
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Urol. , 14 December 2022
Sec. Urologic Oncology
Volume 2 - 2022 | https://doi.org/10.3389/fruro.2022.1064099
Sporadic renal hemangioblastomas (RHBs) are a rare subgroup of extraneurologic hemangioblastomas. They are under-recognized renal tumours whose differential diagnosis remains challenging. Here, we describe a case of RHB in a 61-year-old man was admitted to the hospital two days after the discovery of a right kidney mass. Renal carcinoma was clinically considered, and a radical nephrectomy was performed. Follow-up showed no evidence of postoperative tumour recurrence. Histologically, the tumour boundary is clear and fibrous envelope is visible. The tumour issue was mainly composed of tumour cells and a dendritic capillary network, which consisted of multicellular and oligocellular areas. The tumour cells were polygonal, the cytoplasm was eosinophilic or transparent, and intranuclear pseudoinclusions were found. Immunohistochemically, vimentin, a-inhibin, neurogenic specific enolase (NSE), S-100, smooth muscle actin (SMA), and cluster of differentiation (CD)10 antibodies reacted strongly and were diffused, and Ki-67 was 2% positive. CD31 and CD34 showed vascular morphology. We also summarized related case reports (a total of 41 cases in the Chinese and English literature) to explore the clinicopathological characteristics of RHB and improve the diagnosis and treatment of this disease. RHB is a benign tumour with excellent prognosis; however, it is easily misdiagnosed as other common renal malignancies. Immunohistochemistry is vastly helpful in accurate diagnosis of RHB. Preoperative renal biopsy can effectively avoid misdiagnosis and overtreatment.
Hemangioblastoma, also known as capillary hemangioblastoma, is a rare benign tumour (WHO grade 1) that is sporadic, and approximately 25% are associated with von Hippel-Lindau disease(VHLD) (1, 2). VHLD is an autosomal dominant disease associated with germline mutations in the VHLD tumour suppressor gene located on the short arm of chromosome 3p25 (3). Hemangioblastomas usually occur in the central nervous system (CNS) and occasionally in the bone, liver, soft tissue, lung, skin, pancreas, and kidney (4). They are often accompanied by VHLD. The rest were sporadic and no specific pathogenesis was found. Sporadic hemangioblastoma is more common as isolated lesions located in the cerebellum. The cases of sporadic hemangioblastoma in other sites are rarely reported.
The kidney is a rare site for the growth and development of sporadic hemangioblastomas, and there are few reports on these cases. In addition, renal hemangioblastomas (RHBs) are similar to renal cell carcinoma (RCC) according to pathological examinations. Thus, accurate diagnosis of clinical RHB is greatly challenging. Herein, we report a case of sporadic RHB that was not associated with VHLD. Its microscopic appearance could easily be misdiagnosed as other renal tumours, especially RCC, epithelioid angiomyolipoma, and paraganglioma. Therefore, we further elucidated the clinicopathological features and differential diagnosis of RHB. We also reviewed the literature on RHB to provide a more comprehensive basis for RHB.
A man in his sixties was admitted to the hospital two days after the discovery of a mass in the right kidney. Physical examination revealed positive percussion pain in the right renal area, and the patient denied hematuria, frequent urination, urgency, urination pain, dizziness, headache, and other neurological symptoms. There was no family history of kidney cancer, brain tumours, or VHLD. B-ultrasound showed a slightly hyperechoic and well-defined mass in the lower pole parenchyma of the right kidney with a size of 5.5×4.6 cm (Figure 1A). Computed tomography (CT) revealed a heterogeneous and slightly low-density mass in the lower pole parenchyma of the right kidney. In the arterial enhancement phase, heterogeneous and obvious enhancement was observed at the edge (Figure 1B). CT angiography (CTA) showed a mass of 5.2×4.4 cm in size in the lower pole parenchyma of the right kidney. Renal carcinoma was clinically considered, and a radical nephrectomy was performed. Follow-up for over 13 months showed no evidence of postoperative tumour recurrence.
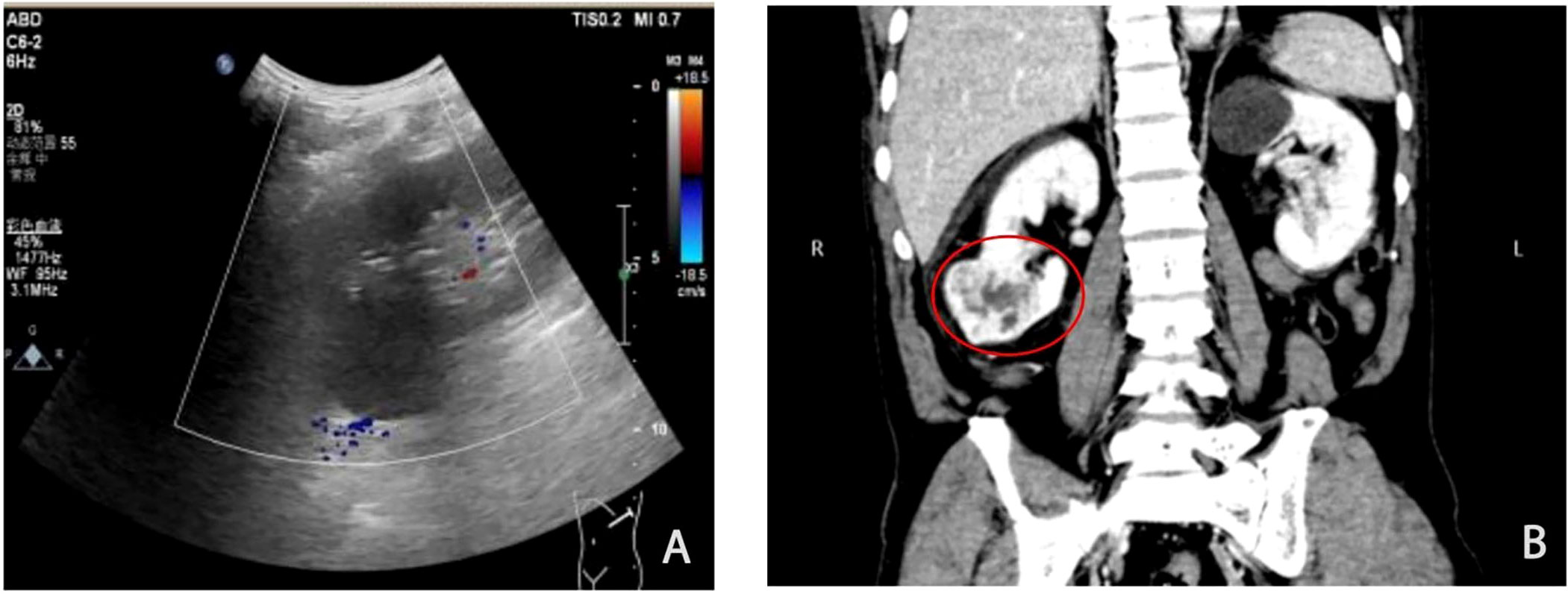
Figure 1 Imagological examinations showing a large tumour in the right kidney. (A) B-Ultrasonic imaging showed a well-defined and slightly hyperechoic mass in the inferior pole of the right kidney, with uneven internal echo protruding toward the kidney surface. (B) Computed tomography (CT). The circles indicate tumours. The CT image shows a heterogeneous soft tissue mass in the inferior pole of the right kidney with heterogeneous enhancement and well-defined boundaries.
The resected tumour specimens were fixed in 10% neutral-buffered formalin and processed for immunohistochemistry by following the standard protocol. Paraffin-embedded blocks were sectioned at a thickness of 5μm and stained with hematoxylin and eosin and various antibodies. The antibody clones, working dilutions, and commercial sources are listed in Table 1.
Macroscopically, the right kidney was 12×7×5 cm in size, with a fat capsule on the surface, and the cut surface was gray-red and dark red. A 5×4×3 cm mass could be seen at the inferior pole surrounded by a complete capsule. The mass protruded towards the surface of the kidney and the section of the mass was gray-red, and some areas were gray-yellow. The mass was tough, without necrosis or cystic changes.
Microscopically, the tumour tissue was clearly demarcated from the surrounding normal renal tissue, which was surrounded by a thick fibrous envelope (Figure 2A). The tumour tissue consisted of multicellular and oligocellular areas (Figure 2B). In some areas, the tumour cells were nested distribution, and abundant thick-walled vascular proliferation, and hyaline degeneration (Figure 2C). The lobulated vascular network separated the tumour tissue into nests or plates. The oligocellular region mainly consisted of abundant hemosiderin, a fibrous interstitium containing reticular vascular channels and rare interstitial cells (Figure 2E). Cytoplasmic staining of the tumour cells was eosinophilic or transparent. Some tumour cells contained foamy lipid droplets. Nucleoli were not obvious and the nuclei were eccentrically displaced and mildly or moderately pleomorphic, with coarse-grained chromatin (Figures 2D, F) and had many distinct intranuclear pseudoinclusion bodies (Figure 2F).
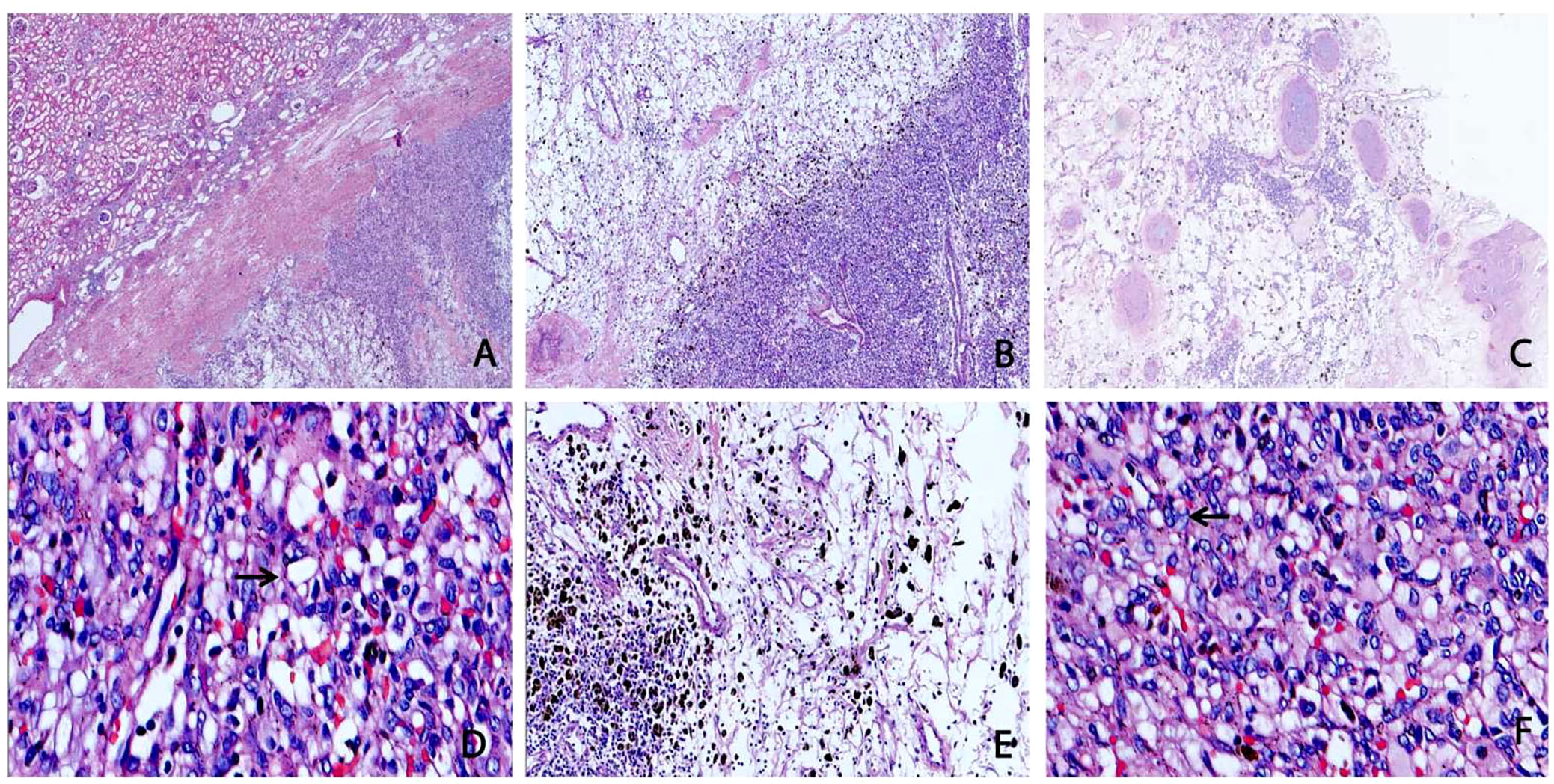
Figure 2 Microscopic architectural features of sporadic renal hemangioblastoma (RHB). (A) The tumour was surrounded by a thick fibrous envelope and clearly demarcated by the surrounding renal tissue (H&E, ×20). (B) Hypercellular areas and hypocellular areas are irregularly intermixed (H&E, ×40). (C) Blood vessels with thick hyalinized walls are apparent in stroma and tumour cells growing in nests with thick-walled vascular proliferation (H&E, ×40). (D) Hypercellular areas are composed of a large number of tumour cells, surrounded by a rich network of capillaries. The cytoplasm was weakly eosinophilic or transparent, and some cells contained lipid droplets (indicated by the arrow) (H&E, ×400). (E) The hypocellular areas were mainly composed of reticular capillaries and fibrous stroma, with a large amount of hemosiderin in the stroma and few tumour cells (H&E, ×100). (F) The nuclei of the tumour were ovoid pleomorphic or singular, with intranuclear pseudoinclusions (indicated by the arrow) (H&E, ×400).
Immunohistochemistry showed that the tumour cells were diffusely positive for vimentin, cluster of differentiation (CD)10, neurogenic specific enolase (NSE), a-inhibin and S-100 (Figures 3A–D), suggesting that the tumour originated from the epithelial tissue. CD31 and CD34 had rich vascular profiles and distributions (Figures 3E, F), consistent with the large capillary network structure of RHB. The Ki-67 index was greater than 2%. The tumour was positive for smooth muscle actin (SMA) immunohistochemical antibody and negative for paired box gene 8 (PAX-8) which ruled out RCC. tumour cells showed diffuse positive immunoreaction to a-inhibin, NSE, and S-100, and negative immunohistochemical antibodies against CD117 and HMB45, ruling out epithelioid angiomyolipoma. The negative reaction of the tumour cells to CD56 and Syn also excluded paragangliomas.
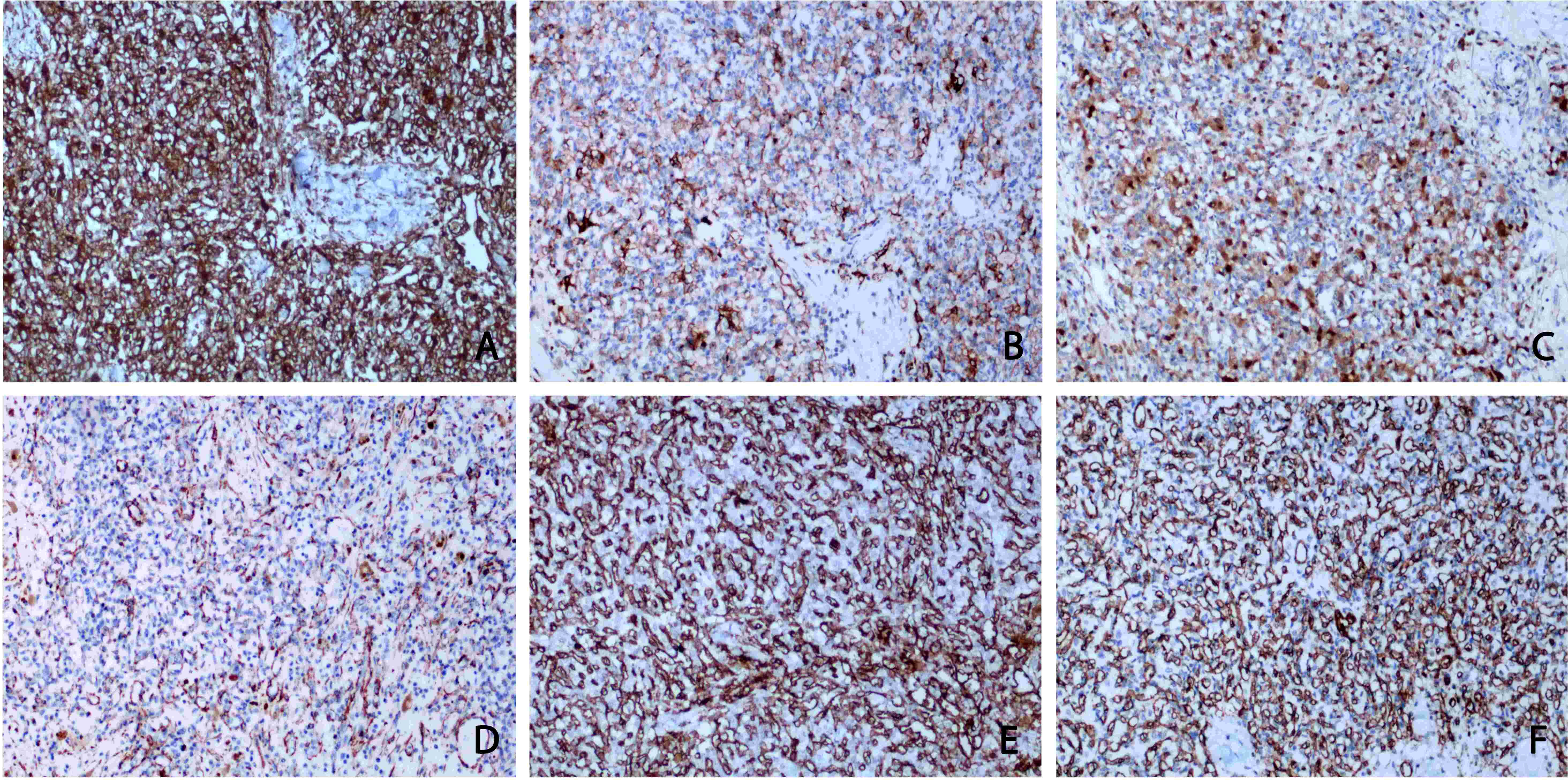
Figure 3 Immunohistochemical staining. (A) Vimentin staining: diffuse and strong positive cytoplasm of tumour cells. (B) CD10 staining: tumour cells were positive. (C) S100 protein staining: some tumour cells were cytoplasmic positive. (D) SMA staining: diffuse positive cytoplasm of tumour cells. (E, F) CD31 and CD34 showed rich and subtle vascular channels, whereas tumour cells were negative. (A-F original magnification is ×100).
Hemangioblastomas are usually sporadic and partly associated with VHLD. Sporadic hemangioblastoma is a rare tumour of unknown etiology, which usually occurs in the CNS, but it can occasionally occur elsewhere.To date, only 40 cases of RHB have been reported in the Chinese and English literature (Table 2) (5–29). Through the analysis and induction of these cases, we found that these tumours mostly occurred in people over 40 years of age (29/41, 71%, ages ranging from 16 to 71 years), and the majority of patients were male (28/41, 68%). All patients had unilateral kidney involvement (41/41, 100%) and the tumours were mostly located in the right kidney (25/41, 61%). Most of the tumours (22/25, 88%) were located in the upper and lower poles, and the remaining 16 cases did not specify the tumour location. The tumours ranged in size from 1.2-15 cm. There was no significant difference in tumour location in the ventral or dorsal kidney, and whether or not they were exophytic. Most of the patients had no special clinical manifestations, and only seven cases (7/41, 17%) developed gross hematuria (6, 9, 10, 17, 23, 29), five patients (5/41, 12%) presented with lumbago or lumbar discomfort (6, 9, 12 23), one patient (1/41, 2%) presented with bilateral subcostal pain (21), and one patient presented with fever (21). Weight loss and other clinical manifestations occurred in only one case (17). Only one patient (1/41, 2%) died of RCC before the next follow-up; all the remaining patients were alive at the next follow-up (18). Only one patient (1/44, 2%) had VHLD (15), with no specific symptoms. CT mostly showed round, high-density, or low-density masses with a clear boundary, which showed heterogeneous enhancement. Forty patients (40/41, 98%) were diagnosed with renal malignant tumours before surgery. Of these 40 patients, two (2/40, 5%) underwent partial nephrectomy (7, 15), and the rest underwent nephrectomy. Only one patient (1/41, 2%) was diagnosed with hamartoma before surgery, and tumour enucleation was performed (16). No recurrence or metastasis was observed, and the prognosis in these cases was good. In the present case, the patient experienced only percussion pain in the right renal region, with no other symptoms. CT showed a mass of slightly low-density shadow in the lower pole parenchyma of the right kidney, and the uneven density was consistent with the characteristics of other RHB cases. The prognosis of our case was good.
Clinically, RHBs are rare and difficult to diagnose. Owing to its similar pathological features and immunophenotype to other renal tumours, such as RCC, rhabdomyosarcoma, oncocytoma, epithelioid angiomyolipoma, etc, it is easily misdiagnosed (7, 8). Nevertheless, the biological behavior, treatment, and prognosis of these diseases vary significantly and require accurate identification. We consider this disease as an example for analyzing the differential diagnosis of RHB.
In this case, the characteristics of tumour cells with abundant pale or eosinophilic cytoplasm and a large number of the capillary network were easily confused with RCC, but the characteristics of tumour cells without hyperchromasia, conspicuous nucleoli, mitotic figures, or intranuclear pseudoinclusions, and cavity lipid in the cytoplasm and nucleus eccentric displacement were not consistent with RCC, but may be useful clues for a correct diagnosis. Immunohistochemical analysis of the tumour cells can largely solve the problem of differential diagnosis, as analysis of RCC cells will show limited expression of a-inhibin, NSE, S-100, and keratin. In this case the positive expression of PAX-8, CD10, and CK, and low Ki-67 index was inconsistent with the high proliferation index in RCC (29). The immunohistochemical results ruled RCC out. The accidental positive expression of CD10 further confirmed that RHB could express renal markers. Therefore, attention should be paid to the unexpected positive expression of RHB nephrogenic markers in the differential diagnosis of RCC.
In addition, a primary diagnosis of epithelioid angiomyolipoma needs to be considered as a possibility. In this case, abundant capillary network, hyperplasia and dilation of thick-walled vessels, interstitial edema, hyperplasia of collagenous fibers, large polygonal cells with abundant cytoplasm, and fat vacuoles were observed. These features support a diagnosis of epithelioid angiomyolipoma; however, the absence of adipose tissue and the smooth muscle component of hyperplasia did not support this diagnosis. Immunohistochemical findings of epithelioid angiomyolipoma are usually positive for HMB45, and negative for NSE, a-inhibin, and S100, which was not observed in this case. Thus, we ruled out epithelioid angiomyolipoma.
Finally, we observed that part of the tumour cells had a nested pattern, the nucleus appeared as intranuclear pseudoinclusions, and some cells had inconspicuous nucleoli. A few cells had a rich capillary network and significant fibrosis, which could be mistaken for a fiber interval that is rich in blood vessels. These pathological changes supported the diagnosis of pheochromocytoma. However, this case lacked mitotic figures and clearly visible nucleoli, and immunohistochemical results were negative for CD56 and Syn; therefore, this diagnosis was ruled out. As mentioned earlier, after excluding the above pathological diagnosis, we re-analyzed the case according to the histological and immunohistochemical characteristics of the case, shifted the diagnosis direction to a relatively rare kidney disease, and finally arrived at a diagnosis of RHB. This also suggests the importance of preoperative renal biopsy to diagnose RHB in clinical practice, so as to avoid overtreatment.
Through the related literature review, we found that RHB mostly had no special clinical manifestations. CT examination showed inhomogeneously enhancing masses which suggest malignant tumours. In addition, RHB was rich in blood flow and easily cause hematoma after renal biopsy (30). The safety and diagnostic accuracy of renal biopsy need to be further improved (31). In clinical practice, preoperative biopsy is not used for the cases of malignant tumour clearly defined by imaging. Therefore, renal biopsy is rarely used for RHB. However, a large number of studies have shown that preoperative renal mass biopsy can avoid misdiagnosis and over-treatment of renal tumours to a great extent, and encourage the expansion of clinical application of renal mass biopsy (32–36). That said, current literature showed that important technical skills in renal biopsy are required to improve the diagnostic accuracy and reduce the risk for patients associated with this procedure (31, 37, 38). When diagnosing malignat renal tumours in the future, we should be aware of RHB and consider renal biopsy in order to avoid misclassification. In the postoperative pathological examination, the pathological manifestations of RHB are also easily misdiagnosed as other renal malignancies. Therefore, whenever pathological diagnosis is not clear, immunohistochemistry should be performed to assist the diagnosis and adjust treatment and follow-up.
RHB is a benign tumour with excellent prognosis and the correct identification of this pathological entity can prevent overtreatment. However, accurate diagnosis of RHB is extremely challenging. In this case, where the morphological features of RHB partially overlap with RCC, epithelioid angiomyolipoma, and pheochromocytoma, as seen in the present cases, the rarity of RHB may lead to misdiagnosis. However, combined immunohistochemistry is helpful in diagnosising renal tumours. Therefore, careful analysis of the gross, histological, and immunohistochemical results of the tumour can effectively lead to a correct diagnosis. Meanwhile, clinicians should be aware of RHB, and renal biopsy should be considered in combination with clinical and imaging findings to avoid misdiagnosis.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethical approval was given by the medical Ethics Committee of the First Affiliated Hospital to Shihezi University School of Medicine, and the patient has signed the informed consent form.The ethical approval number is 2018-011-01. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
JZ, MW and HL performed the experiments and analyzed the data; YQ designed and supervised the study. HJ, WW and LC provided crucial input for the project; JZ, NW and YQ wrote the manuscript. All authors read and approved the final version of the manuscript.
This work was supported by grants from the National Natural Science Foundation of China (grant no. 81860471); Zhanjiang science and technology development special fund Competitive Allocation Project −key projects of disease prevention and control (2021A05145); Provincial Science and technology special fund (“College items+ task list”) project-special topic of basic and applied research (2021A05236).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hussein M. Central nervous system capillary haemangioblastoma: the pathologist's viewpoint. Int J Exp Pathol (2007) 88(5):311–24. doi: 10.1111/j.1365-2613.2007.00535.x
2. Louis D, Ohgaki H, Wiestler O, Cavenee W, Burger P, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta neuropathologica (2007) 114(2):97–109. doi: 10.1007/s00401-007-0243-4
3. Louis D, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee W, et al. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta neuropathologica (2016) 131(6):803–20. doi: 10.1007/s00401-016-1545-1
4. Bisceglia M, Muscarella L, Galliani C, Zidar N, Ben-Dor D, Pasquinelli G, et al. Extraneuraxial hemangioblastoma: Clinicopathologic features and review of the literature. Adv ANAT Pathol (2018) 25(3):197–215. doi: 10.1097/pap.0000000000000176
5. Nonaka D, Rodriguez J, Rosai J. Extraneural hemangioblastoma: a report of 5 cases. Am J Surg Pathol (2007) 31(10):1545–51. doi: 10.1097/PAS.0b013e3180457bfc
6. Ip Y, Yuan J, Cheung H, Chan J. Sporadic hemangioblastoma of the kidney: an underrecognized pseudomalignant tumor? Am J Surg Pathol (2010) 34(11):1695–700. doi: 10.1097/PAS.0b013e3181f2d9b8
7. Verine J, Sandid W, Miquel C, Vignaud J, Mongiat-Artus P. Sporadic hemangioblastoma of the kidney: an underrecognized pseudomalignant tumor? Am J Surg Pathol (2011) 35(4):623–4. doi: 10.1097/PAS.0b013e31820f6d11
8. Wang C-C, Wang S-M, Liau J-Y. Sporadic hemangioblastoma of the kidney in a 29-Year-Old man. Int J Surg Pathol (2012) 20(5):519–22. doi: 10.1177/1066896911434548
9. Liu Y, Qiu X, Wang E. Sporadic hemangioblastoma of the kidney: a rare renal tumor. Diagn Pathol (2012) 7:49. doi: 10.1186/1746-1596-7-49
10. Muscarella L, Bisceglia M, Galliani C, Zidar N, Ben-Dor D, Pasquinelli G, et al. Extraneuraxial hemangioblastoma: A clinicopathologic study of 10 cases with molecular analysis of the VHL gene. Pathology Res Pract (2018) 214(8):1156–65. doi: 10.1016/j.prp.2018.05.007
11. Yin W, Li J, Chan J. Sporadic haemangioblastoma of the kidney with rhabdoid features and focal CD10 expression: report of a case and literature review. Diagn Pathol (2012) 7:39. doi: 10.1186/1746-1596-7-39
12. Zhao M, Williamson S, Yu J, Xia W, Li C, Zheng J, et al. PAX8 expression in sporadic hemangioblastoma of the kidney supports a primary renal cell lineage: implications for differential diagnosis. Hum Pathol (2013) 44(10):2247–55. doi: 10.1016/j.humpath.2013.05.007
13. Jiang J, Rao Q, Xia Q, Tu P, Lu Z, Shen Q, et al. Sporadic hemangioblastoma of the kidney with PAX2 and focal CD10 expression: report of a case. Int J Clin Exp Pathol (2013) 6(9):1953–6.
14. Wang Y, Wei C, Mou L, Zhang Q, Cui Z, Li X, et al. Sporadic renal haemangioblastoma: Case report and review of the literature. Oncol Lett (2013) 5(1):360–62. doi: 10.3892/ol.2012.942
15. Haitao L, Wen K, Yonghui C, Jing Z, Yiran H. Primary renal hemangioblastoma: report of 3 cases and review of literature. J Mod Urol (2015) 20(05):318–21. doi: 10.3969/j.issn.1009-8291.2015.05.008
16. Xiaofeng C, Fan Z, Qi S, Xiaozhi Z, Changwei J, Tieshi L, et al. Sporadic renal hemangioblastoma: report of two cases and review of literature. Chin J OF Surg (2014) 52(07):551–53.
17. Doyle LA, Fletcher CDM. Peripheral hemangioblastoma: Clinicopathologic characterization in a series of 22 cases. Am J Surg Pathol (2014) 38(1):119–27. doi: 10.1097/PAS.0b013e3182a266c1
18. Wu Y, Wang T, Zhang P, Yang X, Wang J, Wang C, et al. Extraneural hemangioblastoma of the kidney: the challenge for clinicopathological diagnosis. J Clin Pathol (2015) 68(12):1020–5. doi: 10.1136/jclinpath-2015-202900
19. Kuroda N, Agatsuma Y, Tamura M, Martinek P, Hes O, Michal M, et al. Sporadic renal hemangioblastoma with CA9, PAX2 and PAX8 expression: diagnostic pitfall in the differential diagnosis from clear cell renal cell carcinoma. Int J Clin Exp Pathol (2015) 8(2):2131–8.
20. YouZongHao, Qingwen, Sheng W. Renal hemangioblastoma: a case report and literature review. J Clin Urology(China) (2016) 31(04):336–39. doi: 10.13201/j.issn.1001-1420.2016.04.012
21. WeiHuiJie, Junwei R, Dingrong L, Dingrong L, Tong W, Feng L, et al. Clinical and pathological observation of sporadic renal hemangioblastoma. J Diag Pathol (2016) 23(03):169–71. doi: 10.3969/j.issn.1007-8096.2016.03.003
22. Xu Z, Xie M, Li X, Chen B, Lu L. Clinicopathological and genetic study of an atypical renal hemangioblastoma. Chin Med Sci J = Chung-kuo i hsueh k'o hsueh tsa chih (2017) 32(3):206–10. doi: 10.24920/j1001-9294.2017.028
23. Weida L, Fenghua Z. Renal hemangioblastoma: a case report. J Wenzhou Med Univ (2019) 49(09):690–91. doi: 10.3969/j.issn.2095-9400.2019.09.014
24. Zhenmin, Qingshui C. Diagnosis and treatment of adult primary renal hemangioblastoma and literature review. J Qiqihar Med Univ (2019) 40(01):132–33. doi: 10.3969/j.issn.1002-1256.2019.01.062
25. Jianqi C, Shuqiang F, Ranwei L, Weitao F, Ming Z. Primary renal hemangioblastoma: a case report and literature review. Chin J Lab Diag (2019) 23(08):1455–56. doi: 103969/jissn1007-4287.2019.08.057
26. Xu Y, Ma X, Ma Y, Li J, Zhang R, Li X, et al. Sporadic hemangioblastoma of the kidney: a clinicopathologic study of three cases and a literature review. J Int Med Res (2021) 49(7). doi: 10.1177/03000605211027774
27. Xueli Z, Rongzhen C, Liyun L, Zhiyong Z. Renal hemangioblastoma: a case report. J Diag Pathol (2021) 28(09):791–92. doi: 10.3969/j.issn.1007-8096.2021.09.025
28. Mingcong G, XuZhifeng, Yiling L. MSCT and PET-CT findings of primary renal hemangioblastoma: A case report. J Mod Med Healt (2021) 37(23):4131–34. doi: 10.3969/j.issn.1009-5519.2021.23.048
29. Wang X, Haines G, Mehrotra M, Houldsworth J, Si Q. Primary hemangioblastoma of the kidney with molecular analyses by next generation sequencing: a case report and review of the literature. Diagn Pathol (2022) 17(1):34. doi: 10.1186/s13000-022-01213-8
30. Weihao Z, Yani Z, Ke L, Tian Y, Lanmei J, Yujia H, et al. Risk factors for bleeding after ultrasound-guided percutaneous renal biopsy. J J Capital Med Univ (2022) 43(05):694–99. doi: 10.3969/j.issn.1006-7795.2022.05.005
31. Capretz T, Patel R, Okhunov Z. Percutaneous renal biopsy: approach, diagnostic accuracy and risks. J Curropin Urol (2018) 28(4):369–74. doi: 10.1097/mou.0000000000000505
32. Azawi N, Tolouee S, Madsen M, Berg K, Dahl C, Fode M, et al. Core needle biopsy clarify the histology of the small renal masses and may prevent overtreatment. Int Urol Nephrol (2018) 50(7):1205–09. doi: 10.1007/s11255-018-1885-y
33. Cotta B, Meagher M, Bradshaw A, Ryan S, Rivera-Sanfeliz G. Percutaneous renal mass biopsy: historical perspective, current status, and future considerations. JExpert Rev Anticancer Ther (2019) 19(4):301–08. doi: 10.1080/14737140.2019.1571915
34. Kutikov A, Smaldone M, Uzzo R, Haifler M, Bratslavsky G, Leibovich BC, et al. Renal mass biopsy: Always, sometimes, or never? J Eur Urol (2016) 70(3):403–6. doi: 10.1016/j.eururo.2016.04.001
35. Ogasawara N, Ueda K, Kurose H, Chikui K, Hayashi S, Nishihara K, et al. Clinical consideration of percutaneous renal biopsy for advanced renal tumor. J Nihon Hinyokika Gakkai Zasshi (2019) 110(2):75–9. doi: 10.5980/jpnjurol.110.75
36. Volpe A, Finelli A, Gill I, Jewett M, Martignoni G, Polascik T, et al. Rationale for percutaneous biopsy and histologic characterisation of renal tumours. J Eur Urol (2012) 62(3):491–504. doi: 10.1016/j.eururo.2012.05.009
37. Bada M, Rapisarda S, Cicero C, Di Mauro M, Sebben M, De Concilio B, et al. The role of renal biopsy to improve small renal mass diagnosis and management: are there predictive factors for a higher detection rate? The first Italian study of 100 cases J Minerva Urol Neprol (2021) 73(1):78–83. doi: 10.23736/s2724-6051.20.03519-5
Keywords: hemangioblastoma, kidney, immunohistochemistry, differential diagnosis, histopathology
Citation: Zhang J, Wang N, Chen L-H, Wang W-J, Wang M, Liu H, Jiang H-G and Qi Y (2022) Primary renal sporadic hemangioblastoma: A case report and literature review. Front. Urol. 2:1064099. doi: 10.3389/fruro.2022.1064099
Received: 07 October 2022; Accepted: 29 November 2022;
Published: 14 December 2022.
Edited by:
Carlo Andrea Bravi, Onze Lieve Vrouwziekenhuis Hospital, BelgiumReviewed by:
Eleonora Balestrazzi, Alma Mater Studiorum (Bologna University), ItalyCopyright © 2022 Zhang, Wang, Chen, Wang, Wang, Liu, Jiang and Qi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Qi, cWl5YW55YW4tMTk5OEAxNjMuY29t; Han-Guo Jiang, MTIzOTkzNjE4MkBxcS5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.