
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Urol., 28 February 2022
Sec. Urologic Oncology
Volume 2 - 2022 | https://doi.org/10.3389/fruro.2022.843597
This article is part of the Research TopicRising Stars in Urologic Oncology: 2023View all 5 articles
Prostate cancer is the second most frequent cancer type among men globally. Herein, the roles of exosomes in prostate cancer tumorigenesis, progress along with metastasis were reviewed. Exosomes are small extracellular vesicles originated from the endosomal system then released to surrounding body fluids. They carry cargo comprising nucleic acids and proteins and deliver then to recipient cells and are implicated in cell-to-cell communications. They regulate the activities of recipient cells by modulating several physiological and pathological processes, such as remodeling the properties of the tumor microenvironment, modulating cancer metabolism and metastases, and regulating drug resistance. Tumor derived exosomes are present in various body fluids and their molecular profile can reflect the real-time status of the cancer cell. These characteristics make them prospective biologic signatures for diagnosis along with prognosis of different cancer types. Besides, exosomes have good biophysical properties, for instance high stability in the lipid bilayer membrane, as well as low immunogenicity which are key parameters for development of novel drug delivery approach.
Prostate cancer (PCa) is the second most frequent tumor in males. PCa is high prevalent in developing countries. Most individuals with prostate cancer present with locally advanced or metastatic disease at diagnosis thus limiting effectiveness of conventional therapies. Prostate specific antigen (PSA) is a widely utilized biomarker for PCa screening, nonetheless, it does not provide precise and accurate diagnostic and prognostic information. Androgen-deprivation therapy (ADT) is the conventional treatment for individuals with mPCa (1–3). Most patients respond to ADT initially, whilst some cases progress eventually to incurable CRPC (castrate-resistance prostate cancer) despite sustained hormonal manipulation (4). Predictive biomarkers are therefore required to ensure early diagnosis and for detection of CRPC thus providing a basis for development of personalized treatment. Liquid biopsies including circulating tumor cells, nucleic acids in circulation, and exosomes have recently been explored as minimally infiltrative biologic signatures for diagnosis, as well as predictive markers for prognosis of individuals with PCa (5, 6).
Exosomes are small extracellular vesicles (EVs) enclosed by a lipid bilayer membrane and are secreted by most eukaryotic cells. The size of EVs range from 50 nm to 1000 nm in diameter, and the size of exosomes range from 30 to 120 nm in diameter (7). Exosomes are present in various biological fluids, for instance blood, urine, milk, semen as well as saliva, and can be purified from cell growth medium. Previous investigations document that exosomes have pivotal roles in diseases through inter-cellular communication and modulation of biological processes. The biological function of an exosome depends on the contents of the cargo, for instance miRNAs, viral particles, mRNAs, proteins, or lipids (8). Exosomes and their cargos play roles as mediators in short- or long-distance communication, as observed in tumor-derived EVs (TDEs). Tumor cells have been documented to be generating, releasing, and utilizing exosomes to enhance tumor growth. Therefore, have an indispensable role in tumorigenesis, proliferation, migration and drug resistance and are prospective signatures for diagnosis along with prognosis and can be used as drug-delivering systems (9, 10).
Exosomes originate from the multivesicular endosome (MVE). The following processes are mainly involved in exosome formation: budding, inward invagination, MVE formation and release. Early endosomes are generated via plasma membrane’s budding inwards. The early endosome membrane buds into the surrounding lumen with cytoplasmic contents through further invaginations leading to generation of numerous intraluminal vesicles (ILV). The late endosomal structures harboring numerous ILVs are termed as MVBS (multivesicular bodies). MVB contains multiple vesicles with each vesicle encapsulating a small portion of the cytosol and comprises numerous proteins, lipids, nucleic acids. Some MVBs are translocated to the trans-Golgi network for recycling in the endosomes and moved to lysosomes for cargo degradation. However, other MVBs fuse with plasma membrane resulting in exosomes’ release into extra-cellular space (11, 12). Exosome biogenesis and secretion is induced via ESCRT (endosomal sorting complex required for transport) and various ESCRT-associated molecules. Four distinct ESCRT (0, I-III) protein complexes and linked proteins (VPS4, Tsg101 and ALIX) have been identified and each protein is implicated in mediating a discrete step in MVB vesicle formation. ESCRT-I along with ESCRT-II triggered deformation of the membrane generating stable membrane neck (13, 14). The mobilization of Vps4 complex to ESCRT-III triggers neck scission of vesicles, and dissociation along with recycling of the ESCRT-III complex. Besides, investigations have proposed an ESCRT-based pathway in bio-generation of exosomes. This cascade is driven by lipids, for instance LBPA (lysobisphosphatidic acid), as well as ceramides. These lipids are transported into specialized endosomal sites, which invaginate and ultimately form vesicles from the local lipid composition. Moreover, proteins are implicated in exosome biogenesis and protein loading. RNA interference-mediated knockdown of RAB27, SLP4 and SLAC2B proteins negatively affects exosome release, indicating that these proteins play important roles in exosomes’ secretion (15).
Exosomes comprise a lipid bilayer membrane and encapsulated molecules (Figure 1). Components of the membrane include lipids and proteins. Raft-forming lipids, for instance cholesterol, sphingolipids and saturated phospholipids are highly enriched in exosomal membranes. These lipids play important role in exosome structure stability and prevent degradation of the molecules in the exosome (16). Proteins that form part of exosome membrane include tetraspanins, (for instance, CD9, CD63 and CD81 which are 4-transmembrane domain-proteins) MHC molecules (a class of proteins linked to antigen’s presentation), cell adherence molecule integrins, and other protein molecules such as PD-L1. Tetraspanin is a relatively specific exosome marker. The contents and surface molecules of the exosome vary under different physiological and pathological conditions. Presence of a specific molecule signature on the surface of exosomes enable them to target recipient cells with high specificity and regulate the biological activities of recipient cells. Encapsulated biomolecules in exosomes include proteins along with nucleic acids. Proteins transported in exosomes mainly include heat-shock proteins (for instance Hsp70, Hsp90), ESCRT-linked proteins (Alix, Tsg101) and cytoskeletal proteins (for instance actin and Tubulin). These proteins are implicated in biogenesis, sorting and secretion of exosomes, organization of membrane microdomains, and form part of the cytoskeleton system, and the endosomal system (17). Proteins in comprise ubiquitous proteins found in most cell types and cell-type specific proteins. Nucleic transported in exosomes include DNA, miRNAs, mRNAs, circRNAs (circular RNAs) and lncRNAs (long noncoding RNAs). Contents of exosomes are released to the cytoplasmic space of recipient cells, when exosomes interact and fuse with recipient cells. This process can be triggered by direct interaction of a receptor with its ligand, can be mediated by adhesion molecules such as integrins which induce fusion and endocytosis, or through phagocytosis of opsonized exosomes (18).
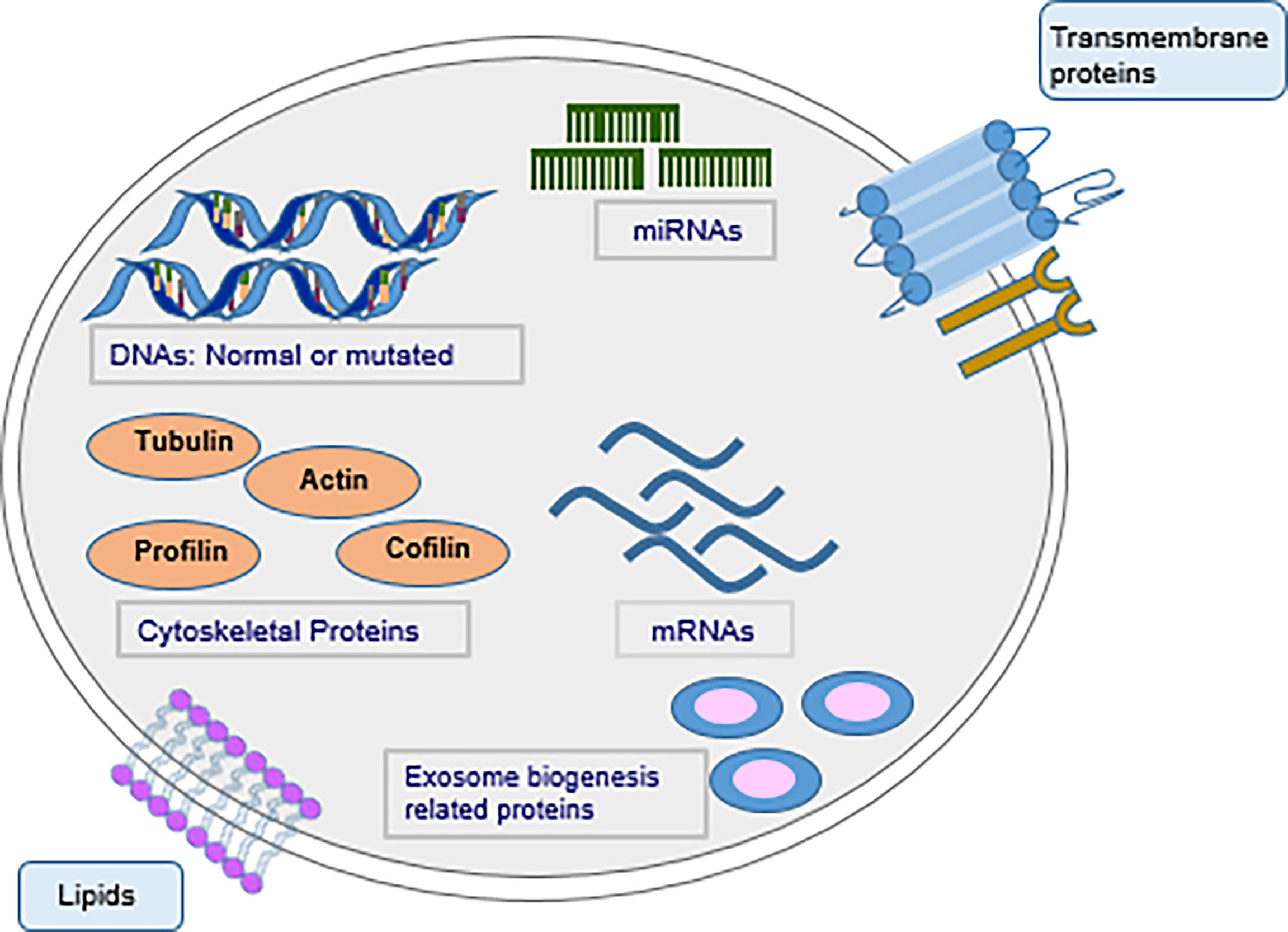
Figure 1 Schematic illustration of an exosome and its composition. Tumor-derived exosomes harbor numerous bioactive molecules, constituting nucleic acids, proteins along with lipids.
Tumor cells from various origins constitutively release exosomes, which play indispensable roles in malignant transformation and progress of tumors. Effects of exosomes are mediated through transfer of cargo that comprises various proteins, RNA (including miRNAs), and DNA. A previous study reports that a favorable prostatic tumoral niche is established through the CXCR1 chemokine receptor present in the exosome membrane derived from the cancer cell in prostate cancer. Tumor derived exosomes activate fibroblasts by upregulating expression of metalloprotein 9 (MMP-9). MMPs are implicated in degrading the extracellular matrix and promote cancer cell invasion. MMP-9 upregulation increases cell motility and promotes increased exosome secretion from activated fibroblasts which ultimately increases cancer cell migration (Figure 2). This cancer-to-fibroblast cell communication mediated by exosomes provides a favorable environment for cancer development (19). Besides, miR-139, miR-21 along with miR-100 trigger migrations of fibroblasts via upregulating expressions of MMP-2, RANKL, MMP-9, as well as MMP-13 (20).
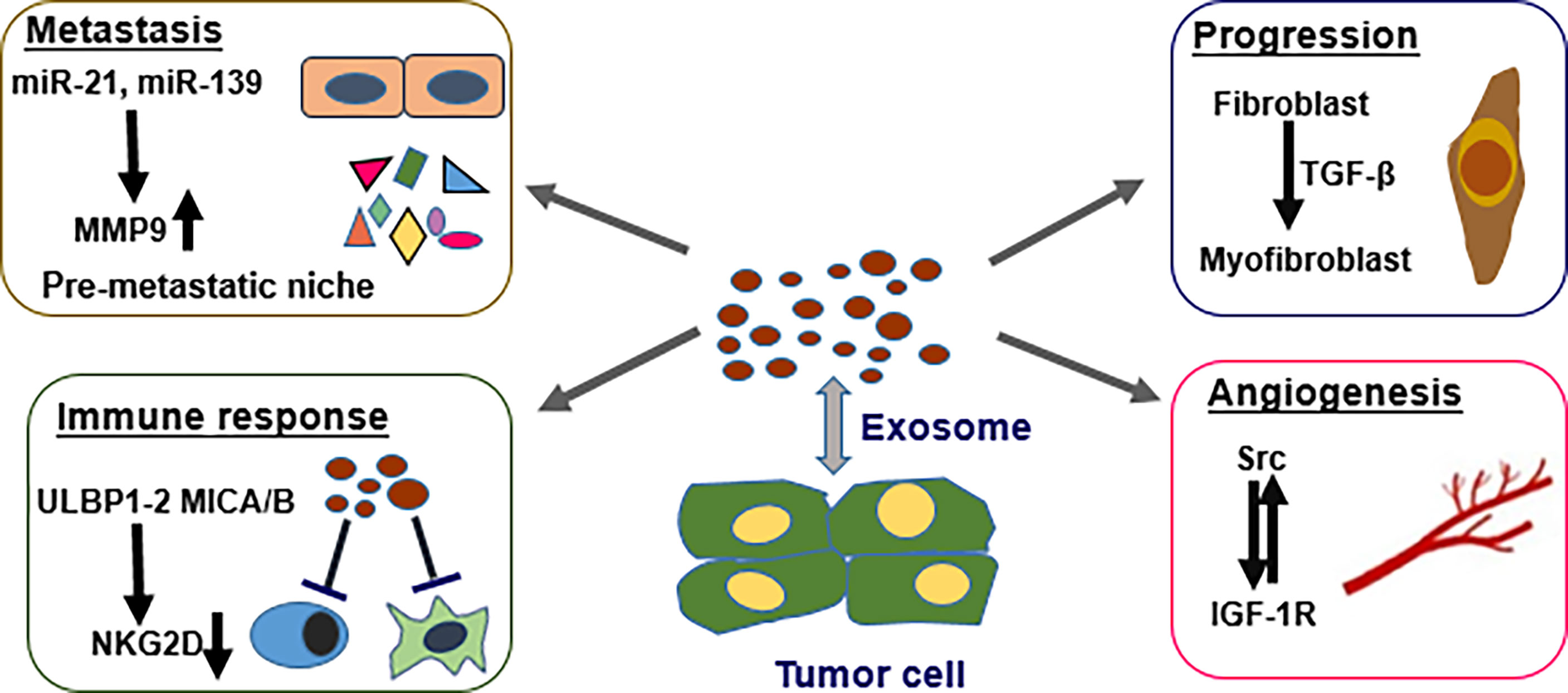
Figure 2 Multiple roles of cancer originated exosomes in PCa. Tumor exosomes can modulate cancer metastasis by forming the niche of pre-metastasis via miRNA regulation of MMP9 expression. TGF-β can induce differentiation of stromal fibroblasts to myofibroblast-like phenotype and promote tumor progression. Tumor exosomes downregulate NKG2D-triggered cytotoxic response in individuals with PC, resulting in immune repression and tumor escape. Cross-talk between tumor originated exosome proteins Src and IGF-1R promotes angiogenesis.
A hallmark of cancer development is transition of the normal stroma cells to reactive stroma cells promoting cancer cell growth along with metastasis. The influenced stromal cells then use exosomes to modulate the tumor microenvironment, as well as enhance tumor growth along with metastasis. A sustained interplay ensues between tumor cells and neighboring stromal cells (21). TGF-β bearing cancer exosomes have pivotal roles in generating tumor-promoting stroma by promoting differentiation of fibroblast to a myofibroblast-like phenotype that induces angiogenesis and tumor growth. This effect is achieved via activating the TGF-β/SMAD3 cascade (Figure 2). Exosomes generated by PCa cells highly express latent TGF-β which bind the exosome surface through proteoglycans thus promote activation of SMAD3 dependent signaling pathways (18).
Metastasis is reported in most advanced PCa patients and is linked to high incidence of death. Pre-metastatic niche (PMN) is a key determinant priming distal site for metastasis. Studies report that exosomes have an indispensable role in triggering tumor cell metastasis via establishing the PMN by acting as cellular communicators. Different populations of PCa cells (bulk and cancer stem cells, CSCs) generate exosomes that harbor miRNAs which modify the pre-metastatic niche. Two miRNA types derived from PCa exosomes including miRNA-21 along with miRNA-139, upregulate protein expressions of MMP-2, MMP-9 coupled with MMP -13, and RANKL in fibroblasts. The balance between RANKL and OPG level at the niche of pre-metastasis can modify tumor microenvironment and promote cell migration. In addition, miRNA-21 upregulates expression of MMPs in normal fibroblasts and triggers cellular alterations involved in activating fibroblast such as alterations in cell shape along with elavated cell migration (22).
Bone metastasis is the most common type of metastasis from of advanced prostate cancer (PCa). Pyruvate kinase M2 (PKM2) is transported through exosomes from PCa cells into BMSCs (bone marrow stromal cells). This feature is a novel mechanism via which primary tumor-originated exosomes enhance pre-metastatic niche formation (8). PCa-derived exosomes upregulate PKM2 expression, which ultimately upregulates CXCL12 expression (C-X-C motif chemokine ligand-12) in BMSCs thus inducing a pre-metastatic niche. Targeting the exosome-triggered CXCL12 axis abrogates exosome-stimulated bone metastasis indicating the therapeutic potential of targeting exosome derived PKM2.
Tumor microenvironment has an indispensable role in cancer development mainly resulting in formation of vascular tumors (13). Angiogenesis constitutes a multistep process for generation of new blood vessels from the already-existing vasculature and is the main cause of tumor metastasis, as well as malignancy (23, 24). Prostate cancer cells generate EVs for transporting sphingomyelin along with CD147 into endothelial cells, and enhance migration coupled with proangiogenic activity of endothelial cells (25, 26). Proteins such as c-Src tyrosine kinase, IGF-R (insulin-like growth factor 1 receptor) as well as FAK (focal adhesion kinase) play pivotal roles in prostate tumor growth along with progression (27). Prostate cancer exosomes have high levels of these proteins. Cross-talk between Src and IGF-1R promotes angiogenesis (28). Src coupled with c-Src are highly expressed in plasma exosomes of prostate tumor-bearing mice, indicating that Src-rich exosomes can enhance angiogenesis in vivo (Figure 2). In addition, Src and IGF1-R modulate angiogenesis by inducing VEGF and VEGF-C, respectively (29, 30). Exosomes derived from menstrual stem cells block prostate tumor-induced angiogenesis through inhibition of reactive oxygen species. Notably, exosomes with anti-angiogenic effect are only derived from menstrual cells (31). These findings indicate that prostate cancer exosomes can stimulate or block angiogenic activity within the tumor microenvironment.
Immune cells present in tumor stroma have a key role in cancer occurrence and development (32). Prostate cancer-originated exosomes enhance evasion of immune responses by cancer cells (33). NKG2D constitutes an activating cell surface receptor that is mainly expressed on cytotoxic immune cells such as NK cells, sub-sets of T cells, NKT cells, as well as CD8+ T cells (34, 35). NKG2D ligands including ULBP1-2 and MICA/B are highly expressed at cancer-originated exosomes’ surfaces. These ligands trigger downregulation of receptors of NKG2D and ultimately impair the cytotoxic role of immune cells. (Figure 2) Individuals with CRPC (Castration-resistant) show a remarkable reduction in expression of surface NKG2D on the circulating NK cells, as well as CD8+ T cells in contrast with the levels in healthy individuals. Cancer-originated exosomes purified from plasma or serum of individuals with CRPC enhance downregulation of the expression of NKG2D in cultured lymphocytes. These in vivo and in vitro findings indicate that prostate tumor-originated exosomes dampen NKG2D-triggered cytotoxic response in individuals with PCa, hence enhancing immune repression and tumor escape.
Moreover, exosome-mediated immune surveillance escape is mediated through impairment of the cytotoxic role of lymphocytes coupled with induction of apoptosis of CD8+ T cells (36, 37). Exosomes stimulate the T cell receptor and upregulate Fas expression on T cells. FasL then triggers apoptosis directly through the CD 95/APO1 receptor or indirectly by modulation of dendritic cells (38). Fas-triggered apoptosis results in immune evasion and can promote tumorigenesis. Furthermore, exosomes transport programmed death ligand 1 (PDL-1). PDL-1 docks to its receptor, PD-1, which is expressed on activated T along with B cells’ surfaces or macrophages and promotes T cell apoptosis (39). Cancer-originated exosomes can affect the role of T cells along with that of NK cells via dampening activation, as well as proliferation or stimulation of apoptosis, and can further dampen antigen-presenting cells along with antitumor immune response (36).
Markers can be determine in liquid biopsies (such as biofluids) thus avoiding use of invasive approaches and can reflect heterogeneity of the tumor effectively compared with prostate needle biopsies (40). Studies are currently exploring biomarkers for PCa that can supplement PSA and improve efficacy of diagnosis. Biologic signatures for early diagnosis, as well as risk stratification should be explored for timely diagnosis and aid in designing more effective therapies. Exosomes generated by cancer cells into biological fluids harbor biomolecules that exhibit the disease condition, and are currently referred as a new kind of liquid biopsies (6, 41).
These molecules include DNA, RNA (such as miRNAs) and proteins. NGS assessment of small amount of urine exosomal RNA showed that miRNA-196a-5p along with miRNA-501-3p harbor diagnostic ability for PCa (42). The elevated content of plasma exosomal miRNA-1290 along with miRNA-375 in CRPC is linked to poor patient prognosis (43). A previous investigation explored tyrosine-protein kinase Met (Met)/miRNA-130b cascade expression in serum and reported that the protein/mRNA expression level is correlated with the risk that individuals with PCa become resistant to castration treatment and present with high metastasis rate. miRNA-1246 constitute an exosomal signature for aggressive PCa whereas miRNA-26a regulates metastasis along with tumor growth of PCa (44, 45). Exosomal survivin protein is a possible signature that can be used for early detection of PCa (46). Besides, PCa antigen 3 (PCA3), flotillin 2, Rab3B as well as LAMTOR1 (late endosomal/lysosomal adaptor, MAPK and mTOR activator-1) present in exosomes are potential diagnostic signatures for PCa (47, 48).
Exosomes have a high potential for targeted drug delivery owing to their excellent biophysical features, for instance high stability, high biocompatibility, high permeability, low toxicity along with low immunogenicity (49). Previous investigations report that stem cell-originated EVs are key mediators of tissue repair, as well as regeneration in diverse animal disease models (50). The endogenous origin and biological properties of exosomes confer them with significant benefits compared with conventional drug delivery approaches, for instance liposome and synthetic nano-particles. Therefore, studies are exploring utility of EVs as drug delivery approaches for chemical drugs, genetic materials along with proteins. EVs contents can be effectively modified through chemical, biological or physical methods. Exosomal miRNA-145 derived from adipose-derived stromal cells (ASCs) reduces the activity of Bcl-xL and promotes apoptosis of prostate cancer cells through caspase-3/7 cascade (37). This implies that RNAs can be encapsulated into exosomes and delivered to target tissue for therapy. Mesenchymal stromal cells (MSCs) can package, then deliver active drugs via their membrane microvesicles (MVs) (51). MSCs can be primed with Paclitaxel (PTX) owing to the properties of exosomes thus transporting and releasing the drug, resulting in effective anti-tumor activities. Recent studies report that loading of Paclitaxel into autologous PCa cell-originated exosomes via an endocytic cascade increases the cytotoxic effect (52). Notably, the effect was independent of the population of EVs.
Exosomes are nano-sized vesicles secreted by mammalian cells into body fluids. Exosomes play several roles in prostate cancer biology, such as promotion of cancer progression and metastasis by inducing the pre-metastatic niche. Exosomes comprise cancer cell-specific protein and nucleic acid contents with immune-regulatory potential and genetic information. Encapsulated molecules of exosomes are potential markers for prostate cancer diagnosis. In addition, these molecules can be used as ana effective approach to explore the response of cancer cells to therapeutic intervention(s) when monitoring cancer progression and treatment. The unique characteristics of exosomes such as high stability and high biocompatibility imply that they are potential effective drug delivery systems. However, further studies on translation of EVs into clinical therapies should be conducted to design standards for exosome classification and manipulation. In summary, exosomes are prospective tools for development of diagnosis, as well as therapy of PCa, however, further studies should explored clinical application of exosomes.
GH and LX: original draft. XC and PG: review and editing. BX: supervision and funding acquisition. All authors contributed to the article and approved the submitted version.
This study was supported by grants for YZ from the National Natural Science Foundation of China (no. 81802524).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sasaki T, Sugimura Y. The Importance of Time to Prostate-Specific Antigen (PSA) Nadir After Primary Androgen Deprivation Therapy in Hormone-Naïve Prostate Cancer Patients. J Clin Med (2018) 7(12):565. doi: 10.3390/jcm7120565
2. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2019. CA Cancer J Clin (2019) 69(1):7–34. doi: 10.3322/caac.21551
3. Wang N, Ye Y, Deng M, Zhao D, Jiang L, Chen D, et al. Prostate Cryoablation Combined With Androgen Deprivation Therapy for Newly Diagnosed Metastatic Prostate Cancer: A Propensity Score-Based Study. Prostate Cancer Prostatic Dis (2021) 24(3):837–44. doi: 10.1038/s41391-021-00335-2
4. Kim SJ, Kim SI. Current Treatment Strategies for Castration-Resistant Prostate Cancer. Korean J Urol (2011) 52(3):157–65. doi: 10.4111/kju.2011.52.3.157
5. Campos-Fernández E, Barcelos LS, de Souza AG, Goulart LR, Alonso-Goulart V. Research Landscape of Liquid Biopsies in Prostate Cancer. Am J Cancer Res (2019) 9(7):1309–28.
6. Pang B, Zhu Y, Ni J, Thompson J, Malouf D, Bucci J, et al. Extracellular Vesicles: The Next Generation of Biomarkers for Liquid Biopsy-Based Prostate Cancer Diagnosis. Theranostics (2020) 10(5):2309–26. doi: 10.7150/thno.39486
7. Gao Y, Raj JU. Extracellular Vesicles as Unique Signaling Messengers: Role in Lung Diseases. Compr Physiol (2020) 11(1):1351–69. doi: 10.1002/cphy.c200006
8. Dai J, Escara-Wilke J, Keller JM, Jung Y, Taichman RS, Pienta KJ, et al. Primary Prostate Cancer Educates Bone Stroma Through Exosomal Pyruvate Kinase M2 to Promote Bone Metastasis. J Exp Med (2019) 216(12):2883–99. doi: 10.1084/jem.20190158
9. Kok VC, Yu CC. Cancer-Derived Exosomes: Their Role in Cancer Biology and Biomarker Development. Int J Nanomedicine (2020) 15:8019–36. doi: 10.2147/IJN.S272378
10. Patil SM, Sawant SS, Kunda NK. Exosomes as Drug Delivery Systems: A Brief Overview and Progress Update. Eur J Pharm Biopharm (2020) 154:259–69. doi: 10.1016/j.ejpb.2020.07.026
11. Hassanpour M, Rezabakhsh A, Rezaie J, Nouri M, Rahbarghazi RJC. Exosomal Cargos Modulate Autophagy in Recipient Cells via Different Signaling Pathways. Cell Biosc (2020) 10:92. doi: 10.1186/s13578-020-00455-7
12. Rezaie J, Aslan C, Ahmadi M, Zolbanin N, Kashanchi F, Jafari RJC, et al. The Versatile Role of Exosomes in Human Retroviral Infections: From Immunopathogenesis to Clinical Application. Cell Biosc (2021) 11(1):19. doi: 10.1186/s13578-021-00537-0
13. Babst M, Katzmann DJ, Estepa-Sabal EJ, Meerloo T, Emr SD. Escrt-III: An Endosome-Associated Heterooligomeric Protein Complex Required for Mvb Sorting. Dev Cell (2002) 3(2):271–82. doi: 10.1016/S1534-5807(02)00220-4
14. Wollert T, Hurley JH. Molecular Mechanism of Multivesicular Body Biogenesis by ESCRT Complexes. Nature (2010) 464(7290):864–9. doi: 10.1038/nature08849
15. Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, et al. Rab27a and Rab27b Control Different Steps of the Exosome Secretion Pathway. Nat Cell Biol (2010) 12(1):19–30; sup pp 11-13. doi: 10.1038/ncb2000
16. Ahmadi M, Rezaie J. Ageing and Mesenchymal Stem Cells Derived Exosomes: Molecular Insight and Challenges. Cell Biochem Funct (2021) 39(1):60–6. doi: 10.1002/cbf.3602
17. Mashouri L, Yousefi H, Aref AR, Ahadi AM, Molaei F, Alahari SK. Exosomes: Composition, Biogenesis, and Mechanisms in Cancer Metastasis and Drug Resistance. Mol Cancer (2019) 18(1):75. doi: 10.1186/s12943-019-0991-5
18. Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, et al. Reassessment of Exosome Composition. Cell (2019) 177(2):428–45.e418. doi: 10.1016/j.cell.2019.02.029
19. Osaki M, Okada F. Exosomes and Their Role in Cancer Progression. Yonago Acta Med (2019) 62(2):182–90. doi: 10.33160/yam.2019.06.002
20. Shimoda M, Khokha R. Metalloproteinases in Extracellular Vesicles. Biochim Biophys Acta Mol Cell Res (2017) 1864(11 Pt A):1989–2000. doi: 10.1016/j.bbamcr.2017.05.027
21. Guo S, Deng CX. Effect of Stromal Cells in Tumor Microenvironment on Metastasis Initiation. Int J Biol Sci (2018) 14(14):2083–93. doi: 10.7150/ijbs.25720
22. Sánchez CA, Andahur EI, Valenzuela R, Castellón EA, Fullá JA, Ramos CG, et al. Exosomes From Bulk and Stem Cells From Human Prostate Cancer Have a Differential microRNA Content That Contributes Cooperatively Over Local and Pre-Metastatic Niche. Oncotarget (2016) 7(4):3993–4008. doi: 10.18632/oncotarget.6540
23. Babaei M, Rezaie J. Application of Stem Cell-Derived Exosomes in Ischemic Diseases: Opportunity and Limitations. J Transl Med (2021) 19(1):196. doi: 10.1186/s12967-021-02863-w
24. Jabbari N, Nawaz M, Rezaie J. Bystander Effects of Ionizing Radiation: Conditioned Media From X-Ray Irradiated MCF-7 Cells Increases the Angiogenic Ability of Endothelial Cells. Cell Commun Signal (2019) 17(1):165. doi: 10.1186/s12964-019-0474-8
25. Kim CW, Lee HM, Lee TH, Kang C, Kleinman HK, Gho YS. Extracellular Membrane Vesicles From Tumor Cells Promote Angiogenesis via Sphingomyelin. Cancer Res (2002) 62(21):6312–7.
26. Millimaggi D, Mari M, D'Ascenzo S, Carosa E, Jannini EA, Zucker S, et al. Tumor Vesicle-Associated CD147 Modulates the Angiogenic Capability of Endothelial Cells. Neoplasia (2007) 9(4):349–57. doi: 10.1593/neo.07133
27. Chang YM, Kung HJ, Evans CP. Nonreceptor Tyrosine Kinases in Prostate Cancer. Neoplasia (2007) 9(2):90–100. doi: 10.1593/neo.06694
28. Min HY, Yun HJ, Lee JS, Lee HJ, Cho J, Jang HJ, et al. Targeting the Insulin-Like Growth Factor Receptor and Src Signaling Network for the Treatment of Non-Small Cell Lung Cancer. Mol Cancer (2015) 14:113. doi: 10.1186/s12943-015-0392-3
29. Lopez T, Hanahan D. Elevated Levels of IGF-1 Receptor Convey Invasive and Metastatic Capability in a Mouse Model of Pancreatic Islet Tumorigenesis. Cancer Cell (2002) 1(4):339–53. doi: 10.1016/S1535-6108(02)00055-7
30. Marx M, Warren SL, Madri JA. Pp60(C-Src) Modulates Microvascular Endothelial Phenotype and In Vitro Angiogenesis. Exp Mol Pathol (2001) 70(3):201–13. doi: 10.1006/exmp.2001.2358
31. Alcayaga-Miranda F, González PL, Lopez-Verrilli A, Varas-Godoy M, Aguila-Díaz C, Contreras L, et al. Prostate Tumor-Induced Angiogenesis Is Blocked by Exosomes Derived From Menstrual Stem Cells Through the Inhibition of Reactive Oxygen Species. Oncotarget (2016) 7(28):44462–77. doi: 10.18632/oncotarget.9852
32. Galli F, Aguilera JV, Palermo B, Markovic SN, Nisticò P, Signore A. Relevance of Immune Cell and Tumor Microenvironment Imaging in the New Era of Immunotherapy. J Exp Clin Cancer Res (2020) 39(1):89. doi: 10.1186/s13046-020-01586-y
33. Lundholm M, Schröder M, Nagaeva O, Baranov V, Widmark A, Mincheva-Nilsson L, et al. Prostate Tumor-Derived Exosomes Down-Regulate NKG2D Expression on Natural Killer Cells and CD8+ T Cells: Mechanism of Immune Evasion. PloS One (2014) 9(9):e108925. doi: 10.1371/journal.pone.0108925
34. Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH. The Role of the NKG2D Immunoreceptor in Immune Cell Activation and Natural Killing. Immunity (2002) 17(1):19–29. doi: 10.1016/S1074-7613(02)00333-3
35. Wu J, Song Y, Bakker AB, Bauer S, Spies T, Lanier LL, et al. An Activating Immunoreceptor Complex Formed by NKG2D and DAP10. Science (1999) 285(5428):730–2. doi: 10.1126/science.285.5428.730
36. Guo Y, Ji X, Liu J, Fan D, Zhou Q, Chen C, et al. Effects of Exosomes on Pre-Metastatic Niche Formation in Tumors. Mol Cancer (2019) 18(1):39. doi: 10.1186/s12943-019-0995-1
37. Lorenc T, Klimczyk K, Michalczewska I, Słomka M, Kubiak-Tomaszewska G, Olejarz W. Exosomes in Prostate Cancer Diagnosis, Prognosis and Therapy. Int J Mol Sci (2020) 21(6):2118. doi: 10.3390/ijms21062118
38. Ichim TE, Zhong Z, Kaushal S, Zheng X, Ren X, Hao X, et al. Exosomes as a Tumor Immune Escape Mechanism: Possible Therapeutic Implications. J Transl Med (2008) 6:37. doi: 10.1186/1479-5876-6-37
39. Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, et al. Exosomal PD-L1 Contributes to Immunosuppression and Is Associated With Anti-PD-1 Response. Nature (2018) 560(7718):382–6. doi: 10.1038/s41586-018-0392-8
40. Neuhaus J, Yang B. Liquid Biopsy Potential Biomarkers in Prostate Cancer. Diagnostics (Basel) (2018) 8(4):68. doi: 10.3390/diagnostics8040068
41. Logozzi M, Mizzoni D, Di Raimo R, Fais S. Exosomes: A Source for New and Old Biomarkers in Cancer. Cancers (Basel) (2020) 12(9):2566. doi: 10.3390/cancers12092566
42. Rodríguez M, Bajo-Santos C, Hessvik NP, Lorenz S, Fromm B, Berge V, et al. Identification of Non-Invasive miRNAs Biomarkers for Prostate Cancer by Deep Sequencing Analysis of Urinary Exosomes. Mol Cancer (2017) 16(1):156. doi: 10.1186/s12943-017-0726-4
43. Huang X, Yuan T, Liang M, Du M, Xia S, Dittmar R, et al. Exosomal miR-1290 and miR-375 as Prognostic Markers in Castration-Resistant Prostate Cancer. Eur Urol (2015) 67(1):33–41. doi: 10.1016/j.eururo.2014.07.035
44. Bhagirath D, Yang TL, Bucay N, Sekhon K, Majid S, Shahryari V, et al. microRNA-1246 Is an Exosomal Biomarker for Aggressive Prostate Cancer. Cancer Res (2018) 78(7):1833–44. doi: 10.1158/0008-5472.CAN-17-2069
45. Wang X, Wang X, Zhu Z, Li W, Yu G, Jia Z, et al. Prostate Carcinoma Cell-Derived Exosomal MicroRNA-26a Modulates the Metastasis and Tumor Growth of Prostate Carcinoma. BioMed Pharmacother (2019) 117:109109. doi: 10.1016/j.biopha.2019.109109
46. Khan S, Jutzy JM, Valenzuela MM, Turay D, Aspe JR, Ashok A, et al. Plasma-Derived Exosomal Survivin, a Plausible Biomarker for Early Detection of Prostate Cancer. PloS One (2012) 7(10):e46737. doi: 10.1371/journal.pone.0046737
47. Nilsson J, Skog J, Nordstrand A, Baranov V, Mincheva-Nilsson L, Breakefield XO, et al. Prostate Cancer-Derived Urine Exosomes: A Novel Approach to Biomarkers for Prostate Cancer. Br J Cancer (2009) 100(10):1603–7. doi: 10.1038/sj.bjc.6605058
48. Wang L, Skotland T, Berge V, Sandvig K, Llorente A. Exosomal Proteins as Prostate Cancer Biomarkers in Urine: From Mass Spectrometry Discovery to Immunoassay-Based Validation. Eur J Pharm Sci (2017) 98:80–5. doi: 10.1016/j.ejps.2016.09.023
49. Kim SM, Kim HS. Engineering of Extracellular Vesicles as Drug Delivery Vehicles. Stem Cell Investig (2017) 4:74. doi: 10.21037/sci.2017.08.07
50. Zhang B, Tian X, Hao J, Xu G, Zhang W. Mesenchymal Stem Cell-Derived Extracellular Vesicles in Tissue Regeneration. Cell Transplant (2020) 29:963689720908500. doi: 10.1177/0963689720908500
51. Pascucci L, Coccè V, Bonomi A, Ami D, Ceccarelli P, Ciusani E, et al. Paclitaxel Is Incorporated by Mesenchymal Stromal Cells and Released in Exosomes That Inhibit In Vitro Tumor Growth: A New Approach for Drug Delivery. J Control Release (2014) 192:262–70. doi: 10.1016/j.jconrel.2014.07.042
Keywords: exosomes, extracellular vesicles, prostate cancer, biomarkers, clinical application
Citation: Hu G, Xie L, Zhou Y, Cai X, Gao P and Xue B (2022) Roles and Clinical Application of Exosomes in Prostate Cancer. Front. Urol. 2:843597. doi: 10.3389/fruro.2022.843597
Received: 26 December 2021; Accepted: 31 January 2022;
Published: 28 February 2022.
Edited by:
James Eastham, Memorial Sloan Kettering Cancer Center, United StatesReviewed by:
Jafar Rezaie, Urmia University of Medical Sciences, IranCopyright © 2022 Hu, Xie, Zhou, Cai, Gao and Xue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Boxin Xue, eGJ4dXJvbEAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.