
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Urol., 13 September 2022
Sec. Male Urology
Volume 2 - 2022 | https://doi.org/10.3389/fruro.2022.986255
This article is part of the Research TopicInsights in Male Urology: Volume IView all 5 articles
 Nisarg V. Gandhi1†
Nisarg V. Gandhi1† Dina N. Murad1†
Dina N. Murad1† Sean A. Hebert1†
Sean A. Hebert1† Monica Morgan2†
Monica Morgan2† Duc T. Nguyen3†
Duc T. Nguyen3† Edward A. Graviss3,4†
Edward A. Graviss3,4† Hassan N. Ibrahim1*†
Hassan N. Ibrahim1*†Some kidney donors experience testicular pain after donation. We studied the presence of testicular pain in male donors from The Renal and Lung Donor Evaluation (RELIVE) Study which investigated short- and long-term outcomes of 8922 kidney donors. Of the 2551 male donors with available testicular status data included in the analysis, 54 (2.12%) developed testicular pain 19 days (IQR 7, 40) after donation: 34 had testicular pain only, 6 had epididymitis, and 14 had both. Donors developing pain were 4 years older and pain occurred more often in those undergoing laparoscopic nephrectomy; 3.6% vs. 1.1% for open nephrectomy. Non-Hispanic White ethnicity and undergoing laparoscopic nephrectomy were associated with increased risk of testicular pain; RR 5.56 (95% CI 1.35, 22.84), p=0.02, RR 3.11 (95% CI 1.71, 5.65), p<0.001, respectively. Laterality of nephrectomy, however, was not associated with increased risk of testicular pain. Testicular pain is not infrequent and contrary to previous reports, left nephrectomy was not associated with a higher incidence of testicular pain. Donors should be routinely asked about this potentially bothersome complication.
Post-operative complications after donor nephrectomy have been studied extensively (1, 2). Short term complications include pain, infection, thrombotic events, anesthesia reactions, hernia, intestinal obstruction, and testicular pain. While many of these complications have been studied, testicular pain has not received much attention.
The etiology of testicular pain has been postulated to be largely due to ligation of the gonadal vein and the prevailing belief is that this complication is less likely to occur after right uninephrectomy (3). Most retrospective studies addressing testicular pain and epididymitis have either reported a low incidence (0.02%-2.86%) or did not address it at all (1, 2, 4–10). In contrast, prospective studies consistently reported a much higher incidence of testicular pain (44- 55%) (3, 11, 12). Since previous studies combined have totaled less than 100 cases of testicular pain after donation, more information is needed to determine the incidence of this complication as this data would aid in the informed consent process for kidney donors. Herein, we describe the incidence of testicular pain after kidney donation in 2551 donors and explore factors associated with its development.
The Renal and Lung Living Donor Evaluation (RELIVE) Study was a National Institutes of Health sponsored effort that studied short- and long-term outcomes of 8922 kidney donors from the University of Minnesota, Mayo Clinic-Rochester and the University of Alabama-Birmingham, as previously described (13). Baseline demographic, laboratory measurements, operative and perioperative complications at donation were abstracted from centers’ records. Family history of hypertension, diabetes mellitus, kidney disease, stroke, or heart disease in donors’ first-degree relatives were recorded. For ascertainment of long-term post-donation events, donors were contacted between 2010-2012 via telephone and multiple mailings for health updates, Quality of Life surveys and any laboratory data that took place after donation was obtained. Post-donation events were also supplemented from centers’ records. The following post-operative variables were abstracted directly from operative reports, hospital stay and post-operative follow-up visits: type of nephrectomy (laparoscopic vs. open), laterality of donated kidney, lowest post-operative hematocrit, intensive care unit admission, time in operating room, requirement for intraoperative blood transfusions, estimated blood loss volume, occurrence, location and timing of testicular pain, epididymitis, or both. Conventional angiograms, computed tomography and magnetic resonance angiogram reports were reviewed by study personnel to provide number of renal arteries in both donated and non-donated kidneys. The donor selection process was highly comparable between centers and none of the centers accepted any donors with proteinuria, measured glomerular filtration rate or creatinine clearance <80 ml/min. This study was exempt from institutional review board approval as it used de-identified data from the publicly available RELIVE Study dataset. The dataset is available at https://immport.org/shared/study/SDY289.
Baseline characteristics are reported as frequencies and proportions for categorical variables and median and interquartile range (IQR) for continuous ones. Differences between donors with and without testicular pain were compared using chi-squared for categorical variables and the Wilcoxon rank-sum test for continuous variables. Univariate, bivariate and multivariate generalized linear model (GLM) were used to determine factors associated with having testicular pain. The variable selection for the GLM model was conducted using the Least Absolute Shrinkage and Selection Operator (LASSO) method with the cross-validation selection option and by the clinical importance of the identified variable (14, 15). All analyses were performed on Stata version 17.0 (StataCorp LLC, College Station, TX, USA). A p-value of <0.05 was considered statistically significant.
There was a total of 8922 live kidney donations between 1963-2007 at the 3 participating centers. After excluding 5023 female donors and an additional 1348 male donors due to missing data in the medical records on the occurrence of testicular pain, a total of 2551 donors were studied (Figure 1). Excluded male donors were generally younger, predominantly White, spent a longer time in the operating room, and were more likely to have undergone open nephrectomy (Table 1). Median age of the 2551 included male donors was 38 years, 81% were non-Hispanic White, 13% were non-Hispanic Black, 1.4% were Hispanic, 0.9% were Asian and 3.7% were categorized as other or unknown. The majority (81%) donated to a family member. The median body mass index (BMI) was 27 kg/m2 and median estimated glomerular filtration rate (eGFR) at donation was 87 ml/min/1.73m2.
Of the 2551 donors included in the study, 54 (2.12%) developed ipsilateral testicular pain 19 days (IQR 7, 40) after donation; 34/54 (63.0%) developed testicular pain only, 6/54 (11.1%) developed epididymitis only and 14/54 (25.9%) had both testicular pain and epididymitis. Donors with testicular pain were older (42 versus 38 years), non-Hispanic White (96%), and were more likely to have undergone a left nephrectomy (82% versus 72%), Table 2. The incidence of testicular pain and trend of open vs. laparoscopic nephrectomy over time is shown in Figure 2. Given that laparoscopic nephrectomy is becoming the norm, a sub-analysis was done including only donors undergoing laparoscopic nephrectomy. The baseline characteristics are shown in Table S1. In kidney donors with testicular pain, 38 (70.4%) underwent laparoscopic nephrectomy and 32 (84%) underwent left nephrectomy, p =0.61 for left vs. right. In the remaining 16 (29.6%) donors who underwent open nephrectomy, 12 (75%) underwent left nephrectomy, p =0.42 for left vs. right (Figure 3).
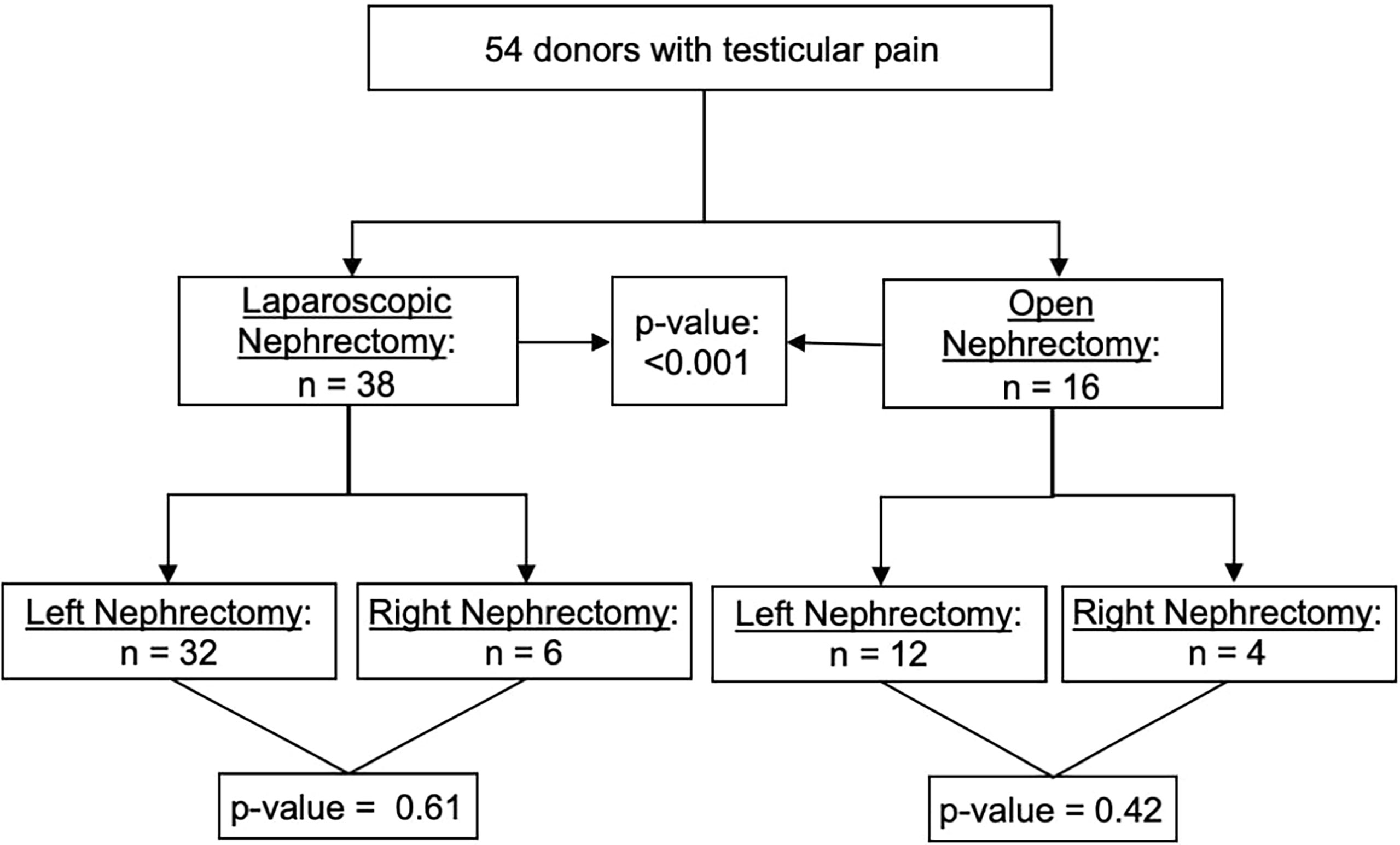
Figure 3 Incidence of testicular pain: right vs. left nephrectomy and open vs. laparoscopic nephrectomy.
In the univariable generalized linear model, there was no significant association between testicular pain and BMI at donation, lipid profile, serum creatinine, eGFR at donation, or lowest post-operative hematocrit. The unadjusted relative risk (RR) of developing testicular pain in non-Hispanic White donors was 6.07 (95% CI 1.48, 24.84), p=0.01. Laparoscopic nephrectomy was associated with a RR of 3.38 (95% CI 1.90, 6.04), p<0.001. Longer operative time was also associated with testicular pain; RR 1.30 (95% CI 1.13, 1.48), p<0.001. Lastly, the presence of multiple renal arteries in the donated kidney was associated a higher likelihood of having testicular pain; RR 2.03 (95% CI 1.11, 3.68), p=0.02 (Table 3).
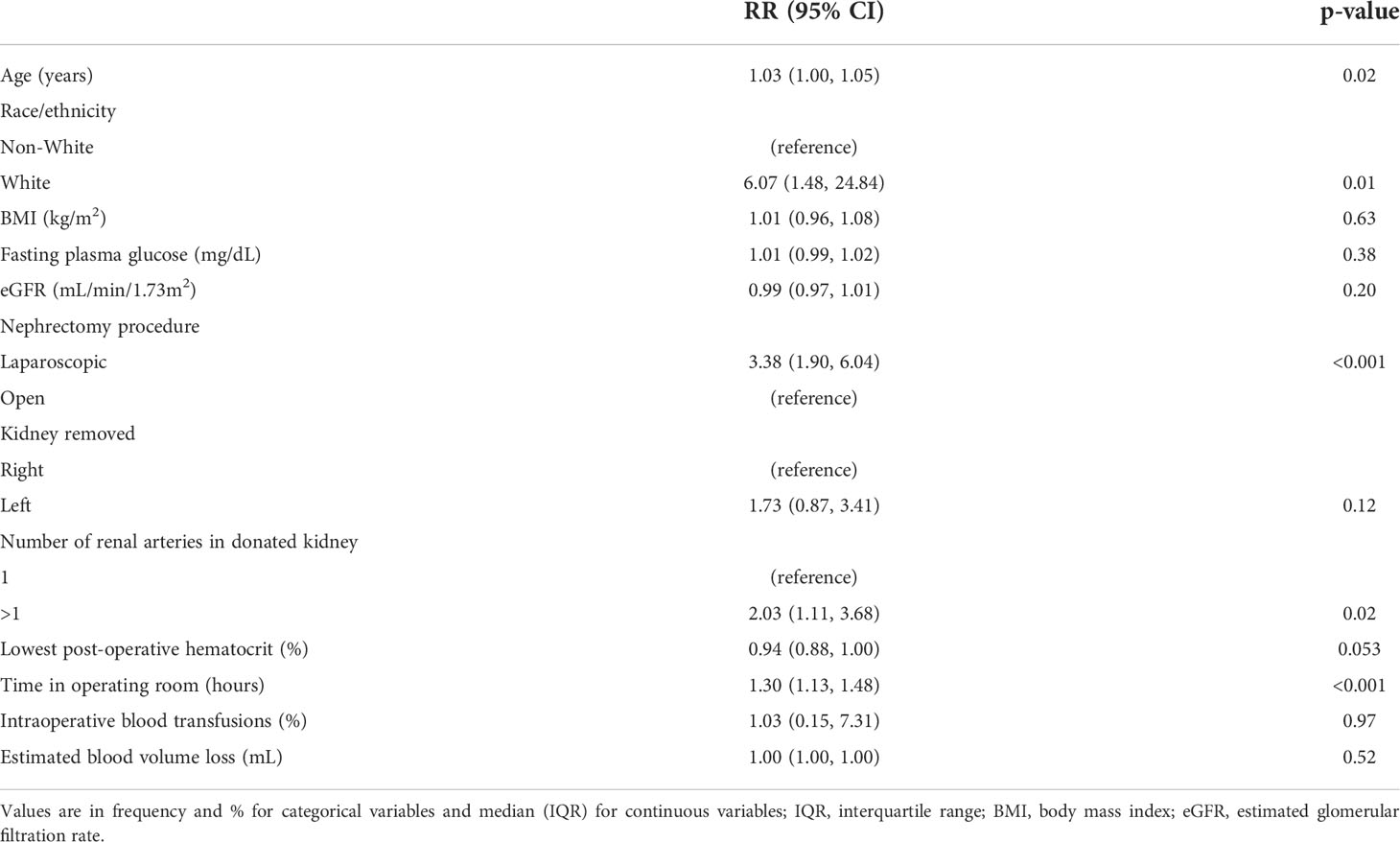
Table 3 Characteristics associated with testicular pain: univariable generalized linear model (GLM).
Multiple renal arteries in the donated kidney was more prevalent in those with testicular pain; 25.9% versus 14.5% (p=0.02). In addition, time spent in the operating room also was longer in donors with testicular pain; 4.2 vs 3.7 hours (p<0.001). Estimated blood loss volume, intensive care unit admission and need for intra-operative blood transfusions were similar in those with and without testicular pain (Table 4).
Post-donation testicular pain was more likely to be experienced by non-Hispanic White donors; RR 5.56 (95% CI 1.35, 22.84), p=0.02 and those undergoing laparoscopic nephrectomy; RR 3.11 (95% CI 1.71, 5.65), p<0.001. Neither laterality of the kidney removed nor the number of renal arteries in the donated kidney were associated with an increased risk of pain; RR 1.35 (95% CI 0.67, 2.72), p=0.40 and 1.73 (95% CI 0.94, 3.18), p=0.08, respectively (Table 5). A sub-analysis is done for only donors undergoing a laparoscopic nephrectomy and shown in Table S2.
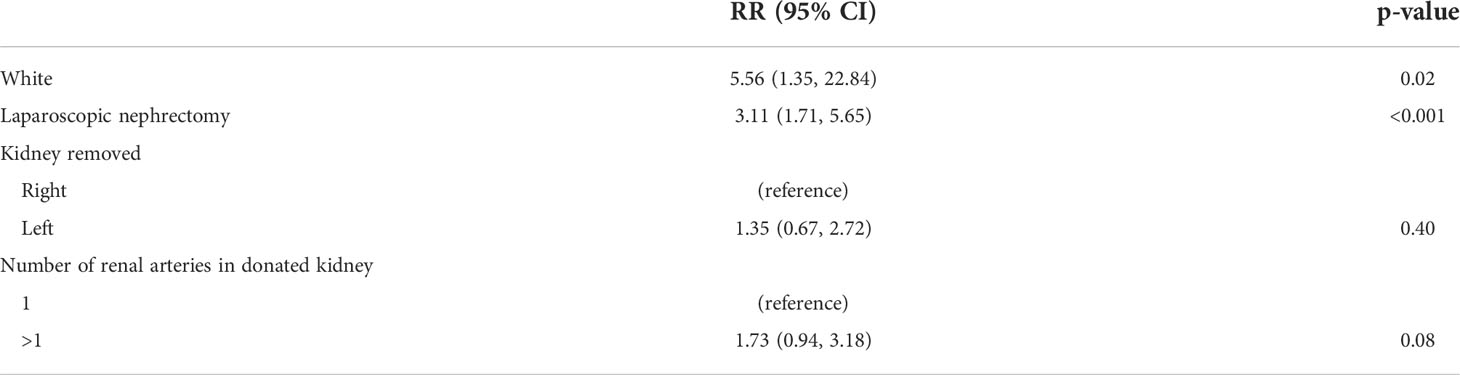
Table 5 Characteristics associated with having testicular pain: multivariable generalized linear model (GLM).
These results show that the incidence of testicular pain is 2.12% in kidney donors in whom the outcome was documented in the medical records. Correlates of testicular pain include being non-Hispanic White and undergoing a laparoscopic nephrectomy. Having a left nephrectomy, the number of renal arteries in the donated kidney or the amount of blood loss intra-operatively were not associated with this outcome.
A few previous reports have studied testicular pain after donation. The results from these studies are summarized in Table 6. Of the 3 largest retrospective registry studies from Switzerland and the United States, only 2 reported on the occurrence of testicular pain; 0.49% (8 donors out of 1649) in the study from Switzerland and 0.02% (3 donors out of 14694) in the U.S. study (1, 2, 8). In 6 other retrospective studies totaling 3837 donors, the incidence ranged from 0.48% to 2.36% (4–7, 9, 10). We are aware of 3 prospective studies addressing peri- and post-operative complications after donation. Garcia-Ochoa et al. studied post-operative complications in 1042 donors from 12 Canadian and 5 Australian centers using structured case report forms, but did not comment on testicular pain (12). The other two prospective studies by Jalali et al. and Gjerston et al. reported an incidence of 44% and 55%, respectively (3, 12). This significant difference in incidence between prospective and retrospective studies is perhaps largely due to recall bias as testicular pain is often mild and self-limited. One should consider the possibility that we do not ask male kidney donors about this complication routinely and perhaps that should change.
Non-Hispanic White donors were more likely to complain of testicular pain. Previously, Lentine et al. found that African American were more likely to have perioperative complications after kidney donation (2). Similar observations were made by Segev et al. (1) Testicular pain, however, was more likely to occur in White donors in this analysis. Of note, the study by Segev et al. did not specifically comment on testicular pain (1).
Donors with multiple renal arteries in the donated kidney were more likely to develop testicular pain (25.9% vs 14.5%, p=0.02). In a recent report by Gandhi et al., donors with multiple renal arteries were less likely to undergo a left nephrectomy (83% vs. 51%, p<0.001) (16). If left nephrectomy is indeed more likely to be associated with testicular pain as commonly held, one would’ve expected the incidence of testicular pain to be lower in individuals with multiple renal arteries. It is conceivable that individuals with multiple renal arteries would likely require a more complex procedure resulting in a longer surgical time. Our study did indeed find that donors who developed testicular pain spent on average 4.2 hours in the operating room versus 3.7 hours in those who did not develop testicular pain (p<0.001).
The biology of the development of testicular pain following nephrectomy is not entirely understood. Several explanations have been offered. In the prospective analysis of testicular pain by Gjerston et al. of 64 individuals who underwent nephrectomy (20 of whom were kidney donors), 55% of donors developed testicular pain compared to 20% in those undergoing nephrectomy for a non-donation related indication (3). It is conceivable that the lesser likelihood of testicular pain after laparoscopic nephrectomy for purposes other than donation stems from the common practice of not dividing the gonadal vein during non-donor nephrectomy and the fact that the ureter is divided early during the surgery thus avoiding the retraction of the ureteric pedicle (10). Of note, in the study by Gjerston et al., 13 (33%) of patients who had ligation of the gonadal vein experienced testicular pain while only 1 (3.4%) of the donors without gonadal vein ligation experienced testicular pain (3). The left gonadal vein drains into the left renal vein while the right gonadal vein drains into the inferior vena cava and current literature suggests that testicular pain is more likely to occur in kidney donors undergoing a left nephrectomy. The removal of the gonadal vein and associated lymphatic channels could lead to vascular congestion resulting in testicular pain. The strong association with laparoscopic nephrectomy may be due to the inability to dissect out the gonadal vein while with an open approach preserving the gonadal vein may be more possible. Alternately, Kim et al. suggested that potential damage to the sensory nerves of the testicle, the superior spermatic nerve, may be responsible for the pain (17). A study by Sureka et al. suggested that testicular pain is related to injury to the spermatic plexus during gonadal vein or ureteral ligation and can be prevented by ligation of the ureter and gonadal vein above the bifurcation of the aorta (18). Similarly, Shirodkar et al. reported an incidence of orchalgia at 6.2% of 129 donors and found this complication to be eradicated after modification of surgical technique to a gonadal vein sparing approach (19). As the development of more robot-assisted minimally invasive techniques for precise dissection of tissue, this potential complication may eventually be preventable (20). Our study did not show any association between nephrectomy laterality and testicular pain. Given that laparoscopic nephrectomy is the norm now, this complication needs to be studied further.
While minor and self-resolving testicular pain may be just a nuisance, in some cases it may prove problematic. Akoh et al. reported a case of a donor who had unrelenting testicular pain following right nephrectomy and eventually required an orchidectomy for symptomatic control (7). Felix et al. also reported a donor who required orchiectomy in their analysis of post-operative complications from the Swiss Organ Living Donor Health Registry (8). Moreover, in the series of 145 donors (95% of whom underwent left nephrectomy), reported by Kim et al., one donor required surgical exploration for undetectable testicular blood flow (17).
This study is limited by its retrospective nature, recall bias and lack of information regarding the severity and the duration of testicular pain. We were unable to analyze 1348 male donors due to missing data on testicular pain which may bias the reported incidence. In fact, Pinar et al. has reported an incidence of testicular pain as high as 31.9% of 69 donors (21). Additionally, the RELIVE Study dataset did not record data on gonadal vein ligation. In addition, the dataset does not have information on how the gonadal artery was handled. The latter is highly relevant as most surgeons would ligate the left gonadal artery and almost universally spare the right gonadal artery. Another potential limitation of these analyses is not knowing how the testicular pain was treated by the participating centers and its severity.
In summary, testicular pain is a complication that is perhaps underappreciated. Factors associated with testicular pain include being non-Hispanic White and undergoing laparoscopic nephrectomy. Importantly, these results do not support the prevailing view that testicular pain is more common in individuals undergoing left nephrectomies. Donors should be routinely asked about this potentially bothersome complication.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
HI conceived of the idea, design, contributed to the analysis and manuscript preparation. NG, DM, SH, and MM contributed to study design and manuscript preparation. DN and EG conducted the analysis and contributed to manuscript preparation. All authors contributed to the article and approved the submitted version.
The authors would like to thank Hana Nguyen, NP for her help with manuscript preparation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fruro.2022.986255/full#supplementary-material
BMI, Body Mass Index; BP, Blood Pressure; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CI, Confidence Interval; eGFR, Estimated Glomerular Filtration Rate; ESKD, End-Stage Kidney Disease; GLM, Generalized Linear Model; IQR, Interquartile Range; LASSO, Least Absolute Shrinkage and Selection Operator; RELIVE, The Renal and Lung Living Donor Evaluation Study; RR, Relative Risk.
1. Segev DL, Muzaale AD, Caffo BS, Mehta SH, Singer AL, Taranto SE, et al. Perioperative mortality and long-term survival following live kidney donation. JAMA J Am Med Assoc (2010) 303(10):959. doi: 10.1001/jama.2010.237
2. Lentine KL, Lam NN, Axelrod D, Schnitzler MA, Garg AX, Xiao H, et al. Perioperative complications after living kidney donation: A national study. Am J Transplant (2016) 16(6):1848–57. doi: 10.1111/ajt.13687
3. Gjertson CK, Sundaram CP. Testicular pain following laparoscopic renal surgery. J Urology (2008) 180(5):2037–41. doi: 10.1016/j.juro.2008.07.045
4. Brook NR, Harper SJ, Waller JR, Nicholson ML. A consecutive series of 70 laparoscopic donor nephrectomies demonstrates the safety of this new operation. Transplant Proc (2005) 37(2):627–8. doi: 10.1016/j.transproceed.2004.12.149
5. Su L-M, Ratner LE, Montgomery RA, Jarrett TW, Trock BJ, Vladimir S, et al. Laparoscopic live donor nephrectomy. Ann Surgery (2004) 240(2):358–63. doi: 10.1097/01.sla.0000133351.98195.1c
6. Pareek G, Hedican SP, Gee JR, Bruskewitz RC, Nakada SY. Meta-analysis of the complications of laparoscopic renal surgery: Comparison of procedures and techniques. J Urology (2006) 175(4):1208–13. doi: 10.1016/S0022-5347(05)00639-7
7. Akoh JA, Rana TA, Stacey SL. Unusual complications following living donor nephrectomy. Dialysis Transplantation (2008) 37(11):446–50. doi: 10.1002/dat.20274
8. Felix B, Huynh-Do U, Hadaya K, Matter M, Muller T, Binet I, et al. Early complications after living donor nephrectomy: analysis of the Swiss organ living donor health registry. Swiss Med weekly (2017) 147:w14497. doi: 10.4414/smw.2017.14497
9. Permpongkosol S, Link RE, Su L-M, Romero FR, Bagga HS, Pavlovich CP, et al. Complications of 2,775 urological laparoscopic procedures: 1993 to 2005. J Urology (2007) 177(2):580–5. doi: 10.1016/j.juro.2006.09.031
10. Gregorio MS, Quilala AC, Cabanayan-Casasola CB, Danguilan RA, Gerial EL Jr. Living donor nephrectomy at the national kidney and transplant institute: Surgical techniques, perioperative complications and outcomes. J Urol Nephrol Open Access (2017) 3(2):1–6. doi: 10.15226/2473-6430/3/2/00129
11. Jalali M, Rahmani S, Joyce AD, Cartledge JJ, Lewis MH, Ahmad N. Laparoscopic donor nephrectomy: An increasingly common cause for testicular pain and swelling. Ann R Coll Surgeons England (2012) 94(6):407–10. doi: 10.1308/003588412X13171221592177
12. Garcia-Ochoa C, Feldman LS, Nguan C, Monroy-Cuadros M, Arnold J, Boudville N, et al. Perioperative complications during living donor nephrectomy: Results from a multicenter cohort study. Can J Kidney Health Dis (2019) 6:2054358119857718. doi: 10.1177/2054358119857718
13. Taler SJ, Messersmith EE, Leichtman AB, Gillespie BW, Kew CE, Stegall MD, et al. Demographic, metabolic, and blood pressure characteristics of living kidney donors spanning five decades. Am J Transplantation (2013) 13(2):390. doi: 10.1111/j.1600-6143.2012.04321.x
14. Hastie T, Tibshirani R WM. Statistical learning with sparsity: The lasso and generalizations. Boca Raton, FL: Chapman & Hall/CRC (2015).
15. StataCorp. Stata lasso. stata reference manual. College Station, TX: Stata Press (2019). Vol Release 16.
16. Gandhi NV, Murad DN, Nguyen DT, Graviss EA, Ibrahim HN. Hypertension and renal outcomes in normotensive kidney donors with multiple renal arteries. Transplant Int (2021) 34(11):2382–93. doi: 10.1111/tri.14024
17. Kim FJ, Pinto P, Su LM, Jarrett TW, Rattner LE, Montgomery R, et al. Ipsilateral orchialgia after laparoscopic donor nephrectomy. J Endourology (2003) 17(6):405–9. doi: 10.1089/089277903767923209
18. Sureka SK, Srivastava A, Agarwal S, Srivastava A, AN S, Singh S, et al. Prevention of orchialgia after left-sided laparoscopic donor nephrectomy–a prospective study. J Endourology (2015) 29(6):696–9. doi: 10.1089/end.2014.0645
19. Shirodkar SP, Gorin MA, Sageshima J, Bird VG, Martinez JM, Zarak A, et al. Technical modification for laparoscopic donor nephrectomy to minimize testicular pain: A complication with significant morbidity. Am J Transplant (2011) 11(5):1031–4. doi: 10.1111/j.1600-6143.2011.03495.x
20. Campi R, Vignolini G, Savi E, Sessa F, Agostini S, Serni S. Robotic kidney transplantation allows safe access for transplant renal biopsy and percutaneous procedures. Transplant Int (2019) 32(12):1333–5. doi: 10.1111/tri.13517
Keywords: retrospective observational study kidney, nephrectomy, testicular pain, kidney donors, outcomes of kidney transplantation
Citation: Gandhi NV, Murad DN, Hebert SA, Morgan M, Nguyen DT, Graviss EA and Ibrahim HN (2022) Incidence and correlates of testicular pain after kidney donation. Front. Urol. 2:986255. doi: 10.3389/fruro.2022.986255
Received: 04 July 2022; Accepted: 18 August 2022;
Published: 13 September 2022.
Edited by:
Ioannis Sokolakis, Martha-Maria Hospital Nuremberg, GermanyReviewed by:
Francesco Sessa, Careggi Hospital, ItalyCopyright © 2022 Gandhi, Murad, Hebert, Morgan, Nguyen, Graviss and Ibrahim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hassan N. Ibrahim, SGFzc2FuLk4uSWJyYWhpbUB1dGgudG1jLmVkdQ==
†ORCID: Nisarg V. Gandhi, orcid.org/0000-0001-6630-2157
Dina N. Murad, orcid.org/0000-0003-1217-8941
Sean A. Hebert, orcid.org/0000-0001-6126-1544
Monica Morgan, orcid.org/0000-0002-4879-6445
Duc T. Nguyen, orcid.org/0000-0002-5059-4404
Edward A. Graviss, orcid.org/0000-0003-1024-5813
Hassan N. Ibrahim, orcid.org/0000-0002-1567-9592
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.