
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Urol. , 11 September 2023
Sec. Female Urology
Volume 3 - 2023 | https://doi.org/10.3389/fruro.2023.1196925
 Berhanu Negese Kebede1*
Berhanu Negese Kebede1* Desta Haftu Hayelom2
Desta Haftu Hayelom2 Gebremaryam Temesgen Birgoda1
Gebremaryam Temesgen Birgoda1 Awol Arega Yimer1
Awol Arega Yimer1 Bezawit Afework Mesfin1
Bezawit Afework Mesfin1 Mesfin Difer Tetema3
Mesfin Difer Tetema3 Solomon Seyife Alemu4
Solomon Seyife Alemu4 Kassaw Beyene Getahun1
Kassaw Beyene Getahun1Background: Globally, millions of women develop pelvic floor disorder. It imposes a considerable emotional, social, and financial burden on women’s lives. Despite this, in developing countries, nearly half of women with pelvic floor disorder do not seek any help due to feelings of isolation, depression, shame, and loss of control. Thus, the magnitude of the problem is largely unknown. The aim of this study was to assess the prevalence of pelvic floor disorder and associated factors among women at Arba Minch Health and Demographic surveillance site.
Methods: A cross-sectional study with a simple random sampling technique was employed on a community basis. The data were entered into EpiData version 3.1 and then exported to Statistical Package for Social Sciences version 25 for data cleaning and analysis. Bivariate and multivariable analyses using binary logistic regressions were carried out to identify factors associated with pelvic floor disorder. The level of significance was declared at a p-value of < 0.05.
Results: The prevalence of pelvic floor disorder was 31.4% (95% CI = 26.9% to 35.8%). Being grand multiparous (AOR = 3.919, 95% CI = 1.495–10.276), having a history of instrumental delivery (AOR = 3.042, 95% CI = 1.483 to 6.241), having a history of perianal tearing (AOR = 2.972, 95% CI = 1.491 to 5.927), and having a medical disease (AOR= 2.698, 95% CI = 1.526 to 4.770) were factors associated with pelvic floor disorder.
Conclusions and recommendations: The prevalence of pelvic floor disorder was high in the study area. Parity, instrumental delivery, perianal tears, and medical problems were factors affecting the prevalence of pelvic floor disorder. There is a need for an improvement of policies and strategies focusing on prevention and treatment services to alleviate the problem.
Pelvic floor disorders (PFDs) are conditions that affect the proper function of a woman’s pelvic organs and can occur when the pelvic floor muscles are too weak, too tight, damaged, or overused (1).
PFDs comprise a collection of conditions, the most notable of which are urinary incontinence (UI), pelvic organ prolapse (POP), and fecal incontinence (FI) (2).
According to a joint report of an international urogynecological association (IUGA/International Continence Society), POP is defined as the descent of the anterior vaginal wall, posterior vaginal wall, uterus (cervix), or vaginal apex (3), and UI is any involuntary loss of urine (4). Fecal incontinence is defined as the involuntary loss of solid and liquid feces (3) and the inability to postpone an evacuation until it is socially acceptable (5).
Stress UI, urge incontinence, and mixed incontinence are all types of urinary incontinence (3, 4).
The prevalence of PFD among women in high-income countries is estimated to be between 25% and 43% (6, 7). The prevalence of POP alone was reported to be between 2.9% and 12.8% (6, 8, 9), with 3.5%–15% of women reporting fecal incontinence in high-income countries (6, 7).
According to a study conducted in developing countries, 20%, one-third, and 7% of parous women have POP, UI, and FI, respectively (10). In low-income countries, UI was reported at a rate of 25%–42% (11, 12), and the prevalence of POP ranged from 2% to 64.8% (13–16).
In Ethiopia, one-eighth to two-fifths of women have one form of PFD (17–19); however, the majority of women with PFDs do not have access to appropriate healthcare (10, 17). The findings of studies conducted in hospitals revealed a high prevalence of POP, with rates ranging from 13.3% to 40.7% (20–22); studies conducted at the community level reported a prevalence rate of 9.5% (17). This variation between the findings of community- and hospital-based studies also reflects a low level of healthcare seeking and the silent suffering of many women with a PFD (17).
The consequences of PFD issues can be disastrous (10). They can cause significant health problems (23), a negative impact on daily quality of life (11), physical activity restriction, emotional affection, and sleep deprivation in 90% of affected women, and role limitations in 70% (12). Women with a PFD, particularly POP, were fearful of disclosure, discrimination, and divorce as a result of community and family perceptions of shameful and strongly prohibited physical and social conditions (24).
In high-income countries, pregnancy and childbirth are well-known risk factors for PFDs (25–27). Furthermore, in developing countries, high parity, home delivery, age, and prolonged heavy lifting are among the factors that increase the risk of PFDs (16, 28).
Ethiopia is a low-income country with limited obstetric care (48% of births are institutionalized) and a high fertility rate (4.6 children per woman) (29). Women in the Arba Minch Health and Demographic Surveillance Site have limited access to obstetric care, putting them at a higher risk of obstetric-related pelvic floor injury, with 76.9% of all births taking place at home prior to 2015 (30). Despite this, many healthcare providers focus on the immediate complications of childbirth, while delayed maternal complications, which may take decades to develop as other lifestyle factors contribute to the development of these disorders, receive less attention. As a result, the purpose of this study was to determine the prevalence of pelvic floor disorder and its associated factors among women in the Arba Minch Health and Demographic Surveillance Site in Gamo Zone, southern Ethiopia.
From 1 to 30 May 2021, a community-based cross-sectional study was conducted at the Arba Minch Health and Demographic Surveillance Site in the Arba Minch Zuria and Gacho Baba districts, Gamo Zone, Southern Ethiopia. According to 2015 census projections, the districts had a total population of 164,529, of which 82,330 were women. The districts had 31 kebeles (the smallest administrative units) in total and were part of the Arba Minch Zuria Demographic and Health Development Program (AM-DHDP). Arba Minch Zuria Demographic and Health Development Program is owned by Arba Minch University, one of six public universities in Ethiopia that has a Health and Demographic Surveillance System (HDSS) (31). The surveillance site is made up of eight rural and one semi-urban kebele, selected from a total of 31 kebeles in the district. The Arba Minch Health and Demographic Surveillance Site contains 24,206 registered households.
As a source population, all women in the Arba Minch Health and Demographic Surveillance Site were considered. Women registered in the Arba Minch HDSS database and living in the kebeles of the Arba Minch Health and Demographic Surveillance Site were the study populations.
The study included women who were registered in the Arba Minch Health and Demographic Surveillance Site database. The study excluded pregnant women and mothers with a postpartum period of fewer than 6 weeks.
The sample size was calculated using the single population proportion formula with the following assumptions: the proportion (p) of pelvic floor disorder in the Kersa district being 20.5% (17), 95% confidence level (, and the degree of precision being 4%. The minimum required sample size was calculated by substituting the above assumptions in the formula: 392. With a 10% response rate, the final minimum required sample size was 431.
Where
n = the required sample size;
z = the value of the standard normal curve score corresponding to the given confidence level, which is 1.96
p = proportion of pelvic floor disorder, which is 20.5%
d = the permissible margin of error, which is 4%
The study took place in the Arba Minch Health and Demographic Surveillance Site’s nine kebeles. A simple random sampling technique was used to select the study sample, which was based on the ID numbers of registered households in each selected kebele. The total number of households in each kebele was determined using the Arba Minch Health and Demographic Surveillance Site database. To adjust the calculated sample size for each kebele, proportional sample allocations were made. Using the Arba Minch HDSS database as a sampling frame, the required number of target women was drawn at random from the sample frame. Finally, the ID numbers of selected households were extracted from the database and printed as hard copies for use in the field.
PFD.
Socio-demographic variables: age, residence, marital status, educational level, ethnicity, occupation, and income.
Obstetrics and gynecologic factors: gravidity, abortion, parity, age at first delivery, place of delivery, perianal tear and episiotomy, mode of delivery, and menopausal status.
Medical and personal factors: heavy lifting, lung disease, diabetes mellitus, urinary tract infection, kind of work or daily activity (working on a farm, carrying water, and preparing false bananas—”kocho”). Heavy physical work and heavy weight lifting increase intra-abdominal pressure, which is believed to play a role in the pathogenesis of PFD (specifically POP). Kocho, an Ethiopian flatbread or bread-like fermented food made from chopped and grated enset pulp, is a traditional and favorite food in the study area. Processing kocho/enset is one of the strenuous tasks women have to perform as one of their daily activities.
Women’s responses to questions about UI, POP, and anal incontinence were used to assess PFDs. The presence of the problem was defined by positive responses to at least one of the questions from each of the PFD categories. Women who reported at least one PFD symptom were classified as “having pelvic floor disorder” (17). A four-point Likert scale was used to assess the levels of distress caused by each PFD’s symptoms. If symptoms were present, the individual was asked, “How much are the symptoms bothering you?” And the response was graded on a scale of “not at all = 1” to “very seriously = 4”. The mean value of symptom distress was multiplied by 25 to obtain the score ranges of 0–100 for each domain of PFD. The severity of the symptoms among women with a PFD was determined based on distress score ranges from 1 to 100 and categorized by tertiles (into three parts) as “mild” if the total score was 1–33, “moderate” if the total score was 34–66, and “severe” if the score was 67–100 (17).
Data were collected using semi-structured, interviewer-administered data collection tools.
Questions about pelvic floor symptoms were adapted from a previous study conducted in Ethiopia (11). The questions assessing other independent variables such as socio-demographic factors, obstetric and gynecologic history, and personal and medical history were adapted primarily from the Epidemiology of Prolapse and Incontinence Questionnaire (32). For data collection, nine midwives with a Bachelor of Science degree who were fluent in Amharic and Gamottho were hired. Three field supervisors with Masters’ degrees in clinical midwifery were also hired to monitor the process and the quality of the data collected.
To ensure consistency, the questionnaire was prepared in English and translated into Amharic and back to English prior to data collection. A pretest was conducted on 22 women (5% of the sample) in the Mirab Abaya woreda. Data collectors received a 2-day training course supported by practical demonstrations on interview methods, with an emphasis on the introduction, communication to correctly assess outcomes, and respecting cultural norms in the community. During the data collection phase, the data collectors thoroughly checked the questionnaire’s completion. The principal investigator and supervisors provided daily intensive supervision throughout the data collection period.
Data were entered into EpiData 3.1 before being exported to Statistical Package for Social Sciences (SPSS) version 25 for cleaning and further analysis.
To describe the characteristics of participants, descriptive statistical analyses such as simple frequencies, percentages, medians, and interquartile ranges were used. In the binary logistic regression model, the relationship between the outcome variable and several independent variables was first examined separately. In the second step, independent variables with a p-value of 0.25 in bivariate analysis were retained and entered into the multivariable logistic regression analysis together. The degree of association between the outcome and independent variables was determined using an OR with a 95% confidence interval and a p-value.
The statistical significance level was set at a p-value of < 0.05.
The institutional review board of Arba Minch University’s College of Medicine and Health Science granted ethical clearance with the ethical clearance number IRB/1066/21. Before collecting data, each study subject provided written consent. Names and identification were not included in the written questionnaires to protect the confidentiality of the information. Each study subject was informed that their participation would be entirely voluntary during the data collection process. Women who were discovered to have pelvic floor symptoms during data collection but were not seeking treatment were counseled and referred to healthcare facilities.
A total of 431 participants were approached and 427 eventually participated in the study, giving a response rate of 99.1%. The respondent’s median age was 29 years [interquartile range (IQR) = 24–45 years]. The majority of the respondents, 361 (84.5%), were in a marital union (married and living with their husband). The majority of respondents (402) (94.1%) were Gamo in ethnicity. Sixty-six percent of those polled were housewives, and 43.3% had no formal education (Table 1).
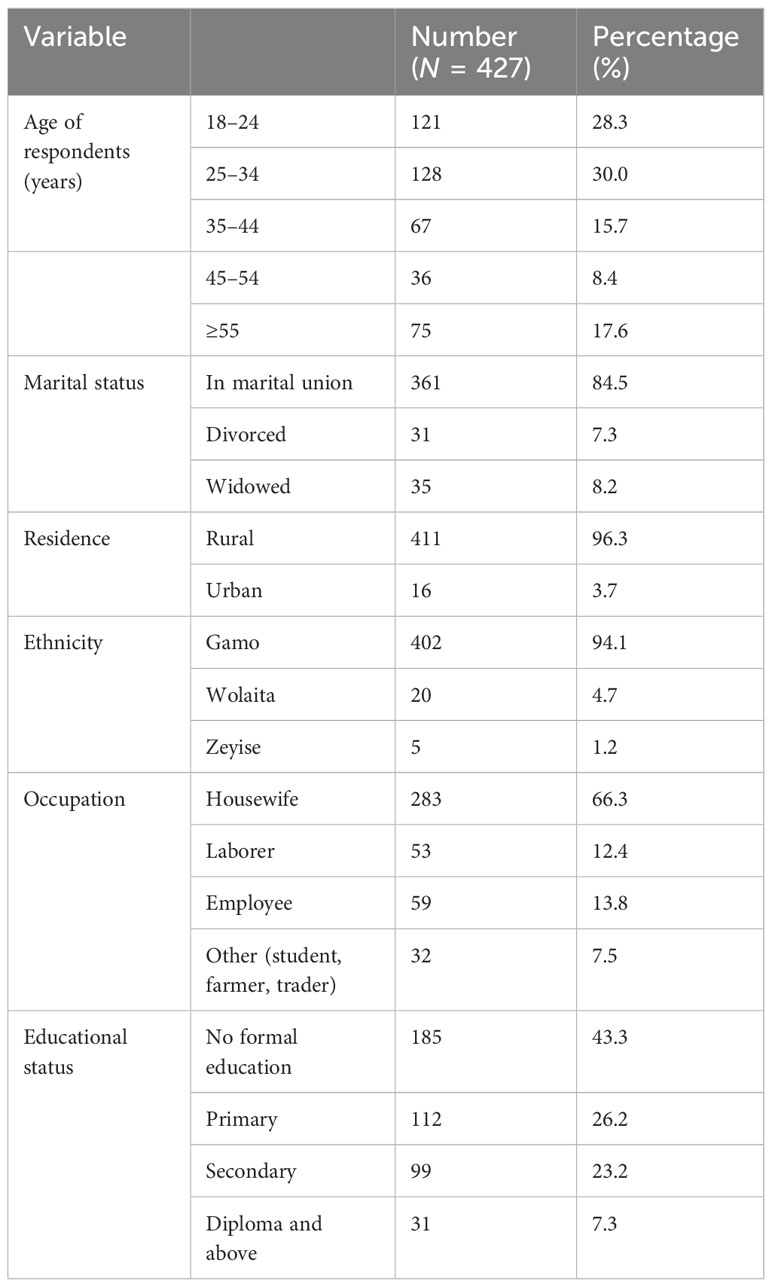
Table 1 Socio-demographic characteristics of the study participants of the study on prevalence of pelvic floor disorder and associated factors in Arba Minch HDSS, Southern Ethiopia, 2021.
Overall, 26.7% of the participants had experienced pregnancy five times or more. In addition, 32% of respondents had a history of abortion, with nearly half having had only one. Almost all study participants were parous, with 22.8% being grand multiparous.
Approximately half of those polled had experience with home delivery. Thirty-five percent of study participants had a history of episiotomy during labor, and 14.7% had a history of instrumental delivery. Regarding perianal tears, regardless of the place of delivery, 18.5% of respondents reported experiencing perianal tears during their delivery. Around 34.3% of respondents had a history of cesarean delivery, with roughly three-quarters having undergone only one cesarean delivery. Menopause had been experienced by 22% of those polled (Table 2).
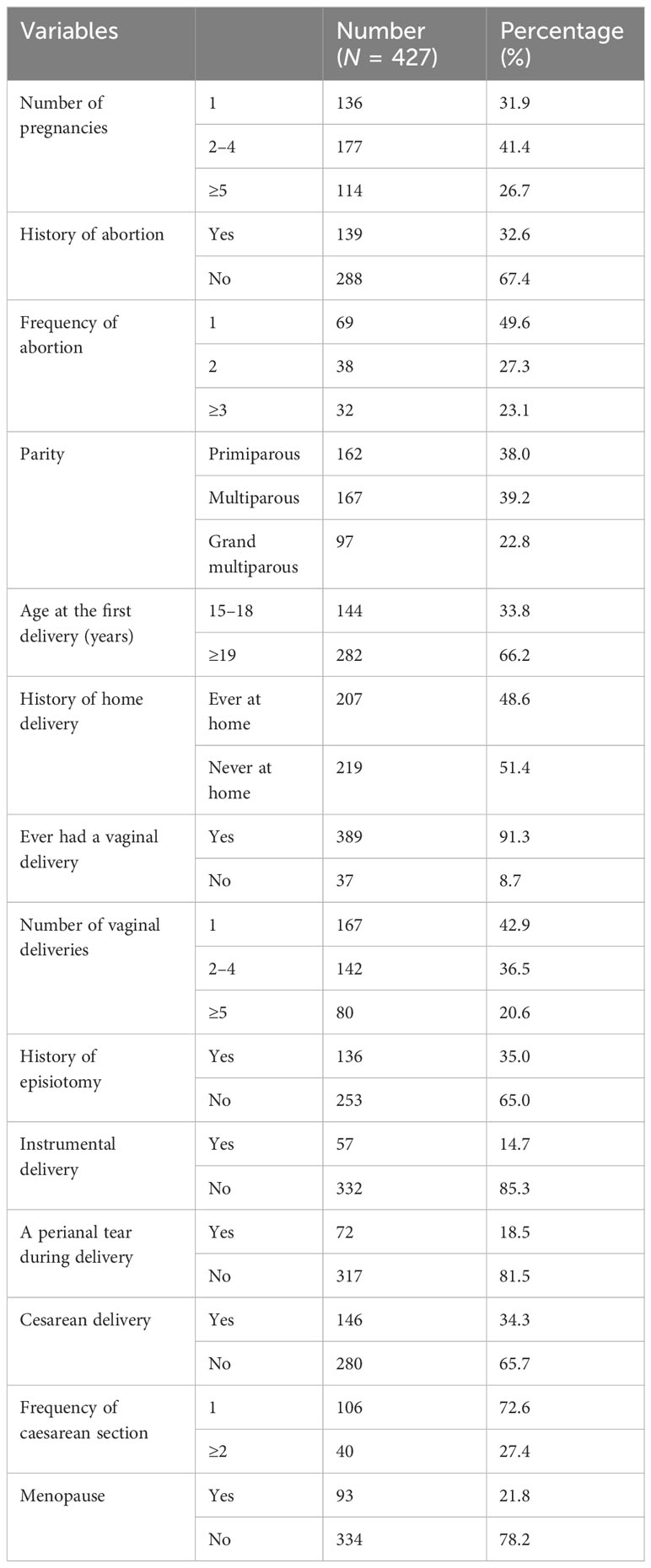
Table 2 Reproductive characteristics of the study participants of the study on the prevalence of PFD and associated factors in Arba Minch HDSS, Southern Ethiopia, 2021.
Forty-two (9.8%) respondents had a history of recurrent urinary tract infections, while 9.4% and 14.8% had diabetes mellitus and lung disease/asthma, respectively. The majority of respondents (90.4%) had been required to lift more than 9 pounds on a regular basis.
In terms of daily activity, 91.1% of respondents carried water for personal use on a daily basis.
Seventy percent of respondents carry water two to three times per day on average, while 65.3% and 37% were involved in farming and false banana preparation, respectively (Table 3).
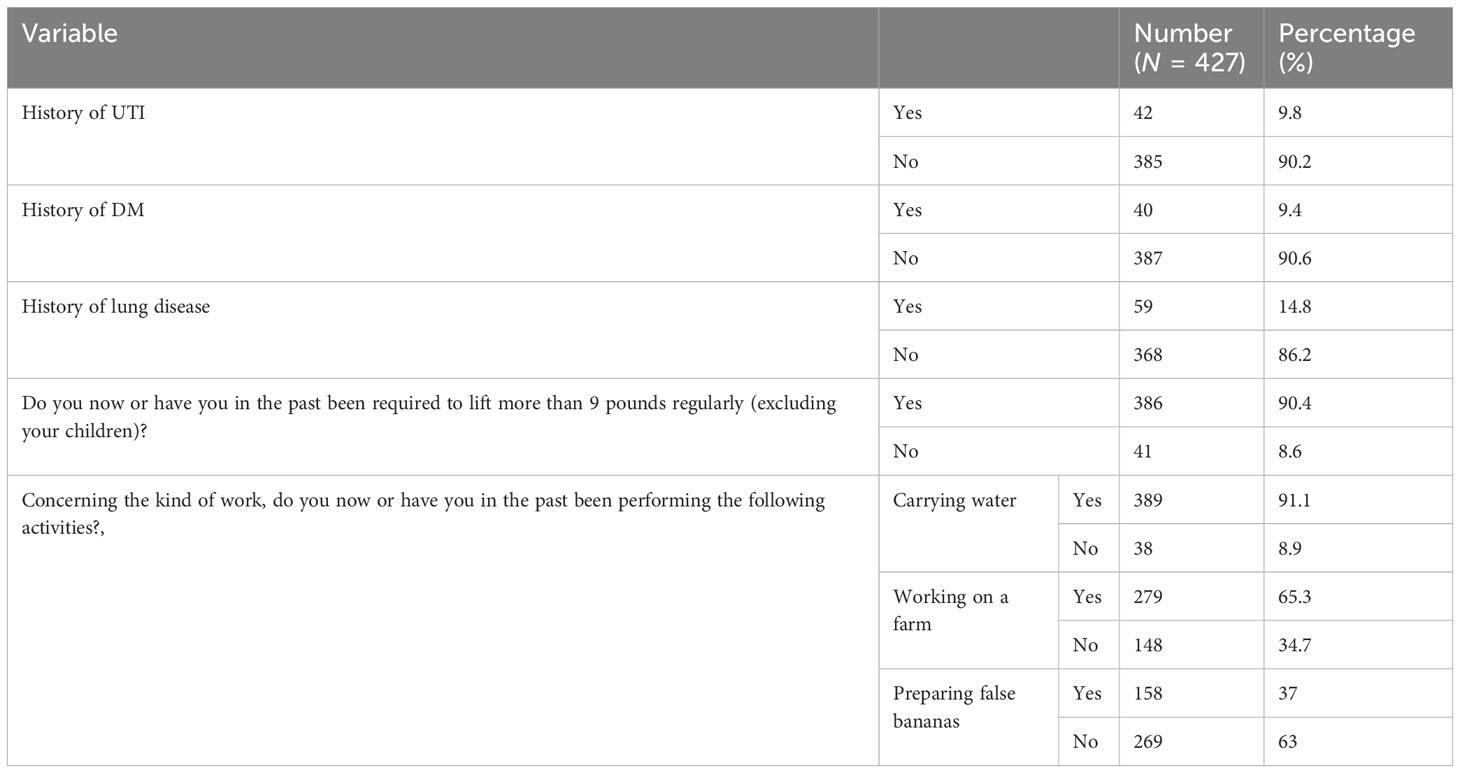
Table 3 Medical and personal history of the study participants of the study on prevalence of PFD and associated factors in Arba Minch HDSS, Southern Ethiopia, 2021.
Overall, 134 (31.4%, 95% CI = 26.9% to 35.8%) of the women reported at least one form of PFD (Figure 1). Incontinence was reported by 122 (28.6, 95% CI = 24.3% to 32.8%) of respondents. The prevalence of each PFD (and corresponding 95% CI) was 19.2% (15.5% to 22.9%) for urge incontinence, 11.2% (8.2% to 14.2%) for stress UI, 8.2% (5.6% to 10.8%) for mixed UI, 12.2% (9.1% to 15.3%) for anal incontinence, and 9.8% (6.9% to 12.6%) for POP.
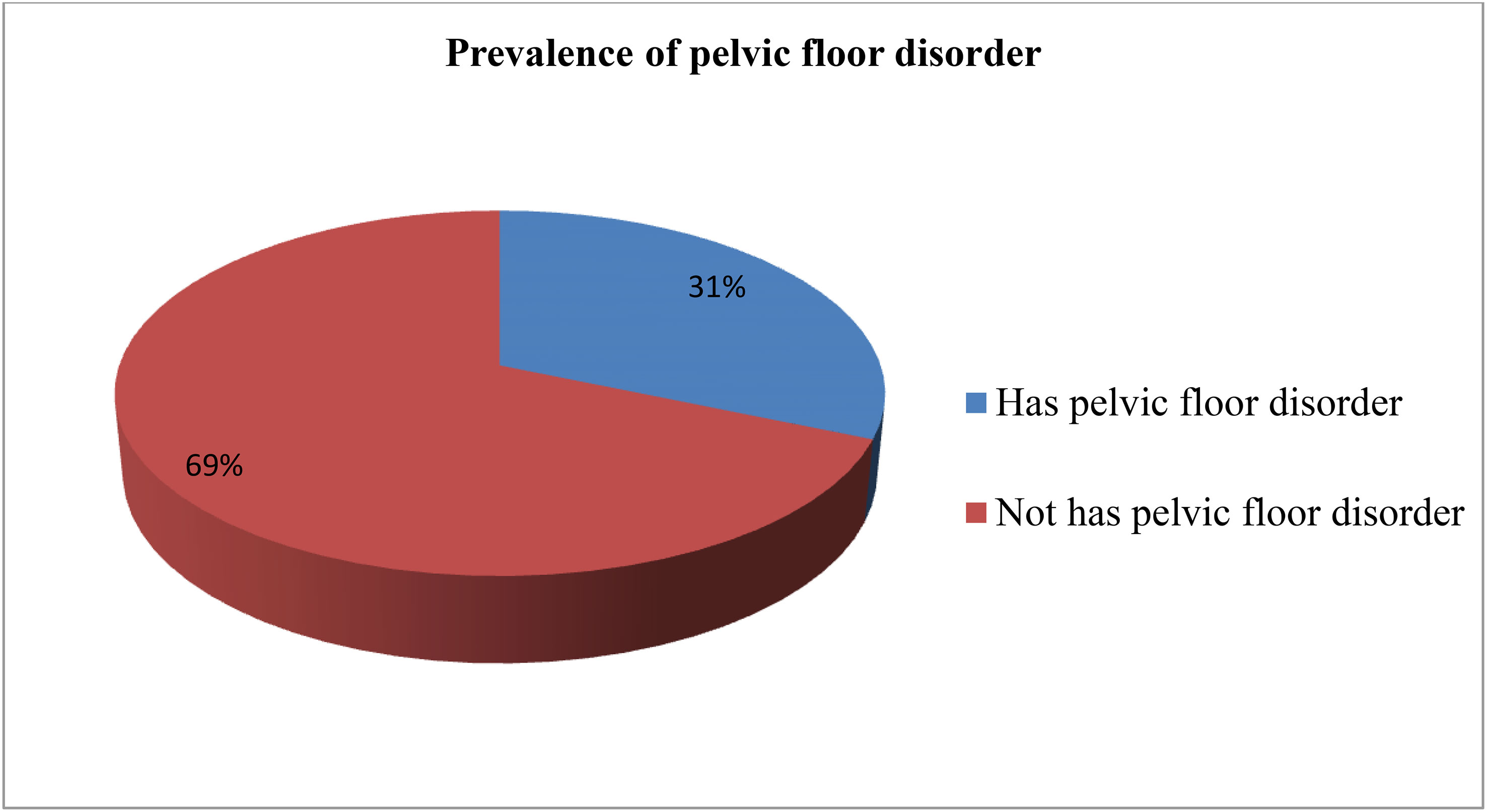
Figure 1 Overall prevalence of pelvic floor disorder among study participants in Arba Minch Health and demographic surveillance site, 2021. Regarding co-occurrence of pelvic floor disorder 19.7%.
Among those who had a PFD, 57.5% reported being seriously worried about their symptoms, whereas 39.6% of women were moderately bothered about their symptoms.
Only 36% of those who reported having a PFD sought medical attention for their symptoms. The most frequently reported reasons for not seeking healthcare were feeling embarrassed/ashamed about the symptoms, being unable to afford it, believing it was a natural part of the aging process, and being too far away (Figure 2).
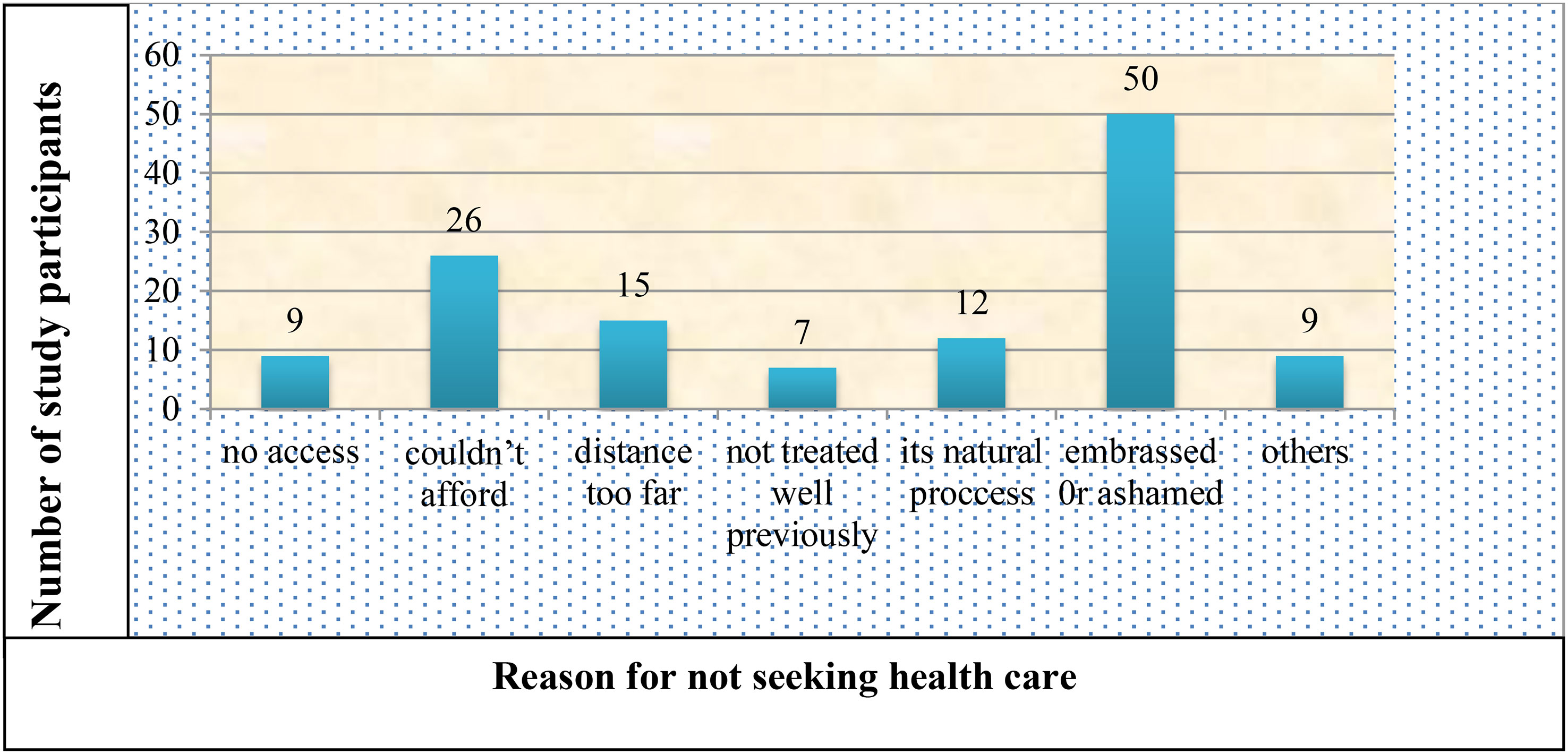
Figure 2 Reason for not seeking health care for pelvic floor disorder among the study participants in Arba Minch HDSS, 2021. Others include transportation problems, not ever seen by the health care provider and no female health care providers.
For the bivariable logistic regression, all variables were computed separately, and variables with a p-value of 0.25 were retrieved and entered into a multivariable logistic regression analysis. The respondents’ age and the number of vaginal delivery variables were excluded because they were highly correlated with the number of delivery variables in the linear logistic regression collinearity diagnostics. Four of the ten variables included in the multivariable logistic regression analysis showed a significant association.
According to the findings, being grand multiparous (AOR = 3.919, 95% CI = 1.495 to 10.276), having a history of instrumental delivery (AOR = 3.042, 95% CI = 1.483 to 6.241), having a history of perianal tearing (AOR=2.972, 95% CI = 1.491 to 5.927), and having a medical disease (AOR=2.698, 95% CI = 1.526 to 4.770) were factors associated with PFD (Table 4).
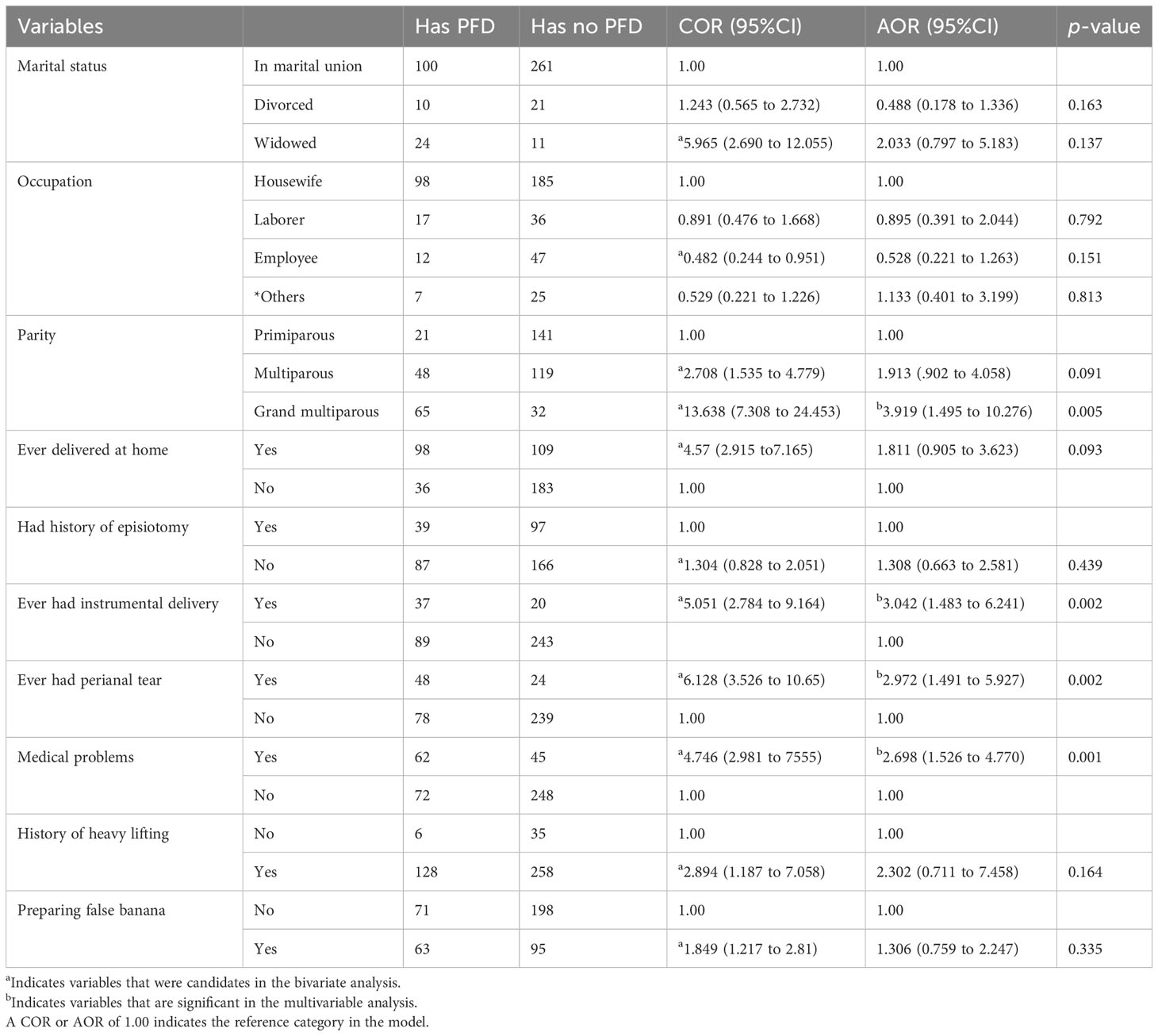
Table 4 Bivariate and multivariable logistic regression analyses as a result of factors associated with PFDs among study participants in Arba Minch HDSS, Ethiopia, 2021.
The prevalence of PFD in this study was 31.4%. This result was higher than a study done in the United States of America (6) and India (14) which reported a prevalence of 25% and 21% respectively. Childbirth is reported to be a well-known risk factor for PFDs (25–27). Ethiopia is among the countries with limited obstetric care (institutional delivery is at 48%) (33) and a high fertility rate (4.6 children per woman) (29). Therefore, the observed difference in the prevalence of pelvic floor disorders could also be partly attributed to the difference in access to obstetric care services and the difference in fertility rates between the countries.
The prevalence of PFD in this study was also higher than the reported 20.5% and 11.9% overall prevalence of PFD in the Kersa (17) and Dabat (18) districts, respectively. The possible reason could be socio-demographic differences among study participants, as the proportion of laborers accounted for 12.6% of this study population but only 2.5% in the Dabat district study population. Prolonged heavy lifting, believed to play a role in the pathogenesis of POP by increasing intra-abdominal pressure, is identified as a risk factor for PFD in developing countries (16, 28, 34). Thus, there could also be other contributing factors for an increase in the prevalence of PFD; more than 90% of study participants were engaged in heavy lifting activities on a regular basis, and more than one-third were involved in preparing false bananas (kocho) in the study area.
The result of this study was lower than that of a study conducted in Iran, which revealed a 42% prevalence of one form of PFD (13). A reported low prevalence could be due to differences in the study population, study setting, and data collection tools. The former was carried out at a hospital and incorporated physical examinations into data collection.
According to the findings of our study, the prevalence of mixed UI was 8.2%. This was in line with the findings of a study conducted in eastern part of Ethiopia, which reported a prevalence of 7.7% for mixed UI (17). However, it was lower than the findings of a study carried out in South-Central Ethiopia, which reported a prevalence of 14% for mixed UI (19). This discrepancy could be owing to the differences in the study population, as the latter included pregnant mothers. This finding in our study was higher than the finding of a study carried out in Norway, which reported a prevalence of 5.9% for mixed UI (35). This difference might be owing to the fact that the Norwegian study was conducted among women younger than 65 years and excluded those who had had more than four vaginal deliveries.
In this study, parity, history of institutional delivery, history of instrumental delivery, history of perianal tearing, previous history of UI during pregnancy, and having medical problems were factors associated with the prevalence of PFD.
The odds of having a PFD were 3.919 times higher among grand multiparous women than primiparous women. This was supported by findings of a study in Germany and Denmark (36), India (12), and Tanzania (16). This could be due to the weakening and damage of the pelvic floor by the excessive strain and stretching caused by repeated births.
Instrumental delivery and perianal lacerations increased the risk of PFDs. Women with at least one forceps delivery and perianal lacerations in two or more deliveries were reported to have increased risks of a PFD (37). This is in accordance with the finding of this study, which revealed that the odds of a PFD were 3.042 times higher among women who had a history of forceps or vacuum deliveries than among those who did not (37). Similarly, the odds of having a PFD were 2.972 times higher among women who had at least one perianal tear than among those who did not have a perianal tear. These birth-related risks could be due to compression, stretching, or the tearing of nerve, muscle, and connective tissue related to vaginal delivery, as intact neuromuscular function and pelvic organ support are both critical to the normal function of pelvic viscera (38).
The prevalence of PFDs was found to be lower in the healthy population group than in those with medical comorbidities (39). Chronic obstructive pulmonary disease (COPD) (36), bronchial asthma (40), and diabetes mellitus have all been linked to an increase in the prevalence of PFDs (41, 42). This is consistent with the study’s findings, which showed that the odds of having a PFD are 2.698 times higher in people who have a history of lung disease, diabetes mellitus, or urinary tract infections than in people who have never had any of these medical problems. Health education on prevention strategies, behavioral change communication to improve community health-seeking behavior, and family planning promotion could all be beneficial.
Women in the study area had a high prevalence of PFDs. Factors associated with PFDs included parity, instrumental delivery, perianal tearing, and medical problems. To alleviate the suffering of women with a PFD, policies and strategies focusing on prevention and treatment services must be improved.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Arba Minch University College of Medicine and Health Science Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
BK, DH, and GB conceived the study and undertook the statistical analysis. AY, SA, and MT supervised the study and statistical analysis. BM and KG contributed to the writing of the manuscript. All authors contributed to the article and approved the submitted version.
The authors thank all the study participants and data collectors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fruro.2023.1196925/full#supplementary-material
AI, anal incontinence; AOR, adjusted odds ratio; BMI, body mass index; CI, confidence interval; COR, crude odds ratio; FI, fecal incontinence; HDSS, Health and Demographics Surveillance Site; MUI, mixed urinary incontinence; OR, odds ratio; PFD, pelvic floor disorder; POP, pelvic organ prolapse; sPOP, symptomatic pelvic organ prolapse; SUI, stress urinary incontinence; UI, urinary incontinence.
1. Neels H. Pelvic floor dysfunction in women: tackling barriers. Beljium University of Antwerp (2018).
2. Patel DA, Xu X, Thomason AD, Ransom SB, Ivy JS, DeLancey JO. Childbirth and pelvic floor dysfunction: an epidemiologic approach to the assessment of prevention opportunities at delivery. Am J Obstet Gynecol (2006) 195(1):23–8. doi: 10.1016/j.ajog.2006.01.042
3. Haylen B, De Ridder D, Freeman R, Swift S, Berghmans B, Lee J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. J Assoc Chartered Physiother Womens Health (2012) 110:33.
4. Bo K, Frawley HC, Haylen BT, Abramov Y, Almeida FG, Berghmans B, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the conservative and nonpharmacological management of female pelvic floor dysfunction. Int Urogynecol J (2017) 28(2):191–213. doi: 10.1007/s00192-016-3123-4
5. Ruiz NS, Kaiser AM. Fecal incontinence-Challenges and solutions. World J Gastroenterol (2017) 23(1):11. doi: 10.3748%2Fwjg.v23.i1.11
6. Wu JM, Vaughan CP, Goode PS, Redden DT, Burgio KL, Richter HE, et al. Prevalence and trends of symptomatic pelvic floor disorders in US women. Obstet Gynecol (2014) 123(1):141–8. doi: 10.1097/AOG.0000000000000057
7. Musa MK, Saga S, Blekken LE, Harris R, Goodman C, Norton C. The prevalence, incidence, and correlates of fecal incontinence among older people residing in care homes: a systematic review. J Am Med Directors Assoc (2019) 20(8):956–62. e8. doi: 10.1016/j.jamda.2019.03.033
8. Milsom I, Gyhagen M. The prevalence of urinary incontinence. Climacteric (2019) 22(3):217–22. doi: 10.1080/13697137.2018.1543263
9. Fritel X, Varnoux N, Zins M, Breart G, Ringa V. Symptomatic pelvic organ prolapse at midlife, quality of life, and risk factors. Obstet Gynecol (2009) 113(3):609. doi: 10.1097/AOG.0b013e3181985312
10. Walker GJ, Gunasekera P. Pelvic organ prolapse and incontinence in developing countries: review of prevalence and risk factors. Int Urogynecol J (2011) 22(2):127–35. doi: 10.1007/s00192-010-1215-0
11. Li T, Zhang Y-J, Zhang H-L, Ding X-H, Yu Z-J, Lu S. Prevalence and risk factors of stress urinary incontinence among perimenopausal women and its influence on daily life in women with sexual desire problem. Curr Med Sci (2019) 39(4):615–21. doi: 10.1007/s11596-019-2082-7
12. Poomalar G, Priyadharshini M. Prevalence of urinary incontinence in reproductive women and its impact on quality of life. Int J Reprod Contracept Obstet Gynecol (2015) 4(5):1353–58. doi: 10.18203/2320-1770.ijrcog20150710
13. Eftekhar T, Ghanbari Z, Kalantari F, Shariat M, Haghollahi F. The frequency of pelvic floor dysfunctions and their risk factors in women aged 40-55. Tehran J Family Reprod Health (2012).
14. Krishna Rao B, Nayak S, Kumar P, Kamath V, Kamath A, Suraj S. Prevalence of pelvic floor dysfunction among married women of Udupi taluk, Karnataka, India. J Women’s Health Care (2015) 4(236):2167–0420.1000. doi: 10.4172/2167-0420.1000236
15. Aytan H, Ertunç D, Tok EC, Yaşa O, Nazik H. Prevalence of pelvic organ prolapse and related factors in a general female population. Turkish J Obstet Gynecol (2014) 11(3):176. doi: 10.4274/tjod.90582
16. Masenga GG, Shayo BC, Msuya S, Rasch V. Urinary incontinence and its relation to delivery circumstances: A population-based study from rural Kilimanjaro, Tanzania. PloS One (2019) 14(1):e0208733. doi: 10.1371/journal.pone.0208733
17. Dheresa M, Worku A, Oljira L, Mengiste B, Assefa N, Berhane Y. One in five women suffer from pelvic floor disorders in Kersa district Eastern Ethiopia: a community-based study. BMC Women’s Health (2018) 18(1):1–8. doi: 10.1186/s12905-018-0585-1
18. Megabiaw B, Adefris M, Rortveit G, Degu G, Muleta M, Blystad A, et al. Pelvic floor disorders among women in Dabat district, northwest Ethiopia: a pilot study. Int Urogynecol J (2013) 24(7):1135–43. doi: 10.1007/s00192-012-1981-y
19. Beketie ED, Tafese WT, Assefa ZM, Berriea FW, Tilahun GA, Shiferaw BZ, et al. Symptomatic pelvic floor disorders and its associated factors in South-Central Ethiopia. PloS One (2021) 16(7):e0254050. doi: 10.1371/journal.pone.0254050
20. Hurissa T, Bekele D. 6. A one-year review of pelvic organ prolapse at St. Paul’s Hospital Millennium Medical College, Addis Ababa Ethiopia. Ethiopian J Reprod Health (2018) 10(1):7–.
21. Akmel M, Segni H. Pelvic organ prolapse in jimma university specialized hospital, southwest Ethiopia. Ethiopian J Health Sci (2012) 22(2):82–92.
22. Henok A. Prevalence and factors associated with pelvic organ prolapse among pedestrian back-loading women in Bench Maji Zone. Ethiopian J Health Sci (2017) 27(3):263–72. doi: 10.4314/ejhs.v27i3.8
23. Cooper J, Annappa M, Quigley A, Dracocardos D, Bondili A, Mallen C. Prevalence of female urinary incontinence and its impact on quality of life in a cluster population in the United Kingdom (UK): a community survey. Primary Health Care Res Dev (2015) 16(4):377–82. doi: 10.1017/S1463423614000371
24. Gjerde JL, Rortveit G, Adefris M, Belayneh T, Blystad A. Life after pelvic organ prolapse surgery: a qualitative study in Amhara region, Ethiopia. BMC Women’s Health (2018) 18(1):1–8. doi: 10.1186/s12905-018-0568-2
25. Handa VL, Blomquist JL, Knoepp LR, Hoskey KA, McDermott KC, Muñoz A. Pelvic floor disorders 5-10 years after vaginal or cesarean childbirth. Obstet Gynecol (2011) 118(4):777. doi: 10.1097/AOG.0b013e3182267f2f
26. Bozkurt M, Yumru AE, Şahin L. Pelvic floor dysfunction, and effects of pregnancy and mode of delivery on pelvic floor. Taiwanese J Obstet Gynecol (2014) 53(4):452–8. doi: 10.1016/j.tjog.2014.08.001
27. Bodner-Adler B, Kimberger O, Laml T, Halpern K, Beitl C, Umek W, et al. Prevalence and risk factors for pelvic floor disorders during early and late pregnancy in a cohort of Austrian women. Arch Gynecol Obstet (2019) 300(5):1325–30. doi: 10.1007/s00404-019-05311-9
28. Masenga GG, Shayo BC, Rasch V. Prevalence and risk factors for pelvic organ prolapse in Kilimanjaro, Tanzania: a population based study in Tanzanian rural community. PloS One (2018) 13(4):e0195910. doi: 10.1371/journal.pone.0195910
29. Central Statistical Agency (CSA) [Ethiopia] and ICF. (2016) Ethiopia Demographic and Health Survey 2016. Addis Ababa, Ethiopia, and Rockville, Maryland, USA: CSA and ICF.
30. Ayele G, Tilahune M, Merdakios B, Animaw Temesgen W. Prevalence and associated factors of home delivery in Arbaminch Zuria District, Southern Ethiopia: community based cross sectional study. Sci J Public Health (2015) 3(1):6–9. doi: 10.11648/j.sjph.20150301.12
31. Worku Sharew, Teshome S. Ethiopian Universities Health and Demographic Surveillance System Network Data Sharing and Release Policy. (2013).
32. Lukacz ES, Lawrence JM, Buckwalter JG, Burchette RJ, Nager CW, Luber KM. Epidemiology of prolapse and incontinence questionnaire: validation of a new epidemiologic survey. Int Urogynecol J (2005) 16(4):272–84.
33. Ethiopian Public Health Institute (EPHI) [Ethiopia] and ICF. (2021) Ethiopia Mini Demographic and HealthSurvey 2019: Final Report. Rockville, Maryland, USA: EPHI and ICF.
34. Lema Z, Berhane Y, Meskele M. Determinants of pelvic organ prolapse among gynecological cases in Wolaita Sodo university referral teaching hospital, southern Ethiopia. J Biol Agric Healthc. (2015) 5(21):1–10.
35. Rortveit G, Daltveit AK, Hannestad YS, Hunskaar S. Urinary incontinence after vaginal delivery or cesarean section. New Engl J Med (2003) 348(10):900–7. doi: 10.1056/NEJMoa021788
36. Schreiber Pedersen L, Lose G, Høybye MT, Elsner S, Waldmann A, Rudnicki M. Prevalence of urinary incontinence among women and analysis of potential risk factors in Germany and Denmark. Acta Obstet Gynecol Scand (2017) 96(8):939–48. doi: 10.1111/aogs.13149
37. Handa VL, Blomquist JL, McDermott KC, Friedman S, Muñoz A. Pelvic floor disorders after childbirth: effect of episiotomy, perineal laceration, and operative birth. Obstet Gynecol (2012) 119(2 Pt 1):233. doi: 10.1097/AOG.0b013e318240df4f
38. Memon HU, Handa VL. Vaginal childbirth and pelvic floor disorders. Women’s Health (2013) 9(3):265–77. doi: 10.2217/WHE.13.17
39. van Meegdenburg MM, Trzpis M, Broens PM. Fecal incontinence and parity in the Dutch population: A cross-sectional analysis. United Eur Gastroenterol J (2018) 6(5):781–90. doi: 10.1177/2050640618760386
40. Ghafouri A, Alnaimi AR, Alhothi HM, Alroubi I, Alrayashi M, Molhim NA, et al. Urinary incontinence in Qatar: A study of the prevalence, risk factors and impact on quality of life. Arab J Urol (2014) 12(4):269–74. doi: 10.1016/j.aju.2014.08.002
41. Ghandour L, Minassian V, Al-Badr A, Abou Ghaida R, Geagea S, Bazi T. Prevalence and degree of bother of pelvic floor disorder symptoms among women from primary care and specialty clinics in Lebanon: an exploratory study. Int Urogynecol J (2017) 28(1):105–18. doi: 10.1007/s00192-016-3080-y
Keywords: pelvic floor disorder, women, factors, Gamo Zone, southern Ethiopia
Citation: Kebede BN, Hayelom DH, Birgoda GT, Yimer AA, Mesfin BA, Tetema MD, Alemu SS and Getahun KB (2023) Prevalence of pelvic floor disorder and associated factors among women in Arba Minch Health and Demographic Surveillance Site, Gamo Zone, Southern Ethiopia, 2021. Front. Urol. 3:1196925. doi: 10.3389/fruro.2023.1196925
Received: 30 March 2023; Accepted: 22 August 2023;
Published: 11 September 2023.
Edited by:
Nazema Siddiqui, Duke University, United StatesReviewed by:
Sakineh Hajebrahimi, Tabriz University of Medical Sciences, IranCopyright © 2023 Kebede, Hayelom, Birgoda, Yimer, Mesfin, Tetema, Alemu and Getahun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Berhanu Negese Kebede, YmVyaGFudS5uZWdlc2VAYW11LmVkdS5ldA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.