- 1Department of Urology, University of Florida College of Medicine, Gainesville, FL, United States
- 2Department of Urology, Carolinas Medical Center, Atrium Health, Charlotte, NC, United States
- 3Department of Urology, University of Kentucky College of Medicine, Lexington, KY, United States
Background: Medical expulsive therapy (MET) is the use of medication to facilitate ureteral stone passage prior to surgical intervention. Practice guidelines for the use of MET in the pediatric population remain limited, primarily due to a scarcity of randomized controlled trials and concerns regarding dosing and side effects. To address this, we conducted a systematic review and meta-analysis to clarify the impact of MET on the spontaneous passage of pediatric stones located within the distal ureter.
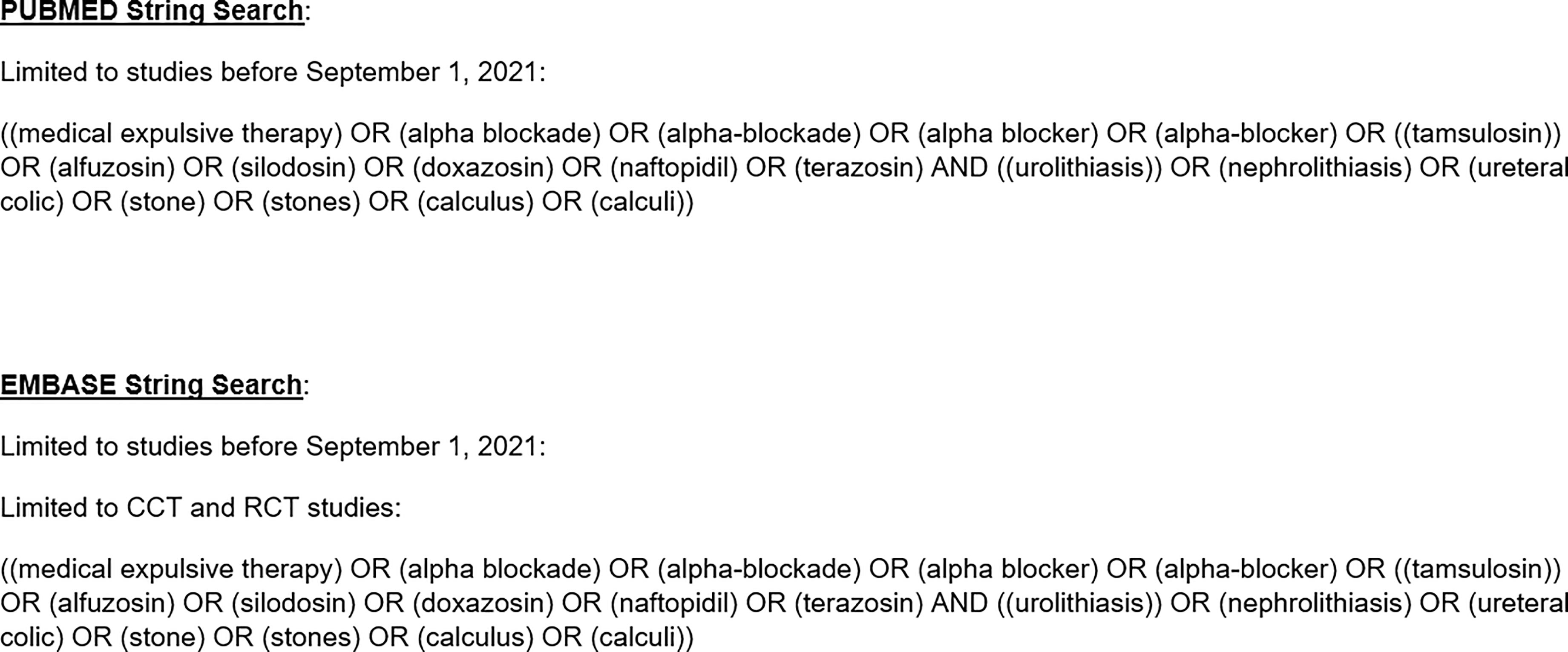
Methods: A narrow scope search using PubMed and Embase with a predefined search strategy was performed in September 2021 to identify all randomized controlled trials involving the use of pediatric MET for stones located in the distal ureter. Raw data from 6 eligible articles were extracted for pooled analysis. Our primary outcome was the overall effect of MET on ureteral calculi passage within 28 days compared to controls.
Results: The mean age of patients in included studies was 7.6 years-old, with a range of ages 2–18 years old. In the pooled analysis of eligible studies, 235 patients received MET and 176 received placebo. The mean stone size was 6.40 mm in the treatment arm and 6.42 mm in the control arm. Children receiving MET were more likely than controls to experience spontaneous stone passage [Relative risk 1.39 (CI 95% 1.21–1.60)]. Considering all included studies, only one child treated with MET withdrew due to medication side effects.
Conclusion: Our systematic review and meta-analysis of the use of pediatric MET on spontaneous distal ureteral stone passage demonstrates a statistically significant benefit. The benefits of MET are diverse and include, possibly, minimizing exposure to anesthesia and radiation alongside improving surgical outcomes if ureteroscopy must be performed. Given the increasing incidence of ureteral stones in children and the nuances inherent to pediatric surgery due to smaller anatomy, MET represents an opportunity for safer and more effective pediatric stone management.
Introduction
Medical expulsive therapy (MET) defines the use of medication to facilitate ureteral stone passage prior to surgical intervention. The two classes of medications generally accepted for use as medical expulsive therapy are alpha-blockers and calcium channel blockers. Current practice guidelines for adults include the use of MET for uncomplicated ureteral calculi (1, 2). Pediatric exclusivity for tamsulosin was granted by the FDA in 2009 and was studied primarily in patients with bladder neck dysfunction due to spina bifida or related spinal dysraphism. In 2012, the FDA’s Pediatric Advisory Committee affirmed this position on tamsulosin and has continued to recommend standard ongoing monitoring processes for adverse effects (3). Formal approval for the use of tamsulosin (or any alpha-blocker) for pediatric stone management has not been granted, and concerns about proper dosing and adverse effects remain as current obstacles to widespread use in pediatric urolithiasis.
In 2019, a meta-analysis was performed which demonstrated the benefit of tamsulosin for adult MET with 2763 patients across 29 randomized control trials (RCTs) (4). In comparison, the evidence base for the use of MET in the pediatric patient is established but less robust. In 2014, Tasian et al. reported a multi-institutional retrospective cohort of 334 eligible children in which the use of tamsulosin improved spontaneous passage of ureteral calculi with an odds ratio (OR) of 3.31 [95% confidence interval (CI) (1.49–7.34)] after controlling for stone size and location (5). A previous meta-analysis by Velázquez et al. in 2015 reported an improved rate of ureteral stone passage in children prescribed MET (6). That meta-analysis was limited by a scarcity of prospective RCTs published at that time (n=3) and synthesized data from 2 retrospective cohorts. In 2017, Tian et al. reported similar findings favoring MET for spontaneous passage in a similar meta-analysis that synthesized 4 RCTs and 1 retrospective cohort (7). Additional RCTs have been published since these two meta-analyses.
We sought to further clarify the impact of MET on spontaneous stone passage in children with obstructive urolithiasis through an updated, selective systematic review and meta-analysis. Preliminary literature review dictated the majority of published data pertained to pediatric patients with distal ureteral calculi. Therefore, we developed a protocol to quantitatively pool results of only the highest quality, prospective evidence to elucidate the effects of MET on pediatric patients with distal ureteral calculi. We hypothesized pooled analysis would demonstrate MET improves spontaneous stone passage rate.
Methods
This study was considered exempt from institutional review as the data used are derived from previously published research. The study was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (8). Supplemental Figure 1 contains the PRISMA checklist. Given the scarcity of high-quality pediatric trials on MET, we did not feel a purely a priori protocol was feasible. Therefore, preliminary literature searches were used to assess the volume of highest-quality publications in the pediatric literature and guide decision-making on endpoint selection.
Eligibility Criteria, Search Strategy, and Identification of Studies
Preliminary literature searches revealed single-center RCTs to be the highest quality of study prevalent in the pediatric literature; therefore, we excluded prospective nonrandomized and retrospective studies. The majority of the pediatric literature reported stones in the distal ureter, so studies that did not specify location of the stone or reported locations other than the distal ureter were excluded. We did not require a specific imaging modality to be used for determining the location of a stone as located within the distal ureter. We also did not require a specific imaging modality to be used for confirmation of stone passage as an endpoint. Studies that did not clearly define randomization, blinding, or placebo groups were excluded. Studies were not excluded based on stone size, publication date, trial medication, trial country, or trial size.
In September 2021, we performed a narrow-scope systematic search using PubMed and Embase with a predefined search strategy (Figure 1) intended to capture all RCTs on MET in the pediatric literature. We defined MET as the use of any alpha-blocker (with or without additional pain management medication) for the purpose of distal stone passage. We reviewed the titles of all articles identified via the initial search for potential eligibility (“first pass”). Articles deemed potentially eligible based on title alone underwent abstract and full-text review (“second pass”). References lists for all articles selected for inclusion after the second pass were reviewed to ensure literature saturation. Two authors (MB and RL) reviewed the abstracts and full text manuscripts during the second pass. In instances of disagreement, the senior author (CB) helped reach a consensus.
Data Collection and Synthesis
For studies meeting eligibility criteria, we extracted raw numerator and denominator data for treatment and control groups. We did not collect adjusted outcome statistics because the methods and variables were inconsistent across studies. Authors were not contacted for unpublished data. Our primary outcome was the overall effect of MET on ureteral calculi passage within 28 days compared to controls.
Data was pooled using both Mantel-Haenszel fixed and random effect meta-analysis, with random effect performed in attempt to explain between study heterogeneity, as per recommendation of the Cochrane Handbook for Systematic Reviews of Interventions (9). Risk ratios (RR) with 95% confidence intervals (CI) were calculated. CIs that did not cross RR 1.0 were considered statistically significant. Statistical heterogeneity was assessed using the I2 statistic. Assessment of risk of bias was conducted using the Cochrane risk-of-bias tool by two authors (RL and CB) for randomized controlled trials (RoB 2) (10). All quantitative data were maintained in and meta-analyses performed with Review Manager (RevMan) 5 (11).
Results
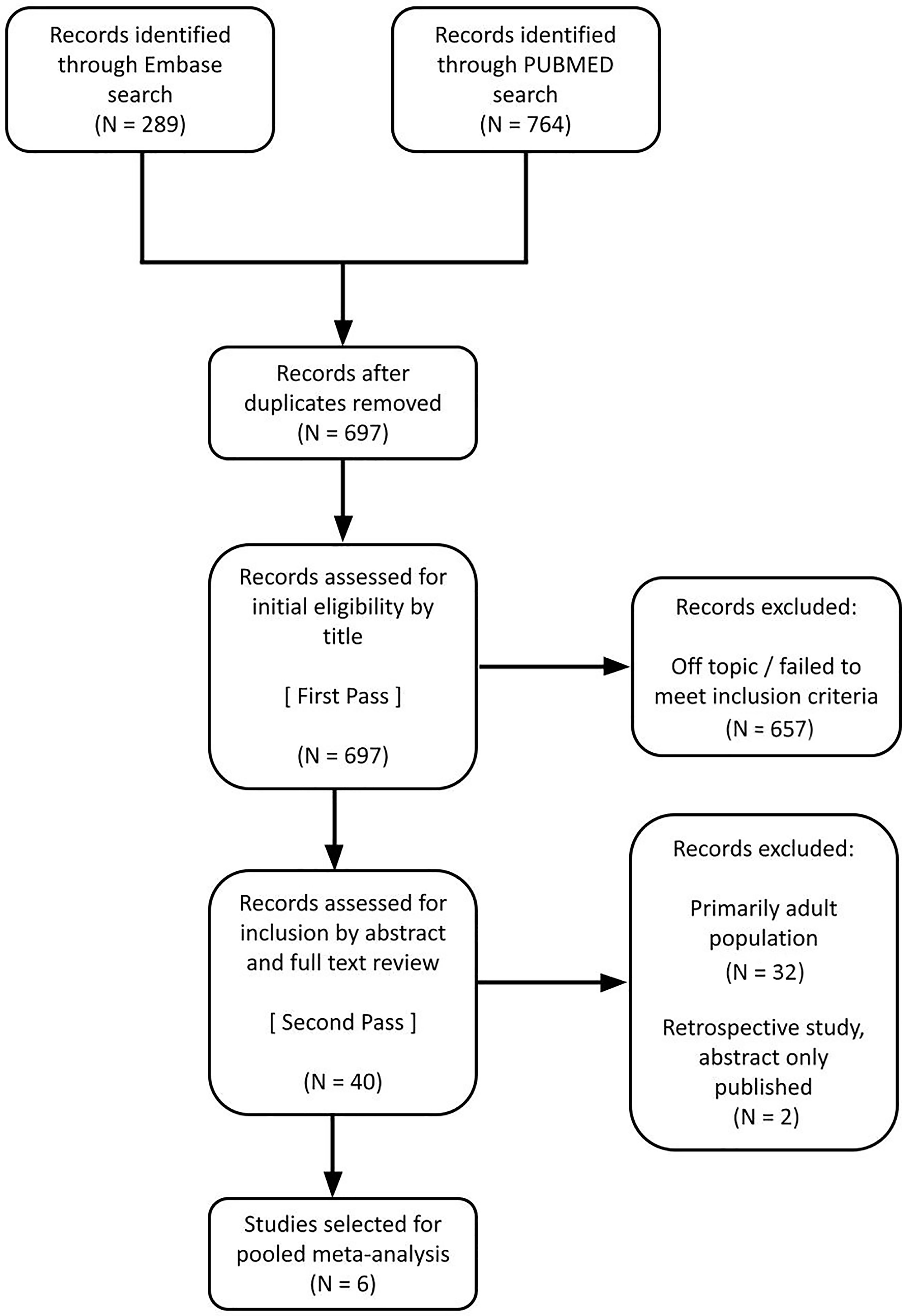
The study selection process is shown in Figure 2. Of the 40 studies reviewed for eligibility, 6 pediatric studies (12–17) met predefined criteria and were suitable for pooled analysis. One retrospective study was excluded, and another study was excluded because the content was reported only as an abstract.
Eligible Studies
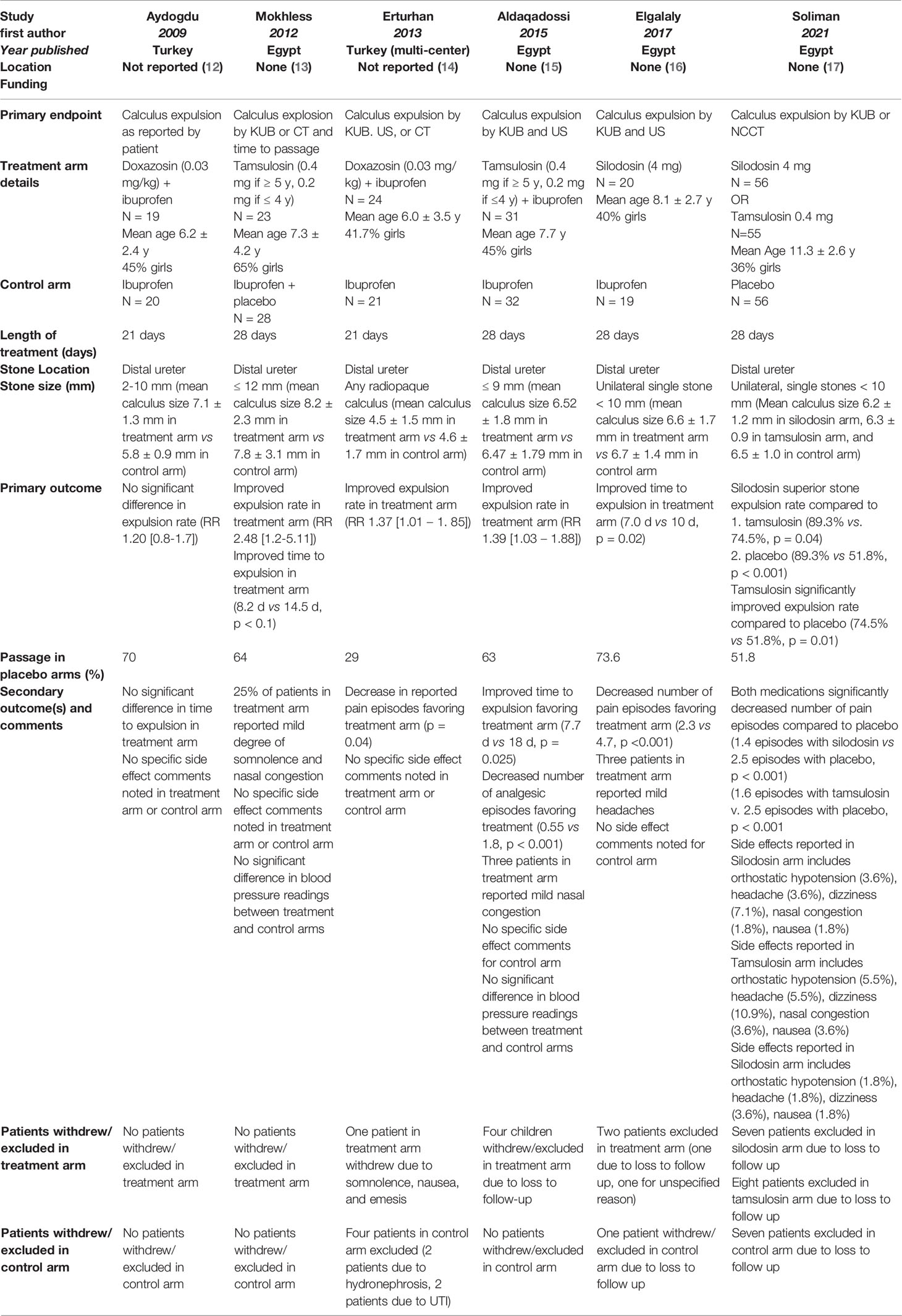
Table 1 identifies key details for all pediatric studies meeting eligibility criteria. Two of the 6 studies used doxazosin at 0.03 mg/kg as the exclusive alpha-blocker in the treatment arm. Both of these studies also included ibuprofen for analgesia alongside the doxazosin (12, 14). Two of the six studies used tamsulosin exclusively at an age-dependent dose (0.4 mg if ≥5 years old or 0.2 mg if ≤4 years old), with one of these studies also including ibuprofen analgesia within the treatment arm (13, 15). One study used silodosin at 4 mg as the alpha-blocker in their treatment arm without accompanying analgesia (16). The remaining study (Soliman et al) included two treatment arms, silodosin at 4 mg and tamsulosin at 0.4 mg (without dose adjustment based on age) (17). Five of the six studies used stone size within the distal ureter of 9 mm (15), 10 mm (12, 16, 17), and 12 mm (13) as an exclusion criterion. The remaining study did not use stone size within the distal ureter as an exclusion criterion (14). Across the six studies, three studies also included ibuprofen alongside the alpha-blocker in the treatment arm (12, 14, 15). The total length of treatment was 28 days in four studies (13, 15–17) and 21 days in the remaining two studies (12, 14).
Pooled Analysis
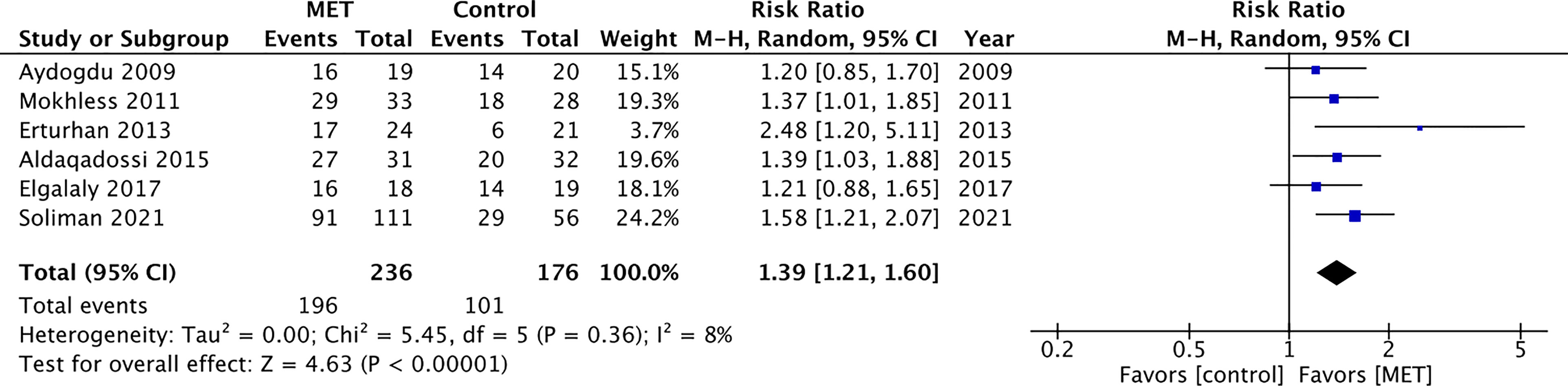
In the pooled pediatric analysis of 6 studies, 235 patients received MET and 176 received placebo or controls. Two studies compared MET versus placebo while 4 compared MET to a control, which was ibuprofen in all cases. The mean age of patients was 7.6 years-old, with a range of ages 2–18 years old. The mean stone size for the treatment group was 6.40 mm with a standard deviation of ± 1.5 mm. The mean stone for the control group was 6.42 with a standard deviation of ± 1.8 mm.
Children receiving MET were more likely than controls to experience spontaneous stone passage [RR 1.39 (CI 95% 1.21–1.60); Figure 3]. Data were pooled using Mantel-Haenszel random effect as both fixed-effect and random effect generated small heterogeneity (I2) of 8% (9).
Adverse Effects and Study Bias
In the pooled analysis, 22 patients were excluded or withdrew from the treatment arms: one due to side effects of somnolence/nausea/emesis, 20 due to loss to follow up, and one for an unknown reason. Eight patients in the control arms were excluded: two due to UTI, two due severe hydronephrosis, and four due to loss to follow up. Four studies discussed the following side effects seen within their respective treatment arms: nasal congestion, headaches, dizziness, orthostatic hypotension, and nausea. None of the studies identified a statistically significant difference in these side-effects compared to the control arm. The full breakdown of each study’s set of excluded patients and side effect profiles is recorded in Table 1.
Studies were assessed qualitatively for bias using five criteria specified in RoB 2. All studies included in pooled analysis were deemed at low or minor concerns for overall risk of bias (Figure 4).
Discussion
We performed a systematic review and meta-analysis to evaluate the effect of MET on spontaneous passage of distal ureteral calculi in pediatric patients. In pooled analysis, children receiving MET were more likely than controls to experience spontaneous stone passage [RR 1.39 (CI 95% 1.21–1.60); Figure 3]. Alpha-blockers were well tolerated in all eligible studies with one child stopping therapy definitely due to adverse effects. We assessed the included studies were at low risk for bias using the RoB 2. These findings are relevant as the incidence in stone disease has increased in children, with some reports indicating a 5-fold increase within a decade and accounting for 1 in 685 pediatric hospitalizations in 2010 in the United States (18). Compared to meta-analyses previously published on the topic, this meta-analysis is more robust in the number of included studies and selective in the level of evidence (i.e. retrospective studies were not included).
The desire to use MET in children stems from the goal of mitigating risk. Pediatric stone surgery is nuanced with smaller anatomy. Safe and effective ureteroscopy often requires preliminary surgery to place a stent in order to facilitate a stage procedure 2–4 weeks later. There are the risks associated with anesthesia and as well as radiation exposure while using fluoroscopy. Furthermore, many of these patients will have more stone events throughout their life, confounding the effects of anesthesia, surgery, and radiation. The finding that MET favors spontaneous stone passage in children is particularly exciting when viewed alongside new data suggesting tamsulosin may improve ureteroscopic access in children. McGee et al. reported higher primary or initial ureteroscopic access rates in school-aged children treated with tamsulosin at one week prior to ureteroscopic stone surgery (19). This preliminary data would suggest MET may stand to improve surgical outcomes and minimize anesthesia and radiation exposure even if not effective in spontaneous stone passage.
Many of the studies included in this analysis reported a decrease in pain episodes in children with usage of MET compared to the control. The questionable objectivity of these results prevents us from drawing substantial conclusions. The safety of these medications in children is promoted by the small number of adverse events reported but is limited due to the sample size and power of this analysis. Alpha-blockers appear to be well tolerated in children but should be used with caution and transparency with future studies to better explore their safety. A search of ClinicalTrials.gov on February 26, 2022, did not identify any active trials using alpha-blockers for the purpose of pediatric stone MET (20).
Review-Level Limitations
This analysis focused on stones located in the distal ureter as this was the location defined by the majority of studies identified in our preliminary literature search. Defining a stone as within the distal ureter can be subjective, may be influenced by the imaging modality used, and likely introduces some between-study variability.
Readers should bear in mind studies on the use of MET in pediatric patients do not have the same level of rigorous evidence as compared to the adult literature: in our 6 included studies, only 2 pediatric were placebo controlled (13, 17), 1 was multicenter (14), 1 was single blinded (17) (and none double blinded), and sample sizes of all pediatric studies are smaller than those in adults. All studies included in this pooled analysis were performed at centers located within Turkey and Egypt, which may also impact the generalizability of these results (12–17).
Outcome-Level Limitations
Our pooled analysis cannot account for differences between study endpoints, treatment medication, and treatment duration. All studies utilized radiographic endpoints to assess stone passage except one (Aydogdu et al), which assessed patient self-reported stone passage (12). Endpoint heterogeneity is a common obstacle in research evaluating MET as anything less than a study-end computed tomography appears to be inadequate. Studies in the adult literature have challenged the accuracy of patient self-reporting in assessing stone passage (21, 22). More worrisome, secondary analysis of a multicenter prospective RCT investigating MET in adults reported over half of patients with a persistent ureteral stone showed resolution of hydronephrosis and reported no pain (23). Four studies treated patients for 28 days (13, 15–17) and 2 studies evaluated 21 days of treatment, demonstrating further variability with these endpoints (12, 14).
Three different alpha-blockers (doxazosin, tamsulosin, and silodosin) were utilized across the 6 included studies. Soliman et al. is especially noteworthy as this was the only study to include two treatment arms (silodosin or tamsulosin) alongside a placebo-controlled arm (17). For the purpose of our analysis, we combined the two treatment arm groups (17). Recent studies using silodosin for adult MET have demonstrated an improved. expulsion rate when compared to tamsulosin, possibly due to increased alpha-blocker selectivity (24, 25). The individual findings of the Soliman et al. are suggestive of similar results in pediatric population (17).
It should be noted one study (Erturhan et al.) demonstrated a spontaneous stone passage rate of 29% in its placebo arm. This contrasts with the placebo passage rate in the 5 other studies (range 51.8%–73.6%) (12–17). It is not clear why this variation exists, and this finding was acknowledged by the authors in the study (14).
Included studies differed in their sample size. Four studies demonstrated statistically significant risk ratios favoring MET, with the studies from Aydogdu et al. and Elgalaly et al. being the only studies that did not (12–17). This difference could be due to the limited sample sizes present within these studies (n=39 in both). The sample size is especially noteworthy for the Elgalaly et al. study when comparing the size of its tamsulosin treatment arm (n=19) to the Soliman et al. study tamsulosin treatment arm (n=56) (16, 17).
Study-Level Limitations
Study arms were inconsistent across all 6 included studies and may not, in most cases, have constituted a “pure” treatment versus placebo comparison. Ibuprofen was part of the treatment arm in 3 of the studies (12, 14, 15). More frequent and optimized use of ibuprofen may allow some patients to tolerate their treatment for longer periods when compared to patients in studies with alpha-blocker only treatment arms. There were no studies that compared the use of alpha-blocker with ibuprofen compared to alpha-blocker exclusively. Only 2 studies included a true placebo in the control arm.
Conclusion
This selective systematic review and meta-analysis of MET in children with distal ureteral stones demonstrated a statistically significant benefit to spontaneous stone passage. Given the increasing incidence of ureteral stones in children and the safety and efficacy of these medications, MET may prove to hold a bigger role in pediatric stone management in the future.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
Study conception and design: CB, RL, and EK. Data acquisition: CB, RL, and EK. Analysis and data interpretation: CB, MB, RL, and EK. Drafting of the manuscript: CB, RL, and EK. Critical revision: CB, MB, CG, and RD. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer RZ declared a past collaboration with authors CG and CB to the handling editor at the time of review.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fruro.2022.866162/full#supplementary-material
References
1. Assimos D, Krambeck A, Miller NL, Monga M, Murad MH, Nelson CP, et al. Surgical Management of Stones: American Urological Association/Endourological Society Guideline, PART I. J Urol (2016) 196:1153–60. doi: 10.1016/j.juro.2016.05.090
2. Türk C, Petřík A, Sarica K, Seitz C, Skolarikos A, Straub M, et al. EAU Guidelines on Diagnosis and Conservative Management of Urolithiasis. Eur Urol (2016) 69:468–74. doi: 10.1016/j.eururo.2015.07.040
3. Karesh A. US Food and Drug Administration Pediatric Advisory Committee Meeting (2012) Available at: https://wayback.archive-it.org/7993/20170114054436/http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM289947.pdf.
4. Lu Z, Dong Z, Ding H, Wang H, Ma B, Wang Z. Tamsulosin for Ureteral Stones: A Systematic Review and Meta-Analysis of a Randomized Controlled Trial. Uro Int (2012) 89(1):107–15. doi: 10.1159/000338909
5. Tasian GE, Cost NG, Granberg CF, Pulido JE, Rivera M, Schwen Z, et al. Tamsulosin and Spontaneous Passage of Ureteral Stones in Children: A Multi-Institutional Cohort Study. J Urol (2014) 192(2):506–11. doi: 10.1016/j.juro.2014.01.091
6. Velázquez N, Zapata D, Wang HH, Wiener JS, Lipkin ME, Routh JC. Medical Expulsive Therapy for Pediatric Urolithiasis: Systematic Review and Meta-Analysis. J Pediatr Urol (2015) 11(6):321–7. doi: 10.1016/j.jpurol.2015.04.036
7. Tian D, Li N, Huang W, Zong H, Zhang Y. The Efficacy and Safety of Adrenergic Alpha-Antagonists in Treatment of Distal Ureteral Stones in Pediatric Patients: A Systematic Review and Meta-Analysis. J Pediatr Surg (2017) 52(2):360–5. doi: 10.1016/j.jpedsurg.2016.10.003
8. Moher D, Liberati A, Tetzlaff J, Altman DG. Prisma Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PloS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
9. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions (2019). John Wiley & Sons. Available at: https://training.cochrane.org/handbook (Accessed September 1 2019).
10. Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ (2019) 366 :l4898. doi: 10.1136/bmj.l4898
11. Review Manager (RevMan) [Computer program]. Version 5.3. The Nordic Cochrane Centre. The Cochrane Collaboration, Copenhagen. (2014).
12. Aydogdu O, Burgu B, Gucuk A, Suer E, Soygur T. Effectiveness of Doxazosin in Treatment of Distal Ureteral Stones in Children. J Urol (2009) 182(6):2880–4. doi: 10.1016/j.juro.2009.08.061
13. Mokhless I, Zahran AR, Youssif M, Fahmy A. Tamsulosin for the Management of Distal Ureteral Stones in Children: A Prospective Randomized Study. J Pediatr Urol (2012) 8(5):544–8. doi: 10.1016/j.jpurol.2011.09.008
14. Erturhan S, Bayrak O, Sarica K, Seckiner I, Baturu M, Sen H. Efficacy of Medical Expulsive Treatment With Doxazosin in Pediatric Patients. Urol (2013) 81(3):640–3. doi: 10.1016/j.urology.2012.11.031
15. Aldaqadossi HA, Shaker H, Saifelnasr M, Gaber M. Efficacy and Safety of Tamsulosin as a Medical Expulsive Therapy for Stones in Children. Arab J Urol (2015) 13:107–11. doi: 10.1016/j.aju.2015.02.007
16. Elgalaly H, Eliwa A, Seleem M, Salem E, Omran M, Shello H, et al. Silodosin in the Treatment of Distal Ureteric Stones in Children: A Prospective, Randomised, Placebo-Controlled Study. Arab J Urol (2017) 15(3):194–8. doi: 10.1016/j.aju.2017.05.005
17. Soliman MG, El-Gamal O, El-Gamal S, Raheem AA, Abou-Ramadan A, El-Abd A. Silodosin Versus Tamsulosin as Medical Expulsive Therapy for Children With Lower-Third Ureteric Stones: Prospective Randomized Placebo-Controlled Study. Urol Int (2021) 105(7-8):568–73. doi: 10.1159/000513074
18. Bush NC, Xu L, Brown BJ Holzer MS, Gingrich A, Schuler B, et al. Hospitalizations for Pediatric Stone Disease in United States, 2002-2007. J Urol (2010) 183:1151–56. doi: 10.1016/j.juro.2009.11.057
19. McGee LM, Sack BS, Wan J, Kraft KH. The Effect of Preoperative Tamsulosin on Ureteroscopic Access in School-Aged Children. J Pediatr Urol (2021) 17(6):795–e1. doi: 10.1016/j.juro.2009.11.057
20. ClinicalTrials.Gov. Bethesda (MD: National Library of Medicine (US). Available at: https://www.clinicaltrials.gov/ct2/results?cond=&term=alpha+blockers+OR+Tamsulosin+OR+doxazosin+OR+silodosin&cntry=&state=&city=&dist=&Search=Search&age=0.
21. McLarty R, Assmus M, Senthilselvan A, Schuler T, Wollin T, De S. Patient Reported Outcomes Predicting Spontaneous Stone Passage May Not Have Acceptable Accuracy. J Urol (2020) 204(3):524–30. doi: 10.1097/JU.0000000000001030
22. Hernandez N, Mozafarpour S, Song Y, Eisner BH. Cessation of Ureteral Colic Does Not Necessarily Mean That a Ureteral Stone Has Been Expelled. J Urol (2018) 199:1011–14. doi: 10.1016/j.juro.2017.10.032
23. Jackman SV, Maganty A, Wolfson AB, Burrows PK, MacPherson C, Vargas NM, et al. Resolution of Hydronephrosis and Pain to Predict Stone Passage for Patients With Acute Renal Colic. Urol (2022) 159:48–52. doi: 10.1016/j.urology.2021.09.017
24. Huang W, Xue P, Zong H, Zhang Y. Efficacy and Safety of Silodosin in the Medical Expulsion Therapy for Distal Ureteral Calculi: A Systematic Review and Meta-Analysis. Br J Clin Pharmacol (2016) 81(1):13–22. doi: 10.1111/bcp.12737
Keywords: medical expulsive therapy, MET, urolithiasis, pediatric urolithiasis, distal ureter calculi, tamsulosin, doxazosin, silodosin
Citation: Bacchus MW, Locke RA, Kwenda EP, DeMarco RT, Grant C and Bayne CE (2022) Medical Expulsive Therapy (MET) for Ureteral Calculi in Children: Systematic Review and Meta-Analysis. Front. Urol. 2:866162. doi: 10.3389/fruro.2022.866162
Received: 30 January 2022; Accepted: 15 March 2022;
Published: 22 April 2022.
Edited by:
Seth A. Alpert, Nationwide Children’s Hospital, United StatesReviewed by:
Rebecca Zee, Virginia Commonwealth University Health System, United StatesJon Ellison, Medical College of Wisconsin, United States
Ching Man Carmen Tong, University of Alabama at Birmingham, United States
Bryan Sack, University of Michigan, United States
Copyright © 2022 Bacchus, Locke, Kwenda, DeMarco, Grant and Bayne. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christopher E. Bayne, Q2hyaXN0b3BoZXIuQmF5bmVAdXJvbG9neS51ZmwuZWR1
 Michael W. Bacchus
Michael W. Bacchus Rachel A. Locke
Rachel A. Locke Elizabeth P. Kwenda1
Elizabeth P. Kwenda1