninum was identified as an abortifacent and histopathology of aborted fetuses undertaken more frequently, the overall diagnostic rate increased to 28% (92).
The parasite has two modes of transmission, vertical from dam to offspring, and horizontal from its definitive host, the dog, to cow. Vertical transmission is the most efficacious, while horizontal transmission plays only a minor role. The immune response in the non-pregnant cow is mediated by inflammatory cytokines that induce growth inhibition, lysis, and cell death to control the growth and spread of the organism. This inflammatory response has been proposed to play a role in inducing abortion in the pregnant cow (93). Infection late in pregnancy in a naïve dam favors transmission without clinical effect, while mid-term infection leads to abortion (94). Quantity and duration of parasitaemia, outcome of the maternal immune response and the ability of the fetus to mount an immune response against the parasite are also important factors in whether abortion ensues or not. For an excellent review on pathogenesis of Neospora abortion see Dubey et al. (95).
At necropsy, observed lesions may include thickened oedematous placental membranes, mild autolysis of the fetus, disseminated inflammatory and necrotic lesions evident by histopathology, and vascular-oriented microgliosis in the brain (96).
Tritrichomonas Fetus
T. fetus is a venereal disease with a similar epidemiology to Campylobacter fetus, commonly transmitted from bull to cow during mating (49). T. fetus infection was confirmed in north-eastern Australia in the 1970's (97, 98) and New Zealand in the 1940's (32), but there are no recently published reports of disease (49). Routine screening of stud bulls used for semen collection is recommended in both countries (99), and is used to successfully prevent venereal transmission of infection by artificial insemination (100). Diagnostic testing on preputial samples is performed using bacteriological culture and molecular testing using polymerase chain reaction (PCR) (101, 102). Reproductive failures caused by T. fetus occur in the first half of gestation, and include early embryonic losses. In cases of abortion, lesions may include placentitis and bronchial pyogranulomatous pneumonia. The organism may be visible by histopathology of the placenta or pulmonary tissues (103), and presence can be confirmed by PCR.
Babesia
In Australia, Babesia bovis and B. bigemina are associated with the distribution of their tick vectors Boophilus and Rhipicephalus between 40°N and 32°S. Babesia species that affect cattle are not reported from New Zealand. Clinical disease in endemic regions of Australia is uncommon due to high levels of resistance, and abortion is a rare occurrence. However, pyrexia associated with infection can result in abortion of late-term pregnant cows and 6–8 weeks of infertility in bulls (34), particularly if high-risk cattle outside of endemic regions are exposed to Babesia. The aborted fetus may present with watery blood, jaundice, an enlarged, discolored liver and focal hemorrhage of the kidneys (34). The intraerythrocytic parasite can be visualized by Giemsa or acridine orange staining (104), and the highest concentration of parasites are found in the erythrocytes of the brain (105).
Fungi
Aspergillus Fumigatus
One of the most common causes of mycotic reproductive failure, abortions due to Aspergillus fumigatus tend to occur in the second and third trimester (35). This fungus proliferates in decomposing hay, poorly preserved silage and soil, and produces a non-airborne, pathogenic spore (106). The spores localize to the uterine caruncle and induce inflammation-induced abortion after 2–5 weeks of proliferation. On gross examination, the placenta often has swollen, necrotic cotyledons, and the intercotyledonary membrane may be diffusely thickened, wrinkled, and leather-like. Occasionally, aborted fetuses have characteristic fungal plaques, 1–10 cm in diameter involving the skin around the eyelids, neck, dorsum, and thorax (37). This is due to fungal proliferation in the amnion, penetration of the epidermis and the fetal inflammatory reaction, as well as hyperkeratosis. Mycotic abortion can be diagnosed by culture, and histologically from placental changes with visible fungal hyphae confirming infection (107).
Mortierella Wolfii
Mortierella wolfii is occasionally reported in Australian cattle, and frequently reported in the North Island of New Zealand (38). The presentation of M. wolfii infection in pregnant cows is the same as A. fumigatus, except that abortion is also followed by fatal pneumonia 4–5 days later in about 20% of cases (108). Spores may be inhaled from contaminated silage, and pass into arterial circulation via the pulmonary vascular bed (109). Pathogenesis is also the same as A. fumigatus, and after haematogenous spread, fungal growth at the uterine caruncle causes widespread tissue necrosis and inflammation, leading to placentitis and abortion. Grossly, the placenta appears thickened, oedematous and necrotic, and other lesions are present consistent with mycotic abortion described above.
Bacteria
A variety of bacteria species have been implicated as causes of bovine abortion, many of them causing sporadic abortion. As a general guide, if no gross lesions are observed in the aborted fetus, microbiological examination of fetal stomach content is recommended to determine if a bacterial agent is responsible.
Leptospira
The serovars of Leptospira are associated with a variety of specific reservoir hosts (110), their respective epidemiology has been extensively reviewed as causes of disease, in particular of abortions (111). A range of serovars have been identified in Australia including L. Hardjo, Tarassovi, Pomona, and Szwajizak (86, 112). In Queensland and Victoria, L. interrogans has been associated with reproductive disease (113, 114). In New Zealand, the first case of bovine abortion associated with leptospirosis was diagnosed in 1953 (115), and fetal losses due to leptospirosis in beef cattle are recorded at 4.7% from L. borgpetersenii serovar Hardjo and 3.6% from L. interrogans serovar Pomona (27). Mild interstitial nephritis may be seen in the aborted fetus. Although diagnosis can be made by histological observation of the organism in the heart, intestine, liver and kidney using silver staining (114), multiplex PCR or quantitative PCR (qPCR) can be performed for a rapid and comprehensive diagnostic test (116, 117, 118). Detection of Leptospira by isolation is not recommended, given the difficulty of culturing this organism (119).
Listeria
L. monocytogenes (120) and L. ivanovii (121) cross the placenta by invading bovine trophoblast cells (122) and cause abortion. Poorly preserved silage can be a source of infection and, if conditions are suitable, contamination of food and water may lead to an outbreak of abortions (120). Gross lesions in the aborted fetus may include pinpoint white foci within the liver (48). Diagnosis of Listeria abortion is based on isolation of Listeria species from culture of fetal stomach contents, lung, and liver, along with placentitis and hepatitis observed by histopathology.
Campylobacter Fetus
Bovine genital campylobacteriosis is a venereal disease without clinical signs in infected bulls, but which causes early embryonic losses in cows. C. fetus subspecies fetus and C. fetus subspecies venerealis infections are causes of reproductive losses in Australia (49). In New Zealand, disease was last reported in 1993 (123) but is now considered absent (52). The PCR test used to detect the presence of C. fetus subspecies venerealis in New Zealand was shown to be cross-reactive with C. hyointestinalis (124). This is now a recognized problem (125), and its sole use is not recommended (126). A diagnosis in the adult can be made using a combination of tests (127), and multiplex PCR assays are available to check for multiple subspecies in one assay (128). Lesions in cases of abortion include pneumonia, encephalitis and inflammation of the placenta, peritoneum and liver (129). An etiological diagnosis can be made using PCR on fetal stomach contents and vaginal or uterine discharges.
Salmonella
Salmonella inhabit the intestine of their host, and infection typically occurs when cattle ingest water and feed contaminated with feces. As a result, infections often occur in outbreaks and abortion storms can sometimes ensue. Bovine abortion as a result of S. Dublin infection has been reported in Australia (55), while S. Brandenburg causes abortions in New Zealand cattle (57), as well as sheep, and has also been associated with disease in humans (130). Clinical signs of salmonellosis in cattle, besides abortion, include diarrhea, a drop in milk production, and dysentery (57, 131). Salmonella infects and proliferates in the placenta and, subsequently, focal necrosis is often observed in the fetal villi after abortion (132). Diagnosis can be achieved by culture of fetal stomach contents or organs, as well as serology (133).
Bacillus
Bacillus species are ubiquitous and may infect the cow from silage. Members of this genus have been reported to cause reproductive failures in Australian and New Zealand cattle (62, 134, 135), the most common of which is Bacillus licheniformis, followed by B. cereus. Abortions caused by infection with this organism usually occur in the third trimester, and sporadically amongst the herd (136). The bacterium causes a necro-suppurative placentitis and fetal broncho-pneumonia. Pneumonia and enteritis occur after the fetus swallows bacteria that infect and proliferate in the amniotic fluid. The suppuration seen microscopically can sometimes be seen grossly at post mortem. Necrotic yellow, 2–3 mm foci can be seen in the placenta, and purulent exudate may be expressed from the pulmonary airways. The leathery, dry, yellowish-brown placentitis observed with B. licheniformis is fairly distinctive, but needs to be differentiated from that caused by fungal infections. Diagnosis is reached by culture of the organism from stomach contents or fresh tissue (60, 137).
Ureaplasma Diversum
Ureaplasma diversum is a commensal of the vagina and prepuce, and in pregnant cows an ascending infection can occur, causing abortion in the last trimester of pregnancy (138). Ureaplasma have also been shown to attach to spermatozoides (139) and may be spread through insemination (140). In Australia, infection in cattle has been confirmed, but without evidence of disease (63, 64), while in New Zealand, U. diversum has been associated with abortions and vaginitis (66). Macroscopic lesions of the placenta include thickening of large areas of the amnion and inter-cotyledonary zones of the chorio-allantois. The thickened regions are opaque white to yellow, with foci of fibrinous exudation and hemorrhage. Microscopic lesions in the placenta, and lymphocytic nodules in the fetal conjunctiva and lung are characteristic and highly suspicious of Ureaplasma infection (141). No gross lesions are seen in the fetus. Diagnosis can be made by multiplex PCR assay on lung and stomach content (117).
Mycoplasma Bovis
Mycoplasma bovis abortion has been reported in Ireland, Germany and Australia (69, 142, 143), but despite this, clinical disease is rarely reported in Australia (67). Until 2009, there was no evidence of M. bovis in New Zealand dairy cattle (144), but infection was detected in 20174 A potential source of transmission was recently described in Finland, when infection was introduced into previously disease-free herds by frozen semen (145). Placentitis, swollen lymph nodes, pulmonary consolidation, interstitial and bronchial pneumonia, fibrinous pericarditis and enlarged liver may be evident in the aborted fetus (142). The organism may be isolated from the stomach content (143), but diagnosis can also be made by multiplex PCR on serum, lung, and liver (117, 146, 147).
Coxiella Burnetii
Coxiella burnetii is the etiological agent of Q-fever, a serious zoonotic infection that affects livestock and wild mammals worldwide, except New Zealand (148). The organism is spread in excretions of infected animals, and can be spread as aerosols or by dust. In humans, for which cattle are only rarely the source of infection (149), the disease is subclinical in >60% of the cases. In ruminants, particularly in sheep and goats, it causes abortions, as evidenced in the recent severe zoonotic outbreak in the Netherlands (150). Experimental confirmation of C. burnetii as the etiological agent in bovine abortion is generally lacking, complicated by the fact that C. burnetti is found in healthy placentas (151). To make an etiological diagnosis in cases of abortion, placentitis should be present alongside the organism, and mild to severe evidence of inflammation should be seen upon histological examination (151). Modified Ziehl-Neelsen (MZN) smears have traditionally been used to detect C. burnetti in the placental cotyledons, but immunohistochemistry, fluorescence in situ hybridization and PCR assay are also possible (151, 152).
Chlamydia
Chlamydia and Chlamydophila are obligate intracellular pathogens that are spread by contact with secretions and excretions of infected animals. These bacteria have been associated with abortions in cattle (153), and Chlamydia psittaci was isolated in a case of abortion in Australia in 1986 (70) but has not been reported since. This may have been a misidentification of a Chlamydophila pecorum infection (154), which does not cause abortion. In New Zealand, C. psittaci has been found in birds (155) but not cattle. In cases of suspected chlamydial abortions, purulent or necrotising placentitis may be observed by histopathology and genetic material may be detected by PCR (117, 156).
Other Bacteria
A range of other opportunistic bacterial infections can lead to abortion, these include: Escherichia coli, Fusobacterium necrophorum, Staphylococcus aureus, Streptococcus bovis, Trueperella pyogenes, and others, which require routine diagnostic procedures to confirm the diagnosis (157).
Viral Abortion
Bovine Viral Diarrhea Virus (BVDV)
The pathogenesis and diagnosis of Bovine Viral Diarrhea has recently been reviewed extensively (158). In both countries, only BVDV Type 1 infections have been recorded. In Australia, the overwhelming majority of isolates are of a Type 1c classification (159), while the diversity of strains observed in New Zealand is much greater (160). The virus has reached a high level of endemicity in both countries, and more than 80% of adult cattle usually show an antibody response. This decreases the potential for major reproductive disease but potentially devastating outbreaks of abortions can still occur when persistently infected cattle are introduced to BVDV naïve groups. The impact in both countries, and the mitigation options available have recently been reviewed (85). BVDV is immunosuppressive, increasing susceptibility to co-infection, so evidence of the virus in fetal and placental samples does not confirm BVDV as the etiological agent. Reproductive failures associated with BVDV are variable in timing and appearance, and lesions associated with BVDV are varied but may include vasculitis, placentitis, tissue necrosis, and atrophy (161–163). The virus can be detected using reverse transcription quantitative PCR (RT-qPCR) or immunohistochemistry. Diagnosis of an acute BVDV infection in the adult can be made using serology on paired serum samples.
Endemic in Either Country but Not Causing Abortion
Bovine Herpes Virus Type 1 (BHV-1)
BHV-1 is a virus with a worldwide distribution, and causes respiratory and genital diseases in cattle. The syndromes are known as infectious bovine rhinotracheitis (IBR) and infectious pustular vulvovaginitis/balanoposthitis (IPV/IPB), respectively. Less commonly, BHV-1 is associated with a variety of other clinical diseases, such as encephalitis, conjunctivitis, enteritis, and a fatal systemic infection of neonatal calves. BHV-1 infection can also induce abortion, and is recognized as such in some overseas countries. Early work carried out in the mid 70's by Durham et al. (164) indicated that the viral strain causing IBR in New Zealand was unlikely to be capable of inducing bovine abortion. From recent studies, it appears that the predominant strain of BHV-1 present in New Zealand and Australia is BHV-1.2b. There is no evidence that more virulent strains of BHV-1, e.g., BHV-1.1 (associated with severe respiratory infections and abortions) or BHV type 5 (associated with neurological infections), have ever been present in New Zealand or Australia (49, 82). In cases of abortion due to infection with virulent BHV-1 subtypes found elsewhere, lesions of the aborted fetus may include inflammation and necrosis of the heart, brain, liver, kidneys, spleen, lungs, and intestine (165). For a sensitive diagnostic test, qPCR may be performed on the liver, which has the highest viral load (166).
Bluetongue Virus (BTV)
BTV is a vector-borne virus spread by Culicoides brevitarsis in Australia. In New Zealand, a complete absence of Culicoides species coincides with the absence of BTV5 (167). Dependence of vector survival and distribution on environmental factors restricts BTV seasonally to the northern and southern coasts. Bluetongue disease occurs when immunologically naïve ruminants are exposed to BTV (168, 169), but disease in cattle is rare. In Australia, clinical Bluetongue disease has not been recorded in livestock to date6 (170). This is partly because BTV is endemic to the region, but also because the virulent BTV serotypes found in other countries are absent from Australia (170–174), and there have been no recorded BTV-induced abortions, due to their inability to cross the placenta (83). Should virulent serotypes emerge, the aborted fetus presents with hydranencephaly that increases in severity the earlier in gestation infection occurs (175). RT-qPCR performed on cerebral tissue is the most suitable diagnostic test (176, 177).
Akabane and Aino Virus
Both Akabane and Aino are members of the genus Orthobunyavirus and are the most pathogenic members of the Simbu serogroup. Like BTV, they are largely transmitted by the biting midge C. brevitarsis, and are therefore absent in New Zealand but endemic to Australia. Antibody surveys suggest a viral distribution north of the “brevitarsis line” in tropical and subtropical parts of the Australia7 (43, 178), associated with the known distribution of their vector (179, 180). As with other endemic viruses, clinical disease is rarely seen in this region. Early gestational exposure [between gestational day (GD) 80–105] is linked to hydranencephaly, whereas later exposure (GD 105–175) more often results in arthrogryposis (181). RT-qPCR is available for detection of, and differentiation between these viruses (182).
Non-infectious and “Undetermined” Causes
Non-infectious causes of abortion include; nitrate toxicity from crops (183), isocupressic acid toxicity (184, 185), twin pregnancy, large placenta syndrome and premature shedding of fetal membranes (186), hydroallantois, drug reactions, physical trauma, inherited lethal abnormalities (187), heat stress, teratogens, iodine deficiency, and endotoxins (188). For a review see Jonker (189).
Diagnostic Approaches
Investigating abortions can be a difficult process, and there is no single diagnostic test to identify all etiologies. However, there are some common signs that indicate an infectious agent is responsible. Inflammation of the placenta, for example, occurs as a result of infection with organisms such as U. diversum or fungi. This results in fetal death due to a disruption of vascularity and oxygen exchange (1). Prostaglandins are also released during the inflammatory response, which results in luteolysis, and the cascade of events leading to fetal expulsion (190, 191). Once the fetus dies, the villous circulation collapses and becomes obliterated, characterized by intra-placental coagulation and endothelial disruption (192). Separation of the cotyledon from the caruncle results in cessation of the pregnancy and the fetus and membranes are expelled, manifesting as an abortion (193).
In the field, it may be necessary to obtain multiple fetuses or dam serum samples because the problem is continuing, or the cause has not been identified. Frequently there are no diagnostic clinical signs or gross lesions, so identification of the etiological agent usually requires a complete diagnostic work-up. This involves post-mortem, histopathology, microbiology, serology and, increasingly, molecular biology. Diagnostic laboratories usually focus on the most likely etiologies, and those with zoonotic potential (194). Diagnosis of the etiological agent has improved with time, from about 33–37% in the 1990s (195, 196), to 44% in the 2000s (197), to 58% (157) 2 years ago, but only if a full range of samples were collected.
An 8 year review (2008–2015) of 544 cases received in one commercial diagnostic laboratory in New Zealand was able to arrive at an etiological diagnosis in 45% of cases. The review showed that Neospora, followed by mycotic infections, were the most common causes of abortion (Figure 1).
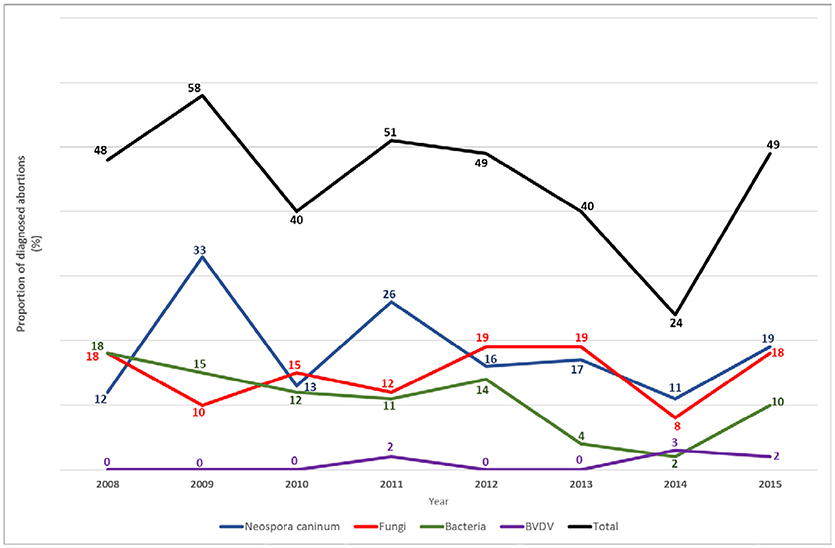
Figure 1. Percentage of aetiological diagnoses made per year, in 544 bovine abortion cases investigated at one veterinary diagnostic laboratory in New Zealand between 2008 and 2015.
The diagnostic rate is affected by:
• Submission of unsuitable samples to the diagnostic laboratory
• Delay between infection and the abortion occurring
• Destruction of the causal organism after fetal death or abortion
• Occurrence of abortion due to non-infectious causes.
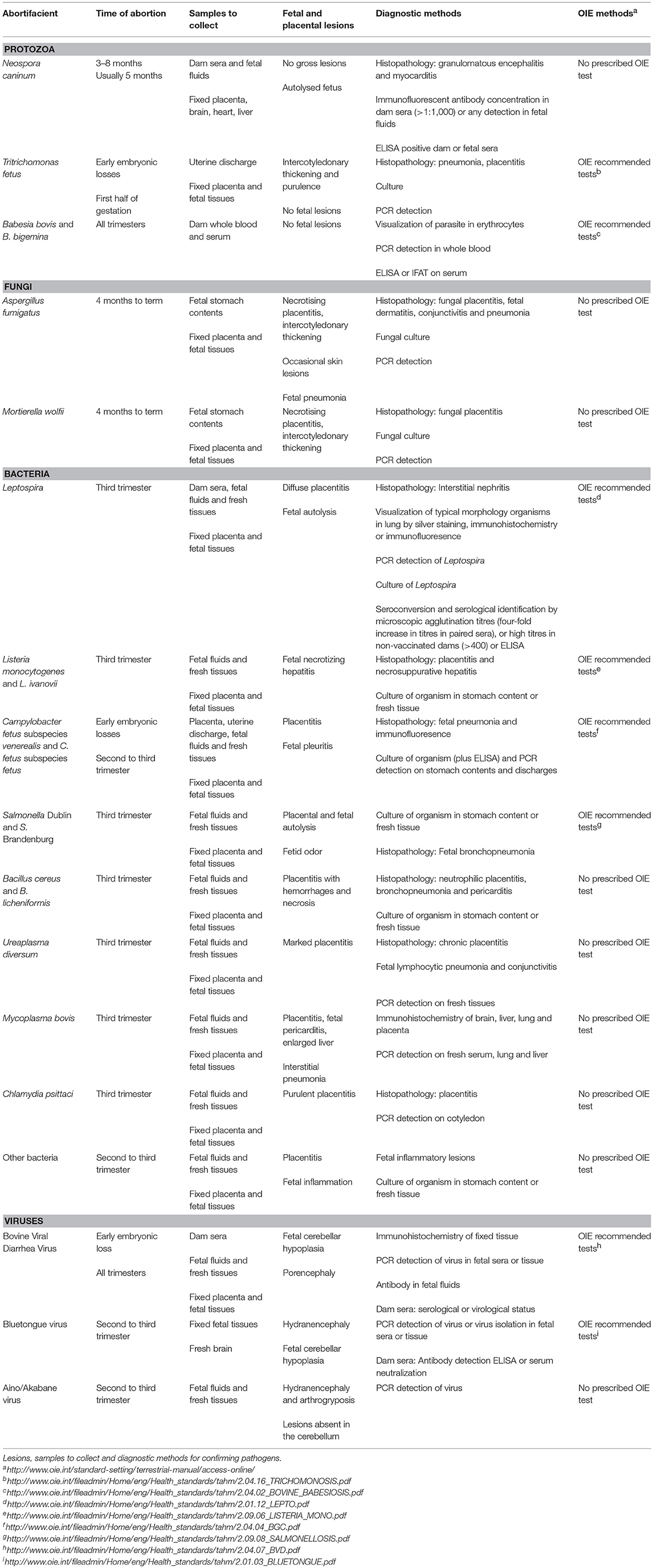
Tissues and samples recommended for submission are summarized in Table 2, and discussed below.
Placenta
Collection and submission of the placenta is recommended, if it can be obtained. Careful gross examination of the placenta can be worthwhile for the observation of lesions such as necrosis and hemorrhage of cotyledons, and intercotyledonary thickening. Fresh placenta samples can be cultured for bacteria and fungi. Multiple sections of any visible lesions should be sampled and placed in 10% neutral buffered formalin. If no lesions are apparent macroscopically, collect a cotyledon and region of intercotyledonary placenta for histopathology analysis.
Fetal Samples
Fetal age can be determined from the length of the fetus in millimeters from the crown of the head to the rump (CR length) (198), or by using on-line calculators (CR length in centimeters)8. Fetal age should be used to classify the trimester in which the abortion occurred. This can be used to refine the likely etiologies, and guide further approaches.
Fixed Samples
First trimester fetuses.
With small, first trimester fetuses (CR length up to 195 mm), the entire fetus may be placed in formalin in toto and dissected at the time of tissue trimming for histopathology.
Second and third trimester fetuses.
In second (195–600 mm CR length) and third (600–1,100 mm CR length) trimester fetuses, post-mortem examination of the fetus and collection of a standard series of tissues samples saved in 10% neutral buffered formalin for histopathology is recommended. Samples to collect include brain, lung, heart, liver, kidney, spleen, and conjunctiva.
The aborted fetus is often in a state of advanced autolysis because death occurred in utero some time before it was expelled. Histopathology is often still worthwhile, even on autolysed fetal tissues, and lesions in the brain can be diagnostic.
In second and third trimester fetuses, conjunctiva should be collected and assessed for any evidence of inflammation. Since the conjunctiva is a mucosal tissue in direct contact with amniotic fluid, an inflammatory response may be visible in the submucosa.
Fresh Samples
Suitable samples to culture for the presence of bacteria or fungal abortion are aseptically collected fetal stomach contents, lung and liver. If stomach contents are available, these should be cultured first, followed by lung and then liver. Stomach content represents a sample of the ingested amniotic fluid (199, 200). Culturing placenta is only recommended if these other samples are not available (195). These fresh samples should be collected routinely, and frozen if not used immediately. Bacteria can move from the vagina to the fetus and placenta when the cervix dilates during an impending abortion. These organisms may rapidly contaminate the placenta, grow in fetal fluids, and even be swallowed by a viable fetus, thereby appearing in the stomach contents, but are unrelated to the cause of the abortion.
If skin lesions are seen on the fetus, these could be scraped and checked for the presence of fungal hyphae, used for fungal culture (195) or collected for histopathology. Fungi isolated from stomach contents are consistent with a fungal cause of abortion (201).
Serology on fetal fluids can be useful in detecting fetal antibody to Leptospira, BTV and BVDV (202). Fetal heart blood or fetal body cavity fluid can be used as fetal sera. The presence of specific antibodies to an infectious agent in fetal heart blood or body cavity fluid provides indisputable evidence of prenatal exposure, though this exposure may not necessarily be the cause of abortion.
Individual or multiplex PCR analyses can be performed on fresh fetal tissues. If bacteria are also isolated from the placenta or cultured from fetal stomach contents, liver or lung, this provides a highly probable positive diagnosis (203). Even more so if lesions consistent with bacterial infection are present, and other causes of abortion have been ruled out.
Dam Samples
Serum from the dam can be tested for the presence of antibodies against various infectious agents such as Leptospira, N. caninum, BHV-1, and BVDV (204). Rationalizing the significance of titres requires knowledge of the pre-pregnancy and pre-abortion disease status of the dam. In a previously serologically disease free herd, any subsequent positive titre would be significant. If quantitative serological values are known, these can also be used for result interpretation. For example, if N. caninum Indirect Fluorescent Antibody Test (IFAT) titres increase to more than 1:1,000 soon after abortion, it is indicative of etiology. The titre then generally decreases to <1:600 over a period of a few weeks. The classical serological diagnosis of leptospirosis rests on a four-fold titre rise in paired sera, which requires two sets of sera to be obtained, increasing the cost of the diagnosis. In addition, titres may be present in response to vaccination or natural infection. However, high titres with no history of vaccination would suggest an etiological diagnosis. Abortions due to BVDV infection should induce antibody titres in the dam, and might allow detection of viral antigen in the aborted fetus. Older fetuses might also have developed an antibody response to the agent themselves.
Cost-Effectiveness
The costs of the diagnostic work-up to the farmers vary a lot in Australia, with some states still subsidizing the cost of outbreak investigations. In most cases, the costs of diagnosis are fully recovered by state-run laboratories or fully charged by commercial providers. For a cost-effective laboratory investigation, submission of a full range of dam, fetal and placental samples is recommended (205). Histopathology is the best first test to employ, and, although the most expensive, it can provide important pathology clues by observation of specific lesions. This can guide subsequent serological testing to check for antibodies in dam or fetal sera against Leptospira spp., N. caninum, and BVDV. Subsequent augmentation of the diagnostic approach with microbiology and mycology enables the isolation of cultivable infectious agents. Modern serology is mostly conducted at relatively low-cost with robust and standardized commercial kits. PCR is a rapid and specific approach to diagnostics which can reduce lead times on diagnoses and can be economical in comparison to conventional techniques.
Conclusions
In order to make a definitive diagnosis in cases of infectious abortion, the gold standard approach should include the following steps: firstly, confirm an infectious etiology is present by observing lesions and/or finding serological evidence. Secondly, identify the presence of the organism using techniques such as culture, PCR, immunohistochemistry or isolation. Finally, exclude all other possibilities. Table 2 outlines the details necessary to follow this method for the abortifacients found in Australia and New Zealand. To streamline the approach, and for cost-effectiveness, the exclusion of as many etiologies as possible should be made first by taking a detailed history, performing a post mortem examination and collecting a wide range of samples.
Useful information includes:
• The estimated abortion rate
• Whether abortion is occurring in both first-calving heifers and multiparous cows
• The estimated gestational age of aborted fetuses
• Relevant findings from physical examinations of aborting cows
• The herd's vaccination status
• Details of any weak or congenitally abnormal calves
• Whether natural or artificial breeding is used, or both
• Whether the fetus is fresh, mummified, or autolysed
This information should be used in conjunction with any characteristic post mortem findings, in order to target the tests used to identify pathogen presence. The validity of this approach holds true for new molecular diagnostic techniques that enhance our ability to detect and identify infectious organisms.
When undertaking a differential diagnosis, the unique ecology of infectious abortifacients in Australia and New Zealand should be taken into account. The notable absence of significant diseases such as brucellosis, and the absence of some pathogenic strains, serotypes and subtypes which are found in other parts of the world, should guide the approaches taken. While the possibility of emergence of exotic diseases should never be excluded, the likelihood of certain etiologies is low, and this can be taken into consideration, along with data from surveillance programs, when performing a cost-effective differential diagnosis.
A sound and systematic diagnostic process can improve the rate of diagnosis, reducing the number of abortions that cannot be reasonably attributed to a definitive cause. Molecular approaches are quickly superseding the classical culture and isolation approach, due to their increased speed, sensitivity, specificity and economy. However, presence of genetic material can rarely be used to make a diagnosis by itself, and results should be interpreted in conjunction with other techniques, and on a case-by-case basis.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
All authors are employees of City University of Hong Kong.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to acknowledge their professional colleagues over the years.
Footnotes
1. ^https://www.mpi.govt.nz/protection-and-response/responding/how-we-respond/
2. ^http://www.agriculture.gov.au/biosecurity/emergency
3. ^https://www.mpi.govt.nz/dmsdocument/10466-statement-absence-of-specified-diseases-from-new-zealand
4. ^https://www.mpi.govt.nz/protection-and-response/responding/alerts/mycoplasma-bovis/.
5. ^https://www.mpi.govt.nz/dmsdocument/10466-statement-absence-of-specified-diseases-from-new-zealand
6. ^https://www.dpi.nsw.gov.au/about-us/services/laboratory-services/veterinary/bluetongue
7. ^https://www.animalhealthaustralia.com.au/wp-content/uploads/AHAJ1707-NAMP-2016-17-Report_Digital_FA2.pdf
8. ^http://www.ansci.wisc.edu/jjp1/ansci_repro/lab/female_anatomy/crown_rump_calculators.htm.
References
1. Schlafer DH, Fisher PJ, Davies CJ. The bovine placenta before and after birth: placental development and function in health and disease. Anim Reprod Sci. (2000) 60–61:145–60. doi: 10.1016/S0378-4320(00)00132-9
2. Sarr D, Bracken TC, Owino SO, Cooper CA, Smith GM, Nagy T, et al. Differential roles of inflammation and apoptosis in initiation of mid-gestational abortion in malaria-infected C57BL/6 and A/J mice. Placenta (2015) 36:738–49. doi: 10.1016/j.placenta.2015.04.007
3. Sanad YM, Jung K, Kashoma I, Zhang X, Kassem II, Saif YM, et al. Insights into potential pathogenesis mechanisms associated with Campylobacter jejuni-induced abortion in ewes. BMC Vet Res. (2014) 10:274. doi: 10.1186/s12917-014-0274-8
4. Maley SWW, Buxton D, Macaldowie CNN, Anderson IEE, Wright SEE, Bartley PMM, et al. Characterization of the immune response in the placenta of cattle experimentally infected with Neospora caninum in early gestation. J Comp Pathol. (2006) 135:130–41. doi: 10.1016/j.jcpa.2006.07.001
5. Bartley PM, Katzer F, Rocchi MS, Maley SW, Benavides J, Nath M, et al. Development of maternal and foetal immune responses in cattle following experimental challenge with Neospora caninum at day 210 of gestation. Vet Res. (2013) 44:91. doi: 10.1186/1297-9716-44-91
6. Kraus TA, Engel SM, Sperling RS, Kellerman L, Lo Y, Wallenstein S, et al. Characterizing the pregnancy immune phenotype: results of the viral immunity and pregnancy (VIP) study. J Clin Immunol. (2012) 32:300–11. doi: 10.1007/s10875-011-9627-2
7. Srinivas SK, Ma Y, Sammel MD, Chou D, McGrath C, Parry S, et al. Placental inflammation and viral infection are implicated in second trimester pregnancy loss. Am J Obstet Gynecol. (2006) 195:797–802. doi: 10.1016/j.ajog.2006.05.049
8. Reichel MP, Alejandra Ayanegui-Alcérreca M, Gondim LFP, Ellis JT. What is the global economic impact of Neospora caninum in cattle - The billion dollar question. Int J Parasitol. (2013) 43:133–42. doi: 10.1016/j.ijpara.2012.10.022
9. Norton JH, Lisle AT, Tranter WP, Campbell RSF. A farming systems study of abortion in dairy cattle on the Atherton Tableland: I. Reproductive performance. Aust Vet J. (1989) 66:161–3. doi: 10.1111/j.1751-0813.1989.tb09791.x
10. McDougall S, Rhodes FM, Verkerk G. Pregnancy loss in dairy cattle in the Waikato region of New Zealand. N Z Vet J. (2005) 53:279–87. doi: 10.1080/00480169.2005.36561
12. Turner AJ. Endemic disease control and regulation in Australia 1901-2010. Aust Vet J. (2011) 89:413–21. doi: 10.1111/j.1751-0813.2011.00811.x
13. Godfroid J, Bishop GC, Bosman PP, Herr S. Bovine brucellosis. In: Coetzer JA, Tustin RC, editors. Infectious Diseases of Livestock. 3rd ed. Cape Town: Oxford University Press (2004).
14. Newton LG. Contagious bovine pleuropneumonia in Australia: some historic highlights from entry to eradication. Aust Vet J. (1992) 69:306–17. doi: 10.1111/j.1751-0813.1992.tb09912.x
15. Davidson RM. Control and eradication of animal diseases in New Zealand. N Z Vet J. (2002) 50(3 Suppl):6–12. doi: 10.1080/00480169.2002.36259
16. Cousins DV, Roberts JL. Australia's campaign to eradicate bovine tuberculosis: the battle for freedom and beyond. Tuberculosis (2001) 81:5–15. doi: 10.1054/tube.2000.0261
17. Tweddle NE, Livingstone P. Bovine tuberculosis control and eradication programs in Australia and New Zealand. Vet Microbiol. (1994) 40:23–39. doi: 10.1016/0378-1135(94)90044-2
18. Crispell J, Zadoks RN, Harris SR, Paterson B, Collins DM, de-Lisle GW, et al. Using whole genome sequencing to investigate transmission in a multi-host system: Bovine tuberculosis in New Zealand. BMC Genomics (2017) 18:180. doi: 10.1186/s12864-017-3569-x
19. Geoghegan JL, Walker PJ, Duchemin J-B, Jeanne I, Holmes EC. Seasonal drivers of the epidemiology of arthropod-borne viruses in Australia. PLoS Negl Trop Dis. (2014) 8:e3325. doi: 10.1371/journal.pntd.0003325
20. Graves SR, Islam A. Endemic Q fever in New South Wales, Australia: a case series (2005-2013). Am J Trop Med Hyg. (2016) 95:55–9. doi: 10.4269/ajtmh.15-0828
23. Moloney BJ, Kirkland PD, Heuer C. Neospora caninum in beef herds in New South Wales, Australia. 1: seroprevalence study. Aust Vet J. (2017) 95:72–9. doi: 10.1111/avj.12561
24. Hall CA, Reichel MP, Ellis JT. Prevalence of Neospora caninum infection in Australian (NSW) dairy cattle estimated by a newly validated ELISA for milk. Vet Parasitol. (2006) 142:173–8. doi: 10.1016/j.vetpar.2006.06.019
25. Dubey JP, Hartley WJ, Lindsay DS. Congenital Neospora caninum infection in a calf with spinal cord anomaly. J Am Vet Med Assoc. (1990) 197:1043–4.
26. Hall CA, Reichel MP, Ellis JT. Neospora abortions in dairy cattle: diagnosis, mode of transmission and control. Vet Parasitol. (2005) 128:231–41. doi: 10.1016/j.vetpar.2004.12.012
27. Sanhueza JM, Heuer C, West D. Contribution of Leptospira, Neospora caninum and bovine viral diarrhea virus to fetal loss of beef cattle in New Zealand. Prev Vet Med. (2013) 112:90–8. doi: 10.1016/j.prevetmed.2013.07.009
28. Tennent-Brown BS, Pomroy WE, Reichel MP, Gray PL, Marshall TS, Moffat PA, et al. Prevalence of Neospora antibodies in beef cattle in New Zealand. N Z Vet J. (2000) 48:149–50. doi: 10.1080/00480169.2000.36182
29. Thornton RN, Gajadhar A, Evans J. Neospora abortion epidemic in a dairy herd. N Z Vet J. (1994) 42:190–1. doi: 10.1080/00480169.1994.35819
30. McCool CJ, Townsend MP, Wolfe SG, Simpson MA, Olm TC, Jayawardhana GA, et al. Prevalence of bovine venereal disease in the Victoria River District of the Northern Territory: likely economic effects and practicable control measures. Aust Vet J. (1988) 65:153–6. doi: 10.1111/j.1751-0813.1988.tb14445.x
31. Vermunt JJ, Parkinson TJ. Infectious diseases of cattle in New Zealand - Part 2 - adult animals. Surveillance (2000) 27:3–9.
32. Docker TM. Bovine trichomonia its diagnosis and control in New Zealand herds. Aust Vet J. (1948) 24:226–34. doi: 10.1111/j.1751-0813.1948.tb04673.x
33. Callow LL, Kanhai GK, Vandenberghe A. Serological comparison of strains of Babesia bovis occurring in Australia and Mozambique. Trop Anim Health Prod. (1981) 13:79–82. doi: 10.1007/BF02237896
34. Trueman KF, McLennan MW. Bovine abortion due to prenatal Babesia bovis infection. Aust Vet J. (1987) 64:63–63. doi: 10.1111/j.1751-0813.1987.tb16136.x
35. McCausland IP, Slee KJ, Hirst FS. Mycotic abortion in cattle. Aust Vet J. (1987) 64:129–32. doi: 10.1111/j.1751-0813.1987.tb09659.x
36. Thompson KG, di Menna ME, Carter ME, Carman MG. Mycotic mastitis in two cows. N Z Vet J. (1978) 26:176–7. doi: 10.1080/00480169.1978.34533
37. Cordes DO, Shortridge EH. Systemic phycomycosis and aspergillosis of cattle. N Z Vet J. (1968) 16:65–80. doi: 10.1080/00480169.1968.33749
38. Gabor LJ. Mycotic pneumonia in a dairy cow caused by Mortierella wolfii. Aust Vet J. (2003) 81:409–10. doi: 10.1111/j.1751-0813.2003.tb11548.x
39. Munday JS, Wolfe AG, Lawrence KE, Pandey SK. Disseminated Mortierella wolfii infection in a neonatal calf. N Z Vet J. (2010) 58:62–3. doi: 10.1080/00480169.2010.65062
40. Carter ME, Cordes DO, di Menna ME, Hunter R. Fungi isolated from bovine mycotic abortion and pneumonia with special reference to Mortierella wolfii. Res Vet Sci. (1973) 14:201–6.
41. Klein M, Brown L, Tucker RW, Ashbolt NJ, Stuetz RM, Roser DJ. Diversity and abundance of zoonotic pathogens and indicators in manures of feedlot cattle in Australia. Appl Environ Microbiol. (2010) 76:6947–50. doi: 10.1128/AEM.01095-10
42. Wynwood SJ, Burns MA, Graham GC, Weier SL, McKay DB, Craig SB. Serological diagnosis of Leptospirosis in bovine serum samples using a microsphere immunoassay. Vet Rec Open (2016) 3:e000148. doi: 10.1136/vetreco-2015-000148corr1
43. Norton JH, Tranter WP, Campbell RSF. A farming systems study of abortion in dairy cattle on the Atherton Tableland: 2. The pattern of infectious diseases. Aust Vet J. (1989) 66:163–7. doi: 10.1111/j.1751-0813.1989.tb09792.x
44. Munday BL, Ryan FB, King SJ, Corbould A. Preparturient infections and other causes of foetal loss in sheep and cattle in Tasmania. Aust Vet J. (1966) 42:189–93. doi: 10.1111/j.1751-0813.1966.tb04687.x
45. Benschop J, Collins-Emerson J, Maskill A, O'Connor P, Tunbridge M, Yupiana Y, et al. Leptospirosis in three workers on a dairy farm with unvaccinated cattle. N Z Med J. (2017) 130:102–8.
46. McAuley CM, McMillan K, Moore SC, Fegan N, Fox EM. Prevalence and characterization of foodborne pathogens from Australian dairy farm environments. J Dairy Sci. (2014) 97:7402–12. doi: 10.3168/jds.2014-8735
47. Fairley RA, Colson M. Enteric listeriosis in a 10-month-old calf. N Z Vet J. (2013) 61:376–8. doi: 10.1080/00480169.2013.809634
48. Gill PA, Boulton JG, Fraser GC, Stevenson AE, Reddacliff LA. Bovine abortion caused by Listeria ivanovii. Aust Vet J. (1997) 75:214–214. doi: 10.1111/j.1751-0813.1997.tb10069.x
49. Hancock AS, Younis PJ, Beggs DS, Mansell PD, Pyman MF. Infectious reproductive disease pathogens in dairy herd bulls. Aust Vet J. (2015) 93:349–53. doi: 10.1111/avj.12369
50. Hum S, Stephens LR, Quinn C. Diagnosis by ELISA of bovine abortion due to Campylobacter fetus. Aust Vet J. (1991) 68:272–5. doi: 10.1111/j.1751-0813.1991.tb03240.x
51. Hum S. Bovine abortion due to Campylobacter fetus. Aust Vet J. (1987) 64:319–20. doi: 10.1111/j.1751-0813.1987.tb07343.x
52. Sanhueza JM, Heuer C, Jackson R, Hughes P, Anderson P, Kelly K, et al. Pregnancy rates of beef cattle are not affected by Campylobacter fetus subsp. venerealis real-time PCR-positive breeding sires in New Zealand. N Z Vet J. (2014) 62:237–43. doi: 10.1080/00480169.2014.898202
53. Vanselow BA, Hum S, Hornitzky MA, Eamens GJ, Quinn K. Salmonella Typhimurium persistence in a Hunter Valley dairy herd. Aust Vet J. (2007) 85:446–50. doi: 10.1111/j.1751-0813.2007.00224.x
54. Trueman KF, Thomas RJ, Mackenzie AR, Eaves LE, Duffy PF. Salmonella Dublin infection in Queensland dairy cattle. Aust Vet J. (1996) 74:367–9. doi: 10.1111/j.1751-0813.1996.tb15447.x
55. Russell RG, Malmo J, Robinson BA, Graaff R. The association of Salmonella Dublin with bovine abortion in Victoria. Aust Vet J. (1973) 49:173–4. doi: 10.1111/j.1751-0813.1973.tb06777.x
56. Davidson KR. Salmonella Dublin abortion in a New South Wales dairy herd. Aust Vet J. (1973) 49:174. doi: 10.1111/j.1751-0813.1973.tb06778.x
57. Clark RG, Fenwick SG, Nicol CM, Marchant RM, Swanney S, Gill JM, et al. Salmonella Brandenburg-emergence of a new strain affecting stock and humans in the South Island of New Zealand. N Z Vet J. (2004) 52:26–36. doi: 10.1080/00480169.2004.36387
58. Cuttance EL. A case definition for Salmonella Brandenburg infection in dairy heifers.N Z Vet J. (2018) 66:52–52. doi: 10.1080/00480169.2017.1396940
59. Wu X-Y, Walker M, Vanselow B, Chao R-L, Chin J. Characterization of mesophilic bacilli in faeces of feedlot cattle. J Appl Microbiol. (2007) 102:872–9. doi: 10.1111/j.1365-2672.2006.03106.x
60. Mitchell G, Barton MG. Bovine abortion associated with Bacillus licheniformis. Aust Vet J. (1986) 63:160–1. doi: 10.1111/j.1751-0813.1986.tb02957.x
61. Parkinson TJ, Merrall M, Fenwick SG. A case of bovine mastitis caused by Bacillus cereus. N Z Vet J. (1999) 47:151–2. doi: 10.1080/00480169.1999.36134
62. Dowling A. B cereus isolated from aborting term beef cows. Proc Sheep Beef Soc New Zeal Vet Assoc. (2006) 36:129–36.
63. Hobson N, Chousalkar K, Chenoweth P. Ureaplasma diversum in bull semen in Australia: its detection and potential effects. Aust Vet J. (2013) 91:469–73. doi: 10.1111/avj.12113
64. Argue B, Chousalkar K, Chenoweth P. Presence of Ureaplasma diversum in the Australian cattle population. Aust Vet J. (2013) 91:99–101. doi: 10.1111/avj.12009
65. Hodges RT, Holland JTS. The recovery of ureaplasmas from the semen and prepuce of bulls. N Z Vet J. (1980) 28:89–90. doi: 10.1080/00480169.1980.34706
67. Morton J, Malmo J, House J, Mein G, Izzo M, Penry J. Mycoplasma bovis in Australian dairy herds. Aust Vet J. (2014) 92:322–3. doi: 10.1111/avj.12243
68. Al-Farha AA-B, Hemmatzadeh F, Khazandi M, Hoare A, Petrovski K. Evaluation of effects of Mycoplasma mastitis on milk composition in dairy cattle from South Australia. BMC Vet Res. (2017) 13:351. doi: 10.1186/s12917-017-1274-2
69. Hum S, Kessell A, Djordjevic S, Rheinberger R, Hornitzky M, Forbes W, et al. Mastitis, polyarthritis and abortion caused by Mycoplasma species bovine group 7 in dairy cattle. Aust Vet J. (2000) 78:744–50. doi: 10.1111/j.1751-0813.2000.tb10444.x
70. Seaman JT, Cockram FA, Scrivener CJ. Isolation of Chlamydia psittaci from an aborted bovine fetus. Aust Vet J. (1986) 63:233–4. doi: 10.1111/j.1751-0813.1986.tb03007.x
71. McLennan MW. Incidence of lameness requiring veterinary treatment in dairy cattle in Queensland. Aust Vet J. (1988) 65:144–7. doi: 10.1111/j.1751-0813.1988.tb14442.x
72. Bennett G, Hickford J, Zhou H, Laporte J, Gibbs J. Detection of Fusobacterium necrophorum and Dichelobacter nodosus in lame cattle on dairy farms in New Zealand. Res Vet Sci. (2009) 87:413–5. doi: 10.1016/j.rvsc.2009.04.001
73. Slee KJ, McOrist S. Mastitis due to a group of pyogenic bacteria. Aust Vet J. (1985) 62:63–5. doi: 10.1111/j.1751-0813.1985.tb14239.x
74. McDougall S. Gross abnormalities, bacteriology and histological lesions of uteri of dairy cows failing to conceive or maintain pregnancy. N Z Vet J. (2005) 53:253–6. doi: 10.1080/00480169.2005.36555
75. Ledgard AM, Smolenski GA, Henderson H, Lee RS-F. Influence of pathogenic bacteria species present in the postpartum bovine uterus on proteome profiles. Reprod Fertil Dev. (2015) 27:395. doi: 10.1071/RD13144
76. de Boer M, Buddle BM, Heuer C, Hussein H, Zheng T, LeBlanc SJ, et al. Associations between intrauterine bacterial infection, reproductive tract inflammation, and reproductive performance in pasture-based dairy cows. Theriogenology (2015) 83:1514–24. doi: 10.1016/j.theriogenology.2015.01.032
77. Weston JF, Heuer C, Parkinson TJ, Williamson NB. Causes of abortion on New Zealand dairy farms with a history of abortion associated with Neospora caninum. N Z Vet J. (2012) 60:27–34. doi: 10.1080/00480169.2011.631171
78. Evans CA, Cockcroft PD, Reichel MP. Antibodies to bovine viral diarrhea virus (BVDV) in water buffalo (Bubalus bubalis) and cattle from the Northern Territory of Australia. Aust Vet J. (2016) 94:423–6. doi: 10.1111/avj.12517
79. Lanyon SR, McCoy R, Bergman E, Reichel MP. Milk as a diagnostic sample for a commercially available ELISA to identify bovine viral diarrhea (BVD) antibodies in dairy herds. Aust Vet J. (2014) 92:269–73. doi: 10.1111/avj.12188
80. Quinn HE, Windsor PA, Kirkland PD, Ellis JT. An outbreak of abortion in a dairy herd associated with Neospora caninum and bovine pestivirus infection. Aust Vet J. (2004) 82:99–101.
81. Cuttance WG, Cuttance EL. Analysis of individual farm investigations into bovine viral diarrhea in beef herds in the North Island of New Zealand. N Z Vet J. (2014) 62:338–42 doi: 10.1080/00480169.2014.928925
82. Wang J, Horner GW, O'Keefe JS. Genetic characterisation of bovine herpesvirus 1 in New Zealand. N Z Vet J. (2006) 54:61–6. doi: 10.1080/00480169.2006.36613
83. Kirkland PD, Hawkes RA. A comparison of laboratory and “wild” strains of bluetongue virus – is there any difference and does it matter? Vet Ital. (2004) 40:448–55.
84. Littlejohns IR, Horner GW. Incidence, epidemiology and control of bovine pestivirus infections and disease in Australia and New Zealand. Rev Sci Tech. (1990) 9:195–205. doi: 10.20506/rst.9.1.480
85. Reichel MP, Lanyon SR, Hill FI. Perspectives on current challenges and opportunities for bovine viral diarrhea virus eradication in Australia and New Zealand. Pathogens (2018) 7:E14. doi: 10.3390/pathogens7010014
86. Black PF, Corney BG, Smythe LD, Dohnt MF, Norris MA, Symonds ML. Prevalence of antibodies to Leptospira serovars in beef cattle in central Queensland. Aust Vet J. (2001) 79:344–8. doi: 10.1111/j.1751-0813.2001.tb12010.x
87. Fang F, Collins-Emerson JM, Cullum A, Heuer C, Wilson PR, Benschop J. Shedding and seroprevalence of pathogenic Leptospira spp. in sheep and cattle at a New Zealand abattoir. Zoonoses Public Health (2015) 62:258–68. doi: 10.1111/zph.12146
88. Thilsted JP, Dubey JP. Neosporosis-like abortions in a herd of dairy cattle. J Vet Diagnostic Investig. (1989) 1:205–9. doi: 10.1177/104063878900100301
89. Reichel MP. Neospora caninum infections in Australia and New Zealand. Aust Vet J. (2000) 78:258–61. doi: 10.1111/j.1751-0813.2000.tb11751.x
93. Quinn HE, Ellis JT, Smith NC. Neospora caninum: a cause of immune-mediated failure of pregnancy? Trends Parasitol. (2002) 18:391–4. doi: 10.1016/S1471-4922(02)02324-3
94. Williams DJL, Guy CS, McGarry JW, Guy F, Tasker L, Smith RF, et al. Neospora caninum-associated abortion in cattle: the time of experimentally-induced parasitaemia during gestation determines foetal survival. Parasitology (2000) 121:347–58. doi: 10.1017/S0031182099006587
95. Dubey JP, Buxton D, Wouda W. Pathogenesis of bovine neosporosis. J Comp Pathol. (2006) 134:267–89. doi: 10.1016/j.jcpa.2005.11.004
96. Barr BC, Rowe JD, Sverlow KW, BonDurant RH, Ardans AA, Oliver MN, et al. Experimental reproduction of bovine fetal neospora infection and death with a bovine neospora isolate. J Vet Diagnostic Investig. (1994) 6:207–15. doi: 10.1177/104063879400600212
97. Dennett DP, Reece RL, Barasa JO, Johnson RH. Observations on the incidence and distribution of serotypes of Tritrichomonas fetus in beef cattle in north-eastern Australia. Aust Vet J. (1974) 50:427–31. doi: 10.1111/j.1751-0813.1974.tb06863.x
98. Christensen HR, Clark BL. Spread of Tritrichomonas fetus in Beef Bulls in an Infected Herd. Aust Vet J. (1979) 55:205. doi: 10.1111/j.1751-0813.1979.tb15291.x
99. Parkinson TJ, Bruère AN. Evaluation of Bulls for Breeding Soundness. Palmerston North: Vetlearn Foundation, Massey University (2007).
100. Eaglesome MD, Garcia MM. Disease risks to animal health from artificial insemination with bovine semen. Rev Sci Tech. (1997) 16:215–25. doi: 10.20506/rst.16.1.1017
101. Cobo ER, Favetto PH, Lane VM, Friend A, VanHooser K, Mitchell J, et al. Sensitivity and specificity of culture and PCR of smegma samples of bulls experimentally infected with Tritrichomonas fetus. Theriogenology (2007) 68:853–60. doi: 10.1016/j.theriogenology.2007.06.019
102. Grahn RA, BonDurant RH, van Hoosear KA, Walker RL, Lyons LA. An improved molecular assay for Tritrichomonas fetus. Vet Parasitol. (2005) 127:33–41. doi: 10.1016/j.vetpar.2004.08.018
103. Rhyan JC, Stackhouse LL, Quinn WJ. Fetal and placental lesions in bovine abortion due to Tritrichomonas fetus. Vet Pathol. (1988) 25:350–5. doi: 10.1177/030098588802500503
104. Yoon E, Vail E, Sann L, Brassel J. New staining technique for diagnosing Babesia species. Am J Clin Pathol. (2015) 144(Suppl. 2):A228. doi: 10.1093/ajcp/144.suppl2.228
105. Callow LL, McGavin MD. Cerebral Babesiosis due to Babesia Argentina. Aust Vet J. (1963) 39:15–21. doi: 10.1111/j.1751-0813.1963.tb04170.x
107. Hill MWM, Whiteman CE, Benjamin MM, Ball L. Pathogenesis of experimental bovine mycotic placentitis produced by Aspergillus fumigatus. Vet Pathol. (1971) 8:175–92. doi: 10.1177/030098587100800206
108. Cordes DO, Carter ME, Di Menna ME. Mycotic pneumonia and placentitis caused by Mortierella-wolfii. II. Pathology of experimental infection of cattle. Vet Pathol. (1972) 9:190–201. doi: 10.1177/030098587200900302
109. Curtis B, Hollinger C, Lim A, Kiupel M. Embolic mycotic encephalitis in a cow following Mortierella wolfii infection of a surgery site. J Vet Diagnostic Investig. (2017) 29:725–8. doi: 10.1177/1040638717710684
111. Marshall RB, Manktelow BW. Fifty years of leptospirosis research in New Zealand: a perspective. N Z Vet J. (2005) 50(3 Suppl):61–3.
112. Lilenbaum W, Martins G. Leptospirosis in cattle: a challenging scenario for the understanding of the epidemiology. Transbound Emerg Dis. (2014) 61:63–8. doi: 10.1111/tbed.12233
113. Carroll AG, Campbell RSF. Reproductive and leptospiral studies on beef cattle in central Queensland. Aust Vet J. (1987) 64:1–5. doi: 10.1111/j.1751-0813.1987.tb06046.x
114. Slee KJ, McOrist S, Skilbeck NW. Bovine abortion associated with Leptospira interrogans serovar hardjo infection. Aust Vet J. (1983) 60:204–6. doi: 10.1111/j.1751-0813.1983.tb09583.x
115. Te Punga WA, Bishop WH. Bovine abortion caused by infection with Leptospira pomona. N Z Vet J. (1953) 1:143–9. doi: 10.1080/00480169.1953.33129
116. Otaka D, Penna B, Martins G, Hamond C, Lilenbaum W, Medeiros MA. Rapid diagnostic of leptospirosis in an aborted bovine fetus by PCR in Rio de Janeiro, Brazil. Vet Microbiol. (2013) 162:1001–2. doi: 10.1016/j.vetmic.2012.11.037
117. Tramuta C, Lacerenza D, Zoppi S, Goria M, Dondo A, Ferroglio E, et al. Development of a set of multiplex standard polymerase chain reaction assays for the identification of infectious agents from aborted bovine clinical samples. J Vet Diagnostic Investig. (2011) 23:657–64. doi: 10.1177/1040638711407880
118. Picardeau M. Diagnosis and epidemiology of leptospirosis. Médecine Mal Infect. (2013) 43:1–9. doi: 10.1016/j.medmal.2012.11.005
119. Chideroli RT, Gonçalves DD, Suphoronski SA, Alfieri AF, Alfieri AA, de Oliveira AG, et al. Culture strategies for isolation of fastidious Leptospira serovar Hardjo and molecular differentiation of genotypes Hardjobovis and Hardjoprajitno. Front Microbiol. (2017) 8:2155. doi: 10.3389/fmicb.2017.02155
120. Kirkbride CA. Bacterial agents detected in a 10-year study of bovine abortions and stillbirths. J Vet Diagn Investig. (1993) 5:64–8. doi: 10.1177/104063879300500114
121. Alexander AV, Walker RL, Johnson BJ, Charlton BR, Woods LW. Bovine abortions attributable to Listeria ivanovii: four cases (1988-1990). J Am Vet Med Assoc. (1992) 200:711–4.
122. Rocha CE, Mol JPS, Garcia LNN, Costa LF, Santos RL, Paixão TA. Comparative experimental infection of Listeria monocytogenes and Listeria ivanovii in bovine trophoblasts. PLoS ONE (2017) 12:e0176911. doi: 10.1371/journal.pone.0176911
123. Loveridge R, Gardner E. Campylobacter fetus venerealis infection in cattle. Surveillance (1993) 20:26–27.
124. Spence RP, Bruce IR, McFadden AMJ, Hill FI, Tisdall D, Humphrey S, et al. Cross-reaction of a Campylobacter fetus subspecies venerealis real-time PCR. Vet Rec. (2011) 168:131. doi: 10.1136/vr.c5264
125. van der Graaf-van Bloois L, Miller WG, Yee E, Rijnsburger M, Wagenaar JA, Duim B. Inconsistency of phenotypic and genomic characteristics of Campylobacter fetus subspecies requires reevaluation of current diagnostics. J Clin Microbiol. (2014) 52:4183–8. doi: 10.1128/JCM.01837-14
126. Waldner CL, Parker S, Gesy KM, Waugh T, Lanigan E, Campbell JR. Application of direct polymerase chain reaction assays for Campylobacter fetus subsp. venerealis and Tritrichomonas fetus to screen preputial samples from breeding bulls in cow-calf herds in western Canada. Can J Vet Res. (2017) 81:91–9.
127. Truyers I, Luke T, Wilson D, Sargison N. Diagnosis and management of venereal campylobacteriosis in beef cattle. BMC Vet Res. (2014) 10:280. doi: 10.1186/s12917-014-0280-x
128. Iraola G, Hernández M, Calleros L, Paolicchi F, Silveyra S, Velilla A, et al. Application of a multiplex PCR assay for Campylobacter fetus detection and subspecies differentiation in uncultured samples of aborted bovine fetuses. J Vet Sci. (2012) 13:371–6. doi: 10.4142/jvs.2012.13.4.371
129. Campero CM, Anderson ML, Walker RL, Blanchard PC, Barbano L, Chiu P, et al. Immunohistochemical identification of Campylobacter fetus in natural cases of bovine and ovine abortions. J Vet Med Ser B (2005) 52:138–41. doi: 10.1111/j.1439-0450.2005.00834.x
130. Baker MG, Thornley CN, Lopez LD, Garrett NK, Nicol CM. A recurring salmonellosis epidemic in New Zealand linked to contact with sheep. Epidemiol Infect. (2007) 135:76–83. doi: 10.1017/S0950268806006534
131. Hinton M. Salmonella Dublin abortion in cattle: studies on the clinical aspects of the condition. Br Vet J. (1974) 130:556–63. doi: 10.1016/S0007-1935(17)35742-1
132. Hall GA, Jones PW. A study of the pathogenesis of experimental Salmonella Dublin abortion in cattle. J Comp Pathol. (1977) 87:53–65. doi: 10.1016/0021-9975(77)90079-2
133. Sánchez-Miguel C, Crilly J, Grant J, Mee JF. Sensitivity, specificity and predictive probability values of serum agglutination test titres for the diagnosis of Salmonella Dublin culture-positive bovine abortion and stillbirth. Transbound Emerg Dis. (2017) 65:676–86 doi: 10.1111/tbed.12784
134. Mason RW, Munday BL. Abortion in sheep and cattle associated with Bacillus spp. Aust Vet J. (1968) 44:297–8. doi: 10.1111/j.1751-0813.1968.tb05000.x
135. Logan NA. Bacillus species of medical and veterinary importance. J Med Microbiol. (1988) 25:157–65. doi: 10.1099/00222615-25-3-157
136. Agerholm JS, Krogh HV, Jensen HE. A retrospective study of bovine abortions associated with Bacillus licheniformis. J Vet Med Ser B (1995) 42:225–34.
137. Agerholm JS, Jensen NE, Dantzer V, Jensen HE, Aarestrup FM. Experimental infection of pregnant cows with Bacillus licheniformis bacteria. Vet Pathol. (1999) 36:191–201. doi: 10.1354/vp.36-3-191
138. Miller R, Chelmonska-Soyta A, Smits B, Foster R, Rosendal S. Ureaplasma diversum as a cause of reproductive disease in cattle. Vet Clin North Am Food Anim Pract. (1994) 10:479–90. doi: 10.1016/S0749-0720(15)30533-8
139. Buzinhani M, Yamaguti M, Oliveira RC, Cortez BA, Marques L, Machado-Santelli GM, et al. Invasion of Ureaplasma diversum in bovine spermatozoids. BMC Res Notes (2011) 4:455. doi: 10.1186/1756-0500-4-455
140. Givens MD. Review: Risks of disease transmission through semen in cattle. Animal (2018) 12:s165–71. doi: 10.1017/S1751731118000708
141. Ruhnke HL, Palmer NC, Doig PA, Miller RB. Bovine abortion and neonatal death associated with Ureaplasma diversum. Theriogenology (1984) 21:295–301. doi: 10.1016/0093-691X(84)90415-1
142. Hermeyer K, Peters M, Brügmann M, Jacobsen B, Hewicker-Trautwein M. Demonstration of Mycoplasma bovis by immunohistochemistry and in situ hybridization in an aborted bovine fetus and neonatal calf. J Vet Diagnostic Investig. (2012) 24:364–9. doi: 10.1177/1040638711435145
143. Byrne WJ, Brennan P, McCormack R, Ball HJ. Isolation of Mycoplasma bovis from the abomasal contents of an aborted bovine fetus. Vet Rec. (1999) 144:211–2. doi: 10.1136/vr.144.8.211
144. McDonald WL, Rawdon TG, Fitzmaurice J, Bolotovski I, Voges H, Humphrey S, et al. Survey of bulk tank milk in New Zealand for Mycoplasma bovis, using species-specific nested PCR and culture. N Z Vet J. (2009) 57:44–9. doi: 10.1080/00480169.2009.36867
145. Haapala V, Pohjanvirta T, Vähänikkilä N, Halkilahti J, Simonen H, Pelkonen S, et al. Semen as a source of Mycoplasma bovis mastitis in dairy herds. Vet Microbiol. (2018) 216:60–6. doi: 10.1016/j.vetmic.2018.02.005
146. Watson P, Mason C, Stevenson H, Scholes S, Schock A, Mearns R, et al. Laboratory diagnosis of Mycoplasma/Ureaplasma abortion in cattle. Vet Rec. (2012) 170:82–4. doi: 10.1136/vr.e495
147. McAuliffe L, Ellis RJ, Lawes JR, Ayling RD, Nicholas RAJ. 16S rDNA PCR and denaturing gradient gel electrophore a single generic test for detecting and differentiating Mycoplasma species. J Med Microbiol. (2005) 54:731–9. doi: 10.1099/jmm.0.46058-0
148. Hilbink F, Penrose M, Ko Vacova E, Kazar J. Q fever is absent from New Zealand. Int J Epidemiol. (1993) 22:945–9. doi: 10.1093/ije/22.5.945
149. Clark NJ, Soares Magalhães RJ. Airborne geographical dispersal of Q fever from livestock holdings to human communities: a systematic review and critical appraisal of evidence. BMC Infect Dis. (2018) 18:218. doi: 10.1186/s12879-018-3135-4
150. Dijkstra F, van der Hoek W, Wijers N, Schimmer B, Rietveld A, Wijkmans CJ, et al. The 2007–2010 Q fever epidemic in The Netherlands: characteristics of notified acute Q fever patients and the association with dairy goat farming. FEMS Immunol Med Microbiol. (2012) 64:3–12. doi: 10.1111/j.1574-695X.2011.00876.x
151. Agerholm JS. Coxiella burnetii associated reproductive disorders in domestic animals-a critical review. Acta Vet Scand. (2013) 55:13. doi: 10.1186/1751-0147-55-13
152. Pritchard GC, Smith RP, Errington J, Hannon S, Jones RM, Mearns R. Prevalence of Coxiella burnetii in livestock abortion material using PCR. Vet Rec. (2011) 169:391. doi: 10.1136/vr.d4693
153. Kauffold J, Wehrend A, Sigmarsson H. Chlamydia and Chlamydophilia in bovine reproduction. Clin Theriogenol. (2014) 6:251–4.
154. Jelocnik M, Islam MM, Madden D, Jenkins C, Branley J, Carver S, et al. Development and evaluation of rapid novel isothermal amplification assays for important veterinary pathogens: Chlamydia psittaci and Chlamydia pecorum. PeerJ (2017) 5:e3799. doi: 10.7717/peerj.3799
155. Gedye KR, Fremaux M, Garcia-Ramirez JC, Gartrell BD. A preliminary survey of Chlamydia psittaci genotypes from native and introduced birds in New Zealand. N Z Vet J. (2018) 66:162–5. doi: 10.1080/00480169.2018.1439779
156. Borel N, Thoma R, Spaeni P, Weilenmann R, Teankum K, Brugnera E, et al. Chlamydia-related abortions in Cattle from Graubunden, Switzerland. Vet Pathol. (2006) 43:702–8. doi: 10.1354/vp.43-5-702
157. Clothier K, Anderson M. Evaluation of bovine abortion cases and tissue suitability for identification of infectious agents in California diagnostic laboratory cases from 2007 to 2012. Theriogenology (2016) 85:933–8. doi: 10.1016/j.theriogenology.2015.11.001
158. Lanyon SR, Hill FI, Reichel MP, Brownlie J. Bovine viral diarrhea: pathogenesis and diagnosis. Vet J. (2014) 199: 201–9. doi: 10.1016/j.tvjl.2013.07.024
159. Ridpath JF, Fulton RW, Kirkland PD, Neill JD. Prevalence and antigenic differences observed between bovine viral diarrhea virus subgenotypes isolated from cattle in Australia and feedlots in the southwestern united states. J Vet Diagnostic Investig. (2010) 22:184–91. doi: 10.1177/104063871002200203
160. Vilček, Björklund HV, Horner GW, Meers J, Belák S. Genetic typing of pestiviruses from New Zealand. N Z Vet J. (1998) 46:35–7. doi: 10.1080/00480169.1998.36048
161. Kirkbride CA. Viral agents and associated lesions detected in a 10-year study of bovine abortions and stillbirths. J Vet Diagn Investig. (1992) 4:374–9. doi: 10.1177/104063879200400402
162. Kendrick JW. Bovine viral diarrhea virus-induced abortion. Theriogenology (1976) 5:91–3. doi: 10.1016/0093-691X(76)90028-5
163. Bistner SI, Rubin LF, Saunders LZ. The ocular lesions of bovine viral diarrhea-mucosal disease. Pathol Vet. (1970) 7:275–86. doi: 10.1177/030098587000700306
164. Durham PJ, Forbes-Faulkner JC, Poole WS. Infectious bovine rhinotracheitis virus: experimental attempts at inducing bovine abortion with a New Zealand isolate. N Z Vet J. (1975) 23:93–4. doi: 10.1080/00480169.1975.34204
165. Sasani F, Vazirian A, Javanbakht J, Hassan MA. Detection of infectious bovine rhinotracheitis in natural cases of bovine abortion by PCR and histopathology assays. Am J Clin Exp Med. (2013) 1:35. doi: 10.11648/j.ajcem.20130102.11
166. Crook T, Benavides J, Russell G, Gilray J, Maley M, Willoughby K. Bovine herpesvirus 1 abortion: current prevalence in the United Kingdom and evidence of hematogenous spread within the fetus in natural cases. J Vet Diagn Investig. (2012) 24:662–70. doi: 10.1177/1040638712448187
167. Mellor PS, Boorman J, Baylis M. Culicoides biting midges: their role as arbovirus vectors. Annu Rev Entomol. (2000) 45:307–40. doi: 10.1146/annurev.ento.45.1.307
168. Maclachlan NJ, Drew CP, Darpel KE, Worwa G. The pathology and pathogenesis of bluetongue. J Comp Pathol. (2009) 141:1–16. doi: 10.1016/j.jcpa.2009.04.003
169. Brand SPC, Keeling MJ. The impact of temperature changes on vector-borne disease transmission: Culicoides midges and bluetongue virus. J R Soc Interface (2017) 14:20160481. doi: 10.1098/rsif.2016.0481
170. Kirkland PD. Bluetongue viruses, vectors and surveillance in Australia - the current situation and unique features. Vet Ital. (2004) 40:47–50.
171. Janowicz A, Caporale M, Shaw A, Gulletta S, Di Gialleonardo L, Ratinier M, et al. Multiple genome segments determine virulence of bluetongue virus serotype 8. J Virol. (2015) 89:5238–49. doi: 10.1128/JVI.00395-15
172. Caporale M, Di Gialleonorado L, Janowicz A, Wilkie G, Shaw A, Savini G, et al. Virus and host factors affecting the clinical outcome of bluetongue virus infection. J Virol. (2014) 88:10399–411. doi: 10.1128/JVI.01641-14
173. Sánchez-Cordón PJ, Pleguezuelos FJ, Pérez de Diego AC, Gómez-Villamandos JC, Sánchez-Vizcaíno JM, Cerón JJ, et al. Comparative study of clinical courses, gross lesions, acute phase response and coagulation disorders in sheep inoculated with bluetongue virus serotype 1 and 8. Vet Microbiol. (2013) 166:184–94. doi: 10.1016/j.vetmic.2013.05.032
174. Hooper PT, Lunt RA, Stanislawek WL. A trial comparing the virulence of some South African and Australian bluetongue viruses. Aust Vet J. (1996) 73:36–7. doi: 10.1111/j.1751-0813.1996.tb09955.x
175. Maclachlan NJ, Osburn BI. Teratogenic bluetongue and related orbivirus infections in pregnant ruminant livestock: timing and pathogen genetics are critical. Curr Opin Virol. (2017) 27:31–5. doi: 10.1016/j.coviro.2017.10.002
176. Mulholland C, McMenamy MJ, Hoffmann B, Earley B, Markey B, Cassidy J, et al. The development of a real-time reverse transcription-polymerase chain reaction (rRT-PCR) assay using TaqMan technology for the pan detection of bluetongue virus (BTV). J Virol Methods (2017) 245:35–9. doi: 10.1016/j.jviromet.2017.03.009
177. Vercauteren G, Miry C, Vandenbussche F, Ducatelle R, Van der Heyden S, Vandemeulebroucke E, et al. Bluetongue virus serotype 8-associated congenital hydranencephaly in calves. Transbound Emerg Dis. (2008) 55:293–8. doi: 10.1111/j.1865-1682.2008.01034.x
178. Agerholm JS, Hewicker-Trautwein M, Peperkamp K, Windsor PA. Virus-induced congenital malformations in cattle. Acta Vet Scand. (2015) 57:54. doi: 10.1186/s13028-015-0145-8
179. Cybinski DH, George TDS, Paull NI. Antibodies to Akabane virus in Australia. Aust Vet J. (1978) 54:1–3. doi: 10.1111/j.1751-0813.1978.tb00256.x
180. Della-Porta AJ, Murray MD, Cybinski DH. Congenital bovine epizootic arthrogryposis and hydranencephaly in Australia. Distribution of antibodies to Akabane virus in Australian Cattle after the 1974 epizootic. Aust Vet J. (1976) 52:496–501. doi: 10.1111/j.1751-0813.1976.tb06983.x
181. Kirkland PD, Barry RD, Harper PA, Zelski RZ. The development of Akabane virus-induced congenital abnormalities in cattle. Vet Rec. (1988) 122:582–6. doi: 10.1136/vr.122.24.582
182. Lee JH, Seo HJ, Park JY, Kim SH, Cho YS, Kim YJ, et al. Detection and differentiation of Schmallenberg, Akabane and Aino viruses by one-step multiplex reverse-transcriptase quantitative PCR assay. BMC Vet Res. (2015) 11:270. doi: 10.1186/s12917-015-0582-7
183. Davison KL, Hansel WM, Krook L, McEntee K, Wright MJ. Nitrate toxicity in dairy heifers. I. Effects on reproduction, growth, lactation, and vitamin A nutrition. J Dairy Sci. (1964) 47:1065–73. doi: 10.3168/jds.S0022-0302(64)88847-0
184. Mason RW. Letter: Foetal cerebral leucomalacia associated with Cupressus macrocarpa abortion in cattle. Aust Vet J. (1974) 50:419. doi: 10.1111/j.1751-0813.1974.tb05356.x
185. Parton K, Gardner D, Williamson NB. Isocupressic acid, an abortifacient component of Cupressus macrocarpa. N Z Vet J. (1996) 44:109–11. doi: 10.1080/00480169.1996.35946
186. Peter AT. Bovine placenta: a review on morphology, components, and defects from terminology and clinical perspectives. Theriogenology (2013) 80:693–705. doi: 10.1016/j.theriogenology.2013.06.004
187. Coates JW, Rousseaux CG, Schmutz SM. Multiple defects in an aborted bovine fetus associated with chromosomal trisomy. N Z Vet J. (1987) 35:173–4. doi: 10.1080/00480169.1987.35432
188. Lee JI, Kim IH. Pregnancy loss in dairy cows: the contributing factors, the effects on reproductive performance and the economic impact. J Vet Sci. (2007) 8:283. doi: 10.4142/jvs.2007.8.3.283
189. Jonker F. Fetal death: comparative aspects in large domestic animals. Anim Reprod Sci. (2004) 82–83:415–30. doi: 10.1016/j.anireprosci.2004.05.003
190. Skarzynski DJ, Jaroszewski JJ, Okuda K. Role of tumor necrosis factor-α and nitric oxide in luteolysis in cattle. Domest Anim Endocrinol. (2005) 29:340–6. doi: 10.1016/j.domaniend.2005.02.005
191. Neuvians TP, Schams D, Berisha B, Pfaffl MW. Involvement of pro-inflammatory cytokines, mediators of inflammation, and basic fibroblast growth factor in prostaglandin F2α-induced luteolysis in Bovine Corpus Luteum1. Biol Reprod. (2004) 70:473–80. doi: 10.1095/biolreprod.103.016154
192. Ornoy A, Crone K, Altshuler G. Pathological features of the placenta in fetal death. Arch Pathol Lab Med. (1976) 100:367–71.
193. Roescher AM, Timmer A, Erwich JJHM, Bos AF. Placental pathology, perinatal death, neonatal outcome, and neurological development: a systematic review. PLoS ONE (2014) 9:e89419. doi: 10.1371/journal.pone.0089419
194. Borel N, Frey CF, Gottstein B, Hilbe M, Pospischil A, Franzoso FD, et al. Laboratory diagnosis of ruminant abortion in Europe. Vet J. (2014) 200:218–29. doi: 10.1016/j.tvjl.2014.03.015
195. Kirkbride CA. Etiologic agents detected in a 10-year study of bovine abortions and stillbirths. J Vet Diagn Investig. (1992) 4:175–80. doi: 10.1177/104063879200400210
196. Jamaluddin AA, Case JT, Hird DW, Blanchard PC, Peauroi JR, Anderson ML. Dairy cattle abortion in California: evaluation of diagnostic laboratory data. J Vet Diagn Invest. (1996) 8:210–8. doi: 10.1177/104063879600800211
197. Anderson ML. Infectious causes of bovine abortion during mid- to late-gestation. Theriogenology (2007) 68:474–86. doi: 10.1016/j.theriogenology.2007.04.001
198. Holler LD. Ruminant abortion diagnostics. Vet Clin North Am Food Anim Pract. (2012) 28:407–18. doi: 10.1016/j.cvfa.2012.07.007
199. Underwood MA, Gilbert WM, Sherman MP. Amniotic fluid: not just fetal urine anymore. J Perinatol. (2005) 25:341–8. doi: 10.1038/sj.jp.7211290
200. Silberstein T, Saphier M, Mashiach Y, Paz-Tal O, Saphier O. Elements in maternal blood and amniotic fluid determined by ICP-MS. J Matern Neonatal Med. (2015) 28:88–92. doi: 10.3109/14767058.2014.905907
201. Knudtson WU, Kirkbride CA. Fungi associated with bovine abortion in the northern plains states (USA). J Vet Diagn Investig. (1992) 4:181–5. doi: 10.1177/104063879200400211
202. Kirkbride CA, Johnson MW. Serologic examination of aborted ovine and bovine fetal fluids for the diagnosis of border disease, bluetongue, bovine viral diarrhea, and leptospiral infections. J Vet Diagn Invest. (1989) 1:132–8. doi: 10.1177/104063878900100208
203. Vidal S, Kegler K, Posthaus H, Perreten V, Rodriguez-Campos S. Amplicon sequencing of bacterial microbiota in abortion material from cattle. Vet Res. (2017) 48:64. doi: 10.1186/s13567-017-0470-1
204. Waldner CL. Serological status for N. caninum, bovine viral diarrhea virus, and infectious bovine rhinotracheitis virus at pregnancy testing and reproductive performance in beef herds. Anim Reprod Sci. (2005) 90:219–42. doi: 10.1016/j.anireprosci.2005.03.017
Keywords: cattle, reproductive failure, abortion, diagnosis, infectious, diagnostic approaches, Australia, New Zealand
Citation: Reichel MP, Wahl LC and Hill FI (2018) Review of Diagnostic Procedures and Approaches to Infectious Causes of Reproductive Failures of Cattle in Australia and New Zealand. Front. Vet. Sci. 5:222. doi: 10.3389/fvets.2018.00222
Received: 30 June 2018; Accepted: 28 August 2018;
Published: 02 October 2018.
Edited by:
Dadin Prando Moore, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), ArgentinaReviewed by:
Veronica Risco-Castillo, École Nationale Vétérinaire d'Alfort, FranceFaten Abdelaal Okda, St. Jude Children's Research Hospital, United States
Copyright © 2018 Reichel, Wahl and Hill. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael P. Reichel, bXJlaWNoZWxAY2l0eXUuZWR1Lmhr
 Michael P. Reichel
Michael P. Reichel Lloyd C. Wahl
Lloyd C. Wahl Fraser I. Hill
Fraser I. Hill