
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Urol. , 15 June 2022
Sec. Urologic Oncology
Volume 2 - 2022 | https://doi.org/10.3389/fruro.2022.891798
This article is part of the Research Topic Rising Stars in Urologic Oncology: 2023 View all 5 articles
 Mohammed Shahait1*
Mohammed Shahait1* Nadine Hamieh2
Nadine Hamieh2 Ryan W. Dobbs3
Ryan W. Dobbs3 Tuan Nguyen4
Tuan Nguyen4 Hamzeh Alshannaq5
Hamzeh Alshannaq5 Jessica Kim6
Jessica Kim6 Ayah El-Fahmawi6
Ayah El-Fahmawi6 Daniel J. Lee6
Daniel J. Lee6 David I. Lee4
David I. Lee4Objective: To compare the association between previous local treatment modalities and the progression to castrate-resistant prostate cancer (CRCP) and overall survival (OS) in men with newly diagnosed metastatic prostate cancer.
Methods: We conducted a retrospective analysis using a nationwide, de-identified electronic health record (EHR)-derived database (Flatiron). Eligible patients had previously received radiation therapy (RT) or radical prostatectomy (RP) for their local disease, and had progressed to metastatic disease. Stratified Kaplan-Meier estimates by local treatment were used to measure OS from the date of metastasis diagnosis. Cox proportional models were used to test the association between prior local treatment, progression to CRPC, and death, after adjusting for patient-and disease-specific parameters. Also, we conducted a propensity score-matched analysis.
Results: Of the 1,338 patients who met the inclusion criteria, 46% underwent RP with or without adjuvant RT and 54% received RT. Median follow up for RP group and RT group were 38.6 months (32.6-45.4) and 26.0 months (I24.3-29.9), respectively. After adjusting for patient-and disease-specific parameters, the patients who received RT had a higher risk of developing CRPC than those in the RP group 1.36 [1.05-1.76]. After propensity score matching and adjusting for patient and disease-specific parameters, men who received RT had higher risk of death compared to their counterparts (HR:1.36, 95% CI:1.1-1.65, P= 0.003)
Conclusion: Real-world data suggest that patients with metastatic disease who had undergone prior RP might have a lower risk of developing a castrate-resistant state and improved OS compared to patients who had received RT. Significant amount of bias limits validity and strength of our findings. Whether type of local treatment influence the disease behavior remains open question and should be answered only within randomized trial.
Metastatic prostate cancer is a heterogeneous disease that encompasses both de novo castrate-sensitive metastatic prostate cancer at initial presentation and its progression following definitive therapy for localized disease with either radical prostatectomy (RP) or radiation therapy (RT). Mounting evidence suggests that patients with de novo castrate-sensitive metastatic prostate cancer at initial presentation have worse prostate cancer-specific and overall survival (OS) than patients who progress to metastatic disease following definitive therapy for localized disease (1–4).
There is conflicting evidence on the association of different types of primary treatment on time to develop castrate-resistant prostate cancer (CRPC) and OS, although recent studies have suggested a possible benefit for men undergoing RP as compared to RT (3–5). This might be attributed due to selection bias, and retrospective nature of these studies. However, some authors have theorized that tumor self-seeding of the irradiated prostate leads to increased metastatic potential of the cancer cells within the prostate. The potential benefit of local treatment of the prostate on subsequent response to androgen deprivation therapy (ADT) and number of treatment lines before death have not yet been evaluated (3–5).
The objective of this study was to assess the association of previous local treatment modalities with progression to a castrate-resistant state and OS in men who progressed to metastatic prostate cancer by utilizing real-world registry data.
During the study period, the de-identified data originated from approximately 280 US cancer clinics (~800 sites of care) with more than 2.2 million cancer patients. The database includes 10,210 patients with prostate cancer between January 1975-June 2018. De-identified EHR-derived patient information was constructed to allow for longitudinal patient-level data from real-world practice settings, and datasets were prospectively constructed by technological abstraction supplemented by manual review by centrally trained oncology nurses and tumor registry specialists with an oncologist overview. All datasets undergo statistical and scientific review for the purpose of quality control to ensure the accuracy and validity of factors. Computer algorithms have been developed to account for missing data elements of patient records, a common pitfall for real-world data studies (6).
Following approval from the University of Pennsylvania and Copernicus Group Institutional Review Board, we conducted an ad hoc analysis of a real-world prostate cancer longitudinal database for patients with metastatic prostate cancer between January 1, 2013 (first date of metastasis) and June 31, 2018 (last date of follow-up).
Patients with non-metastatic CRPC (n=520) and those enrolled in clinical trials (n=371) were excluded. We then focused on patients who were diagnosed with metastatic prostate cancer between January 1, 2013-December 31, 2017 (n=8,455) after excluding patients with erroneous data (n=126), with a diagnosis of metastatic prostate cancer before January 2013, and with unknown CRPC (n=107). In this population, patients who had less than six months of follow-up (n=3,281) and/or fewer than one clinical visit (n=65) were excluded to allow for a minimum of six months of follow-up and at least one clinical visit after metastasis diagnosis. Patients diagnosed with localized prostate cancer before January,2000 were excluded (n=919). In this analysis cohort, patients with preexisting or synchronous primary malignancy (n=280) and those who developed leukemia at any point during the study period (n=52) were excluded. Patients with no documented treatments and those who received alternative treatment options for localized disease, such as high-intensity focal ultrasound (HIFU), cryotherapy, or primary ADT (n=2,470), were also excluded (7).. Therefore, our final population consisted of 1,388 patients who were followed up for a castrate-resistant state and OS from January 1, 2013-June to 31, 2018 (Supplementary Figure 1).
The type of local treatment was assessed based on the diagnostic criteria for metastatic prostate cancer based of the International Classification of Diseases (ICD) 9th and 10th editions, with subsequent manual review to confirm prior localized prostate cancer treatment. Local treatments before metastasis diagnosis, including RP ± ADT ± RT and RT ± ADT, were assessed. For this analysis, we selected patients who had previously received RT or RP for localized PCa. Receipt of ADT as an adjuvant treatment before metastasis was noted. In addition, the types of systematic treatments, such as chemotherapy, immunotherapy, and second-line hormone treatment (abiraterone and enzalutamide), were compared.
Patient-and disease-specific baseline parameters were abstracted. These parameters included age, race, PSA levels at the index date in ng/mL (<10, ≥10 and <20, ≥20), Gleason score (<8 or ≥8), Eastern Cooperative Oncology Group (ECOG) performance score (<2 or ≥2), year of primary treatment (2000-2005; 2006-2008; 2009-2011; 2012-2017), and site of metastasis. Regarding the year of primary treatment, the year 2000 was the first year of treatment, as the number of patients who underwent RP before 2000 was less than 10,and this variable was assessed as quartiles while considering the first quartile (2000-2005) as the reference group.
To study the association between treatment type and progression to a castrate-resistant state, the endpoint was from the index date (first date of metastatic disease, January 1, 2013) to the date of CRPC. Patients were considered to have progressed to CRPC if there was documentation in the database based on clinical practitioner notes. To study OS, the endpoint was from the index date to a) the end of the study (June 31, 2018) and b) the last activity date. The difference between the endpoints and the index date was divided by 30 because the unit was in days.
All P-values were two-sided (α P = 0.05). All statistical analyses were performed using SAS software (version 9.4, SAS Institute, Cary, NC, USA). Each participant contributed person-time from the index date to a) CRPC, b) death, c) last follow-up, and d) the end of the study, whichever came first.
Descriptive estimates of the baseline patient and disease-specific parameters by type of local treatment were computed using chi-square tests and independent t-tests for discrete and continuous variables, respectively.
To minimize selection bias, we conducted a propensity score-matched analysis. For our study, the propensity of treatment type (RP and RT) was estimated. The propensity score was calculated from a multivariable logistic model considering the following variables: age, race, Gleason score, PSA, site of metastasis, ECOG score, treatment lines and year of primary treatment. Based on the resulting propensity score, patients with RP were matched 1:1 to patients with RTy using nearest neighbor matching without replacement. After 1:1 propensity score matching, the final analysis included 468 cases each in the RP and RT groups. Patient’s characteristics and outcomes were compared between the two groups before and after propensity-score matching. All propensity score matching analyses were conducted in R (R Core Team, 2014).
After propensity score matching, survival analysis for OS according to the type of local treatment was performed to estimate the median and interquartile range (IQR) (i.e., the interval between the 25th and 75th percentiles) and survival probabilities from the index date for both types of treatment using the Kaplan-Meier (KM) method (8). Patients who did not die were censored on the date of their last follow-up. Weighted log-rank tests were performed to assess survival between the two types of local treatments.
After propensity score matching, Cox proportional hazards regression was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between the type of local treatment and a) CRPC and b) death. To study the association between the type of local treatment and death, we used Cox proportional hazards regression to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). The roughly parallel stratified Kaplan-Meier curves log (-log (survival)) versus log (time) transformation verified the proportionality of the hazards.
Table 1 summarizes the patient characteristics. Of the 1,388 included patients meeting the inclusion criteria, 644 (46%) underwent RP with or without adjuvant radiation and 744 (54%) received RT alone. Compared to the RT group, men who developed metastasis in the RP group were younger and had a better ECOG performance status (all P <0.01). There was no significant difference in the administration of adjuvant ADT between the two groups (P=0.08). Propensity score matching significantly reduces baseline differences between groups (Table 1). The matching plot (Supplementary Figure 2) shows the mean difference in all variables between two groups before and after PS matching. From this plot, it is clear that balance was relatively poor before matching, but complete matching improved balance on all covariates, and most within a threshold of 0.1.
The median follow-up time was 38.6 months (IQR: 32.6-45.4) for the RP group and 26.0 months (IQR: 24.3-29.9) for the RT group (Supplementary Table 1). The median time to develop metastasis from time of initial diagnosis was 78.9 (IQR: 43.2-126.3) months for the RP group, and 73.1 (IQR: 39.6-113.6) months for the RT group. Among patients identified as having CRPC, the median time from metastasis diagnosis to CRPC was 20.0 months (IQR: 13.7-28.6) for the RP group and 13.1 months (IQR: 11.5-14.8) for the RT. After propensity score matching, patients who received RT had 1.31 times higher risk of developing a castrate-resistant state from metastasis diagnosis compared to the RP group (HR: 1.36, 95% CI: 1.07-1.61, P= 0.009) (Table 2).
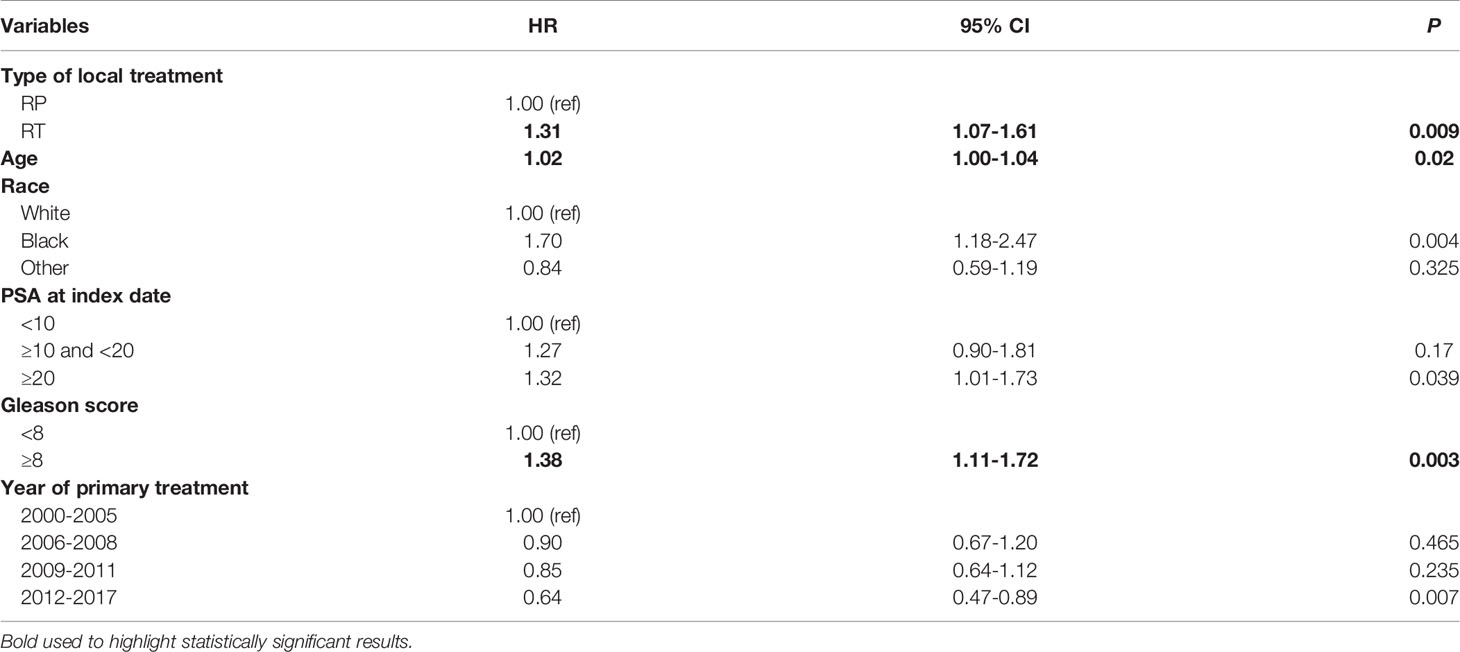
Table 2 Association between a) type of local treatment, b) patient and disease-specific parameters and castrate-resistant state in the de-identified database after propensity score matching, 2013-2018 (Hazard ratios (HR), 95% confidence intervals (CI), P-value).
The OS of the RP group was higher than that of the RT group (Supplementary Figure 3). Indeed, the medians of OS for the RP group were 38.6 months and 27months for the RT group (P <0.0001) (Supplementary Table 1) After propensity score matching and adjusting for patient and disease-specific parameters, men who received RT had higher risk of death compared to their counterparts (HR:1.35, 95% CI:1.1-1.65, P= 0.003) (Table 3).
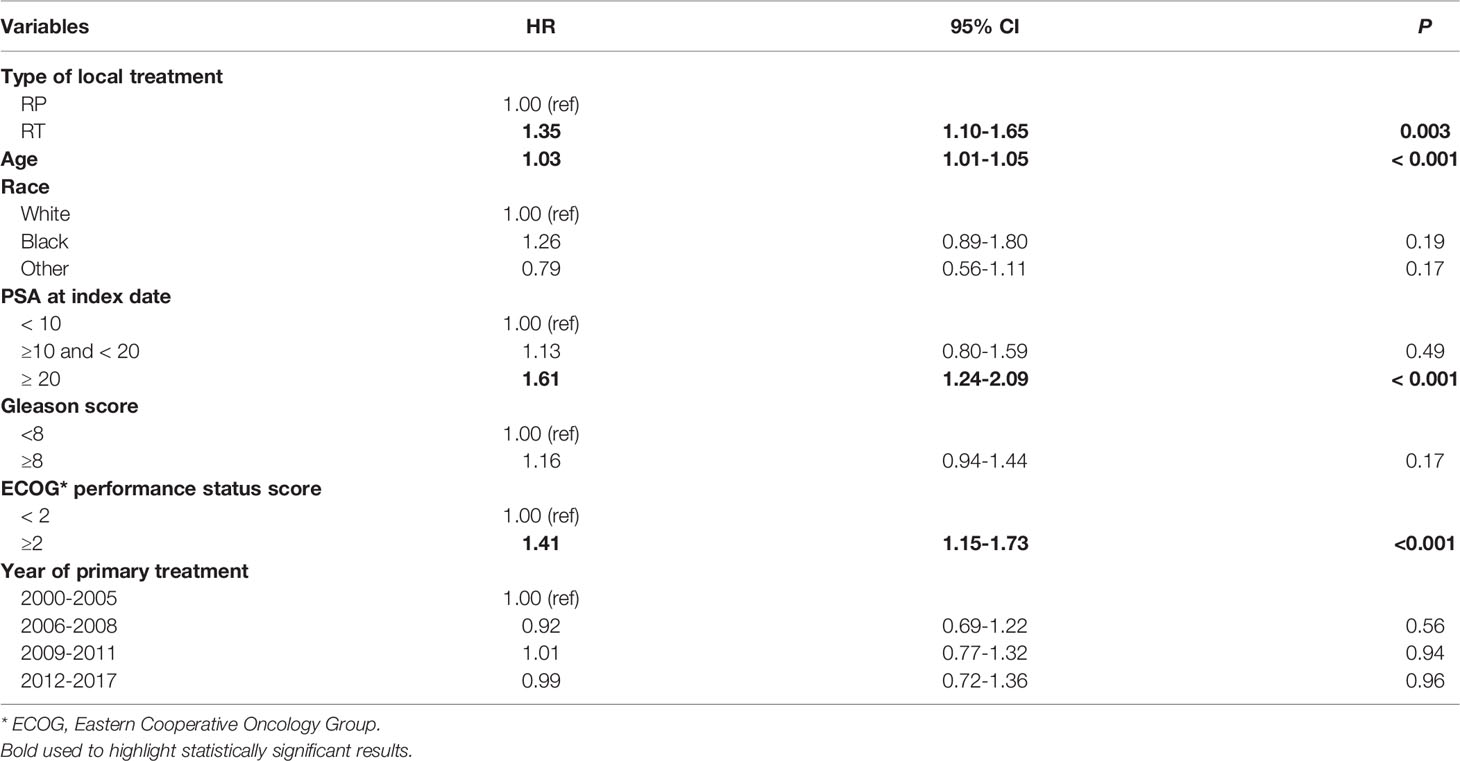
Table 3 Association between a) type of local treatment, b) patient and disease-specific parameters and death in the de-identified database after propensity score matching, 2013-2018 (Hazard ratios (HR), 95% confidence intervals (CI), P-value).
In this cohort of patients who developed metastatic disease, prior definitive local treatment with RT was associated with an increased risk of developing CRPC and decreased OS compared with RP. There is increasing evidence in the literature that patients with metastatic prostate cancer who received prior local treatment had improved OS compared to those who did not (1–5, 9). However, the results from these studies should be interpreted cautiously, as they compared patients who progressed to metastatic disease following definitive therapy for localized disease to those presenting with de novo metastatic prostate cancer. Patients with de novo metastatic disease tend to have more biologically aggressive disease than those who develop metastasis after primary treatment, which might explain why the prospective data showed no benefit of RT or cytoreductive radical prostatectomy (CRP) on the OS of patients with de novo metastatic disease compared to the best therapy group (10, 11). Therefore it is prudent not to mix these two biologically distinct populations in comparative studies that assess cancer-specific or OS (12, 13). In this study, we excluded patients with de novo metastatic disease a priori, to make the cohort population more biologically homogeneous.
The development of metastatic disease is an uncommon event after primary treatment for prostate cancer, and it might be challenging to recruit such patients in randomized clinical trials. In a prior study of the Surveillance Epidemiology and End Results (SEER) database, in a cohort of 66,492 men who underwent curative local treatment for prostate cancer, only 2,802 (4.2%) of men developed metastatic disease within a 7.3 year median follow up (14). Similarly, over a shorter period of 5 years, only 250 men out of 30,936 (0.8%) in the Center for Prostate Disease Research and Johns Hopkins University database were found to have metastatic disease after RP (15). In modeled incidence studies, it has been estimated that in 2020, patients with biochemical failure after local therapy (nmCRPC) comprised approximately 58,960 men annually with a 34% annual progression rate to metastatic disease (16).
The finding of decreased OS in men with metastatic prostate cancer who had undergone prior RT is in line with the post-hoc analysis of the Southwest Oncology Group (SWOG) Study 8894 trial, although the SWOG trial was performed in the early era of prostate specific antigen (PSA) screening and represented a cohort of patients with an earlier stage of disease and without prior ADT treatment (1). Also, In our analysis of such cohort, RP was associated with a decreased risk of developing CRPC after adjusting for patient-and disease-specific parameters. In addition to the selection bias at the time of administering local treatment before metastasis, these findings might be explained as follows. First, the PSA at metastasis diagnosis for the RP group was lower than that of the RT group, which might account for a lead time bias associated with the early administration of life-prolonging treatments. In addition to the differences in sensitivity for detecting biochemical recurrence following RP, as PSA is anticipated to be undetectable following successful treatment as compared to RT,where residual PSA following treatment is expected. Second, radiation might play a role in potentiating the epithelial-mesenchymal transition (EMT), which enhances cell migration, invasion, and poor response to ADT and docetaxel (17–19). Also, this might be attributed to the intratumoral heterogeneity of radiosensitivity; as a result, there is rapid selection of radiation-resistant cells over the course of fractionated radiation therapy (20, 21). Finally, the patients in the RT group were older and had poorer performance status, and higher comorbidities than those in the RP group, which might preclude the administration of docetaxel as well as the direct association of these factors with OS. Nevertheless, we did not notice any difference in the proportion of patients who received docetaxel or second-line hormonal therapy between the two groups.
To date, level 1 evidence comparing the efficacy of RP and RT for localized disease is lacking. Wallis et al. conducted a well-designed meta-analysis that pooled 118,830 patients with localized prostate cancer and found that RT had a significantly higher risk of death (OM: HR, 1.63; 95% CI, 1.54–1.73; PCM: HR, 2.08; 95% CI, 1.76–2.47) (22). Definitely, the data used in this metanalysis are heterogeneous, and should be cautiously interpreted as it lacks details of tumor and patients related factors that might affect overall survival. The results from our study are consistent with these findings and show that the type of local treatment used before metastasis might influence disease behavior after metastasis, and this is usually unaddressed during patient counseling about local treatment options.
Interestingly, the median overall survival for the RP group was 38.6 months for the RP group and 27 months for the RT group, which are comparable to the OS noted in SWOG 8894, and lower than OS reported by CHAARTED, ENZAMET, and ARCHES trials (1, 23). This might reflect the fact that over half of metastatic hormone-sensitive PCa (mHSPC) patients treated in real-world settings in the USA do not receive intensified first-line treatment (ADT plus docetaxel or second-line hormone treatment) (24, 25).
Our study had several limitations that warrant discussion. First, the Flatiron metastatic prostate cancer database was not designed to compare the different types of local treatments. In other words, no detailed information on whether the individual had RP only or RP followed by RT was available; hence, no further analyses were conducted to determine the differences between the two groups. We only assessed whether an individual had undergone RP or RT. In addition, it does not capture any data on treatment from the onset of biochemical recurrence until the development of metastasis. Second, this database included selection bias, incomplete documentation of the duration and type of ADT, cause of death, sequence of systematic treatment, indications to change from one treatment line to another, and unmeasured confounding factors (education level, medical literacy, income, and social support). Third, there were missing data on metastasis location, which precludes the possibility of assessing the pattern of metastasis after each type of local treatment and correlating it with the primary endpoints. Finally, the performance status and medical comorbidities of the patients at different time points were not available, and this may have influenced the decision to receive the type of local treatment and subsequent treatment; however, we did not notice any difference in the proportion of patients who received docetaxel or second-line hormone therapy between the two groups.
In this study, real-world data indicated that patients with metastatic prostate cancer who had undergone prior RP might have a lower risk of developing a castrate-resistant state and improved OS compared to patients who had received RT. Significant amount of bias limits validity and strength of our findings. Whether type of local treatment influence the disease behavior remains open question and should be answered only within randomized trial.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the University of Pennsylvania and Copernicus Group Institutional Review Board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
MS: Protocol/project development, Manuscript writing. NH: Data analysis, manuscript writing. TN: Data analysis, manuscript writing. RD: Manuscript editing. HA: Data analysis. JK: Data management. AE-F: Data management. DJL: Manuscript editing. DIL: Protocol/project development, Manuscript editing All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fruro.2022.891798/full#supplementary-material
Supplementary Figure 1 | Cohort Flow chart in the real-world database.
Supplementary Figure 2 | Matching plot showing covariate balance measured by standardized mean difference before and after propensity score matching.
Supplementary Figure 3 | The Kaplan Meier survival curves by type of local treatment before propensity score matching.
Supplementary Table 1 | The follow-up time and the adjusted Kaplan Meier survival by type of local treatment after propensity score matching.
ADT, Androgen deprivation therapy; BCR, Biochemical recurrence; CRPC, Castrate-resistant prostate cancer; ECOG, Eastern Cooperative Oncology Group; EHR, Electronic health record; LT, Local treatment; OS, Overall survival; PSA, Prostate Specific Antigen; RP, Radical Prostatectomy; RT, Radiation therapy.
1. Thompson IM, Tangen C, Basler J, Crawford ED. Impact of Previous Local Treatment for Prostate Cancer on Subsequent Metastatic Disease. J Urol (2002) 168(3):1008–12. doi: 10.1016/S0022-5347(05)64562-4
2. Halabi S, NJ V, SS Ou, Small EJ. The Impact of Prior Radical Prostatectomy in Men With Metastatic Castration Recurrent Prostate Cancer: A Pooled Analysis of 9 Cancer and Leukemia Group B Trials. J Urol (2007) 177(2):531–4. doi: 10.1016/j.juro.2006.09.050
3. Patel DN, Jha S, LE H, CL A, WJ A, MR C, et al. Impact of Prior Local Therapy on Overall Survival in Men With Metastatic Castration-Resistant Prostate Cancer: Results From Shared Equal Access Regional Cancer Hospital. Int J Urol (2018) 25(12):998–1004. doi: 10.1111/iju.13806
4. Zabell JR, Adejoro O, SL J, SP E, Konety BR. Impact of Initial Local Therapy on Survival in Men Later Receiving Chemotherapy for Prostate Cancer: A Population-Based, Propensity-Weighted Multivariable Analysis. World J Urol (2016) 34(10):1397–403. doi: 10.1007/s00345-016-1790-x
5. Antwi S, Everson TM. Prognostic Impact of Definitive Local Therapy of the Primary Tumor in Men With Metastatic Prostate Cancer at Diagnosis: A Population-Based, Propensity Score Analysis. Cancer Epidemiol (2014) 38(4):435–41. doi: 10.1016/j.canep.2014.04.002
6. George DJ, Sartor O, Miller K, Saad F, Tombal B, Kalinovský J, et al. Treatment Patterns and Outcomes in Patients With Metastatic Castration-Resistant Prostate Cancer in a Real-World Clinical Practice Setting in the United States. Clin Genitourin Cancer (2020) 18(4):284–94. doi: 10.1016/j.clgc.2019.12.019
7. Cooperberg MR, JM B, Carroll PR. Time Trends and Local Variation in Primary Treatment of Localized Prostate Cancer. J Clin Oncol (2010) 28(7):1117. doi: 10.1200/JCO.2009.26.0133
8. Bland JM, Altman DG. Survival Probabilities (the Kaplan-Meier Method). Bmj (1998) 317(7172):1572–80. doi: 10.1136/bmj.317.7172.1572
9. Löppenberg B, Dalela D, Karabon P, Sood A, JD S, CP M, et al. The Impact of Local Treatment on Overall Survival in Patients With Metastatic Prostate Cancer on Diagnosis: A National Cancer Data Base Analysis. Eur Urol (2017) 72(1):14–9. doi: 10.1016/j.eururo.2016.04.031
10. Parker CC, ND J, CD B, NW C, AP H, Ali A, et al. Radiotherapy to the Primary Tumour for Newly Diagnosed, Metastatic Prostate Cancer (STAMPEDE): A Randomised Controlled Phase 3 Trial. Lancet (2018) 392(10162):2353–66. doi: 10.1016/S0140-6736(18)32486-3
11. Steuber T, KD B, MA R, Brasso K, Iversen P, Huland H, et al. Does Cytoreductive Prostatectomy Really Have an Impact on Prognosis in Prostate Cancer Patients With Low-Volume Bone Metastasis? Results From a Prospective Case-Control Study. Eur Urol Focus (2017) 3(6):646–9. doi: 10.1016/j.euf.2017.06.016
12. Finianos A, Gupta K, Clark B, SJ S, Aragon-Ching JB. Characterization of Differences Between Prostate Cancer Patients Presenting With De Novo Versus Primary Progressive Metastatic Disease. Clin Genitourin Cancer (2018) 16(1):85–9. doi: 10.1016/j.clgc.2017.08.006
13. Strock J, KP G, Nakabayashi M, Evan C, O'Donnell E, Pomerantz M, et al. Characterization of Patients Who Present With De Novo Metastatic Prostate Cancer: Single-Institution Database Analysis. J Clin Oncol (2013) 31:6_suppl:33–3. doi: 10.1200/jco.2013.31.6_suppl.33
14. Shao YH, Kim S, DF M, Shih W, Lin Y, Stein M, et al. Cancer-Specific Survival After Metastasis Following Primary Radical Prostatectomy Compared With Radiation Therapy in Prostate Cancer Patients: Results of a Population-Based, Propensity Score-Matched Analysis. Eur Urol (2014) 65(4):693–700. doi: 10.1016/j.eururo.2013.05.023
15. Markowski MC, Chen Y, Feng Z, Cullen J, BJ T, Suzman D, et al. And Absolute PSA Predict Metastasis-Free Survival in Men With Biochemically Recurrent Prostate Cancer After Radical Prostatectomy. Clin Genitourin Cancer (2019) 17(6):470–475.e1. doi: 10.1016/j.clgc.2019.08.002
16. Scher HI, Solo K, Valant J, MB T, Mehra M. Prevalence of Prostate Cancer Clinical States and Mortality in the United States: Estimates Using a Dynamic Progression Model. PloS One (2015) 10(10):e0139440. doi: 10.1371/journal.pone.0139440
17. Lee SY, EK J, MK J, HM J, MY K, CH K, et al. Induction of Metastasis, Cancer Stem Cell Phenotype, and Oncogenic Metabolism in Cancer Cells by Ionizing Radiation. Mol Cancer (2017) 16(1):1–25. doi: 10.1186/s12943-016-0577-4
18. Deng X, BD E, JM P, WB M, SC K, NM H, et al. Ionizing Radiation Induces Neuroendocrine Differentiation of Prostate Cancer Cells In Vitro, In Vivo and in Prostate Cancer Patients. Am J Cancer Res (2011) 1(7):834.
19. Khan T, KF S, TM B, Lock J, Nimir M, Ma Y, et al. The Prospect of Identifying Resistance Mechanisms for Castrate-Resistant Prostate Cancer Using Circulating Tumor Cells: Is Epithelial-To-Mesenchymal Transition a Key Player? Prostate Cancer (2020) 30:2020. doi: 10.1155/2020/7938280
20. Paczkowski M, Kretzschmar WW, Markelc B, Liu SK, Kunz-Schughart LA, Harris AL, et al. Reciprocal Interactions Between Tumour Cell Populations Enhance Growth and Reduce Radiation Sensitivity in Prostate Cancer. Commun Biol (2021) 4:6. doi: 10.1038/s42003-020-01529-5
21. Alfonso J, Berk L. Modeling the Effect of Intratumoral Heterogeneity of Radiosensitivity on Tumor Response Over the Course of Fractionated Radiation Therapy. Radiat Oncol (2019) 14:88. doi: 10.1186/s13014-019-1288-y
22. Wallis CJ, Saskin R, Choo R, Herschorn S, RT K, Satkunasivam R, et al. Surgery Versus Radiotherapy for Clinically-Localized Prostate Cancer: A Systematic Review and Meta-Analysis. Eur Urol (2016) 70(1):21–30. doi: 10.1016/j.eururo.2015.11.010
23. Parker C, Castro E, Fizazi K, Heidenreich A, Ost P, Procopio G, et al. ESMO Guidelines Committee. Electronic Address: Clinicalguidelines at Esmo.Org. Prostate Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol (2020) 31(9):1119–34. doi: 10.1016/j.annonc.2020.06.011
24. Swami U, Hong A, NN E-C, Nimke D, Ramaswamy K, EJ B, et al. Real-World First-Line (1L) Treatment Patterns in Patients (Pts) With Metastatic Castration-Sensitive Prostate Cancer (mCSPC) in a US Health Insurance Database. J Clin Oncol (2021) 39(15_suppl):5072. doi: 10.1200/JCO.2021.39.15_suppl.5072
Keywords: prostate cancer, radical prostatectomy, radiation, metastatic, local treatment
Citation: Shahait M, Hamieh N, Dobbs RW, Nguyen T, Alshannaq H, Kim J, El-Fahmawi A, Lee DJ and Lee DI (2022) Comparative Analysis of Primary Prostate Cancer Treatment and Subsequent Metastatic Disease. Front. Urol. 2:891798. doi: 10.3389/fruro.2022.891798
Received: 08 March 2022; Accepted: 16 May 2022;
Published: 15 June 2022.
Edited by:
Shawn Dason, The Ohio State University, United StatesReviewed by:
Jure Murgic, Sisters of Charity Hospital, CroatiaCopyright © 2022 Shahait, Hamieh, Dobbs, Nguyen, Alshannaq, Kim, El-Fahmawi, Lee and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammed Shahait, bXNoYWhhaXRAeWFob28uY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.