
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Urol., 15 September 2023
Sec. Urologic Oncology
Volume 3 - 2023 | https://doi.org/10.3389/fruro.2023.1206398
Muscle-invasive bladder tumors pose a grave mortality risk due to their propensity for distant metastases. The therapeutic spectrum for such tumors encompasses surgery, chemotherapy, and radiation, tailored to the cancer’s severity. In the context of high-risk Bacillus Calmette-Guérin (BCG)-unresponsive non-muscle invasive bladder cancer (NMIBC), a novel treatment has emerged as a beacon of hope. Nadofaragene firadenovec, a pioneering gene therapy, has gained worldwide approval for combating this condition, marking a watershed moment in bladder cancer therapy. Nadofaragene firadenovec is ingeniously designed to address high-risk BCG-unresponsive NMIBC, particularly carcinoma in situ (CIS) with or without papillary tumors, in adult patients. Rooted in a vector DNA, this therapy encodes interferon (IFN)-2b, which imparts urothelial cells with the ability to generate IFN-2b. The resulting cascade of events triggers a multifaceted assault on cancer, characterized by its immunostimulatory, antiangiogenic, and apoptotic effects. The therapeutic efficacy of nadofaragene firadenovec rests on its capacity to exploit the transformed urothelial cells to deliver these targeted anticancer activities. The evolutionary trajectory of nadofaragene firadenovec culminated in its monumental approval in December 2022 by the United States, signifying a pivotal juncture in the field. Notably, a segment of patients, approximately 30%, prove refractory to BCG treatment. For these individuals, alternative therapeutic avenues are imperative. Presently, the landscape for patients with non-muscle invasive bladder cancer lacks a definitive, enduring solution. Against this backdrop, the introduction of nadofaragene firadenovec heralds a momentous stride toward the global availability of an authorized therapeutic intervention.
Muscle-invasive bladder tumors carry a high risk of mortality due to distant metastases (1). Treatment can range from surgery, and chemotherapy to radiation therapy, depending on the severity of the cancer. In some cases, a combination of treatments may be recommended to most effectively treat cancer. With an estimated 1.6 million people affected worldwide in 2018, bladder cancer was a major global health concern. This figure included 549,000 new cases and 200,000 deaths. Rates of bladder cancer were highest in Southern and Western Europe at 15 cases per 100,000 people, followed by North America at 15, 13, and 12 cases per 100,000 people. Northern Africa and Western Asia experienced the highest rates of bladder cancer deaths, followed by Southern Europe (2). About 90% of bladder cancer diagnoses occur in people aged 55 and over, and the disease is four times more common in males than females (3). Approximately 550,000 new cases of bladder cancer are diagnosed each year, making it one of the world’s top ten most common cancer types (4).
About 20-40% of bladder cancer cases are non-muscle invasive bladder cancer (1) in which the malignant cells typically form flat, papillary lesions on the bladder wall. These papillary tumors can grow in size or number and have the potential to spread to other organs in the body which can lead to invasive bladder cancer, a more serious form of the disease. The most common treatment for NMIBC is Bacillus Calmette-Guérin (BCG) immunotherapy, which can provide long-term remission in up to 70% of patients. However, a subset of patients does not respond to BCG treatment, considering it to be more aggressive and difficult to treat than other types of bladder cancer. While the cause of BCG-unresponsive NMIBC is not known, it is thought to be due to a combination of genetic and environmental factors.
The immune system recognizes and eliminates foreign invaders and cancer cells. In NMIBC, the tumor cells can evade the immune system, allowing them to grow and proliferate. BCG immunotherapy is used to stimulate the immune system to recognize and destroy bladder cancer cells. The BCG vaccine is administered directly into the bladder, where it triggers an inflammatory response that stimulates the immune system to attack tumor cells. This therapy is effective in up to 70% of NMIBC cases. However, the remaining 30% of patients are unresponsive to BCG treatment. These patients may require alternative treatments, such as chemotherapy and radiation therapy, to manage their disease.
In cases with BCG unresponsive non-muscle invasive bladder cancer (NMIBC), the Bacillus Calmette-Guérin (BCG) treatment is unable to successfully treat the malignancy. The features of the tumor, such as its bigger size, higher grade, multifocality, and presence of in situ carcinoma, frequently have a role in its resistance to BCG therapy. The immune system’s reaction, which includes TLRs and CD4+ and CD8+ T cells, is also important in the ineffectiveness of BCG. Immune tolerance strategies can prevent immune recognition and destruction, such as by expressing immune checkpoint molecules like PD-L1. BCG resistance can also result from genetic alterations, such as those in the mitogen-activated protein kinase (MAPK) pathway. BCG response may also be impacted by the tumor microenvironment, which is made up of several cell types, extracellular elements, and signaling chemicals. In this microenvironment, regulatory T cells and myeloid-derived suppressor cells provide an immunosuppressive environment that impairs the immune response and promotes tumor progression (5). BCG resistance is also influenced by host-related variables such as age, general health state, and immunological capability. Patients who have immune systems that are impaired by underlying illnesses or immunosuppressive drugs are more likely to show decreased response to BCG treatment. Treatment results may also be affected by variations in the BCG strain utilized or the delivery method (6). To improve treatment outcomes for patients who do not respond to standard BCG therapy, new therapeutic approaches, such as immune checkpoint inhibitors, targeted therapies, or combinations, must take into account the complex pathophysiological mechanisms underlying BCG unresponsive NMIBC.
1. Radical Cystectomy: For patients with high-risk, BCG-unresponsive, and for aggressive or muscle-invasive bladder cancer, radical cystectomy is the gold standard therapy, providing efficient care and long-term disease management (7). It lessens the chance of development and recurrence by removing the malignant bladder and surrounding tissues. The operation also aids in determining the stage, relieving symptoms, and removing lymph nodes, preventing cancer from spreading to other body areas. The bladder and lymph nodes must be removed in order to make treatment choices after the operation (8). The major operation known as a radical cystectomy entails medical risks, a loss of bladder function, emotional effects, and a protracted recovery period. It may result in urine leakage, urethral narrowing, urinary tract infections, and problems with urinary diversion. To adjust to the changed urinary system, patients may need to change their regular habits, hydration consumption, and food. Urinary leaks, strictures, urinary tract infections, and problems with urine diversion are possible postoperative consequences. In order to adjust to the changed urinary system, lifestyle adjustments could also be required, which would make some activities more difficult. Due to changes in anatomy and body image, radical cystectomies can influence sexual performance. For males, the removal of the bladder may necessitate the removal of the prostate gland, which might have an impact on erectile function. Long-term observation is necessary for further complications and recurrence (9) (Figure 1).
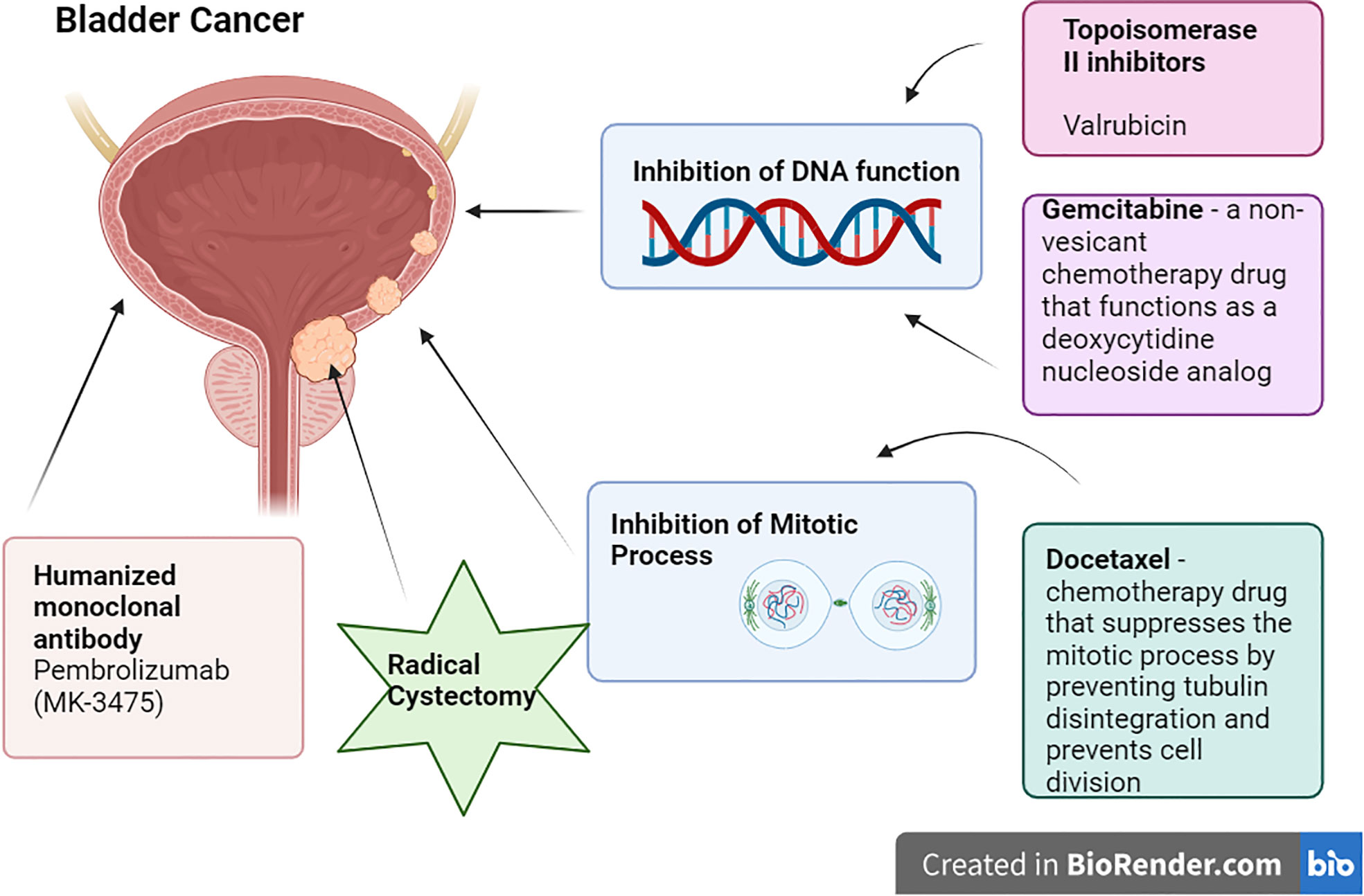
Figure 1 Current Treatment Options for NMIBC (created from Biorender.com).
2. Valrubicin: Valrubicin (second-generation anthracycline) is a cell cytostatic medication that produces its antitumorigenic action by contacting the bladder wall and then being absorbed by cancer cells, where it exerts its cytostatic effect, reducing the growth of tumoral cells (10). According to the FDA, patients for whom urgent cystectomy would be associated with unacceptable morbidity or death are expressly mentioned as candidates. The acceptance of valrubicin in modern treatment has been very slow due to its relatively restricted indication and subpar overall long-term effectiveness as salvage therapy (7).
3. Gemcitabine/Docetaxel: Gemcitabine is a non-vesicant chemotherapy drug that functions as a deoxycytidine nucleoside analog. It prevents the production of DNA, which causes cell death. Whereas Docetaxel is a chemotherapy drug that suppresses the mitotic process by preventing tubulin disintegration and prevents cell division (11). According to a 2019 retrospective research, Relapse Free Survival rates were 60% and 46% at 1 and 2 years, respectively, with 15.6% of patients advancing to Radical Cystectomy at a median duration of 11.3 months following induction (7).
4. Pembrolizumab: Pembrolizumab (MK-3475) is a humanized monoclonal antibody that binds to the PD-L1 receptor on immunological (T) cells, blocking them from engaging with their ligands (12). In a phase 2 trial, 18.4% of people had immune-related adverse events (AEs), and 1 treatment-related death from colitis following the drug (7) (Table 1).
There is an imperative need for innovative medicines in the treatment of NMIBC because of the high rates of progression and recurrence associated with existing therapy, and the significant side effects and morbidity associated with definitive therapies. Nadofaragene firadenovec (nadofaragene firadenovec-vncg; Adstiladrin®) is a Ferring Pharmaceuticals-developed non-replicating adenoviral vector-based gene therapy for the treatment of high-risk BCG-unresponsive NMIBC (13). The recombinant adenovirus Nadofaragene firadenovec delivers human interferon-cDNA to the bladder epithelium together with Syn3, a polyamide surfactant that speeds up viral transduction of the urothelium. Local interferon production causes tumor regression (7). In December 2022, nadofaragene firadenovec gained its first worldwide approval in the United States for the treatment of high-risk BCG-unresponsive NMIBC with carcinoma in situ (CIS) with or without papillary tumors in adults (13, 14). It is the first gene therapy to be approved for the treatment of bladder cancer (15). Individuals with immunocompromised and immunodeficient conditions should not administer nadofaragene firadenovec because it may spread disseminated adenovirus infection (16). Multiple trials were conducted which were efficacious, with a positive benefit-to-risk ratio. In a study conducted between 2016 and 2019, 25 (45%) of 55 patients with carcinoma in situ had their response sustained at 12 months. After the 103 patients who participated, 55 (53%) achieved a full response within 3 months of the first dosage, making it a breakthrough drug (17) (Table 2).
In a phase III clinical trial involving patients with BCG-unresponsive non-muscle invasive bladder cancer (NMIBC), characterized by an open-label, single-arm design and conducted across multiple centers, a remarkable complete response (CR) rate of 53.4% (95% CI 43.3%–63.3%) was achieved among a cohort of 103 patients with carcinoma in situ (CIS) [primary endpoint] (ClinicalTrials.gov identifier: NCT02773849) (17). Notably, this CR rate significantly surpassed the predetermined rate of 27% established for this specific cohort (p < 0.0001). The study enrolled 157 participants aged 18 years or older, possessing an Eastern Cooperative Oncology Group status of 2 or lower. These individuals received intravesical treatment with nadofaragene firadenovec at a concentration of 3 × 1011 viral particles/mL in 75 mL doses. Subsequent doses were administered at 3, 6, and 9 months for patients who exhibited an absence of high-grade recurrent disease. Exclusion criteria encompassed conditions such as upper urinary tract disease, urothelial carcinoma within the prostatic urethra, lymphovascular invasion, micropapillary bladder cancer, and hydronephrosis stemming from T1 disease.
In a phase II clinical trial carried out across multiple centers and employing an open-label design, patients diagnosed with BCG-refractory or relapsed non-muscle invasive bladder cancer (NMIBC) were investigated. The primary endpoint of interest pertained to the 12-month relapse-free survival rate in cases of high-grade disease. Among the study participants, a cohort of 21 individuals received a low dose (1 × 1011 viral particles/mL) of nadofaragene firadenovec, while another group of 19 patients received a higher dose (3 × 1011 viral particles/mL) of the same therapy [ClinicalTrials.gov identifier: NCT01687244] (18). Impressively, the observed 12-month relapse-free survival rate was 33.3% for those who received the low dose and 36.8% for those who received the high dose. In this trial, all patients were subjected to a single intravesical administration of nadofaragene firadenovec, infused in a 75 mL volume. Patients who remained free from recurrence of high-grade disease subsequently underwent additional treatment sessions with nadofaragene firadenovec at months 4, 7, and 10 (18).
In phase I studies, a phase Ib trial observed that 29% (2 out of 7) of patients with BCG-refractory NMIBC achieved a complete response (CR) when subjected to intravesical treatment involving nadofaragene firadenovec at a concentration of 3 × 1011 viral particles/mL in a 75 mL volume. The primary focus of this trial was to assess the transfection efficacy of a second dose (19). Another phase I trial explored intravesical treatment with nadofaragene firadenovec at concentrations ranging from 3 × 109 to 3 × 1011 viral particles/mL in a 75 mL volume. Within this trial, a complete response was attained in 41% (7 out of 17) of patients with BCG-refractory NMIBC. The primary objective of this study was centered around safety assessment. These findings highlight the potential efficacy of nadofaragene firadenovec as a therapeutic avenue for BCG-refractory NMIBC in phase I trials (20) (Tables 3, 4).
Nadofaragene (rAd-IFN/Syn3) is a gene therapy used for the treatment of bladder cancer. A single intravesical instillation of Nadofaragene, which contains x10 11 viral particles, is administered every 3 months for a total of four doses (17). Nadofaragene is administered via intravesical instillation, which involves the injection of the therapy directly into the bladder. It acts on tumor cells and expresses INF alpha-2b intracellularly which further indirectly activates antiviral, antiproliferative, antitumor and immune-modulating effects (5). The most commonly reported side effects of Nadofaragene therapy include bladder spasms, hematuria, micturition urgency, fatigue, and urinary tract infection (17) (14). In the phase III clinical trial, the safety population included all treated patients in the CIS and the high-grade Ta or T1 illness groups. Sixty-six percent of patients had drug-related adverse events (AEs) of grade 1 or 2 whereas four percent of patients suffered grade 3 AEs. Grade 4 or 5 AEs were not documented. The most frequent grade 1 or 2 TRAEs (incidence 10%) were pyrexia (incidence 10%), weariness (incidence 20%), bladder spasm (incidence 15%), micturition urgency (incidence 14%), chills (incidence 12%), and discharge surrounding the catheter during instillation (incidence 25%). Urinary incontinence, bladder spasm, syncope, hypertension, and urgency to urinate (all two patients; incidence 1%), as well as syncope and syncope-like symptoms, were all grade 3 drug-related adverse events that were observed during the experiment (17). These side effects are generally mild to moderate in severity and resolve within a few days to weeks. In addition, Nadofaragene therapy may cause serious adverse events, including bladder contracture, urinary fistula, and bladder perforation, although these events are rare. Patients should be closely monitored for signs of adverse events following Nadofaragene administration (14, 17).
The treatment landscape for patients with non-muscle invasive bladder cancer (NMIBC) is undergoing rapid and transformative evolution. Recent advancements in medical research and technology have led to a dynamic shift in therapeutic options, offering new hope and possibilities for patients facing this challenging condition. Emerging treatments, such as gene therapies like nadofaragene firadenovec, are reshaping the way NMIBC is managed, aiming to provide more effective and targeted interventions. Additionally, the integration of immunotherapies and combination therapies, like pembrolizumab and gemcitabine/docetaxel, respectively, is altering the traditional approach to NMIBC treatment, harnessing the power of the immune system and multi-faceted drug combinations for improved outcomes. These breakthroughs not only expand the array of available therapies but also underline the remarkable progress being made in addressing the unmet medical needs of NMIBC patients, marking a pivotal era of innovation and promise in bladder cancer care. Table 5 illustrates a diverse range of phase II-IV clinical trials aimed at exploring innovative treatments, combinations of drugs, and methods of administration for NMIBC. These phase II-IV trials showcase the relentless pursuit of improved therapies, innovative drug combinations, and advanced delivery approaches. The breadth of investigations underscores the medical community’s dedication to addressing the challenges posed by NMIBC, particularly in cases that are unresponsive to conventional treatments. As these trials progress and yield valuable insights, they offer the potential to revolutionize the management and outcomes of NMIBC patients, ushering in a new era of more effective and tailored therapeutic interventions.
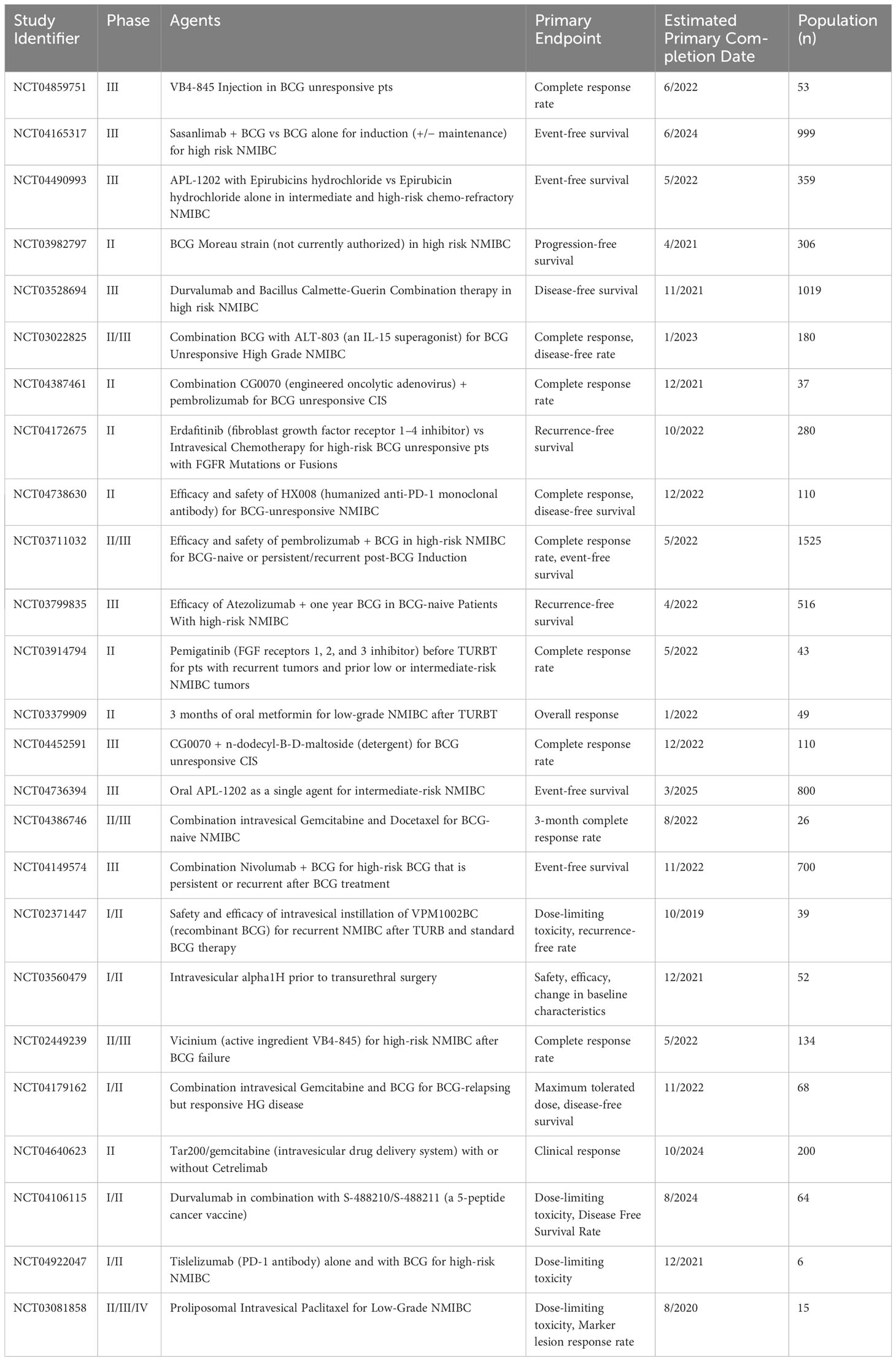
Table 5 Trial data obtained from clinicaltrials.gov.
In the realm of bladder oncology, the emergence of Nadofaragene firadenovec stands as a watershed moment, symbolizing a transformative breakthrough in the treatment of high-risk, BCG-unresponsive non-muscle invasive bladder cancer (NMIBC). This novel gene therapy, marked by its ingenious design and innovative mechanism of action, holds the promise of reshaping the landscape of NMIBC treatment. With its ability to stimulate interferon production within urothelial cells, Nadofaragene firadenovec orchestrates a multifaceted assault on cancer, invoking immunostimulatory, antiangiogenic, and apoptotic effects. Against the backdrop of the current therapeutic limitations and challenges in addressing high-risk BCG-unresponsive NMIBC, the approval of Nadofaragene firadenovec in December 2022 by the United States regulatory authorities signifies a momentous turning point. Approximately 30% of patients remain refractory to BCG treatment, necessitating alternative therapeutic pathways.
The advent of Nadofaragene firadenovec introduces an optimistic stride towards providing a potent and authorized therapeutic intervention, filling the void in the treatment landscape for these individuals. As the clinical trials of Nadofaragene firadenovec progress and demonstrate its potential efficacy, this groundbreaking therapy brings renewed hope to patients and medical practitioners alike. The evolution of NMIBC treatment is now marked by innovative gene therapies that aim to revolutionize patient outcomes and redefine the approach to bladder cancer therapy. This new era of personalized and targeted interventions holds immense promise, heralding an era of improved clinical management and enhanced quality of life for those affected by high-risk BCG-unresponsive NMIBC.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
AN, WB and KQ were involved in the study concept, the collection of the data, drafting, literature review, data validation, supervision, and editing of the manuscript. LV, AA and RT were responsible for the literature review and revising the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet (London England) (2009) 374(9685):239–49. doi: 10.1016/S0140-6736(09)60491-8
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. Saginala K, Barsouk A, Aluru JS, Rawla P, Padala SA, Barsouk A. Epidemiology of bladder cancer. Med Sci (Basel Switzerland) (2020) 8(1):15. doi: 10.3390/medsci8010015
4. Richters A, Aben KKH, Kiemeney LALM. The global burden of urinary bladder cancer: an update. World J Urol (2020) 38(8):1895–904. doi: 10.1007/s00345-019-02984-4
5. Definition of nadofaragene firadenovec-vncg - NCI Drug Dictionary - NCI. Available at: https://www.cancer.gov/publications/dictionaries/cancer-drug/def/nadofaragene-firadenovec.
6. Martini A, Tholomier C, Mokkapati S, Dinney CP. Interferon gene therapy with nadofaragene firadenovec for bladder cancer: from bench to approval. Front Immunol (2023) 14:1260498. doi: 10.3389/fimmu.2023.1260498
7. Smelser WY. BCG-unresponsive NMIBC: Current evidence and options. In: Urology times (Urology Times Urologists in Cancer Care) (2021) 10 (4). Available at: https://www.urologytimes.com/view/bcg-unresponsive-nmibc-current-evidence-and-options.
8. Froehner M, Brausi MA, Herr HW, Muto G, Studer UE. Complications following radical cystectomy for bladder cancer in the elderly. Eur Urol (2009) 56(3):443–54. doi: 10.1016/j.eururo.2009.05.008
9. Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol (2001) 19(3):666–75. doi: 10.1200/JCO.2001.19.3.666
10. Hajian R, Hossaini P, Mehrayin Z, Woi PM, Shams N. DNA-binding studies of valrubicin as a chemotherapy drug using spectroscopy and electrochemical techniques. J Pharm Anal (2017) 7(3):176–80. doi: 10.1016/j.jpha.2017.01.003
11. Steinberg RL, Thomas LJ, O'Donnell MA, Nepple KG. Sequential intravesical gemcitabine and docetaxel for the salvage treatment of non-muscle invasive bladder cancer. Bladder Cancer (Amsterdam Netherlands) (2015) 1(1):65–72. doi: 10.3233/BLC-150008
12. Rayn KN, Hale GR, Grave GP, Agarwal PK. New therapies in nonmuscle invasive bladder cancer treatment. Indian J Urol IJU J Urological Soc India (2018) 34(1):11–9. doi: 10.4103/iju.IJU_296_17
13. U.S. Food & Drug Administration. FDA approves first gene therapy for the treatment of high-risk, non-muscle-invasive bladder cancer [media release]. Available at: https://www.fda.gov/ (Accessed 16 Dec 2022).
14. Lee A. Nadofaragene firadenovec: first approval. Drugs (2023) 83:353–7. doi: 10.1007/s40265-023-01846-z
15. Ferring Pharmaceuticals. Ferring receives approval from U.S. FDA for Adstiladrin for high-risk, BCG-unresponsive non-muscle invasive bladder cancer [media release]. Available at: https://www.ferring.com/ (Accessed 16 Dec 2022).
16. Ferring Pharmaceuticals. ADSTILADRIN® (nadofaragene firadenovec-vncg) suspension, for intravesical use: US prescribing information (2022). Available at: https://www.fda.gov/ (Accessed 27 Jan 2023).
17. Boorjian SA, Alemozaffar M, Konety BR, Shore ND, Gomella LG, Kamat AM, et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: a single-arm, open-label, repeat-dose clinical trial. Lancet Oncol (2021) 22(1):107–17. doi: 10.1016/S1470-2045(20)30540-4
18. Shore ND, Boorjian SA, Canter DJ, Ogan K, Karsh LI, Downs TM, et al. Intravesical rAd–IFNα/Syn3 for patients with high-grade, Bacillus Calmette-Guerin–refractory or relapsed non–muscle-invasive bladder cancer: a phase II randomized study. J Clin Oncol (2017) 35(30):3410–6. doi: 10.1200/JCO.2017.72.3064
19. Navai N, Benedict WF, Zhang G, Abraham A, Ainslie N, Shah JB, et al. Phase 1b trial to evaluate tissue response to a second dose of intravesical recombinant adenoviral interferon α2b formulated in Syn3 for failures of Bacillus Calmette-Guerin (BCG) therapy in nonmuscle invasive bladder cancer. Ann Surg Oncol (2016) 23(12):4110–4. doi: 10.1245/s10434-016-5300-6
20. Dinney CPN, Fisher MB, Navai N, O'Donnell MA, Cutler D, Abraham A, et al. Phase I trial of intravesical recombinant adenovirus mediated interferon-α2b formulated in Syn3 for Bacillus Calmette-Guérin failures in nonmuscle invasive bladder cancer. J Urol (2013) 190(3):850–6. doi: 10.1016/j.juro.2013.03.030
21. Mitra AP, Narayan VM, Mokkapati S, Miest T, Boorjian SA, Alemozaffar M, et al. Antiadenovirus antibodies predict response durability to nadofaragene firadenovec therapy in BCG-unresponsive non-muscle-invasive bladder cancer: secondary analysis of a phase 3 clinical trial. Eur Urol (2022) 81(3):223–8. doi: 10.1016/j.eururo.2021.12.009
Keywords: Nadofaragene firadenovec, BCG-unresponsive non-muscle invasive bladder cancer, gene therapy, interferon-2b, carcinoma in situ, immunostimulatory, antiangiogenic
Citation: Nadeem A, Qamar K, Bilal W, Vohra LI, Ahsan A and Tariq R (2023) Nadofaragene firadenovec: a breakthrough in the field of bladder oncology. Front. Urol. 3:1206398. doi: 10.3389/fruro.2023.1206398
Received: 15 April 2023; Accepted: 29 August 2023;
Published: 15 September 2023.
Edited by:
Gian Maria Busetto, University of Foggia, ItalyReviewed by:
Lawrence Karsh, The Urology Center of Colorado, United StatesCopyright © 2023 Nadeem, Qamar, Bilal, Vohra, Ahsan and Tariq. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdullah Nadeem, aWFtLmFiZHVsbkBvdXRsb29rLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.