- 1Urology Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, NY, United States
- 2Department of Urology, Mayo Clinic, Rochester, MN, United States
Radical prostatectomy (RP) remains a standard treatment option for clinically localized high-risk prostate cancer. While RP provides excellent local control, patients with high-risk disease remain at considerable risk for recurrence after surgery. Disease relapse may be the result of occult distant metastases or regional micrometastatic disease at the time of surgery. Accordingly, the role of systemic (neoadjuvant) therapy prior to RP has been investigated. Proposed neoadjuvant regimens: include monotherapy or combinations of chemotherapy, hormonal deprivation, and immunologic agents. Randomized trials using androgen deprivation have demonstrated improved pathologic outcomes, including pathologic downstaging and decreased risk of positive surgical margins, extracapsular extension, and seminal vesical invasion. However, these, albeit early, trials did not reliably demonstrate improved post-prostatectomy oncologic outcomes. More recent trials have evaluated novel combinations of chemo-hormonal therapy and immunologic based therapies. These studies are currently maturing and offer the promise, pending findings, of potentially informing future practice. In this review, we highlight the pathophysiologic basis and contemporary evidence for neoadjuvant therapy prior to RP for clinically localized high-risk prostate cancer.
Introduction
Prostate cancer remains as one of the commonest cancers in the developed world, of which a majority are clinically organ-confined at diagnosis (1). In the setting of low and intermediate-risk prostate cancer, active surveillance, radical prostatectomy (RP), and radiotherapy results in excellent prostate cancer specific survival (2, 3). In the setting of higher-risk disease, the role of local treatment with RP remains controversial. However, over the past decade, there has been a gradual shift to performing RP on increasingly higher risk patient cohorts (4, 5).
Controversy regarding RP in clinically localized high-risk prostate cancer exists due to a higher risk biochemical-recurrence (BCR) compared to RP performed in men with lower-risk prostate cancer. A high-volume multi-center study reported BCR of 50% and a salvage therapy rate of 37% after RP in high-risk patients (6). Such recurrences result from either post-RP residual local disease and/or undiagnosed occult metastatic disease at the time of surgery.
Increasingly, research has been directed to improving outcomes in patients with clinically localized high-risk prostate cancer treated with RP. Administration of systematic therapies prior to surgery (neoadjuvant therapy) may downstage the local tumor and improve local cancer control and further, these therapies may address occult micrometastatic disease improving oncologic outcomes.
In this review, we highlight the pathophysiologic basis and contemporary evidence assessing neoadjuvant therapy prior to RP for clinically localized high-risk prostate cancer.
Rationale
Neoadjuvant therapy is proposed to provide benefit by treating occult distant disease (micrometastases) and as well as to improve local disease control by downstaging the primary tumor. Additionally, monitoring the in-vivo disease response to systemic agents may provide prognostic information (7). Neoadjuvant therapies have been successfully introduced into other malignancies including esophageal (8) and bladder cancer (9).
As a general principal, neoadjuvant therapies may be considered feasible to investigate if (7):
● a successful local treatment already exists
● the risk of recurrence or progression is high, despite local therapy
● the drug candidate(s) is active against the disease
Following these principals above, high-risk prostate cancer appears particularly relevant for consideration of neoadjuvant therapies. In the PSA-era, high risk prostate cancer represents up to 30% of new prostate cancer diagnoses (10, 11). While subtle variations exist in the precise definition of high-risk disease, consensus suggests that this refers to patients with a Prostate Specific Antigen (PSA) over >20ng/mL, or Grade Group 4 or 5, or clinical stage of >T2c (12–15). Curative local treatment for high-risk clinically localized prostate cancer exists including RP. Nevertheless, in the setting of post-prostatectomy clinically localized high-risk disease, the 10 year BCR-free, cancer-recurrence-free survival and salvage therapy rates are 50%, 87% and 37%, respectively (6). Registry data, based on the SEER database, suggests a 5-year prostate cancer specific mortality of 2.3% and 3.5% in patients post-prostatectomy with high risk and very-high risk disease, respectively (16).
Several systemic therapies have proven beneficial in the setting of metastatic prostate cancer and are thereby potential candidates to be used earlier in the disease process to improve oncologic outcomes. These agents represent candidates as neoadjuvant therapies. Firstly, agents that manipulate the intra-tumoral hormonal environment are the mainstay of treatment in men with metastatic prostate cancer (14). Secondly, traditional chemotherapeutic agents that target prostate cancer cell cycle and replication have improved survival in men with advanced disease (17). Finally, novel immunotherapy agents that upregulate host immune response, such as checkpoint inhibitors, are of current interest and may prove beneficial in the neoadjuvant setting.
Based on the above principals for neoadjuvant therapy, investigation of RP combined with neoadjuvant systemic therapy in men with clinically localized high-risk prostate cancer is worthwhile, due to the high risk of recurrence after standard treatment options and the activity of various drug groups against this disease.
Neoadjuvant therapies, specifically hormonal deprivation, has been considered accepted practice prior to radiotherapy for localized prostate cancer (14). Indeed, in high-risk disease, hormonal deprivation in combination with radiotherapy is superior to radiotherapy along (18, 19). The proposed theory of mechanism of this is that hormonal deprivation may sensitize prostate cancer cells to radiotherapy. This sensitization is thought to be mediated by inhibition of androgen-receptor mediated repair of damaged DNA following radiotherapy injury. Given this proposed mechanism, the principals of neoadjuvant therapy prior to radiotherapy are not directly extrapolatable to the prostatectomy setting.
Neoadjuvant Hormonal Therapy
Hormonal deprivation, or androgen deprivation therapy (ADT), has long been a therapeutic strategy for mitigating progression of prostate cancer since the works of Huggins in the 1940s (20). This early work recognized that prostate cancer is largely a hormone-driven tumor through the effects of physiologic and pathologic androgens (21). Androgen receptor (AR) expression by prostate cancer cells, and subsequent stimulation by androgens (including testosterone and dihydroxytestosterone), results in AR nuclear translocation and activation of pathways that promote cellular proliferation and cell survival (22).
AR stimulation primarily occurs as a result of dihydroxy-testosterone, following conversion from testosterone by 5α-reductase. A majority of testosterone production occurs in the testes and, to a lesser extent, the adrenal glands and the tumor itself. Testicular testosterone production is regulated via the hypothalamic-pituitary-gonadal axis, and thus this is the primary target to modulate testosterone production in the setting of ADT. Specifically, these agents include Luteinizing Hormone Releasing Hormone (LHRH) analogues. In the setting of metastatic prostate cancer, sustained castration eventually leads to progression to castration-resistance, which is variously mediated by upregulation of AR expression, AR gain-of-function mutations, and tumoral androgen production (23, 24).
While LHRH analogues reduce circulating serum testosterone by 90-95%, these agents only limit intratumoral testosterone production by 75% (25). Accordingly, novel anti-androgen agents have been developed. Such agents include rationally designed AR inhibitors that limit AR nuclear translocation and downstream effects, including enzalutamide, apalutamide, durolutamide. Further, agents such as abiraterone act by irreversibly inhibiting CYP17A1 and thus blocking the production of androgens, including both adrenal and intratumoral production.
The potential benefit of neoadjuvant ADT must be considered in the context of potential adverse effects from these therapies. Adverse effects of LHRH analogues may be categorized as either sexual dysfunction (impotence, reduced libido), endocrine abberations (including weight gain, diabetes mellitus, obesity, gynecomastia, increased fracture risk, hot flushes), cardiovascular effects (acute myocardial infarction, ischemic heart disease or thrombosis), and compromised quality of life outcomes (mental health, mood, physical capacity) (24). In addition to these, novel anti-androgens may harbor specific adverse effects depending on the agent, including seizures (enzalutamide) and hypertension (abiraterone).
Neoadjuvant Hormonal Deprivation With Conventional ADT
Multiple trials have evaluated conventional ADT agents (LHRH analogues with or without first generation antiandrogens) in the setting of neoadjuvant therapy before radical prostatectomy for patients with clinically localized high risk-prostate cancer. Trials assessing these agents are summarized in Table 1.
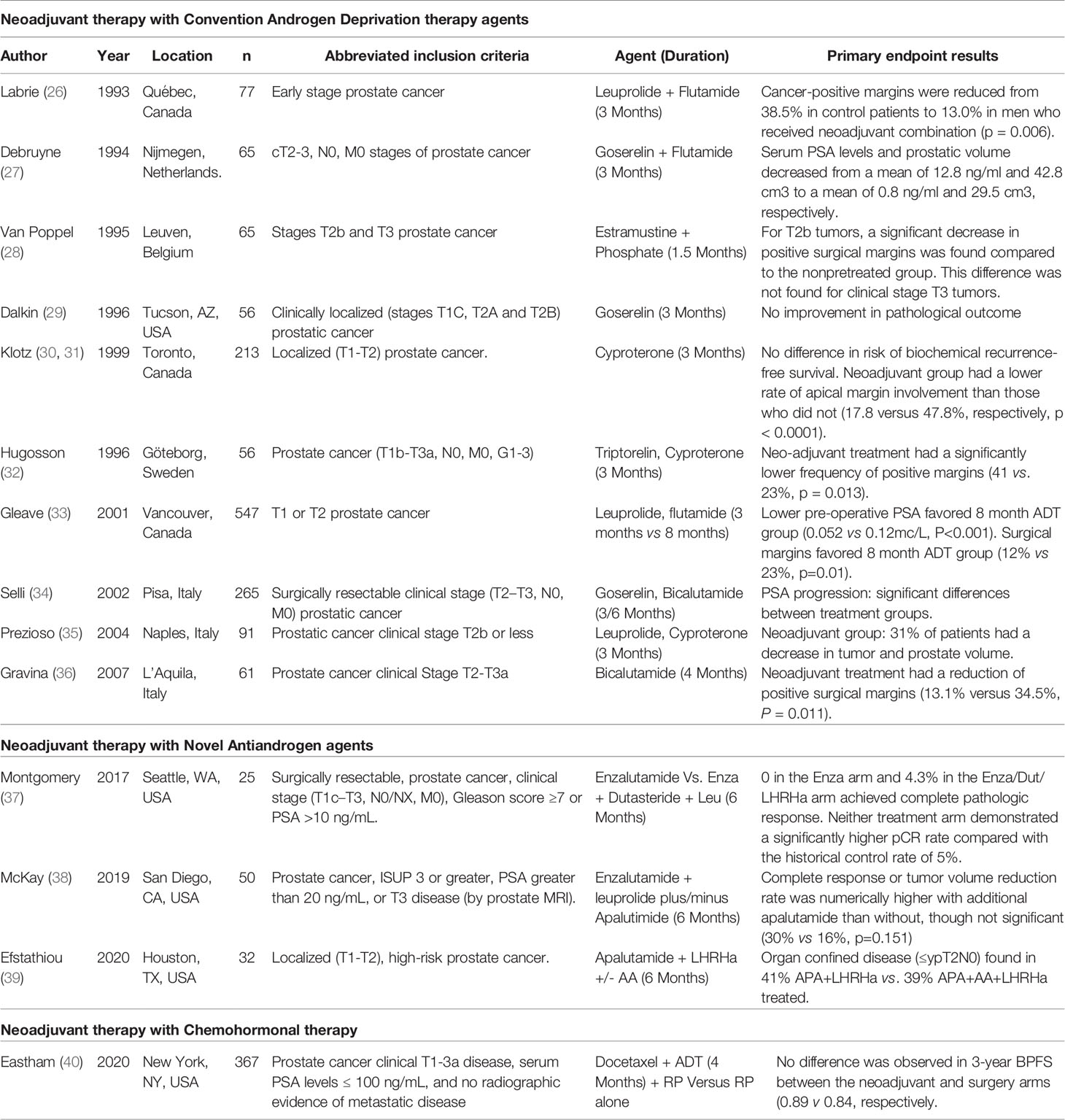
Table 1 Completed, published randomized trials assessing the role of neoadjuvant hormonal or chemohormonal therapy prior to prostatectomy.
Among the earliest of these trials was conducted by Labrie et al. in 1993, which demonstrated pathological downstaging and a reduction in positive surgical margins in the clinically localized high-risk population (26). These findings were corroborated by subsequent trials assessing goserelin monotherapy (29), cyproterone monotherapy (30) and combination therapy with either leuprolide plus flutamide (41) or goserelin plus flutamide (42). A systematic review and meta-analysis of trials demonstrated that, compared to surgery alone, neoadjuvant ADT resulted in reduced positive surgical margin rates (RR 0.49, 95% CI 0.42-0.56, p<0.001) and a higher likelihood of organ confined disease (RR 1.63, 95% 1.36-1.95, p<0.001) (43).
While a consistent effect on adverse pathologic features has been found with neoadjuvant ADT prior to RP, a benefit with regard to clinical oncologic outcomes has not been established. For example, Schulman et al. noted no difference in the risk of BCR between the groups receiving of not receiving neoadjuvant ADT prior to RP (44). Subsequent studies assessing varying conventional ADT agents with longer term follow-up have similarly reported no difference in the rates of BCR or overall survival (45–47). Moreover, in the aforementioned meta-analysis, no difference was observed between these groups with regard to prostate cancer specific- or overall-survival (43).
Of note, the duration of neoadjuvant therapy in these studies was typically limited to 3 to 4 months. Interestingly, Sayyid et al. compared 3 months and 8 months of leuprolide and a prior to RP and demonstrated that patients receiving 8 months of ADT demonstrated reduced risk of adverse pathologic features than those receiving 3 months of treatment (48). This notion was corroborated by Selli et al. using goserelin and flutamide (34). However, these studies did not report post-prostatectomy clinical outcomes and thus, the relevance of these pathologic findings is unclear.
Neoadjuvant Hormonal Deprivation Novel Anti-Androgens
The aforementioned development of more potent antiandrogens has reinvigorated interest of the role of neoadjuvant therapy for clinically localized high risk prostate cancer. However, due to the limited maturity of these agents, minimal long term oncologic clinical data exists.
Abiraterone, an irreversible CYP17A inhibitor, functions by reducing testicular, tumoral, and adrenal androgen production (49). In the setting of neoadjuvant therapy, abiraterone was the first novel antiandrogen assessed in high risk disease prior to RP (50). Taplin et al. randomized 58 patients to either 3-months of neoadjuvant abiraterone plus LHRH agonist or LHRH agonist monotherapy prior to prostatectomy. In the group with abiraterone, patients demonstrated a lower intratumoral testosterone (0.061pg/mg vs 0.098pg/mg, p=0.02) and DHT (0.180 pg/mg vs 1.307 pg/mg, p<0.001). This study reported reduced adverse pathologic features with the addition of abiraterone with higher proportions of complete response or minimally residual disease (MRD) (62% vs 48%). Efstathiou et al. performed a comparable Phase II open label trial, randomizing 65 men to 3-months of LHRH agonist with and without abiraterone in the neoadjuvant setting. This study similarly reported significant reduction in tumor volume measures favoring the abiraterone + LHRH agonist group (50).
Enzalutamide is an androgen receptor inhibitor that prevents androgen binding and receptor-ligand translocation. Similar to the findings observed in the abiraterone neoadjuvant trials, Phase II trials assessing neoadjuvant enzalutamide with LHRH agonist have reported outcomes regarding pathologic features. Montegomery randomized 52 patients, of which, 48 underwent prostatectomy after 6-months of enzalutamide monotherapy or enzalutamide with dutasteride and a LHRH analogue (37). Patients administered the combination therapy demonstrated lower intratumoral DHT (0.04pg/mg vs 3.34pg/mg, p<0.001) and intratumoral testosterone (0.18pg/mg vs 0.90pg/mg, p<0.001). The combination therapy group also demonstrated improved pathologic features including higher rates of either pathologic complete response or MRD (17.3% vs 0%) and lower residual cancer burden (0.41cm3 vs 0.06cm3) (37). Results of this study suggests neoadjuvant monotherapy with enzalutamide, and perhaps other novel anti-androgens, may not produce sufficient castration for clinical benefit.
McKay et al. recently assessed 6-months of neoadjuvant androgen blockade by means of either LHRH agonist with enzalutamide plus/minus the additional of abiraterone. This trial enrolled 75 patients and reported a trend of complete pathologic response or MRD favoring the addition of abiraterone, without reaching statistical significance (30% v 16%, p=0.263). Rates of adverse pathology were comparable between the groups, including pT3 disease, positive surgical margins and positive lymph nodes (38). As such, data from these trials suggest intense castration with two novel anti-androgen agents plus LHRH analogue may provide no additional benefit when compared to single novel anti-androgen therapy plus LHRH analogue.
Apalutamide, like enzalutamide, is an androgen receptor inhibitor and has recently been trialed in the neoadjuvant setting (39, 51, 52). While no randomized data comparing apalutamide with LHRH versus LHRH monotherapy exists, data assessing apalutamide in combination with other novel anti-androgens has been reported. Results of two phase II trials suggested no improvement in rates of complete pathologic response or reduction in residual tumor in patients treated with apalutamide in addition to abiraterone and LHRH agonist (39, 51). A single-arm Phase II trial reported pathologic features after 3-months of neoadjuvant apalutamide, abiraterone, degarelix and indomethacin prior to prostatectomy (53). The addition of indomethacin may further decrease production of testosterone by inhibiting AKR1C3. Despite maximal blockade, 5% of patients had complete pathologic response, 30% had MRD and 90% had T3 disease at prostatectomy.
In sum, neoadjuvant ADT with novel antiandrogens consistently reduces intratumoral testosterone and inconsistently improves rates of pathologic complete response or tumor volume reduction. Current data suggests that more intense hormonal blockade with multiple novel anti-androgens does not appear to result in meaningful improvements in risk of adverse pathology at prostatectomy. Longer follow-up including clinical and oncologic outcomes is required to further define the role of novel antiandrogens in the neoadjuvant setting prior to RP.
Chemohormonal Therapy
Previous groups have suggested that residual tumor may exist following neoadjuvant ADT due to a proportion of tumor clones exhibiting a degree of castration-resistance (54, 55). Accordingly, the addition of a cytotoxic chemotherapy agent has been proposed as a mechanism to target such cells (56). Regarding specific cytotoxic agents, as the efficacy of docetaxel has been demonstrated (with ADT) in metastatic prostate cancer (17, 57, 58), it is not surprising that this agent has thus been tested in the neoadjuvant setting as well. Specifically, neoadjuvant chemohormonal therapy has been investigated in several trials to date (40, 59–64). Despite the potential for therapeutic benefit of chemohormonal therapy, the potential morbidity must be carefully considered it the context of dual treatment pathways. In addition to the aforementioned adverse effects of ADT, docetaxel therapy is morbid and may be associated with fatigue, neuropathy, myelosuppression and rarely death (17).
Of the available studies assessing neoadjuvant chemohormonal therapy, a majority of these earlier trials represented single-arm Phase II safety and feasibility assessments of neoadjuvant chemohormonal therapy (59–64). Broadly speaking, these safety and feasibility trials reported acceptable tolerability of chemohormonal therapy with encouraging outcomes pertaining to pathologic downstaging and recurrence-free survival. Chi et al. performed one such Phase II trial and recruited 72 patients with high-risk disease (59). Prior to prostatectomy, patients received ADT and docetaxel (three cycles of docetaxel weekly for 6 weeks). Of these patients, two patients demonstrated complete pathologic response at the time of prostatectomy and at a median follow-up of 42.7 months, 30% had disease relapse. These findings have been corroborated by comparable Phase II single arm trials (59–64).
Subsequent comparative series have performed post-hoc comparative analysis of these patients enrolled on the single arm Phase II trials, matching patients with those that did not receive neoadjuvant therapies (63, 65, 66). For example, Narita et al. performed a propensity score matched analysis used patients from an aforementioned Phase II trial with a subgroup of patients from an existing database (66). In this analysis, neoadjuvant chemohormonal therapy was associated with a reduced risk of biochemical recurrence, compared to RP alone (p=0.021). Despite varying neoadjuvant chemohormonal regimes, comparable analysis by other groups has demonstrated improved BCR free-survival (63, 66), progression-free survival (65) and metastases-free survival (63) was observed when compared to RP alone.
A recent contemporary randomized trial examining the role of neoadjuvant chemohormonal therapy was published by Eastham et al. (40). The Preoperative Use of Neoadjuvant Chemotherapy (PUNCH) Alliance 90203 trial compared RP plus/minus neoadjuvant chemohormonal therapy with six cycles of docetaxel every 3-weeks and an LHRH analogue therapy for 18-24 weeks. The trial recruited a 788 patients with clinically localized high-risk disease (pT1-T3,NxM0) (40). At the time of prostatectomy, men receiving neoadjuvant chemohormonal therapy had lower pathologic stage (p<0.001), fewer positive surgical margins (p<0.001), and fewer metastatic lymph nodes (p=0.05) (40). However, the trial did not meet its primary endpoint of reducing BCR at 3 years. Though, longer term follow-up subsequently reported improved BCR-free survival (HR 0.69, 95% CI 0.48-0.99) and overall survival (80% vs 74%, HR 0.61, 95% CI 0.40-0.94), although the low event rate limits interpretation of these data. Additionally, rates of adjuvant or salvage treatment were lower in the neoadjuvant chemohormonal therapy group (HR 0.61, 95% 0.48-0.78).
Studies assessing chemohormonal therapy with novel anti-androgens are currently underway (Table 1).
Immunotherapy
Immunotherapy agents have been developed and introduced into cancer care in alternate organs, such as kidney (67–70) and bladder (71, 72). Such agents act by improving the host immune response to cancer cells. Broadly speaking, this may be achieved by either upregulating the host immune response, or conversely, by limiting tumor cells escape pathways. The effectiveness of immunotherapy is determined by the immunogenicity of cancer cells, specifically by the characteristic expression of unique tumor associated antigens (73). Accordingly, immunotherapy in prostate cancer may have utility given the prostate expresses specific proteins (e.g. PSA, PSMA) and further, given it is not a vital organ, collateral damage to physiologic tissue is of minimal consequence (74). Additionally, patients with prostate cancer are known to have a disrupted immune system, particularly in the later stages of the disease, as characterized by a reduction in Natural Killer (NK) cell activity (75, 76).
Despite increasing interest, only limited data currently exists in the setting of neoadjuvant immunotherapy for high risk prostate cancer. Of the available data to date, agents assessed include GVAX, rituximab and Sipuleucel-T. GVAX is a granulocyte-macrophage colony stimulating factor (GM CSF) secreting vaccine which has been studied in a Phase II trial by the Johns Hopkins group (NCT01696877) (77). While data pertaining to clinical outcomes were limited, GVAX showed improved immunologic infiltrates such as CD8+ and CD4+ T cells. Rituximab is an anti-CD20 antibody that results in B-cell ablation and has been trailed in an exploratory Phase II setting (NCT01804712) with primary immunologic end points. This trial demonstrated neoadjuvant administration of rituximab resulted in an altered immune tumor microenvironment; the implications on clinical outcomes from this therapy is unclear (78). Similar neoadjuvant exploratory analyses have been performed using Sipuleucel-T, a systemic agent that induces CD4 and CD8 T cell recruitment. Given the exploratory nature, a lack of clinical data exists; however, this study did demonstrate a upregulation of immune response scores in the tumor microenvironment following Sipuleucel-T administration (79)
Checkpoint inhibitors, such as CTLA4, PD1 and PDL1 inhibitors, act by directly or indirectly upregulating host T cell response to tumor cells (80). Such agents have been of intense interest in renal cell carcinoma and other tumor types (69, 70, 81). To date, in the setting of advanced prostate cancer, the efficacy of monotherapy with these agents has been underwhelming, with the exception of pembrolizumab in patients with mismatch repair (MMR) deficiency or a high microsatellite instability status (82, 83). Data is lacking assessing the role of immunotherapy in the earlier stages of high risk disease. Accordingly, current trials are currently in progress, assess in the role of these novel checkpoint inhibitors as neoadjuvant agents, including nivolumab (NCT02933255), atezolizumab (NCT03821246) and other CTLA4 agents (NCT04301414).
Future Directions/Current Active Trials
There is significant interest in neoadjuvant therapies prior to RP for clinically localized high-risk prostate cancer exists, as evidenced by numerous active and recruiting trials (summarized in Table 2). Per the principals of neoadjuvant therapy, future directions of research may focus on alternate treatments that have known activity in prostate cancer. Emerging trials are underway assessing therapies such as novel antiandrogens and immunotherapy agents. Trials assessing pertinent immunotherapy agents such as PD1, PDL1 and CTLA4 inhibitors are underway.
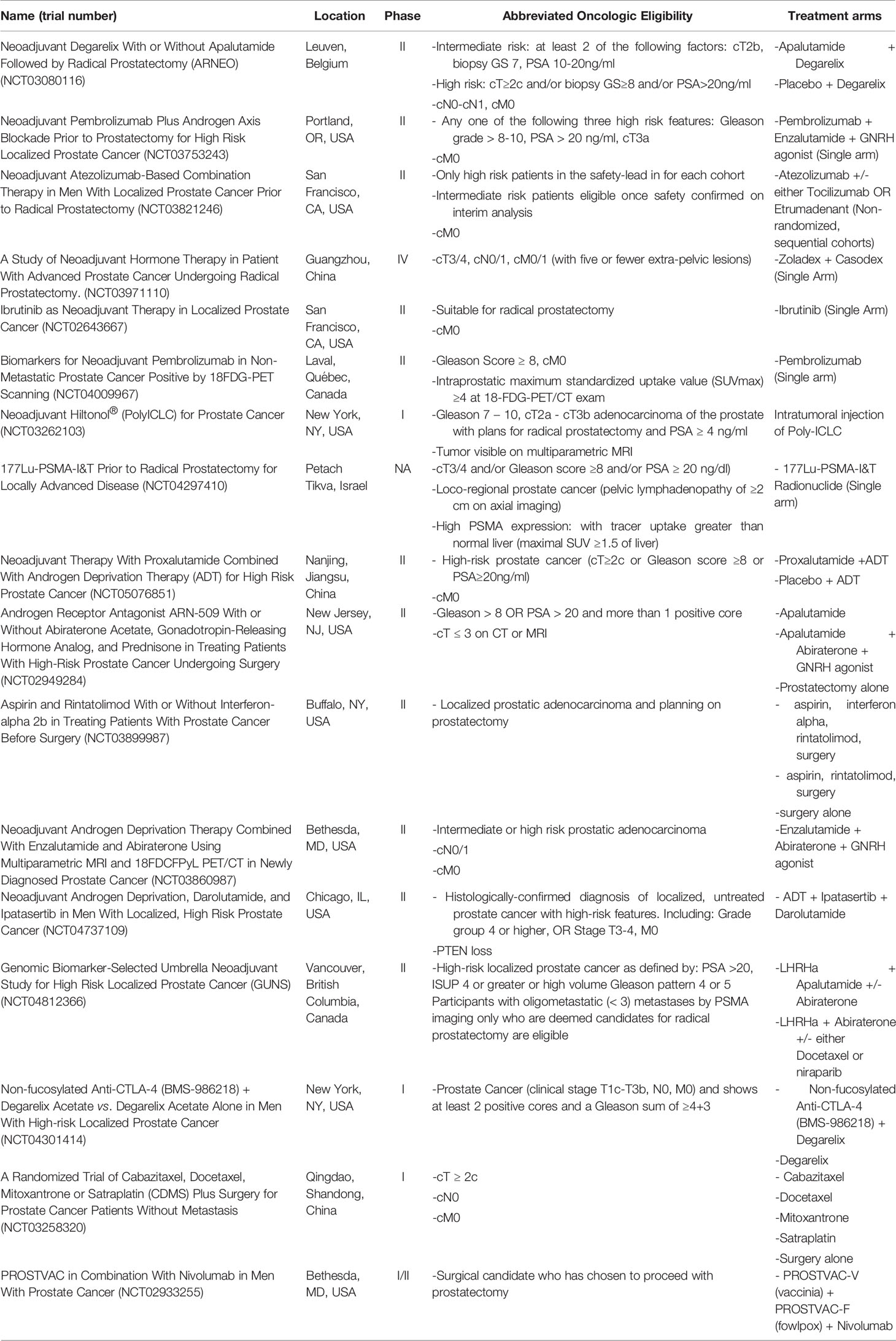
Table 2 Current active trials assessing neoadjuvant therapies prior to radical prostatectomy for high-risk, localized disease.
Gene targeted therapy remains an area of intense research, particularly in the era of genomic profiling in the setting of high risk disease, per the NCCN guidelines (15). Recently, PARP inhibitors including olaparib, is recommended for patients with metastatic castrate resistance prostate cancer with pathogenic mutations in BRCA1, BRCA2, ATM, MARD1, BRIP1, CDK12, CHEK1, CHEK2, PANCL, PALB2, RAD51B, RAD51C, RAD51D and RAD54L. While the role of gene-targeted therapy in earlier disease is yet to be defined, it may represent a particular focus as experience with such technologies mature.
A further focus of interest pertains to the potential consideration of radiopharmaceuticals and theranostics. For example, in the setting of castration-resistance prostate cancer, the recently published VISION trial demonstrated efficacy in Lu177-PSMA (84). The inclusion criteria of comparable active trials assessing Lu177-PSMA, suggest a minimum SUV avidity of 20 for metastases (85). Such values are plausible in intraprostatic disease, suggesting possible efficacy for PSMA rich intraprostatic disease prior to local definitive therapy.
Recommendations
At present, neoadjuvant therapy is not recommended prior to RP in men with clinically localized high-risk prostate cancer outside of a clinical trial. Robust studies including long term oncologic outcomes will be required to establish a role for initial systemic therapy in the management of clinically localized high-risk prostate cancer. Thus, current standard of care includes RP or radiotherapy with neoadjuvant/concurrent/adjuvant ADT. Patients eligible for such ongoing neoadjuvant clinical trials should be encouraged to consider participation.
Conclusions
Patients with clinically localized high-risk prostate cancer is remain at significant risk of recurrence despite current local treatment options. Neoadjuvant therapy prior to RP is designed to reduce the risk of post-operative residual local disease and address micrometastatic disease prior to definitive treatment. However, evidence to date has demonstrated that neoadjuvant ADT or chemohormonal therapy may be associated with reduced adverse pathologic features but not oncologic outcomes. Clinical trials assessing novel therapeutic regimens are ongoing.
Author Contributions
MP: manuscript preparation/drafting. BB: data collection and manuscript revision. ME: data collection and manuscript draft. CG: data collection. WY: data collection and manuscript revision. SB: supervision and manuscript revision. JE: supervision and manuscript revision. All authors contributed to the article and approved the submitted version.
Funding
MP is sponsored by the Australian-America Fulbright Commission administered through a 2021-2022 Fulbright Future Scholarship funded by The Kinghorn Foundation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA: A Cancer J Clin (2021) 71:7–33. doi: 10.3322/caac.21654
2. Roberts MJ, Papa N, Perera M, Scott S, Teloken PE, Joshi A, et al. A Contemporary, Nationwide Analysis of Surgery and Radiotherapy Treatment for Prostate Cancer. BJU Int (2019) 124 Suppl 1:31–6. doi: 10.1111/bju.14773
3. Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-Year Outcomes After Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med (2016) 375:1415–24. doi: 10.1056/NEJMoa1606220
4. Sathianathen NJ, Lamb AD, Lawrentschuk NL, Goad JR, Peters J, Costello AJ, et al. Changing Face of Robot-Assisted Radical Prostatectomy in Melbourne Over 12 Years. ANZ J Surg (2018) 88:E200–e3. doi: 10.1111/ans.14169
5. Boehm K, Borgmann H, Ebert T, Höfner T, Khaljani E, Schmid M, et al. Stage and Grade Migration in Prostate Cancer Treated With Radical Prostatectomy in a Large German Multicenter Cohort. Clin Genitourin Cancer (2021) 19:162–6.e1. doi: 10.1016/j.clgc.2020.12.004
6. Abdollah F, Sood A, Sammon JD, Hsu L, Beyer B, Moschini M, et al. Long-Term Cancer Control Outcomes in Patients With Clinically High-Risk Prostate Cancer Treated With Robot-Assisted Radical Prostatectomy: Results From a Multi-Institutional Study of 1100 Patients. Eur Urol (2015) 68:497–505. doi: 10.1016/j.eururo.2015.06.020
7. Kent EC, Hussain MH. Neoadjuvant Therapy for Prostate Cancer: An Oncologist’s Perspective. Rev Urol (2003) 5 Suppl 3:S28–37.
8. Kidane B, Coughlin S, Vogt K, Malthaner R. Preoperative Chemotherapy for Resectable Thoracic Esophageal Cancer. Cochrane Database Syst Rev (2015) 2015:Cd001556. doi: 10.1002/14651858.CD001556.pub3
9. Petrelli F, Coinu A, Cabiddu M, Ghilardi M, Vavassori I, Barni S. Correlation of Pathologic Complete Response With Survival After Neoadjuvant Chemotherapy in Bladder Cancer Treated With Cystectomy: A Meta-Analysis. Eur Urol (2014) 65:350–7. doi: 10.1016/j.eururo.2013.06.049
10. Meng MV, Elkin EP, Latini DM, Duchane J, Carroll PR. Treatment of Patients With High Risk Localized Prostate Cancer: Results From Cancer of the Prostate Strategic Urological Research Endeavor (CaPSURE). J Urol (2005) 173:1557–61. doi: 10.1097/01.ju.0000154610.81916.81
11. Cooperberg MR, Broering JM, Carroll PR. Time Trends and Local Variation in Primary Treatment of Localized Prostate Cancer. J Clin Oncol (2010) 28:1117–23. doi: 10.1200/JCO.2009.26.0133
12. D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical Outcome After Radical Prostatectomy, External Beam Radiation Therapy, or Interstitial Radiation Therapy for Clinically Localized Prostate Cancer. JAMA (1998) 280:969–74. doi: 10.1001/jama.280.11.969
13. Sanda MG, Cadeddu JA, Kirkby E, Chen RC, Crispino T, Fontanarosa J, et al. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part I: Risk Stratification, Shared Decision Making, and Care Options. J Urol (2018) 199:683–90. doi: 10.1016/j.juro.2017.11.095
14. Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment With Curative Intent. Eur Urol (2021) 79:243–62. doi: 10.1016/j.eururo.2020.09.042
15. Mohler JL, Antonarakis ES, Armstrong AJ, D’Amico AV, Davis BJ, Dorff T, et al. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2019) 17:479–505. doi: 10.6004/jnccn.2019.0100
16. Chierigo F, Wenzel M, Würnschimmel C, Flammia RS, Horlemann B, Tian Z, et al. Survival After Radical Prostatectomy Versus Radiation Therapy in High-Risk and Very High-Risk Prostate Cancer. J Urol (2022) 207:375–84. doi: 10.1097/JU.0000000000002250
17. Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med (2015) 373:737–46. doi: 10.1056/NEJMoa1503747
18. Bolla M, Van Tienhoven G, Warde P, Dubois JB, Mirimanoff RO, Storme G, et al. External Irradiation With or Without Long-Term Androgen Suppression for Prostate Cancer With High Metastatic Risk: 10-Year Results of an EORTC Randomised Study. Lancet Oncol (2010) 11:1066–73. doi: 10.1016/S1470-2045(10)70223-0
19. Roach M 3rd, Bae K, Speight J, Wolkov HB, Rubin P, Lee RJ, et al. Short-Term Neoadjuvant Androgen Deprivation Therapy and External-Beam Radiotherapy for Locally Advanced Prostate Cancer: Long-Term Results of RTOG 8610. J Clin Oncol (2008) 26:585–91. doi: 10.1200/JCO.2007.13.9881
20. Huggins C, Hodges CV. Studies on Prostatic Cancer. I. The Effect of Castration, of Estrogen and Androgen Injection on Serum Phosphatases in Metastatic Carcinoma of the Prostate. CA Cancer J Clin (1972) 22:232–40. doi: 10.3322/canjclin.22.4.232
21. Crawford ED, Heidenreich A, Lawrentschuk N, Tombal B, Pompeo ACL, Mendoza-Valdes A, et al. Androgen-Targeted Therapy in Men With Prostate Cancer: Evolving Practice and Future Considerations. Prostate Cancer Prostatic Dis (2019) 22:24–38. doi: 10.1038/s41391-018-0079-0
22. Davey RA, Grossmann M. Androgen Receptor Structure, Function and Biology: From Bench to Bedside. Clin Biochem Rev (2016) 37:3–15.
23. Dai C, Heemers H, Sharifi N. Androgen Signaling in Prostate Cancer. Cold Spring Harb Perspect Med (2017) 7:1–18. doi: 10.1101/cshperspect.a030452
24. Perera M, Roberts MJ, Klotz L, Higano CS, Papa N, Sengupta S, et al. Intermittent Versus Continuous Androgen Deprivation Therapy for Advanced Prostate Cancer. Nat Rev Urol (2020) 17:469–81. doi: 10.1038/s41585-020-0335-7
25. Mostaghel EA, Page ST, Lin DW, Fazli L, Coleman IM, True LD, et al. Intraprostatic Androgens and Androgen-Regulated Gene Expression Persist After Testosterone Suppression: Therapeutic Implications for Castration-Resistant Prostate Cancer. Cancer Res (2007) 67:5033–41. doi: 10.1158/0008-5472.CAN-06-3332
26. Labrie F, Dupont A, Cusan L, Gomez J, Diamond P, Koutsilieris M, et al. Downstaging of Localized Prostate Cancer by Neoadjuvant Therapy With Flutamide and Lupron: The First Controlled and Randomized Trial. Clin Invest Med (1993) 16:499–509.
27. Debruyne FMJ, Witjes WPJ, Schulman CC, van Cangh PJ. Oosterhof GON. A Multicentre Trial of Combined Neoadjuvant Androgen Blockade With Zoladex and Flutamide Prior to Radical Prostatectomy in Prostate Cancer. Eur Urol (1994) 26(suppl 1):4–. doi: 10.1159/000475423
28. van Poppel H. Neoadjuvant Hormone Therapy and Radical Prostatectomy: The Jury Is Still Out. Eur Urol (2001) 39(suppl 1):10–4. doi: 10.1159/000052544
29. Dalkin BL, Ahmann FR, Nagle R, Johnson CS. Randomized Study of Neoadjuvant Testicular Androgen Ablation Therapy Before Radical Prostatectomy in Men With Clinically Localized Prostate Cancer. J Urol (1996) 155:1357–60. doi: 10.1016/S0022-5347(01)66266-9
30. Goldenberg SL, Klotz LH, Srigley J, Jewett MA, Mador D, Fradet Y, et al. Randomized, Prospective, Controlled Study Comparing Radical Prostatectomy Alone and Neoadjuvant Androgen Withdrawal in the Treatment of Localized Prostate Cancer. Can Urologic Oncol Group J Urol (1996) 156:873–7. doi: 10.1016/S0022-5347(01)65645-3
31. Klotz LH, Goldenberg SL, Jewett M, Barkin J, Chetner M, Fradet Y, et al. CUOG Randomized Trial of Neoadjuvant Androgen Ablation Before Radical Prostatectomy: 36-Month Post-Treatment PSA Results. Can Urologic Oncol Group Urol (1999) 53:757–63. doi: 10.1016/S0090-4295(98)00616-5
32. Hugosson J, Abrahamsson PA, Ahlgren G, Aus G, Lundberg S, Schelin S, et al. The Risk of Malignancy in the Surgical Margin at Radical Prostatectomy Reduced Almost Three-Fold in Patients Given Neo-Adjuvant Hormone Treatment. Eur Urol (1996) 29:413–9. doi: 10.1159/000473789
33. Gleave ME, Goldenberg SL, Chin JL, Warner J, Saad F, Klotz LH, et al. Randomized Comparative Study of 3 Versus 8-Month Neoadjuvant Hormonal Therapy Before Radical Prostatectomy: Biochemical and Pathological Effects. J Urol (2001) 166:500–6; discussion 6-7. doi: 10.1016/S0022-5347(05)65971-X
34. Selli C, Montironi R, Bono A, Pagano F, Zattoni F, Manganelli A, et al. Effects of Complete Androgen Blockade for 12 and 24 Weeks on the Pathological Stage and Resection Margin Status of Prostate Cancer. J Clin Pathol (2002) 55:508–13. doi: 10.1136/jcp.55.7.508
35. Prezioso D, Lotti T, Polito M, Montironi R. Neoadjuvant Hormone Treatment With Leuprolide Acetate Depot 3.75 Mg and Cyproterone Acetate, Before Radical Prostatectomy: A Randomized Study. Urol Int (2004) 72:189–95. doi: 10.1159/000077113
36. Gravina GL, Festuccia C, Galatioto GP, Muzi P, Angelucci A, Ronchi P, et al. Surgical and Biologic Outcomes After Neoadjuvant Bicalutamide Treatment in Prostate Cancer. Urology (2007) 70:728–33. doi: 10.1016/j.urology.2007.05.024
37. Montgomery B, Tretiakova MS, Joshua AM, Gleave ME, Fleshner N, Bubley GJ, et al. Neoadjuvant Enzalutamide Prior to Prostatectomy. Clin Cancer Res (2017) 23:2169–76. doi: 10.1158/1078-0432.CCR-16-1357
38. McKay RR, Ye H, Xie W, Lis R, Calagua C, Zhang Z, et al. Evaluation of Intense Androgen Deprivation Before Prostatectomy: A Randomized Phase II Trial of Enzalutamide and Leuprolide With or Without Abiraterone. J Clin Oncol (2019) 37:923–31. doi: 10.1200/JCO.18.01777
39. Efstathiou E, Boukovala MA, Spetsieris N, Wen S, Hoang A, Weldon JA, et al. Neoadjuvant Apalutamide (APA) Plus Leuprolide (LHRHa) With or Without Abiraterone (AA) in Localized High-Risk Prostate Cancer (LHRPC). J Clin Oncol (2020) 38:5504–. doi: 10.1200/JCO.2020.38.15_suppl.5504
40. Eastham JA, Heller G, Halabi S, Monk JP 3rd, Beltran H, Gleave M, et al. Cancer and Leukemia Group B 90203 (Alliance): Radical Prostatectomy With or Without Neoadjuvant Chemohormonal Therapy in Localized, High-Risk Prostate Cancer. J Clin Oncol (2020) 38:3042–50. doi: 10.1200/JCO.20.00315
41. Soloway MS, Sharifi R, Wajsman Z, McLeod D, Wood DP Jr., Puras-Baez A. Randomized Prospective Study Comparing Radical Prostatectomy Alone Versus Radical Prostatectomy Preceded by Androgen Blockade in Clinical Stage B2 (T2bNxM0) Prostate Cancer. The Lupron Depot Neoadjuvant Prostate Cancer Study Group. J Urol (1995) 154:424–8. doi: 10.1016/S0022-5347(01)67067-8
42. Witjes WP, Schulman CC, Debruyne FM. Preliminary Results of a Prospective Randomized Study Comparing Radical Prostatectomy Versus Radical Prostatectomy Associated With Neoadjuvant Hormonal Combination Therapy in T2-3 N0 M0 Prostatic Carcinoma. The European Study Group on Neoadjuvant Treatment of Prostate Cancer. Urology (1997) 49:65–9. doi: 10.1016/S0090-4295(97)00171-4
43. Shelley MD, Kumar S, Wilt T, Staffurth J, Coles B, Mason MD. A Systematic Review and Meta-Analysis of Randomised Trials of Neo-Adjuvant Hormone Therapy for Localised and Locally Advanced Prostate Carcinoma. Cancer Treat Rev (2009) 35:9–17. doi: 10.1016/j.ctrv.2008.08.002
44. Schulman CC, Debruyne FM, Forster G, Selvaggi FP, Zlotta AR, Witjes WP. 4-Year Follow-Up Results of a European Prospective Randomized Study on Neoadjuvant Hormonal Therapy Prior to Radical Prostatectomy in T2-3N0M0 Prostate Cancer. European Study Group on Neoadjuvant Treatment of Prostate Cancer. Eur Urol (2000) 38:706–13. doi: 10.1159/000020366
45. Klotz L, Vesprini D, Sethukavalan P, Jethava V, Zhang L, Jain S, et al. Long-Term Follow-Up of a Large Active Surveillance Cohort of Patients With Prostate Cancer. J Clin Oncol (2015) 33:272–7. doi: 10.1200/JCO.2014.55.1192
46. Aus G, Abrahamsson PA, Ahlgren G, Hugosson J, Lundberg S, Schain M, et al. Three-Month Neoadjuvant Hormonal Therapy Before Radical Prostatectomy: A 7-Year Follow-Up of a Randomized Controlled Trial. BJU Int (2002) 90:561–6. doi: 10.1046/j.1464-410X.2002.02982.x
47. Yee DS, Lowrance WT, Eastham JA, Maschino AC, Cronin AM, Rabbani F. Long-Term Follow-Up of 3-Month Neoadjuvant Hormone Therapy Before Radical Prostatectomy in a Randomized Trial. BJU Int (2010) 105:185–90. doi: 10.1111/j.1464-410X.2009.08698.x
48. Sayyid RK, Evans A, Hersey K, Maloni R, Hurtado-Coll A, Kulkarni G, et al. A Phase II, Randomized, Open-Label Study of Neoadjuvant Degarelix Versus LHRH Agonist in Prostate Cancer Patients Prior to Radical Prostatectomy. Clin Cancer Res (2017) 23:1974–80. doi: 10.1158/1078-0432.CCR-16-1790
49. O’Donnell A, Judson I, Dowsett M, Raynaud F, Dearnaley D, Mason M, et al. Hormonal Impact of the 17alpha-Hydroxylase/C(17,20)-Lyase Inhibitor Abiraterone Acetate (CB7630) in Patients With Prostate Cancer. Br J Cancer (2004) 90:2317–25. doi: 10.1038/sj.bjc.6601879
50. Taplin ME, Montgomery B, Logothetis CJ, Bubley GJ, Richie JP, Dalkin BL, et al. Intense Androgen-Deprivation Therapy With Abiraterone Acetate Plus Leuprolide Acetate in Patients With Localized High-Risk Prostate Cancer: Results of a Randomized Phase II Neoadjuvant Study. J Clin Oncol (2014) 32:3705–15. doi: 10.1200/JCO.2013.53.4578
51. McKay RR, Xie W, Ye H, Fennessy FM, Zhang Z, Lis R, et al. Results of a Randomized Phase II Trial of Intense Androgen Deprivation Therapy Prior to Radical Prostatectomy in Men With High-Risk Localized Prostate Cancer. J Urol (2021) 206:80–7. doi: 10.1097/JU.0000000000001702
52. Yang* X, Aslim Edwin J, Ngo Nye T, Khor Li Y, Chong Tsung W, Peng Yuen John S, et al. MP79-05 Neoadjuvant Apalutamide (ARN-509) and Radical Prostatectomy in Treatment of Intermediate to High Risk Prostate Cancer (Near) Phase II Trial. J Urol (2020) 203:e1216–e7. doi: 10.1097/JU.0000000000000971.05
53. Graham LS, True LD, Gulati R, Schade GR, Wright J, Grivas P, et al. Targeting Backdoor Androgen Synthesis Through AKR1C3 Inhibition: A Presurgical Hormonal Ablative Neoadjuvant Trial in High-Risk Localized Prostate Cancer. Prostate (2021) 81:418–26. doi: 10.1002/pros.24118
54. Sowalsky AG, Ye H, Bhasin M, Van Allen EM, Loda M, Lis RT, et al. Neoadjuvant-Intensive Androgen Deprivation Therapy Selects for Prostate Tumor Foci With Diverse Subclonal Oncogenic Alterations. Cancer Res (2018) 78:4716–30. doi: 10.1158/0008-5472.CAN-18-0610
55. Devos G, Devlies W, De Meerleer G, Baldewijns M, Gevaert T, Moris L, et al. Neoadjuvant Hormonal Therapy Before Radical Prostatectomy in High-Risk Prostate Cancer. Nat Rev Urol (2021) 18:739–62. doi: 10.1038/s41585-021-00514-9
56. Deng Q, Tang DG. Androgen Receptor and Prostate Cancer Stem Cells: Biological Mechanisms and Clinical Implications. Endocr Relat Cancer (2015) 22:T209–20. doi: 10.1530/ERC-15-0217
57. Eigl BJ, Eggener SE, Baybik J, Ettinger S, Chi KN, Nelson C, et al. Timing is Everything: Preclinical Evidence Supporting Simultaneous Rather Than Sequential Chemohormonal Therapy for Prostate Cancer. Clin Cancer Res (2005) 11:4905–11. doi: 10.1158/1078-0432.CCR-04-2140
58. Kyriakopoulos CE, Chen YH, Carducci MA, Liu G, Jarrard DF, Hahn NM, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J Clin Oncol (2018) 36:1080–7. doi: 10.1200/JCO.2017.75.3657
59. Chi KN, Chin JL, Winquist E, Klotz L, Saad F, Gleave ME. Multicenter Phase II Study of Combined Neoadjuvant Docetaxel and Hormone Therapy Before Radical Prostatectomy for Patients With High Risk Localized Prostate Cancer. J Urol (2008) 180:565–70; discussion 70. doi: 10.1016/j.juro.2008.04.012
60. Koie T, Ohyama C, Yamamoto H, Hatakeyama S, Yoneyama T, Hashimoto Y, et al. Safety and Effectiveness of Neoadjuvant Luteinizing Hormone-Releasing Hormone Agonist Plus Low-Dose Estramustine Phosphate in High-Risk Prostate Cancer: A Prospective Single-Arm Study. Prostate Cancer Prostatic Dis (2012) 15:397–401. doi: 10.1038/pcan.2012.29
61. Zurita AJ, Pisters LL, Wang X, Troncoso P, Dieringer P, Ward JF, et al. Integrating Chemohormonal Therapy and Surgery in Known or Suspected Lymph Node Metastatic Prostate Cancer. Prostate Cancer Prostatic Dis (2015) 18:276–80. doi: 10.1038/pcan.2015.23
62. Thalgott M, Horn T, Heck MM, Maurer T, Eiber M, Retz M, et al. Long-Term Results of a Phase II Study With Neoadjuvant Docetaxel Chemotherapy and Complete Androgen Blockade in Locally Advanced and High-Risk Prostate Cancer. J Hematol Oncol (2014) 7:20. doi: 10.1186/1756-8722-7-20
63. Silberstein JL, Poon SA, Sjoberg DD, Maschino AC, Vickers AJ, Bernie A, et al. Long-Term Oncological Outcomes of a Phase II Trial of Neoadjuvant Chemohormonal Therapy Followed by Radical Prostatectomy for Patients With Clinically Localised, High-Risk Prostate Cancer. BJU Int (2015) 116:50–6. doi: 10.1111/bju.12676
64. Narita S, Tsuchiya N, Kumazawa T, Maita S, Numakura K, Obara T, et al. Short-Term Clinicopathological Outcome of Neoadjuvant Chemohormonal Therapy Comprising Complete Androgen Blockade, Followed by Treatment With Docetaxel and Estramustine Phosphate Before Radical Prostatectomy in Japanese Patients With High-Risk Localized Prostate Cancer. World J Surg Oncol (2012) 10:1. doi: 10.1186/1477-7819-10-1
65. Pan J, Chi C, Qian H, Zhu Y, Shao X, Sha J, et al. Neoadjuvant Chemohormonal Therapy Combined With Radical Prostatectomy and Extended PLND for Very High Risk Locally Advanced Prostate Cancer: A Retrospective Comparative Study. Urol Oncol (2019) 37:991–8. doi: 10.1016/j.urolonc.2019.07.009
66. Narita S, Nara T, Kanda S, Numakura K, Saito M, Inoue T, et al. Radical Prostatectomy With and Without Neoadjuvant Chemohormonal Pretreatment for High-Risk Localized Prostate Cancer: A Comparative Propensity Score Matched Analysis. Clin Genitourin Cancer (2019) 17:e113–e22. doi: 10.1016/j.clgc.2018.09.019
67. Lee CH, Shah AY, Rasco D, Rao A, Taylor MH, Di Simone C, et al. Lenvatinib Plus Pembrolizumab in Patients With Either Treatment-Naive or Previously Treated Metastatic Renal Cell Carcinoma (Study 111/KEYNOTE-146): A Phase 1b/2 Study. Lancet Oncol (2021) 22:946–58. doi: 10.1016/S1470-2045(21)00241-2
68. Uemura M, Tomita Y, Miyake H, Hatakeyama S, Kanayama HO, Numakura K, et al. Avelumab Plus Axitinib vs Sunitinib for Advanced Renal Cell Carcinoma: Japanese Subgroup Analysis From JAVELIN Renal 101. Cancer Sci (2020) 111:907–23. doi: 10.1111/cas.14294
69. Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel TM, Harrison MR, et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J Clin Oncol (2014) 33:1430–7. doi: 10.1200/JCO.2014.59.0703
70. Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, et al. Nivolumab Plus Ipilimumab Versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med (2018) 378:1277–90. doi: 10.1056/NEJMoa1712126
71. Massard C, Gordon MS, Sharma S, Rafii S, Wainberg ZA, Luke J, et al. Safety and Efficacy of Durvalumab (MEDI4736), an Anti-Programmed Cell Death Ligand-1 Immune Checkpoint Inhibitor, in Patients With Advanced Urothelial Bladder Cancer. J Clin Oncol (2016) 34:3119–25. doi: 10.1200/JCO.2016.67.9761
72. Balar AV, Kamat AM, Kulkarni GS, Uchio EM, Boormans JL, Roumiguié M, et al. Pembrolizumab Monotherapy for the Treatment of High-Risk non-Muscle-Invasive Bladder Cancer Unresponsive to BCG (KEYNOTE-057): An Open-Label, Single-Arm, Multicentre, Phase 2 Study. Lancet Oncol (2021) 22:919–30. doi: 10.1016/S1470-2045(21)00147-9
73. Chabanon RM, Pedrero M, Lefebvre C, Marabelle A, Soria JC, Postel-Vinay S. Mutational Landscape and Sensitivity to Immune Checkpoint Blockers. Clin Cancer Res (2016) 22:4309–21. doi: 10.1158/1078-0432.CCR-16-0903
74. Gulley JL, Drake CG. Immunotherapy for Prostate Cancer: Recent Advances, Lessons Learned, and Areas for Further Research. Clin Cancer Res (2011) 17:3884–91. doi: 10.1158/1078-0432.CCR-10-2656
75. Liu G, Lu S, Wang X, Page ST, Higano CS, Plymate SR, et al. Perturbation of NK Cell Peripheral Homeostasis Accelerates Prostate Carcinoma Metastasis. J Clin Invest (2013) 123:4410–22. doi: 10.1172/JCI69369
76. Healy CG, Simons JW, Carducci MA, DeWeese TL, Bartkowski M, Tong KP, et al. Impaired Expression and Function of Signal-Transducing Zeta Chains in Peripheral T Cells and Natural Killer Cells in Patients With Prostate Cancer. Cytometry (1998) 32:109–19. doi: 10.1002/(SICI)1097-0320(19980601)32:2<109::AID-CYTO6>3.0.CO;2-G
77. Antonarakis ES, Zahurak M, Schaeffer EM, Partin AW, Ross A, Allaf M, et al. Neoadjuvant Randomized Trial of Degarelix (Deg) ± Cyclophosphamide/GVAX (Cy/GVAX) in Men With High-Risk Prostate Cancer (PCa) Undergoing Radical Prostatectomy (RP). J Clin Oncol (2017) 35:5077–. doi: 10.1200/JCO.2017.35.15_suppl.5077
78. Ryan ST, Zhang J, Burner DN, Liss M, Pittman E, Muldong M, et al. Neoadjuvant Rituximab Modulates the Tumor Immune Environment in Patients With High Risk Prostate Cancer. J Transl Med (2020) 18:214. doi: 10.1186/s12967-020-02370-4
79. Hagihara K, Chan S, Zhang L, Oh DY, Wei XX, Simko J, et al. Neoadjuvant Sipuleucel-T Induces Both Th1 Activation and Immune Regulation in Localized Prostate Cancer. Oncoimmunology (2019) 8:e1486953. doi: 10.1080/2162402X.2018.1486953
80. Kim TJ, Koo KC. Current Status and Future Perspectives of Checkpoint Inhibitor Immunotherapy for Prostate Cancer: A Comprehensive Review. Int J Mol Sci (2020) 21:5484. doi: 10.3390/ijms21155484
81. Rini BI, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, et al. Atezolizumab Plus Bevacizumab Versus Sunitinib in Patients With Previously Untreated Metastatic Renal Cell Carcinoma (IMmotion151): A Multicentre, Open-Label, Phase 3, Randomised Controlled Trial. Lancet (2019) 393:2404–15. doi: 10.1016/S0140-6736(19)30723-8
82. Abida W, Cheng ML, Armenia J, Middha S, Autio KA, Vargas HA, et al. Analysis of the Prevalence of Microsatellite Instability in Prostate Cancer and Response to Immune Checkpoint Blockade. JAMA Oncol (2019) 5:471–8. doi: 10.1001/jamaoncol.2018.5801
83. Antonarakis ES, Shaukat F, Isaacsson Velho P, Kaur H, Shenderov E, Pardoll DM, et al. Clinical Features and Therapeutic Outcomes in Men With Advanced Prostate Cancer and DNA Mismatch Repair Gene Mutations. Eur Urol (2019) 75:378–82. doi: 10.1016/j.eururo.2018.10.009
84. Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med (2021) 385:1091–103. doi: 10.1056/NEJMoa2107322
Keywords: prostatectomy, neoadjuvant, hormonal deprivation, chemohormonal therapy, immunotherapy
Citation: Perera M, Beech BB, De Jesus Escano M, Gmelich C, Yip W, Boorjian SA and Eastham JA (2022) Neoadjuvant Systemic Therapy Prior to Radical Prostatectomy for Clinically Localized High-Risk Prostate Cancer. Front. Urol. 2:864646. doi: 10.3389/fruro.2022.864646
Received: 28 January 2022; Accepted: 16 February 2022;
Published: 10 March 2022.
Edited by:
Shawn Dason, The Ohio State University, United StatesReviewed by:
Cristina Magi-Galluzzi, University of Alabama at Birmingham, United StatesBrian Shinder, The State University of New Jersey, United States
Copyright © 2022 Perera, Beech, De Jesus Escano, Gmelich, Yip, Boorjian and Eastham. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marlon Perera, UGVyZXJhbTFAbXNrY2Mub3Jn
 Marlon Perera
Marlon Perera Benjamin B. Beech
Benjamin B. Beech Manuel De Jesus Escano
Manuel De Jesus Escano Caroline Gmelich1
Caroline Gmelich1 Stephen A. Boorjian
Stephen A. Boorjian James A. Eastham
James A. Eastham