
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Vet. Sci. , 28 June 2018
Sec. Animal Behavior and Welfare
Volume 5 - 2018 | https://doi.org/10.3389/fvets.2018.00131
Measuring and understanding personality in animals is a rising scientific field. Much research has been conducted to assess distinctive individual differences in behavior in a large number of species in the past few decades, and increasing numbers of studies include farm animals. Nevertheless, the terminology and definitions used in this broad scientific field are often confusing because different concepts and methods are used to explain often synonymously applied terms, such as personality, temperament and coping style. In the present review we give a comprehensive overview of the concepts and terms currently used in animal personality research and critically reveal how they are defined and what they measure. First, we shortly introduce concepts describing human personality and how these concepts are used to explain animal personality. Second, we present which concepts, methods and measures are applied in farm animal personality research and show that the terminology used seems to be somehow species-related. Finally, we discuss some findings on the possible impact of personality on the welfare of farm animals. The assessment of personality in farm animals is of growing scientific and practical interest. Differences in theoretical frameworks and methodological approaches may also entail the diverse use of the different concepts between basic and applied research approaches. We conclude that more consistency is needed in using different theoretical concepts, terms and measures, especially in farm animal personality research. The terms coping style and temperament, which are used in different ways, should not be examined as independent concepts, but rather should be considered as different aspects of the whole personality concept. Farm animal personality should be increasingly considered for the improvement of animal housing, management, breeding and welfare.
Measuring animal personality has become quite important in the past few decades, but many studies use different concepts and terminology to explain individual differences in behavior and physiology. Nevertheless, studies on farm animals do measure personality traits with methods that have been used to assess animal behavior. It must be born in mind that the concepts and terms such as personality, temperament, coping style used in animal personality research have their origin in human personality research.
In human personality research, the term personality is used to describe a distinctive and relatively stable set of mental traits that explain the organism's behavior, and the concept of personality is explained with the “Five-Factor Model of Personality.” The five factors describing human personality are extraversion, agreeableness, conscientiousness, neuroticism, and openness (1). Goldberg (2) describes which traits define each factor—extraversion (or surgency) comprises traits like talkativeness, assertiveness and activity levels on one end of the spectrum and silence, passivity and reserve on the other end; agreeableness (or pleasantness) includes traits like kindness, trust and warmth and the opposite traits of hostility, selfishness and distrust; conscientiousness (or dependability) comprises traits like organization, thoroughness and reliability on one end of the spectrum and carelessness, negligence and unreliability on the other end; neuroticism (or emotional stability) includes traits like nervousness, moodiness and temperamentality; and openness to experience (or intellect) describes traits like imagination, curiosity and creativity on one end of the spectrum and shallowness and imperceptiveness on the other end.
The term temperament in human psychology research is closely related to personality. Temperament is defined as the inherited, early appearing tendencies that continue throughout life and serve as the foundation for personality (3–5) because temperament is observable in infants and is tied to basic psychological processes (6, 7). According to Buss (8), the term temperament is used to reflect genetic behavioral differences, while the term personality, which is based on temperament, seems to be used to reflect non-genetic differences. Rothbart et al. (7) suggested that understanding temperament is central to understanding personality because it influences and is influenced by the experiences of each individual and because one of its outcomes is the adult personality. However, most theorists assume that temperament provides the starting place for personality development [reviewed by McCrae et al. (6)]. Temperament provides process-oriented models by establishing links between individual differences in behavior and their psychological and biological substrates in humans as well as in non-human animals, while personality provides subject-oriented models (7).
Coping is a very broad and a multidimensional concept with a long and complex history. In human psychology research, coping strategies refer to intentional cognitive or behavioral attempts by the individual to manage a stressor (9). Lazarus (10) has emphasized coping as a key concept for research on adaptation and health. Because coping is a behavioral reaction to aversive situations that induce several physiological stress reactions (11), individual ways of dealing with stress have an enormous impact on human health (12). Lazarus (10) suggests to differentiating between two approaches to coping, one that treats coping as a personality characteristic (style) and another that refers to the efforts to manage stress (process). Whereas the process approach primarily addresses the behavioral and physiological mechanisms involved in the adaptational response to stress, coping style rather seems to describe a personal or individual consistency in this response. The classification of coping into an “approach-avoidance” category can also be found in animal personality research. This category classifies behavior and cognitive abilities that are used by the individual to face the problem (approach) or to divert the individual's attention away (avoidance) from the problem (13).
Aim of the present review was to give a comprehensive overview how these concepts and terms, which have their origin in human psychology, are currently used in animal and especially farm animal personality research and to critically reveal how they are defined and what they measure. To obtain an overview of published studies in selected mammalian farm animal species we carried out a systematic literature search using the Web of Science (state December 2017) with keywords including the respective animal species (cattle, goat, sheep, horse, pig) and different synonyms applied in personality research (temperament, personality, coping). Then we exemplarily assessed the found studies within the context of terms and methodological approaches in general personality research. Finally, we discuss some findings on the possible impact of personality on the welfare of farm animals to especially highlight a future direction of personality research.
In animal personality research, many terms are used to explain individual differences in behavior, besides personality itself [e.g., behavioral syndromes (14) and coping style (15, 16)]. Definitions of the different terms used in animal personality research are shown in Table 1: Personality is defined as a correlated set of individual behavioral and physiological traits that are consistent over time and contexts (15, 29).
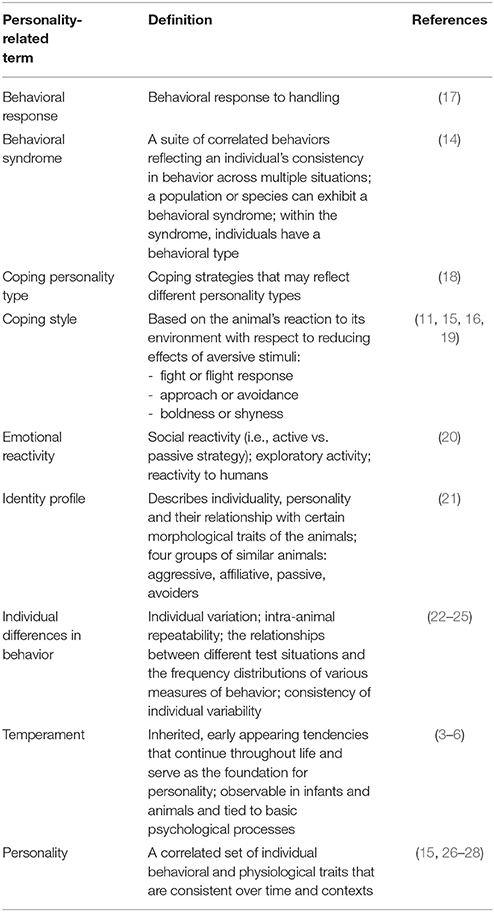
Table 1. Different personality-related terms in alphabetical order used to describe individual differences in animal behavior and their definitions.
Personality may explain individual differences in dominance rank, coping, cognitive abilities and physiology. At least one of the five factors which are used to describe human personality (i.e., extraversion, neuroticism, agreeableness, openness, and conscientiousness) has been investigated in many different species. The most commonly measured personality factors in animal personality research are exploration, activity, aggressiveness, sociability, and boldness (29–31). Exploration seems to resemble the human factor openness, aggressiveness seems to resemble agreeableness, and sociability seems to resemble extraversion, while boldness and activity seem to be combined in the human factor neuroticism [reviewed by Gosling and John (32)]. According to Gosling and John (32), the human factor conscientiousness can only be found in chimpanzees and gorillas. Figure 1 shows the five personality factors in humans and how they describe the personality type of two imaginary individuals [adapted from (33)] and the equivalent personality factors found in animals. While individual A scores highly in exploration (openness), sociability (extraversion) and aggressiveness (agreeableness), individual B scores highly on boldness and activity (neuroticism) and, if this individual is a human, in conscientiousness (Figure 1). A discussed factor in animals is the dominance factor that seems to be defined by boldness, physical aggression and low levels of fearfulness (32). In humans, these traits seem to be related to extraversion because humans participate in multiple dominance hierarchies that are less clearly defined and involve widely divergent skills (32). This means that species not only differ in the type of relevant factors, but also in the number of such factors (34): While chimpanzees share a common six-factor structure, including dominance, extraversion, agreeableness, conscientiousness, neuroticism, and openness (35), mountain gorillas seem to have only four personality factors: dominance, openness, sociability and proto-agreeableness (36). As in human personality research, sex differences can be found in the expression of animal personality traits. For example, hyena (Crocuta crocuta) males are more fearful and nervous than females. This effect seems to be influenced by the social organization. In hyenas, the dominance rank is transmitted through a matrilineal system, and females are larger than males. Sex differences in personality seem to be related to the ecological niches occupied by the two sexes [reviewed by Gosling and John (32)].
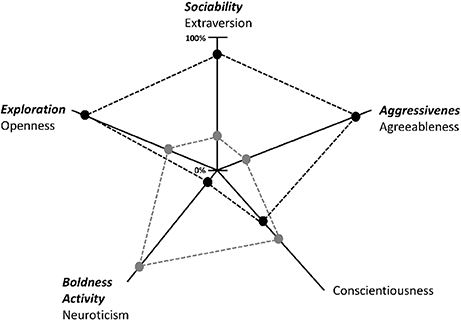
Figure 1. The five personality factors in humans and how they describe the personality type of two imaginary individuals [adapted from (33)]. The equivalent personality factors described in animals are written in bold and italics. Scores are given from zero to 100 in percentages. For example, Individual A (black dashed lines and points) scored high in openness, extraversion and agreeableness, while Individual B (gray dashed lines and points) scored high on neuroticism and conscientiousness.
David and Dall (37) state in their review that most of the confusion about terminology in animal personality studies is due to two main concepts, which can also be used complementarily: the intra-individual variability and the life-history approach. The first concept tries to explain the occurrence of between-individual differences in the consistency of any behavior (34), which means that studies employing this concept focus on the consistency and flexibility of behavioral expression. The second concept focuses only on boldness-like behaviors, which means that studies using this concept concentrate on the individual average behavior and the between-individual relationships between life history and behavior (37). One approach using both concepts is the pace-of-life syndrome (POLS) [reviewed by Réale et al. (26)]. This concept includes the idea that between-individual differences in behavior are associated with individual variations in life-history traits [e.g., habitat use, predation avoidance, dispersal and/or social behavior (14, 38–40)] because both may contribute to evolutionary trade-offs and may have co-evolved. And physiological traits that differ between individuals, such as growth, metabolic rate, reproductive value and hormone levels have also become important for animal personality research (28, 40–42) because they can be influenced by genetic predispositions and early environmental effects (27, 43).
Another important aspect of personality research in animals is that despite the consistency of some factors, others seem to be plastic and help the organism adapt to changing environments. This aspect is termed behavioral and/or physiological plasticity (43). Responsiveness to environmental conditions may span from short-term (phenotypic flexibility) to long-term effects (44), and the early environment an individual experiences contributes to between-individual differences in life histories and personality factors (41, 45). For example, in coral reef fish, individual scores on activity, boldness and aggressiveness increased from 2.5- to 6-fold when induced by an increase in water temperature, indicating individual coping abilities and/or behavioral plasticity (46). Moreover, photoperiodic changes influenced fearlessness, the stress response, the timing of maturation and resting metabolic rate (RMR) in wild cavies (Cavia aperea) and showed differences between sexes (47, 48). Behavioral and physiological plasticity is an important factor for the survival of an organism. Even if most of the personality factors proved to be consistent over time and contexts, factors like RMR and fearlessness were not consistent in cavies, whereas exploration and boldness were consistent regardless of the photoperiodic treatment (47). One might argue that factors such as RMR and fearlessness should not count as personality factors. However, an example in the European mink (Mustela lutreola) showed that traits that describe factors like boldness and exploration can change between seasons but that these changes remain consistent over time (49), which matches the definition of a personality factor. These examples indicate that the plasticity and consistency of personality factors are not exact opposites and indicate interesting asymmetries in the adjustments of personality factors that seem somehow species-related.
In farm animal studies, the term personality is scarcely used, even if the methods employed (e.g., repeated measurements) and measured variables (e.g., curiosity) can be described as personality factors. In those studies, the term temperament is used as an equivalent to the term personality. This could be due to the fact that farm animals are used to get meat, milk and other products and that it is easier to consume farm animal products when scientists do not attribute human-like characteristics to explain individual differences in farm animal behavior. Table 2 lists selected studies assessing personality factors using different experimental designs in mammalian farm animals (Table 2A: cattle; Table 2B: goats; Table 2C: sheep; Table 2D: horses; Table 2E: pigs) and the behavioral tests used. Some of these studies only measured and/or analyzed a small aspect of personality, measured just one or two personality traits or are examples of the very beginning of farm animal personality research, while other studies, shown in this table, show consistency and/or cross-context correlations between traits. One can see that the use of a certain personality-related term is dependent on the species studied. In cattle, the term temperament is mostly used to explain individual differences in behavior, while in pigs, the term coping style is mainly used (Tables 2A,E). In horses and more recent farm animal personality studies, one can find the term personality as well [e.g., (18, 76)] (Table 2D). In most of the farm animal studies investigating temperament, different personality factors can be found [e.g., curiosity shown in the general response to unfamiliar humans or the exit from a restraint device (50, 100)]. These can be considered as personality factors when they are measured in at least two different time periods of an animals' life. Like in other taxa, some farm animal studies can show that the personality factor boldness is related to other investigated behavioral traits. Boldness was found to be related to reactivity to humans in horses (77), cattle (50), and sheep (69): Individuals that interacted more with a novel stimulus were more interested in novel humans as well. In sheep, bold individuals proved to be more explorative and the distance between individuals while grazing was greater than in shy individuals (69). Moreover, the personality factor activity proved to be related to boldness and reactivity to humans in sheep: More active sheep seem to be bolder und more reactive to humans than shy sheep (65, 101). Calves can be categorized in four different personality types based on a combination of behavioral and heart rate variability (HRV) data (50).
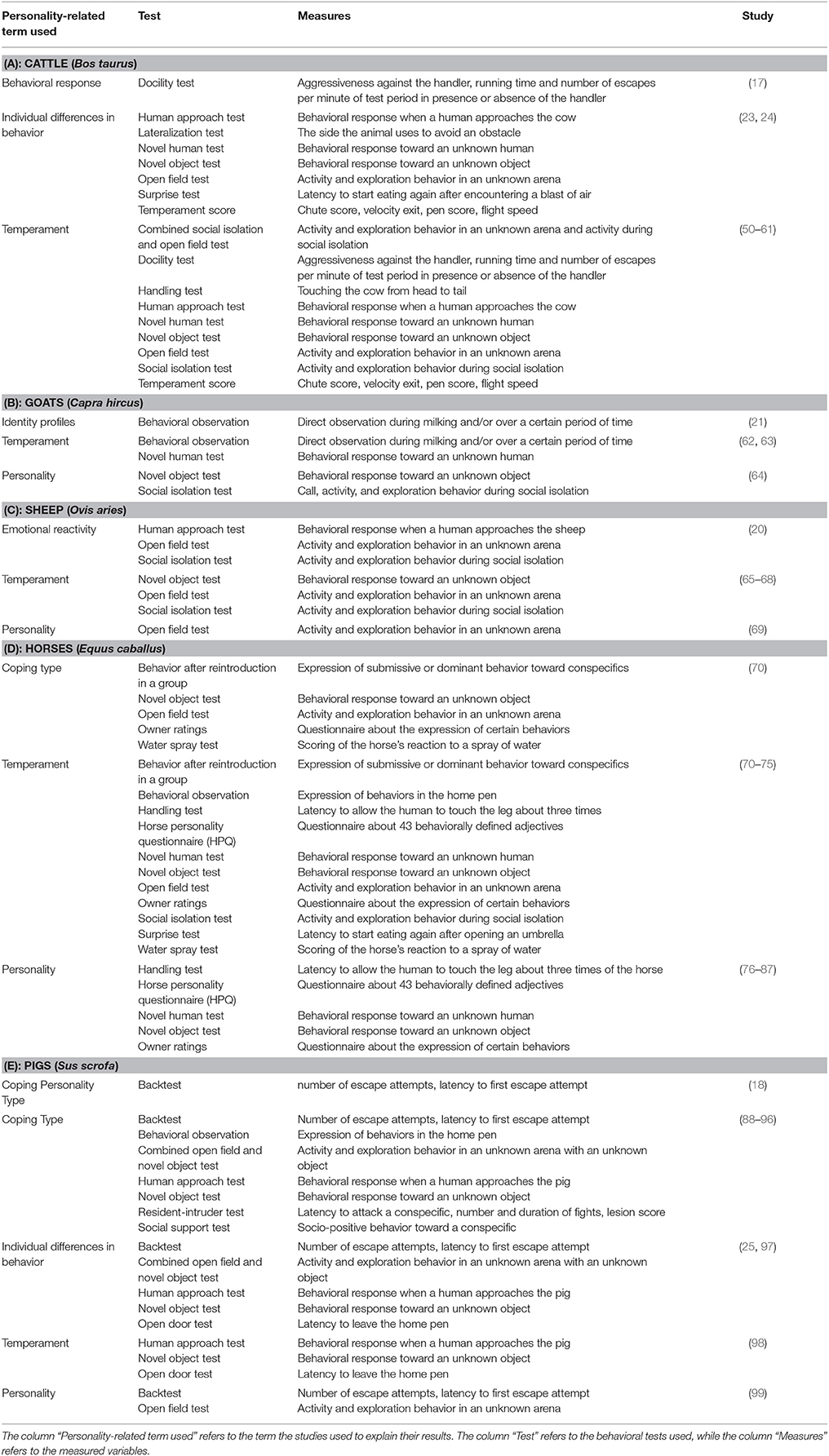
Table 2. Selected studies assessing personality factors using different experimental designs in mammalian farm animals [2 (A): cattle; 2 (B): goats; 2 (C): sheep; 2 (D): horses; 2 (E): pigs].
In animal personality research, the term temperament is closely related to personality. Temperament often matches the definition found in human personality research and is defined as inherited, early-appearing tendencies that continue throughout life and that serve as the foundation for personality (6) (Table 1). Since the distinction of both terms by definition is often vague, some animal researchers simply treat them as synonyms (29), while others argue that the term temperament is used simply to avoid using the term personality with regard to animals, which might be associated with anthropomorphism by some ethologists (102). While temperament is assumed to be based on early, stable predispositions in the emotional and behavioral responses of an individual like boldness, aggressiveness and pleasantness, personality is often reserved for patterns of reflection and reaction to environmental circumstances acquired during a lifetime in organisms with sophisticated cognitive capacities. The behavioral factors that are the focus of research on animal temperament in many different species are reactivity, fearfulness, sociability, responsiveness, and aggression. Temperament in non-human animals is often described on a one-dimensional scale using expressions such as “proactive vs. reactive,” “aggressive vs. non-aggressive,” “bold vs. shy,” etc. (103, 104). The actual theoretical frameworks on temperament in non-human animals argue for scoring reactions in more than one dimension to reflect the entire nature of temperament (29, 105). A multivariate approach has been used by Meager et al. (106) to investigate whether distinct temperaments are present in Atlantic cod (Gadus morhua). Their results refer to the multidimensionality of animal temperament and provide a clear indication that two distinct behavioral phenotypes are evident in fish. A study in non-human primates has shown that temperament, based on a factor analysis including behavior in different dimensions in yearling rhesus monkeys (Macaca mulatta), seems to be an important factor in an individual's ability to select friends and is related to later variations in the animals' social networks (107). The subjects preferred peers that had similar temperament scores to their own even after accounting for sex, rank and kinship. This example shows that the definition of temperament from the human domain can be used for non-human animals as well. Foyer et al. (108) investigated the impact of the level of maternal care in dogs on the offspring's behavior at approximately 18 months of age. The authors conducted a set of temperament tests to score the behavior of the dogs in different factors, such as sociability and fearfulness. The study showed that the level of maternal care differed consistently and that it has a significant impact on the adult temperament of the offspring, mainly in terms of sociability and dominance. The responses of cats across different behavioral factors were assessed to calculate a feline temperament profile (109). Later, the reactions of the cats in a 3-min stress test were measured. The authors did not find correlations between temperament scores and measures of behavioral and adrenocortical responses in the stress test. However, many studies concerning individual differences in animal behavior do not make the distinction between temperament and personality consistently, which makes it more difficult to understand the results with regard to temperament and/or personality.
In many farm animal studies, the term temperament is used as an equivalent to the term personality, which also can be seen in Table 2. General responses to unfamiliar humans or situations are quite important for handling, management and selective breeding (65). And traits such as fearfulness, happiness, alertness and docility [(51, 52, 110) can be considered to describe temperament when measured during the juvenile period of an animal and/or once in an animal's life. Animals that are more likely to cope with handling procedures are considered to have a “good” temperament (51) and dairy temperament (generally defined as the animal's response to milking) has been included in the breeding objectives of some countries [reviewed by Gibbons (111)]. Furthermore, high reactive bulls (reactivity was measured with a temperament score) had a greater rectal temperature and a higher cortisol and epinephrine concentration prior to and after transportation than low reactive bulls (53). As these examples measure personality, because measured in adult animals, these examples show how complex it is to describe individual personality in animals and that temperament reflects a sub-aspect of the whole concept of animal personality.
The concept of coping styles has also been used in the past few decades to better understand animal personality and is based on the animal's reaction to its environment with respect to reducing the effect of aversive stimuli (16, 19) (Table 1). Like in humans, animals show individual differences in coping with different environmental changes, confirming that personality and coping style are closely linked [(11, 15, 16, 19, 112); but see Zidar et al. (113) for an example of a missing relationship in the red junglefowl]. According to the boldness-shyness continuum, animals can be categorized into three sub-groups based on their risk-taking behavior: bold, intermediate and shy (112) or proactive, intermediate and reactive based on the animal's reaction to its environment with regard to altering the effect of aversive stimuli (fight/flight response) (11, 15, 16, 19). Proactive individuals are considered to be more aggressive toward conspecifics, show more dominant behavior, and are considered more explorative, bold and active. Moreover, they respond with a strong sympathetic activation and an increase in noradrenergic stimulation when confronted with a challenging situation, while reactive animals are considered the opposite behavioral phenotype, with behavior that is more submissive, less explorative and less active and responding with a strong hypothalamic-pituitary-adrenocortical reactivity toward challenging situations (31, 114–117).
Figure 2 shows a possible multidimensional approach to describing the five basic factors in animal personality research applied to the concept of animal coping style using an example of two imaginary animals. In addition, there is a potential sixth factor, dominance. There is an ongoing discussion as to whether dominance is a factor by itself or just a social outcome, and it also represents other species-related factors that are often missed in measurements (34, 102). Considering the description of proactive and reactive individuals above, the animal that scores low on the aggressiveness, activity and sociability axes would be described as being more peaceful, inactive and highly social and would represent a reactive coping style. The animal that scores high on the boldness, exploration and aggressiveness axes would be described as being more aggressive, explorative and bold and would be considered as a proactive individual. Intermediate individuals would express behavior described by a mixture of the scores on the factors resulting in individual differences on a continuum. Some factors show only low inter-correlations and are relatively independent from one another, which means that they must not co-occur [reviewed by Uher (118)]. Each factor summarizes the shared variation of diverse inter-correlating traits. The rank-orders of the same individuals on the same factor can vary considerably across different situations. An individual can have different scores on each factor [reviewed by Uher (118)]. The individual-oriented perspective (like in humans and shown in Figure 1) can also be applied to the analyses of multiple individuals such that individuals sharing a similar profile can be grouped statistically into configurational types. Such groups represent distinct and discontinuous categories of prototypes (e.g., proactive, intermediate and reactive) [reviewed by Uher (118)]. According to Koolhaas (119), proactive individuals perform better under highly predictable conditions, while reactive individuals perform better under variable and unpredictable environmental conditions. Proactive individuals also grow faster and have a higher RMR, which they can afford due to their higher rates of food acquisition. When food is abundant, proactive individuals may perform better than reactive individuals [reviewed by Careau et al. (120)]. Many studies have shown that different coping styles correlate with dominance rank, cognitive abilities and physiological measures [e.g., immunology (119)]. In fish, bold female guppies (Poecilia reticulate) learned a task more quickly than shy females (121), and in zebra finches (Taeniopygia guttata), more active birds were faster at solving a task, while less active individuals needed longer or did not solve the task at all, which indicates that higher activity may lead to routine-forming behavior instead of being attentive to external cues (122). Proactive Senegalese sole (Solea senegalensis) juveniles exhibited a shorter feeding latency, a higher duration of escape attempts and a lower cortisol level than reactive individuals (123). An example in mammals revealed that different coping styles in wild alpine marmots (Marmota marmota) are accompanied by different baseline and stress-induced plasma oxidative statuses (28). In male rainbowfish (Melanotaenia duboulayi), personality factors like aggression, activity and boldness covaried with the male's position in the hierarchy, directly influencing their reproductive success (124). Moreover, an example of spiders living in colonies showed that individuals with different coping styles do different tasks. While aggressive spiders perform prey capture and colony defense, less aggressive spiders perform brood care (22).
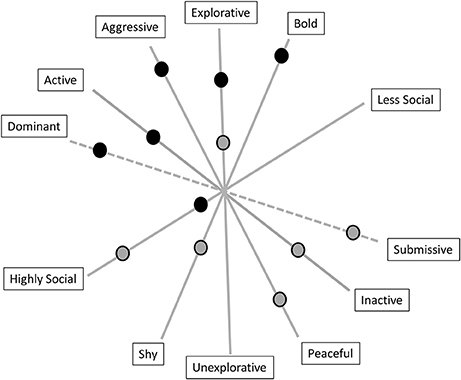
Figure 2. The five basic factors in animal personality research (bold lines) that can also be used to describe coping style. The dominance factor is presented in dashed lines because there are insufficient data to consider it as a fully accepted sixth factor in animal personality. The dots represent two imaginary animals with different coping styles. The animal behaving as described by the black-gray dots would represent a reactive coper, while the animal behaving as described by the black dots would be considered as a proactive individual. Intermediate individuals would express behavior described by a mixture of scores on the factors resulting in individual differences along a continuum.
Farm animal research on coping styles became more and more important because the coping style covers the aspect of an animal's skill in coping with environmental challenges. Farm animals like pigs (Sus scrofa), cattle (Bos taurus), and horses (Equus caballus) have to cope with numerous challenging situations (e.g., regrouping, housing, management) and technical material during their lives. Therefore, investigating their coping strategies and cognitive abilities appears to be a very important research topic, because like temperament, it reflects sub-aspects of the whole concept of animal personality. Aggression, boldness and exploration seem to be related to results in backtests that determine the specific coping-type in pigs assessed by the early escape behavior of piglets: Proactive pigs proved to be more aggressive, explorative and bolder than reactive pigs (88, 97). Recently, a number of studies were conducted to investigate the impact of different coping styles on psychophysiological measures and molecular correlates later in life. Reactive cows can be distinguished by their baseline HRV (54) and pigs characterized as belonging to different coping styles differed in their general autonomic reaction (e.g., HR, HRV) and in their affective appraisal in relation to common husbandry-related situations like feeding or human handling (125), indicating personality-depended emotional assessment of environmental situations. There is also evidence that individual coping styles of pigs are reflected in their immune responses indicating that proactive pigs might favor molecular pathways enabling a more effective strategy for defense and recovery than in reactive pigs (89, 90). Moreover, a recently conducted genome-wide association study revealed that hypothalamic genes known to be involved in pathways regarding the immune system, telomere function signaling, neurotransmitter receptors as well as circadian rhythms were differently expressed in pigs with different coping styles (126).
Personality, temperament and/or coping style and how they are interrelated with a diversity of other external or internal stimuli are investigated using different experimental setups. The main problem is to know exactly which personality factor or variable is being investigated using a certain experimental design (e.g., tests of exploration in an open field, test of boldness in investigating a novel object). Murphy et al. (127) identify in their review the most important aspects of experiments used to investigate farm animal personality. A test should be ecologically valid and the animal should be able to display its natural emotion-related behaviors. Since emotions have recently been discussed to be a personality factor by itself [see below reviewed by Koolhaas et al. (128)], tests should be sensitive enough to capture slight differences in levels of emotional arousal (bodily activation or excitation, the first dimension of emotion) or valence (negative or positive, the second dimension of emotion) (129, 130), which is important for longitudinal studies. Furthermore, the test itself and the variables measured should be standardized to allow for comparisons between studies (127). In his review, Gosling (3) describes the variables that can be used to analyse personality factors: (i) reactivity, emotionality, fearfulness: e.g., measurement of the defecation rate in an open field; (ii) exploration/boldness: e.g., interactions with a novel object; (iii) sociability: e.g., frequency of social encounters; (iv) aggression: e.g., latency to attack another individual; (v) activity: e.g., amount of enclosures (e.g., open field or arena test) covered; (vi) dominance/assertiveness: e.g., the individual's rank in the dominance hierarchy. Of course, these variables can be used to analyse other personality factors as well, and the mentioned personality factors can be measured by other variables or even a mixture thereof.
The most important aspect is to find the most appropriate test with the most appropriate variables to record the behaviors that are desired to analyse the intended personality factors for each species. Different species have different environmental requirements because they have different habitats and different life-histories. Koski (34) indicated that most studies about personality focus on boldness, aggressiveness, activity, exploration and sociability as the most prominent personality factors. However, she writes that the focus on these personality factors derived from human personality research may ignore the possibility of other factors that are more important for the investigated species and that such a focus limits our understanding of the full repertoire of personality factors [also reviewed by Gosling and John (32)]. Therefore, it is possible that species not only differ in the type of relevant factors, but also in the number of such factors (34): A study done on 1223 horses of eight different breeds with a Horse Personality Questionnaire also identified approximately six personality factors (78). Carter et al. (131) found boldness to be one of the most commonly measured personality factors in animal personality research, and it has been interpreted as the propensity to take risks, especially in novel situations, and as an individual's response to a risky situation faced alone. Boldness is often tested by quantifying behavioral responses to novel objects, responses to a novel environment, and/or responses to predation risk. However, these three types of tests are not necessarily comparable and demonstrate a lack of standardization for quantifying this behavior. For example, measuring boldness in cavies is slightly different than measuring boldness in pigs and cattle. For the three species, one can use the novel object test. While in cavies, the novel object test is conducted in the home enclosure (30, 47, 48), with pigs and cattle, the test is normally conducted in an arena outside the home pen after a habituation period or after an open field test [reviewed by Forkman et al. (132)]. Tests like the novel human test or the open-door test are conducted with farm animal species and measure the reactivity of an individual toward unknown persons or places (55, 71, 98). This type of test is only feasible with domesticated species that are used to human manipulation such as farm animals. In most cases, the measured trait is suggested to mirror fearfulness when describing an animal having a higher latency in approaching an unknown person [reviewed by Murphy et al. (127)]. In rodents, tests like the elevated plus maze [e.g., in mice and rats (133, 134)] or leaving a plastic shelter in an open field [e.g., in cavies (47)] are used to measure fearfulness or its opposite, fearlessness. Exploration is measured in most mammal studies and in most farm animal species with an open field test [reviewed by Forkman et al. (132), but see recent critiques by Perals et al. (135)].
To measure different coping strategies in pigs, Hessing et al. (136) suggested conducting the backtest at an early age to measure defensive reactions like struggling while the piglet is turned over on its back. Zebunke et al. (91) used more than 3,000 individuals and repeated the test four times with each individual. The results showed a moderate consistency of behavioral reactions across repeated testing, which cannot be attributed to a randomly occurring pattern. The authors concluded that the backtest does not provide phenotypic evidence for definitive coping styles that are clearly separable. Instead, they found pronounced individual dispositions along a continuum from proactive to reactive coping behaviors. The results of an equivalent to the backtest conducted with juvenile cavies before weaning (the struggle test) show a similar pattern: the time spent struggling was not significantly repeatable but showed a trend (137). Therefore, the backtest may indicate a certain coping style in pigs, which is discussed to be a personality factor by itself (113, 128). The backtest should not be used as a reference on its own but rather should be used as an addition to other tests measuring behavior and a certain personality type. Moreover, these examples confirm that one test can simultaneously be influenced by and therefore measure two or more personality factors (29).
Besides the fear of anthropomorphism in using the term personality, to know what is measured is one of the biggest lacking points in the field of farm animal personality research (see Table 2). Inspired by the definitions in Table 1, Figure 3 shows how researchers could proceed to know which personality related term they should use for their interpretation. Temperament and coping style overlap with personality, because they are sub-aspects of the whole personality concept. With all the behavioral tests used in farm animal personality research (e.g., open field test, novel object test, open door test, backtest etc.) applied ethologist can measure different aspects of personality: It depends of the approach they use. For instance, (i) using a behavioral test to measure a response to an aversive or stressful stimulus or situation (e.g., approach or avoidance) means that primarily a specific coping style is assessed, (ii) conducting various behavioral tests in a combination and/or conducted at least twice during lifetime, measures cross-context correlations and/or consistency and therefor assesses personality, (iii) conducting behavioral tests during the juvenile stage and only once during lifetime, would assess temperament.
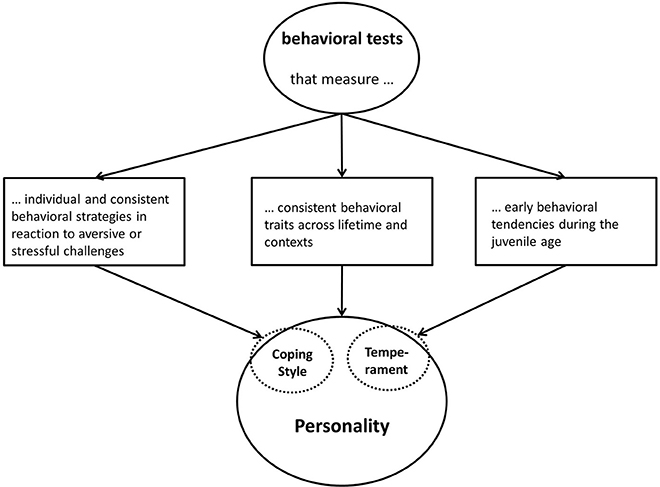
Figure 3. Various behavioral tests that can be used to assess different aspects of personality. It becomes evident that coping style and temperament are sub-aspects of the concept of personality.
In human psychology, studies show that subjective well-being is associated with health (138) and is linked to personality [reviewed by Weiss et al. (139)]. Well-being correlates with the five-factor model of personality, especially with neuroticism, extraversion and conscientiousness (140). In zoo animals, studies indicate that zoo keepers are able to reliably rate animal personality traits and that those ratings are implemented into zoo management practices to improve the welfare of captive animals (141). Personality research in farm animals has become important as well because welfare not only comprises the actual health status of an animal but also is affected by individual differences in behavior and physiology (142). Dawkins (143) already stated that individual behavior has a big advantage in welfare studies because it might become an “early warning system” of trouble yet to come. Changes in behavior (e.g., aggressiveness toward the caretaker) can be a hint of pain or other problems (144). Therefore, investigating and understanding individual differences in behavior, respective to personality, in farm animals is a possible means of measuring states of welfare and can also help to increase welfare. Individuals that are less well adapted to their environment may have reduced welfare, which in turn can lead to reduced productivity (145, 146). Figure 4 represents the influence of personality on individual welfare. Personality directly influences behavior and physiology and therefore influences individual welfare, while as in a feedback-loop, welfare can directly influence behavior and physiology. Behaviors can influence physiology and vice versa in a sort of positive feedback system as well. Especially in farm animals, domestication has an impact on behavior and physiology and directly influences breeding. During domestication of most farm animal species, behavior changed to lower levels of aggression and activity (147). Artificial selection for the improvement of production traits may have resulted in the selection of animals that would count as reactive copers (147). Genetic studies of captive animals often rely on the selection of specific production traits (148–150), because specific production traits are emphasized in the individual pedigree of an animal used for breeding [(151, 152); reviewed by Laine and van Oers (153)]. Recently, personality traits have been considered to a greater extent in the calculation of breeding indices because some of these traits show moderate to high heritability (150, 154–156). Aggression is highly heritable in pigs [ranging from h2 = 0.32 to h2 = 0.48 (157–159)]. In pigs as well as in cattle, aggressive behavior toward stockpersons and group-members is related to increased maternal behavior, which can be problematic [reviewed by Haskell et al. (155, 158)]. In beef and dairy cattle, handling shows moderate to high heritability scores: e.g., chute score (h2 = 0.24), flight speed (h2 = 0.36), and docility (h2 = 0.26) [reviewed by Haskell et al. (56, 155, 160)]. Especially in dairy cattle, milking temperament shows a moderate heritability (e.g., on average h2 = 0.19), but is also related to production traits such as milk yield [reviewed by Haskell et al. (56, 155)]. In France the docility test has been used to select for improved temperament in Limousin cattle since 1992, and dairy temperament (generally defined as the animal's response to milking) and milking speed have been included in the breeding objectives of some countries [e.g., United Kingdom and Norway reviewed by Gibbons (111)]. Horses, especially stallions considered for breeding purposes, are judged for performance (e.g., the gaits under the rider, jumping ability, rideability, fitness, health, stamina) and personality (e.g., behavior during handling (labeled as character), attention and reactivity (labeled as temperament) and braveness, willingness and ability to learn [labeled as willingness to work (72)].
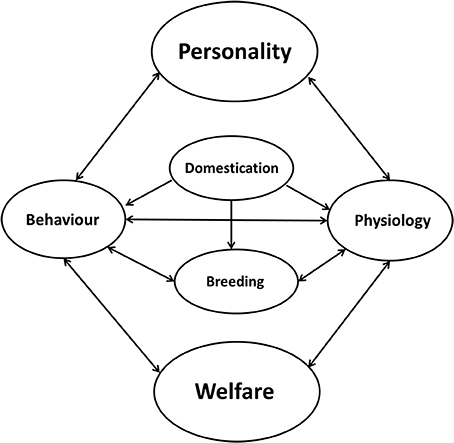
Figure 4. Relationship between personality and welfare. Personality directly influences behavior and physiology and therefore influences individual welfare, while as in a feedback-loop, welfare can directly influence behavior and physiology. Behavior can influence physiology and vice versa in a sort of positive feedback system. Especially in farm animals, domestication has an impact on behavior and physiology and directly influences breeding.
Another approach to explain the relationship between personality and welfare has been reviewed by Koolhaas and van Reenen (128), and it describes a three-dimensional model with coping style, emotionality and sociability as independent factors. These factors are defined as being stable over time and across contexts within the individual. Emotionality seems to be one important aspect to increasing welfare because it makes the distinction between fearful animals that are highly emotionally aroused by a challenging situation and non-fearful animals that do not perceive the same situation as stressful or alarming (128). A highly emotionally aroused animal would therefore exhibit activation of neuroendocrine systems while a non-fearful animal do not show any enhanced biological responses (128).
Some studies have shown evidence that emotionality, and therefore personality, seem to have an impact on production traits, such as meat quality and milk production. Studies on steers show that individual differences in stress responses (e.g., increase of cortisol in a stressful situation) or in blood lactate levels have an impact on meat tenderness (57, 58). In dairy cows, personality has an impact on behavioral and physiological responses to milking and on the stress associated with being milked in a novel environment (50, 161). A study on horses shows that personality has an influence on pain expression. Horses that were highly affected by vertebral problems showed more aggressive behavior toward humans than horses with no vertebral problems (144). Lameness was more expressed by highly extraverted horses even if the severity level of the injury threshold was lower when compared to more neurotic individuals (76). A study on female sheep (Ovis aries) showed that animals typed as “nervous” [measurement of the behavioral reactivity to the psychosocial stress of social isolation and selected for “calm” or “nervous” temperament for 17 generations (66)] seem to show a decrease in agitation score, the frequency of vocalizations and the plasma concentrations of cortisol when treated with lavender oil as an alternative treatment to alleviate anxiety compared to the response of “calm” sheep (66). Moreover, these “nervous” sheep produced a lower volume of higher viscosity colostrum than “calm” sheep, and this disparity could be corrected by nutritional supplementation (with barley), which only had an effect on “nervous” sheep (162). These examples indicate that personality seems to be an important factor in the efficacy of certain substances and nutritional supplementations. Personalized medicine in animals and humans already indicates that personality is a strong indicator for pathology development, medical treatment and substance efficacy (163). Understanding the individual personalities of farm animals is not only important for their welfare but also has an impact on economic factors for farmers [reviewed by Clark et al. (164)]. Therefore, it is important to think about considering to draw an individual personality profile like in Figure 1 [adapted from Costa and McCrae (33)] to picture farm animal personality and in concordance to improve management, handling, breeding, medical treatment and the design of housing systems that allow the animal to perform effective coping behavior [reviewed by Wechsler (11)].
This review gives an impression of how diverse farm animal personality research is and which aspects have to be considered in investigating personality in different farm animal species. Terminology in farm animal personality research is somewhat confusing and in some cases difficult to compare because the terms used seem somehow to be species-related. While studies in different mammals, birds, and other taxa widely use the term personality to describe between-individual consistency in behavioral variation, in farm animal research the terms temperament and coping style are predominantly used, probably because personality might be associated with anthropomorphism. The broad field of personality research generally needs more consistency in using theoretical concepts, terms and measures and we recommend that the terms should neither be regarded as synonyms nor as independent terms for consistent behavioral responses in animals, but as different aspects of the whole personality concept. Research on personality of farm animals is currently far from covering all possible aspects, but focuses in particular on the phenotyping of personality traits and potential relationships with cognition, emotion and welfare. We conclude that the assessment of personality in farm animals is of growing scientific, practical and economic interest, because it has an obvious verifiable impact on the individual behavioral reaction to different housing systems, management practices and veterinary interventions and is therefore important for the improvement of animal welfare.
M-AF, JL, and BP contributed to the conception and structure of the manuscript. Main parts of the manuscript were written by M-AF. The final version of the manuscript was prepared and edited by M-AF, JL, and BP.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Christian Nawroth, Katrin Siebert, Evelin Normann and the reviewers for valuable comments and corrections on earlier versions of the manuscript. The publication of this article was funded by the Open Access Fund of the Leibniz Association and the Open Access Fund of the Leibniz Institute for Farm Animal Biology (FBN).
1. John OP, Srivastava S. The Big Five trait taxonomy: history, measurement and theoretical perspectives. In: Lawrence AP, Oliver PJ, editors. Handbook of Personality: Theory and Research. New York: Guilford (1999). p. 102–38.
2. Goldberg LR. The structure of phenotypic personality traits. Am Psychol. (1993) 48:26–34. doi: 10.1037/0003-066X.48.1.26
3. Gosling SD. From mice to men: what we can learn about personality from animal research? Psychol Bull. (2001) 127:45–86. doi: 10.1037/0033-2909.127.1.45
4. Buss AH. Personality: Temperament, Social Behavior and the Self. Needham Heights, MA: Allyn & Bacon (1995).
5. Goldsmith H, Buss A, Plomin R, Rothbart M, Thomas A, Chess S, et al. Roundtable: what is temperament? Four approaches. Child Dev. (1987) 58:505–29. doi: 10.2307/1130527
6. McCrae RR, Costa JR, Paul T, Ostendorf F, Angleitner A, Hrebíčkovà M, et al. Nature over nurture: temperament, personality and life span development. J Pers Soc Psychol. (2000) 78:173–86. doi: 10.1037/0022-3514.78.1.173
7. Rothbart MK, Ahadi SA, Evans DE. Temperament and personality: origins and outcomes. J Pers Soc Psychol. (2000) 78:122–35. doi: 10.1037/0022-3514.78.1.122
8. Buss AH. Personality: primate heritage and human distinctiveness. In: Aronoff J, Rabin A, editors. Emergence of Personality. New York, NY: Springer Publishing Co. (1987) p. 57–101.
9. Affleck G, Tennen H. Construing benefits from adversity: adaptional significance and dispositional underpinnings. J Pers. (1996) 64:899–922. doi: 10.1111/j.1467-6494.1996.tb00948.x
10. Lazarus RS. Coping theory and research: past, present, and future. Psychosom Med. (1993) 55:234–47. doi: 10.1097/00006842-199305000-00002
11. Wechsler B. Coping and coping strategies: a behavioural view. Appl Anim Behav Sci. (1995) 43:123–34. doi: 10.1016/0168-1591(95)00557-9
12. Henry JP, Stephens PM. Stress, Health, and the Social Environment. New York, NY: Springer (1977)
13. Moos RH. Coping Response Inventory Youth Form: Professional Manual. Odessa, FL: Psychological Assessment Resources, Inc. (1993).
14. Sih A, Bell A, Chadwick Johnson J. Behavioural syndromes: an ecological and evolutionary overview. Trends Ecol Evol. (2004) 19:372–8. doi: 10.1016/j.tree.2004.04.009
15. Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, et al. Coping styles in animals: current status in behaviour and stress-physiology. Neurosci Biobehav Rev. (1999) 23:925–35. doi: 10.1016/S0149-7634(99)00026-3
16. Korte SM, Koolhaas JM, Wingfield JC, McEwen BS. The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci Biobehav Rev. (2005) 29:3–38. doi: 10.1016/j.neubiorev.2004.08.009
17. Grignard L, Boissy A, Boivin X, Garel JP, Le Neindre P. The social environment influences the behavioural responses of beef cattle to handling. Appl Anim Behav Sci. (2000) 68:1–11. doi: 10.1016/S0168-1591(00)00085-X
18. Melotti L, Oostindjer M, Bolhuis JE, Held S, Mendl M. Coping personality type and environmental enrichment affect aggression at weaning in pigs. Appl Anim Behav Sci. (2011) 133:144–53. doi: 10.1016/j.applanim.2011.05.018
20. Boissy A, Bouix J, Orgeur P, Poindron P, Bibe B, Le Neindre P. Genetic analysis of emotional reactivity in sheep: effects of the genotypes of the lambs and of their dams. Genet Select Evol. (2005) 37:381–401. doi: 10.1186/1297-9686-37-5-381
21. Miranda-de la Lama GC, Sepúlveda WS, Montaldo HH, María GA, Galindo F. Social strategies associated with identity profiles in dairy goats. Appl Anim Behav Sci. (2011) 134:48–55. doi: 10.1016/j.applanim.2011.06.004
22. Holbrook CT, Wright CM, Pruitt JN. Individual differences in personality and behavioural plasticity facilitate division of labour in social spider colonies. Anim Behav. (2014) 97:177–83. doi: 10.1016/j.anbehav.2014.09.015
23. Kilgour RJ, Melville GJ, Greenwood PL. Individual differences in the reaction of beef cattle to situations involving social isolation, close proximity of humans, restraint and novelty. Appl Anim Behav Sci. (2006) 99:21–40. doi: 10.1016/j.applanim.2005.09.012
24. Boissy A, Bouissou MF. Assessment of individual differences in behavioural reactions of heifers exposed to various fear-eliciting situations. Appl Anim Behav Sci. (1995) 46:17–31. doi: 10.1016/0168-1591(95)00633-8
25. Reimert I, Rodenburg TB, Ursinus WW, Kemp B, Bolhuis JE. Responses to novel situations of female and castrated male pigs with divergent social breeding values and different backtest classifications in barren and straw-enriched housing. Appl Anim Behav Sci. (2014) 151:24–35. doi: 10.1016/j.applanim.2013.11.015
26. Réale D, Garant D, Humphries MM, Bergeron P, Careau V, Montiglio PO. Personality and the emergence of the pace-of-life syndrome concept at the population level. Philos Trans R Soc B. (2010) 365:4051–63. doi: 10.1098/rstb.2010.0208
28. Constantini D, Ferrari C, Pasquaretta C, Cavallone E, Carere C, Von Hardenberg A, et al. Interplay between plasma oxidative status, cortisol and coping styles in wild alpine marmots, Marmota marmota. J Exp Biol. (2012) 215:374–83. doi: 10.1242/jeb.062034
29. Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. Integrating animal temperament within ecology and evolution. Biol Rev Camb Philos Soc. (2007) 82:291–318. doi: 10.1111/j.1469-185X.2007.00010.x
30. Guenther A, Finkemeier MA, Trillmich F. The ontogeny of personality in the wild guinea pig. Anim Behav. (2014) 90:131–9. doi: 10.1016/j.anbehav.2014.01.032
31. Verbeek MEM, Boon A, Drent PJ. Exploration, aggressive behavior and dominance in pair-wise confrontations of juvenile male great tits. Behaviour (1996) 113:945–63. doi: 10.1163/156853996X00314
32. Gosling SD, John OP. Personality dimensions in nonhuman animals: a cross-species review. Curr Dir Psychol Sci. (1999) 8:69–75. doi: 10.1111/1467-8721.00017
33. Costa PT, McCrae RR. Revised NEO Personality Inventory (NEO PI-R) and NEO Five-Factor Inventory (NEO-FFI): Professional Manual. Oxford: Psychological Assessment Resources, Incorporated (1992).
34. Koski SE. Broader horizons for animal personality research. Front Ecol Evol. (2014) 2:70. doi: 10.3389/fevo.2014.00070
35. Altschul DM, King JE, Inoue-Murayama M, Ross SR, Weiss A. Longevity and personality in captive chimpanzees (Pan troglodytes). Peer J. Preprints (2016). 4:e1916w2. doi: 10.7287/peerj.preprints.1916v2
36. Eckardt W, Steklis HD, Steklis NG, Fletcher AW, Stoinski TA, Weiss A. Personality dimensions and their behavioral correlates in wild virunga mountain gorillas (Gorilla beringei beringei). J Comp Psychol. (2015) 129:26–41. doi: 10.1037/a0038370
37. David M, Dall SRX. Unravelling the philosophies underlying ‘animal personality’ studies: a brief re-appraisal of the field. Ethology (2016) 122:1–9. doi: 10.1111/eth.12445
38. Dall SRX, Houston AI, McNamara JM. The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol Lett. (2004) 7:734–9. doi: 10.1111/j.1461-0248.2004.00618.x
39. Dingemanse NJ, Both C, Van Noordwijk AJ, Rutten AL, Drent PJ. Natal dispersal and personalities in great tits (Parus major). Proc R Soc Lond Biol. (2003) 270:741–7. doi: 10.1098/rspb.2002.2300
40. Dingemanse NJ, Réale D. Natural selection and animal personality. Behaviour (2005) 142:1159–84. doi: 10.1163/156853905774539445
41. Biro PA, Stamps JA. Are animal personality traits linked to life-history productivity? Trends Ecol Evol. (2008) 23:361–8. doi: 10.1016/j.tree.2008.04.003
42. Bouwhuis S, Quinn JL, Sheldon BC, Verhulst S. Personality and basal metabolic rate in a wild bird population. Oikos (2013) 123:56–62. doi: 10.1111/j.1600-0706.2013.00654.x
43. West-Eberhard MJ. Phenotypic accommodation: adaptive innovation due to developmental plasticity. J Exp Zool. (2005) 304B:610–8. doi: 10.1002/jez.b.21071
44. Piersma T, Drent J. Phenotipic flexibility and the evolution of organismal design. Trends Ecol Evol. (2003) 18:228–33. doi: 10.1016/S0169-5347(03)00036-3
45. Groothuis TGG, Maestripieri D. Parental influences on offspring personality. In: Carere, C, Maestripieri D, editors. Animal Personalities, Chicago. IL: University of Chicago Press (2013). p. 317–52.
46. Biro PA, Beckmann C, Stamps JA. Small within-day increases in temperature affects boldness and alters personality in coral reef fish. Proc R Soc Lond Biol. (2009) 277:71–7. doi: 10.1098/rspb.2009.1346
47. Finkemeier MA, Trillmich F, Guenther A. Match-mismatch experiments using photoperiod expose developmental plasticity of personality traits. Ethology (2016) 122:80–93. doi: 10.1111/eth.12448
48. Guenther A, Trillmich F. Photoperiod influences the behavioral and physiological phenotype during ontogeny. Behav Ecol. (2013) 24:402–11. doi: 10.1093/beheco/ars177
49. Haage M, Bergvall UA, Maran T, Kiik K, Angerbjörn A. Situation and context impacts the expression of personality: the influence of breeding season and test context. Behav Process. (2013) 100:103–9. doi: 10.1016/j.beproc.2013.08.009
50. Sutherland MA, Rogers AR, Verkerk GA. The effect of temperament and responsiveness towards humans on the behavior, physiology and milk production of multi-parous dairy cows in a familiar and novel milking environment. Physiol Behav. (2012) 107:329–37. doi: 10.1016/j.physbeh.2012.07.013
51. Sant'Anna AC, Paranhos da Costa MJR. Validity and feasibility of qualitative behavior assessment for the evaluation of Nellor cattle temperament. Livest Sci. (2013) 157:254–62. doi: 10.1016/j.livsci.2013.08.004
52. Webb LE, van Reenen CG, Jensen MB, Schmitt O, Bokkers EAM. Does temperament affect learning in calves? Appl Anim Behav Sci. (2015) 165:33–9. doi: 10.1016/j.applanim.2015.01.013
53. Burdick NC, Carroll JA, Hulbert LE, Dailey JW, Willard ST, Vann RC, et al. Relationships between temperament and transportation with rectal temperature and serum concentrations of cortisol and epinephrine in bulls. Livest Sci. (2010) 129:166–72. doi: 10.1016/j.livsci.2010.01.020
54. Frondelius L, Järvenranta K, Koponen T, Mononen J. The effects of body posture and temperament on heart rate variability in dairy cows. Physiol Behav. (2015) 139:437–41. doi: 10.1016/j.physbeh.2014.12.002
55. Gibbons J, Lawrence A, Haskell M. (2009). Responsiveness of dairy cows to human approach and novel stimuli. Appl Anim Behav Sci. (2009) 116:163–73. doi: 10.1016/j.applanim.2008.08.009
56. Graunke KL, Nürnberg G, Repsilber D, Puppe B, Langbein J. Describing temperament in an ungulate: a multidimensional approach. PLoS ONE (2013) 8:1–12. doi: 10.1371/journal.pone.0074579
57. Boles JA, Kohlbeck KS, Meyers MC, Perz KA, Davis KC, Thomson JM. The use of blood lactate concentration as an indicator of temperament and its impact on growth rate and tenderness of steaks from Simmental × Angus steers. Meat Sci. (2015) 103:68–74. doi: 10.1016/j.meatsci.2015.01.003
58. Cafe LM, Robinson DL, Ferguson DM, Geesink GH, Greenwood PL. Temperament and hypothalamic-pituitary-adrenal axis function are related and combine to affect growth, efficiency, carcass, and meat quality traits in Brahman steers. Domest Anim Endocrinol. (2011) 40:230–40. doi: 10.1016/j.domaniend.2011.01.005
59. Phocas F, Boivin X, Sapa J, Trillat G, Boissy A, Le Neindre P. Genetic correlations between temperament and breeding traits in Limousin heifers. Anim Sci. (2006) 82:805–11. doi: 10.1017/ASC200696
60. Brand B, Hadlich F, Brandt B, Schauer N, Graunke KL, Langbein J, et al. (2015). Temperament type specific metabolite profiles of the prefrontal cortex and serum in cattle. PLoS ONE (2015) 10:e0125044. doi: 10.1371/journal.pone.0125044
61. del Campo M, Brito G, Soares de Lima J, Hernández P, Montossi F. Finishing diet, temperament and lairage time effects on carcass and meat quality traits in steers. Meat Sci. (2010) 86:908–14. doi: 10.1016/j.meatsci.2010.07.014
62. Lyons DM, Price EO, Moberg GP. Individual differences in temperament of domestic dairy goats: constancy and change. Anim Behav. (1988) 36:1323–33. doi: 10.1016/S0003-3472(88)80201-X
63. Lyons DM. Individual differences in temperament of dairy goats and the inhibition of milk ejection. Appl Anim Behav Sci. (1989) 22:269–82. doi: 10.1016/0168-1591(89)90022-1
64. Nawroth C, Prentice PM, McElligott AG. Individual personality differences in goats predict their performance in visual learning and non-associative cognitive tasks. Behav Process. (2017) 134:43–53. doi: 10.1016/j.beproc.2016.08.001
65. Beausoleil NJ, Blache D, Stafford KJ, Mellor DJ, Noble ADL. Selection for temperament in sheep: domain-general and context-specific traits. Appl Anim Behav Sci. (2012) 139:74–85. doi: 10.1016/j.applanim.2012.02.020
66. Hawken PAR, Fiol C, Blache D. Genetic differences in temperament determine whether lavender oil alleviates or exacerbates anxiety in sheep. Physiol Behav (2012a) 105:1117–23. doi: 10.1016/j.physbeh.2011.12.005
67. Hazard D, Moreno C, Foulquie D, Delval E, Francois D, Bouix J, et al. Identification of QTLs for behavioral reactivity to social separation and humans in sheep using the OvineSNP50 BeadChip. BMC Genomics (2014) 15:778. doi: 10.1186/1471-2164-15-778
68. McBride SD, Wolf B. Using multivariate statistical analysis to measure ovine temperament; stability of factor construction over time and between groups of animals. Appl Anim Behav Sci. (2007) 103:45–58. doi: 10.1016/j.applanim.2006.04.030
69. Sibbald AM, Erhard HW, McLeod JE, Hooper RJ. Individual personality and the spatial distribution of groups of grazing animals: an example with sheep. Behav Process. (2009) 82:319–26. doi: 10.1016/j.beproc.2009.07.011
70. Seaman SC, Davidson HPB, Waran NK. How reliable is temperament assessment in the domestic horse (Equus caballus)? Appl Anim Behav Sci. (2002) 78:175–91. doi: 10.1016/S0168-1591(02)00095-3
71. Lansade L, Simon F. Horses' learning performances are under the influence of several temperamental dimensions. Appl Anim Behav Sci. (2010) 125:30–37. doi: 10.1016/j.applanim.2010.02.010
72. von Borstel UK, Pasing S, Gauly M. Towards a more objective assessment of equine personality using behavioural and physiological observations from performance test training. Appl Anim Behav Sci. (2011) 135:277–85. doi: 10.1016/j.applanim.2011.10.007
73. Lansade L, Bouissou MF, Erhard HW. Fearfulness in horses: a temperament trait stable across time and situations. Appl Anim Behav Sci. (2008) 115:182–200. doi: 10.1016/j.applanim.2008.06.011
74. Graf P, von Borstel UK, Gauly M. Practical considerations regarding the implementation of a temperament test into horse performance tests: results of a large-scale test run. J Vet Behav. (2014) 9:329–40. doi: 10.1016/j.jveb.2014.08.004
75. Hausberger M, Muller C, Lunel C. Does work affect personality? A study in horses. PLoS ONE (2011) 6:e14659. doi: 10.1371/journal.pone.0014659
76. Ijichi C, Collins LM, Elwood RM. Pain expression is linked to personality in horses. Appl Anim Behav Sci. (2014) 152:38–42. doi: 10.1016/j.applanim.2013.12.007
77. Górecka-Bruzda A, Jastrzebska E, Sosnowska Z, Jaworski Z, Jezierski T, Chruszczewski MH. Reactivity to humans and fearfulness tests: field validation in Polish Cold Blood Horses. Appl Anim Behav Sci. (2011) 133:207–15. doi: 10.1016/j.applanim.2011.05.011
78. Lloyd AS, Martin JE, Bornett-Gauci HLI, Wilkinson RG. Horse personality: variation between breeds. Appl Anim Behav Sci. (2008) 112:369–83. doi: 10.1016/j.applanim.2007.08.010
79. Visser EK, van Reenen CG, Hopster H, Schilder MBH, Knaap JH, Barneveld A, et al. Quantifying aspects of young horses' temperament: consistency of behavioural variables. Appl Anim Behav Sci. (2001) 74:241–58. doi: 10.1016/S0168-1591(01)00177-0
80. Visser EK, van Reenen CG, Engel B, Schilder MBH, Barneveld A, Blokhuis HJ. The association between performance in show-jumping and personality traits earlier in life. Appl Anim Behav Sci. (2003) 82:279–95. doi: 10.1016/S0168-1591(03)00083-2
81. Olsen HF, Klemetsdal G. Temperament of the Norwegian horse breeds-a questionnaire based study. Appl Anim Behav Sci. (2017) 193:60–6. doi: 10.1016/j.applanim.2017.03.015
82. Lansade L, Bouissou MF. Reactivity to humans: a temperament trait of horses which is stable across time and situations. Appl Anim Behav Sci. (2008) 114:492–508. doi: 10.1016/j.applanim.2008.04.012
83. Lansade L, Bouissou MF, Boivin X. Temperament in preweanling horses: development of reactions to humans and novelty, and startle responses. Dev Psychobiol. (2007) 49:501–13. doi: 10.1002/dev.20233
84. Krüger K, Farmer K, Heinze J. The effects of age, rank and neophobia on social learning in horses. Anim Cogn. (2014) 17:645–55. doi: 10.1007/s10071-013-0696-x
85. Lansade L, Philippon P, Herve L, Vidament M. Development of personality tests to use in the field, stable over time and across situations, and linked to horses' show jumping performance. Appl Anim Behav Sci. (2016) 176:43–51. doi: 10.1016/j.applanim.2016.01.005
86. Duberstein KJ, Gilkeson JA. Determination of sex differences in personality and trainability of yearling horses utilizing a handler questionnaire. Appl Anim Behav Sci. (2010) 128:57–63. doi: 10.1016/j.applanim.2010.09.012
87. Roberts K, Hemmings AJ, Moore-Colyer M, Parker MO, McBride SD. Neural modulators of temperament: a multivariate approach to personality trait identification in the horse. Physiol Behav. (2016) 167:125–31. doi: 10.1016/j.physbeh.2016.08.029
88. Ruis MAW, te Brake JHA, Engel B, Buist WG, Blokhuis HJ, Koolhaas JM. Adaptation to social isolation acute and long-term stress responses of growing gilts with different coping characteristics. Physiol Behav. (2001) 73:541–51. doi: 10.1016/S0031-9384(01)00548-0
89. Oster M, Scheel M, Muráni E, Ponsuksili S, Zebunke M, Puppe B, et al. The fight-or-flight response is associated with PBMC expression profiles related to immune defence and recovery in swine. PLoS ONE (2015) 10:e0120153. doi: 10.1371/journal.pone.0120153
90. Bolhuis JE, Parmentier HK, Schouten WGP, Schrama JW, Wiegant VM. Effects of housing and individual coping characteristics on immune responses of pigs. Physiol Behav. (2003) 79:289–96. doi: 10.1016/S0031-9384(03)00090-8
91. Zebunke M, Repsilber D, Nürnberg G, Wittenburg D, Puppe B. The backtest in pigs revisited–an analysis of intra-situational behaviour. Appl Anim Behav Sci. (2015) 169:17–25. doi: 10.1016/j.applanim.2015.05.002
92. Bolhuis JE, Schouten WGP, de Leeuw JA, Schrama JW, Wiegant VM. Individual coping characteristics, rearing conditions and behavioural flexibility in pigs. Behav Brain Res. (2004) 152:351–60. doi: 10.1016/j.bbr.2003.10.024
93. Reimert I, Bolhuis JE, Kemp B, Rodenburg TB. Social support in pigs with different coping styles. Physiol Behav. (2014) 129:221–9. doi: 10.1016/j.physbeh.2014.02.059
94. Spake JR, Gray KA, Cassady JP. Relationship between backtest and coping styles in pigs. Appl Anim Behav Sci. (2012) 140:146–53. doi: 10.1016/j.applanim.2012.06.007
95. Zebunke M, Nürnberg G, Melzer N, Puppe B. The backtest in pigs revisited – inter-situational behaviour and animal classification. Appl Anim Behav Sci. (2017) 194:7–13. doi: 10.1016/j.applanim.2017.05.011
96. Janczak AM, Pedersen LJ, Bakken M. Aggression, fearfulness and coping styles in female pigs. Appl Anim Behav Sci. (2003) 81:13–28. doi: 10.1016/S0168-1591(02)00252-6
97. Kooij EvErp-vd, Kuijpers AH, Schrama JW, van Eerdenburg FJCM, Schouten WGP, Tielen MJM. Can we predict behaviour in pigs? Searching for consistency in behaviour over time and across situations. Appl Anim Behav Sci. (2002) 75:293–305. doi: 10.1016/S0168-1591(01)00203-9
98. Brown JA, Dewey C, Delange CFM, Mandell IB, Purslow PP, Robinson JA, et al. Reliability of temperament tests on finishing pigs in group-housing and comparison to social tests. Appl Anim Behav Sci. (2009) 118:28–35. doi: 10.1016/j.applanim.2009.02.005
99. Ruis MAW, te Brake JHA, van de Burgwal JA, de Jong IC, Blokhuis HJ, Koolhaas JM. Personalities in female domesticated pigs: behavioural and physiological indications. Appl Anim Behav Sci. (2000) 66:31–47. doi: 10.1016/S0168-1591(99)00070-2
100. Randle HD. Facial hair whorl position and temperament in cattle. Appl Anim Behav Sci. (1998) 56:139–47. doi: 10.1016/S0168-1591(97)00086-5
101. Beausoleil NJ, Blach D, Stafford KJ, Mellor DJ, Noble ADL. Exploring the basis of divergent selection for “temperament” in domestic sheep. Appl Anim Behav Sci. (2008) 109:261–74. doi: 10.1016/j.applanim.2007.03.013
102. Jones AC, Gosling SD. Temperament and personality in dogs (Canis familiaris): a review and evaluation of past research. Appl Anim Behav Sci. (2005) 95:1–53. doi: 10.1016/j.applanim.2005.04.008
103. Benus RF, Bohus B, Koolhaas JM, van Oortmerssen GA. Behavioral strategies of aggressive and nonaggressive male-mice in active shock avoidance. Behav Processes (1989) 20:1–12. doi: 10.1016/0376-6357(89)90008-9
104. Boersma GJ, Scheurink AJ, Wielinga PY, Steimer TJ, Benthem L. The passive coping Roman Low Avoidance rat, a non-obese rat model for insulin resistance. Physiol Behav. (2009) 97:353–8. doi: 10.1016/j.physbeh.2009.03.005
105. Posner J, Russell JA, Peterson BS. The circumplex model of affect: an integrative approach to affective neuroscience, cognitive development, and psychopathology. Dev Psychopathol. (2005) 17:715–34. doi: 10.1017/S0954579405050340
106. Meager JJ, Fernö A, Skjæraasen JE, Järvi T, Rodewald P. Multidimensionality of behavioural phenotypes in Atlantic cod, Gadus morhua. Physiol Behav. (2012) 106:462–70. doi: 10.1016/j.physbeh.2012.03.010
107. Weinstein TAR, Capitanio JP. Individual differences in infant temperament predict social relationships of yearling rhesus monkeys, Macaca mulatta. Anim Behav. (2008) 76:455–65. doi: 10.1016/j.anbehav.2008.01.024
108. Foyer P, Wilsson E, Jensen P. Levels of maternal care in dogs affect adult offspring temperament. Sci Rep. (2016) 6:19253. doi: 10.1038/srep19253
109. Iki T, Ahrens F, Pasche KH, Bartels A, Erhard MH. Relationships between scores of the feline temperament profile and behavioural and adrenocortical responses to a mild stressor in cats. Appl Anim Behav Sci. (2011) 132:71–80. doi: 10.1016/j.applanim.2011.03.008
110. Le Neindre P, Trillat G, Sapa J, Ménissier F, Bonnet JN, Chupin JM. Individual differences in docility in limousin cattle. J Anim Sci. (1995) 73:2249–53. doi: 10.2527/1995.7382249x
111. Gibbons J. The Effect of Selecting for “Robustness” on Temperament in Dairy Cows. Ph.D., thesis, The University of Edinburgh (2009).
112. Coleman K, Wilson DS. Shyness and boldness in pumpkinseed sunfish: individual differences are context-specific. Anim Behav. (1998) 56:927–36. doi: 10.1006/anbe.1998.0852
113. Zidar J, Balogh A, Favati A, Jensen P, Leimar O, Løvlie H. A comparison of animal personality and coping styles in the red junglefowl. Anim Behav. (2017) 130:209–20. doi: 10.1016/j.anbehav.2017.06.024
114. Carere C, Drent PJ, Privitera L, Koolhaas JM, Groothuis TGG. Personalities in great tits, Parus mayor: stability and consistency. Anim Behav. (2005) 70:795–805. doi: 10.1016/j.anbehav.2005.01.003
115. Carere C, Caramaschi D, Fawcett T. Covariation between personalities and individual differences in coping with stress: converging evidence and hypotheses. Curr Zool. (2010) 56:728–40.
116. Coppens CM, De Boer SF, Koolhaas JM. Coping styles and behavioural flexibility: towards underlying mechanisms. Philos Trans R Soc B. (2010) 365:4021–8. doi: 10.1098/rstb.2010.0217
117. Koolhaas JM, De Boer SF, Coppens CM, Buwalda B. Neuroendocrinology of coping styles: towards understanding the biology of individual variation. Front Neuroendocrinol. (2010) 31:307–21. doi: 10.1016/j.yfrne.2010.04.001
118. Uher J. Individual behavioural phenotypes: an integrative meta-theoretical framework. Why “Behavioral Syndromes” are not analogs of “personality”. Dev Psychobiol. (2011) 53:521–48. doi: 10.1002/dev.20544
119. Koolhaas JM. Coping style and immunity in animals: making sense of individual variation. Brain Behav Immun. (2008) 22:662–7. doi: 10.1016/j.bbi.2007.11.006
120. Careau V, Thomas D, Humphries M, Réale D. Energy metabolism and animal personality. Oikos (2008) 117:641–53. doi: 10.1111/j.0030-1299.2008.16513.x
121. Trompf L, Brown C. Personality affects learning and trade-offs between private and social information in guppies, Poecilia reticulate. Anim Behav. (2014) 88:99–106. doi: 10.1016/j.anbehav.2013.11.022
122. Brust V, Wuerz Y, Krüger O. Behavioural flexibility and personality in zebra finches. Ethology (2013) 119:559–69. doi: 10.1111/eth.12095
123. Silva PIM, Martins CIM, Engrola S, Marino G, Øverli Ø, Conceição LEC. Individual differences in cortisol levels and behaviour of Senegalese sole (Solea senegalensis) juveniles: evidence for coping styles. Appl Anim Behav Sci. (2010) 124:75–81. doi: 10.1016/j.applanim.2010.01.008
124. Colléter M, Brown C. Personality traits predict hierarchy rank in male rainbowfish social groups. Anim Behav. (2011) 81:1231–7. doi: 10.1016/j.anbehav.2011.03.011
125. Krause A, Puppe B, Langbein J. Coping style modifies general and affective autonomic reactions of domestic pigs in different behavioral contexts. Front Behav Neurosci. (2017) 11:103. doi: 10.3389/fnbeh.2017.00103
126. Ponsuksili S, Zebunke M, Murani E, Trakooljul N, Krieter J, Puppe B, et al. Integrated genome-wide association and hypothalamus eQTL studies indicate a link between the circadian rhythm-related gene PER1 and coping behaviour. Sci Rep. (2015) 5:16264. doi: 10.1038/srep16264
127. Murphy E, Nordquist RE, van der Staay FJ. A review of behavioural methods to study emotion and mood in pigs, Sus scrofa. Appl Anim Behav Sci. (2014) 159:9–28. doi: 10.1016/j.applanim.2014.08.002
128. Koolhaas JM, van Reenen CG. Animal Behavior and Well-Being Symposium: interaction between coping style/personality, stress, and welfare: relevance for domestic farm animals. J Anim Sci. (2016) 94:2284–96. doi: 10.2527/jas.2015-0125
129. Mendl M, Burman OHP, Paul ES. An integrative and functional framework for the study of animal emotion and mood. Proc R Soc Lond Biol. (2010) 277:2895–904. doi: 10.1098/rspb.2010.0303
130. Russell J. A circumplex model of affect. J Pers Soc Psychol. (1980) 39:1161–78. doi: 10.1037/h0077714
131. Carter AJ, Feeney WE, Marshall HH, Cowlishaw G, Heinsohn R. Animal personality: what are behavioural ecologists measuring? Biol Rev Camb Philos Soc. (2013) 88:465–75. doi: 10.1111/brv.12007
132. Forkman B, Boissy A, Meunier-Salaün MC, Canali E, Jones RB. A critical review of fear tests used on cattle, pigs, sheep, poultry and horses. Physiol Behav. (2007) 92:340–74. doi: 10.1016/j.physbeh.2007.03.016
133. Handley SL, Mithani S. Effects of alpha-adrenoceptor agonists and antagonists in a maze-exploration model of ‘fear’-motivated behaviour. Naunyn Schmiedebergs Arch Pharmacol. (1984) 327:1–5.
134. Montgomery KC. The relation between fear induced by novel stimulation and exploratory drive. J Comp Physiol Psychol. (1955) 48:254–60. doi: 10.1037/h0043788
135. Perals D, Griffin AS, Bartomeus I, Sol D. Revisiting the open-field test: what does it really tell us about animal personality? Anim Behav. (2017) 123:69–79. doi: 10.1016/j.anbehav.2016.10.006
136. Hessing MJC, Hagelso AM, Vanbeek JAM., Wiepkema PR, Schouten WGP, Krukow R. Individual behavioral characteristics in pigs. Appl Anim Behav Sci. (1993) 37:285–95. doi: 10.1016/0168-1591(93)90118-9
137. Guenther A, Trillmich F. Within-litter differences in personality and physiology relate to size differences among siblings in cavies. Physiol Behav. (2015) 145:22–8. doi: 10.1016/j.physbeh.2015.03.026
138. Diener E, Suh EM, Lucas RE, Smith HL. Subjective well-being: three decades of progress. Psychol Bull. (1999) 125:276–302. doi: 10.1037/0033-2909.125.2.276
139. Weiss A, Bates TC, Luciano M. Happiness is a personal(ity) thing: the genetics of personality and well-being in a representative sample. Psychol Sci. (2008) 19:205–10. doi: 10.1111/j.1467-9280.2008.02068.x
140. DeNevre KM, Cooper H. Origins and functions of positive and negative affect: a control-process view. Psychol Rev. (1998) 124:197–229.
141. Tetley CL, O'Hara SJ. Ratings of animal personality as a tool for improving the breeding management and welfare of zoo animals. Animal Welf. (2012) 21:463–76. doi: 10.7120/09627286.21.4.463
144. Fureix C, Menguy H, Hausberger M. Partners with bad temper: reject or cure? A study of chronic pain and aggression in horses. PLoS ONE (2008) 5:e12434. doi: 10.1371/journal.pone.0012434
145. Burrow HM, Dillon RD. Relationship between temperament and growth in a feedlot and commercial carcass traits in Bos indicus crossbreeds. Aust J Exp Agric. (1997) 37:407–11. doi: 10.1071/EA96148
146. Voisinet BD, Grandin T, Tatum JD, O'Connor SF, Struthers JJ. Feedlot cattle with calm temperaments have higher average daily gains than cattle with excitable temperaments. J Anim Sci. (1997) 75:892–6.
147. Rauw WM., Johnson AK, Gomez-Raya L, Dekkers JCM. A hypothesis and review of the relationship between selection for improved production efficiency, coping behavior and domestication. Front Genet. (2017) 8:134. doi: 10.3389/fgene.2017.00134
148. Drent PJ, van Oers K, Noordwijk AJ. Realized heritability of personalities in the great tit (Parus major). Proc Royal Soc B. (2003) 270:45–51. doi: 10.1098/rspb.2002.2168
149. van Oortmerssen GA, Bakker TC. Artificial selection for short and long attack latencies in wild Mus musculus domesticus. Behav Genet. (1981) 11:115–26. doi: 10.1007/BF01065622
150. van Oers K, Drent PJ, Jong GD, Noordwijk AJ. Additive and nonadditive genetic variation in avian personality traits. Heredity (2004) 93:496–503. doi: 10.1038/sj.hdy.6800530
151. Henderson CR. Applications of Linear Models in Animal Breeding. Guelph, ON: University of Guelph (1984).
152. Kruuk LE. Estimating genetic parameters in natural populations using the ‘animal model’. Proc R Soc B. (2004) 359:873–90. doi: 10.1098/rstb.2003.1437
153. Laine VN, van Oers K. The quantitative and molecular genetics of individual differences in animal personality. In: Vonk J, Weiss A, Kuczaj S, editors. Personality in Nonhuman Animals. Cham: Springer (2017). p. 55–72.
154. Careau V, Thomas D, Pelletier F, Turki L, Landry F, Garant D, et al. Genetic correlation between resting metabolic rate and exploratory behaviour in deer mice (Peromyscus maniculatus). J Evol Biol. (2011) 24:2153–63. doi: 10.1111/j.1420-9101.2011.02344.x
155. Haskell MJ, Simm G, Turner SP. Genetic selection for temperament traits in dairy and beef cattle. Front Genet. (2014) 5:368. doi: 10.3389/fgene.2014.00368
156. Stirling DG, Réale D, Roff DA. Selection, structure and the heritability of behaviour. J Evol Biol. (2002) 15:277–89. doi: 10.1046/j.1420-9101.2002.00389.x
157. D'Eath RB, Rohe R, Turner SP, Ison SH, Farish M, Jack MC, et al. Genetics of animal temperament: aggressive behaviour at mixing is genetically associated with the response to handling in pigs. Animal (2009) 3:1544–54. doi: 10.1017/S1751731109990528
158. Hellbrügge B, Tölle KH, Bennewitz J, Henze C, Presuhn U, Krieter J. Genetic aspects regarding piglet losses and the maternal behaviour of sows. Part 2. Genetic relationship between maternal behaviour on sows and piglet mortality. Animal (2008) 2:1281–8. doi: 10.1017/S1751731108002516
159. Stukenborg A, Traulsen I, Stamer E, Puppe B, Presuhn U, Krieter J. Heritabilities of agonistic behavioural traits in pigs and their relationships within and between different age groups. Livest Sci. (2012) 149:25–32. doi: 10.1016/j.livsci.2012.06.020
160. Hoppe S, Brandt HR, König S, Erhardt G, Gauly M. Temperament traits of beef calves measured under field conditions and their relationships to performance. J Anim Sci. (2010) 88:1982–9. doi: 10.2527/jas.2008-1557
161. Hedlund L, Løvlie H. Personality and production: nervous cows produce less milk. J Dairy Sci. (2015) 98:5819–28. doi: 10.3168/jds.2014-8667
162. Hawken PAR, Williman M, Milton J, Kelly R, Nowak R, Blache D. Nutritional supplementation during the last week of gestation increased the volume and reduced the viscosity of colostrum produced by twin bearing ewes selected for nervous temperament. Small Rumin Res. (2012b) 105:308–14. doi: 10.1016/j.smallrumres.2012.01.011
163. Boersma GJ, Benthem L, van Beek AP, van Dijk G, Scheurink AJW. Personality, a key factor in personalized medicine? Eur J Pharmacol. (2011) 667:23–25. doi: 10.1016/j.ejphar.2011.05.079
Keywords: personality, temperament, coping style, welfare, farm animals
Citation: Finkemeier M-A, Langbein J and Puppe B (2018) Personality Research in Mammalian Farm Animals: Concepts, Measures, and Relationship to Welfare. Front. Vet. Sci. 5:131. doi: 10.3389/fvets.2018.00131
Received: 06 March 2018; Accepted: 29 May 2018;
Published: 28 June 2018.
Edited by:
Edna Hillmann, Humboldt-Universität zu Berlin, GermanyReviewed by:
T. Bas Rodenburg, Wageningen University and Research, NetherlandsCopyright © 2018 Finkemeier, Langbein and Puppe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jan Langbein, bGFuZ2JlaW5AZmJuLWR1bW1lcnN0b3JmLmRl
Birger Puppe, cHVwcGVAZmJuLWR1bW1lcnN0b3JmLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.