- Urology and Nephrology Center, Mansoura University, Mansoura, Egypt
Purpose: This is an experimental preclinical study testing the applicability of autologous skeletal muscle-derived cells as a treatment of SUI in a canine model.
Methods: Ten mongrel dogs were included in this study. Skeletal muscle was harvested for biopsy in 4 dogs. One month later, incontinence was induced in 8 dogs through urethrolysis. Biopsied muscle cells were incubated and expanded for 8 weeks. Muscle-derived cells were collected and covered with a polyglycolic acid (PGA) scaffold immersed in culture medium and coated with Matrigel to be used as a sling, which was placed suburethrally in 8 dogs; 4 had cell seeding, and 4 had scaffolds only. Urethral pressure (UP) measurements were performed at baseline and 2 & 6 weeks after sling insertion. The urethra was harvested 4 weeks after sling insertion for histopathology.
Results: One month, a statistically significant increase of mean urethral pressure values compared to baseline was observed in all dogs with a scaffold inserted. The increase ranged from 5 to 40 cmH2O. Histopathology showed significant periurethral proliferation of skeletal muscles in 4 dogs with cell-seeded scaffolds. These levels were the maximum levels in dogs # 1 & 2. This was not the case in the 4 dogs that had slings only.
Conclusion: Based on the outcome of this preliminary experience, the use of skeletal muscle-seeded PGA scaffolds seems to be an easy and reproducible procedure which preserves histological differentiation and integrity in a canine model
Introduction
The treatment of stress urinary incontinence is challenging (1). Different surgical techniques have evolved throughout numerous clinical trials to efficiently treat this condition (2). To date, no procedure is considered the “gold standard”. However, a midurethral sling (MUS) is the most widely used procedure and is considered the standard of care (3). Synthetic MUS have been used for decades but are not without risks and sometimes can be life threatening (4). Although many trials, both experimental and human, have attempted to provide an autologous, efficacious and durable tissue-engineered sling (5, 6), none of these presented results that would allow their use in clinical practice and consequently to date no optimal autologous sling is available. So, we conducted this experimental trial with the purpose of assessing the potential of autologous cell seeding of a biodegradable scaffold in treating stress-induced urinary incontinence in canines.
This study evaluated a biodegradable seeded (PGA) scaffold enriched with autologous skeletal muscles without the need for a harvest procedure such as that used during the harvest of the rectus fascia sling (7), which is the prototype for autologous slings (8).
Materials and methods
The study comprised 10 mongrel female dogs. Eight dogs were the subject matter of the study and 2 were controls. Median weight was 17 Kg. Approval from the local ethics committee was obtained (Research Center Ethics Panel, Urology & Nephrology Center registration # 2018/Exp. 01).
All methods were carried out in accordance with relevant regulations and in accordance with ARRIVE guidelines, including housing, anesthesia and surgical procedures in dogs.
Group 1 comprised 4 dogs in which a cell-seeded sling was applied, while in Group 2, only the PGA sling was applied.
Isolation and expansion of muscle-derived cells
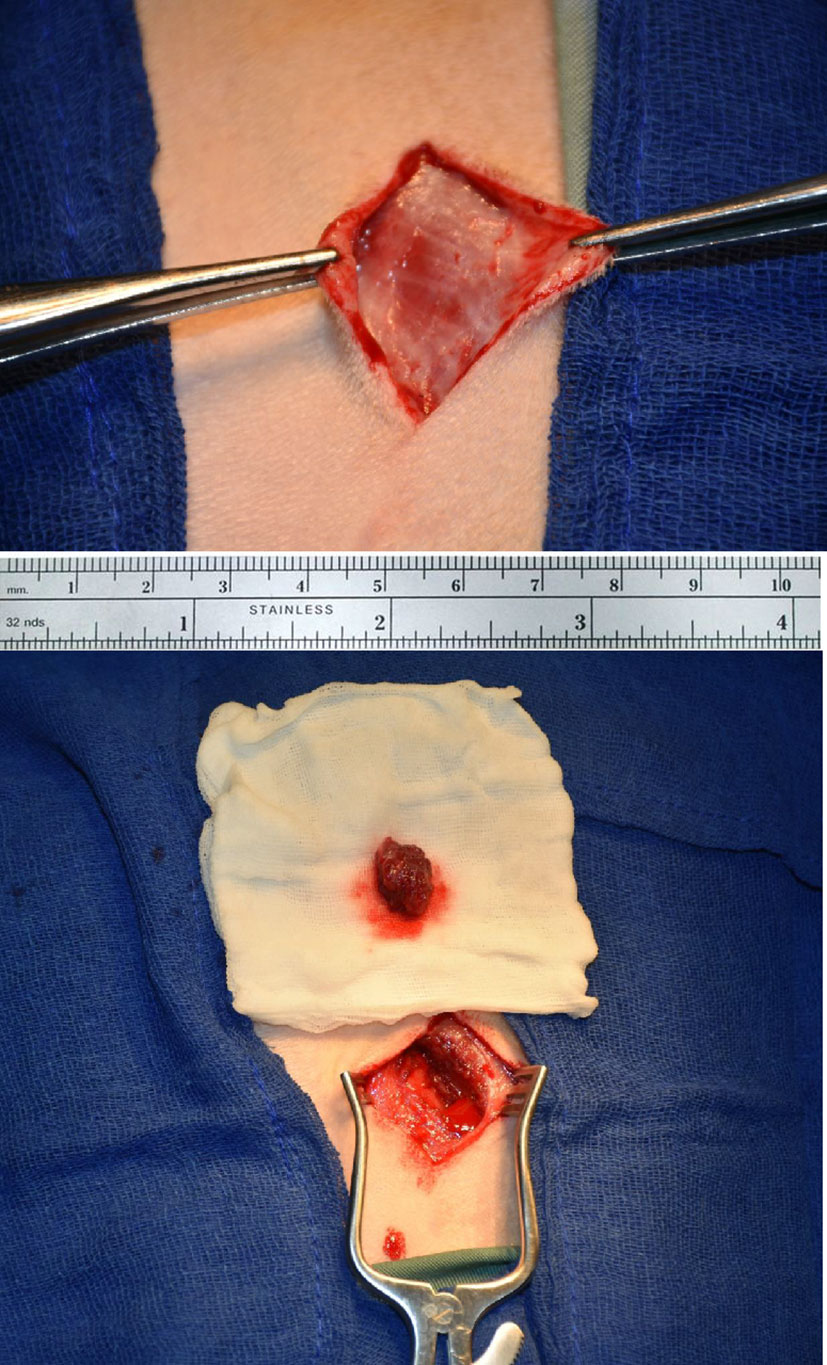
In 4 dogs (Group 1), a muscle biopsy was harvested from the biceps under general anesthesia. Figure 1 shows the biopsy sampling. The obtained biopsy averaged 0.2 g and was incubated in 0.2% collagenase type 1A in Dulbecco’s modified Eagle’s medium (DMEM) for 20 minutes at 37°C (Sigma–Aldrich®, St. Louis, USA) digestion. Cells were cultured on laminin-coated tissue culture flasks in SKGM-2 medium (Lonza®, Valkersville, MD). The medium was replaced every 3 days. At confluence, cells were passaged and split 1:2 into tissue culture flasks. After 8 weeks and 4 passages, MDCs were collected for transplantation.
Characterization of MDCs
The morphology of the cells was evaluated using phase-contrast microscopy. The expression of Desmin was assessed by immunohistochemistry (IHC) using monoclonal anti-Desmin antibodies (DakoCytomation®, Glostrub, Denmark). Detailed description of the skeletal muscle culturing, propagation and characterization is explained in appendix.
Cell seeding on scaffolds
A Neoveil absorbable PGA sheet (Gunze Limited®, Kyoto, Japan) was used in 2x3 cm segments as a scaffold. The scaffold material is completely degenerated in 2-3 months. In sling- only group, an identical segment of the PGA sheet is used as a sling without cell seeding. Seeding was started by immersion in culture medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin for 24 h at 37°C before seeding. The scaffold was coated with Matrigel, and MDCs were seeded at a concentration of 1 million cells/cm2. Matrigel was used in our study to increase cell adherence to the scaffold, as it is a promising material for this application (9). After 24 h, the seeding side was flipped, and the other side was treated the same way. Both surfaces were fully immersed in medium during the seeding process. The seeded scaffold was cultured for an additional 3 days in the same culture medium.
● Induction of incontinence: Open episiotomy was carried out 2 weeks prior to the study to facilitate the vaginal approach in inserting the scaffold/sling.
One month after biopsy, incontinence was induced in 8 dogs through midline suprapubic incision and identification of the urethra and bladder. The urethra was sharply dissected all around the structure until disruption of the periurethral sphincter was achieved. This method is similar to that previously described by Rodriguez et al. (10) with the addition of pubourethral ligament disruption.
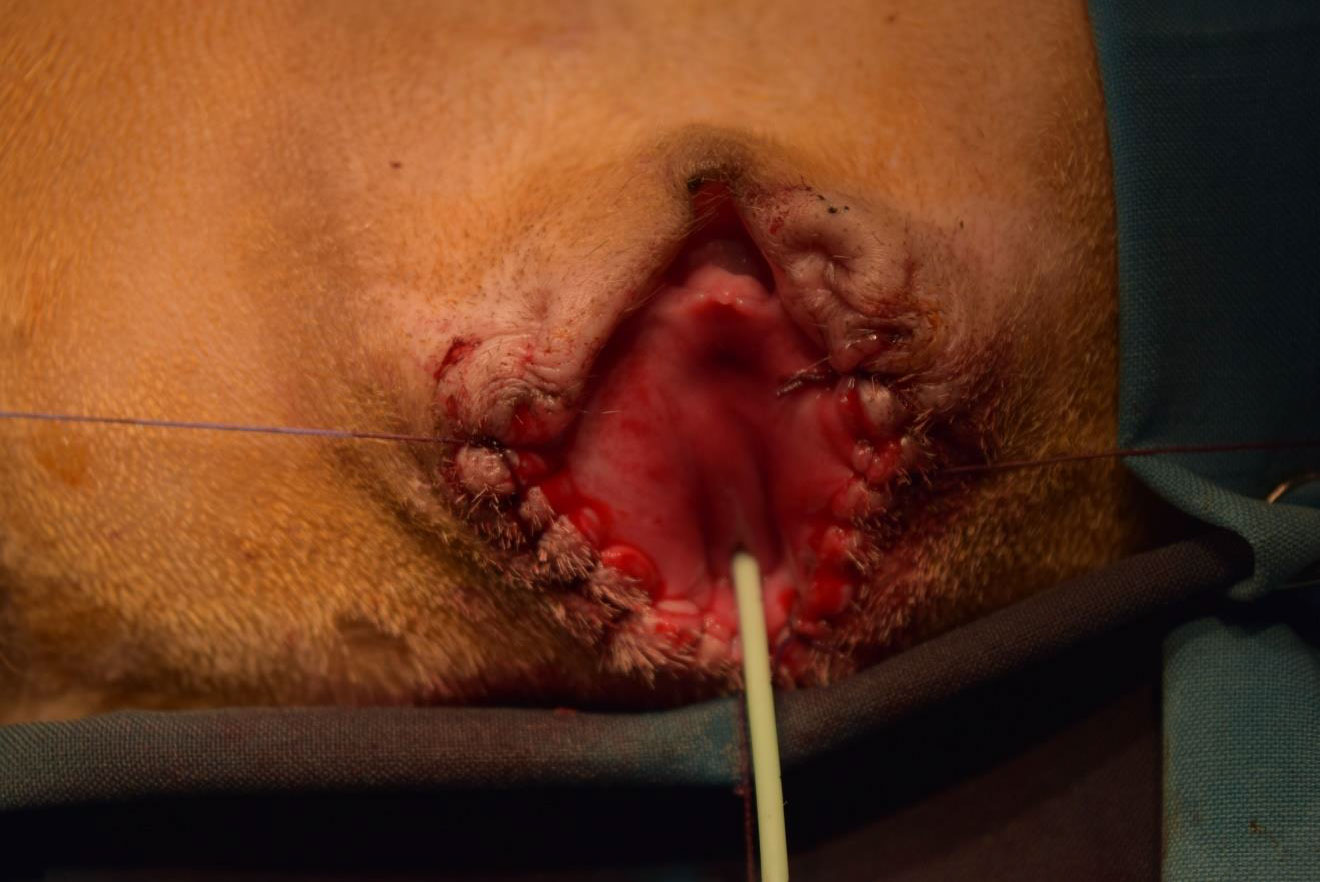
• Two weeks after induction of incontinence, the sling was applied through a vaginal incision in the suburethral position in all 8 dogs: cell-seeded scaffold in the first 4 dogs and scaffold only in the other 4 dogs. The sling was fixed to the periurethral tissue.
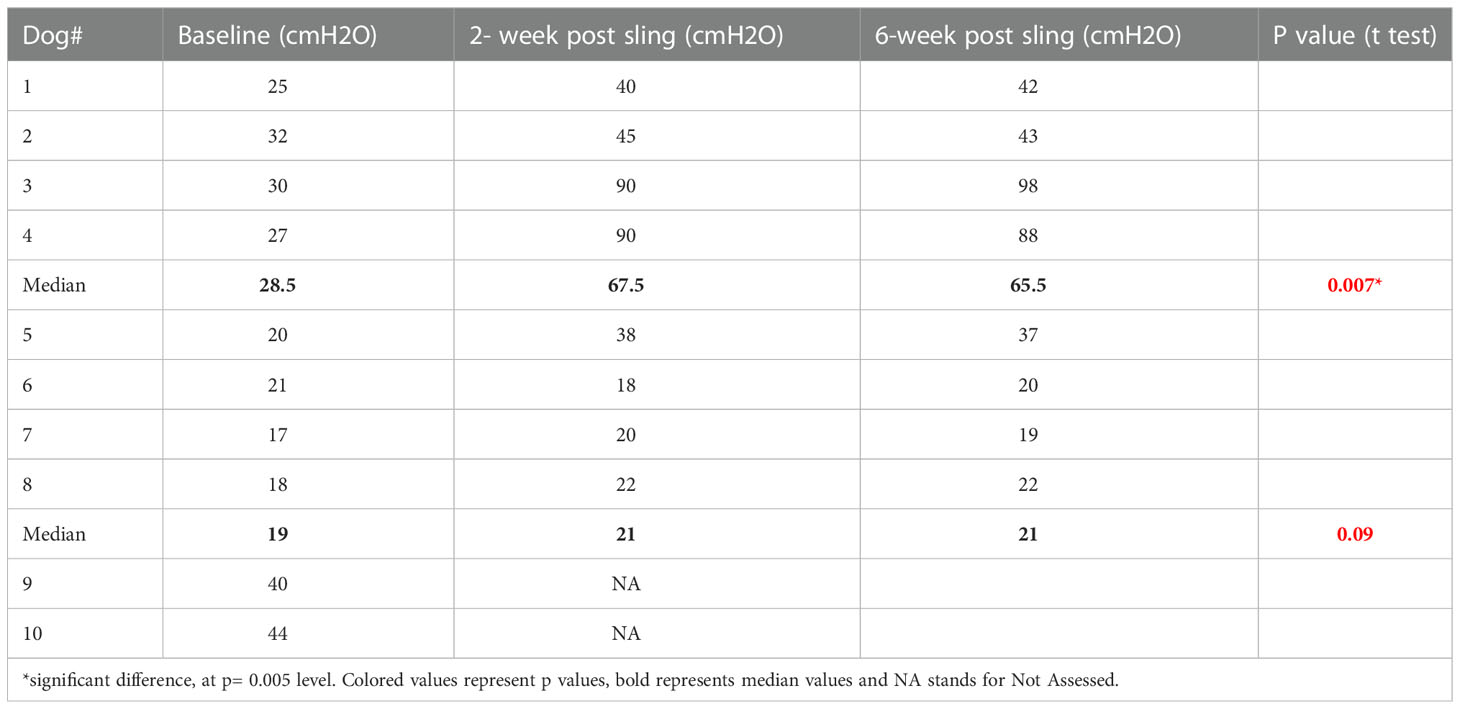
• Urethral pressure (UP) assessment: Urethral pressure measurements were performed before (baseline) and 2 and 6 weeks after insertion of the sling. A 6 F dual-lumen catheter was used for pressure recording and Laborie ®, Goby machine was used for urethral pressure measurement with external pressure water- filled transducers. Student’s t test was used for significance testing of the urethra pressure differences. Figure 2 demonstrates urodynamic testing of one of the dogs.
• The urethra with its surrounding tissue was harvested, and the dogs were sacrificed 4 weeks after sling insertion. Figure 3 shows the scaffold and steps of the surgical procedure.
• The urethra was fixed in 10% buffered formalin and processed in paraffin blocks, sectioned at 5 μm and stained with hematoxylin and eosin (H&E), methenamine silver (for reticuline fiber detection) and Masson trichrome stain (for fibrosis detection and muscle bundle delineation).
• Two dogs were considered controls, in whom no urethrolysis or insertion of slings was carried out. UP was carried out in these dogs before they were sacrificed, and their urethras were taken as controls.
• For IHC, Desmin antibodies were used (Abcam, Cambridge, UK) at a dilution of 1:100. Nerve fibers were identified through staining with polyclonal anti-S100 (DAKO, Carpinteria, CA) at a dilution of 1:200. Immunolabeling was performed using an avidin–biotin detection kit (Vectastain Elite ABC, Vector, Bulingame, CA). All sections were counterstained with Gill’s hematoxylin.

Figure 3 (A) Agar plate with sterile medium and the scaffold immersed. The mesh of the scaffold is seen impregnated with seeded muscle cells, (B) Dog urethra exposed and dissected clear (C) PGA sling inserted sub-urethral and fixed by 5/0 stitches (D) Whole urethra is being harvested after 4 weeks after insertion.
A flowchart for the research procedures has been provided (Supplement).
Results
● UP results showed an increase in the mean maximum urethral pressure in dogs with a scaffold inserted. Those with seeded scaffold exhibited significant increase in their UP as compared to those with scaffold- only sling. Table 1 demonstrates the change in UP over time. The median maximum urethral closure pressure (MUCP) in group 1, where the scaffold was seeded with skeletal muscle cells, was 28.5 cmH2O at baseline, which increased to 67.5 at 2 weeks and 65.5 at 6 weeks. In Group 2 (scaffold-only group), the median MUCP increased from a basal value of 19 to 21 cmH2O and remained at this level at 6 weeks. The increase in the MUCP ranged from 13 to 60 cmH2O (median 39 cmH2O) in group 1. In group 2, the increase in MUCP ranged from -3 to 18 (median 5.5 cmH2O). Table 1 shows UP measurements in the 2 groups.
● Histopathology
The urethra was fixed in 10% buffered formalin and processed in paraffin blocks, sectioned at 5 µm and stained with hematoxylin and eosin (H&E), methenamin silver (for reticuline fiber detection) and Masson trichrome stain (for fibrosis detection and muscle bundle delineation). The area positive for collagen (blue in Masson trichrome) and muscle (lavender red in Masson trichrome and brown in IHC) was calculated using Image Pro Plus software (Media Cybernetics, Bethesda, MD, USA).
For analysis, 10 fields of view of muscle layer fibres and collagen bundles were used from each animal
Luminal diameter, epithelial lining, lamina propria thickness with its elastic and collagenous fiber content (normally approximately 70% of whole sphincter thickness), fibrous tissue deposition, thick-walled vein condition (stratum cavernosum), and smooth and striated muscle (both constituting approximately 30% of total urethral thickness) (11) were assessed.
Group 1: the urethral lumen was slightly dilated in comparison to the control group; the mucosal lining was of near normal thickness. The lamina propria was slightly thicker, and there was no evidence of fibrous tissue deposition, as evidenced with Masson trichrome staining. The reticular fibers were the same as those in the control group. Thick-walled veins were mildly increased in number and caliber compared to the control group. The overall muscle layer appears as lavender red bundles in Masson trichrome and was normal in thickness with some degrees of crowding and disorganization. Areas of newly formed muscle bundles with central nuclei were confirmed.
Group 2: the damaged sphincter shows a mildly dilated luminal area, with an atrophic urothelial lining. The lamina propria thickness was increased with moderate fibrous tissue deposition in addition to lower reticular fiber content and mild inflammatory lymphocytic infiltrate. The vascular component is unremarkable. There was substantial loss of the muscle layer with an overall decreased wall thickness.
IHC for anti-desmin showed significantly higher sphincter muscle mass in seeded scaffold specimens than in the other group. Newly formed muscle bundles are wider and more randomly aligned than the normal sphincter. Staining for S100 showed the presence of nerve fibers between the regenerated sphincter muscles. Figures 4, 5 show the histopathological changes in the two groups.
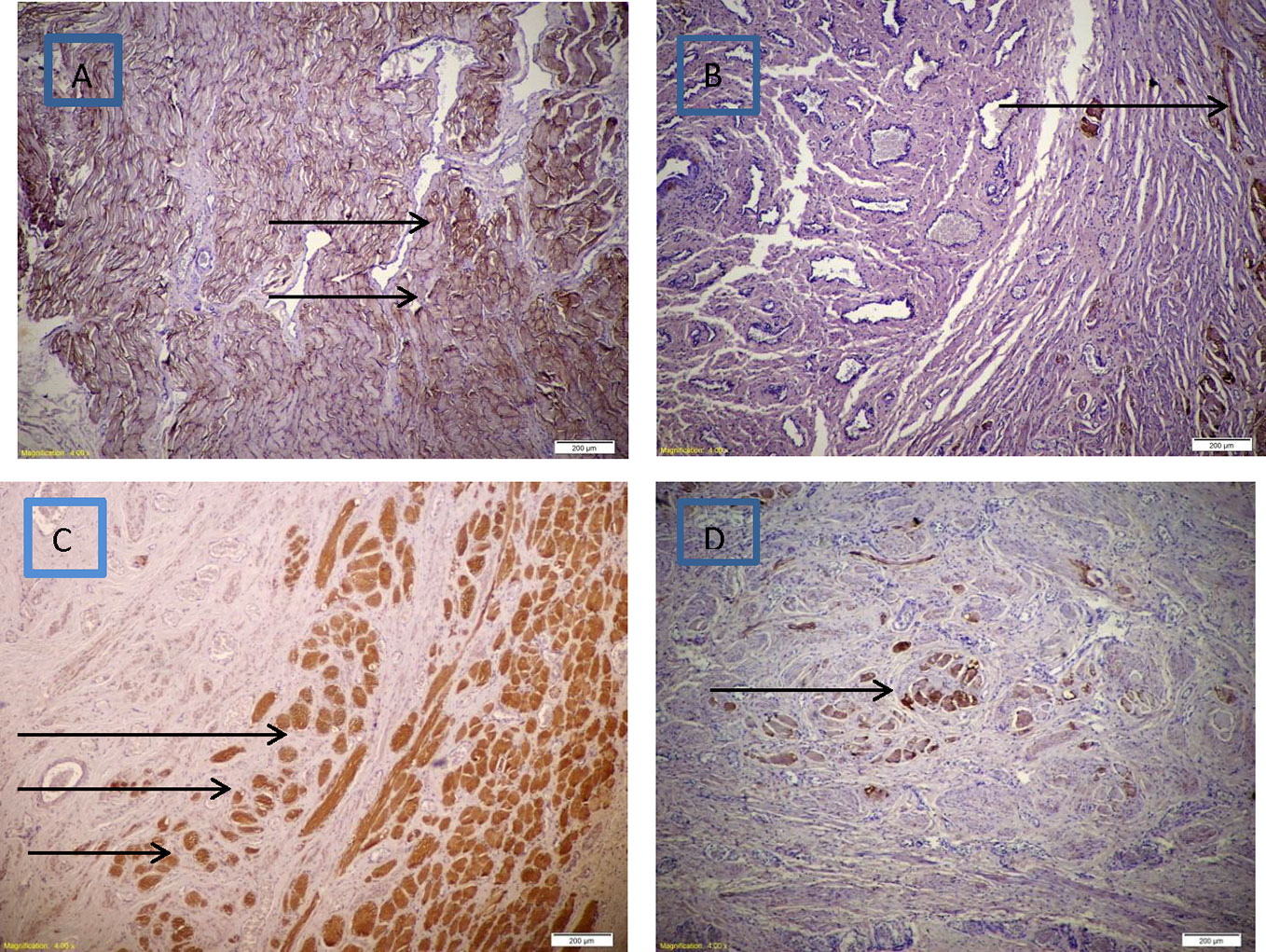
Figure 4 (A) Diffuse increase in thickness of skeletal muscle bundles with mild disorganization in PGA seeded with MDC using IHC for desmin stain (brown), (B) Minimal increase in thickness of skeletal muscle bundles in PGA only using IHC for desmin stain (brown), (C) Abundant nerve fibers in between muscle bundles in PGA seeded with MDC using IHC for S100 stain (brown), (D) Minimal increase in nerve fibers in between muscle bundles in PGA only using IHC for S100 stain (brown).
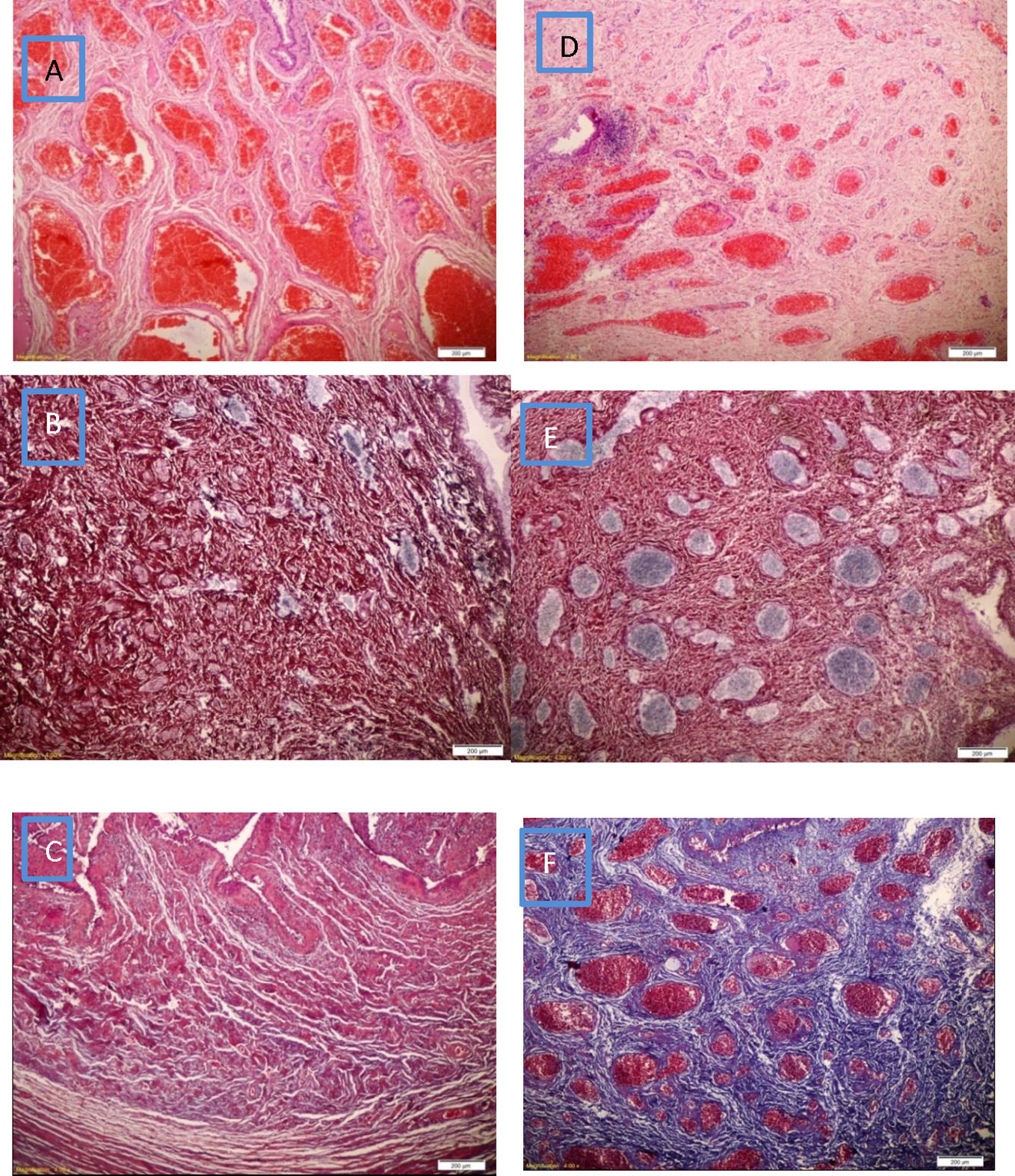
Figure 5 (A) Venous plexus is increased in number with marked congestion and mild edema in PGA seeded with MDC, (D) Venous plexus is near normal in number with mild congestion with mild inflammatory infiltrate in PGA only, (B) Silver stain shows near normal amount of reticular fibers (black) in PGA seeded with MDC, (E) Silver stain shows lower amount of reticular fibers (black) in PGA only, (C) The lamina propria expressed absent fibrous tissue (blue) in PGA seeded with MDC using Masson trichrome stain, (F) The lamina propria showed diffuse moderate fibrous tissue (blue) in PGA only using Masson trichrome stain.
Discussion
Induction of stress urinary incontinence by urethrolysis with pubourethral ligament injury was our approach of choice. It results in disruption of the native support of the urethra and durable loss of urethral resistance (12). Incontinence was confirmed by a lower maximum urethral closure pressure compared to control dogs, in which no urethrolysis was carried out.
Myoblasts have been used as a treatment for stress urinary incontinence in animal models by means of intraurethral injection (13). In 2014, a similar approach was used in 35 adult women. Gras et al. (14) described a technique performed largely under local anesthesia/intravenous analgesia. An open muscle biopsy was obtained from the vastus lateralis muscle and was “minced” and injected as a suspension in the same session via the periurethral route in 35 women under vaginal US. Cure/improvement was noted in 7% to 63% of the patients.
Other human trials involving intraurethral injection of muscle-derived stem cells have been published in both adult women (6) and children (15). Although the results were promising, some reports turned out to be unfounded (16).
Another research strategy involved making structured skeletal muscle tissue in vitro, with the potential of making an all-autologous sling. Many studies have evolved to reconstruct skeletal muscle tissue. Some focused on developing self-organizing tissues without artificial scaffolds (17); others preferred seeding cells on natural or synthetic biodegradable substrates, e.g., collagen matrices (18).
We preferred polyglycolic acid as a biodegradable scaffold, as used by Saxena et al. (19, 20), where myoblasts derived from neonatal rats, Fisher CDF-F344 cells, were seeded onto polyglycolic acid meshes and implanted into the omentum of syngeneic adult Fisher CDF-F344 rats with promising results and a comprehensive description of the approach. The advantage of the sling approach is that it applies a potentially successful technique (21) with the additional effect of the autologous skeletal muscle fibers to the mid-urethra.
To our knowledge, no human study involving biodegradable slings seeded with autologous muscle-derived cells has yet been reported. Such a study will be of paramount importance, considering that the current treatment options for women with SUI are far from optimum (22).
The question we opted to answer is whether muscle-seeded biodegradable scaffold is better than a plain scaffold.
Cell-seeded slings proved to be more efficacious than plain PGA. Urethral pressure measurement 6 weeks after sling insertion showed that the median increase in urethral closure pressure in the cell-seeded sling was over 40 cm H2O, while in the other group where only PGA sling was applied, it was 5.5 cmH2O.
Histopathological study of harvested urethral segments 4 weeks after insertion of slings proved the persistence of viable skeletal muscle and nerve fibers.
This sling design avoids polypropylene-related adverse events. Application of this technique in humans is probably easy and practical, based on functional and urodynamic outcomes. It is definitely supportive of the urethra but it is not clear from our study whether or not the scaffold helps regeneration of the sphincter.
One of the limits of our study is reliance on the measurement of maximum urethral pressure in female dogs, which might not be very reproducible. This is probably true according to one study (23); however, others (24) have adopted the same parameter we used. However, the use of static urethral pressure has its limitations in human studies, and was found to be neither diagnostic of stress urinary incontinence nor prognostic of the surgical outcome (25). The small number os animals and the relatively short follow up period is another limitation
Conclusion
In our preliminary experience, a biodegradable urethral sling with autologous myoblasts survived 6 weeks after insertion in animal model and provided an increase of mean urethral pressure values compared to baseline. The use of skeletal muscle-seeded PGA scaffolds could represent a practical technique with preserved histological differentiation in a canine model in the short term.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical review and approval was not required for the animal study however, IRB of the hospital has approved the study that was done in our research lab with the aid of our veterinarians under optimum conditions for the animals (mongrel dogs) subject matter of the study.
Author contributions
BW: Performance of the surgical procedures and urodynamics for all dogs. Shared in the design and drafting of the manuscript. HA: Care for the animals during the period of the experiment and assisted in performing surgical procedures. SK: Histopathology. MG: Preparation of the cell culture and propagation of skeletal muscle cells as well as seeding of PGA scaffolds. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by Urology and Nephrology Center, Mansoura University.
Acknowledgments
The staff of the research lab and animal house of UNC helped with the experiment.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fruro.2022.959583/full#supplementary-material
Supplementary Figure | Flowchart of the study procedures.
Abbreviations
- DMEM, Dulbecco’s modified Eagle’s medium; SUI, stress urinary incontinence; IHC, immunohistochemistry; PGA, polyglycolic acid; MDCs, muscle-derived cells; UPP, urethral pressure profile.
References
1. Nadeau G, Herschorn S. Management of recurrent stress incontinence following a sling. Curr Urol Rep (2014) 15(8):427. doi: 10.1007/s11934-014-0427-0
2. Hinoul P, Roovers JP, Ombelet W, Vanspauwen R. Surgical management of urinary stress incontinence in women: a historical and clinical overview. Eur J Obstet Gynecol Reprod Biol (2009) 145(2):219–25. doi: 10.1016/j.ejogrb.2009.04.020
3. Abrams P, Andersson KE, Birder L, Brubaker L, Cardozo L, Chapple C, et al. Fourth international consultation on incontinence recommendations of the international scientific committee: evaluation and treatment of urinary incontinence, pelvic organ prolapse, and fecal incontinence. Neurourol.Urodyn (2010) 29(1):213–40. doi: 10.1002/nau.20870
4. Daneshgari F, Kong W, Swartz M. Complications of mid urethral slings: Important outcomes for future clinical trials. J Urol (2008) 180(5):1890–7. doi: 10.1016/j.juro.2008.07.029
5. Williams JK, Eckman D, Dean A, Moradi M, Allickson J, Cline JM, et al. The dose-effect safety profile of skeletal muscle precursor cell therapy in a dog model of intrinsic urinary sphincter deficiency. Stem Cell Transl Med (2015) 4:286–94. doi: 10.5966/sctm.2014-0114
6. Carr LK, Steele D, Steele S, Wagner D, Pruchnic R, Jankowski R, et al. 1-year follow-up of autologous muscle-derived stem cell injection pilot study to treat stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct (2008) 19:881–3. doi: 10.1007/s00192-007-0553-z
7. Wadie BS, Edwan A, Nabeeh AM. Autologous fascial sling vs. polypropylene tape at short-term followup: a prospective randomized study. J Urol. (2005) 174(3):990–3. doi: 10.1097/01.ju.0000169492.96167.fe
8. Blaivas JG, Simma-Chiang V, Gul Z, Dayan L, Kalkan S, Daniel M. Surgery for stress urinary incontinence : Autologous fascial sling. Urol Clin North Am (2019) 46(1):41–52. doi: 10.1016/j.ucl.2018.08.014
9. Lam NT, Lam H, Sturdivant NM, Balachandran K. Fabrication of a matrigel-collagen semi-interpenetrating scaffold for use in dynamic valve interstitial cell culture. BioMed Mater (2017) 12(4):045013. doi: 10.1088/1748-605X/aa71be
10. Rodríguez LV, Chen S, Jack GS, de Almeida F, Lee KW, Zhang R. New objective measures to quantify stress urinary incontinence in a novel durable animal model of intrinsic sphincter deficiency. Am J Physiol Regul Integr Comp Physiol (2005) 288(5):R1332–8. doi: 10.1152/ajpregu.00760.2004
11. Eberli D, Aboushwareb T, Soker S, Yoo JJ, Atala A. Muscle precursor cells for the restoration of irreversibly damaged sphincter function. Cell Transplant (2012) 21(9):2089–98. doi: 10.3727/096368911X623835
12. Sajadi KP, Gill BC, Damaser MS. Neurogenic aspects of stress urinary incontinence. Curr Opin Obstet Gynecol. (2010) 22(5):425–9. doi: 10.1097/GCO.0b013e32833e499d
13. Chancellor MB, Yokoyama T, Tirney S, Mattes CE, Ozawa H, Yoshimura N, et al. Preliminary results of myoblast injection into the urethra and bladder wall: a possible method for the treatment of stress urinary incontinence and impaired detrusor contractility. Neurourol. Urodyn. (2000) 19(3):279–87. doi: 10.1002/(SICI)1520-6777(2000)19:3<279::AID-NAU9>3.0.CO;2-M
14. Gräs S, Klarskov N, Lose G. Intraurethral injection of autologous minced skeletal muscle: a simple surgical treatment for stress urinary incontinence. J Urol (2014) 192(3):850–5. doi: 10.1016/j.juro.2014.04.005
15. Kajbafzadeh AM, Elmi A, Payabvash S, Salmasi AH, Saeedi P, Mohamadkhani A, et al. Transurethral myoblast injection for treatment of urinary incontinence in children with classic bladder exstrophy. J Urol. (2008) 180:1098–105. doi: 10.1016/j.juro.2008.05.057
16. Kleinert S, Horton R. Retraction–autologous myoblasts and fibroblasts versus collagen [corrected] for treatment of stress urinary incontinence in women: A [corrected] randomised controlled trial. Lancet (2008) 372:789790. doi: 10.1016/S0140-6736(08)61320-3
17. Dennis RG, Kosnik P, Gilbert ME, Faulkner JA. Excitability and contractility of skeletal muscle engineered from primary cultures and cell lines. Am J Physiol Cell Physiol (2001) 280:C288–95. doi: 10.1152/ajpcell.2001.280.2.C288
18. Kosnik PE Jr, Faulkner JA, Dennis RG. Functional development of engineered skeletal muscle from adult and neonatal rats. Tissue Eng (2001) 7(5):573–84. doi: 10.1089/107632701753213192
19. Saxena AK, Marler J, Benvenuto M, Willital GH, Vacanti JP. Skeletal muscle tissue engineering using isolated myoblasts on synthetic biodegradable polymers: preliminary studies. Tissue Eng. (1999) 5(6):525–32. doi: 10.1089/ten.1999.5.525
20. Saxena AK, Willital GH, Vacanti JP. Vascularized three dimensional skeletal muscle tissue-engineering. BioMed Mater Eng. (2001) 11(4):275–81.
21. Richter HE, Albo ME, Zyczynski HM, Kenton K, Norton PA, Sirls LT, et al. Retropubic versus transobturator midurethral slings for stress incontinence. N Eng. J Med (2010) 362(22):2066–76. doi: 10.1056/NEJMoa0912658
22. Albo ME, Richter HE, Brubaker L, Norton P, Kraus SR, Zimmern PE, et al. Burch Colposuspension versus fascial sling to reduce urinary stress incontinence n. Eng. J Med (2007) 356(21):2143–55. doi: 10.1056/NEJMoa070416
23. Holt PE. 'Simultaneous' urethral pressure profilometry in the bitch: methodology and reproducibility of the technique. Res Vet Sci (1989) 47(1):110–6.
24. Claeys S, de Leval J, Hamaide A. Transobturator vaginal tape inside out for treatment of urethral sphincter mechanism incompetence: preliminary results in 7 female dogs. Vet Surg (2010) 39(8):969–79. doi: 10.1111/j.1532-950X.2010.00737.x
Keywords: sling, skeletal muscle, dogs, incontinence, seeded scaffold
Citation: Wadie BS, Aamer HG, Khater SM and Gabr MM (2023) The use of autologous skeletal muscle-derived cells as a sling in the treatment of stress-induced urinary incontinence: An experimental study in dogs. Front. Urol. 2:959583. doi: 10.3389/fruro.2022.959583
Received: 01 June 2022; Accepted: 15 December 2022;
Published: 05 January 2023.
Edited by:
Giovanni Palleschi, Medica San Carlo, ItalyReviewed by:
Rui Almeida Pinto, Centro Hospitalar São Joao, PortugalJames K. Williams, Wake Forest University, United States
Copyright © 2023 Wadie, Aamer, Khater and Gabr. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bassem S. Wadie, YndhZGllQGFvbC5jb20=; QmFzc2VtX3dhZGllQG1hbnMuZWR1LmVn
 Bassem S. Wadie
Bassem S. Wadie Haytham G. Aamer
Haytham G. Aamer Mahmoud M. Gabr
Mahmoud M. Gabr