
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Urol., 12 August 2022
Sec. Pediatric, Adolescent and Developmental Urology
Volume 2 - 2022 | https://doi.org/10.3389/fruro.2022.893822
This article is part of the Research TopicWomen in Pediatric, Adolescent, and Developmental Urology: Volume IView all 11 articles
Background: Children, especially adolescents, constitute the most rapid growing demographic of nephrolithiasis. Due to the risks of recurrent stone disease, a 24-h urine analysis is recommended for the evaluation of children at risk of recurrent nephrolithiasis or those who are otherwise interested in further evaluation. However, data regarding patients most likely to have abnormal urine studies are sparse. We aim to identify predictors of abnormal 24-h urine studies in children presenting for evaluation of nephrolithiasis.
Methods: A retrospective review of children ≤17 years of age with a diagnosis of nephrolithiasis at both primary children’s hospitals within our state from 2012 to 2017 was performed. Children with an adequate initial 24-h urine study (creatinine ≥9 mg/kg/24 h) not on a thiazide or potassium citrate during the study were included. Factors associated with any abnormality [calcium ≥4 mg/kg; oxalate ≥45 mg/1.73 m2; citrate ≤310 mg/1.73 m2 (girls) or ≤365 mg/1.73 m2 (boys)] were evaluated as well as magnesium, uric acid, volume, sodium, and phosphorus.
Results: A total of 111 children were included, 69 of whom (62%) had at least one abnormal result. Of factors hypothesized to be associated with an abnormal 24-h urine study, only sex was significant (p = 0.001). Boys had a greater proportion of hypercalciuria (55%) and hypocitraturia (73%) and a slightly lower proportion of hyperoxaluria (48%) than those in girls.
Conclusion: Male sex was the only factor associated with an abnormal 24-h urine study, largely driven by increased rates of hypercalciuria and hypocitraturia in boys.
Pediatric nephrolithiasis has seen a steady rise in the past several decades, with adolescents showing the greatest rise in incidence across the entire age spectrum (1). These children have a 50% recurrence rate within 3 years, although risk stratification across the pediatric spectrum of disease has not been well defined (2). Each recurrent event poses additional risk for radiation exposure, anesthetic need, and surgical intervention, each of which carries unique morbidity in the pediatric population (3–6). Historically, there is limited research on the etiology of nephrolithiasis in pediatric patients.
Additionally, even in the absence of a known genetic cause of stones, nephrolithiasis may be a symptom of a more systemic disease process. Kidney stones may be one element of a larger disorder of calcium homeostasis. Preliminary work has suggested links between atherosclerosis or precursors to vascular disease in children with nephrolithiasis (7, 8). Furthermore, low bone density and an increased risk of skeletal fractures have also been associated with pediatric stone disease (7, 9, 10). Thus, evaluating the risk factors for urinary stone disease may have value in further understanding these more insidious disease processes and future health consequences in addition to assessing modifiable factors for risk reduction of future stone disease.
Traditionally, a 24-h urine analysis is recommended for the evaluation of patients at risk of recurrent nephrolithiasis, which includes all children diagnosed with stone disease (11). Children performing a 24-h urine analysis have been shown to have a 60% lower risk of stone recurrence, although the driving factors behind this association are unclear (2). While this evaluation can be beneficial in terms of highlighting underlying metabolic factors and guiding or reinforcing dietary strategies, they are expensive and logistically tasking on patients and their families (12, 13). Consequently, those who are economically disadvantaged are at risk of not completing the evaluation (12). The combination of these barriers has led to low completion rates and inadequate collections of 24-h urine in pediatric patients (12). Furthermore, even when a sample is adequate, a normal collection may not lead to a change in management (2, 13).
Due to the financial strain and the difficulty of obtaining a 24-h urine, the identification of patients most likely to have an abnormal urine study could avoid the unnecessary testing burden on patients and families. However, data regarding pre-evaluation factors indicative of a greater probability for 24-h urine abnormalities are sparse. To address this, we investigated patients with nephrolithiasis with 24-h urine studies in order to identify predictors of abnormal urine studies known prior to such an evaluation. These results could ultimately allow for a more effective selection of patients for urinary metabolic analysis. Because previous data have suggested that the highest rate of rise of kidney stone disease is in female subjects and adolescents, we hypothesized that female subjects and adolescents would be more likely to have abnormal 24-h urine studies.
We performed a retrospective chart review of children aged 0–17 years presenting with an index visit of nephrolithiasis at either of the two tertiary pediatric hospitals in the state, defined as the first visit for nephrolithiasis on or following January 1, 2012 (Site A), or January 1, 2014 (Site B) (based on the initiation of the electronic health record at each institution) and before January 1, 2018. The institutional review boards of each institution independently approved the study for research purposes. Given the minimal risk nature of this retrospective review, waivers of assent and consent were requested and granted by each institution. Children were included if they submitted a 24-h urine study for their evaluation during the study period. The decision to obtain a 24-h urine study was made through shared decision-making with providers and families. Children were excluded if they were on inhibitory medications for nephrolithiasis (i.e., thiazide, potassium citrate) or were lacking a height and weight at the time of the index visit (2). Additionally, 24-h urine samples were excluded if inadequately collected based on a primary and more restrictive definition, as outlined below.
The electronic health records including clinic visits, operative reports, lab results, and imaging were reviewed for data abstraction and entered into the Research Electronic Data Capture (REDCap) system housed at the data coordinating center (14). Patient demographics, comorbidities, personal and family history of nephrolithiasis, and 24-h urine results were analyzed. Comorbidities known or hypothesized to increase the risk of nephrolithiasis were captured including immobility, epilepsy, dependence on enteral feeding, neurogenic bladder, recurrent urinary tract infections (≥three infections per year documented preceding the index stone event), and prematurity. Medications known to or hypothesized to promote kidney stone formation (zonisamide, topiramate, furosemide, and prednisone) were recorded from the medication administration record at the time of the index clinic visit. Imaging review recorded the largest documented calculus. The follow-up interval was defined as the time to the most recent imaging follow-up.
The need for a 24-h urine study was determined by the treating provider. All 24-h urine studies were performed via one of two centralized laboratories [Litholink Corp. (Chicago, IL, USA) and UroRisk, Quest Diagnostics (Secaucus, NJ, USA)]. Abnormal urine studies were defined a priori as follows: calcium >4 mg/kg; oxalate >45 mg/1.73 m2; citrate <310 mg/1.73 m2 (girls) or <365 mg/1.73 m2 (boys) (15). Inadequate samples were defined as previously described by Saitz et al. (16) as ≤9 mg/kg/creatinine for our primary analysis. Acknowledging variable definitions for inadequate urine specimens, a sensitivity analysis used a more restrictive definition utilized by Litholink Inc., specifically, adequate creatinine/kg in boys of 11.9–24.4 and in girls of 8.7–20.3. If two consecutive 24-h urine studies were received (i.e., 48-h urine collection), the results for each analyzed component were averaged over the time period and counted as one sample. Post-hoc analyses for additional 24-h urine findings [volume, supersaturation of calcium oxalate (SS CaOx), supersaturation of calcium phosphate (SS CaP), urinary magnesium, urinary phosphate, and urinary uric acid levels] evaluated these variables as continuous, as opposed to categorical variables.
Body mass index (BMI) was calculated in standard fashion from height and weight available within 6 months of the index visit. BMI and BMI-for-age percentile were determined using the publicly available calculator via the Centers for Disease Control (17). Obesity was defined as BMI percentile ≥85%.
Primary outcome was defined as any abnormality in the 24-h urine sample. Secondary outcomes were defined as the number and characterization of the specific 24-h urine abnormalities based on the abnormal definitions referenced above.
Based upon the findings of our a priori hypothesis-driven analysis, we proceeded to perform several post-hoc analyses based on the primary finding of sex-associated differences in 24-h urine studies including comparisons of specific urinary citrate abnormalities by obese status and comparison of a more comprehensive set of urinary parameters as continuous variables.
Descriptive and comparative statistics were performed using Stata v15.1 (College Station, TX, USA). Single or averaged consecutive samples were utilized, and no-repeat samples from patients were included to avoid clustering of data. Comparative statistics were performed between those children with normal and abnormal 24-h urine studies using an unpaired two-tailed t-test for continuous variables and a chi-square test for categorical variables. A priori hypothesized factors influencing 24-h urine findings were as follows: age (<12 years; ≥12 years); sex (male/female); race (white/all others); ethnicity (Hispanic or Latino/all others); comorbidity (present/absent); recurrent stone disease (present/absent); family history of stone disease (present/absent); obesity (18). As only sex was significant on univariate analysis, no multivariate regressions were performed. Statistical significance was defined as a p-value <0.05. Assessment of urinary parameter distribution for each variable as a continuous variable was performed using a test for skewness, revealing that none of these parameters were normally distributed. As such, descriptive statistics are reported using medians with interquartile ranges and comparative statistics by sex were performed using a Wilcoxon rank sum test.
Of the 127 children <18 years of age from both sites with a 24-h urine study, 16 were excluded for lack of weight or height data (4), inadequate collection (5), or on potassium citrate or thiazide medications (7). No children had a diagnosis of primary hyperoxaluria. The remaining 111 children were eligible for analysis. Our cohort ranged from ages 3 to 17 years. Among the 111 children, 69 (62.2%) had at least one urine abnormality. Table 1 shows the demographic data of the included cohort. Thirty-eight (81%) boys had abnormalities in their 24-h urine studies, whereas 31 (48%) girls had abnormalities. Thirty-nine (74%) patients from Site A showed abnormal 24-h urine studies, whereas Site B had 30 patients (52%) with abnormalities, although this difference did not reach statistical significance. Comorbidities of patients with normal and abnormal 24-h urine studies are displayed in Table 2. Over half of the included population (60%) had one or more of the associated comorbidities. Substratified by comorbidity type, eight (100%) patients with immobility had abnormal 24-h urine studies. Nine children with gastrostomy tube (90%) had abnormal 24-h urine studies. Out of 10 children with epilepsy, seven (70%) of them had abnormal 24-h urine studies. Nine out of 11 children (82%) had abnormal 24-h urine studies while on a stone-promoting medication. Table 3 displays stone characteristics and presenting symptoms in children with nephrolithiasis during the initial evaluation. Eight children (67%) with obstruction had abnormal 24-h urine studies. Out of 15 asymptomatic children, 11 (73%) had abnormal 24-h urine studies. There were no significant differences in unilateral stone location vs. bilateral stone location for abnormal 24-h urine studies, with 53 children (63%) with unilateral and seven (64%) with bilateral kidney stones. Table 4 displays associating factors in abnormal 24-h urine studies. Thirty-eight male children (81%, p < 0.001) had abnormal 24-h urine results as compared to 31 (48%) female patients with abnormal 24-h urine studies. The remaining characteristics showed no statistical differences between normal and abnormal 24-h urine studies. Figure 1 displays the specific 24-h urine abnormalities based on sex. There was no difference in results after an additional subanalysis of all factors performed with the exclusion of patients with known comorbid factors. Table 5 displays the specific urinary parameter differences between male and female sex. There was a statistical difference in citrate and oxalate across sexes but no statistically significant differences in the comparison to other urinary parameters stratified by sex. Additional analysis showed no statistical difference between BMI and citrate levels (data not shown). Median 24-h urine volume/kg was stratified by any metabolite abnormality (14.5 ml/kg) vs. no abnormalities (8.6 ml/kg) (p = 0.0318, Wilcoxon rank sum test).
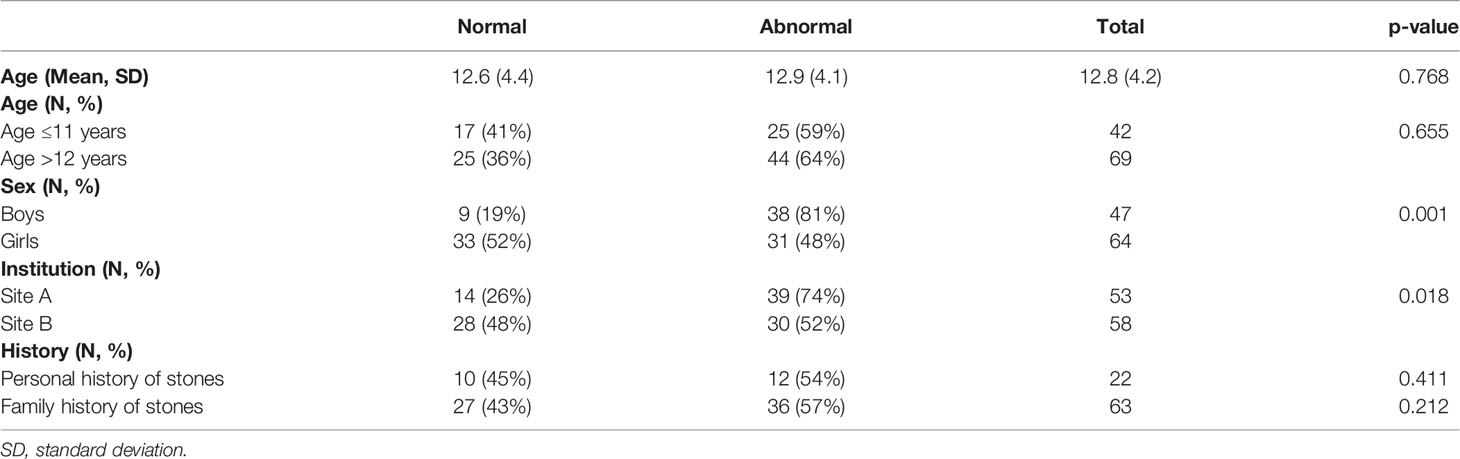
Table 1 Patient demographics, institution location, and history of nephrolithiasis of the included cohort.
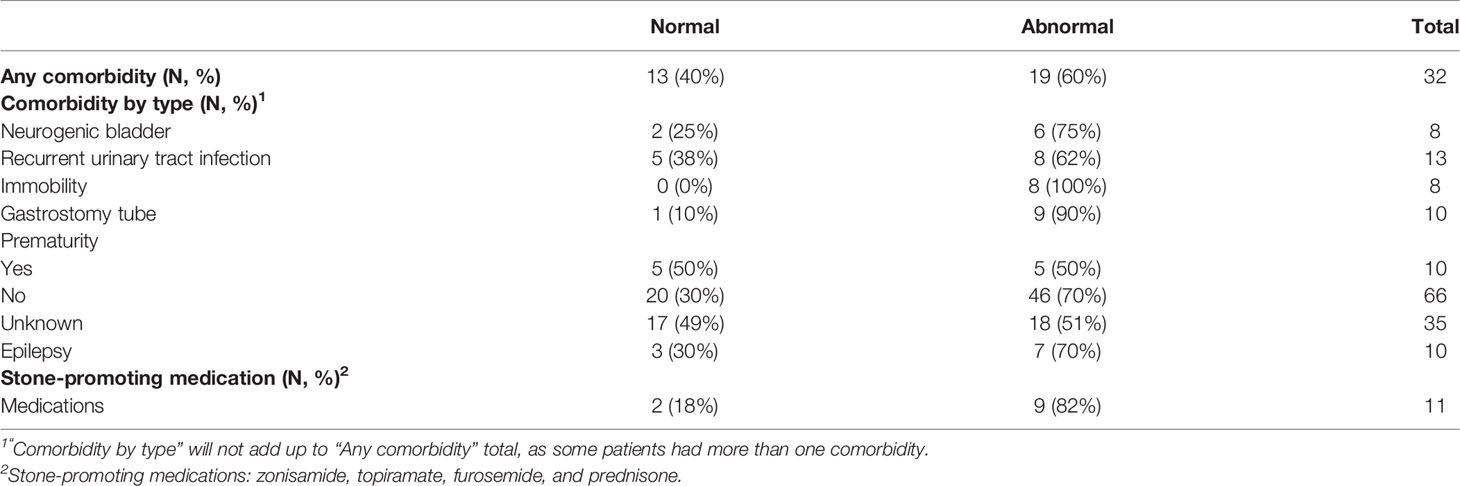
Table 2 Medical comorbidities of the included cohort stratified by any comorbidity and comorbidity by type.
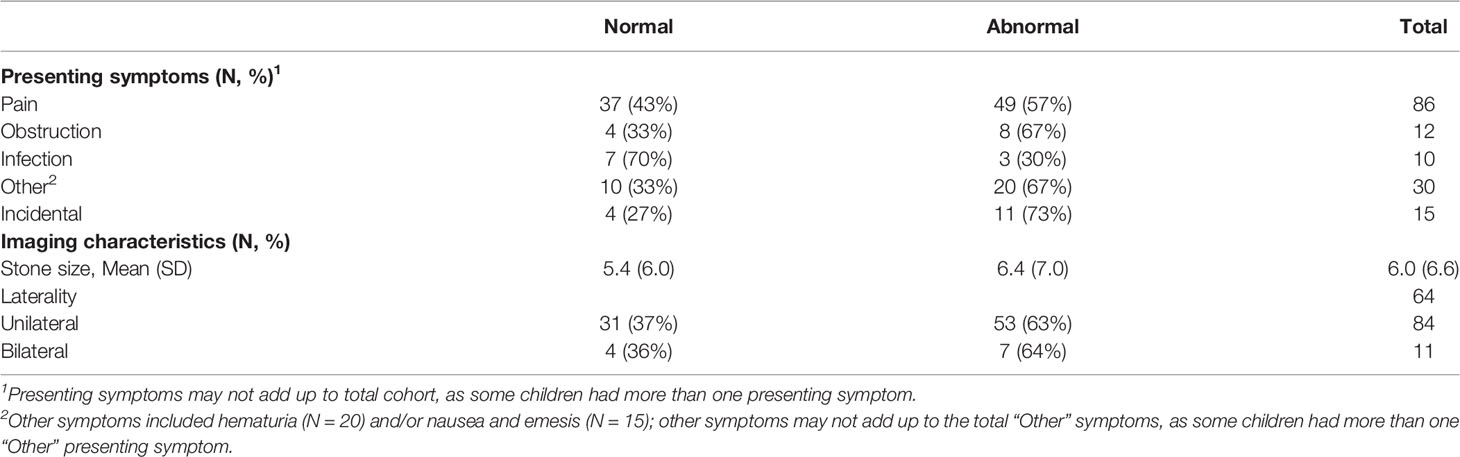
Table 3 Presentation of nephrolithiasis symptoms in children with 24-h urine studies with associated imaging characteristics of their most recent kidney stone(s).
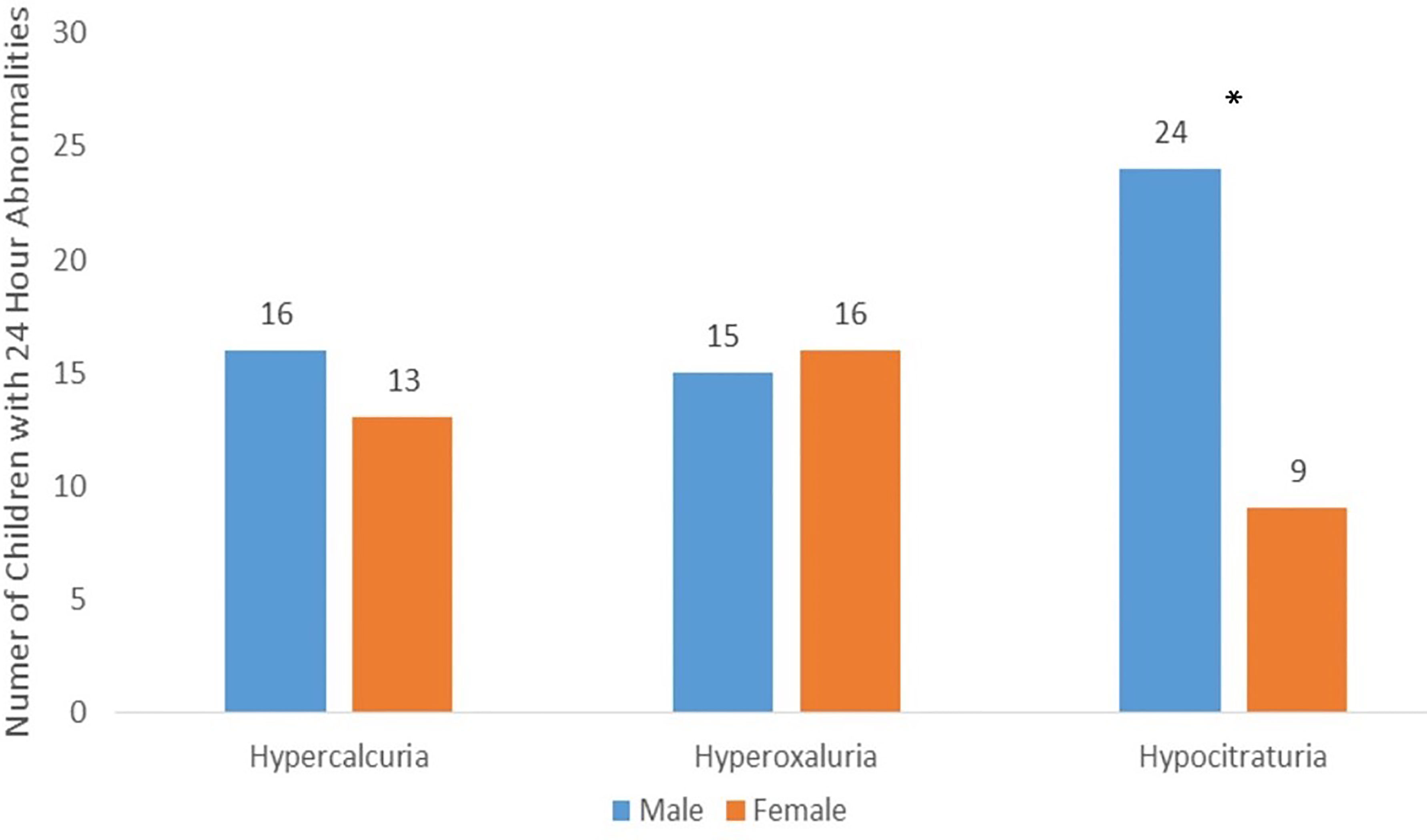
Figure 1 The 24-Hour Urine Abnormalities: The three most common abnormalities in 24-h urine results stratified by sex. Hypocitraturia was found to be significantly higher (p < 0.001) in boys than that in girls. There were no significant differences in 24-h urine abnormalities by sex for hypercalciuria and hyperoxaluria.
A subanalysis of 63 individuals who met more stringent criteria for 24-h urine collection adequacy found that sex-based differences in abnormal 24-h urine parameters remained significant between boys and girls (78% vs. 42%, p = 0.004), while other comparisons remained nonsignificant (data not shown).
This study analyzed initial 24-h urine studies of 111 eligible children with nephrolithiasis from both tertiary care pediatric hospitals in Wisconsin. Evaluation with a 24-h urine analysis is recommended by the American Urological Association (AUA) for all high-risk and interested patients, and completion of this test has been associated with a reduction in the recurrence of nephrolithiasis in pediatric patients (19). Indeed, Tasian et al. (2) reported a 60% risk reduction associated with the completion of 24-h urine studies in children and adolescents. Nonetheless, 24-h urine assessments are costly and tedious for patients to complete (12, 13). The tension between the potential benefit of these evaluations weighed against the burden of 24-h urine collections emphasizes the importance to risk-stratify children who may benefit from this testing. Chan et al. (20) found that a limited 24-h urine metabolic evaluation consisting only of urinary oxalate, calcium, citrate, and urine volume combined with a stone analysis would detect many significant metabolic abnormalities in the pediatric population. While a limited urinary metabolic evaluation is lower in cost, the additional data supplied with a complete 24-h urine collection (such as urinary salt supersaturation and urinary measurements of dietary factors) are critical in determining what further evaluation is necessary and often assist in devising a treatment strategy. Additionally, such an approach still does not eliminate the need for a 24-h urine collection. For these reasons, the concept of a limited 24-h urine evaluation has not been widely adopted.
Improving the identification of clinical (i.e., prior to testing) factors associated with 24-h urine anomalies could help to target pediatric patients who clearly require further metabolic evaluations and therefore avoid requiring select children to perform the cumbersome 24-h urine collection process altogether. Because previous data have shown rising incidence rates of kidney stone diseases in female subjects and adolescents, we hypothesized that these groups would be more likely to have an abnormal 24-h urine evaluation and therefore more expansive metabolic evaluations could be targeted to these groups (1, 21). Our cohort is demographically representative of other reported studies on pediatric nephrolithiasis in terms of both female predilection and the proportion of adolescents represented. Surprisingly, of the pre-evaluation identifiers we studied, male sex was the only factor associated with abnormal 24-h urine analysis. As such, our study emphasizes the importance of considering sex-based differences in kidney stone etiology. Additionally, it strongly suggests that all children, including boys, presenting with nephrolithiasis should undergo a 24-h urine collection to evaluate metabolic abnormalities, as we did not identify a distinct pattern predictive of abnormalities beyond male sex.
When a 24-h urine analysis is used in the initial evaluation of pediatric patients, the presence of a metabolic risk factor on a 24-h urine evaluation is seen in as many as 69%–90% of pediatric patients (22, 23). Prior studies have demonstrated that the most common abnormalities in the pediatric population are hypercalciuria and hypocitraturia in pediatrics (22–25). These findings are consistent with our study’s results with hypocitraturia (73% of boys, 27% of girls) and hypercalciuria (55% of boys, 44% of girls) as the most common abnormalities in our cohort. Hypocitraturia was the largest driver of differences in 24-h urine abnormalities across sexes.
Other studies have also identified sex-based differences in nephrolithiasis among various ages, although a specific focus on urinary abnormalities has been limited (26). Specifically, boys tend to have nephrolithiasis more commonly in the first decade of life, while girls more commonly exhibit nephrolithiasis in the second decade of life (27). Adolescent female subjects, in particular, have recently been identified as having the greatest increase in the incidence of nephrolithiasis across the entire life span of the disease (1). The variation in incidence rates seen across sexes may indicate a metabolic difference between male subjects and female subjects that contributes to previously reported differences in stone composition and urine studies (26). Female subjects tend to have struvite and calcium phosphate stones more commonly (28, 29). Healthy women have also been shown to have lower urinary calcium and higher citrate compared to men, which are likely protective factors against the formation of common calcium oxalate stones (30). Indeed, Kirejczyk et al. (31) found that healthy girls have been shown to have a large spike in urinary citrate when they reach adolescence. In the context of our findings, we believe that there may be an underappreciation of the protective effect of this urinary citrate surge, vis-à-vis typically reported “normative” values of citrate in adolescent female subjects. Hypocitraturia has also been found to correlate to lower consumption of potassium and magnesium suggestive of a possible dietary component to this change in urinary abnormalities. However, the specific drivers for alterations in urinary citrate levels as children age have not been well defined. Our data derive from a region where Western diets heavy on animal proteins, dairy, and processed food are prevalent, although it should be noted that we did not evaluate dietary parameters in our study specifically. Components of these diets have been linked to childhood obesity and may contribute to the risk of nephrolithiasis in children by altering the acid–base status of the urine, thereby reducing levels of buffered urinary citrate (32). Indeed, animal models focused on the specific impact of fructose consumption on urinary parameters demonstrated a decrease in inhibitors of urinary stone formation (33).
Although 24-h urine parameters are often evaluated in a dichotomized “normal” vs. “abnormal” fashion in clinical care, we acknowledge that risk assessment of urinary stone parameters exists on a continuum. As such, our secondary analysis evaluated urinary parameters as a continuous variable and stratified these by sex. Based on our findings, urinary citrate (higher in girls) and urinary oxalate (higher in boys) differed significantly across sexes. Urinary citrate is known to differ in adolescent male subjects and adolescent female subjects, which was taken into account when we generated our definitions of hypocitraturia for this analysis (31). However, urinary oxalate has not been previously reported to differ in the pediatric population. Otto et al. (34) reported on differences in urinary oxalate excretion in adults, with men having a higher proportion of hyperoxaluria in their series. While our dichotomized analysis showed no difference in hyperoxaluria, evaluating oxalate as a continuous risk factor certainly raises the opportunity to further investigate sex-related differences in oxalate homeostasis in the pediatric population as well.
Dietary modifications and fluid intake are mainstays in secondary kidney stone prevention for both children and adults. Fluid intake goals are directed by the Institute of Medicine recommendations, although children with nephrolithiasis likely require augmented fluid intake beyond these recommendations (35). While data suggest that pediatric and adolescent male subjects may be more likely to reach their fluid intake goals within the healthy population, the relationship of these data to the kidney stone-forming population is unknown (36). Our data suggest no significant difference in weight-adjusted fluid intake as stratified by sex and support a broad-based approach to fluid intake assessments. Acknowledging the importance of fluid intake (and therefore volume) on urinary stone risk, we assessed 24-h urine volume/kg as a function of normal vs. abnormal parameters and found that those children with a documented 24-h urine metabolic abnormality had higher urinary volumetric outputs than those with no known abnormality. This finding may suggest that for those children with no defined metabolic abnormality, fluid intake is a greater driver of risk than for those children who have a defined metabolic abnormality. However, we would emphasize that management strategies regardless of the 24-h urine study findings should include fluid intake as a mainstay of prevention.
Strategies to improve fluid intake are of great interest to children with urinary stone disease, their families, and their providers. As such, the results of trials such as the Prevention of Urinary Stones with Hydration study may illuminate successful strategies in further detail and specifically have enrolled an adolescent subcohort to focus on the pediatric population (37). Dietary modifications may influence a number of urinary stone parameters. High dietary protein intake may impact urinary pH, uric acid, and citrate excretion, while a diet high in sodium may influence urinary magnesium and calcium homeostasis (38). Although sex-related differences in diet may exist, some data suggest that dietary changes are more age-related, with both peripubertal boys and girls eating greater amounts of fast food, which could influence both dietary protein and sodium intake (39). Our clinics do not routinely recommend limiting protein intake in children, given the low rates of uric acid-based nephrolithiasis in children and the need for dietary protein in longitudinal growth and development throughout childhood. However, dietary salt limitation is a routine focus of our clinics’ standard dietary recommendations (15). While our study found no differences in assessment of unadjusted urinary magnesium, phosphorus, or uric acid across sexes, given the current paucity of data surrounding the importance of these factors in assessing urinary stone risk in children, further study is still needed. Unfortunately, we did not have standard dietary assessment documentation available to correlate these factors with dietary intake, although this focus would be ripe for future studies.
Our study has several limitations including its retrospective nature. Additionally, our institutions are tertiary care centers with a large referral population that may represent children more likely to have a 24-h urine evaluation recommended in their workup. Prior studies have also identified specific barriers to 24-h urine study completion, such as socioeconomic status and insurance status (12, 13). However, our study was unable to evaluate those children for whom a 24-h urine study was recommended but not completed. Furthermore, while including patients from multiple institutions expands the scope of representative patients, there should be caution in the generalizability of our results to patients who do not consume a western diet. The pediatric population also poses a challenge for the determination of adequate 24-h urine samples with urinary creatinine excretion varying significantly in pediatrics compared to adults due to their lower muscle mass, especially in those children with chronic disease states and muscle wasting. Importantly, our results were still significant after initial criteria were instituted and maintained even after subsequent analysis with a more stringent definition for adequate collections. Additionally, our study used only calcium, oxalate, and citrate to indicate an abnormal 24-h urine in a dichotomized fashion. Although there is a concern for possible missed abnormal 24-h urines, Chan et al. (20) demonstrated a limited urinary metabolic evaluation still detects the vast majority of clinically significant abnormalities. Lastly, we acknowledge that the 24-h urine analysis is not truly a dichotomized study, and the variation seen between urinary studies is quite significant. As such, further secondary analysis on a broader range of 24-h urine parameters was also performed. Additionally, the choice to act on these results is provider-dependent and can impact the usability of these data in clinical practice. However, the potential benefits of a 24-h urine evaluation due to its association with a 60% decreased risk of recurrence for pediatric nephrolithiasis cannot be ignored (2). While we cannot determine the clinical implications of our results, we believe that the significance of exploring sex-based differences is important in understanding the epidemiology of stone disease and the usefulness of 24-h urine evaluations going forward.
Despite these limitations, our multi-institutional study provides a large cohort to study 24-h urine analysis across pediatric stone formers. Our study sought to identify critical pre-evaluation factors to better select participants for 24-h urine screening, which can be tedious and costly, thereby maximizing their diagnostic potential. We discovered that male sex was the only pre-evaluation factor associated with an abnormal 24-h urine evaluation.
Recent studies have shown the relationship between sex and stone disease changes throughout the life span. This shift to assessing sex-based differences in kidney stone disease and our own study’s results add to the growing literature of biologic differences between sexes and propose the need for further evaluation of sex-based differences among pediatric stone formers. Our study also emphasizes the need for further investigation to delineate the value of 24-h urine evaluations and the possible benefits of risk stratification based on either 24-h urine anomalies or the risk of recurrent disease.
Our data also suggest that certain at-risk populations, such as children with epilepsy, gastrostomy feedings, or immobility, have a very high likelihood of having metabolic abnormalities on 24-h urine stone risk analysis, albeit underpowered in our analysis to detect a difference. Obtaining a 24-h urine collection from these patients is traditionally very logistically challenging; however, given the increased risk of identifying a potentially treatable urinary stone risk factor, it is important to pursue 24-h urine testing whenever feasible.
In conclusion, this study showed that male sex was significantly associated with an increased risk of an abnormal 24-h urine evaluation. Our study highlights the importance of evaluating sex-based differences in stone disease, especially in the pediatric population. With rising incidence rates and increased risk of recurrence in pediatric patients, further understanding of the pediatric stone disease is crucial in developing management strategies. Our data were unable to isolate other patient characteristics that could safely identify those patients who could avoid undergoing a 24-h urine metabolic workup. This finding reaffirms the current AUA recommendation that all patients at risk for future stone events, which at present time would include all children who present with nephrolithiasis, be offered a 24-h urine assessment to identify lithogenic risk factors. Future studies delineating sex-based differences will allow for a better understanding of 24-h urine evaluations and lead to a standardization of evaluation of pediatric stone formers, decreasing the use of unnecessary diagnostic evaluations in pediatric patients and overall care of pediatric stone formers.
De-identified raw data will be made available upon request, pending institutional data use agreement and regulatory review. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Children’s Wisconsin IRB and American Family Children’s Hospital IRB. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
The authors confirm contribution to the paper as follows: study conception and design: JE, NP data collection: AM, RM Author; analysis and interpretation of results: JE, NP, AM draft manuscript preparation: AM, JE, NP, KS All authors reviewed the results and approved the final version of the manuscript.
JE is a consultant for Dicerna, Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor EJ declared a shared advisory council Pediatric Urology Specialty Advisory Council for NSQIP-pediatric with the author JE at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to acknowledge the Division of Pediatric Urology at Children’s Wisconsin for providing internal divisional funding to support this project. This work would not have been possible without Edmond Bedjeti, Division of Pediatric Urology Clinical Research Coordinators, who provided regulatory and data management support for this project.
1. Tasian GE, Ross ME, Song L, Sas DJ, Keren R, Denburg MR, et al. Annual Incidence of Nephrolithiasis Among Children and Adults in South Carolina From 1997 to 2012. Clin J Am Soc Nephrol (2016) 11(3):488–96. doi: 10.2215/CJN.07610715
2. Tasian GE, Kabarriti AE, Kalmus A, Furth SL. Kidney Stone Recurrence Among Children and Adolescents. J Urol (2017) 197(1):246–52. doi: 10.1016/j.juro.2016.07.090
3. Miglioretti DL, Johnson E, Williams A, Greenlee RT, Weinmann S, Solberg LI, et al. The Use of Computed Tomography in Pediatrics and the Associated Radiation Exposure and Estimated Cancer Risk. JAMA Pediatr (2013) 167(8):700–7. doi: 10.1001/jamapediatrics.2013.311
4. Graham MR, Brownell M, Chateau DG, Dragan RD, Burchill C, Fransoo RR. Neurodevelopmental Assessment in Kindergarten in Children Exposed to General Anesthesia Before the Age of 4 Years: A Retrospective Matched Cohort Study. Anesthesiology (2016) 125(4):667–77. doi: 10.1097/ALN.0000000000001245
5. O'Leary JD, Janus M, Duku E, Wijeysundera DN, To T, Li P, et al. A Population-Based Study Evaluating the Association Between Surgery in Early Life and Child Development at Primary School Entry. Anesthesiology (2016) 125(2):272–9. doi: 10.1097/ALN.0000000000001200
6. Kokorowski PJ, Hubert K, Nelson CP. Evaluation of Pediatric Nephrolithiasis. Indian J Urol (2010) 26(4):531–5. doi: 10.4103/0970-1591.74453
7. Kovacevic L, Lu H, Caruso JA, Kovacevic N, Lakshmanan Y. Urinary Proteomics Reveals Association Between Pediatric Nephrolithiasis and Cardiovascular Disease. Int Urol Nephrol (2018) 50(11):1949–54. doi: 10.1007/s11255-018-1976-9
8. Kusumi K, Smith S, Barr-Beare E, Saxena V, Schober MS, Moore-Clingenpeel M, et al. Pediatric Origins of Nephrolithiasis-Associated Atherosclerosis. J Pediatr (2015) 167(5):1074–80.e2. doi: 10.1016/j.jpeds.2015.08.014
9. Denburg MR, Leonard MB, Haynes K, Tuchman S, Tasian G, Shults J, et al. Risk of Fracture in Urolithiasis: A Population-Based Cohort Study Using the Health Improvement Network. Clin J Am Soc Nephrol (2014) 9(12):2133–40. doi: 10.2215/CJN.04340514
10. Denburg MR. Skeletal Manifestations of Renal Disease in Childhood. Curr Opin Nephrol Hypertens (2016) 25(4):292–300. doi: 10.1097/MNH.0000000000000233
11. Pearle MS, Goldfarb DS, Assimos DG, Curhan G, Denu-Ciocca CJ, Matlaga BR, et al. ADe. Medical Management of Kidney Stones. AUA Guideline: J Urol (2014) 316–24. doi: 10.1016/j.juro.2014.05.006
12. Lee AS, McGarry L, Bowen DK, Tasian GE. Patient Characteristics Associated With Completion of 24-Hour Urine Analyses Among Children and Adolescents With Nephrolithiasis. Urology (2019) 127:102–6. doi: 10.1016/j.urology.2019.02.008
13. Carnes K, Howe A, Feustel PJ, Listman JA, White M, Kogan BA. 24-Hour Urine Collection for First Time Pediatric Stone Formers: Is It Worth it? J Pediatr Urol (2020) 387.e1–7. doi: 10.1016/j.jpurol.2020.12.001
14. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (Redcap)—a Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support. J Biomed Inf (2009) 42(2):377–81. doi: 10.1016/j.jbi.2008.08.010
15. Hernandez JD, Ellison JS, Lendvay TS. Current Trends, Evaluation, and Management of Pediatric Nephrolithiasis. JAMA Pediatr (2015) 169(10):964–70. doi: 10.1001/jamapediatrics.2015.1419
16. Saitz TR, Mongoue-Tchokote S, Sharadin C, Giel DW, Corbett S, Kovacevic L, et al. 24 Hour Urine Metabolic Differences Between Solitary and Multiple Stone Formers: Results of the Collaboration on Urolithiasis in Pediatrics (Cup) Working Group. J Pediatr Urol (2017) 13(5):506. doi: 10.1016/j.jpurol.2017.03.015
17. Prevention CfDCa. Healthy Weight, Nutrition, and Physical Activity: Child & Teen Bmi Calculator Centers for Disease Control and Prevention (2021). Available at: https://www.cdc.gov/healthyweight/bmi/calculator.html.
18. Chan KH, Moser EA, Whittam BM, Misseri R, Cain MP, Krambeck A, et al. Initial Collection of an Inadequate 24-Hour Urine Sample in Children Does Not Predict Subsequent Inadequate Collections. J Pediatr Urol (2019) 15(1):74.e1–.e7. doi: 10.1016/j.jpurol.2018.10.019
19. Pearle MS, Goldfarb DS, Assimos DG, Curhan G, Denu-Ciocca CJ, Matlaga BR, et al. Medical Management of Kidney Stones American Urological Association (2019). Available at: https://www.auanet.org/guidelines/kidney-stones-medical-mangement-guideline.
20. Chan KH, Moser EA, Whittam BM, Misseri R, Cain MP, Krambeck A. The Ability of a Limited Metabolic Assessment to Identify Pediatric Stone Formers With Metabolic Abnormalities. J Pediatr Urol (2018) 14(4):331.e1– e6. doi: 10.1016/j.jpurol.2018.08.005
21. Dwyer ME, Krambeck AE, Bergstralh EJ, Milliner DS, Lieske JC, Rule AD. Temporal Trends in Incidence of Kidney Stones Among Children: A 25-Year Population Based Study. J Urol (2012) 188(1):247–52. doi: 10.1016/j.juro.2012.03.021
22. Karabacak OR, Ipek B, Ozturk U, Demirel F, Saltas H, Altug U. Metabolic Evaluation in Stone Disease Metabolic Differences Between the Pediatric and Adult Patients With Stone Disease. Urology (2010) 76(1):238–41. doi: 10.1016/j.urology.2010.01.036
23. Kovacevic L, Wolfe-Christensen C, Edwards L, Sadaps M, Lakshmanan Y. From Hypercalciuria to Hypocitraturia–a Shifting Trend in Pediatric Urolithiasis? J Urol (2012) 188(4 Suppl):1623–7. doi: 10.1016/j.juro.2012.02.2562
24. DeFoor W, Minevich E, Jackson E, Reddy P, Clark C, Sheldon C, et al. Urinary Metabolic Evaluations in Solitary and Recurrent Stone Forming Children. J Urol (2008) 179(6):2369–72. doi: 10.1016/j.juro.2008.01.151
25. Spivacow FR, Del Valle EE, Boailchuk JA, Sandoval Díaz G, Rodríguez Ugarte V, Arreaga Álvarez Z. Metabolic Risk Factors in Children With Kidney Stone Disease: An Update. Pediatr Nephrol (2020) 35(11):2107–12. doi: 10.1007/s00467-020-04660-x
26. Ellison JS, Tasian GE. The Impact of Sex and Gender on Clinical Care and Research Design in Nephrolithiasis. Urology (2021) 151:54–7. doi: 10.1016/j.urology.2020.04.089
27. Novak TE, Lakshmanan Y, Trock BJ, Gearhart JP, Matlaga BR. Sex Prevalence of Pediatric Kidney Stone Disease in the United States: An Epidemiologic Investigation. Urology (2009) 74(1):104–7. doi: 10.1016/j.urology.2008.12.079
28. Gabrielsen JS, Laciak RJ, Frank EL, McFadden M, Bates CS, Oottamasathien S, et al. Pediatric Urinary Stone Composition in the United States. J Urol (2012) 187(6):2182–7. doi: 10.1016/j.juro.2012.01.124
29. Daudon M, Dore JC, Jungers P, Lacour B. Changes in Stone Composition According to Age and Gender of Patients: A Multivariate Epidemiological Approach. Urol Res (2004) 32(3):241–7. doi: 10.1007/s00240-004-0421-y
30. Sarada B, Satyanarayana U. Urinary Composition in Men and Women and the Risk of Urolithiasis. Clin Biochem (1991) 24(6):487–90. doi: 10.1016/s0009-9120(05)80007-4
31. Kirejczyk JK, Porowski T, Konstantynowicz J, Kozerska A, Nazarkiewicz A, Hoppe B, et al. Urinary Citrate Excretion in Healthy Children Depends on Age and Gender. Pediatr Nephrol (2014) 29(9):1575–82. doi: 10.1007/s00467-014-2806-7
32. Paiste HJ, Moradi L, Assimos DG, Wood KD, Dangle PP. Is There an Association Between Childhood Obesity and Pediatric Kidney Stone Disease? A Literature Review. Uro (2021) 1(3):108–17. doi: 10.3390/uro1030014
33. Ross SS, Masko EM, Abern MR, Allott EH, Routh JC, Wiener JS, et al. The Effect of Dietary Sodium and Fructose Intake on Urine and Serum Parameters of Stone Formation in a Pediatric Mouse Model: A Pilot Study. J Urol (2013) 190(4 Suppl):1484–9. doi: 10.1016/j.juro.2013.02.3199
34. Otto BJ, Bozorgmehri S, Kuo J, Canales M, Bird VG, Canales B. Age, Body Mass Index, and Gender Predict 24-Hour Urine Parameters in Recurrent Idiopathic Calcium Oxalate Stone Formers. J Endourol (2017) 31(12):1335–41. doi: 10.1089/end.2017.0352
35. Tasian GE, Copelovitch L. Evaluation and Medical Management of Kidney Stones in Children. J Urol (2014) 192(5):1329–36. doi: 10.1016/j.juro.2014.04.108
36. Kant AK, Graubard BI. Contributors of Water Intake in Us Children and Adolescents: Associations With Dietary and Meal Characteristics–National Health and Nutrition Examination Survey 2005-2006. Am J Clin Nutr (2010) 92(4):887–96. doi: 10.3945/ajcn.2010.29708
37. Scales CD Jr., Desai AC, Harper JD, Lai HH, Maalouf NM, Reese PP, et al. Prevention of Urinary Stones With Hydration (Push): Design and Rationale of a Clinical Trial. Am J Kidney Dis (2020) 898–906.e1. doi: 10.1053/j.ajkd.2020.09.016
38. Penniston KL, Nakada SY. Updates in the Metabolic Management of Calcium Stones. Curr Urol Rep (2018) 19(6):41. doi: 10.1007/s11934-018-0791-2
Keywords: pediatric, urolithiasis, risk factors, urinalysis, 24-hour urine analysis
Citation: Moyer A, Ellison JS, Medairos R, Sheridan KR and Paloian NJ (2022) Factors Associated With Abnormal Initial 24-Hour Urine Studies in Pediatric Nephrolithiasis: Can We Better Select Patients for Evaluation? Front. Urol. 2:893822. doi: 10.3389/fruro.2022.893822
Received: 10 March 2022; Accepted: 10 June 2022;
Published: 12 August 2022.
Edited by:
Emilie K Johnson, Ann & Robert H. Lurie Children’s Hospital of Chicago, United StatesReviewed by:
Janelle Fox, Children’s Hospital of The King’s Daughters, United StatesCopyright © 2022 Moyer, Ellison, Medairos, Sheridan and Paloian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Moyer, YW1veWVyQGFsdW1uaS5tY3cuZWR1; Jonathan S. Ellison, amVsbGlzb25AY2h3LmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.