
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Urol., 23 September 2022
Sec. Pediatric, Adolescent and Developmental Urology
Volume 2 - 2022 | https://doi.org/10.3389/fruro.2022.993710
This article is part of the Research TopicReconstruction, Repairing and Tissue Engineering Of The Urinary TractView all 4 articles
Introduction: Calyceal diverticula (CD) are cystic structures within the renal parenchyma that likely result from inappropriate interaction between the ureteric bud and metanephric blastema during development and, if obstructed, may cause problems that include flank pain, urinary tract infections, gross hematuria, or nephrolithiasis. Herein, we present the results of percutaneous management of an obstructed calyceal diverticulum causing recurrent flank pain and infections in a 17-year-old girl.
Case summary: The patient initially presented with febrile pyelonephritis, at which time an ultrasound was concerned about an abscess versus an infected cyst. She underwent percutaneous drain placement with IV antibiotics and improved. Three years later, she re-presented with similar symptoms. After a similar treatment course, a sinogram revealed communication of the cystic-appearing lesion with the proximal ureter, confirming a diagnosis of a calyceal diverticulum. The patient opted for endoscopic management. An initial attempt at ureteroscopy was unsuccessful due to both the small ureteral caliber as well as the stenotic infundibular os. A repeat attempt at percutaneous management revealed a stenotic infundibulum that could not accommodate a wire. A holmium laser fiber was used to create a neoinfundibulum from the diverticulum to the renal pelvis adjacent to the stenotic infundibular os. Follow-up retrograde pyelography and ultrasonography confirmed an interval decrease in size of the infundibulum, and the patient’s infectious symptoms and pain resolved.
Discussion: CD is a rare anomaly and is infrequently reported in the pediatric population. Percutaneous management of CD in adults has been well described in the literature. Direct percutaneous management of CD involves access to the CD and ablation of the CD cavity to prevent re-accumulation of urine. Previous studies done in adults have suggested that stone-free rates from this management range from about 80% to 100%. Diverticular obliteration, as measured by postoperative CT scans, also appears to be feasible in a percutaneous, antegrade approach, with complete diverticular obliteration rates ranging from 60% to 100%. Our case demonstrated a successful decrease in size from the original diverticulum (9 cm) to a smaller size (2 cm). Future studies with longer follow-ups will need to ascertain if percutaneous management of symptomatic CD is effective in the pediatric population.
Calyceal diverticula are cystic structures within the renal parenchyma that likely result from inappropriate interaction between the ureteric bud and metanephric blastema during development (1). These nonsecretory structures communicate with the calyceal or pelvic system through a narrow infundibulum and are passively filled with urine in a retrograde fashion (1). Often mistaken for simple renal cysts on ultrasound, calyceal diverticula were traditionally diagnosed on intravenous urograms, but more recently on CT scans or MRI in an excretory phase (2). Most cases are asymptomatic; however, some patients do present with symptoms that include flank pain, urinary tract infections, or gross hematuria (3). Herein, we present the case of a teenager who initially presented with what was thought to be an infected cyst and was eventually found to have calyceal diverticulum requiring percutaneous endoscopic management.
The patient, a 14-year-old girl, initially presented to the emergency department with right-sided flank pain, nausea, and emesis. On presentation to the hospital, she was found to be febrile to 102.1°F and tachycardic to 120 bpm. She subsequently underwent a renal ultrasound, which was notable for an 8.1-cm cystic lesion in the right superior upper pole without hydronephrosis (Figure 1A). The considered differential included an obstructed upper pole moiety of a duplicated renal collecting system versus an infected renal cyst or renal abscess. She was admitted to the hospital for the initiation of IV antibiotics.
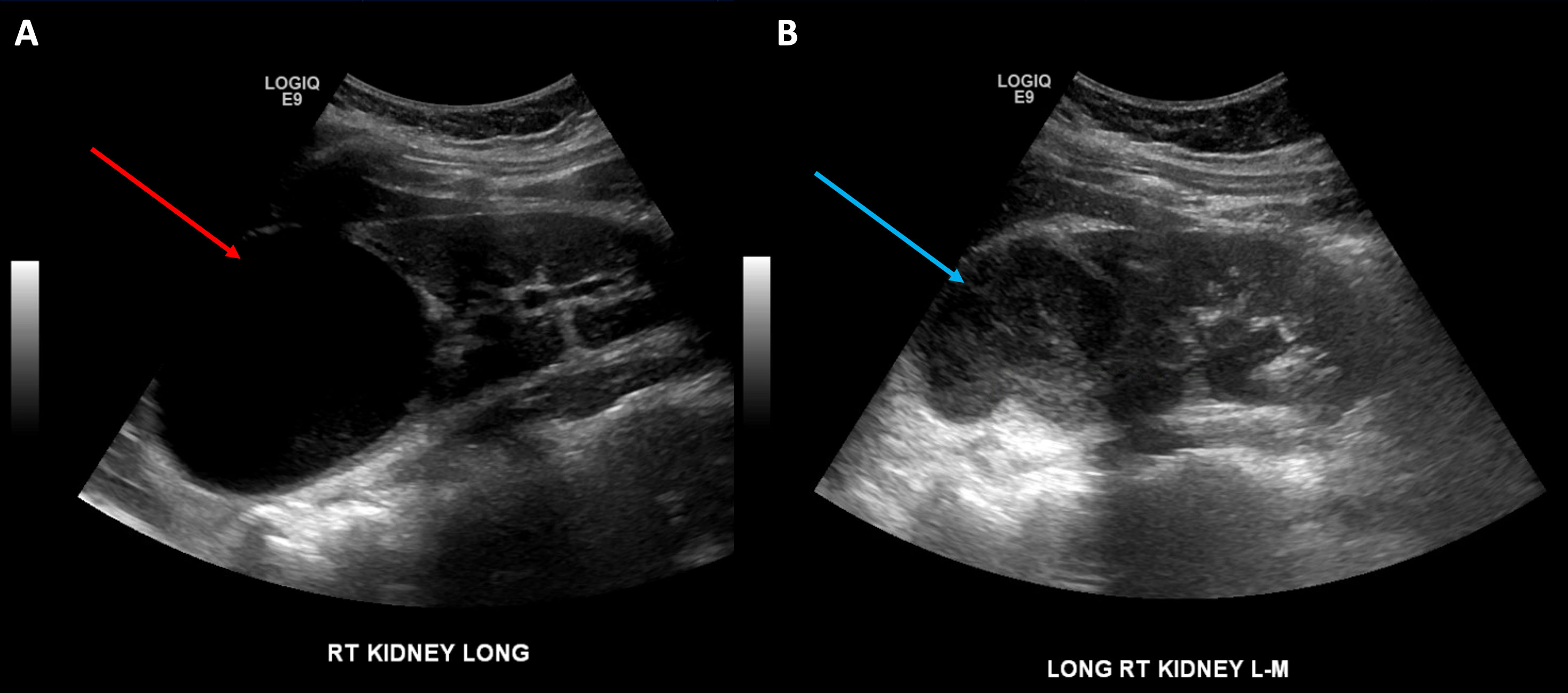
Figure 1 View of the infected cyst: (A) initial presentation prior to drain placement (red arrow) and (B) second presentation prior to drain placement (blue arrow).
However, despite the IV antibiotics, the patient continued to have high-grade fevers with worsening leukocytosis and worsening flank pain. Because of this, the decision was made to place an image-guided drain into the upper pole fluid collection. Under ultrasound guidance, a 10-Fr APD drain was placed into the upper pole fluid collection with a return of about 200cc of seropurulent fluid. Following the drain placement, her flank pain resolved, and she remained afebrile on antibiotics. Her urine and drain culture both grew over 100,000 colony-forming units of Proteus mirabilis, and she was discharged on a 10-day course of culture-appropriate antibiotics. Two weeks after drain placement, the presumed abscess cavity was evaluated under fluoroscopy, which showed resolution. The drain was then subsequently removed.
She then re-presented to the emergency department 3 years later with similar symptoms of flank pain, nausea, emesis, and a recently diagnosed urinary tract infection (Proteus mirabilis). Renal ultrasound at that time demonstrated a 6.8-cm cystic upper pole lesion with complex external fluid concerning repeat infection of an upper pole cyst versus internal hemorrhage (Figure 1B). A CT angiography of the abdomen and pelvis was obtained to determine if there was any active hemorrhage into the renal cyst. This scan demonstrated a 5.6-cm complex cystic lesion in the right upper pole without any active extravasation of contrast (Figures 2A, B). She was again admitted for IV antibiotics and underwent ultrasound-guided placement of a 12-Fr pigtail drain into her right upper pole abscess with an aspiration of 75 cm3 of purulent fluid. Drain cultures again grew >100,000 colony-forming units of Proteus mirabilis, and she was discharged on a 14- day course of culture-appropriate antibiotics.
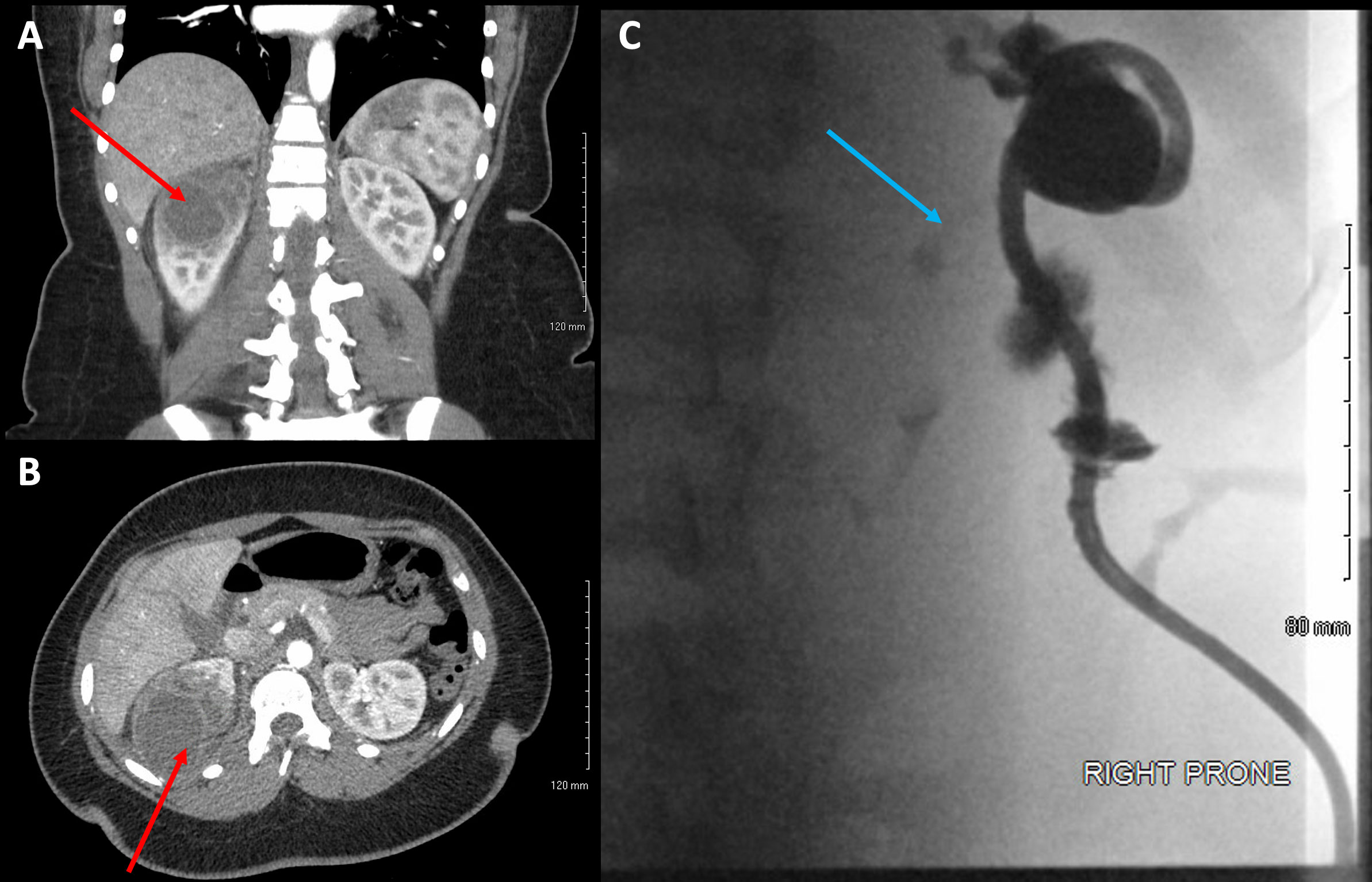
Figure 2 (A) Views of the diverticulum on coronal CT imaging (red arrow), (B) axial CT imaging (red arrow), and (C) ureteral contrast on fluoroscopic sinogram (blue arrow).
In order to prevent future recurrence, she was brought back to the hospital 7 days after drain placement for planned sclerotherapy of the cyst. However, the sinogram at that time demonstrated a trace amount of contrast reaching the proximal ureter, raising concern for a calyceal diverticulum rather than a true cyst and precluding sclerotherapy (Figure 2C). The treatment plans were discussed with the patient and her family, which included retrograde and antegrade ablation of the diverticulum versus laparoscopic calyceal diverticulectomy. The patient and her family had opted to start off with retrograde calyceal diverticulum ablation, with or without possible percutaneous antegrade ablation, due to the shortened required convalescence from work.
We initially attempted retrograde diverticulum ablation but were unable to pass 5–9 Fr instruments or ureteroscopes due to a narrow caliber distal ureter. This appeared to be an intrinsically narrow ureter, without focal evidence of stricture. Attempts to fluoroscopically cannulate the calyceal diverticulum with a wire in a retrograde fashion were similarly unsuccessful, due to a long, narrow, and circuitous infundibulum seen on retrograde pyelography. Therefore, a ureteral stent and nephrostomy tube were placed for planned antegrade ablation in the future.
About 2 weeks later, we brought the patient back for the antegrade ablation. We began our antegrade ablation by exchanging her ureteral stent for a 5-Fr open-ended ureteral access catheter. Her existing renal access was dilated and exchanged for a 30-Fr sheath to accommodate a 26-Fr rigid nephroscope. Dilute betadine was injected retrograde through the ureteral access catheter, which showed that the infundibular os was at the inferior aspect of the diverticulum. However, this was severely stenotic and was unable to be cannulated using a variety of wires or catheters. Therefore, using the betadine injection as a guide, we used a 400-nm holmium laser fiber to ablate diverticular tissue adjacent to the os until the renal pelvis was visualized. Once this communication had been established, we passed a wire from below and brought it through our access sheath, giving us through-and-through access. The infundibular os was then dilated using an 8-Fr fascial dilator. We then proceeded to ablate the diverticulum tissue using a Bugbee electrode, with care to avoid any collecting system injury. Once complete, we placed a 6-Fr-×-28-cm double-J ureteral stent in antegrade fashion across the infundibulum to keep the os open (Figure 3A). A 22-Fr Foley was placed through our percutaneous access site for maximal drainage and subsequently removed on postoperative day 2. She was discharged on postoperative day 2.
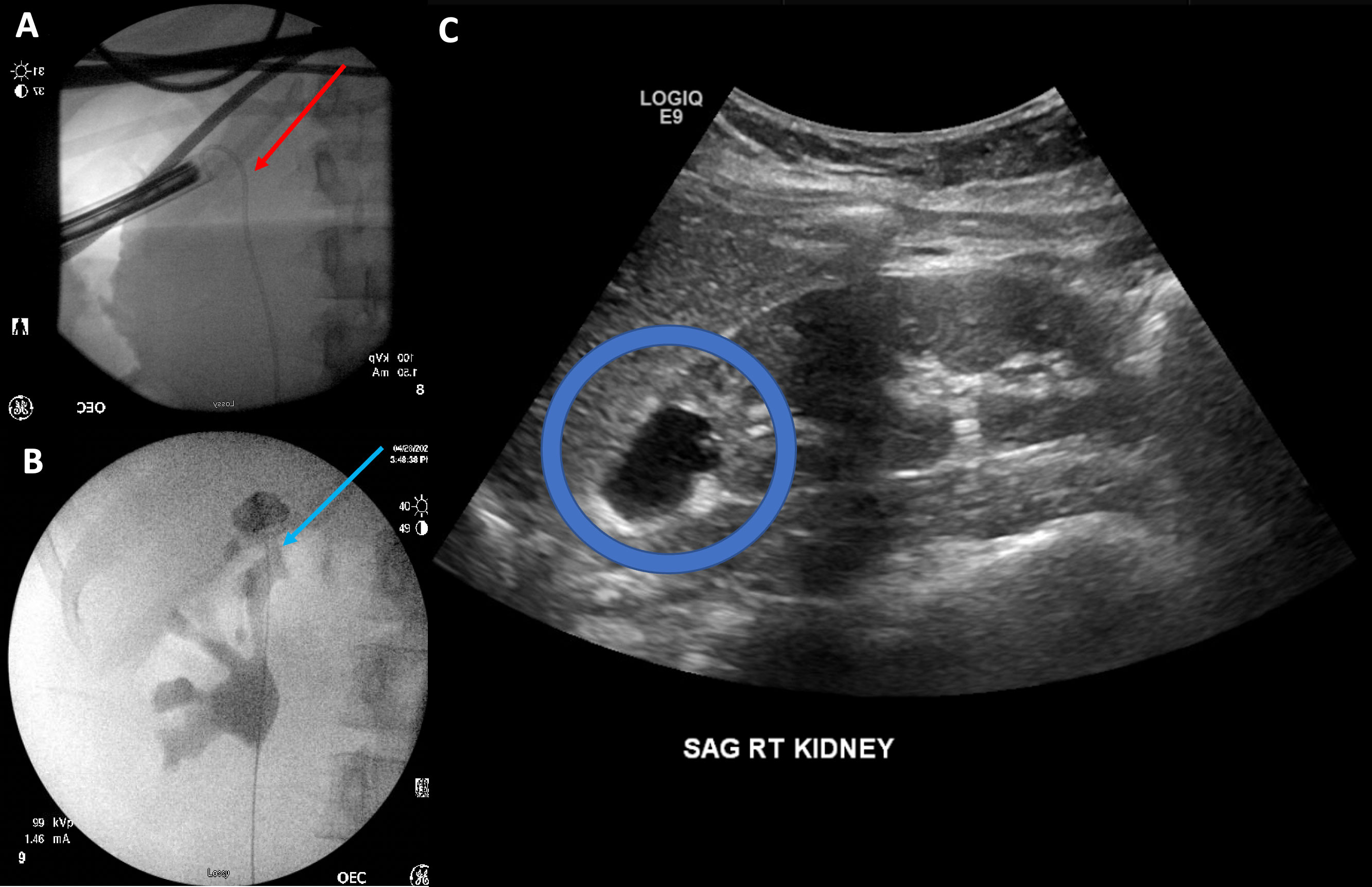
Figure 3 (A) Stent placement across the neoinfundibulum (red arrow). (B) View of the infundibulum after stent removal (blue arrow). (C) Appearance of the diverticulum at follow-up ultrasound (blue circle).
Two months following the ablation, the patient was brought back to the operating room for retrograde pyelograms. This demonstrated opacification of the entire renal pelvis along with the diverticulum (Figure 3B). The infundibulum was noted to have a wide neck, and the diverticulum appeared to drain well along with the rest of the kidney. The stent was then subsequently removed. Repeat kidney ultrasound 2 months following ablation showed that this diverticulum was smaller, measuring 2 cm compared to the original 9 cm when compared to previous images (Figure 3C). The patient has since been asymptomatic. She will undergo a repeat ultrasound in another 6 months.
Calyceal diverticula (CD) are rare congenital cystic structures often mistaken for simple cysts. These nonsecretory cystic lesions passively fill with urine via a narrow infundibulum that communicates with the main collecting system (1). While the exact etiology of these lesions is unknown, it is generally accepted that these structures form due to inappropriate interactions between the ureteric bud and metanephric blastema (4, 5). Despite being a congenital malformation, the data regarding the management of CD in the pediatric population remain sparse.
CD are classically divided into two types, as described by Wulfoshn (5). Type 1 CD communicates with a minor calyx, generally occurs at the poles, and is the most common type. Type II tends to be larger and communicates directly with the renal pelvis or a major calyx. An alternative classification schema was proposed by Dretler based on the length and width of the infundibulum (ranging from type I with an open mouth and short neck to type IV with an obliterated neck) (6). In addition to anatomic descriptions, his classification method came with suggested interventions for each type.
Despite these classification schemas, establishing a diagnosis of CD is often unclear. Physical exams, history, or laboratory exams are not specific to the diagnosis of CD. Clinicians must rely on imaging to make the diagnosis. Even then, differentiating between CD and other cystic structures can be difficult and requires a high index of suspicion. On ultrasound, CD appears nearly identical to cysts, with similar echotexture and appearance (1). Increasing size, stones, or sludge in the cyst can suggest a CD, but none of these features are diagnostic. Cross-sectional imaging with computed tomography (CT) or magnetic resonance imaging (MRI) urograms can be particularly helpful in diagnosing CD. Given that CD rely on the infundibulum to passively fill with urine, a CD can appear on urograms to fill in a delayed fashion compared to the rest of the collecting system. However, in patients whose CD have an obliterated infundibular neck, this may not even occur (7).
The majority of patients with CD are asymptomatic and do not require treatment. However, in patients with recurrent urinary tract infections, gross hematuria, nephrolithiasis, chronic pain, or a decrease in renal function, surgical intervention may be required. Adult literature has primarily focused on stone clearance. Pediatric data remain limited. The largest series comes from McGarry and colleagues from the Children’s Hospital of Philadelphia (8). Of the 757 patients they saw for renal cysts, 43 (5%) were ultimately found to have a CD and 25 of these 43 (58%) ultimately required surgical intervention. Pain and increasing size of CD were the two clinical variables associated with an increased likelihood of requiring intervention. Surgical intervention in their study included ureteroscopic ablation (17 patients) and laparoscopic/robotic deroofing, ablation, and decortication (eight patients). They found that the success rate of ureteroscopic intervention was 40% compared to 100% among the eight patients who underwent laparoscopic/robotic intervention (p = 0.016). However, despite being the largest series, this remains a relatively small, retrospective cohort with a high likelihood of significant bias. Despite the differences in success rates, our patient opted to attempt endoscopic intervention due to the shorter required convalescence from her employment, with the understanding that laparoscopic excision could be required if her initial treatment was unsuccessful.
Percutaneous management of CD in adults has been well described in the literature (9–12). Direct percutaneous management of CD involves direct percutaneous access into the CD with the extraction of stones and ablation of the CD cavity to promote closure of the CD and prevent re-accumulation of urine. Previous studies done in adults have suggested that stone-free rates from this management range from about 80% to 100% (2). Diverticular obliteration, as measured by postoperative CT scans, also appears to be feasible in a percutaneous, antegrade approach, with complete diverticular obliteration rates ranging from 60% to 100% (9, 12, 13).
However, as of 2022, there have been no published studies or reports regarding the use of percutaneous, antegrade ablation of a calyceal diverticulum. Herein, we present the case of a young adolescent girl who was initially thought to have an infected renal cyst. She represented 3 years later with a similar presentation. Further evaluation at that time demonstrated the presence of a calyceal diverticulum, which was successfully managed with percutaneous ablation. We must acknowledge that our patient was 17 years old at the time of intervention, and we would need to investigate further the utility of percutaneous endoscopic ablation in the prepubertal population and the associated challenges. Future studies are needed to determine the most effective method of management of pediatric calyceal diverticula.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Waingankar N, Hayek S, Smith AD, Okeke Z. Calyceal diverticula: A comprehensive review. Rev Urol (2014) 16:29.
2. Ochoa Santiago Y, Sanguesa Nebot C, Pico Aliaga S, Serrano Durba A., Ortega Lopez P. Calyceal diverticula in children: Imaging findings and presentations. Radiologia (2018) 60:5. doi: 10.1016/j.rxeng.2018.05.001
3. Timmons JW Jr., Malek RS, Hattery RR, Deewerd J. H. Caliceal diverticulum. J Urol (1975) 114:6. doi: 10.1016/S0022-5347(17)66930-1
4. Middleton AW Jr., Pfister RC. Stone-containing pyelocaliceal diverticulum: Embryogenic, anatomic, radiologic and clinical characteristics. J Urol (1974) 111:2. doi: 10.1016/S0022-5347(17)59872-9
6. Dretler S. A new useful endourologic classification of calyceal diverticula. J Endourol (1992) 6:81.
7. Davidson AJ. Davidson's radiology of the kidney and genitourinary tract. Philadelphia, PA: WB Saunders Company (1999).
8. McGarry L, Sahadev R, Hogan G, Otero H, Srinivasan A.K, Shukla A.R. Calyceal diverticula in children: laparoscopic marsupialization is the optimal intervention. J Pediatr Urol (2020) 16:221.e1. doi: 10.1016/j.jpurol.2020.01.014
9. Canales B, Monga M. Surgical management of the calyceal diverticulum. Curr Opin Urol (2003) 13:255. doi: 10.1097/00042307-200305000-00015
10. Alwaal A, Azhar RA, Andonian S. Percutaneous holmium laser fulguration of calyceal diverticula. Case Rep Urol (2012) 716786:2012. doi: 10.1155/2012/716786
11. Monga M, Smith R, Ferral H, Thomas R. Percutaneous ablation of caliceal diverticulum: long-term followup. J Urol (2000) 163:28. doi: 10.1089/089277902320913233
12. Kim SC, Kuo RL, Tinmouth WW, Watkins S, Lingeman J.E. Percutaneous nephrolithotomy for caliceal diverticular calculi: A novel single stage approach. J Urol 173 (1194) 2005:1194–8. doi: 10.1155/2012/716786
Keywords: calyceal diverticulum, PCNL, infundibulum, nephroscopy, CAKUT (Congenital Anomalies of the Kidney and Urinary Tract)
Citation: Das A, Blumenthal Z, Monroe E, Farhat WA and Bhatia VP (2022) Percutaneous endoscopic creation of a neoinfundibulum for a calyceal diverticulum: A case report and review of the literature. Front. Urol. 2:993710. doi: 10.3389/fruro.2022.993710
Received: 14 July 2022; Accepted: 23 August 2022;
Published: 23 September 2022.
Edited by:
Alessandro Morlacco, Università di Padova, ItalyReviewed by:
Lisieux Jesus, Hospital Universitário Antônio Pedro, BrazilCopyright © 2022 Das, Blumenthal, Monroe, Farhat and Bhatia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vinaya P. Bhatia, YmhhdGlhQHVyb2xvZ3kud2lzYy5lZHU=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.