- 1Moriah Hospital, Oncology Department, São Paulo, Brazil
- 2First Clinic, Oncology Department, São Paulo, Brazil
- 3COI Institute, Clinical Research Department, Americas Oncology Cancer Center, Rio de Janeiro, Brazil
- 4Department of Biochemistry, Institute of Chemistry, University of São Paulo, São Paulo, Brazil
Previous studies suggested that obesity pro-inflammatory state could improve immune checkpoint inhibitors (ICI) clinical efficacy. This is a retrospective, multicenter, and observational study that included patients treated in a private Brazilian Oncology Group. Primary outcomes were the association of body mass index (BMI) category with overall survival (OS) and progression free survival (PFS). Secondary outcomes were association between BMI and objective response rate (ORR). In the total cohort, 448 patients were classified as a normal weight (43%), overweight (36%), obese (17%) and underweight (4%). The patients were predominantly male gender (62%), with stage IV lung cancer (57%) and melanoma (19%). The obese group (BMI ≥ 30 kg/m2) had a not statistically significant longer median OS than the non-obese group (BMI < 30 kg/m2) - 21.8 months (95% CI NR - NR) versus 14.9 months (95% CI 8.3 - 21.5); HR = 0.82, (95% CI 0.57 - 1.18, P = 0.28). Obese patients treated with anti-CTLA4 did not reach the mOS, while the non-obese group had a mOS of 23.1 months (P = 0.04). PFS did not differ between subgroups. Obese patients had also lower ORR, but without reaching statistical significance. In conclusion, this study did not report an improved OS among high BMI patients treated with ICI.
Introduction
There is substantial and prospective evidence to support the link between obesity and an increased risk of cancer development (1). Excess body fat seems to be associated with alterations in hormonal, metabolic, and inflammatory pathways that may lead to activation of the carcinogenesis process (2). In this process, the cancer cells also exhibit the hallmark of evasion of the immune system through immune checkpoint activation, which normally mediates immune self-tolerance. Therefore, the latest breakthrough in oncology was the development of monoclonal antibodies against immune checkpoints such as PD-1 (programmed death protein 1), PD-L1 (programmed death ligand 1), and CTLA-4 (cytotoxic T-lymphocyte antigen-4). These immune checkpoint inhibitors (ICIs) can shift the tumor microenvironment from immunosuppressed to inflammatory and unleash the antitumor immune response (3–5).
Recently published data have suggested that a high body mass index (BMI) may be associated with an improved prognosis in advanced, metastatic, or unresectable cancer settings (6, 7), however, there is no definitive evidence on whether this association is causal or correlative. As obesity is associated with a self-sustaining pro-inflammatory state, some studies have hypothesized that this pattern could be associated with improved clinical efficacy of immune checkpoint inhibitors. A large retrospective analysis of six independent cohorts of 2046 patients with metastatic melanoma who were treated with targeted therapy, immunotherapy or chemotherapy in randomized controlled trials (RCT) or retrospective cohorts. In this study, McQuade et al. demonstrated that obesity was associated with better progression-free survival and overall survival compared with normal BMI. However, the obesity-associated survival benefit was restricted to patients treated with targeted therapy and immunotherapy (8). Interestingly, a retrospective cohort of 203 MRC patients treated with ICB also showed that obese and overweight patients had statistically significantly longer overall survival than those of normal weight, but this difference did not reach statistical significance after adjusting for the International Metastatic Renal Cell Carcinoma Database. Consortium (IMDC) risk criteria (9).
The potential correlation between high BMI and more favorable treatment outcome for PD-1/PD-L1 blocking therapy has also been demonstrated. Retrospective data have suggested a survival benefit among obese patients with advanced cancer treated with ICI versus anti-PD-1/anti-PD-L1 and anti-CTLA-4 (6–16). In an interesting retrospective series of 313 MRC patients treated with nivolumab, the results suggested that both low BMI and high systemic immuno-inflammation were significantly associated with worse overall survival (14). In a smaller, single-center retrospective cohort of 198 patients with advanced cancer with mixed primary sites and treated with anti-PD-1/anti-PD-L1 immunotherapy agents, the authors evaluated the association of BMI and serum albumin levels. with clinical outcomes. Albumin level was not associated with OS or PFS. Although a higher BMI (≥ 30 kg/m2) was associated with improved OS as a continuous variable in univariate analysis, in multivariate analysis BMI was not significantly associated with OS or PFS (17). Finally, in a large retrospective Italian multicenter analysis of 976 patients with advanced cancer with multiple primary sites and treated with anti-PD-1/PD-L1 drugs, the authors also reported that obesity significantly improved clinical outcomes. According to BMI, patients were classified into two groups: overweight/obese (≥ 25 kg/m2) and not overweight (< 25 kg/m2). Time to treatment failure (TTF), PFS and OS were significantly longer for overweight/obese patients in univariate and multivariate models. The ORR was also significantly higher in the high BMI groups (11).
In this way, we carried out the first retrospective, multicenter, observational study to assess the clinical outcome of Brazilian patients with advanced cancer treated with ICI according to baseline BMI. Since there are no previous studies published on this topic with the Brazilian population, our data are interesting and have the potential to be further studied to understand the reasons that support a possible positive association between high BMI and clinical outcomes in patients with advanced cancer.
Materials and methods
Patient eligibility
We included patients with a diagnosis of stage IV cancer, regardless of primary tumor site, who underwent treatment with ICI (anti-PD-1, anti-PD-L1, anti-CTLA-4, or combo) as first or subsequent lines (2 to 4), in two medical oncology centers of Americas Oncology Group – Americas Oncology Cancer Center, in Rio de Janeiro/RJ, and Paulistano Hospital in São Paulo/SP, Brazil - between January 2014 and November 2019. We excluded from our study patients with more than one primary tumor site, those without weight and height information available.
Study design and outcomes
The primary outcomes were the association of BMI category with overall survival (OS) - defined as the time from treatment initiation until death from any cause - and progression free survival (PFS) - defined as the time from treatment initiation until disease progression or death from any cause. The primary outcomes were stratified by gender, age, treatment agent, and primary tumor site. Secondary outcomes were the association of BMI category with objective response rate - ORR (complete and partial responses). Patients who were alive or did not experience disease progression during immunotherapy treatment were censored on the date of their last contact. The ORR was calculated as the proportion of patients who developed an objective response (complete or partial response) as their best response to therapy according to the Response Evaluation Criteria in Solid Tumors version 1.1 (18). This study followed the ethical principles and good practices of clinical research mentioned in Helsinki Declaration and Good Clinical Practice from the ICH, and local regulations, such as the Brazilian Resolution CNS/MS 466/12 and the Document of Americas 2005. The Institutional Review Board of COI Institute, the research department of Americas Oncology Group, and the central ethics committee from Hospital Procardiaco, Rio de Janeiro/RJ, Brazil, approved the study protocol (CAAE:89354418.0.0000.5533).
Data extraction
All patient’s data were only collected from their medical charts and no biological samples were obtained. Weight and height were obtained from patients’ medical charts at the time of the first cycle of immunotherapy. Demographic variables were analyzed using descriptive and analytic statistics. BMI was calculated using the formula weight/height2 (kilograms per square meters) and then classified according to the World Health Organization (WHO) standard categories: underweight, BMI < 18.5 kg/m2; normal, 18.5 kg/m2 ≤ BMI ≤ 24.9 kg/m2; overweight, 25 kg/m2 ≤ BMI ≤ 29.9 kg/m2; obesity, BMI ≥ 30 kg/m2.
Regarding the definition of a cut-off for BMI, few published studies compared obese against normal overweight or normal weight individuals in a conservative analysis while most studies used a different categorization of obese/overweight (BMI ≥ 25 kg/m2) and normal weight patients (BMI < 25 kg/m2). In contrast, in our analysis, we categorized the BMI values into two groups: obese (BMI ≥ 30 kg/m2) and non-obese (BMI < 30 kg/m2) because this approach would more conservative and better assess the isolated effect of obesity. The combination of obese and overweight patients would likely overestimate the real effect of BMI in comparison to using group with only obese patients.
Statistical analysis
The correlation between categorical variables was assessed using the chi-square or Fisher’s exact test. The PFS and OS were analyzed with the use of Kaplan-Meier Methods, and survival curves were compared using the Log-rank test. The following covariates were considered as potential risk factors for PFS or OS using Cox regression as univariate and multivariate analysis: age (≥ 65 years vs. ≤ 65 years), sex (female vs. male), Eastern Cooperative Oncology Group Performance Status - ECOG-PS (≥ 1 vs. 0), visceral disease (present vs. absent), and obesity (overweight and obesity) (19). All statistical tests were two-sided, with a significance level at p < 0.05 using IBM SPSS Statistics Version 19 (IBM Corp. IBM SPSS Statistics for Windows, Version 19.0. Armonk, NY: IBM Corp).
Results
Patients’ characteristics
We collected data from 448 patients with advanced disease treated with ICI. The median age was 64 years (range: 21 - 94 years), and 62% were male patients. ECOG-PS was 0 in 57 patients (34%), 1 in 104 patients (61%), and ≥ 2 in 9 patients (5%). The most common primary tumors were lung cancer (253 patients, 57%), melanoma (83 patients, 19%), and kidney cancer (40 patients, 9%). In our study group, a total of 288 (64%) patients had visceral disease. Most patients (89%) were treated with a single anti-PD-1/anti-PD-L1 drug (nivolumab, pembrolizumab, or atezolizumab). Regarding the line of therapy, 128 patients (28%) were treated in the first-line setting, 200 patients (44%) in the second-line setting, 60 patients (14%) in the third-line setting, and 60 patients (14%) in the fourth line setting. Tumor PD-L1 expression was available for 94 patients: 43 patients (46%) had a PD-L1 < 1%, 21 patients (22%) had a PD-L1 between 1% and 49%, and 30 patients (32%) had a PD-L1 ≥ 50%. Most patients (86%) whose tumor sample was tested for PD-L1 expression had a primary diagnosis of lung cancer. The median BMI was 25.5 kg/m2 (range: 16.3 - 47.0). According to the WHO classification, 19 patients (4%) were defined as underweight, 192 patients (43%) were normal weight, 159 patients (36%) were overweight, and 78 patients (17%) were obese. Information about BMI was not available for 4 patients (1%), who were excluded from our statistical analysis.
Based on BMI values, we categorized the 448 patients into two groups: obese (BMI ≥ 30 kg/m2) and non-obese (BMI < 30 kg/m2). A total of 370 patients (83%) were included in the non-obese group, and the remaining 78 (17%) patients in the obese group. There was no significant difference between these two groups (Table 1).
Response rate analysis
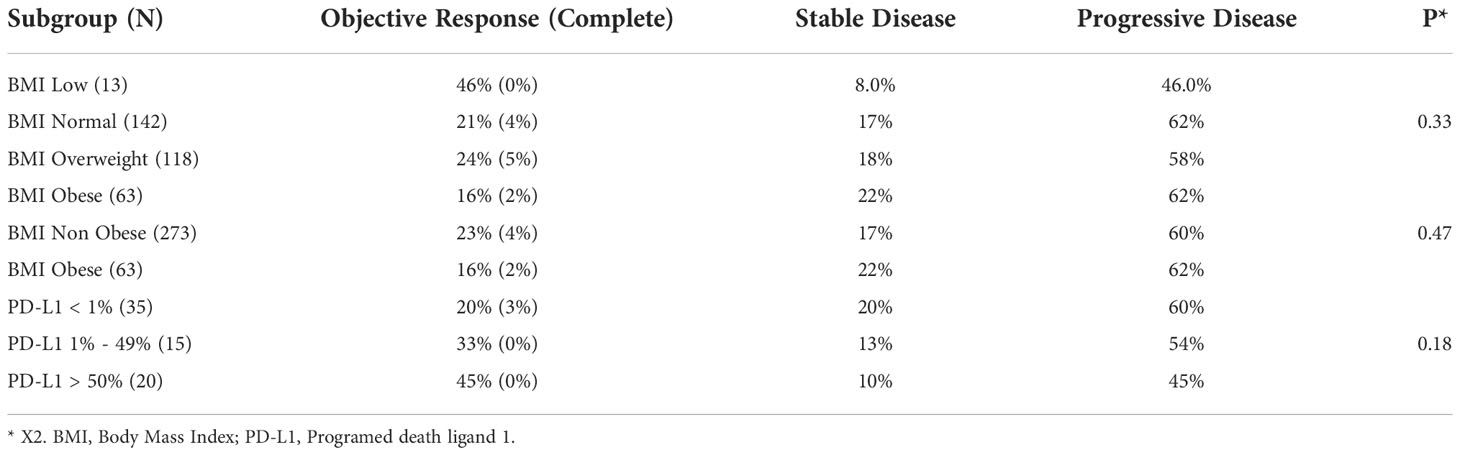
In the entire cohort, 334 patients were evaluated for response rate outcomes. We assessed whether the ORR was associated with any of the BMI categories as well as the categorical classification of obese and non-obese patients. There was no significant difference among the patients’ ORR according to BMI categories: low, normal overweight, and obese (P = 0.07). Despite a trend for lower ORR in the obese group compared to the non-obese Group (16% versus 23%), we did not find any significant difference in ORR between the two groups (P = 0.33).
In the response rate analysis among patients with PD-L1 expression available, we found a trend for better ORR with higher PD-L1 expression, which was not statistically significant (P = 0.47) (Table 2). Tumor PD-L1 expression was available for 94 patients: 43 patients (46%) had a PD-L1 < 1%, 21 patients (22%) had a PD-L1 between 1% and 49%, and 30 patients (32%) had a PD-L1 ≥ 50%. Most patients (86%) whose tumor sample was tested for PD-L1 expression had a primary diagnosis of lung cancer.
Survival analysis
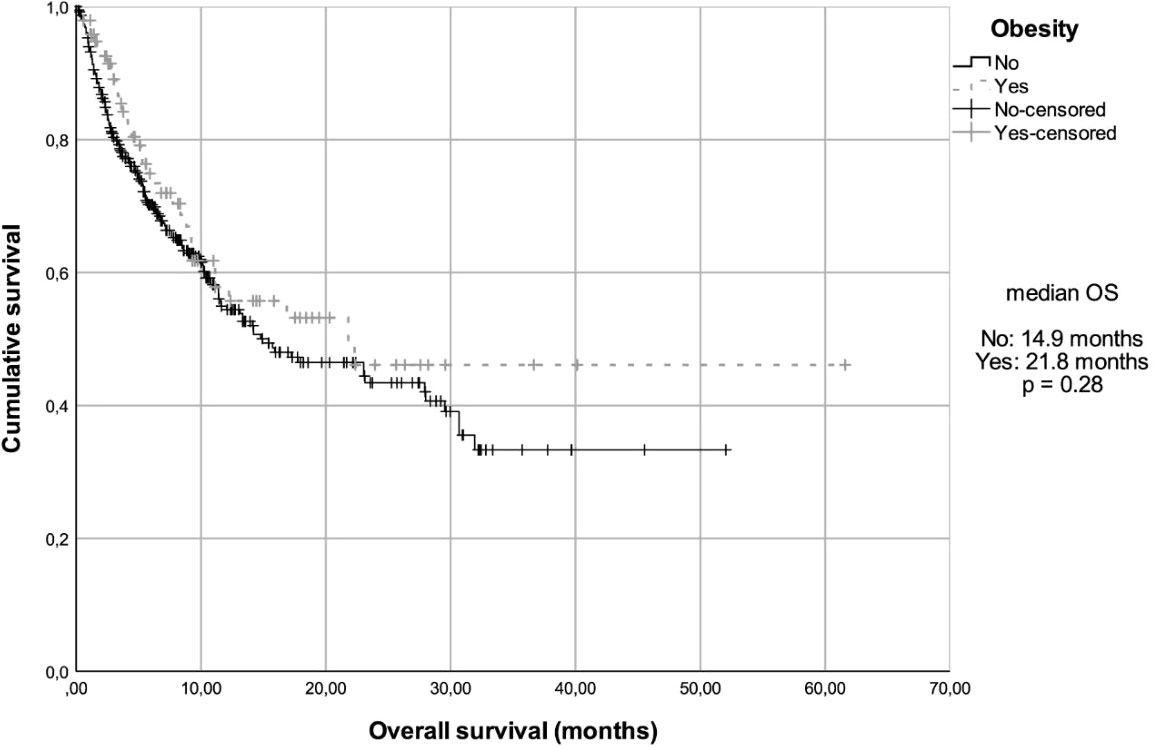
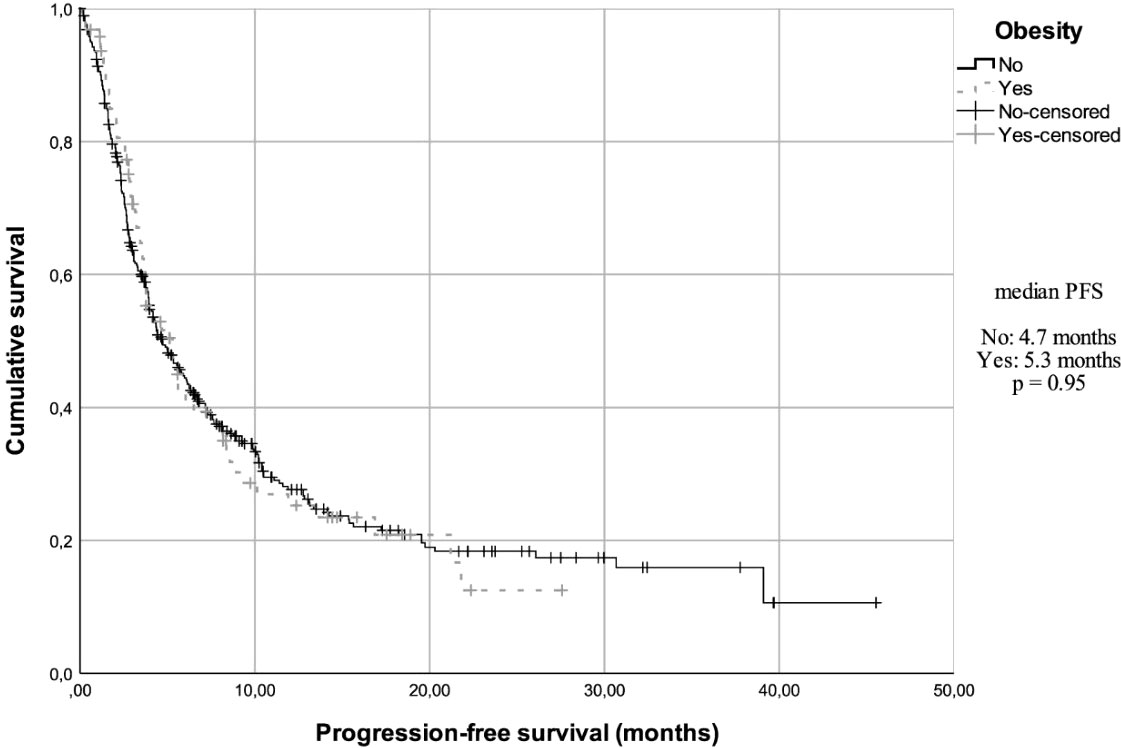
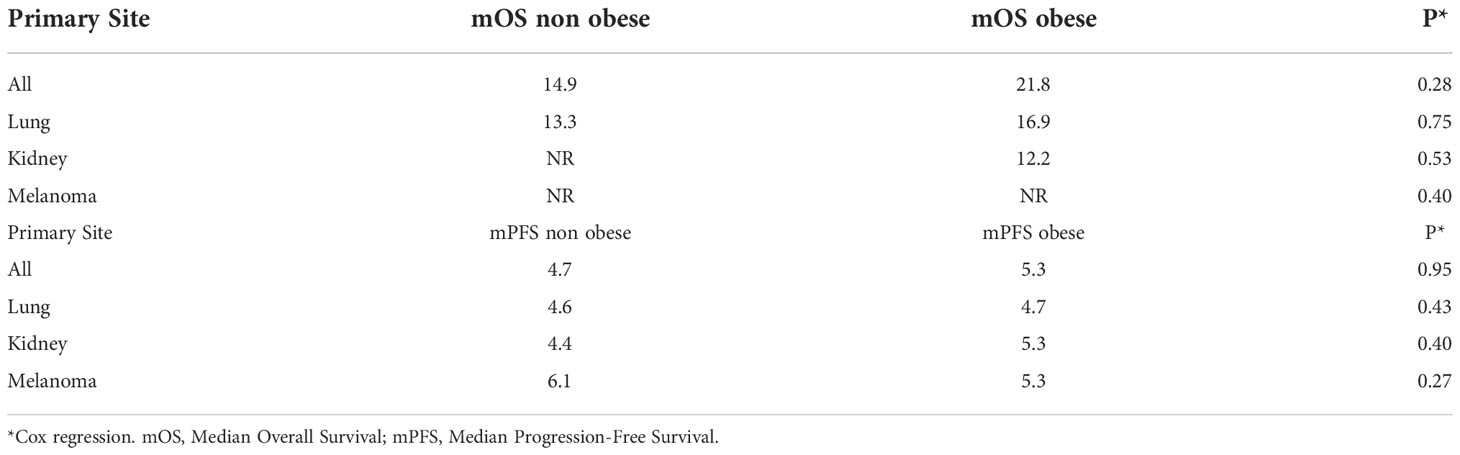
At a median follow-up of 14.8 months (95% confidence interval (CI), 11.5 - 17.3), the median overall survival (mOS) in the total cohort was 15.9 months (95% CI, 9.87 - 21.94) and the median progression-free survival (mPFS) was 4.7 months (95% CI, 3.9 - 5.6). The OS in the three most prevalent primary sites were also analyzed. Patients with lung cancer had a mOS of 13.33 months (95% CI, 6.55 - 20.12); patients with kidney cancer and melanoma did not reach the mOS value. In addition, when the OS data were analyzed according to BMI, after 35 events (36%), the mOS was 14.9 months (95% CI, 8.3 - 21.5) among non-obese patients, while the obese group had a mOS of 21.8 months (95% CI, NR-NR) after 165 events (42%) (Figure 1). However, this difference was not statistically significant (Hazard ratio (HR) = 0.82, 95% CI, 0.57 - 1.18, P = 0.28). When assessing mOS by BMI groups stratified by primary site, there was no statistically significant difference. In the obese patients’ group, after 65 events (68%), the mPFS was 4.7 months (95% CI, 3.8 - 5.7), while in non-obese group, after 266 events (69%), the mPFS was 5.3 months (95% CI, 3.45 - 7.15) (Figure 2). This difference was not statistically significant (HR = 0.99, 95% CI, 0.76 - 1.30, P = 0.95). When assessing mPFS by BMI groups stratified by primary site, there was also no statistically significant difference (Table 3).
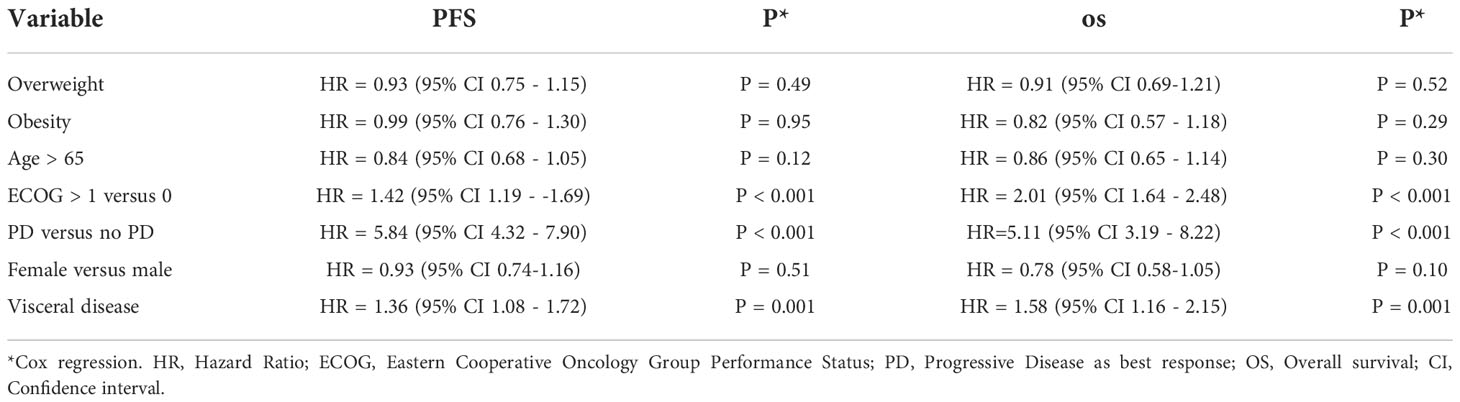
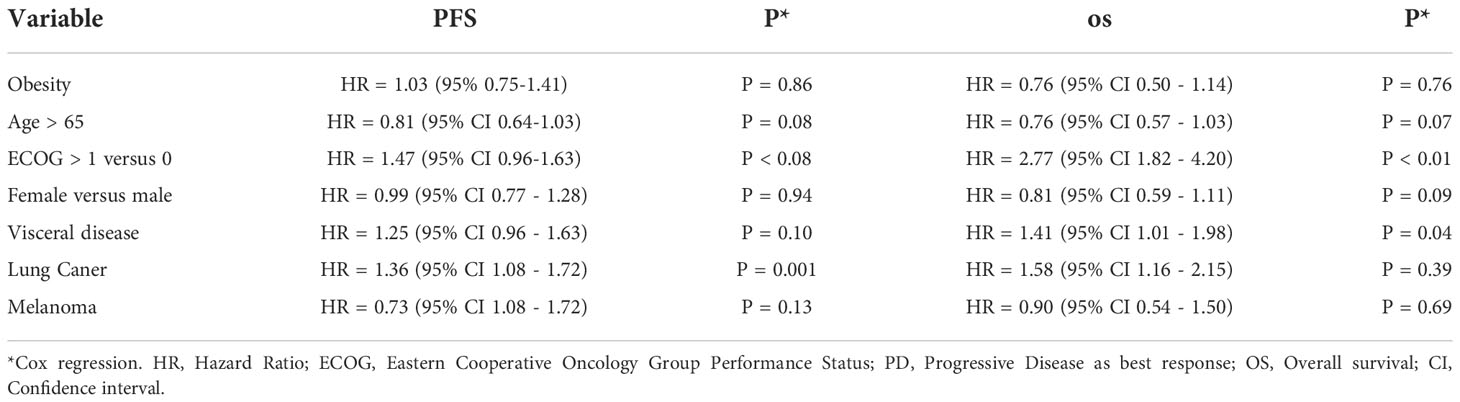
In the univariate analysis of risk factors for PFS and OS, the presence of visceral disease, progressive disease as the best response, and reduced performance status were significantly associated with worse PFS and OS (Table 4). In the multivariate analysis, visceral disease and reduced performance were significantly associated with worse OS (Table 5).
Association between class of immune checkpoint inhibitor and obesity
We assessed obesity as a risk factor for PFS and OS according to the class of ICIs used (anti-PD-1/anti-PD-L1 and anti-CTLA4). Due to the small number of patients treated with combination immunotherapy, they were excluded from the analysis. In a total of 401 patients treated with anti-PD-1/anti-PD-L1 drugs, we categorized them into obese (71 patients) and non-obese (330 patients). The obese group had a mOS of 16.9 months (95% CI, 5.4 - 28.3) and the non-obese group achieved a mOS of 14.8 months (95% CI, 8.0 - 21.6) (P = 0.67). The group of patients treated with anti-CTLA-4 (N = 31) were also categorized as obese (N = 5) or non-obese (N = 26). The obese group did not reach the mOS value, while the non-obese group had a mOS of 23.1 months (P = 0.04).
Discussion
The International Agency for Research on Cancer (IARC) of the WHO has recently gathered substantial evidence of the strong association between obesity and an increased risk of several cancer types such as esophageal adenocarcinoma, gastric cardia adenocarcinoma, colorectal cancer, liver cancer, gallbladder cancer, pancreas cancer, postmenopausal breast cancer, uterine cancer, ovarian cancer, renal cell carcinoma, meningioma - as well as limited evidence of obesity’s association with three other cancer types - fatal prostate cancer, male breast cancer, and diffuse large B-cell lymphoma (2). In fact, obesity may promote carcinogenesis through the promotion of several mechanisms such as insulin resistance, hyperinsulinemia, oxidative stress, and inflammation. However, in the metastatic setting, obesity is regarded as a positive prognostic factor and predictive of longer overall survival (2).
Retrospective studies suggest a possible positive association between high BMI and clinical outcomes. In patients with advanced renal cell carcinoma treated with targeted therapy, an elevated BMI (≥ 25 kg/m2) was considered a prognostic factor for better survival and progression-free survival (7). A small study of 147 MRC patients treated with immune checkpoint blockade (ICB) alone or with combination therapy demonstrated that patients with a high BMI (> 25 kg/m2) achieved significantly better overall survival compared to those with a low BMI (13). When evaluating a selective group of patients with metastatic non-small cell lung cancer (NSCLC), Kichenadasse et al. demonstrated that high BMI was independently associated with improved survival in a large, pooled analysis of four RCTs that included individual-level data from 2,110 atezolizumab-treated subjects, and this survival benefit was more pronounced in cases of high PD-L1 expression (15). In a study of patients with castration-resistant prostate cancer in the post-docetaxel setting who were treated with enzalutamide or abiraterone, there was a significant association between an increased amount of subcutaneous adipose tissue and improved overall survival (19), however, this longer OS obesity paradox for high BMI patients can be considered a result of relative protection against cancer cachexia. The BMI definition of obesity can be misleading because it is not a true marker of total body fat or abnormal distribution. Also, in some cases, a high BMI can hide a sarcopenic state. Furthermore, adipose tissue plays a significant role in the interface between the metabolic changes of obesity and the inflammatory response leading to a “meta-inflammatory” state, where there is low-grade systemic immune activation triggered by increased pro-inflammatory cytokines (20, 21).
Given the recent advancement in immune checkpoint inhibitors against anti-PD-1/anti-PD-L1 and anti-CTLA-4, there is growing interest in the search for biomarkers that can predict a patient’s clinical outcome after immunotherapy. Most of the research on this topic has focused on evaluating tumor characteristics such as tumor mutation load, intratumoral levels of PD-1/PD-L1, and the presence of T-cell infiltration stains (22, 23). Real-world data from 703 patients with advanced NSCLC treated with nivolumab or pembrolizumab revealed a significant association between longer overall survival and high BMI (16). In another smaller retrospective cohort of 44 patients with MRC treated with nivolumab, higher BMI was statistically significant associated with improved overall clinical survival (12). On the other hand, a retrospective analysis of 324 patients with advanced NSCLC treated with PD-1 inhibitors (nivolumab or pembrolizumab) showed no significant differences in the clinical outcomes of patients classified as overweight (BMI ≥ 25 kg/m2) and not overweight (BMI < 25 kg/m2) (10).
However, some provocative data suggest that obesity may be a relevant prognostic factor in patients with advanced cancer treated with ICIs. In a large retrospective analysis of six independent cohorts of 2046 patients with metastatic melanoma, McQuade et al. demonstrated that obesity was associated with better progression-free survival and overall survival compared with normal BMI. Interestingly, this survival benefit was not seen among patients treated with chemotherapy (8). The lack of benefit among chemotherapy-treated patients favors the hypothesis that obese patients have a pro-inflammatory state that may improve immunotherapy outcomes, although this hypothesis does not explain the observed benefit among patients treated with target agents.
An analysis of real-world data from 703 patients with advanced NSCLC treated with nivolumab or pembrolizumab revealed that low BMI was associated with shorter OS. However, the major concern in interpreting this finding is whether BMI is the main cause of the observed outcome or whether low BMI is another consequence related to cachexia and worse performance status, which are known factors of worse prognosis (16). On the other hand, a retrospective analysis of 324 patients with advanced NSCLC treated with PD-1 inhibitors (nivolumab or pembrolizumab) showed no significant differences in the clinical outcomes of patients classified as overweight (BMI ≥ 25 kg/m2) and not overweight (BMI < 25 kg/m2) (10). Still, the body of evidence points out that high BMI patients are more likely to derive more clinical benefit from immunotherapy than their normal or low BMI counterparts. An association between high BMI and better performance status not only explains the better results achieved, but there is also a supposed role of obesity in the regulation of the inflammatory response that is still not well understood.
There are several limitations in our study. First, the relatively short follow-up time and smaller sample size compared to the most relevant data on this subject may partially explain why our results did not reproduce previous findings of improved OS in obese patients. The retrospective design may cause bias and restrict the interpretation of our results, given the strong likelihood of bias, especially selection and recall bias. Second, the broad inclusion criteria for patients with advanced disease from any primary site may have led to a heterogeneous sample, which makes our analyzes less powerful. Given the mixed primary tumor sites in our sample and the many different prognostic factors involved, it would be difficult to gauge the true significance of baseline BMI for the clinical benefit of immune checkpoint inhibitors. In contrast, most literature data with positive results have homogeneous cohorts with a single primary tumor. Third, given that only 28% of our patients received ICI as first-line therapy, this may have negatively impacted outcomes. The clinical benefit of ICI is usually more evident in the first line than in the later lines. Unlike other studies that included patients enrolled in RCTs, our study included real-world patients who were treated according to drugs approved by the Brazilian regulatory agency and available to the population at the time of the study. Thus, the lower proportion of patients treated in the first line with ICI may point to barriers in the access to immunotherapy and this issue is discussed in the literature (24). Finally, the fact that most of our patients had primary lung cancer may have influenced our analysis, whereas most studies have shown positive results in cohorts of patients with melanoma, renal cell carcinoma, and lung cancer (8, 9, 12, 14–16). The design and methodological limitations of our study may not allow a definitive conclusion. However, we speculate that a longer follow-up period, a larger sample size and a more homogeneous population could provide more information.
Despite limitations, this retrospective study was the first to assess the association of BMI and ICI clinical outcomes in a Brazilian group of patients with advanced cancer. We found a trend towards higher mOS among obese patients treated with immune checkpoint inhibitors, but this result did not reach statistical significance. Among anti-CTLA4-treated patients, obese patients had improved OS compared to non-obese patients; however, the sample size was too small to have sufficient statistical power. Although our results did not reach statistical significance, there was a trend towards a clinical benefit of ICI in obese patients, which has already been suggested in other populations. In the future, we hope that prospective trials with a homogeneous population with a primary tumor site and BMI as a stratification variable will adequately address the role of BMI in the clinical benefit of immunotherapy.
Conclusions
This study did not report a statistically significant improvement in terms of overall survival among high BMI patients, although there was a trend toward a survival benefit with immune checkpoint blockade, especially anti-CTLA-4 agents. Design and methodological limitations of our study may not allow a definitive conclusion. Since most of the data in this area comes from retrospective studies, BMI should be explored as a stratification variable in the design of further prospective trials with advanced cancer patients receiving immunotherapy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics committee from Hospital Procardiaco, Rio de Janeiro/RJ (CAAE:89354418.0.0000.5533). The ethics committee waived the requirement of written informed consent for participation.
Author contributions
Conception and design: RM, MM, PNA. Development of methodology: RM, MF, MRM, PNA. Acquisition of data: MF, MM, FL, ES, PMA, MP, LL, AS. Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): RM, MF, MM, PNA. Writing, review, and/or revision of the manuscript: RM, MF, MM, FL, ES, PMA, MP, LL, AS, JN, DF, LA, PNA, CT. Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): RM, MM, PMA, DF, LA, PNA, CT. Study supervision: RM, MM, PNA. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors are grateful to all UnitedHealth Group professionals who worked on supportive care of cancer patients. CVTR is grateful for FAPESP fellowships (Project No. 2019/08999-3-DD).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Arnold M, Leitzmann M, Freisling H, Bray F, Romieu I, Renehan A, et al. Obesity and cancer: An update of the global impact. Cancer Epidemiol (2016) 41:8–15. doi: 10.1016/j.canep.2016.01.003
2. Lauby-Secretan B, Ph D, Scoccianti C, Ph D, Loomis D, Ph D. Body fatness and cancer — viewpoint of the IARC working group. N Engl J Med (2016) 375(8):794–8. doi: 10.1056/NEJMsr1606602
3. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell (2011) 144(5):646–74. doi: 10.1016/j.cell.2011.02.013
4. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer (2012) 12(4):252–64. doi: 10.1038/nrc3239
5. Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci (2010) 107(9):4275–80. doi: 10.1073/pnas.0915174107
6. Richtig G, Hoeller C, Wolf M, Wolf I, Rainer BM, Schulter G, et al. Body mass index may predict the response to ipilimumab in metastatic melanoma: An observational multi-centre study. PloS One (2018) 13(10):1–9. doi: 10.1371/journal.pone.0204729
7. Albiges L, Ari Hakimi A, Xie W, McKay RR, Simantov R, Lin X, et al. Body mass index and metastatic renal cell carcinoma: Clinical and biological correlations. J Clin Oncol (2016) 34(30):3655–63. doi: 10.1200/JCO.2016.66.7311
8. McQuade JL, Daniel CR, Hess KR, Mak C, Wang DY, Rai RR, et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: A retrospective, multicohort analysis. Lancet Oncol (2018) 19(3):310–22. doi: 10.1016/S1470-2045(18)30078-0
9. Ged Y, Lee C-H, Sanchez A, Duzgol C, Chaim J, Carlo MI, et al. Association of body mass index (BMI) with clinical outcomes in 203 metastatic clear cell renal cell carcinoma (ccRCC) patients (pts) treated with immuno-oncology (IO) agents. J Clin Oncol (2019) 37(suppl):e16103. doi: 10.1200/JCO.2019.37.15_suppl.e16103
10. Tateishi A, Horinouchi H, Yoshida T, Masuda K, Jo H, Shinno Y, et al. Correlation between body mass index and efficacy of anti-PD-1 inhibitor in patients with non-small cell lung cancer. Respir Investig (2021) 60(2):234–40. doi: 10.1016/j.resinv.2021.11.003
11. Cortellini A, Bersanelli M, Buti S, Cannita K, Santini D, Perrone F, et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: When overweight becomes favorable. J Immunother Cancer (2019) 7(1):1–11. doi: 10.1186/s40425-019-0527-y
12. Herrmann T, Mione C, Montoriol P-F, Molnar I, Ginzac A, Durando X, et al. Body mass index, sarcopenia, and their variations in predicting outcomes for patients treated with nivolumab for metastatic renal cell carcinoma. Oncology (2022) 100(2):114–23. doi: 10.1159/000520833
13. Lalani A-KA, Xie W, Flippot R, Steinharter JA, Harshman LC, McGregor BA, et al. Impact of body mass index (BMI) on treatment outcomes to immune checkpoint blockade (ICB) in metastatic renal cell carcinoma (mRCC). J Clin Oncol (2019) 37(suppl 7S):abstr 566. doi: 10.1200/JCO.2019.37.7_suppl.566
14. De Giorgi U, Procopio G, Sabbatini R, Caserta C, Mitterer M, Ortega C, et al. Association of body mass index and systemic inflammation index with survival in patients with renal cell cancer treated with nivolumab. J Clin Oncol (2019) 37(15_suppl):e16077–7. doi: 10.1200/JCO.2019.37.15_suppl.e16077
15. Kichenadasse G, Miners JO, Mangoni AA, Rowland A, Hopkins AM, Sorich MJ. Association between body mass index and overall survival with immune checkpoint inhibitor therapy for advanced non-small cell lung cancer. JAMA Oncol (2019) 15:1–7. doi: 10.1001/jamaoncol.2019.5241
16. Zhi J, Khozin S, Kuk D, Torres AZ, Sorg R, Lee SE, et al. Association of baseline body mass index (BMI) with overall survival (OS) in patients (pts) with metastatic non-small cell lung cancer (mNSCLC) treated with nivolumab (N) and pembrolizumab (P). J Clin Oncol (2018) 36(suppl):6553. doi: 10.1200/JCO.2018.36.15_suppl.6553
17. Ibrahimi S, Mukherjee S, Roman D, King C, Machiorlatti M, Aljumaily R. Effect of body mass index and albumin level on outcomes of patients receiving anti PD-1/PD-L1 therapy. J Clin Oncol (2018) 36(suppl 5S):213. doi: 10.1200/JCO.2018.36.5_suppl.213
18. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
19. Antoun S, Bayar A, Ileana E, Laplanche A, Fizazi K, di Palma M, et al. High subcutaneous adipose tissue predicts the prognosis in metastatic castration-resistant prostate cancer patients in post chemotherapy setting. Eur J Cancer (2015) 51(17):2570–7. doi: 10.1016/j.ejca.2015.07.042
20. Wang Z, Aguilar EG, Luna JI, Dunai C, Khuat LT, Le CT, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med (2019) 25(1):141–51. doi: 10.1038/s41591-018-0221-5
21. Navab M, Gharavi N, Watson AD. Inflammation and metabolic disorders. Curr Opin Clin Nutr Metab Care (2008) 11(4):459–64. doi: 10.1097/MCO.0b013e32830460c2
22. Steuer CE, Ramalingam SS. Tumor mutation burden: Leading immunotherapy to the era of precision medicine? J Clin Oncol (2018) 36(7):631–2. doi: 10.1200/JCO.2017.76.8770
23. Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol (2016) 17(12):e542–51. doi: 10.1016/S1470-2045(16)30406-5
24. Ferreira CG, Abadi MD, de Mendonça Batista P, Serra FB, Peixoto RB, Okumura LM, et al. Demographic and clinical outcomes of Brazilian patients with stage III or IV non–Small-Cell lung cancer: Real-world evidence study on the basis of deterministic linkage approach. JCO Glob Oncol (2021) 7):1454–61. doi: 10.1200/GO.21.00228
Keywords: body mass index, checkpoint inhibitors, immunotherapy, metastatic cancer, obesity
Citation: Moreira RB, Fernandes M, Monteiro MR, Luiz FMA, Silva ES, Andrade PdM, Pinto MB, Lima L, Silva A, Nunez J, Freitas D, de Lima Araujo LH, Rossini CVT and Aguiar PN Jr. (2022) Body mass index and immune checkpoint inhibitor efficacy in metastatic cancer patients: A Brazilian retrospective study. Front. Urol. 2:1069045. doi: 10.3389/fruro.2022.1069045
Received: 13 October 2022; Accepted: 28 November 2022;
Published: 12 December 2022.
Edited by:
Muhannad Alsyouf, University of Southern California, United StatesReviewed by:
Alvaro Pinto, University Hospital La Paz, SpainSeyedeh Sanam Ladi Seyedian, University of Southern California, United States
Copyright © 2022 Moreira, Fernandes, Monteiro, Luiz, Silva, Andrade, Pinto, Lima, Silva, Nunez, Freitas, de Lima Araujo, Rossini and Aguiar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caio Vinicius Teles Rossini, Y2Fpb19yb3NzaW5pQHVzcC5icg==
 Raphael Brandao Moreira1,2
Raphael Brandao Moreira1,2 Mariana Ribeiro Monteiro
Mariana Ribeiro Monteiro Astrid Silva
Astrid Silva Luiz Henrique de Lima Araujo
Luiz Henrique de Lima Araujo Caio Vinicius Teles Rossini
Caio Vinicius Teles Rossini