
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Urol., 06 May 2022
Sec. Urologic Oncology
Volume 2 - 2022 | https://doi.org/10.3389/fruro.2022.863784
This article is part of the Research TopicInsights in Urologic Oncology, Volume IView all 5 articles
 Bernadett Szabados1
Bernadett Szabados1 Sarah Duncan2
Sarah Duncan2 Julia Choy1
Julia Choy1 Francesca Jackson-Spence1
Francesca Jackson-Spence1 Charlotte Toms2
Charlotte Toms2 Giorgia Trevisan2
Giorgia Trevisan2 Daniel M. Berney2
Daniel M. Berney2 Thomas Powles1
Thomas Powles1 Charlotte Ackerman1*
Charlotte Ackerman1*Background: Several preclinical and clinical studies demonstrated a strong correlation between androgen receptor (AR) signaling and bladder tumorigenesis. This study aims to evaluate the prognostic significance of AR expression in metastatic urothelial carcinoma (mUC).
Methods: Samples from a phase III trial (LaMB, NCT00949455) which compared maintenance lapatinib versus placebo after completion of first-line platinum-based chemotherapy in patients with HER 1/2-positive mUC of the bladder were collected. Corresponding baseline and follow-up data included patients enrolled in the study and those who screen failed. AR expression was assessed independently by a single pathologist who was blinded to the study. Samples were grouped according to AR expression (negative vs. positive) and correlated with baseline tumor characteristics and survival.
Results: Of the 446 screened samples in the LaMB study, 90 were retrospectively analyzed for AR expression. There were no correlations between AR expression and tumor stage (r = −0.10), tumor grade (r = 0.05) at diagnosis, or subsequent treatment with lapatinib (r = −0.04). The median progression-free survival was 6 months (95% CI, 3.20–6.80) in the AR-negative group and 5 months (95% CI, 3.41–6.59) in the AR-positive group [HR 0.54 (95% CI, 0.31–0.92), p = 0.02]. Similarly, patients with AR-negative disease had more favorable overall survival (OS) with 16 months (95% CI, 6.6–25.4) and 11 months (95% CI, 7.0–15.0) in the AR-positive group [HR 0.55 (95% CI, 0.31–0.98), p = 0.04]. In the multivariate analysis, AR expression was significantly associated with worse OS (p = 0.045).
Conclusions: AR expression is a predictor of poor outcome and presents a targetable alteration in patients with mUC.
Although the exact mechanism of urothelial carcinoma development remains unknown, increasing evidence suggests that the androgen receptor (AR) signaling pathway plays a potential role (1). While traditionally not thought to be an androgen-driven malignancy, urothelial carcinoma is associated with a strong male preponderance, and preclinical studies demonstrated an association between AR signaling and the development and progression of urothelial carcinoma (1–3). Furthermore, The Cancer Genome Atlas (TCGA) bladder cancer analysis showed that while somatic alterations in the AR gene are rare (4), a higher expression of genes involved in AR signaling was observed in luminal tumors (5). Together, these findings suggest that AR could be a rational therapeutic target in bladder cancer.
Here, we performed AR staining on prospectively collected tissue samples of patients with metastatic urothelial carcinoma (mUC) of the bladder treated with first-line platinum-containing chemotherapy and assessed AR expression and its prognostic significance.
Tissue samples from a phase III, randomized, double-blind trial (LaMB) which compared maintenance lapatinib versus placebo after completion of first-line platinum-based chemotherapy in patients with human epidermal growth factor receptor 1/2 (HER1/2)-positive mUC of the bladder (NCT00949455) were prospectively collected. Patients with metastatic upper tract urothelial carcinoma or other concomitant malignancies were excluded from the study. A total of 446 patients were screened for HER1/2 expression. Those who tested positive and obtained disease control or response after first-line chemotherapy (4–8 cycles) were randomly assigned to lapatinib or placebo (1:1). Tissue and the corresponding baseline and follow-up data were collected both from patients enrolled in the study and those who screen failed due to either disease progression or biomarker-negative disease.
The study did not meet its primary endpoint of progression-free survival (PFS) [HR 1.07 (95% CI, 0.81–1.43), p = 0.63)] or secondary endpoint of overall survival (OS) [HR 0.96 (95% CI, 0.70–1.31), p = 0.80]; thus, the addition of maintenance lapatinib did not contribute to outcomes compared with standard of care only. (6) Of the 446 screened tumor samples in the LaMB study, 90 were retrospectively analyzed for AR expression and correlated with treatment outcome. All patients gave informed consent for translational research as part of the study, which had appropriate ethical approval (07/H1102/73).
Archived, paraffin-embedded tissue blocks from patients with histologically confirmed urothelial carcinoma of the bladder were used. Androgen receptor immunohistochemistry (IHC) staining was performed on 4-µm-thick sections, using the clone AR441 (Dako, California, USA). Scoring was performed independently by a single pathologist who was blinded to the study. The intensity of staining was graded as IHC 0 to 3+. Any amount of staining was considered positive, and an average was used for the final score for each patient. Samples were stratified according to AR expression and divided into two groups: AR negative (IHC 0) and AR positive (IHC 1+, weakly positive; 2+, moderately positive; 3+, strongly positive) (7).
Data from patients with mUC who were screened in the LaMB study were used to determine baseline characteristics. Progression-free survival was defined as the time from the end of first-line chemotherapy until disease progression or death due to disease, following the primary endpoint of the study. Overall survival was defined as the time between the end of chemotherapy and death of any cause. Patients alive at the date of last disease assessment were censored. Progression-free survival and OS were compared using the log-rank test stratified by AR expression levels, and the corresponding two-sided 95% CIs were presented. Multivariate analysis was conducted to correlate clinical and pathological factors with PFS and OS. Correlations between AR expression and tumor or treatment characteristics were measured by Pearson product-moment correlation coefficient. p-values of ≥0.05 were considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 25.0.
In total, 90 samples from patients with mUC of the bladder were stained for AR expression (Figure 1). Of these, 79% (71/90) were men, with a median age of 71 years (IQR 66–78). Seventy-five percent (68/90) had an ECOG performance status of 0–1 at the time of diagnosis and 35% (32/90) had visceral metastatic disease (Table 1). Patients received standard-of-care first-line chemotherapy mainly with platinum-containing regimens. Among the 90 patients, 12% (11/90) had disease progression on first-line chemotherapy and screen failed in the LaMB study. A furthe0r 27% (24/90), though benefiting from chemotherapy, screen failed due to not meeting all eligibility criteria of the study. The remaining 61% (55/90) of patients achieved either response or stable disease on first-line chemotherapy and were enrolled in the LaMB study. Of these, 53% (29/55) of patients were randomized to the lapatinib maintenance therapy and the remaining 47% (26/55) of patients received placebo.
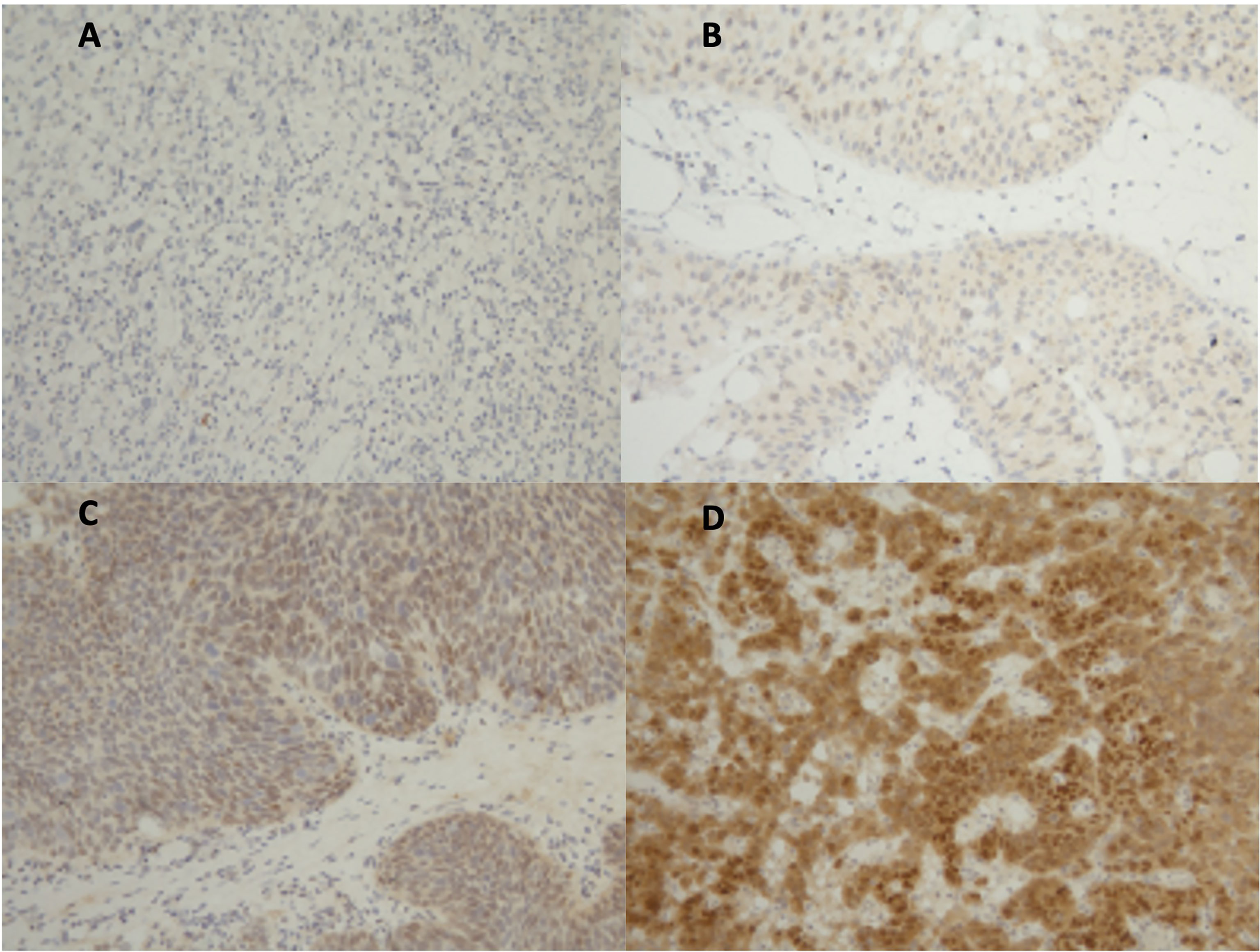
Figure 1 Androgen receptor immunohistochemistry (IHC) staining. (A) IHC negative; (B) 1+, weakly positive; (C) 2+, moderately positive; (D) 3+, strongly positive.
Among the 90 stained samples, 62% (56/90) were AR negative and 38% (34/90) demonstrated AR expression at various intensities. Positive AR expression rates in samples originating from male and female patients were found in 38% (27/71) and 37% (7/19) of cases, respectively. There were no correlations between AR expression and tumor stage (r = −0.10), tumor grade (r = 0.05) at diagnosis, or subsequent treatment with lapatinib (r = −0.04).
Next, we explored the relationship between AR expression and survival. The median PFS from the end of chemotherapy was 5 months (95% CI, 3.8–6.2) and the median OS was 13 months (95% CI, 8.1–17.8) for the whole cohort. The median PFS was 6 months (95% CI, 3.20–6.80) in the AR-negative group and 5 months (95% CI, 3.41–6.59) in the AR-positive group (Figure 2A). The hazard ratio for disease progression or death from disease was 0.54 (95% CI, 0.31–0.92; p = 0.02) (Figure 2A). Similarly, patients with AR-negative disease had a more favorable OS with 16 months (95% CI, 6.6–25.4) in the AR-negative group and 11 months (95% CI, 7.0–15.0) in the AR-positive group (Figure 2B). The hazard ratio for death was 0.55 (95% CI, 0.31–0.98; p = 0.04) (Figure 2B).
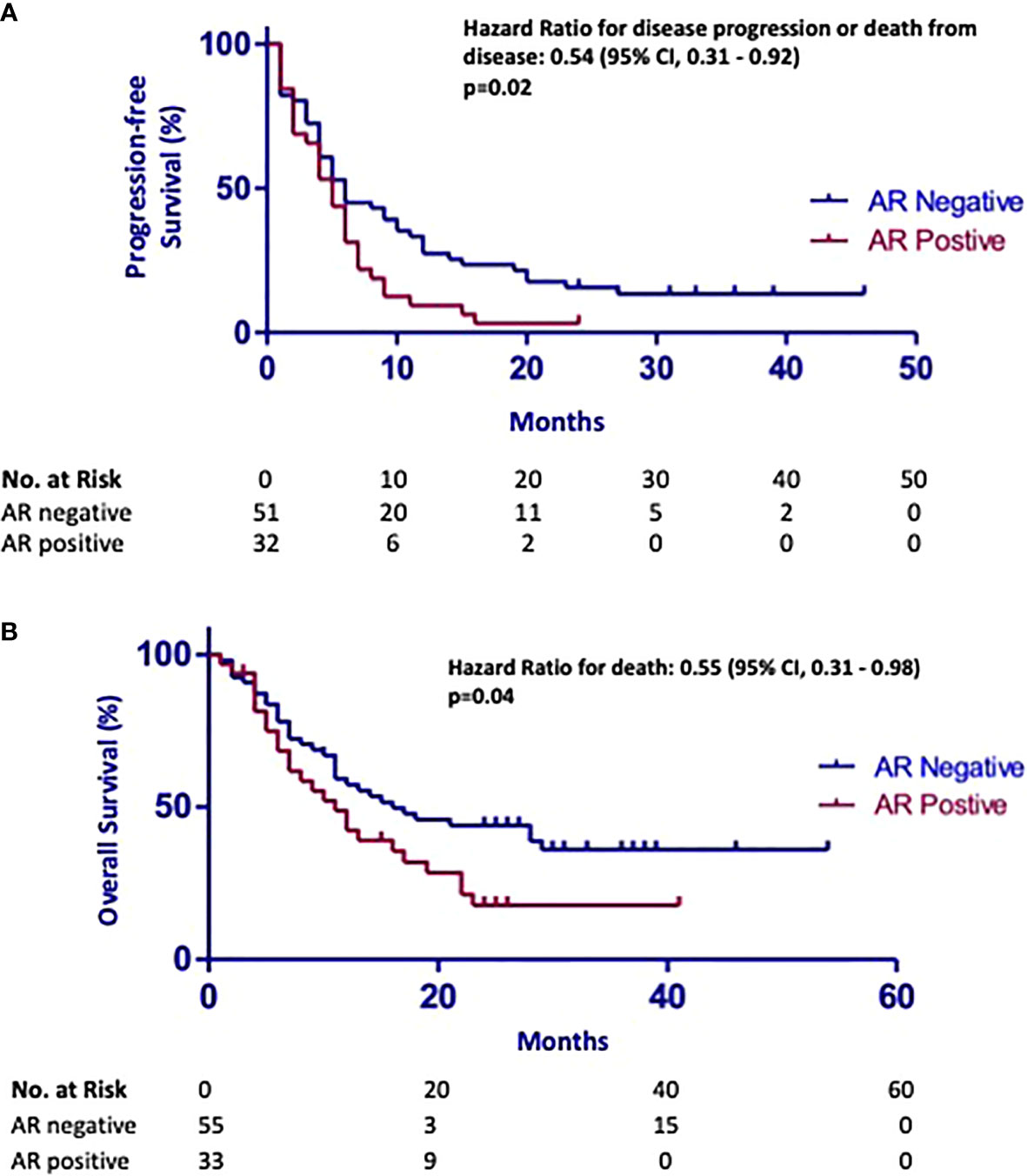
Figure 2 (A) Kaplan–Meier curves demonstrating that high expression of androgen receptor (AR) in metastatic urothelial carcinoma is associated with shorter progression-free survival [HR 0.54 (95% CI, 0.31–0.92), p = 0.02]. (B) Kaplan–Meier curves showing that high expression of androgen receptor (AR) in urothelial carcinoma samples is associated with poor overall survival [HR 0.55 (95% CI, 0.31–0.98), p = 0.04].
Subgroup analysis showed no associations between the intensity of staining (IHC 1+, 2+, 3+) and PFS (p = 0.13). However, samples with moderately (IHC 2+) and strongly positive (IHC 3+) AR expression (n = 19) were associated with worse OS [7 months (95% CI, 3.12–10.88), p = 0.014]. In the multivariate analysis, no factors were significantly associated with better PFS. Androgen receptor expression was significantly associated with worse OS (p = 0.045).
In recent years, there has been a significant progress in the treatment landscape of mUC with the advent of immune checkpoint inhibitors and antibody–drug conjugates. Compared with other solid tumors, (8) there is a lack of targeted therapy options. The only currently approved treatment is erdafitinib, a pan-fibroblast growth factor receptor (FGFR) tyrosine kinase inhibitor (9); however, only 20% of patients with advanced urothelial carcinoma have FGFR alterations (10). Furthermore, targeted therapy options are necessary to improve the prognosis of patients with mUC.
In this retrospective study, we showed that AR expression is a predictor of poor outcome in patients with mUC receiving first-line platinum-containing chemotherapy. Both PFS and OS were poorer in the AR-positive patient group [HR 0.54 (95% CI, 0.31–0.92), p = 0.02 and HR 0.55 (95% CI, 0.31–0.98), p = 0.04, respectively].
Several preclinical studies demonstrated a link between AR expression and the development of bladder cancer, especially in men (3, 11, 12). A recent meta-analysis of immunohistochemical studies including 2,049 patients from 13 retrospective studies showed a strong correlation between AR expression and gender (male vs. female: OR = 0.658; p = 0.027) or tumor grade (low grade vs. high grade: OR = 0.575; p < 0.001) (13). Our study showed no correlation between AR expression and baseline tumor stage or grade which is in line with previously published conflicting data (14).
Another study compared AR expression in normal urothelium and urothelial carcinoma tissue and demonstrated that AR-positive tumors had a poorer prognosis than those with no AR expression (15). We observed similar findings with AR-positive samples demonstrating significantly shorter PFS and OS. These results indicate that AR expression is a predictor of poor outcome in mUC. Further work is needed to explore these findings.
A rising number of therapeutic agents targeting AR are approved or under development, making these preliminary findings clinically relevant. A systematic review of preclinical and clinical studies evaluating the impact of AR modulation on outcomes of urothelial carcinoma demonstrated that AR modulators have the potential to interfere with the activity of multiple anticancer agents used for the treatment of urothelial carcinoma. Moreover, the relative risk of non-muscle-invasive bladder cancer recurrence is significantly lower in patients undergoing therapy with five alpha reductase inhibitors or androgen deprivation therapy [RR 0.50 (95% CI, 0.30–0.82), p = 0.006] (16).
The limitations of our study include the retrospective data analysis and the relatively small number of patients with AR-positive disease. Furthermore, data were analyzed from the completion of chemotherapy in line with the maintenance approach of the study. Maintenance targeted therapy is not standard of care, thus justifying this approach. Nevertheless, the effects from a more orthodox frontline perspective are unknown. A large, prospective randomized study may be justified to validate these findings and potentially repurpose AR-modulating agents in this context.
Androgen receptor expression is a predictor of poor outcome in mUC and presents a targetable alteration in bladder cancer. Further exploration is needed to confirm these findings.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The original study involving human participants were reviewed and approved by London – City and East (07/H1102/73). The patients/participants included here provided their written informed consent to participate in future research
CA: conceptualization, methodology, validation, writing—original draft, and visualization. BS: conceptualization and writing—original draft. SD, JC, FJ-S, and CT: data curation and visualization. GT and DB: formal analysis. TP: supervision. All authors read and approved the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank the patients of this study, their family members, and the nurses and medical specialists for their contribution to this study. The authors acknowledge the Centre for Experimental Cancer Medicine, Barts Cancer Institute for running the LaMB study and collecting the samples used in this project.
1. Miyamoto H, Yang Z, Chen YT, Ishiguro H, Uemura H, Kubota Y, et al. Promotion of Bladder Cancer Development and Progression by Androgen Receptor Signals. Jnci J Natl Cancer Inst (2007) 99:558–68. doi: 10.1093/jnci/djk113
2. Wu J-T, Han B-M, Yu S-Q, Wang H-P, Xia S-J. Androgen Receptor Is a Potential Therapeutic Target for Bladder Cancer. Urology (2010) 75:820–7. doi: 10.1016/j.urology.2009.10.041
3. Zheng Y, Izumi K, Yao JL, Miyamoto H. Dihydrotestosterone Upregulates the Expression of Epidermal Growth Factor Receptor and ERBB2 in Androgen Receptor-Positive Bladder Cancer Cells. Endocr-Relat Cancer (2011) 18:451–64. doi: 10.1530/ERC-11-0010
4. Research NCGA. Comprehensive Molecular Characterization of Urothelial Bladder Carcinoma. Nature (2014) 507:315–22. doi: 10.1038/nature12965
5. Trilla-Fuertes L, Gámez-Pozo A, Prado-Vázquez G, Zapater-Moros A, Díaz-Almirón M, Arevalillo JM, et al. Biological Molecular Layer Classification of Muscle-Invasive Bladder Cancer Opens New Treatment Opportunities. BMC Cancer (2019) 19:636. doi: 10.22037/uj.v11i06.2617
6. Powles T, Huddart RA, Elliot T, Sarker SJ, Ackerman C, Jones R, Phase III. Double-Blind, Randomized Trial That Compared Maintenance Lapatinib Versus Placebo After First-Line Chemotherapy in Patients With Human Epidermal Growth Factor Receptor 1/2–Positive Metastatic Bladder Cancer. J Clin Oncol (2017) 35:48–55. doi: 10.1200/JCO.2015.66.3468
7. Gårdmark T, Wester K, Torre MDL, Carlsson J, Malmström P. Analysis of HER2 Expression in Primary Urinary Bladder Carcinoma and Corresponding Metastases. Bju Int (2005) 95:982–6. doi: 10.1111/j.1464-410X.2005.05452.x
8. Yuan M, Huang L-L, Chen J-H, Wu J, Xu Q. The Emerging Treatment Landscape of Targeted Therapy in Non-Small-Cell Lung Cancer. Signal Transduct Target Ther (2019) 4:61. doi: 10.1038/s41392-019-0099-9
9. Loriot Y, Necchi A, Park SH, Garcia-Donas J, Huddart R, Burgess E, et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N Engl J Med (2019) 381:338–48. doi: 10.1056/NEJMoa1817323
10. Knowles MA, Hurst CD. Molecular Biology of Bladder Cancer: New Insights Into Pathogenesis and Clinical Diversity. Nat Publishing Group (2015) 15:25–41. doi: 10.1038/nrc3817
11. Bertram JS, Craig AW. Specific Induction of Bladder Cancer in Mice by Butyl-(4-Hydroxybutyl)-Nitrosamine and the Effects of Hormonal Modifications on the Sex Difference in Response. Eur J Cancer 1965 (1972) 8:587–94. doi: 10.1016/0014-2964(72)90137-5
12. Chang C, Lee SO, Yeh S, Chang TM. Androgen Receptor (AR) Differential Roles in Hormone-Related Tumors Including Prostate, Bladder, Kidney, Lung, Breast and Liver. Oncogene (2014) 33:3225–34. doi: 10.1038/onc.2013.274
13. Ide H, Inoue S, Miyamoto H. Histopathological and Prognostic Significance of the Expression of Sex Hormone Receptors in Bladder Cancer: A Meta-Analysis of Immunohistochemical Studies. PloS One (2017) 12:e0174746. doi: 10.1371/journal.pone.0174746
14. Ide H, Miyamoto H. Steroid Hormone Receptor Signals as Prognosticators for Urothelial Tumor. Dis Markers (2015) 2015:840640. doi: 10.1155/2015/840640
15. Mashhadi R, Pourmand G, Kosari F, Mehrsai A, Salem S, Pourmand MR, et al. Role of Steroid Hormone Receptors in Formation and Progression of Bladder Carcinoma: A Case-Control Study. Urol J (2014) 11:1968–73.
16. Creta M, Celentano G, Napolitano L, La Rocca R, Capece M, Califano G, et al. Inhibition of Androgen Signalling Improves the Outcomes of Therapies for Bladder Cancer: Results From a Systematic Review of Preclinical and Clinical Evidence and Meta-Analysis of Clinical Studies. Diagnostics (2021) 11:351. doi: 10.3390/diagnostics11020351
Keywords: androgen receptor, AR, urothelial carcinoma, bladder cancer, survival
Citation: Szabados B, Duncan S, Choy J, Jackson-Spence F, Toms C, Trevisan G, Berney DM, Powles T and Ackerman C (2022) Androgen Receptor Expression Is a Predictor of Poor Outcome in Urothelial Carcinoma. Front. Urol. 2:863784. doi: 10.3389/fruro.2022.863784
Received: 27 January 2022; Accepted: 14 March 2022;
Published: 06 May 2022.
Edited by:
Trushar Patel, University of South Florida, United StatesReviewed by:
Alvaro Pinto, University Hospital La Paz, SpainCopyright © 2022 Szabados, Duncan, Choy, Jackson-Spence, Toms, Trevisan, Berney, Powles and Ackerman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Charlotte Ackerman, Yy5hY2tlcm1hbkBxbXVsLmFjLnVr, orcid.org/0000-0001-8583-1695
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.