- 1International Collaboration on Repair Discoveries (ICORD), Faculty of Medicine, University of British Columbia (UBC), Vancouver, BC, Canada
- 2Doctor of Medicine (MD) Undergraduate Program, Faculty of Medicine, UBC, Vancouver, BC, Canada
- 3Master of Science (MSc) Graduate Program, School of Population and Public Health, Faculty of Medicine, UBC, Vancouver, BC, Canada
- 4Department of Urology, University Hospital Basel, University of Basel, Basel, Switzerland
- 5Division of Physical Medicine and Rehabilitation, Faculty of Medicine, UBC, Vancouver, BC, Canada
- 6George Frederick (GF) Strong Rehabilitation Centre, Vancouver Coastal Health, Vancouver, BC, Canada
The aim of this pilot study was to assess the short-term microbiological burden and surface damage of catheters for intermittent catheterization (IC) in adult individuals with chronic (> 1-year) spinal cord injury (SCI). Three participants (two females, one male mean age 52 years) were asked to clean and reuse polyvinyl chloride catheters for IC over three days. Urine and catheter swab samples were collected on each day for microbiological analysis. After reuse, all catheters were analyzed via electron microscopy. Of all catheter swab cultures, 14 were negative, 12 were contaminated (i.e. skin or mixed flora), and one had growth of Haematomicrobium sanguinis. All urine cultures revealed either growth of Escherichia coli (n=10) in participants 1 and 2, or Klebsiella pneumoniae (n=4) or mixed enteric flora (n=1) in participant 3. Since all participants had asymptomatic bacteriuria with a significant number of colony-forming units per liter (CFU/L, i.e. > 100’000’000) prior to the study, we could not observe additional increases in the microbial growth (i.e. urine culture). Electron microscopy showed signs of surface damage, accumulation of debris and bacterial colonization on the exterior surface and lumen of the reused catheters. Thus, future studies should exclude participants with pre-study bacteriuria. Furthermore, a longer study duration as well as conducting electron microscopy of catheters after varied days of reuse could provide even better evidence on how structural and microbial changes of reused catheters progress over time.
Introduction
For individuals with spinal cord injury (SCI), neurogenic lower urinary tract dysfunction (NLUTD) and episodic increases of systolic blood pressure, known as autonomic dysreflexia (AD), are two chronic conditions that present as significant clinical problems (1). Complications arising from NLUTD, such as irritation of the urinary bladder due to a urinary tract infection (UTI) (2, 3) or neurogenic detrusor overactivity (4) are leading triggers of AD episodes. Additionally, NLUTD and its management is of high priority for individuals living with SCI as it significantly reduces quality of life and interferes with their daily life (5, 6) as incontinence, renal impairment, UTI and renal/bladder stones are all complications of this condition (7). Consequently, reducing the incidence of UTI is crucial in individuals with SCI. Intermittent catheterization is considered the gold standard for bladder management for individuals with SCI with sufficient dexterity (1, 8). It is well known that many users chose to inappropriately reuse single-use catheters for intermittent catheterization because of convenience and reduced cost (9–11), however the reuse of a catheter has yet to be proven to be as safe as single use (12, 13) due to the increased risk of bacterial colonization on the catheter and damage to the physical structure of the catheter itself. Structural damage to the catheter can cause urethral and bladder trauma (14) which in addition to the increased bacterial colonization may put the user at increased risk of UTI. With this in mind, we sought to uncover the short-term microbiological burden and surface damage of catheters for intermittent catheterization in adult individuals with chronic SCI.
Materials and methods
Study design
The study was performed and approved by the Research Ethics Board (REB) at the University of British Columbia (ethics number H17-03228). The study was a pilot case series investigating the burden of intermittent catheterization in adult individuals with NLUTD following SCI. Individuals were recruited through email and phone from a target population that included both females and males with chronic SCI performing intermittent catheterization.
Participants were either sourced from a database including individuals who were previously involved in research at the International Collaboration on Repair Discoveries (ICORD), or were sourced from existing patients with SCI at the GF Strong Rehabilitation Centre, both located in Vancouver, Canada. Inclusion criteria were: being male or female, 18 years of age or older, any SCI, able to perform intermittent catheterization with sufficient hand function or have a committed caregiver, family member or partner who will perform intermittent catheterization for management of urinary bladder drainage, able to rinse, dry and store catheter in clean clothes for next catheterization, able to comply with all clinic visits and study-related procedures, provide informed consent and be able to understand and complete study related instructions (must be able to understand and speak English), must not be pregnant and be clear of undergoing any urinary diversion procedure, such as bladder augmentation, cystectomy, neobladder, pouch reservoir, ileal conduit, Mitrofanoff appendicovesicostomy. Participants who had presence of a severe acute medical issue that in the investigator’s judgement would adversely affect the individual’s participation in the study, do not have others to perform intermittent catheterization for them, is a member of the investigational team or his/her immediate family, underwent any urinary diversion procedure in the past, had a UTI, had recent antibiotic use or used high temperature catheter cleaning methods (i.e. boiling or microwaving catheters) or any method that could compromise the integrity of the catheter or the biofilm were excluded. Three participants were eligible and were enrolled between October 2020 and February 2021. All three participants completed the study by February 2021.
Participants were provided with standard SpeediCath® Coloplast polyvinyl chloride (PVC) study catheters as well as all study-related materials including: sterile rayon swabs containing liquid amies medium (Transystem™ 138C, COPAN Diagnostics Inc., California, United States of America), sterile urine specimen jars (STARPLEX® 3 oz/90 mL Specimen Container, STARPLEX Scientific Inc., Etobicoke, Ontario Canada) benzalkonium chloride wipes (LORIS™ 0.13% BZK Wipe, Lernapharm Inc., Quebec, Canada), bacteriostatic lubricating jelly (MUKO®, 3.5g package, Cardinal Health Canada Inc, Toronto, Canada), sterile towels, sterile scissors, sterile forceps and a glass jar filled with sterile saline.
Sample collection and analysis
Three participants completed this pilot case study. Participants were instructed to clean their catheters as they otherwise normally would between uses but were asked to always utilize the same cleaning procedure after each catheterization. Five urine samples were collected for culture and sensitivity analyses and nine catheter swab samples were collected for gram smear test and culture analysis from each participant. The timeline of the sample collection can be seen in Figure 1. All swab and urine samples were analyzed by a standardized clinical laboratory testing company (Lifelabs, Canada). In case of unidentifiable microorganisms, samples were sent to the British Columbia Centre for Disease Control (BCCDC) for further analysis and identification. The presence of microbial organisms and their quantity (i.e. colony forming units per litre [CFU/L]) as well as their antibiotic susceptibilities were analyzed.
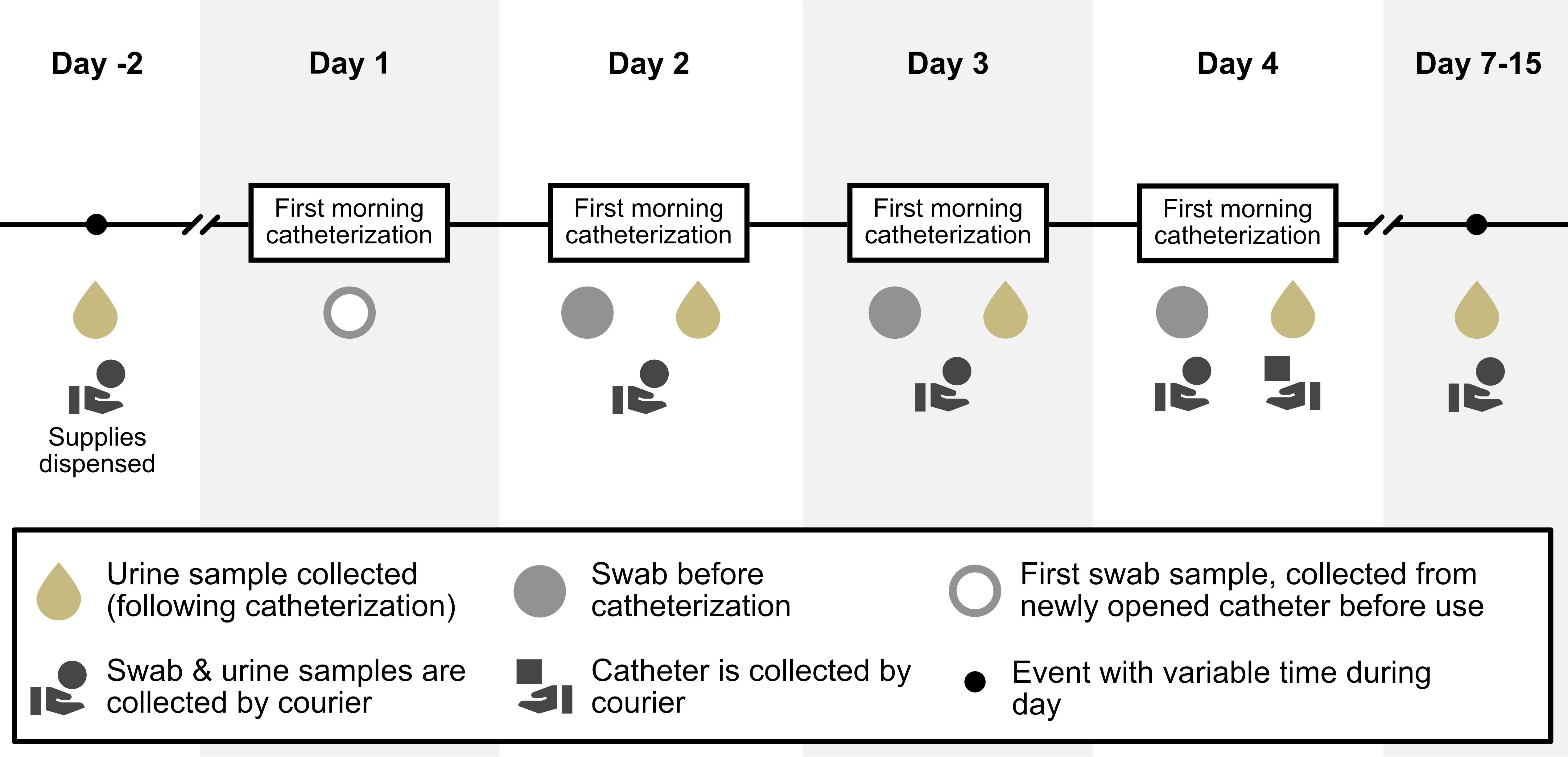
Figure 1 Timeline of events. This figure provides an overview of the length of the study and the timeline events.
Following the completion of the third day of the study, 9 cm sections, which included the tip and eyelets, of the reused catheters from of the three participants were collected along with an identical section of a new unused control catheter. Catheter samples were analyzed via Scanning Electron Microscopy (SEM) by the Centre for High-Throughput Phenogenomics located at the University of British Columbia, Vancouver, Canada. These catheter sections were imaged by SEM using either a Hitachi SU-3500 SEM (Hitachi High Technologies Corporation) or Helios NanoLab 650 Focused Ion Beam SEM (FEI) in conjunction with Zig Zag large area view software. The images were stitched by Image Composite Editor to create a high-resolution montage (Appendix 1).
Results
Demographics
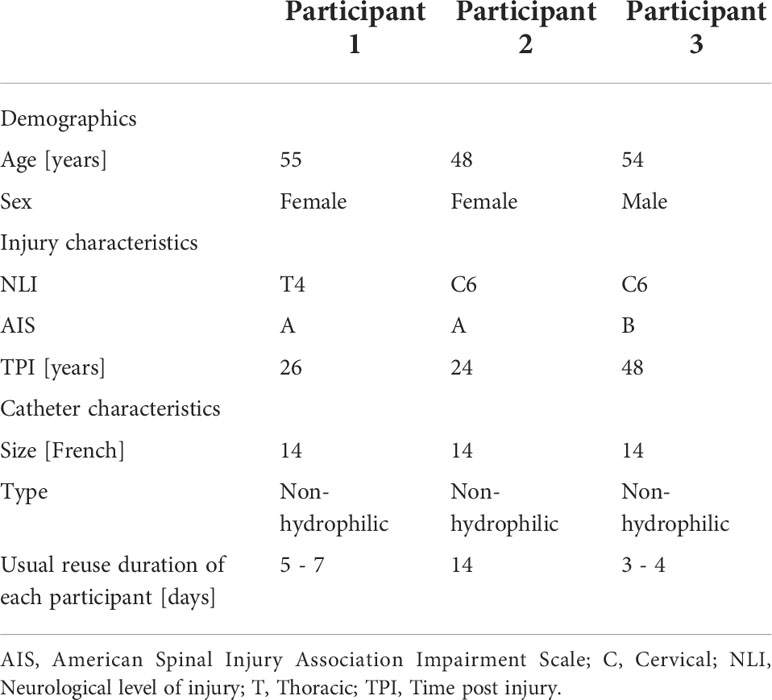
Overall, three participants (two females and one male) with a mean age of 52 years (range 48 – 55 years) completed this pilot case study. Participants demographics as well as injury and catheter characteristics are shown in Table 1.
Catheter swab sample – gram smear test and routine culture
Of all catheter swab cultures, 52% (14/27) were negative. 41% (11/27) of catheter swab samples contained contamination from skin flora, 4% (1/27) contained mixed flora and 4% (1/27) had growth of Haematomicrobium sanguinis (Supplementary Table S1). Notably, samples from Day 1 of the study had the lowest percentage of cellularity in the swab gram stains 11% (1/9) and growth in the cultures 33% (3/9). In contrast, samples from Day 3 had the highest rates of gram stain cellularity and culture growth with 67% (6/9) positive results for each. No further analysis was performed on cultures that grew skin or mixed flora.
Urine culture and sensitivity analysis
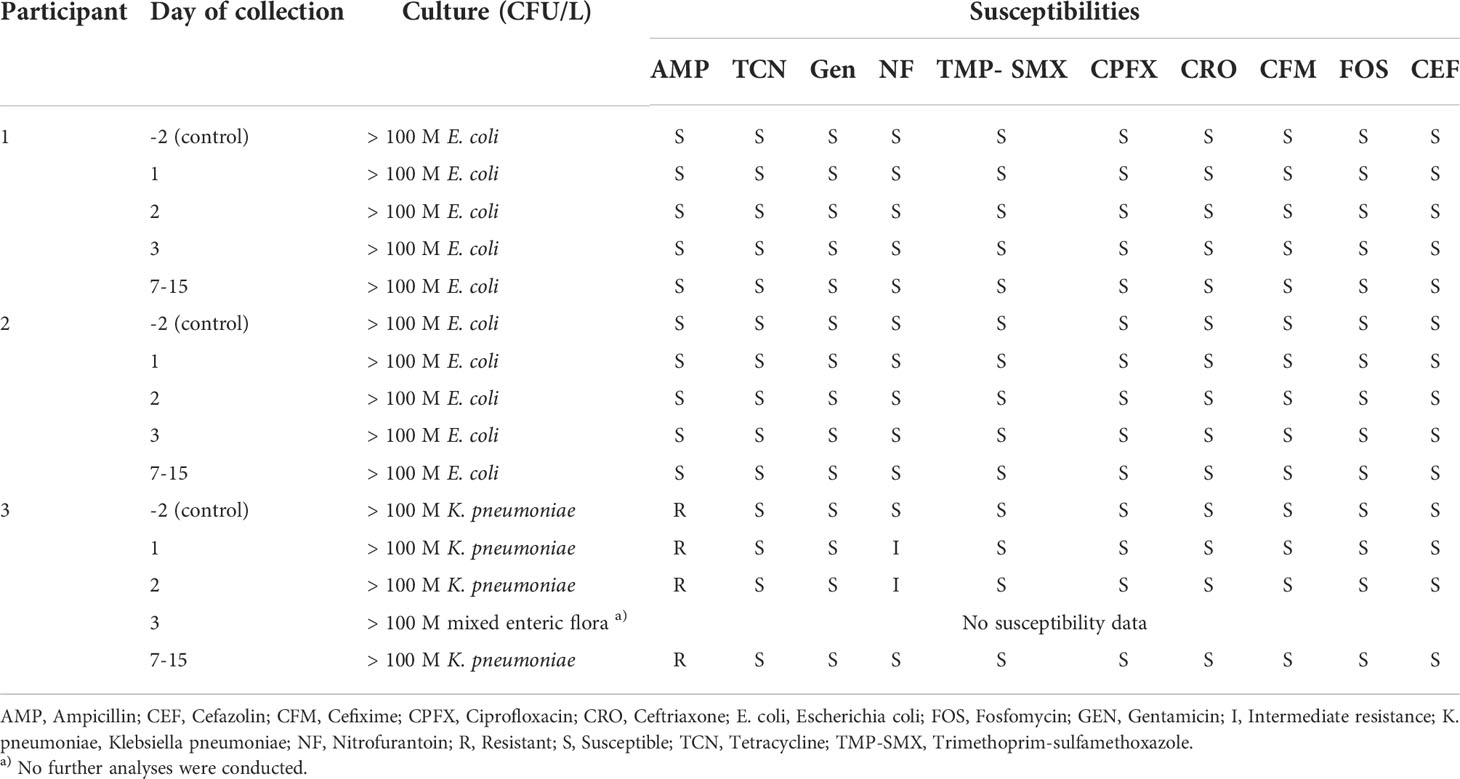
All urine cultures, including the pre-study control samples, cultured Escherichia coli in participants 1 and 2, and Klebsiella pneumoniae in participant 3. Escherichia coli had full susceptibility whereas Klebsiella pneumoniae had resistance to ampicillin only. Mixed enteric flora was also cultured on Day 3 in participant’s 3 urine. Rates of positive urine cultures throughout the course of the study did not change as all urine cultures recorded bacterial presence greater than 100 M CFU/L, including the control samples which were sampled two days prior to the beginning of the study period (Table 2).
Electron microscopy of external surface, lumen and eyelets of catheters
Electron microscopy images of the catheter surface clearly show clear signs of surface damage, accumulation of debris and bacterial colonization both on the exterior surface and in the lumen of the catheters after just 3 days. At low magnification there was appreciable surface erosion and debris to the external surface and eyelets. At higher magnification some rods, cocci and microfractures were visible in addition to the surface erosion and debris seen at the lower magnification. Debris, bacterial deposition, microfractures and surface erosion were not present in the unused control catheter (Figure 2).
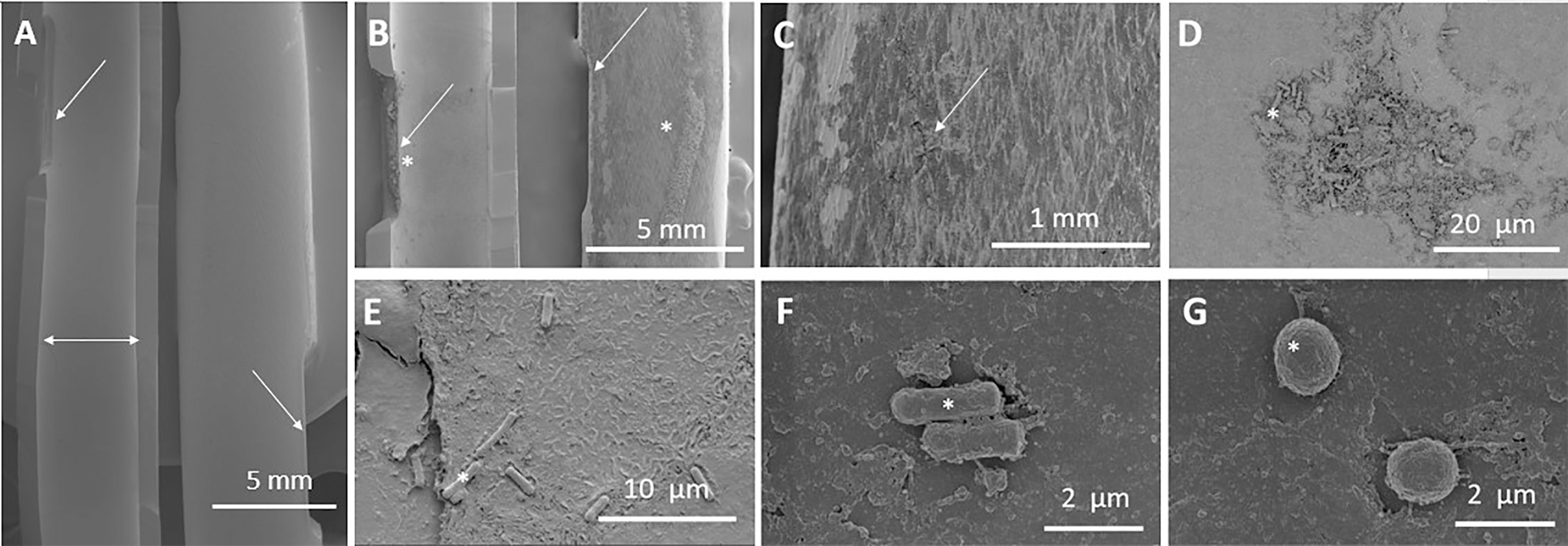
Figure 2 Microscopic analysis of catheter. This figure includes several images of both used and reused catheters at different magnifications. (A) Luminal (Left side, horizontal double headed arrow) and external (right side) surface of unused control catheter eyelets (angled arrows) at 6-fold magnification. (B-G) Display of a reused catheter over a period of 3 days. (B) Luminal (left) and external (right) surface eyelets at 10-fold magnification. Evidence of surface eyelet damage and debris (stars). (C) Catheter eyelets at 50-fold magnification. Microcracks in the external surface of the catheter (arrow). (D) Lumen of catheter at 2,000-fold magnification. Evidence of surface damage with debris collections and bacteria. (E) Rods and debris seen in the lumen at 5,000-fold magnification. (F) Rods seen in the lumen of the catheter at 20000-fold magnification. (G) Cocci seen in the lumen of the catheter at 20,000-fold magnification. Images (A-D) taken on the Hitachi SU3500 instrument and (E-G) taken on Helios 650 high magnetic instrument.
Discussion
Although it was not possible to quantify the daily increase in surface damage of catheters, it appears that daily catheter reuse in individuals with preexisting significant bacteriuria can place a burden as evidenced by the progressively increasing rates of gram stain cellularity and culture growth on the catheter swab samples taken over each of the three days of the study. Cells seen on gram stain included gram-positive bacilli and gram-positive cocci in addition to epithelial cells could have been be introduced into the bladder during IC and thus, represent a potential contamination. Additionally, this was supported by the fact that rods and cocci were visible on the internal and external surfaces of the catheter tips following the completion of the study. It was noteworthy that all three participants from our pilot case series exhibited no signs of clinical infection before, during or after the study, hence the presence of asymptomatic bacteriuria. Prevalence of asymptomatic bacteriuria among people with spinal cord injury who intermittently use catheters is usually between 23% and 69%, and such instances are usually advised against in regards to screening and treatment (15).
These are important preliminary results as there is currently no consensus as to whether cleaning and reusing intermittent catheters is both safe and effective, owing mainly due to a lack of published data on the topic. A recent systematic review (16) reported two studies (17, 18) that proposed cleaning methods which both completely sterilize and do not damage the structure of the intermittent catheter. A limitation to these studies was that these outcomes were assessed after just one reuse; therefore, their generalizability is questionable as many that chose to reuse do so more than once before disposing of their catheters. For example, the participants in our pilot case series indicated that they usually reuse their catheters for at least three days with one participant reporting reuse for up to fourteen days. To make our preliminary observations more generalizable we examined the impact of cleaning and reuse over the course of three days and allowed the participants to clean their catheters by their preferred method.
The gram stains of the catheter swab samples were negative for all samples from three participants on the first day of sampling; however, two of the three participants had evidence of red blood cells and epithelial cells in the second and third days of reuse. It is possible that this could be due to microtrauma during catheterization as SEM imaging showed that the catheter surfaces were damaged following the third day of catheterization (Figure 2).
Most of the catheter swab cultures were negative for growth except for contamination from skin flora. This may have been a consequence of contamination from improper technique when self-catheterizing. Once skin flora was seen on one catheter swab sample, most of the remaining swab samples from that same catheter were also positive for skin flora. It is possible that the cleaning method used between catheterizations was not bactericidal and therefore inadequate for its removal. Consequently, the microbial burden of the surface of the catheter increased daily with each reuse although this must have been due to contamination as neither Escherichia coli nor Klebsiella pneumoniae were present. In addition, it is possible that there could be viable but non-culturable bacterial contamination on the surface of the catheter which has been reported in previous literature following catheter cleaning and reuse (18). There was one catheter swab sample from the third participant, which after three days of reuse, had growth of Haematomicrobium sanguinis in addition to skin flora; however, the clinical significance of this was unknown. While it would be useful to know exactly which day bacterial burden was significant enough to result in positive swab cultures that reveal the bacterial species found in urine, our results bring into question the utility of performing multiple daily catheter swab samples. In future studies it may be more practical to perform catheter swab samples once daily and with a longer surveillance period.
The results of the urine cultures remained unchanged over the course of the study as every urine sample including the control samples collected prior to the start of the trial were positive for either Escherichia coli or Klebsiella pneumoniae. Consequently, we were unable to use these results to appreciate an increase in the microbial burden in the participant’s urine as a consequence of catheter reuse. One participant did grow mixed enteric flora on the final day of the study. It is possible that the additional enteric flora was a consequence of contamination from incomplete sterilization from cleaning and reuse, but there may be other explanations for this such as poor sterilization of the urethral meatus prior to insertion. In future studies it would be more useful to eradicate asymptomatic bacteriuria in participants (i.e. through antibiotics or prophylactic measures) prior to participation in the study or only include participants that have less than 100,000 CFU/L of urine, in order to get a better picture of when bacterial colonization occurs in the urine following catheter reuse. Alternatively, more precise bacterial colonization concentration lab testing could be done to observe the increase in CFU over time. Moreover, although there were changes present on the surface of the catheter from participants, it can be argued that the impacts of these changes on the culture results appears to be little and that there were no changes in culture composition given the high number of CFU/L among our participants.
The catheter tips that were imaged by SEM after the completion of the three-day study showed clear signs of surface damage, accumulation of debris and bacterial colonization both on the exterior surface and in the lumen of the catheters (Figure 2). This is consistent with other studies that had a longer duration of catheter reuse. For example, Kovindha et al. (19) found encrustation in the lumen of catheters that had been reused for 1.5 years. A more recent study by Newman et al. (10) saw bacterial contamination, biofilm and debris on the surface of catheters that had been reused for a mean of 21 days. In both of these studies, the users were able to clean their catheters by their method of choice. Finally, Wilks et al. (18) reported bacterial colonization and surface damage of their catheters; however, the catheters they used were contaminated artificially and each were cleaned by a different cleaning method.
While the results from the catheter swab samples and SEM images indicate that the process of cleaning and reuse may increase microbial burden and catheter surface damage in the short-term, these are preliminary data and there was no clinical detriment due to the increase in microbiological burden of the catheters, therefore determining whether these findings are clinically significant will require further inquiry. Our study was limited by the fact that we only were able to recruit 3 participants due to logistical difficulties of completing this study while adhering to the local COVID-19 guidelines at the time; therefore, we were unable to make causal inferences from this data. The participants included in the study routinely reused their catheters already, so attempting to determine a difference using catheter swab samples and urine cultures by comparing control samples with samples collected during the study was difficult. In addition, the presence of asymptomatic bacteriuria in the control samples is a confounding factor. A potential way to overcome these limitations could be to increase the size of the cohort, standardize the catheter cleaning method, ensure that participants do not have a significant bacteriuria at the beginning of the trial, and require that participants not reuse catheters for a certain period prior to starting the trial. Future studies should be longer and image catheters after varied days of reuse to evaluate when initial changes are seen and how surface damage and bacterial attachment progress over time. Ideally, randomizing patients to single use and reuse groups and analyzing bacterial counts through regular urine analysis of catheter catch urine specimens would best assess the impact of reusing catheters on bacterial colonization.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Research Ethics Board (REB) at the University of British Columbia (ethics number H17-03228). The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization: MG, RL, MW, and AK. Data curation: MG, RL, MW, and AK. Formal analysis: MG, RL, MW, and AK. Funding acquisition: MG, MW, and AK. Methodology: MG, RL, MW, and AK. Project administration: MG, RL, MW, and AK. Visualization: MG, RL, MW, and AK. Writing- original draft: MG and RL. Writing- review and editing: MW and AK. All authors contributed to the article and approved the submitted version.
Funding
MG (University of British Columbia – Faculty of Medicine Summer Student Research Program Award recipient) and AK (University of British Columbia – Faculty of Medicine, Department of Medicine, Endowed Chair in Rehabilitation Medicine). Matthias Walter – Michael Smith Foundation for Health Research (MSFHR) and Rick Hansen Foundation Postdoctoral Research Trainee Award (Grant number 17110).
Acknowledgments
We thank all three individuals for their participation in this study. Furthermore, we thank Gethin Owen (Center for High-Throughput Phenogenomics, UBC, Faculty of Dentistry, Vancouver, BC, Canada) for the electron microscopy images.
Conflict of interest
Author MW receives or has received research support from the Rick Hansen Institute & Foundation, the Michael Smith Foundation for Health Research, Pfizer Canada, Coloplast, and Wellspect. MW serves on advisory board for Coloplast. AK receives or has received research support from the Craig Neilsen Foundation, the Rick Hansen Institute & Foundation, the Canadian Institutes of Health Research, the Canadian Foundation for Innovation, the Heart and Stroke Foundation of Canada, the Michael Smith Foundation for Health Research, the Minnesota Spinal Cord Injury and Traumatic Brain Injury Research Grant Program, Wings For Life Spinal Cord Research Foundation, Pfizer, Allergan, Coloplast, and Purdue; serves on advisory boards for Coloplast, Wellspect, and the Craig H. Neilsen Foundation; and is Past-President of the American Spinal Injury Association (ASIA).
The authors declare that this study was supported by an unrestricted research grant from Coloplast A/S, Humlebaek, Denmark (grant number COLOAK- NLUTD-SCI: F18-03036). The funder was involved in the study design, interpretation of the data, and preparation as well as review of the manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fruro.2022.938968/full#supplementary-material
References
1. Groen J, Pannek J, Castro Diaz D, Del Popolo G, Gross T, Hamid R, et al. Summary of European association of urology (EAU) guidelines on neuro-urology. Eur Urol (2016) 69(2):324–33. doi: 10.1016/j.eururo.2015.07.071
2. Liu N, Fougere R, Zhou M-W, Nigro MK, Krassioukov AV. Autonomic dysreflexia severity during urodynamics and cystoscopy in individuals with spinal cord injury. Spinal Cord (2013) 51(11):863–7. doi: 10.1038/sc.2013.113
3. Teasell RW, Arnold JM, Krassioukov A, Delaney GA. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil (2000) 81(4):506–16. doi: 10.1053/mr.2000.3848
4. Walter M, Knüpfer SC, Leitner L, Mehnert U, Schubert M, Curt A, et al. Autonomic dysreflexia and repeatability of cardiovascular changes during same session repeat urodynamic investigation in women with spinal cord injury. World J Urol (2016) 34(3):391–7. doi: 10.1007/s00345-015-1589-1
5. Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma (2004) 21(10):1371–83. doi: 10.1089/neu.2004.21.1371
6. Krassioukov A, Cragg JJ, West C, Voss C, Krassioukov-Enns D. The good, the bad and the ugly of catheterization practices among elite athletes with spinal cord injury: a global perspective. Spinal Cord (2015) 53(1):78–82. doi: 10.1038/sc.2014.208
7. Taweel WA, Seyam R. Neurogenic bladder in spinal cord injury patients. Res Rep Urol (2015) 7:85–99. doi: 10.2147/RRU.S29644
8. Wyndaele J-J, Brauner A, Geerlings SE, Bela K, Peter T, Bjerklund-Johanson TE. Clean intermittent catheterization and urinary tract infection: review and guide for future research. BJU Int (2012) 110(11 Pt C):E910–917. doi: 10.1111/j.1464-410X.2012.11549.x
9. Avery M, Prieto J, Okamoto I, Cullen S, Clancy B, Moore KN, et al. Reuse of intermittent catheters: a qualitative study of IC users’ perspectives. BMJ Open (2018) 8(8):e021554. doi: 10.1136/bmjopen-2018-021554
10. Newman DK, New PW, Heriseanu R, Petronis S, Håkansson J, Håkansson MÅ, et al. Intermittent catheterization with single- or multiple-reuse catheters: clinical study on safety and impact on quality of life. Int Urol Nephrol (2020) 52(8):1443–51. doi: 10.1007/s11255-020-02435-9
11. Walter M, Krassioukov AV. Single-use versus multi-use catheters: Pro single-use catheters. Eur Urol Focus (2020) 6(5):807–8. doi: 10.1016/j.euf.2019.10.001
12. Prieto J, Murphy CL, Moore KN, Fader M. WITHDRAWN: Intermittent catheterisation for long-term bladder management. Cochrane Database Syst Rev (2017) 8:CD006008. doi: 10.1002/14651858.CD006008.pub4
13. Christison K, Walter M, Wyndaele J-JJM, Kennelly M, Kessler TM, Noonan VK, et al. Intermittent catheterization: The devil is in the details. J Neurotrauma (2018) 35(7):985–9. doi: 10.1089/neu.2017.5413
14. Kennelly M, Thiruchelvam N, Averbeck MA, Konstatinidis C, Chartier-Kastler E, Trøjgaard P, et al. Adult neurogenic lower urinary tract dysfunction and intermittent catheterisation in a community setting: Risk factors model for urinary tract infections. Adv Urol (2019) 2019:2757862. doi: 10.1155/2019/2757862
15. Nicolle LE, Gupta K, Bradley SF, Colgan R, DeMuri G, Drekonja D, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the infectious diseases society of America. Clin Infect Dis (2019) 68(10):e83–110. doi: 10.1093/cid/ciy1121
16. Grasdal M, Walter M, Krassioukov AV. The microbiological and physical properties of catheters for intermittent catheterization: A systematic review on the impact of reuse and cleaning methods. Spinal Cord (2022) 60(7):581-593. doi: 10.1038/s41393-021-00740-3
17. Bogaert GA, Goeman L, de Ridder D, Wevers M, Ivens J, Schuermans A. The physical and antimicrobial effects of microwave heating and alcohol immersion on catheters that are reused for clean intermittent catheterisation. Eur Urol (2004) 46(5):641–6. doi: 10.1016/j.eururo.2004.06.016
18. Wilks SA, Morris NS, Thompson R, Prieto JA, Macaulay M, Moore KN, et al. An effective evidence-based cleaning method for the safe reuse of intermittent urinary catheters: In vitro testing. Neurourol Urodyn (2020) 39(3):907–15. doi: 10.1002/nau.24296
19. Kovindha A, Mai WNC, Madersbacher H. Reused silicone catheter for clean intermittent catheterization (CIC): Is it safe for spinal cord-injured (SCI) men? Spinal Cord (2004) 42(11):638–42. doi: 10.1038/sj.sc.3101646
Appendix 1 Catheter sample preparation and fixation for Scanning Electron Microscopy (SEM) imaging protocol.
1. Cut the catheter and immersed in sterile cold saline.
2. Delivered to Center for high Throughput Genomics on ice.
3. Split the catheter in half using a scalpel.
4. Rinsed in 0.1M PIPES buffer pH 7.4 x3.
5. Fixed in 2.5% Glutaraldehyde (EM Grade) in 0.1M PIPES pH 7.4 for 30 min.
6. Rinsed in 0.1M PIPES buffer pH 7.4 x3.
7. Post fix in 1% Osmium Tetroxide in 0.1M PIPES pH 6.8 for 1h.
8. Rinsed x3 in dH2O.
9. Dehydrated in a graded ethanol (EM Grade) series 50, 60, 70,80 90, 100% for 5 min each then a further 2x in 100% Ethanol for 5 min each.
10. Critical point drying.
11. Mounted the sample in configurations so that the outer and inner surface is visible for analysis.
12. Sputter coated the samples with 20nm Iridium using a rotary device to make sure that all surfaces are electrically conductive.
13. Whole samples were imaged by SEM (Hitachi SU-3500 or Helios NanoLab 650 Focused Ion Beam SEM) using tiles each with equivalent magnifications of x500 and Zig Zag large area view software. The images were stitched by Image Composite Editor to create a high-resolution montage of the whole sample in JPEG.
Keywords: asymptomatic colonization, biofilm, electron microscopy, intermittent catheterization, spinal cord injury
Citation: Grasdal M, Lai R, Walter M and Krassioukov AV (2022) Short-term reuse of catheters is associated with microbiological and structural burden: A prospective pilot case series. Front. Urol. 2:938968. doi: 10.3389/fruro.2022.938968
Received: 08 May 2022; Accepted: 29 August 2022;
Published: 23 September 2022.
Edited by:
Vaidyanathan Subramanian, Southport and Ormskirk Hospital NHS Trust, United KingdomReviewed by:
Bakulesh Soni, Southport and Ormskirk Hospital NHS Trust, United KingdomLuisa Jordao, Instituto Nacional de Saúde Doutor Ricardo Jorge (INSA), Portugal
Copyright © 2022 Grasdal, Lai, Walter and Krassioukov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthias Walter, ZHIubWF0emVAZ214LmRl; Andrei V. Krassioukov, a3Jhc3Npb3Vrb3ZAaWNvcmQub3Jn
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share senior authorship
§ORCID: Mark Grasdal, orcid.org/0000-0002-8864-709X
Matthias Walter, orcid.org/0000-0001-5347-1584
Andrei V. Krassioukov, orcid.org/0000-0002-0022-7972
 Mark Grasdal1,2†§
Mark Grasdal1,2†§ Matthias Walter
Matthias Walter Andrei V. Krassioukov
Andrei V. Krassioukov