
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Urol., 14 July 2022
Sec. Neurourology, Behavioural Urology, and Urodynamics
Volume 2 - 2022 | https://doi.org/10.3389/fruro.2022.890990
 Baylie Hochstedler-Kramer1
Baylie Hochstedler-Kramer1 Cara Joyce2
Cara Joyce2 Omar Abdul-Rahim1
Omar Abdul-Rahim1 Hayley C. Barnes3,4†
Hayley C. Barnes3,4† Elizabeth R. Mueller3,4
Elizabeth R. Mueller3,4 Alan J. Wolfe1
Alan J. Wolfe1 Linda Brubaker5,6
Linda Brubaker5,6 Lindsey A. Burnett5,6*
Lindsey A. Burnett5,6*The discovery of the urinary microbiome prompted researchers to begin characterizing microbiota associated with various health and disease states; however, the etiology of bladder infections, the most common urinary tract infection (UTI), is still simplistically attributed to the invasion of a uropathogen, mainly Escherichia coli, without regard for the resident microbial community. In addition, the clinical variability of UTI symptoms remains poorly understood. Very little research has been done to investigate the role of baseline microbial communities in development and resolution of UTI symptoms. The goal of this study was to identify associations between urinary microbiota and lower urinary tract symptoms profiles in adult women with UTI symptoms. This is a secondary analysis of a previously published IRB-approved study that included 225 women who reported having acute UTI symptoms, submitted catheterized urine specimens for analysis by an enhanced urine culture method and were assessed for symptom resolution 7-10 days after receiving culture-directed antibiotic treatment. In this UTI population, we identified six distinct symptom profiles, termed symptotypes, that were characterized by varying severity and degree of bother of certain lower urinary tract symptoms. These symptotypes were not associated with urotype or the presence of specific microbes. In participants with pain on presentation, the presence of non-E. coli and non-uropathogens was associated with persistence of symptoms at follow up; however, this was not true for those with E. coli urotype. These data suggest that the presence of E. coli may not account for the underlying cause of typical UTI symptoms; instead, co-existence of a uropathogen in the context of the existing urinary microbiota and the host may be responsible for these symptom profiles.
Urinary tract infections (UTIs) are common bacterial infections, impacting 150 million people every year and causing significant societal and financial costs, with growing global anti-microbial resistance concerns (1–3). Most research on UTI preceded the discovery of the urinary microbiome (urobiome) (4, 5). Conventionally, a UTI is diagnosed based on patient reported symptoms, sometimes with a urine dipstick assessment, laboratory urinalysis and/or culture. The diagnosis is dichotomous, despite the growing evidence that various states of dysbiosis can exist in the bladder microbial community. The term “sporadic UTI” is used to describe a UTI event that occurs less than twice in 6 months (6), and recurrent UTI is diagnosed when culture-documented UTI events occur more than twice in 6 months or at least three times in a year. Recurrent UTI is often characterized by persistent symptoms even in the absence of the sensation of acute infection (7). In contrast, sporadic UTI symptoms are considered episodic with symptom resolution after treatment.
The clinical diagnosis and empiric treatment is often initiated based on symptom profiles without laboratory testing. Oral antibiotics are the default treatment for UTI and antibiotic selection is often empiric or informed by results of antibiotic sensitivity profiles for uropathogens detected on standard urine culture. Standard urine culture has decreased sensitivity for uropathogen detection compared to enhanced culture techniques (8, 9). Currently, clinical care is not routinely based on results of culture-independent microbial detection methods (i.e., high throughput sequencing techniques) (10). The value of these sensitive microbial detection methods in the diagnosis and treatment of both recurrent and sporadic UTI is still under investigation.
There is existing evidence that E. coli-predominant infections appear less prevalent in recurrent compared to sporadic UTI patients and drug resistances are more common in the recurrent UTI population (11, 12). In sporadic UTI, patient symptoms are episodic and assumed to be associated with an acute alteration of the urobiome. However, the relationship between patient symptoms and bladder microbial content in the sporadic UTI population has not been studied. Clinicians consider symptoms as a foundational diagnostic element for a UTI diagnosis. Classic “UTI symptoms” are typically associated with an abrupt change in baseline urinary sensations and/or function; often dysuria, urinary urgency and/or frequency are present. The UTI Symptoms Assessment questionnaire (UTISA) (13) is a validated UTI symptoms questionnaire that includes these key symptoms. However, as patients age, chronic urinary conditions, such as overactive bladder make it more difficult to clearly identify meaningful changes in urinary symptoms (14). Also, patients may report more unique personal symptoms, such change in urine odor or appearance, onset of generalized symptoms, such as unusual fatigue, or other “atypical” symptoms.
In adult women with recurrent UTI, there is a relationship between clinical profiles, including UTISA quantified symptoms, and the microbial content of the bladder as detected by expanded quantitative urine culture (EQUC) (7). Urinary microbial content also has been linked to other clinical symptoms including incontinence (9, 15–17), suggesting a relationship between clinical phenotypes and the urobiome. Barnes and colleagues conducted a randomized controlled trial in women with UTI symptoms were randomized to either standard urine culture or EQUC to guide clinical treatment of UTI (NCT03190421) (18). The primary outcome was symptom resolution 7-10 days after culture and treatment. The role of more sensitive microbial detection methods when non-E. coli uropathogens were present was confirmed, a potentially important finding to guide clinical care (18). In this secondary analysis of data from that trial, we examined the relationship between patient reported symptoms (by clinical profile and UTISA) and the microbial content of the bladder as characterized by EQUC. Additionally, we investigated whether microbial content impacted symptom resolution.
In this secondary analysis of the previously reported, IRB-approved clinical trial, we included data and lab findings from all previously reported participants (18). Briefly, participants were adult women ≥18 years of age who presented to Loyola University Medical Center urogynecology clinic and responded “yes” to the question “do you feel you have a UTI?”. Exclusion criteria included women who were on antibiotics, pregnant, had an indwelling urinary catheter, were performing intermittent self-catheterization, declined to be catheterized, or were treated empirically on the day of enrollment. Following verbal and written consent for research, participants completed the validated UTISA questionnaires (13, 19).
The UTISA (13), a validated 14-item patient administered questionnaire, was used to characterize symptoms in the enrolled population. Each item on the UTISA ranges from 0-3 (higher scores indicate worse symptoms). Seven items relate to symptom severity and are summed to calculate the UTISA-Presence scale (0-21) and seven items related to bothersomeness comprise the UTISA-Bother scale (0-21). In the validation of UTISA, four domains were described: “urination regularity”, “problems with urination”, “pain associated with UTI”, and “blood in urine”. UTISA domain scores for urinary regularity, problems with urination, and pain associated with UTI are the sum of four items each (0-12); the UTISA domain score for blood in the urine is the sum of two items (0-6).
Demographic and clinical factors also were collected at the initial visit. Following clinically directed UTI treatment, women who consented to a follow-up phone call were contacted 7-10 days after culture collection, and asked “do you continue to have UTI symptoms?” UTI symptoms were considered resolved if patients answered “no” to this question.
Using standard aseptic technique, a urine specimen was collected via transurethral catheterization. Urine specimens were assessed by EQUC (20) performed by trained laboratory investigators. EQUC technique used an inoculation of 100 microliters of urine onto 5% sheep blood, MacConkey, and colistin and nalidixic acid agar plates. All plates were incubated in 5% CO2 at 37°CC for 48 hours. After incubation, morphologically distinct bacterial colonies were enumerated and identified by Matrix-Assisted Laser Desorption/Ionization Time-of Flight (MALDI-TOF) mass spectroscopy (Bruker MALDI Biotyper).
Urine culture results were used to determined urotypes based on the following criteria: E. coli-predominant (≥50% of total CFU/mL cultured from a sample were E. coli), non-E. coli uropathogen-predominant ((≥50% of total CFU/mL were known uropathogens other than E. coli including Klebsiella, Proteus, Streptococcus and Enterococcus species), and non-uropathogen-predominant ((≥50% of total CFU/mL were Lactobacillus or Gardnerella species). Non-uropathogen-predominant and culture negative were grouped into a single urotype as neither was treated with antibiotics based on clinical protocol.
Symptom profile (symptotype) identification was determined using the QIIME2 platform (https://view.qiime2.org/) by performing principal component analysis (PCoA) on results of the self-reported UTISA questionnaire. The three main points of variance between test samples were used to cluster datapoints based on their three-dimensional position in space. Biplot analysis overlaid a vector demonstrating the direction and magnitude of variance contributed by a specific factor to identify the main contributors of variance between symptotypes. Symptotype assignments for each participant were then added to existing metadata for subsequent analysis. This combinatorial approach does not assume symptoms would be within a single subdomain and instead examined natural clustering of all symptoms reported on the UTISA.
Microbiota prevalence and abundance were compared across symptotypes. Alpha diversity measures, including species richness, abundance, and evenness, were calculated for each participant.
The associations of symptotype with urotype and microbiota presence were assessed using chi-square or Fisher’s exact tests, as appropriate. The UTISA presence, bother, and symptom type scales were compared by urotype using analysis of variance. In univariable analyses, alpha diversity and UTISA scores were compared by UTI symptom persistence using Wilcoxon rank sum tests and two-sample t-tests, respectively. The crude associations of UTI symptom persistence with symptotype and urotype were assessed using chi-square tests. Then, multivariable logistic models predicting UTI symptom persistence were developed from symptom and urobiome characteristics with model fit statistics (Akaike information criterion) and likelihood ratio tests used to guide variable selection. Adjusted odds ratios were reported from logistic regression models with urotype, UTISA score, an interaction term, and treatment with antibiotics as a covariate. Statistical analysis was performed using SAS 9.4 and microbiome data analysis and data visualization was done in R 4.1.2.
The 225 participants (mean age 66 years, range 20-99 years) contributed 225 catheterized urine samples. 215 of 225 participants provided data on follow-up phone call (143 [95%] of standard urine culture group, 72 [97%] of EQUC group). Table 1 displays the cohort demographics. The study population identified as 0.9% as Asian, 13.3% as Black, 14.2% as Hispanic and 70.7% as White. The average body mass index was 31.2 ± 8.2. Relevant medication use includes bladder control medication (22 [9.8%]) and vaginal estrogen use in the past month (76 [34.1%]).
The overall UTISA score was 10.3 ± 4.4 for UTISA-Presence and 10.3 ± 4.7 for UTISA-Bother. The mean (SD) subdomain scores were pain associated with UTI (range 0-6) was 5.2 ± 3.8, urination regularity (range 0-12) was 8.7 ± 3.3, problems with urination (range 0-12) 6.2 ± 3.7 and blood in urine (range 0-6) 0.6 ± 1.4.
Unsupervised clustering of symptom UTISA responses using PCoA resulted in 6 significantly different groupings of participant symptom profiles annotated as A-F. The biplot analysis (Figure 1) demonstrates the drivers of differences between UTISA responses. Some UTISA symptoms commonly occurred together, such as incomplete emptying and urgency. Other symptoms were less likely to be present together, as those with incomplete emptying and urgency experienced less dysuria and vice versa. Similarly, frequency was rarely seen in participants with abdominal pain. Frequency and dysuria tended to be present together predominantly in those with severe overall scores. Profiles of UTISA symptoms for each symptotype group are seen in Table 2. The predominant symptoms for each symptotype are as follows: A: severe overall, B: severe frequency and urgency, C: moderate overall, D: mild overall, E: frequency, F: incomplete emptying.
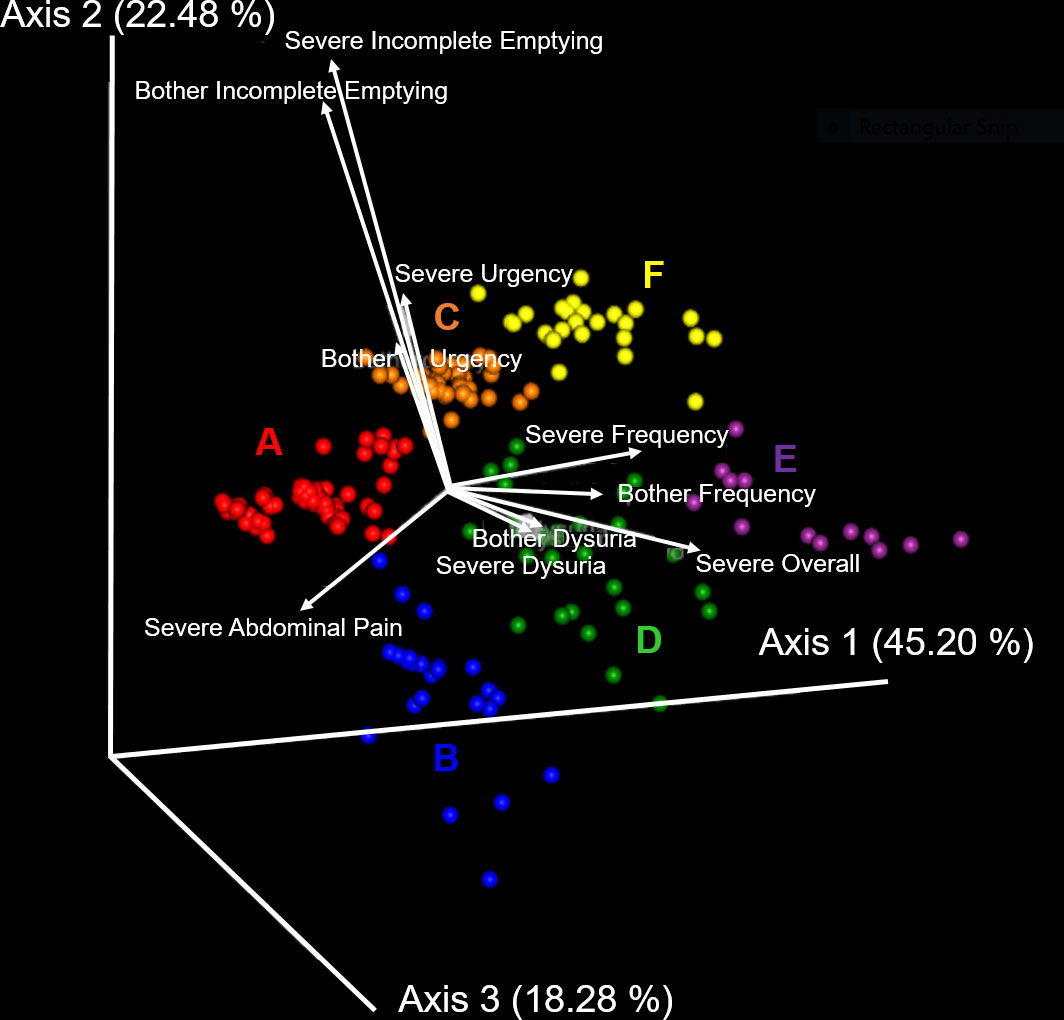
Figure 1 Principal component analysis of UTISA responses. Unsupervised clustering of UTISA responses demonstrated six distinct symptom profiles (symptotypes) annotated as A-F. Axis 1 accounting for 45.20% of symptom variability described the spectrum of severe abdominal pain, severe frequency, bothersome frequency and severe overall symptoms. Abdominal pain was seen more frequently in A (red) and B (blue) symptotype groups. Severe frequency, bothersome frequency and severe overall symptoms were seen most commonly in the E (purple) symptotype group. Axis 2 accounting for 22.48% of variability in symptoms described bothersome incomplete emptying and severe incomplete emptying and these symptoms were more common in C (orange) and F (yellow) symptotype groups. Axis 3 described 18.28% of variability which predominantly described bother and severity of dysuria symptoms. Biplot analysis (white arrows) indicate the UTISA domains driving separation of symptom groups. Symptom categories that are responsible for clustering, shown as vectors, denote the direction (arrow) and strength of magnitude (length of line) contributed by each symptom category.
Figure 2 displays the categorization of the participants’ EQUC results into the three urotype categories: E. coli uropathogen (ECU, N=100), non-E. coli uropathogen (NECU, N=77), and non-uropathogen (NU, N=35). There was no growth on the remaining cultures (N=13). Of positive cultures, the urotype distribution was ECU (47%), NECU (40%) and NU (17%). Polymicrobial cultures were common (69% of ECU, 74% of NECU, 66% of NU). Monocultures were less frequent (31% of ECU, 26% of NECU, 34% of NU) and prevalence did not differ between urotypes (p >0.05). E. coli monocultures accounted for only 15% of positive cultures. The prevalence of the ECU, NECU and NU/culture negative urotypes did not differ among the 6 symptom profile groups (Table 3). The UTISA domain scores did not differ between urotypes. (Table 3).
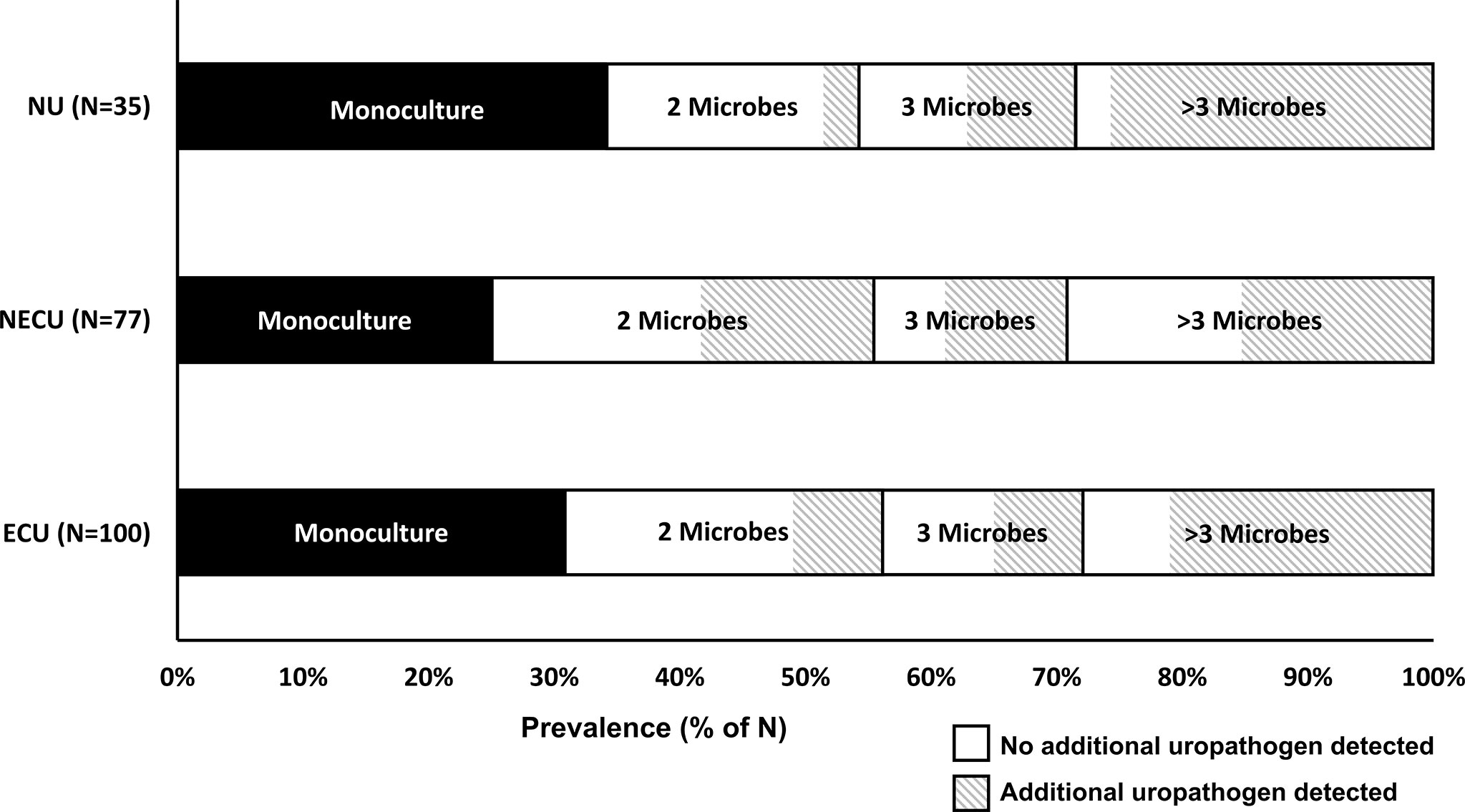
Figure 2 Prevalence of polymicrobial cultures by urotype. The main urotype categories for positive cultures (y-axis, N=212) in this population were E. coli uropathogen (ECU), non-E. coli uropathogen (NECU), and non-uropathogen (NU) dominant cultures. The prevalence of monocultures, cultures with two microbes, cultures with three microbes, and cultures with more than three microbes (range 4-11) is displayed on the x-axis. The detection prevalence for additional and/or non-dominant uropathogens is denoted by the diagonal line patterned area for each polymicrobial category.
No specific microbes were significantly enriched in any symptom profile group. After correction for multiple testing, the initial findings that Cornybacterium was significantly enriched and Aerococcus was approaching significant enrichment in the largest and most symptomatic symptom profile group (Group A), were no longer significant (Table 4).
No significant differences were also seen between symptotypes for any microbiome diversity index (Table 4). When compared by urotype, Shannon and Simpson indices were significantly decreased between ECU compared to NECU and NU (Figures 3A, B). Richness did not differ between urotypes (Figure 3C). Evenness was significantly increased in NU compared to ECU and NECU, which did not differ (Figure 3D).
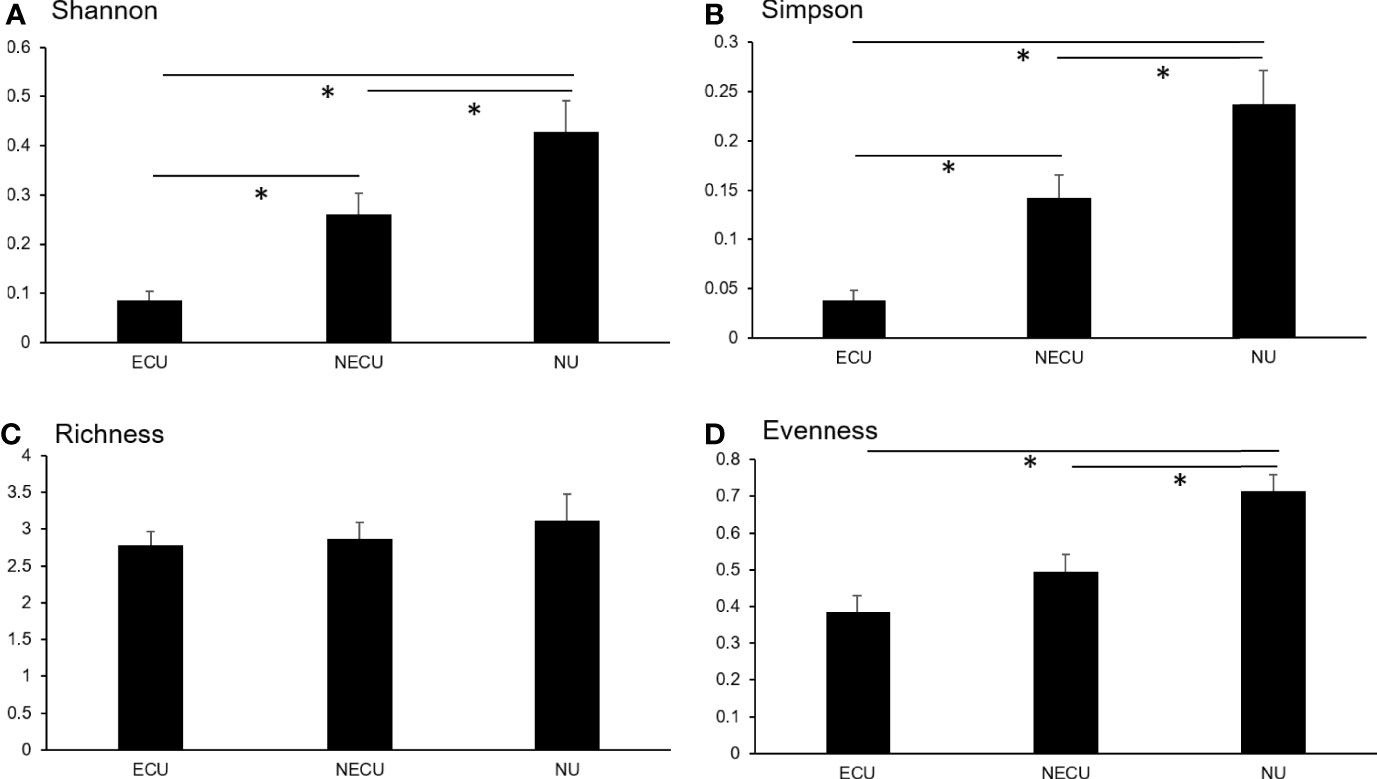
Figure 3 Microbial diversity as assessed by symptotype. (A) Shannon diversity was significantly different between all groups. (B) Simpson diversity was significantly different between all groups. (C) Richness diversity was not significantly different between groups. (D) Evenness diversity was not significantly different between ECU and NECU groups but increased in NU. *p-values based on Kruskal-Wallis; significant at p < 0.05.
Symptoms were present in 73/215 participants (34.0%) at follow-up 7-10 days after baseline and the persistence rate did not vary significantly by urotype (p=0.57) nor by symptotype (p=0.40). Alpha diversity measures were similar in those with symptom persistence compared to those with symptom resolution (p>0.05 for all comparisons). UTISA overall and subscale scores were generally higher in those with UTI symptom persistence, with largest differences seen for the UTISA-presence scale (11.2 ± 4.5 vs 9.9 ± 4.4, p=0.03), problems with urination (6.9 ± 3.6 vs 5.8 ± 3.6, p=0.03), and pain associated with UTI (5.9 ± 4.0 vs 4.9 ± 3.6, p=0.08). (Table 5)
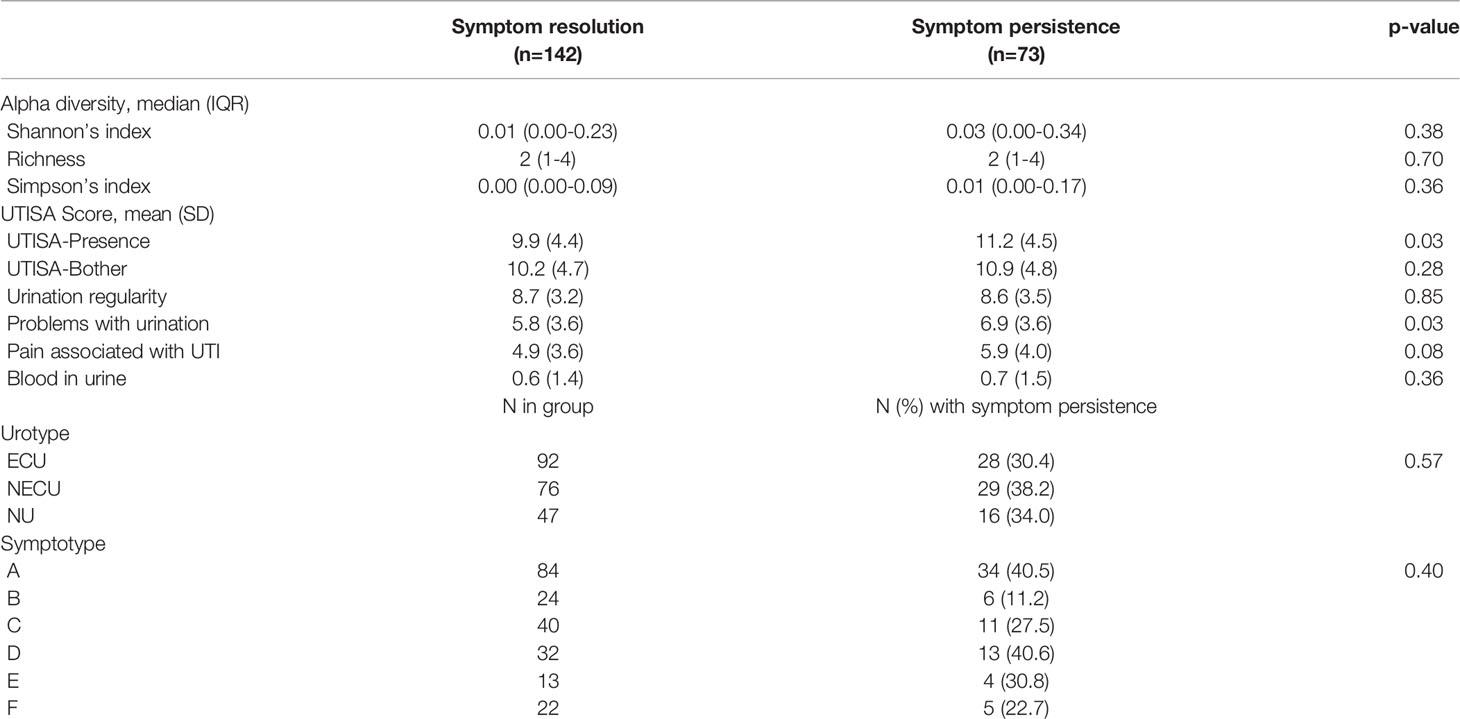
Table 5 Univariable comparisons of presenting symptoms, symptotype, urotype, and alpha diversity by UTI symptom persistence at follow up.
Univariate analysis identified an association between UTISA overall and subcategory scores with symptom persistence. In multivariable analysis, higher UTISA-Presence scores on initial assessment were associated with higher odds of UTI symptoms at follow up in the NECU (OR 1.13 95% CI: 1.01-1.33, p=0.03) and NU (OR 1.16 95% CI: 1.01-1.26, p=0.03) cohorts (p=0.03 for interaction). This association was not present in the ECU group (OR 0.95 95% CI: 0.85-1.06, p=0.35). Problems with urination was associated with higher odds of UTI symptoms at follow up in the NECU (OR 1.15 95% CI: 1.02-1.31, p=0.03); however the p-value for interaction was not significant (p=0.29). Pain with UTI on presentation was also associated with higher odds of UTI symptoms at follow up in the NECU (OR 1.15 95% CI: 1.02-1.31, p=0.03) and NU (OR 1.12 95% CI: 1.00-1.38, p=0.047) cohorts (p=0.02 for interaction). This association was not seen in the ECU group (OR 0.90 95% CI: 0.78-1.04, p=0.16) (Table 6).
This analysis has two key findings: In adults with acute UTI symptoms, 1) the clinical symptom profile is not associated with urotype or the presence of specific microbes, 2) in participants with pain associated with UTI, the presence of NECU and NU/culture negative urotype is associated with the persistence of UTI symptoms after culture-directed antibiotic treatment for UTI.
Our finding that the clinical symptom profiles in this cohort assessed by UTISA domain or symptom profile were not associated with urotype or the presence of specific microbes contrasts with previous findings in a population of adult women with recurrent UTI population (7). In that cohort, a relationship between the presence of specific microbes and patient symptom profiles was detected. This difference may be due to cohort characteristics, as the current analytic cohort is comprised of adult women who were not diagnosed with recurrent UTI and treated using clinical algorithms for sporadic UTI. Further studies will be needed to rigorously compare symptom profiles between cohorts with sporadic versus recurrent UTI, as women affected by recurrent UTI may have ongoing disruption of homeostatic mechanisms and chronic urothelial disruption that may not occur in sporadic UTI (21). Symptoms associated with sporadic UTI may be related to acute inflammation, which resolves with antibiotic treatment in most cases and may not be microbe-specific. In contrast, recurrent UTI symptom profiles that are associated with microbes may represent the chronic effects of these microbes on host urothelium.
The association of symptom persistence with presence of pain on initial evaluation differed by urotype, with a clear association for NECU or NU/negative culture urotypes but not for ECU cultures. One possible interpretation is that UTI treatment intended to eradicate E. coli is effective when the urotype is ECU, but not when the urotype is NECU or NU. Persistent pain may be caused by untreated non-E. coli uropathogens or non-uropathogens/not recognized uropathogens. Consistent with this possibility, the index randomized controlled trial treatment found that, in the subset of women with non-E. coli uropathogens, there was a trend toward more symptom resolution in the EQUC arm (21%, p=0.08) (18). These data suggest inadequate treatment of non-E. coli uropathogens or non-uropathogens may be associated with persistence of pain with UTI.
Previous studies have indicated E. coli presence, abundance, or genomic content as weak predictors of UTI status (22). Although it is accepted that UTI can result from E. coli overgrowth, an accumulating body of evidence suggests multiple different bacteria can cause UTI symptoms (22). Existing literature suggests E. coli is more frequent in the sporadic UTI population compared to recurrent UTI participants (7, 12). E. coli detection in our population was more frequent, consistent with a likely predominantly sporadic UTI population; however, E. coli monocultures were more infrequent than reported by studies utilizing less sensitive bacterial detection methods (18, 23). This study has several limitations. First, although previous studies in the recurrent UTI population found relationships between urinary symptoms and urinary microbes with less than a quarter of the sample size of this study, this cohort is relatively small. Second, there was limited characterization of the previous UTI history. Thus, this cohort likely includes participants that may meet criteria for recurrent UTI diagnosis. Third, despite having longitudinal data on participant symptoms, we lack longitudinal data regarding the presence and absence of urinary microbes which is needed to better characterize the relationship between microbes and their host response and sensitivity.
Clearly, one size does not fit all. The insights from this analysis offer an early description of the relationship between microbes and the urinary bladder in adult women with symptomatic UTI. The increasing evidence that UTI, despite the presence of E. coli, can be a polymicrobial event warrants consideration of a more personalized, urobiome-centric approach to UTI treatment. The baseline, pre-treatment urobiome appears to be a potential biomarker for risk of persistent symptoms. Investigators are encouraged to include baseline urobiome characterization in future studies of UTI for both sporadic and recurrent UTI populations.
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
This is a secondary analysis of a study involving human participants that was reviewed and approved by Loyola University Medical Center Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
LAB, LB, AW and BH-K contributed to conception and design of the study. BH-K, OA-R, HB and EM collected data for study. LAB, CJ and BH-K performed the data analysis and statistical analysis. LAB wrote the first draft of the manuscript. LB, BH-K, AW and CJ wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors gratefully acknowledge funding by NIH/NIDDK award numbers R01 DK10718 (LB and AW), U2CDK129917/TL1DK132769 (BH-K) and the International Urogynecological Association Basic Science Research Grant (LAB) for the conduct of this research.
AW discloses research support from the NIH, the DOD, and Pathnostics. He also discloses membership on the advisory boards of Urobiome Therapeutics and Pathnostics. LB discloses research funding from NIH and editorial stipends from Female Pelvic Medicine and Reconstructive Surgery, UpToDate and JAMA. EM discloses research funding from NIH. LAB discloses funding from the International Urogynecological Association.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to acknowledge the participants in these studies, and the clinical and research teams that made this study possible. This includes the research coordinator Mary Tulke, clinicians, and nursing staff in urogynecology at Loyola University Chicago who recruited participants and collected samples and performed the follow-ups and the members of the Wolfe lab that processed these samples.
1. Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary Tract Infections: Epidemiology, Mechanisms of Infection and Treatment Options. Nat Rev Microbiol (2015) 13(5):269–84. doi: 10.1038/nrmicro3432
2. Stamm WE, Norrby SR. Urinary Tract Infections: Disease Panorama and Challenges. J Infect Dis (2001) 183(Suppl 1):S1–4. doi: 10.1086/318850
3. Antimicrobial Resistance C. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet (2022) 399(10325):629–55. doi: 10.1016/S0140-6736(21)02724-0
4. Wolfe AJ, Toh E, Shibata N, Rong R, Kenton K, Fitzgerald M, et al. Evidence of Uncultivated Bacteria in the Adult Female Bladder. J Clin Microbiol (2012) 50(4):1376–83. doi: 10.1128/JCM.05852-11
5. Fouts DE, Pieper R, Szpakowski S, Pohl H, Knoblach S, Suh MJ, et al. Integrated Next-Generation Sequencing of 16s Rdna and Metaproteomics Differentiate the Healthy Urine Microbiome From Asymptomatic Bacteriuria in Neuropathic Bladder Associated With Spinal Cord Injury. J Transl Med (2012) 10:174. doi: 10.1186/1479-5876-10-174
6. Foxman B. Epidemiology of Urinary Tract Infections: Incidence, Morbidity, and Economic Costs. Am J Med (2002) 113 Suppl:1A:5S–13S.
7. Burnett LA, Hochstedler BR, Weldon K, Wolfe AJ, Brubaker L. Recurrent Urinary Tract Infection: Association of Clinical Profiles With Urobiome Composition in Women. Neurourol Urodyn (2021) 40(6):1479–89. doi: 10.1002/nau.24707
8. Hochstedler BR, Burnett LA, Price TK, Jung CE, Wolfe AJ, Brubaker L. Urinary Microbiota of Women With Recurrent Urinary Tract Infection: Collection and Culture Methods. Int Urogynecol J (2021) 33:563–70.
9. Price TK, Lin H, Gao X, Thomas-White KJ, Hilt EE, Mueller ER, et al. Bladder Bacterial Diversity Differs in Continent and Incontinent Women: A Cross-Sectional Study. Am J Obstet Gynecol (2020) 223(5):729.e1– e10. doi: 10.1016/j.ajog.2020.04.033
10. Mueller ER, Wolfe AJ, Brubaker L. Female Urinary Microbiota. Curr Opin Urol (2017) 27(3):282–6. doi: 10.1097/MOU.0000000000000396
11. Hisano M, Bruschini H, Nicodemo AC, Gomes CM, Lucon M, Srougi M. The Bacterial Spectrum and Antimicrobial Susceptibility in Female Recurrent Urinary Tract Infection: How Different They Are From Sporadic Single Episodes? Urology (2015) 86(3):492–7. doi: 10.1016/j.urology.2015.05.033
12. Bradley MS, Cabrera C, Clark SG, Sassani J, Venuti K, Ackenbom MF. Sporadic Compared to Recurrent Urinary Tract Infections: Considerations for Urogynecologic Patients. Neurourol Urodyn (2020) 39(8):2186–91. doi: 10.1002/nau.24471
13. Clayson D, Wild D, Doll H, Keating K, Gondek K. Validation of a Patient-Administered Questionnaire to Measure the Severity and Bothersomeness of Lower Urinary Tract Symptoms in Uncomplicated Urinary Tract Infection (Uti): The Uti Symptom Assessment Questionnaire. BJU Int (2005) 96(3):350–9. doi: 10.1111/j.1464-410X.2005.05630.x
14. Dune TJ, Price TK, Hilt EE, Thomas-White KJ, Kliethermes S, Brincat C, et al. Urinary Symptoms and Their Associations With Urinary Tract Infections in Urogynecologic Patients. Obstet Gynecol (2017) 130(4):718–25. doi: 10.1097/AOG.0000000000002239
15. Govender Y, Gabriel I, Minassian V, Fichorova R. The Current Evidence on the Association Between the Urinary Microbiome and Urinary Incontinence in Women. Front Cell Infect Microbiol (2019) 9:133. doi: 10.3389/fcimb.2019.00133
16. Pearce MM, Hilt EE, Rosenfeld AB, Zilliox MJ, Thomas-White K, Fok C, et al. The Female Urinary Microbiome: A Comparison of Women With and Without Urgency Urinary Incontinence. MBio (2014) 5(4):e01283–14. doi: 10.1128/mBio.01283-14
17. Pearce MM, Zilliox MJ, Rosenfeld AB, Thomas-White KJ, Richter HE, Nager CW, et al. The Female Urinary Microbiome in Urgency Urinary Incontinence. Am J Obstet Gynecol (2015) 213(3):347.e1–11. doi: 10.1016/j.ajog.2015.07.009
18. Barnes HC, Wolff B, Abdul-Rahim O, Harrington A, Hilt EE, Price TK, et al. A Randomized Clinical Trial of Standard Versus Expanded Cultures to Diagnose Urinary Tract Infections in Women. J Urol (2021) 206(5):1212–21. doi: 10.1097/JU.0000000000001949
20. Hilt EE, McKinley K, Pearce MM, Rosenfeld AB, Zilliox MJ, Mueller ER, et al. Urine Is Not Sterile: Use of Enhanced Urine Culture Techniques to Detect Resident Bacterial Flora in the Adult Female Bladder. J Clin Microbiol (2014) 52(3):871–6. doi: 10.1128/JCM.02876-13
21. Horsley H, Malone-Lee J, Holland D, Tuz M, Hibbert A, Kelsey M, et al. Enterococcus Faecalis Subverts and Invades the Host Urothelium in Patients With Chronic Urinary Tract Infection. PloS One (2013) 8(12):e83637. doi: 10.1371/journal.pone.0083637
22. Garretto A, Miller-Ensminger T, Ene A, Merchant Z, Shah A, Gerodias A, et al. Genomic Survey of E. Coli Bladders Women Without Lower Urinary Tract Symptoms Front Microbiol (2020) 11:2094. doi: 10.3389/fmicb.2020.02094
Keywords: symptomatic urinary tract infection, urinary symptoms, urinary microbiome, urinary microbiota, enhanced urine culture
Citation: Hochstedler-Kramer B, Joyce C, Abdul-Rahim O, Barnes HC, Mueller ER, Wolfe AJ, Brubaker L and Burnett LA (2022) One Size Does Not Fit All: Variability in Urinary Symptoms and Microbial Communities. Front. Urol. 2:890990. doi: 10.3389/fruro.2022.890990
Received: 07 March 2022; Accepted: 16 June 2022;
Published: 14 July 2022.
Edited by:
Blayne Welk, Western University, CanadaReviewed by:
Sara Lenherr, University of Utah Hospital, United StatesCopyright © 2022 Hochstedler-Kramer, Joyce, Abdul-Rahim, Barnes, Mueller, Wolfe, Brubaker and Burnett. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lindsey A. Burnett, bGlidXJuZXR0QGhlYWx0aC51Y3NkLmVkdQ==
†Present address: Hayley C. Barnes, Department of Obstetrics and Gynecology, University of Cincinnati Health, Cincinnati OH, United States
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.