- 1Department of ICER, National Institutes of Health-National Institute of Allergy and Infectious Diseases-International Center for Excellence in Research, Chennai, India
- 2Department of Immunology, Indian Council of Medical Research (ICMR)-National Institute for Research in Tuberculosis, Chennai, India
- 3Infectious Diseases, Dignity Health, Chandler, AZ, United States
- 4Prof. M. Viswanathan Diabetes Research Center, Chennai, India
- 5Department of Bacteriology, ICMR-National Institute for Research in Tuberculosis, Chennai, India
- 6Department of Clinical Research, ICMR-National Institute for Research in Tuberculosis, Chennai, India
- 7ICMR-Regional Medical Research Centre, Port Blair, India
- 8Medicine, UMass Chan Medical School, Worcester, MA, United States
- 9Laboratory of Parasitic Diseases, National Institutes of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, United States
Introduction: Anemia has been shown to be an independent predictor of disease progression and death in tuberculosis (TB) patients, significantly impacting TB in several ways. This dual burden poses significant challenges for TB control efforts. However, the mechanism by which anemia influences disease severity, bacterial burden, and TB treatment outcomes remains poorly understood.
Methods: In this study, we aimed to compare bacterial burdens, disease severity, and TB treatment outcomes in TB patients with or without anemia. Participants were recruited from Chennai, South India, as part of the prospective Effect of Diabetes on Tuberculosis Severity (EDOTS) study conducted from February 2014 to August 2018. Anemia was defined as hemoglobin (Hb) levels <13 g/dL and <12 g/dL for males and females, respectively. We employed chest X-rays to assess bilateral lung and cavitary diseases and sputum smear grades to measure bacterial loads in TB subjects. Treatment outcomes were defined as favorable or unfavorable. Cytokine profile was measured using multiplex ELISA.
Results: The study comprised of 483 culture-confirmed TB individuals, with 288 positives for anemia {Median Hb was 11.0 [interquartile range (IQR)], 10.3–12.3} and 195 negatives [Median Hb was 14.3 (IQR), 13.5–15.2]. The study revealed that TB patients with anemia had significantly higher bacterial loads [adjusted prevalence ratio (aPR), 4.01; 95% CI, 2.22–6.63; p < 0.001], cavitary lung lesions [aPR, 3.36; 95% CI, 1.95–5.68; p < 0.001] and unfavorable treatment outcomes [aPR, 1.61; 95% CI, 1.31–2.19; p = 0.046] compared to those without anemia. Our data also show that TB is associated with significantly lower levels of type-1 cytokines (IFNγ and IL-2) but significantly higher levels of pro-inflammatory cytokines (IL-6, IFNα, and IFNβ) and pro-fibrotic factors (VEGF, EGF, FGF-2, and PDGF-AB/BB) in anemic individuals compared to those without anemia.
Conclusions: These findings highlight a clear association between anemia and increased TB severity, elevated bacterial loads, and poor treatment outcomes. Our data also suggest that anemia might be associated with the modulation of cytokine responses, which could impart a detrimental effect on TB pathogenesis.
Introduction
Tuberculosis (TB) remains a major global health threat, resulting in millions of new cases and fatalities annually (1). Anemia is a common risk factor and hematological abnormality associated with TB, with a prevalence of 20–94% in TB patients (2, 3). On the contrary, the likelihood of TB among anemic patients is higher than non-anemic patients (2–4). Anemia is defined as the insufficiency of erythrocyte mass to deliver adequate oxygen to peripheral tissues (5). TB is known to cause “anemia of inflammation” a condition in which systemic inflammation may change iron metabolism and lower red blood cell counts (6). The reduction of erythropoiesis by inflammatory indicators, malabsorption syndrome, and nutritional inadequacies are elucidated as the underlying pathophysiology of anemia in TB patients (7).
Anemia profoundly impacts the course and severity of TB in several ways and has been found to be an independent predictor of disease progression and fatality in TB patients (8, 9). TB patients with anemia have heavy sputum bacillary load and worsened pulmonary infection (10, 11). Studies indicate that anemia is associated with more severe forms of TB and unfavorable disease outcomes, including increased mortality rates and extended treatment periods (12–17). Anemia may increase the risk of complications like pulmonary dysfunction due to larger infectious zones in the lungs, further aggravating TB outcomes (18, 19). Additionally, anemia exacerbates the adverse impacts of TB medications, including gastrointestinal disorders and hepatotoxicity (20, 21).
Cytokines from the innate and adaptive immune systems play crucial roles in orchestrating the immune response to TB. Immune alterations favor the survival, multiplication, and dissemination of Mycobacterium tuberculosis (Mtb) and associated sequelae (22, 23). Cell-mediated Th1 immunity, coordinated by Interferon (IFN)-γ, is required to suppress Mtb inside macrophages at the infection site in the lung (24, 25). Th1 cytokines typically activate macrophages and cytolytic T cells to kill intracellular Mtb via the induction of reactive oxygen and nitrogen species, antimicrobial peptides, and autophagy (26). Conversely, Th2 cytokines, such as Interleukin (IL)-4 and IL-13, induce anti-inflammatory reactions that impede pathological inflammation while concurrently impeding macrophage and T cell capacity to efficiently eliminate Mtb (27). Pro-inflammatory cytokines such as IL-6 are multifunctional cytokines that play a crucial role in regulating the immune response, inflammation, and hematopoiesis and are key mediators of anemia of inflammation (24, 28). However, expression of these cytokines in immune responses to TB in anemic individuals have not been explored in detail, and clear data on their impact on bacterial burdens, disease severity, and treatment outcomes are lacking.
To address this knowledge gap, our study aimed to compare bacterial burdens, disease severity, and TB treatment outcomes in TB patients with or without anemia. Moreover, to explore the immunological underpinnings of the interaction between anemia and TB, we examined circulating plasma levels of a large panel of cytokines and pro-fibrotic factors in TB patients with or without anemia.
Materials and methods
Ethics statement
The study was approved by the ethics committees of the National Institute for Research in Tuberculosis (NIRT) and the Prof. M. Viswanathan Diabetes Research Center (MVDRC; ECR/51/INST/TN/2013/MVDRC/01).
Patient consent statement
Informed written consent was obtained from all participants, and study procedures adhered to institutional ethical guidelines.
Study population and data variables
Participants were recruited from Chennai, South India, as part of the prospective Effect of Diabetes on Tuberculosis Severity (EDOTS) study conducted from February 2014 to August 2018. Anemia was diagnosed based on WHO criteria (hemoglobin concentration < 12 g/dL in women and < 13 g/dL in men) (29). The study included adult individuals aged 25–73 who were newly diagnosed with positive sputum smears and culture. All the participants were screened for diabetes and nutritional indices. Smoking and alcohol consumption status were recorded. Exclusion criteria were previous TB episodes, prior TB treatment, drug-resistant TB, positive HIV status, use of immunosuppressive medications, pregnancy, and lactation. A complete blood count was done on all samples in a DxH 520 hematology analyzer (Beckman Coulter). Anthropometric measurements (height, and weight), and biochemical parameters were procured using standardized techniques. Low body mass index (LBMI) was described based on the American Heart Association/American College of Cardiology guidelines (LBMI ≤ 18.5 kg/m2), overweight by body mass index (BMI) 25–29.9 kg/m2, and obesity defined by BMI threshold of ≥30.0 kg/m2. Diabetes was defined as an glycated hemoglobin (HbA1c) reading of 6.5% or greater and a fasting blood glucose of ≥126 mg/dl, according to the American Diabetes Association criteria. A sample of the individuals with a result of total cholesterol (TC) < 130 mg/dl, triglyceride (TG) < 90 mg/dl, low-density lipoprotein cholesterol (LDL-C) < 100 mg/dl, and high-density lipoprotein cholesterol (HDL-C) < 40 mg/dl were considered as hypolipidemic while individuals with the result of TC ≥ 200, TG ≥ 150, LDL-C ≥ 130 mg/dl, or HDL-C > 40 mg/dl were classified as hyperlipidemic. Vitamin D deficiency was defined as < 30 ng/mL. High or low serum albumin were determined according to serum albumin level of ≥ or < 3.9 g/dl. Chest X-rays were utilized to assess the presence of bilateral lung disease and cavitary lesions and chest x-rays were read by two independent radiologists. Sputum smear grades were used to measure bacterial loads in individuals with TB and classified as 0, 1+, 2+, and 3+ with 0 being no bacteria in microscopy and 3+ the highest number of bacteria. The laboratory investigators were blinded to the chest x-ray and bacteriology results. All recruited TB patients received anti-TB treatment through Directly Observed Treatment Short Course (DOTS) therapy as per WHO recommendations, monitored by the National Tuberculosis Elimination Program (NTEP). Follow-up extended through 6 months of treatment and 1-year post-treatment completion. Treatment outcomes were defined as favorable or unfavorable. Favorable treatment outcome (cure) was defined as negative results of sputum cultures at months 5 and 6 of treatment without recurrent disease during follow-up. Unfavorable treatment outcomes included treatment failure defined as positive sputum culture results at month 5 or 6, all-cause mortality, or recurrent TB within 12 months after initial cure. These participants did not receive any treatment for anemia.
Multiplex assays
Circulating plasma cytokines and pro-fibrotic levels were measured in a subset of anemic (n = 288) and non-anemic (n = 195) TB individuals using multiplex Luminex assay (Bio-Rad Laboratories, Inc.). The analytes measured included cytokines [Interferon (IFN)-γ, Interleukin (IL)-2, Tumor Necrosis Factor (TNF-α), IL-4, IL-5, IL-6, IL-13, IFN-α, and IFN-β] and pro-fibrotic factors (Vascular endothelial growth factor (VEGF), Epidermal growth factor (EGF), Fibroblast growth factor (FGF-2), and Platelet-derived growth factor (PDGF)-AB/BB). The experiment was conducted according to the manufacturer's instructions (R&D Systems).
Statistical analysis
Before analysis, the data was thoroughly checked for completeness and consistency. Continuous variables were examined for normality using the Shapiro-Wilks test and were found not to be normal. The data was then presented using frequency, percentages, median and quartiles. Measurements of central tendency utilized geometric means (GMs). Differences in continuous variables between the two groups were examined using the Wilcoxon rank sum test, while the relationship between groups and factors such as sputum smear grade, bilateral lung lesion, cavitary lesion, and TB treatment failure and relapse were examined using the Pearson chi-square test. Statistically significant differences between two groups were analyzed using the non-parametric Mann-Whitney U-test with Holm's correction for multiple comparisons. Generalized linear models with binomial regression and log-link functions were used to identify key factors. The selection of covariates for the regression model was determined based on data availability, a review of relevant literature, and the opinions of subject matter experts. Prevalence ratios (PR) and adjusted prevalence ratios (aPR) were calculated along with the corresponding 95% confidence intervals (CIs). Covariates with significant PR, were considered when adjusting for aPR. Data analysis was performed using STATA software, version 15.0 (StataCorp., Texas, USA), with all P-values considered two-sided and statistical significance set at the 0.05 α level.
Results
Study population characteristics
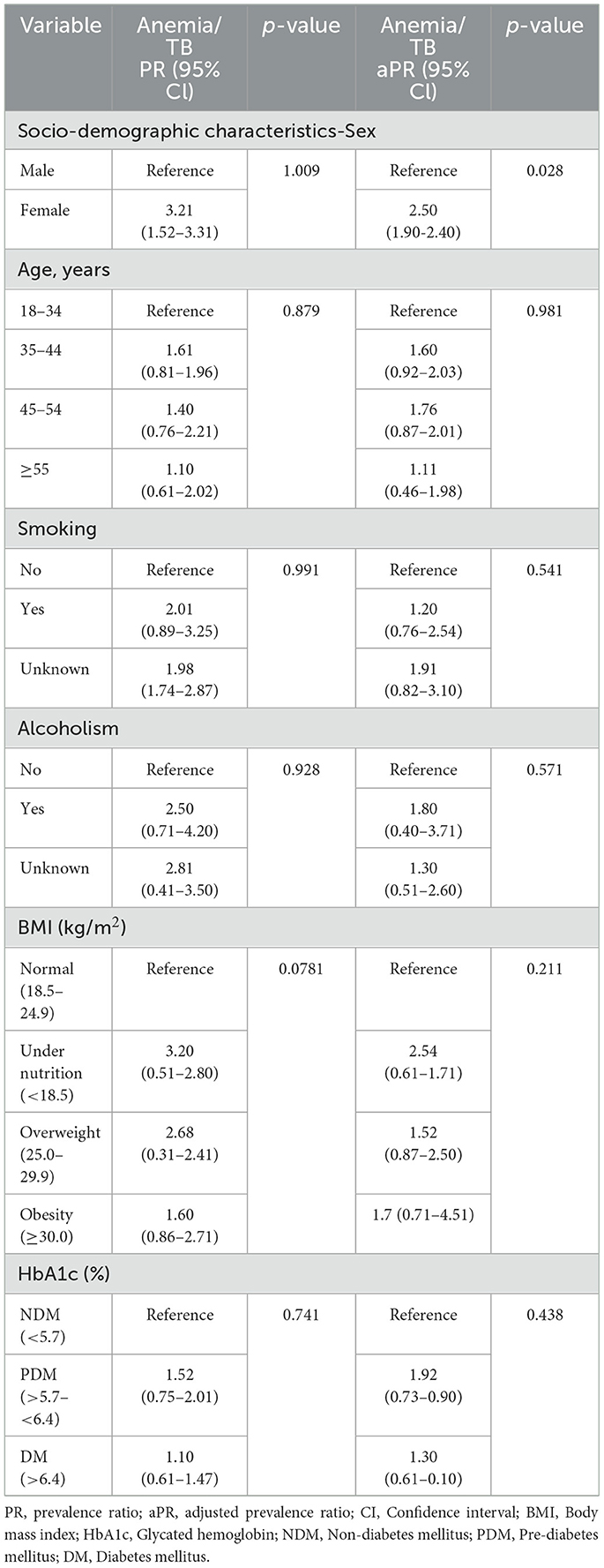
The study comprised 483 culture-confirmed TB individuals, with 288 positives (101 male, 187 female) for anemia and 195 negatives (90 male, 105 female). Median age was 45 years [interquartile range (IQR), 36.0–53.0] for participants with anemia and 47 years (IQR, 36.3–52.0) for participants without anemia. There were no statistically significant differences in age, BMI, smoking, alcohol use, and HbA1c between the TB subjects with anemia and those without anemia. However, significant differences were observed in gender (p = 0.0175; Table 1) and notable differences in certain hematological and biochemical parameters (Table 2). Individuals with anemia exhibited significantly lower levels of red blood cells (RBC; GM of 4.4 g/dL vs. 5.1 mg/dL; p < 0.0001), hemoglobin (Hb; GM of 11 vs. 14.3 g/dL; p < 0.0001), and hematocrit (HCT; GM of 34.9 vs. 42.4%; p < 0.0001), and elevated monocyte counts (GM of 708.2 vs. 620.1 cells/μL; p = 0.0404) compared to subjects without anemia. Additionally, biochemical parameters such as triglycerides (GM of 97.1 vs. 104.1 mg/dL; p = 0.0310), total cholesterol (GM of 157.3 vs. 168.2 mg/dL; p = 0.0083), LDL (GM of 89.6 vs. 95.4 mg/dL; p < 0.0001), total protein (GM of 7.9 vs. 8.2 g/dL; p = 0.0048), serum albumin (GM of 3.8 vs. 4.2 g/dL; p < 0.0001), and Vitamin D (GM of 15.4 vs. 17.4 IU; p = 0.0269) were significantly lower in individuals with anemia compared to subjects without anemia.
Association of clinical co-morbidities with anemia in TB individuals
No significant differences were observed in age, BMI, smoking, alcoholism, or HbA1c) between the two groups (Table 3). However, significant differences were noted in gender (female). The PR for female individuals with anemia was 3.21 (95% CI: 1.52–3.31; p = 0.009), and this association remained significant after adjusting for possible confounders (aPR 2.50, 95% CI: 1.90–2.40; p = 0.028).
Anemia is associated with increased radiographic TB disease severity and greater bacterial burdens
Anemia was significantly associated with an increased risk of cavitary disease (PR, 4.62; 95% CI, 3.04–7.08; p < 0.001) but not of bilateral lung lesions (PR, 2.21; 95% CI, 0.98–3.12; p = 0.287). After adjusting for confounding variables, anemia remained significantly associated with a higher risk of cavitation (aPR, 3.36; 95% CI, 1.95–5.68; p < 0.001), indicating increased TB disease severity in individuals with anemia. Additionally, anemia was significantly associated with an elevated risk of higher smear grades (PR, 5.51; 95% CI, 3.45–9.34; p < 0.001). This association persisted after adjusting for confounders, with anemia remaining significantly associated with increased smear grades (aPR, 4.01; 95% CI, 2.22–6.63; p < 0.001), indicating higher bacterial burdens in TB patients with anemia (Table 4).
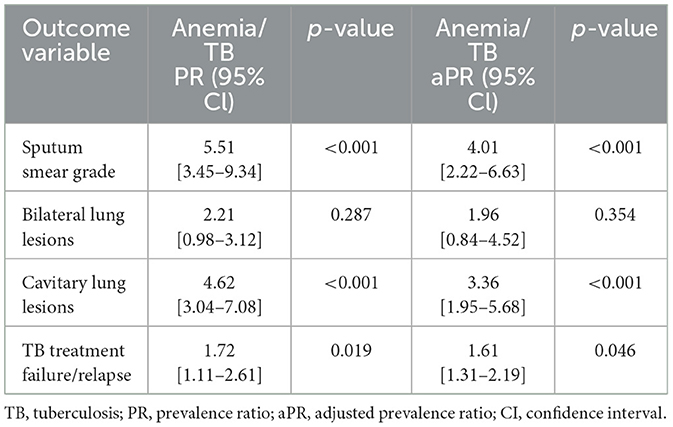
Table 4. Association of anemia with bacterial burden, disease severity, and treatment failure/relapse in TB.
Anemia is associated with increased risk of unfavorable TB treatment outcomes
Anemia was significantly associated with an increased risk of unfavorable treatment outcomes (PR, 1.72; 95% CI, 1.11–2.61; p = 0.019). This association persisted even after adjusting for confounding variables, with anemia remaining significantly associated with unfavorable treatment outcomes (aPR, 1.61; 95% CI, 1.31–2.19; p = 0.046). These findings indicate a heightened risk of treatment failure or TB recurrence in TB patients with anemia (Table 4).
Anemia is associated with altered levels of cytokines and pro-fibrotic factors in TB
The circulating levels of TNF-α, IL-4, IL-5, and IL-13 did not significantly differ between the two groups. However, pro-inflammatory cytokines [IFN-α (GM of 15.18 vs. 13.91 pg/ml, p < 0.0001), IFN-β (GM of 6.69 vs. 6.53 pg/ml, p < 0.0001), IL-6 (GM of 146.53 vs. 133.69 pg/ml, p = 0.0032)], and pro-fibrotic factors [VEGF (GM of 217 vs. 154.71 pg/ml, p < 0.0001), EGF (GM of 384.22 vs. 317.56 pg/ml, p < 0.0001), FGF-2 (GM of 2,584.82 vs. 2,000.96 pg/ml, p = 0.0011), and PDGF-AB/BB (GM of 1,758.71 vs. 1,601.31 pg/ml, p = 0.0093)] were significantly elevated in TB individuals with anemia compared to those without anemia. Conversely, the circulating plasma levels of type 1 cytokines [IFN-γ (GM of 275.56 vs. 313.81 pg/ml, p < 0.0001), IL-2 (GM of 104.75 vs. 121.96 pg/ml, p = 0.0006)] were significantly diminished in TB individuals with anemia compared to those without anemia (Figures 1, 2). Thus, anemia is associated with heightened levels of pro-inflammatory cytokines and pro-fibrotic factors and diminished levels of type 1 cytokines in TB individuals.
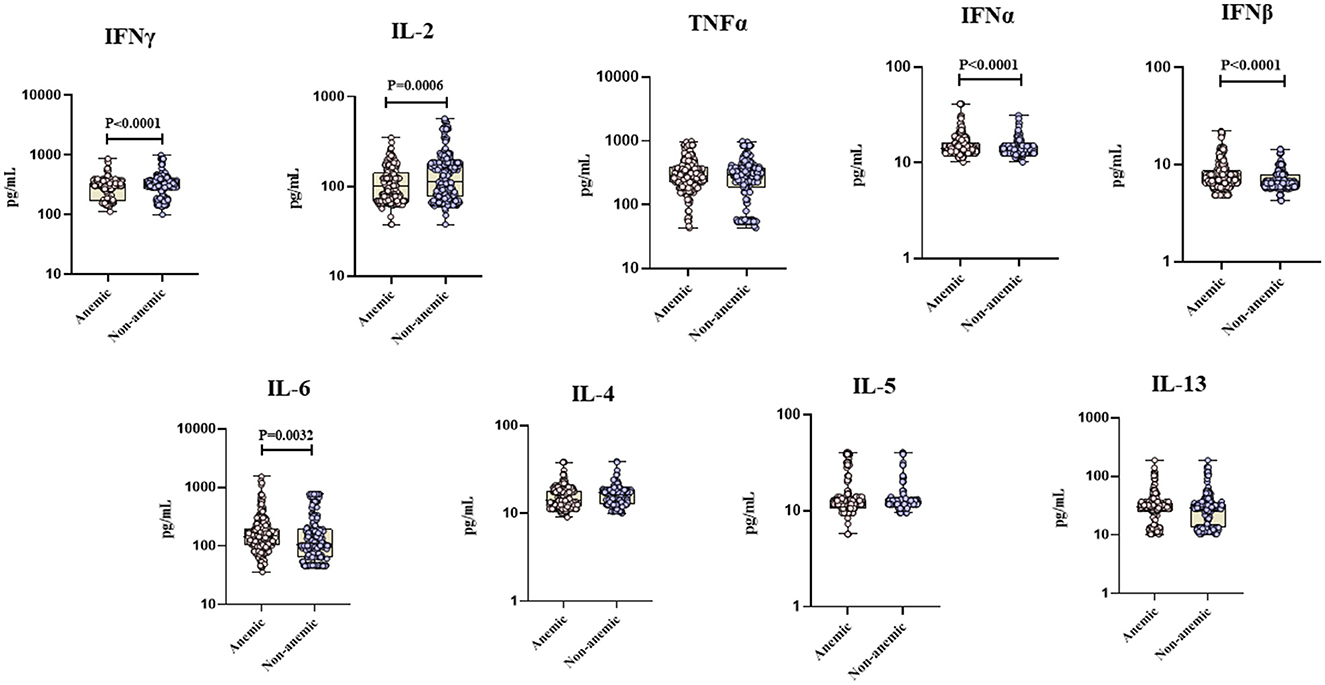
Figure 1. Anemia is associated with altered levels of cytokines in TB individuals. The figure illustrates the cytokine profile in anemic and non-anemic TB individuals. Circulating plasma cytokines including Interferon gamma (IFN γ), Interleukin-2 (IL-2), Tumor necrosis factor alpha (TNF-α), Interferon alpha (IFN α), Interferon beta (IFN β), Interleukin-6 (IL-6), Interleukin-4 (IL-4), Interleukin-5 (IL-5), and Interleukin-13 (IL-13) were measured. Each data point represents an individual subject, with the bar indicating the geometric mean (GM) cytokine level. Statistical analysis was performed using the Mann–Whitney U-test.
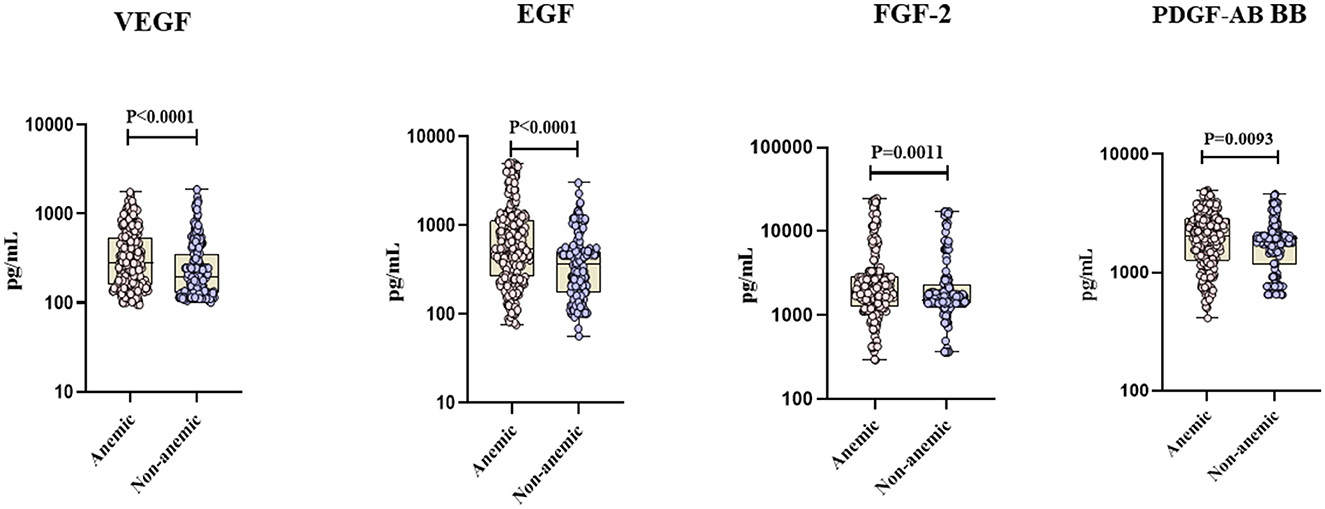
Figure 2. Anemia is associated with heightened levels of pro-fibrotic factors in TB individuals. The figure illustrates the pro-fibrotic factors profile in anemic and non-anemic TB individuals. Circulating plasma pro-fibrotic factors including Vascular endothelial growth factor (VEGF), Epidermal growth factor (EGF), Fibroblast growth factor 2 (FGF-2), and Platelet-derived growth factor (PDGF-AB BB) were measured. Each data point represents an individual subject, with the bar indicating the geometric mean (GM) cytokine level. Statistical analysis was performed using the Mann–Whitney U-test.
Discussion
To enhance TB management, targeted interventions must investigate the risk factors associated with disease progression and poor treatment outcomes (30). Anemia is a prominent comorbidity with TB (19). However, existing literature on the relationship between anemia and disease severity is scarce and inconsistent. While some studies suggest that anemia does not significantly predict TB risk (31–33), others identify it as a potential risk factor (34–36). This discrepancy underscores the necessity for robust, well-designed studies with larger sample sizes and standardized methodologies.
Consistent with prior research, our study revealed a substantial burden of anemia among TB patients in our cohort (37, 38). Notably, we observed higher rates of anemia among female TB patients compared to males, likely attributed to physiological differences, dietary habits, and variation in health-seeking behavior between genders (39). In contrast to non-anemic subjects, individuals with anemia exhibited a marked increase in monocyte levels and significant decreases in Hb, HCT, and RBC. Recent studies have linked elevated monocytes to poor prognosis and delayed pulmonary cavity closure in TB patients with anemia (10). Experimental evidence suggests that reduced Hb levels in anemic TB patients may result from the severity of TB infection and inflammation, impacting erythropoiesis and exacerbated by iron deficiency (40, 41). Hence, individuals with TB-related anemia may have a longer time for the proliferation and accumulation of Mtb, exposing them to inflammation for a longer time (12). The decreased production of RBC might result in reduced oxygen-carrying capacity and tissue hypoxia, which may have an impact on cytokine levels, leukocyte function, bone marrow function, and tissue destruction in TB (42, 43). Furthermore, our findings revealed that subjects with anemia had significantly lower levels of vitamin D and serum albumin compared to non-anemic subjects. Low serum albumin levels serve as a predictor of anemia and indicate the severity of inflammation (44, 45). The biological plausibility of lower vitamin D in anemia is supported by evidence suggesting that vitamin D regulates hepcidin production, thereby controlling iron homeostasis and erythropoiesis (46, 47).
Biomarkers for TB unfavorable treatment outcomes can play a major role in identifying novel TB intervention strategies (48–54). Cytokines are critical in the host defense against mycobacterial infections, serving as markers of disease severity and bacterial burden in active TB (55–57). Research shows that LBMI significantly impacts both acquired and innate host defense mechanisms, increasing susceptibility to TB (58–62). Our findings add to this knowledge by demonstrating that TB with coexistent anemia is associated with reduced levels of type 1 cytokines and increased pro-inflammatory and pro-fibrotic factors, potentially heightening TB risk. Our data indicate that TB patients with anemia have lower circulating levels of type 1 cytokines (IFNγ and IL-2), suggesting impaired protective immunity (63, 64). The reduced production of these cytokines in anemic individuals suggests a higher risk for severe TB due to weakened cell-mediated immunity, aligning with studies reporting lower type 1 cytokine levels in individuals with LBMI and TB compared to those with normal or high BMI (58, 62).
Loss of immune control in TB often results from excessive pro-inflammatory cytokine production, leading to neutrophil infiltration and pathological inflammation. This promotes granuloma remodeling and lung tissue destruction (65). We found significantly elevated pro-inflammatory cytokines (IL-6, IFNα, and IFNβ) in TB patients with anemia compared to non-anemic individuals. This aligns with previous research linking high IL-6 levels to inflammation-related anemia, which inhibits iron absorption and exacerbates TB progression (66, 67). Additionally, high IL-6 concentrations are associated with anemia in TB/HIV co-infected patients (68). Pro-fibrotic factors are crucial in bacterial infection processes. Our study showed increased levels of pro-fibrotic factors (VEGF, EGF, FGF-2, and PDGF-AB BB) in anemic individuals compared to non-anemic individuals. VEGF, associated with pleural inflammation and fibrosis in TB patients, has been found at elevated levels in smear-positive and culture-positive TB subjects (69). Systemic VEGF levels also rise significantly in TB patients with cavitations and bilateral disease involvement (70).
In this study, rigorous control was exercised over several factors known to influence disease severity and bacterial burdens, such as age, BMI, diabetes, smoking status, and alcohol use. The findings of this study provide valuable insights into the association between anemia and TB disease severity. Notably, our study revealed several key findings that warrant further exploration. We observed that TB patients with coexistent anemia exhibit more severe disease manifestations, including lung cavitation, indicative of advanced TB disease. These findings align with previous research suggesting that such lesions negatively impact patients and may lead to poor treatment outcomes, relapses, and drug resistance (71). Our results revealed a strong correlation between anemia and elevated bacterial burdens in TB patients, a key indicator of transmission (10). Our data further confirm that TB individuals with anemia were at a significantly higher risk of experiencing unfavorable treatment outcomes, including treatment failure or TB recurrence. This finding aligns with previous research indicating that anemic patients with TB-HIV co-infection exhibit poor treatment outcomes and a heightened degree of inflammatory perturbation (72).
Our study suffers from the limitation of not measuring red cell indices (MCV, MCH, and MCHC) or biochemical measures (iron, ferritin, hepcidin, and transferrin) to assess the type of anemia. Another limitation of our study is that cytokine levels exhibit a great degree of overlap between groups and that there is variability in the responses of different individuals in the same group. It is theoretically possible that other factors not examined in this study could have contributed to the differential responses. Nevertheless, our study offers novel insights into the immunological underpinnings of the anemia-TB comorbidity.
Conclusion
Our study reveals intricate interactions between anemia and disease severity, bacterial burdens, and treatment outcomes in TB patients. Importantly, our data highlights the significant association of anemia with the cytokine milieu in TB, suggesting a plausible biological mechanism for the increased disease severity observed in TB individuals with coexistent anemia. Our findings highlight the critical need for further research and interventions aimed at addressing the complex interplay between anemia and TB to optimize patient outcomes and advance TB control efforts.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by National Institute for Research in Tuberculosis (NIRT) and Prof. M. Viswanathan Diabetes Research Center (MVDRC). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
BD: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. SM: Data curation, Formal analysis, Validation, Writing – review & editing. NP: Data curation, Formal analysis, Investigation, Project administration, Supervision, Validation, Writing – review & editing. KM: Data curation, Investigation, Methodology, Writing – review & editing. AP: Data curation, Investigation, Methodology, Writing – review & editing. SN: Resources, Validation, Writing – review & editing. VV: Conceptualization, Investigation, Project administration, Resources, Supervision, Writing – review & editing. SS: Conceptualization, Investigation, Project administration, Resources, Supervision, Writing – review & editing. SH: Resources, Supervision, Writing – review & editing, Conceptualization, Investigation, Project administration. KT: Data curation, Formal analysis, Writing – review & editing. HK: Conceptualization, Investigation, Project administration, Resources, Supervision, Writing – review & editing. SB: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by federal funds (in whole or in part) from the Government of India's (GOI) Department of Biotechnology (DBT), the Indian Council of Medical Research (ICMR), the United States National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID), Office of AIDS Research (OAR), and distributed in part by CRDF Global [grant USB1-31149-XX-13]. This work was also funded by CRDF Global RePORT India Consortium Supplemental Funding [grant OISE-17-62911-1]. This work was supported in part by DIR, NIAID, and NIH.
Acknowledgments
We thank and acknowledge the help rendered by Shruthi BS, Mothi Shankar, Srinivasan, and other members of EDOTS Team. We also thank the Greater Chennai Corporation, Senthil Kumar (City Health Officer), and Lavanya J. (Revised National Tuberculosis Elimination Program Officer) for providing permission to conduct the study in the Tuberculosis Units of North Chennai. We thank the Department of Clinical Research, NIRT for assistance with radiology and the Department of Bacteriology, NIRT for bacterial smears and cultures. We thank the staff of MVDRC for recruiting participants and samples collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. WHO. Global Tuberculosis Report (2024). Available at: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2024 (accessed October 10, 2024).
2. Gelaw Y, Getaneh Z, Melku M. Anemia as a risk factor for tuberculosis: a systematic review and meta-analysis. Environ Health Prev Med. (2021) 26:13. doi: 10.1186/s12199-020-00931-z
3. Barzegari S, Afshari M, Movahednia M, Moosazadeh M. Prevalence of anemia among patients with tuberculosis: a systematic review and meta-analysis. Indian J Tuberc. (2019) 66:299–307. doi: 10.1016/j.ijtb.2019.04.002
4. Oliveira MG, Delogo KN, Oliveira HM, Ruffino-Netto A, Kritski AL, Oliveira MM. Anemia in hospitalized patients with pulmonary tuberculosis. J Bras Pneumo. (2014) 40:403–10. doi: 10.1590/S1806-37132014000400008
5. Harmening D. Clinical Hematology and Fundamentals of Hemostasis. Philadelphia, PA: FA Davis Co. (2009).
6. Nagu TJ, Spiegelman D, Hertzmark E, Aboud S, Makani J, Matee MI, et al. Anemia at the initiation of tuberculosis therapy is associated with delayed sputum conversion among pulmonary tuberculosis patients in Dar-es-Salaam, Tanzania. PLoS ONE. (2014) 9:e91229. doi: 10.1371/journal.pone.0091229
7. Hella J, Cercamondi CI, Mhimbira F, Sasamalo M, Stoffel N, Zwahlen M, et al. Anemia in tuberculosis cases and household controls from Tanzania: contribution of disease, coinfections, and the role of hepcidin. PLoS ONE. (2018) 13:e0195985. doi: 10.1371/journal.pone.0195985
8. de Mendonça EB, Schmaltz CA, Sant'Anna FM, Vizzoni AG, Mendes-de-Almeida DP, et al. Anemia in tuberculosis cases: a biomarker of severity? PLoS ONE. (2021) 16:e0245458. doi: 10.1371/journal.pone.0245458
9. O'Brien ME, Kupka R, Msamanga GI, Saathoff E, Hunter DJ, Fawzi WW. Anemia is an independent predictor of mortality and immunologic progression of disease among women with HIV in Tanzania. J Acquir Immune Defic Syndr. (2005) 40:219–25. doi: 10.1097/01.qai.0000166374.16222.a2
10. Luo M, Liu M, Wu X, Wu Y, Yang H, Qin L, et al. Impact of anemia on prognosis in tuberculosis patients. Ann Transl Med. (2022) 10:329. doi: 10.21037/atm-22-679
11. Baluku JB, Mayinja E, Mugabe P, Ntabadde K, Olum R, Bongomin F, et al. Prevalence of anaemia and associated factors among people with pulmonary tuberculosis in Uganda. Epidemiol Infect. (2022) 150:e29. doi: 10.1017/S0950268822000103
12. Gil-Santana L, Cruz LAB, Arriaga MB, Miranda PFC, Fukutani KF, Silveira-Mattos PS, et al. Tuberculosis-associated anemia is linked to a distinct inflammatory profile that persists after initiation of antitubercular therapy. Sci Rep. (2019) 9:1381. doi: 10.1038/s41598-018-37860-5
13. Sahiratmadja E, Wieringa FT, van Crevel R, de Visser AW, Adnan I, Alisjahbana B, et al. Iron deficiency and NRAMP1 polymorphisms (INT4, D543N and 3′UTR) do not contribute to severity of anaemia in tuberculosis in the Indonesian population. Br J Nutr. (2007) 98:684–90. doi: 10.1017/S0007114507742691
14. Isanaka S, Mugusi F, Urassa W, Willett WC, Bosch RJ, Villamor E, et al. Iron deficiency and anemia predict mortality in patients with tuberculosis. J Nutr. (2012) 142:350–7. doi: 10.3945/jn.111.144287
15. Araújo-Pereira M, Schutz C, Barreto-Duarte B, Barr D, Villalva-Serra K, Vinhaes CL, et al. Interplay between systemic inflammation, anemia, and mycobacterial dissemination and its impact on mortality in TB-associated HIV: a prospective cohort study. Front Immunol. (2023) 14:1177432. doi: 10.3389/fimmu.2023.1177432
16. Araújo-Pereira M, Nogueira BMF, Spener-Gomes R, Carvalho ACC, Sant'Anna FM, Figueiredo MC, et al. RePORT-Brazil Consortium Anemia and anti-tuberculosis treatment outcome in persons with pulmonary tuberculosis: a multi-center prospective cohort study. J Infect Public Health. (2023) 16:974–80. doi: 10.1016/j.jiph.2023.04.009
17. Araújo-Pereira M, Barreto-Duarte B, Arriaga MB, Musselwhite LW, Vinhaes CL, Belaunzaran-Zamudio PF, et al. Relationship between anemia and systemic inflammation in people living with HIV and tuberculosis: a sub-analysis of the CADIRIS clinical trial. Front Immunol. (2022) 13:916216. doi: 10.3389/fimmu.2022.916216
18. Ashenafi S, Bekele A, Aseffa G, Amogne W, Kassa E, Aderaye G, et al. anemia is a strong predictor of wasting, disease severity, and progression, in clinical tuberculosis (TB). Nutrients. (2022) 14:3318. doi: 10.3390/nu14163318
19. Dasaradhan T, Koneti J, Kalluru R, Gadde S, Cherukuri SP, Chikatimalla R. Tuberculosis-associated anemia: a narrative review. Cureus. (2022) 14:e27746. doi: 10.7759/cureus.27746
20. Kassa E, Enawgaw B, Gelaw A, Gelaw B. Effect of anti-tuberculosis drugs on hematological profiles of tuberculosis patients attending at University of Gondar Hospital, Northwest Ethiopia. BMC Hematol. (2016) 16:1. doi: 10.1186/s12878-015-0037-1
21. Agarwal AK, Chugh IM, Panjabi C, Dewan S, Shah A. Asymptomatic aplastic anaemia in a patient receiving anti-tuberculosis treatment. Indian J Tuberc. (2001) 48:97–100.
22. Gong JH, Zhang M, Modlin RL, Linsley PS, Iyer D, Lin Y, et al. Interleukin-10 downregulates Mycobacterium tuberculosis-induced Th1 responses and CTLA-4 expression. Infect Immun. (1996) 64:913–8. doi: 10.1128/iai.64.3.913-918.1996
23. Pessanha AP, Martins RA, Mattos-Guaraldi AL, Vianna A, Moreira LO. Arginase-I Expression in Granulomas of Tuberculosis Patients. Hoboken, NJ: Blackwell Publishing Ltd. (2012).
24. O'Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP. The immune response in tuberculosis. Annu Rev Immunol. (2013) 31:475–527. doi: 10.1146/annurev-immunol-032712-095939
25. Saunders BM, Britton WJ. Life and death in the granuloma: immunopathology of tuberculosis. Immunol Cell Biol. (2007) 85:103–11. doi: 10.1038/sj.icb.7100027
26. Arranz-Trullen J, Lu L, Pulido D, Bhakta S, Boix E. Host antimicrobial peptides: the promise of new treatment strategies against tuberculosis. Front Immunol. (2017) 8:1499. doi: 10.3389/fimmu.2017.01499
27. Brighenti S, Joosten SA. Friends and foes of tuberculosis: modulation of protective immunity. J Intern Med. (2018) 10:125–44. doi: 10.1111/joim.12778
28. Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol. (2009) 27:393–422. doi: 10.1146/annurev.immunol.021908.132703
29. Izaks G, Westendorp R, Knook D. The definition of anemia in older persons. J Am Med Assoc. (1999) 281:1714–7. doi: 10.1001/jama.281.18.1714
30. Narasimhan P, Wood J, Macintyre CR, Mathai D. Risk factors for tuberculosis. Pulm Med. (2013) 2013:828939. doi: 10.1155/2013/828939
31. Kerkhoff AD, Wood R, Cobelens FG, Gupta-Wright A, Bekker LG, Lawn SD. The predictive value of current haemoglobin levels for incident tuberculosis and/or mortality during long-term antiretroviral therapy in South Africa: a cohort study. BMC Med. (2015) 13:1–13. doi: 10.1186/s12916-015-0320-9
32. Iroezindu M, Ofondu E, Mbata G, van Wyk B, Hausler HP, Dh A, et al. Factors associated with prevalent tuberculosis among patients receiving highly active antiretroviral therapy in a Nigerian tertiary hospital. Ann Med Health Sci Res. (2016) 6:120–8. doi: 10.4103/2141-9248.181837
33. Li N, Manji KP, Spiegelman D, Muya A, Mwiru RS, Liu E, et al. Incident tuberculosis and risk factors among HIV-infected children in Tanzania. AIDS. (2013) 27:1273–81. doi: 10.1097/QAD.0b013e32835ecb24
34. Liu E, Makubi A, Drain P, Spiegelman D, Sando D, Li N, et al. Tuberculosis incidence rate and risk factors among HIV-infected adults with access to antiretroviral therapy. AIDS. (2015) 29:1391–9. doi: 10.1097/QAD.0000000000000705
35. Alemu YM, Andargie G, Gebeye E. High incidence of tuberculosis in the absence of isoniazid and cotrimoxazole preventive therapy in children living with HIV in Northern Ethiopia: a retrospective follow-up study. PLoS ONE. (2016) 11:e0152941. doi: 10.1371/journal.pone.0152941
36. Phyo K, Oo M, Harries A, Saw S, Aung TK, Moe J, et al. High prevalence and incidence of tuberculosis in people living with the HIV in Mandalay, Myanmar, 2011-2017. Int J Tuberc Lung Dis. (2019) 23:349–57. doi: 10.5588/ijtld.18.0436
37. Karyadi E, Schultink W, Nelwan RH, Gross R, Amin Z, Dolmans WM, et al. Poor micronutrient status of active pulmonary tuberculosis patients in Indonesia. J Nutr. (2000) 130:2953–8. doi: 10.1093/jn/130.12.2953
38. Lee SW, Kang YA, Yoon YS, Um SW, Lee SM, Yoo CG, et al. The prevalence and evolution of anemia associated with tuberculosis. J Korean Med Sci. (2006) 21:1028–32. doi: 10.3346/jkms.2006.21.6.1028
39. Bentley ME, Griffiths PL. The burden of anemia among women in India. Eur J Clin Nutr. (2003) 57:52–60. doi: 10.1038/sj.ejcn.1601504
40. Adzani M, Dalimoenthe NZ, Wijaya I. Profile of anaemia on lung tuberculosis at Dr. Hasan Sadikin General Hospital and Community Lung Health Center. Bandung Althea Med J. (2016) 3:137–40. doi: 10.15850/amj.v3n1.473
41. Kumar NP, Banurekha VV, Nair D, Dolla C, Kumaran P, Babu S. Modulation of iron status biomarkers in tuberculosis-diabetes co-morbidity. Tuberculosis. (2018) 108:127–35. doi: 10.1016/j.tube.2017.11.011
42. Nagaraju N, Varma A, Taksande A, Meshram RJ. Bone marrow changes in septic shock: a comprehensive review. Cureus. (2023) 15:e42517. doi: 10.7759/cureus.42517
43. Ong CWM, Fox K, Ettorre A, Elkington PT, Friedland JS. Hypoxia increases neutrophil-driven matrix destruction after exposure to Mycobacterium tuberculosis. Sci Rep. (2018) 8:11475. doi: 10.1038/s41598-018-29659-1
44. Heidari B, Taheri H, Hajian-Tilaki K, Yolmeh M, Akbari R. Low baseline serum albumin as a predictor of anemia in chronic hemodialysis patients. Caspian J Intern Med. (2015) 6:161–4.
45. de Mutsert R, Grootendorst DC, Indemans F, Boeschoten EW, Krediet RT, Dekker FW, et al. Association between serum albumin and mortality in dialysis patients is partly explained by inflammation, and not by malnutrition. J Ren Nutr. (2009) 19:127–35. doi: 10.1053/j.jrn.2008.08.003
46. Nur-Eke R, Özen M. The relationship between vitamin D levels and iron deficiency and anemia in adults applied for periodic medical examination. Clin Lab. (2020) 66:190918. doi: 10.7754/Clin.Lab.2019.190918
47. Smith EM, Tangpricha V. Vitamin D and anemia: insights into an emerging association. Curr Opin Endocrinol Diabetes Obes. (2015) 22:432–8. doi: 10.1097/MED.0000000000000199
48. Moideen K, Nathella PK, Madabushi S, Renji RM, Srinivasan P, Ahamed SF, et al. Plasma Vitamin D levels in correlation with circulatory proteins could be a potential biomarker tool for pulmonary tuberculosis and treatment monitoring. Cytokine. (2023) 168:156238. doi: 10.1016/j.cyto.2023.156238
49. Kumar NP, Nancy A, Viswanathan V, Sivakumar S, Thiruvengadam K, Ahamed SF, et al. Chitinase and indoleamine 2, 3-dioxygenase are prognostic biomarkers for unfavorable treatment outcomes in pulmonary tuberculosis. Front Immunol. (2023) 14:1093640. doi: 10.3389/fimmu.2023.1093640
50. Kumar NP, Moideen K, Viswanathan V, Sivakumar S, Ahamed SF, Ponnuraja C, et al. Heightened microbial translocation is a prognostic biomarker of recurrent tuberculosis. Clin Infect Dis. (2022) 75:1820–6. doi: 10.1093/cid/ciac236
51. Kumar NP, Moideen K, Nancy A, Viswanathan V, Thiruvengadam K, Sivakumar S, et al. Acute phase proteins are baseline predictors of tuberculosis treatment failure. Front Immunol. (2021) 12:731878. doi: 10.3389/fimmu.2021.731878
52. Gupte AN, Kumar P, Araújo-Pereira M, Kulkarni V, Paradkar M, Pradhan N, et al. Baseline IL-6 is a biomarker for unfavourable tuberculosis treatment outcomes: a multisite discovery and validation study. Eur Respir J. (2022) 59:2100905. doi: 10.1183/13993003.00905-2021
53. Kumar NP, Moideen K, Nancy A, Viswanathan V, Thiruvengadam K, Sivakumar S, et al. Association of plasma matrix metalloproteinase and tissue inhibitors of matrix metalloproteinase levels with adverse treatment outcomes among patients with pulmonary tuberculosis. J Am Med Assoc Netw Open. (2020) 3:e2027754. doi: 10.1001/jamanetworkopen.2020.27754
54. Kumar NP, Moideen K, Nancy A, Viswanathan V, Thiruvengadam K, Nair D, et al. Plasma chemokines are baseline predictors of unfavorable treatment outcomes in pulmonary tuberculosis. Clin Infect Dis. (2021) 73:e3419–27. doi: 10.1093/cid/ciaa1104
55. Cooper AM, Khader SA. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunol Rev. (2008) 226:191–204. doi: 10.1111/j.1600-065X.2008.00702.x
56. Kumar NP, Moideen K, Banurekha VV, Nair D, Babu S. Plasma proinflammatory cytokines are markers of disease severity and bacterial burden in pulmonary tuberculosis. Open Forum Infect Dis. (2019) 6:ofz257. doi: 10.1093/ofid/ofz257
57. Kumar NP, Moideen K, Nancy A, Viswanathan V, Shruthi BS, Sivakumar S, et al. Plasma chemokines are biomarkers of disease severity, higher bacterial burden and delayed sputum culture conversion in pulmonary tuberculosis. Sci Rep. (2019) 9:18217. doi: 10.1038/s41598-019-54803-w
58. Anuradha R, Munisankar S, Bhootra Y, Kumar NP, Dolla C, Kumaran P, et al. Coexistent malnutrition is associated with perturbations in systemic and antigen-specific cytokine responses in latent tuberculosis infection. Clin Vaccine Immunol. (2016) 23:339–45. doi: 10.1128/CVI.00009-16
59. Anuradha R, Munisankar S, Bhootra Y, Dolla C, Kumaran P, Babu S. High body mass index is associated with heightened systemic and mycobacterial antigen—specific pro-inflammatory cytokines in latent tuberculosis. Tuberculosis. (2016) 101:56–61. doi: 10.1016/j.tube.2016.08.004
60. Rajamanickam A, Munisankar S, Dolla CK, Babu S. Undernutrition is associated with perturbations in T cell-, B cell-, monocyte- and dendritic cell- subsets in latent Mycobacterium tuberculosis infection. PLoS ONE. (2019) 14:e0225611. doi: 10.1371/journal.pone.0225611
61. Kathamuthu GR, Sridhar R, Baskaran D, Babu S. Low body mass index has minimal impact on plasma levels of cytokines and chemokines in tuberculous lymphadenitis. J Clin Tuberc Other Mycobact Dis. (2020) 20:100163. doi: 10.1016/j.jctube.2020.100163
62. Kumar NP, Nancy AP, Moideen K, Menon PA, Banurekha VV, Nair D, et al. Low body mass index is associated with diminished plasma cytokines and chemokines in both active and latent tuberculosis. Front Nutr. (2023) 10:1194682. doi: 10.3389/fnut.2023.1194682
63. Zhang SY, Boisson-Dupuis S, Chapgier A, Yang K, Bustamante J, Puel A, et al. Inborn errors of interferon (IFN)-mediated immunity in humans: insights into the respective roles of IFN-alpha/beta, IFN-gamma, and IFN-lambda in host defense. Immunol Rev. (2008) 226:29–40. doi: 10.1111/j.1600-065X.2008.00698.x
64. Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. (2001) 19:93–129. doi: 10.1146/annurev.immunol.19.1.93
65. Kaufmann SH, Dorhoi A. Inflammation in tuberculosis: interactions, imbalances and interventions. Curr Opin Immunol. (2013) 25:441–9. doi: 10.1016/j.coi.2013.05.005
66. Araújo-Pereira M, Krishnan S, Salgame P, Manabe YC, Hosseinipour MC, Bisson G, et al. Effect of the relationship between anaemia and systemic inflammation on the risk of incident tuberculosis and death in people with advanced HIV: a sub-analysis of the REMEMBER trial. EClinicalMedicine. (2023) 60:102030. doi: 10.1016/j.eclinm.2023.102030
67. Cercamondi CI, Stoffel NU, Moretti D, Zoller T, Swinkels DW, Zeder C, et al. Iron homeostasis during anemia of inflammation: a prospective study of patients with tuberculosis. Blood. (2021) 138:1293–303. doi: 10.1182/blood.2020010562
68. van Lettow M, West CE, van der Meer JW, Wieringa FT, Semba RD. Low plasma selenium concentrations, high plasma human immunodeficiency virus load and high interleukin-6 concentrations are risk factors associated with anemia in adults presenting with pulmonary tuberculosis in Zomba district, Malawi. Eur J Clin Nutr. (2005) 59:526–32. doi: 10.1038/sj.ejcn.1602116
69. Ferrian S, Manca C, Lubbe S, Conradie F, Ismail N, Kaplan G, et al. A combination of baseline plasma immune markers can predict therapeutic response in multidrug resistant tuberculosis. PLoS ONE. (2017) 12:e0176660. doi: 10.1371/journal.pone.0176660
70. Kumar NP, Banurekha VV, Nair D, Babu S. Circulating angiogenic factors as biomarkers of disease severity and bacterial burden in pulmonary tuberculosis. PLoS ONE. (2016) 11:e0146318. doi: 10.1371/journal.pone.0146318
71. Urbanowski ME, Ordonez AA, Ruiz-Bedoya CA, Jain SK, Bishai WR. Cavitary tuberculosis: the gateway of disease transmission. Lancet Infect Dis. (2020) 20:e117–28. doi: 10.1016/S1473-3099(20)30148-1
Keywords: anemia, tuberculosis, cytokines, disease severity, treatment outcomes
Citation: Dasan B, Munisankar S, Pavan Kumar N, Moideen K, Pandiarajan AN, Nott S, Viswanathan V, Shanmugam S, Hissar S, Thiruvengadam K, Kornfeld H and Babu S (2025) Coexistent anemia modulates systemic inflammation and exacerbates disease severity and adverse treatment outcomes in tuberculosis. Front. Tuberc. 2:1462654. doi: 10.3389/ftubr.2024.1462654
Received: 10 July 2024; Accepted: 20 December 2024;
Published: 14 January 2025.
Edited by:
Mariana Araújo-Pereira, Gonçalo Moniz Institute (IGM), BrazilReviewed by:
Guadalupe Delgado-Sánchez, National Institute of Public Health, MexicoFelipe Ridolfi, Oswaldo Cruz Foundation (Fiocruz), Brazil
Copyright © 2025 Dasan, Munisankar, Pavan Kumar, Moideen, Pandiarajan, Nott, Viswanathan, Shanmugam, Hissar, Thiruvengadam, Kornfeld and Babu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saravanan Munisankar, c2FyYXZhbmFuLm1AaWNlcmluZGlhLm9yZw==
 Bindu Dasan1
Bindu Dasan1 Saravanan Munisankar
Saravanan Munisankar Arul Nancy Pandiarajan
Arul Nancy Pandiarajan Vijay Viswanathan
Vijay Viswanathan Sivakumar Shanmugam
Sivakumar Shanmugam Syed Hissar
Syed Hissar Kannan Thiruvengadam
Kannan Thiruvengadam Hardy Kornfeld
Hardy Kornfeld Subash Babu
Subash Babu