- 1Parasites and Vector Research Unit (PAVBRU), Department of Microbiology and Parasitology, University of Buea, Buea, Cameroon
- 2Research Foundation for Tropical Diseases and the Environment (REFOTDE), Buea, Cameroon
- 3Department of Biology, Mai Nefhi College of Science, Mai Nefhi, Eritrea
- 4Children's Investment Fund Foundation, London, United Kingdom
- 5Department of Global Health and Infection, Brighton and Sussex Medical School, University of Sussex, Brighton, United Kingdom
- 6Armauer Hansen Research Institute (AHRI), Addis Ababa, Ethiopia
- 7Institute for Medical Microbiology, Immunology and Parasitology (IMMIP), University Hospital Bonn (UKB), Bonn, Germany
- 8German Center for Infection Research (DZIF), Partner Site Bonn-Cologne, Bonn, Germany
- 9German-West African Centre for Global Health and Pandemic Prevention (G-WAC), Partner Site Bonn, Bonn, Germany
Introduction: Comorbid non-communicable diseases (NCDs) like diabetes, cardiovascular diseases (CVD), kidney diseases, and hypertension, could have implications for tuberculosis (TB) treatment management and increase the disease burden amongst active TB patients.
Methods: This cross-sectional study aimed at profiling comorbidities amongst sputum-positive TB patients in the South West and Littoral regions of Cameroon and was relevant for improving disease management and public health interventions. Diabetes was defined by elevated blood glucose, body mass index (underweight: <18.5 kg/m2, normal: 18.5–<25.0 kg/m2, overweight: 25–<30 kg/m2 and obese: ≥30.0 kg/m2) and hypertension by elevated blood pressure levels (i.e., systolic ≥130 mmHg or diastolic ≥80 mmHg). Socio-demographic and clinical data were collected using case report forms. Descriptive analysis was performed, bivariate logistic regression analysis was computed with at least one comorbidity as the dependent variable (global model) and a multivariable logistic regression analysis was done to provide adjusted odds ratios (final model). The covariate with the highest p-value was removed until p < 0.25 cut-off, using R software version 4.3.1. p-value <0.05 at 95% confidence interval was considered statistically significant.
Results: Five hundred and forty-nine sputum-positive microscopically confirmed active TB patients were enrolled into this study. Two-thirds (65.8%) of the total patients were male. Overall, 56 sputum-positive TB patients had at least one non-communicable disease, thus a prevalence of 10.2% (95% CI = 7.9–13.0). The most frequently recorded NCD was diabetes 4.4% (95% CI = 3.1–6.7) followed by kidney disease 2% (95% CI = 1.1–3.6), hypertension 0.9% (95% CI = 0.4–2.2), and CVD 0.91% (95% CI = 0.4–2.2). Three TB patients (0.6%) had all four comorbidities examined. Age group (p < 0.001), and level of education (p = 0.049) were factors significantly associated with having at least one comorbidity.
Discussion: Our findings showed that diabetes was significantly the most prevalent comorbid NCD amongst sputum-positive TB patients (p < 0.001). HIV status, occupation, body mass index (BMI), and alcohol intake were not significantly associated with having at least one comorbidity. Implementing public health intervention programmes such as systematic screening of TB patients for NCDs especially diabetes is highly recommended for better control of these diseases.
Introduction
Tuberculosis (TB) is caused by the bacillus Mycobacterium tuberculosis and is the world's second leading cause of death from a single infectious agent after COVID-19 (1). TB is among the oldest endemic diseases affecting humans and still poses a significant global public health problem (2, 3). Transmission of tuberculosis usually happens when an individual with active pulmonary tuberculosis coughs, sneezes, speaks or sings, releasing aerosolized droplets containing infectious tubercle bacilli that are inhaled by an uninfected person (4, 5). The global number of people newly diagnosed with TB in 2022 was 7.5 million, this represents the highest number since the World Health Organization (WHO) began global TB monitoring in 1995 (1). On the other hand, non-communicable diseases (NCDs) have long-term health consequences and are non-transmissible. They are caused by a combination of genetic, physiological, environmental, and behavioral factors and disproportionately affect people in low and middle-income countries, where more than three-quarters of global NCD deaths (31.4 million) occur (6). NCDs and tuberculosis are among the top 10 causes of and share common risk factors like smoking, alcohol abuse, physical inactivity and inadequate vegetable and fruit consumption (6–8). NCDs like heart disease, hypertension, chronic obstructive pulmonary diseases (COPD), cancer, diabetes mellitus, alcohol use disorders and smoking-related conditions are most common in TB patients (9, 10). Pulmonary tuberculosis is increasingly diagnosed among individuals with common mental disorders and alcohol and substance abuse, contributing substantially to the rise of multi-drug-resistant tuberculosis (MDR-TB) in many countries as a result of non-compliance (11–14). Meta-analysis of studies from all continents calculated a two–four fold increased risk for TB in patients with diabetes mellitus, with the concurrence of TB and diabetes mellitus being more prevalent than TB and HIV (15). People with diabetes also show impaired sputum conversion and cure rates on tuberculosis treatment, with increased risk of death and relapse (16, 17). Furthermore, the estimated prevalence of hypertension among patients with TB ranges between 0.7 and 38.3% (18) and TB patients have also been reported to significantly have higher risks of developing chronic kidney disease (CKD) (19). Although, the mechanism of how NCDs might negatively affect the dynamics of the TB epidemic is still under research (20). It is mandatory to consider NCDs as an important factor with regards to TB management by control programmes. Indeed, it has been shown that the absence of reports on NCD co- and multi-morbidity amongst TB patients may harm the success of TB control programmes (14, 21). Currently, there are limited studies that have described the prevalence of comorbidities amongst TB patients in Cameroon. Additionally, TB treatment is lengthy and other chronic comorbid diseases could have significant consequences on disease burden as well as implications for treatment management. This study therefore aimed at profiling non-communicable disease comorbidities amongst sputum-positive TB patients diagnosed by microscopy in the South West and Littoral regions of Cameroon.
Methods
Study site
This study was carried out in the Littoral and South West regions of Cameroon in the cities of Douala, Limbe and Buea. Douala is the economic capital of Cameroon. The city and its surrounding area have an estimated population of 3.9 million (22), with twenty TB treatment and diagnostic centers. Limbe and Buea are towns located in Fako Division-South West region of Cameroon, with seven TB treatment and diagnostic centers. As of 2015, the population of the South West region was 1,534,232 (23). Eight out of 27 TB diagnostics and treatment centers were randomly selected for the study: two from the South West and six from the Littoral regions (Figure 1).
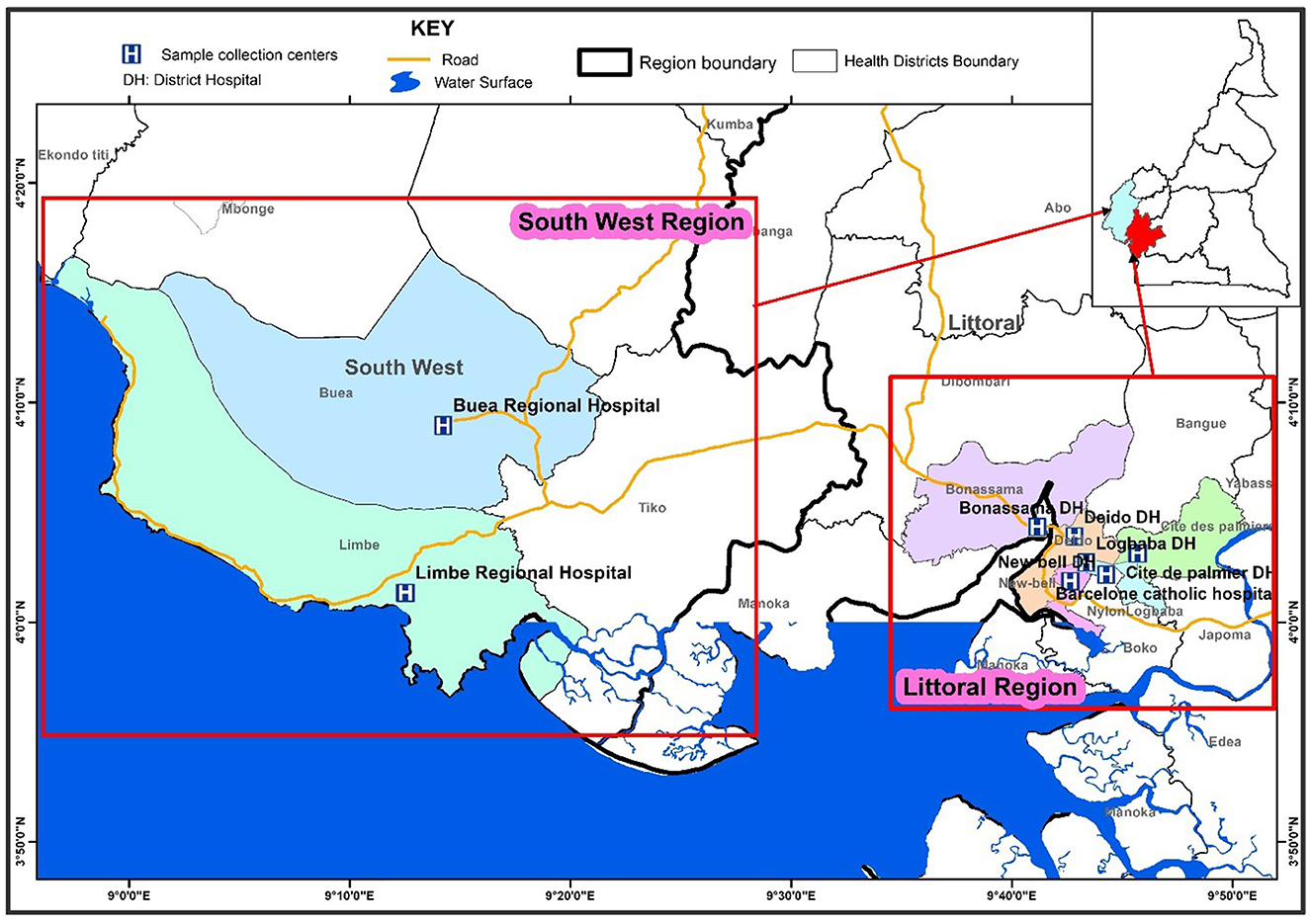
Figure 1. Map showing the eight TB diagnostic and treatment centers selected in the South West and Littoral regions of Cameroon.
Study design
This was a cross-sectional study carried out among newly diagnosed sputum-positive TB cases aged 15 years and above from February 2020 to February 2022. Demographic information, vital signs, previous TB infection history, medical history of NCDs [diabetes, hypertension, cardiovascular diseases (CVD), and kidney disease], as well as other infections, alcohol consumption and cigarette smoking were prospectively collected by trained healthcare personnel from individual hospital records and verbal self-reports using structured questionnaires that partially followed WHO guidelines for NCDs self-reporting (dietary habits and physical activity were not recorded) before the onset of TB treatment.
TB and NCD diagnosis
TB diagnosis and treatment in Cameroon is the responsibility of the National TB Control Programme under the Ministry of Public Health. Individuals with symptoms suggestive of TB (such as cough, fever, night sweats and weight loss) were sent to tuberculosis diagnostic and treatment centers for laboratory diagnosis, including sputum smear microscopy and Xpert MTB/RIF assay or the TB-LAM assay according to national guidelines. Only TB patients tested sputum-positive by microscopy were enrolled into this study. CRFs were used to collect sociodemographic information. Vital signs, height, and weight were measured and recorded on the CRFs. NCD information was obtained from self-reports of the patients based on current medical history. In that regard, medical hospital records information about diabetes, hypertension, cardiovascular diseases (CVD), kidney disease, alcohol consumption and cigarette smoking were obtained. Diabetes was defined by increased blood glucose levels (fasting glucose: >126 mg/dl, random glucose: >200 mg/dl), obesity by Body Mass Index (BMI; underweight: < 18.5 kg/m2, normal: 18.5– < 25.0 kg/m2, overweight: 25– < 30 kg/m2 and obese: ≥30.0 kg/m2 (24), hypertension by increased blood pressure (systolic ≥130 mmHg or diastolic ≥80 mmHg), kidney disease (albumin-to-creatinine ratio >30 mg/g in two of three spot urine specimens) and CVD (records of heart and blood vessel disorders). Participants of the study and also individuals who declined the enrolment started their anti-TB treatment based on national guidelines.
Study population and selection criteria
For enrolment into the study, the participants or legal guards had to provide informed consent. Moreover, participants have to be above 15 years old, had a sputum-positive smear and did not start treatment for TB. Pregnant women and individuals who were disoriented, in severe distress, severely anemic, unconscious or mentally incapacitated individuals, or those with bleeding tendencies were not enrolled in the study but received TB treatment according to the national guidelines.
Sample size calculation
The sample size was calculated based on the formula for sample size calculation for cross-sectional studies as follows
Where n is the sample size, z = 1.96 is the critical value of the confidence interval for a standard normal distribution (for 95% confidence intervals). P = 0.5 is an estimated population proportion that produces the largest sample size (for a given value of m). The value of 50% was used because, at the time of this study, there was no previous study on comorbidity prevalence rates in bacteriologically confirmed TB patients in Cameroon, and m = 0.05 is the required precision. The minimum estimated sample size was 384 cases. However, this study enrolled 549 sputum-positive TB patients.
Data processing
Data were entered into Microsoft Excel 2016 and checked for completeness. R software version 4.3.1 was used for simple and multiple binary logistic regression analysis. Descriptive analysis was performed, and frequency distribution of comorbidity combinations included only sputum-positive TB patients having at least one comorbidity. The outcome variable was the presence of at least one NCD (diabetes, hypertension, CVD, or kidney disease) amongst sputum-positive TB patients. The predictor variables assessed as associated risk factors for comorbidity were sex, age group, occupation, education level, health facility, cigarette smoking, BMI, alcohol intake, and HIV status. Multiple binary logistic regression analysis including all the covariates was performed for the global model. The final model was obtained by a step-wise backward deletion of covariates from the global model. For each step of deletion, the covariate with the highest p-value was removed until p < 0.25 cutoff. Crude and adjusted odds ratios, as well as their 95% confidence intervals were deduced from those models. A statistically significant difference was set at p < 0.05. Results were presented using frequencies (percentages) in tables and charts.
Ethics
The study was cleared by the National Ethics Committee for Human Research, Yaoundé (CNERSH) No. 2019/03/1154/CE/CNERSH/SP. Written informed consent was sought and obtained for all participants before enrolment.
Results
General characteristics of the study population
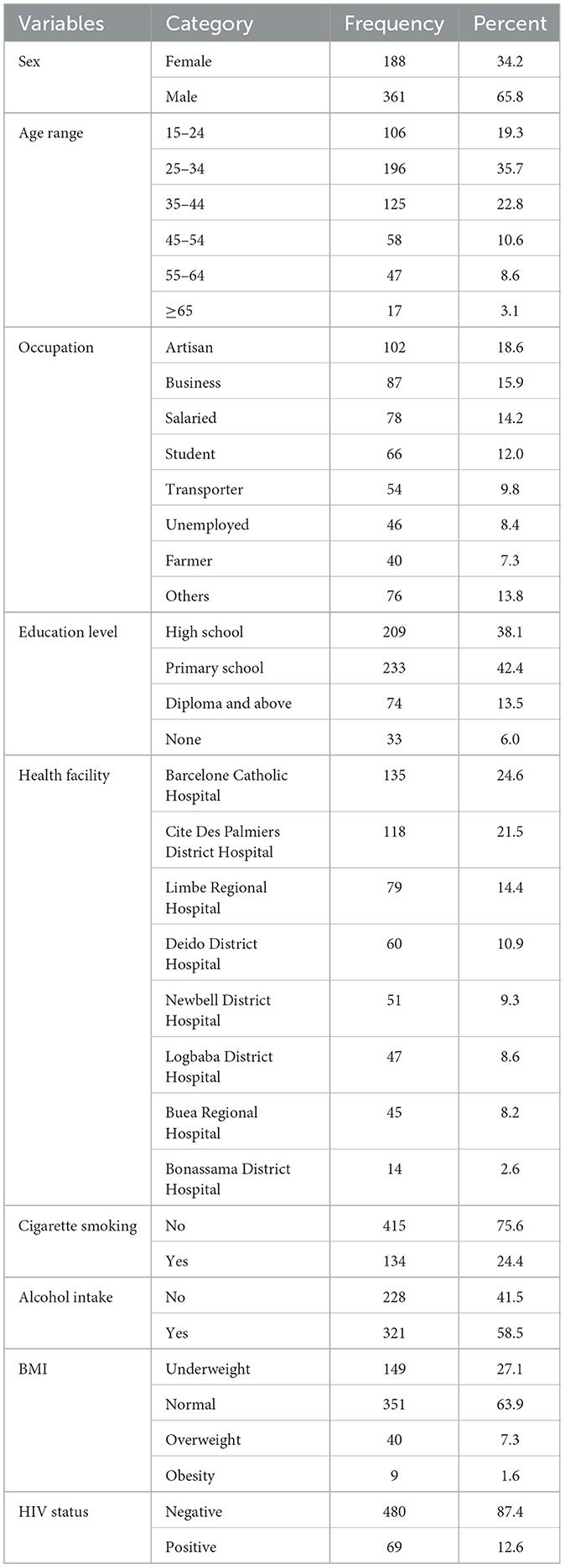
Five hundred and forty-nine sputum-positive TB patients were enrolled into this study. Two-thirds (65.8%) of the total patients were male. The distribution of participants according to their sex, age groups, occupation, education level, health facility, cigarette smoking, alcohol intake, BMI and HIV status are shown in Table 1. The highest number of sputum-positive TB patients came from Barcelone Catholic Hospital (135/459), and 87.4% of all sputum-positive TB patients were HIV-negative. Most of these patients (75.6%) did not smoke cigarettes, (63.9%) had normal BMI, and the majority of them were artisans and businessmen/women (18.6 and 15.9%, respectively).
Frequency distribution of comorbidity combinations in sputum-positive TB patients having at least one comorbidity
Overall, 56 sputum-positive TB patients had at least one non-communicable disease, thus a prevalence of 10.2% (95% CI = 7.9–13.0). The most frequently recorded NCD was diabetes with 4.4% (95% CI = 3.1–6.7); p < 0.001, followed by kidney disease with 2% (95% CI = 1.1–3.6), hypertension with 0.91% (95% CI = 0.4–2.2), and CVD with 0.91% (95% CI = 0.4–2.2). Three sputum-positive TB patients (0.6%) had all four comorbidities examined as shown in Figure 2. Diabetes and hypertension multi-morbidity was recorded in four TB patients. CVD and kidney disease multi-morbidity were also found in one TB patient. This was also the case for diabetes and kidney disease multi-morbidity as well as hypertension and CVD multi-morbidity.
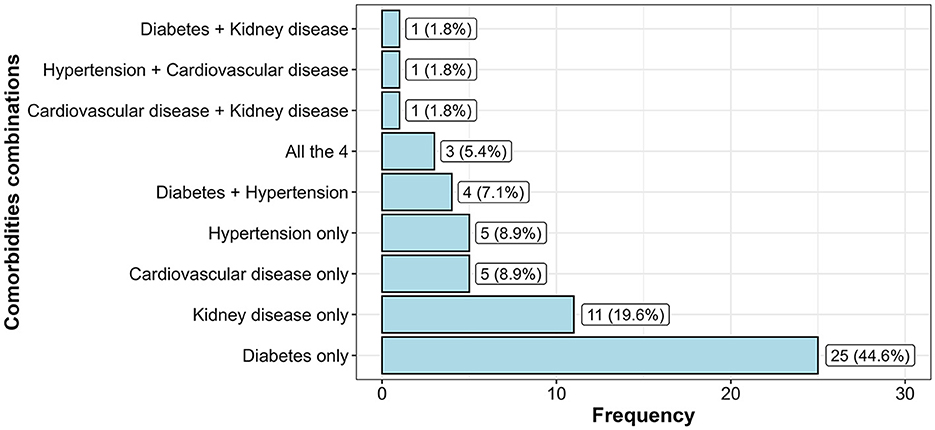
Figure 2. Frequency distribution of comorbidity combinations in sputum-positive TB patients having at least one comorbidity.
Socio-demographic, clinical, and behavioral factors associated with tuberculosis and comorbidities
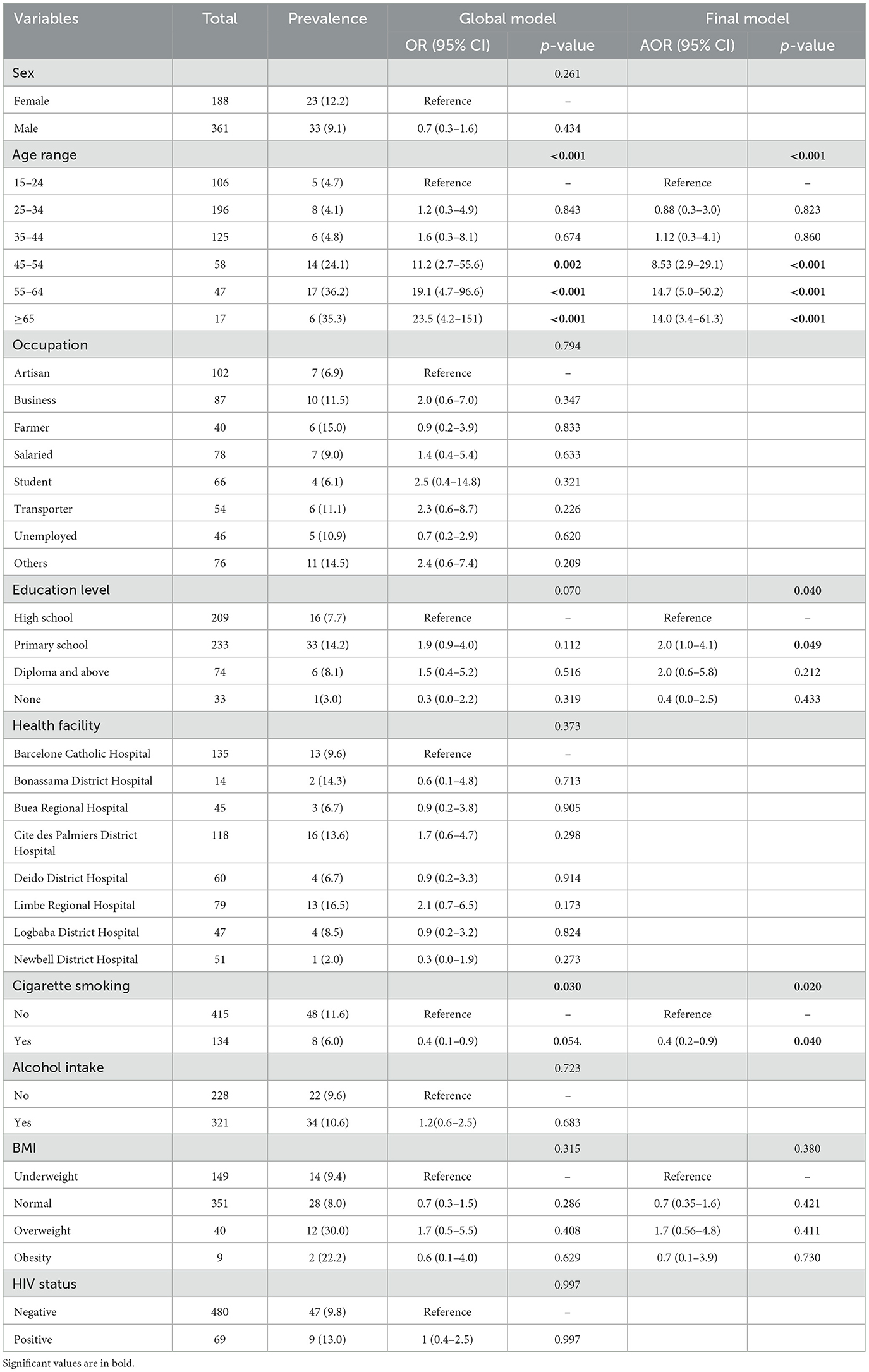
Based on the global model, age group (p < 0.001), and level of education (p = 0.049) were two factors associated with having at least one comorbidity. From the final model, sputum-positive TB patients who were 45 years and above had significantly higher odds of having at least one comorbidity (p < 0.001) when compared to patients between 15 and 24 years of age. TB patients with primary school education had significantly higher odds of having at least one comorbidity when compared to TB patients with high school education (AOR, 2.0; 95% CI: 1.0–4.1). TB patients who smoke cigarettes were less likely to be found with at least one comorbidity as compared to sputum-positive TB patients who did not smoke cigarettes (AOR, 0.4; 95% CI: 0.2–0.9, p = 0.040). Sex, occupation, health facility, alcohol intake, BMI, and HIV status were not seen as factors associated with having at least one comorbidity in sputum-positive TB patients as shown in Table 2.
Discussion
Tuberculosis, as well as a wide range of comorbid NCDs are prevalent and overlapping in most developing countries. There are already some studies reporting comorbidities among TB cases in Sub-Saharan Africa, particularly diabetes and hypertension. However, data sets about distinct NCDs, especially cardiovascular and kidney diseases are limited and almost no reports have been published from rural areas as shown here with study sites in the South West and Littoral regions of Cameroon. Thus, we aim to fill this gap of knowledge and assessed comorbid diabetes, hypertension, kidney disease, and cardiovascular diseases among sputum-positive TB patients before their enrolment into the direct observed treatment (DOT) in tuberculosis diagnostic and treatment centers. Our study found that 10.2% were comorbid with at least one NCD assessed. This figure is higher than 7.22% reported in Ethiopia (25) and lower than 26.9% in South Africa (26). These variations might be due to differences in NCD prevalence in different communities. This study found a higher proportion of males suffering from TB than females. The higher share of TB cases among men is consistent with evidence from global reports showing similar patterns (1, 27, 28), as well as surveys conducted in low- and middle-income countries (29).
The most frequently recorded comorbid NCD amongst TB patients in this study was diabetes with a prevalence of 4.6%. These findings are higher than reports of 1.6% in Coutonou-Benin (30), and lower than the 5.7% prevalence in Nigeria (31), 6.7% in Tanzania and Kenya (32, 33), 8.5% in Uganda (34), 9.5% in Cameroon (35), and 16% in Ethiopia (36). Reasons for these differences could be variations in sample size and lifestyle of TB patients in the study sites. Kidney disease was the second most frequently recorded comorbid NCD amongst sputum-positive TB patients in this study with a prevalence of 2%. These results are lower than the 5.24% prevalence of chronic kidney disease from a study carried out amongst pulmonary and extra-pulmonary TB patients in Cameroon (37). Hypertension was the third most frequently recorded comorbid NCD amongst TB patients in this study with a prevalence of 0.9%. These findings are higher than reports of 0.7% in Brazil (38) and lower than reports of 19% in Angola (39). The very low prevalence of comorbid hypertension in TB patients in Brazil could be because data entry on comorbidity in that study was optional. This study recorded a 0.91% prevalence of cardiovascular diseases. These findings are lower than reports of 11% pooled prevalence from a systematic review of CVD among TB patients (40).
Sputum-positive TB patients aged 45 years and above in this study were more likely at risk for at least one comorbidity when compared to the 15–24 years age group. Several studies have reported that older age increases the risk of TB and diabetes comorbidity (41, 42). These findings are in line with other studies where older age was also associated with hypertension in tuberculosis patients (39, 43). Generally, other underlying factors likely influence the blood pressure dynamics in active TB patients. In addition, a study carried out in Taiwan also showed that the incidence of chronic kidney disease was higher in TB patients with age over 50 years (19). These comorbid conditions among active TB patients could have implications for treatment management. Our findings showed that sputum-positive TB patients with primary school education had significantly higher odds of having at least one comorbidity when compared to TB patients with high school education. Contrary to these findings, another study indicated that having education beyond primary schooling was an associated factor to TB-diabetes comorbidity (44).
We found that active TB patients who smoke cigarettes had lower odds for at least one comorbidity. Contrary to these findings, smoking has been reported as risk factor for NCD (45) and diabetes in TB patients (46). BMI, HIV status and alcohol intake were not seen as factors associated to having at least one comorbidity in sputum-positive TB patients. Contrary to these findings, a study carried out in China reported overweight and obesity to be significantly associated with diabetes in pulmonary TB patients (47), similar to a study carried out in Angola where BMI was associated with hypertension (39). Furthermore, other studies have reported HIV status (48, 49) and alcohol intake as factors associated with comorbid hypertension (43) in TB and diabetes patients (50). The reasons for these differences in associated risk factors to comorbidities in active TB patients in various studies could range from behavioral and societal factors amongst individuals in study areas as well as differences in study designs. TB treatment success is very crucial for TB management strategies, especially in low- and middle-income countries, as treatment failure increases the incidence of active TB as well as multidrug resistance. Comorbid NCDs generally have implications for the management of TB disease. One important factor contributing to TB treatment failure is comorbidity which may eventually lead to death (51, 52). Comorbid diabetes in TB patients leads to a number of negative TB treatment consequences including increased drug resistance in TB patients, TB treatment prolongation and failure (53–55). The “WHO Collaborative Framework of Care and Control of Tuberculosis and Diabetes” (56) recommended for healthcare professionals to ensure effective management of both diseases, should be diligently followed in TB diagnostic and treatment centers nation-wide. Administering the appropriate treatment for TB patients with chronic kidney disease is of great importance in reducing mortality, as inappropriate dosage of anti-TB drugs can result in drug-related side effects or unsuccessful treatment (57). Hypertension has been associated with increased mortality in patients with tuberculosis (58). Tuberculosis may also contribute to the pathogenesis of CVD (59). Given that undiagnosed comorbid NCDs in tuberculosis patients might negatively influence the success of TB control programmes (21), developing an integrated platform for systematic screening of active TB patients for comorbid NCDs is a great strategy for targeting comorbidities in TB patients and hence enhance disease management as well as treatment success rates.
Limitations
Although we confirmed the diagnosis of tuberculosis, as in many observational studies, information on NCDs was obtained from individual hospital record books and verbal self-reports. It is possible that several cases were missed because of no concurrent diagnosis of comorbid NCDs, and the unwillingness of patients to declare their health status. Information based on self-reports could not be verified for accuracy. In addition, other risk factors such as dietary habits, and physical activity were omitted from the data collection and information about concomitant medication against NCDs have been not collected. Therefore, these findings should be interpreted with caution. Another limitation of the study results is that only sputum-positive TB patients were enrolled into the study. It is known that coinfections like HIV can reduce the positivity of sputum smears and thus it might be that some TB cases were not detected. However, Xpert MTB/RIF or TB-LAMP assay was not available in most of the TB centers, we were forced to rely on the microscopical diagnosis. Altogether, the limitations of the study especially in regards to the undetected TB and NCD cases might even lead to an underestimation of the overlapping prevalences of TB and NCDs, highlighting the need for improved TB and NCD diagnosis.
Conclusions
Sputum-positive TB patients aged 45 years and above in this study had higher odds of having at least one comorbidity. Diabetes was the most frequently recorded comorbid NCD amongst sputum-positive TB patients. HIV status, occupation, BMI, and alcohol intake were not significantly associated with having at least one comorbidity. Concurrent diagnosis of NCDs amongst TB patients is highly recommended since it will shed more light on these overlapping disease conditions and might support disease management and control, ultimately reducing the disease burden and contributing to the WHO End TB Strategy.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by National Ethics Committee for Human Research, Yaoundé (CNERSH, No. 2019/03/1154/CE/CNERSH/SP) and the Ethics Committee of the University Hospital Bonn (Lfd. Nr. 021/18). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
CM: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. LN: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. MW: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. DN: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. NT: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. HM: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. KD: Writing – original draft, Writing – review & editing. FN: Investigation, Methodology, Writing – original draft, Writing – review & editing. JF: Investigation, Methodology, Writing – original draft, Writing – review & editing. FF: Investigation, Methodology, Writing – original draft, Writing – review & editing. TK: Investigation, Methodology, Writing – original draft, Writing – review & editing. JC: Investigation, Methodology, Writing – original draft, Writing – review & editing. EIG: Investigation, Methodology, Writing – original draft, Writing – review & editing. SWad: Investigation, Writing – original draft, Writing – review & editing. KB: Investigation, Writing – original draft, Writing – review & editing. MN: Investigation, Writing – original draft, Writing – review & editing. AH: Conceptualization, Funding acquisition, Resources, Supervision, Writing – original draft, Writing – review & editing. MR: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. SWan: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by the Human Heredity and Health in Africa (H3Africa) (grant number: H3A-18-003). H3Africa is managed by the Science for Africa (SFA) foundation in partnership with the Wellcome Trust (UK), the National Institute of Health (US), and the African Society of Human Genetics (AfSHG). This work was also supported through a grant by the Deutsche Forschungsgemeinschaft (DFG) within the “African-German Cooperation Projects in Infectiology” (HO 2009/14-1). AH was additionally supported by the DFG under Germany's Excellence Strategy – EXC2151 – 390873048 and SWan is the Senior Fellow Plus of the European Developing Clinical Trial Partnership (EDCTP2).
Acknowledgments
We are grateful to all study participants for providing the necessary data and the staff of the different tuberculosis diagnostic and treatment centers in the South West and Littoral regions for facilitating sample collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
TB, tuberculosis; HIV, Human Immunodeficiency Virus; NCDs, non-communicable diseases; OR, odds ratio; AOR, adjusted odds ratio; WHO, World Health Organization; COPD, chronic obstructive pulmonary diseases; DM, diabetes mellitus; MDR-TB, multi-drug-resistant tuberculosis; CKD, chronic kidney disease; CVD, cardiovascular disease; ZN, Ziehl-Nelson; AFB, acid-fast bacilli; LJ, Lowenstein-Jensen; BMI, body mass index; PBS, phosphate buffer saline.
References
2. Comas I, Coscolla M, Luo T, Borrell S, Holt KE, Kato-Maeda M, et al. Out-of-Africa migration and Neolithic coexpansion of Mycobacterium tuberculosis with modern humans. Nat Genet. (2013) 45:1176–82. doi: 10.1038/ng.2744
3. Kyu HH, Maddison ER, Henry NJ, Mumford JE, Barber R, Shields C, et al. The global burden of tuberculosis: results from the Global Burden of Disease Study 2015. Lancet Infect Dis. (2018) 18:261–84. doi: 10.1016/S1473-3099(17)30703-X
4. CDC. Transmission and pathogenesis of tuberculosis. Self-Study Modules on Tuberculosis, 2019 Module 1: Transmission and Pathogenesis of Tuberculosis. Atlanta, GA: CDC (2024), p. 40.
5. Glickman MS, Jacobs WR. Microbial pathogenesis of Mycobacterium tuberculosis: dawn of a discipline. Cell. (2001) 104:477–85. doi: 10.1016/S0092-8674(01)00236-7
6. WHO. Non-communicable diseases. (2023). Available at: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed December 16, 2023).
7. Begum N, Nawshin I, Rahman MA. Behavioural risk factors of non-communicable diseases among rural population in a selected area of Dhaka City. J Curr Adv Med Res. (2022) 9:9–15. doi: 10.3329/jcamr.v9i1.59738
8. Esmailnasab N, Moradi G, Delaveri A. Risk factors of non-communicable diseases and metabolic syndrome. Iran J Public Health. (2012) 41:77–85.
9. Wang Y, Wang J. Modelling and prediction of global non-communicable diseases. BMC Public Health. (2020) 20:1–13. doi: 10.1186/s12889-020-08890-4
10. Lönnroth K, Castro KG, Chakaya JM, Chauhan LS, Floyd K, Glaziou P, et al. Tuberculosis control and elimination 2010–50: cure, care, and social development. Lancet. (2010) 375:1814–29. doi: 10.1016/S0140-6736(10)60483-7
11. deAraújo GS, Pereira SM, dos Santos DN, Marinho JM, Rodrigues LC, Barreto ML. Common mental disorders associated with tuberculosis: a matched case-control study. PLoS ONE. (2014) 9:e99551. doi: 10.1371/journal.pone.0099551
12. Simet SM, Sisson JH. Alcohol's effects on lung health and immunity. Alcohol Res. (2015) 37:199–208.
13. Armenta RF, Collins KM, Strathdee SA, Bulterys MA, Munoz F, Cuevas-Mota J, et al. Mycobacterium tuberculosis infection among persons who inject drugs in San Diego, California. Int J Tuberc Lung Dis. (2017) 21:425–31. doi: 10.5588/ijtld.16.0434
14. Marais BJ, Lönnroth K, Lawn SD, Migliori GB, Mwaba P, Glaziou P, et al. Tuberculosis comorbidity with communicable and non-communicable diseases: integrating health services and control efforts. Lancet Infect Dis. (2013) 13:436–48. doi: 10.1016/S1473-3099(13)70015-X
15. Al-Rifai RH, Pearson F, Critchley JA, Abu-Raddad LJ. Association between diabetes mellitus and active tuberculosis: a systematic review and meta-analysis. PLoS ONE. (2017) 12:e0187967. doi: 10.1371/journal.pone.0187967
16. Faurholt-Jepsen D, Range N, Praygod G, Kidola J, Faurholt-Jepsen M, Aabye MG, et al. The role of diabetes co-morbidity for tuberculosis treatment outcomes: a prospective cohort study from Mwanza, Tanzania. BMC Infect Dis. (2012) 12:165. doi: 10.1186/1471-2334-12-165
17. Baker MA, Harries AD, Jeon CY, Hart JE, Kapur A, Lönnroth K, et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med. (2011) 9:81. doi: 10.1186/1741-7015-9-81
18. Seegert AB, Rudolf F, Wejse C, Neupane D. Tuberculosis and hypertension – a systematic review of the literature. Int J Infect Dis. (2017) 56:54–61. doi: 10.1016/j.ijid.2016.12.016
19. Shen TC, Huang KY, Chao CH, Wang YC, Muo CH, Wei CC, et al. The risk of chronic kidney disease in tuberculosis: a population-based cohort study. QJM. (2015) 108:397–403. doi: 10.1093/qjmed/hcu220
20. Puchner KP, Rodriguez-Fernandez R, Oliver M, Solomos Z. Non-communicable diseases and tuberculosis: anticipating the impending global storm. Glob Public Health. (2019) 14:1372–81. doi: 10.1080/17441692.2019.1580760
21. Creswell J, Raviglione M, Ottmani S, Migliori GB, Uplekar M, Blanc L, et al. Tuberculosis and noncommunicable diseases: neglected links and missed opportunities. Eur Respir J. (2011) 37:1269–82. doi: 10.1183/09031936.00084310
22. Population Stat. Douala, Cameroon Population 2020 - Population Stat. Available at: https://populationstat.com/cameroon/douala (accessed September 13, 2024).
23. World Data Atlas Cameroon South West population persons. Available at: https://knoema.com/atlas/Cameroon/South-West (accessed September 13, 2024).
24. World Health Organization. BMI. Classification 2023. Available at: https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/body-mass-index?introPage=intro_3.html (accessed September 13, 2024).
25. Nunemo MH, Gidebo KD, Woticha EW, Lemu YK. Predictors of tuberculosis and non-communicable disease comorbidities among newly enrolled tuberculosis patients, Southern Ethiopia. Integr Blood Press Control. (2023) 16:95–109. doi: 10.2147/IBPC.S432251
26. Peltzer K. Tuberculosis non-communicable disease comorbidity and multimorbidity in public primary care patients in South Africa. Afr J Prim Health Care Fam Med. (2018) 10:1–6. doi: 10.4102/phcfm.v10i1.1651
29. Horton KC, MacPherson P, Houben RM, White RG, Corbett EL. Sex differences in tuberculosis burden and notifications in low- and middle-income countries: a systematic review and meta-analysis. PLoS Med. (2016) 13:e1002119. doi: 10.1371/journal.pmed.1002119
30. Ade S, Affolabi D, Agodokpessi G, Wachinou P, Faïhun F, Toundoh N, et al. Low prevalence of diabetes mellitus in patients with tuberculosis in Cotonou, Benin. Public Health Action. (2015) 5:147–9. doi: 10.5588/pha.14.0110
31. Olayinka AO, Anthonia O, Yetunde K. Prevalence of diabetes mellitus in persons with tuberculosis in a tertiary health Centre in Lagos, Nigeria. Indian J Endocrinol Metab. (2013) 17:486. doi: 10.4103/2230-8210.111646
32. Mugusi F, Swai A, Alberti K, McLarty D. Increased prevalence of diabetes mellitus in patients with pulmonary tuberculosis in Tanzania. Tubercle. (1990) 71:271–6. doi: 10.1016/0041-3879(90)90040-F
33. Owiti P, Keter A, Harries A, Pastakia S, Wambugu C, Kirui N, et al. Diabetes and pre-diabetes in tuberculosis patients in western Kenya using point-of-care glycated haemoglobin. Public Health Action. (2017) 7:147–54. doi: 10.5588/pha.16.0114
34. Kibirige D, Ssekitoleko R, Mutebi E, Worodria W. Overt diabetes mellitus among newly diagnosed Ugandan tuberculosis patients: a cross sectional study. BMC Infect Dis. (2013) 13:122. doi: 10.1186/1471-2334-13-122
35. Fonkeng LS, Ali IM, Noubom M, Bamou R, Sterve AH, Leo A, et al. Prevalence, predictors and treatment outcome of type 2 diabetes among newly diagnosed sputum positive pulmonary tuberculosis patients in Western Cameroon. J Infect Dis Epidemiol. (2017) 3:2. doi: 10.23937/2474-3658/1510031
36. Damtew E, Ali I, Meressa D. Prevalence of diabetes mellitus among active pulmonary tuberculosis patients at St. Peter specialized hospital, Addis Ababa, Ethiopia. World J Med Sci. (2014) 11:389–96. doi: 10.5829/idosi.wjms.2014.11.3.85152
37. Mangamba LM, Halle MP, Onana CL, Tochie JN, Ngamby V, Noubibou JC, et al. Impact of chronic kidney disease on the mortality of tuberculosis patients: a cross-sectional study in the City of Douala. Health Sci Dis. (2023) 24. doi: 10.5281/hsd.v24i2.4184
38. Reis-Santos B, Gomes T, Macedo LR, Horta BL, Riley LW, Maciel EL. Prevalence and patterns of multimorbidity among tuberculosis patients in Brazil: a crosssectional study. Int J Equity Health. (2013) 12:61. doi: 10.1186/1475-9276-12-61
39. Segafredo G, Kapur A, Robbiati C, Joseph N, de Sousa JR, Putoto G, et al. Integrating TB and noncommunicable diseases services: pilot experience of screening for diabetes and hypertension in patients with Tuberculosis in Luanda, Angola. PLoS ONE. (2019) 14:e0218052. doi: 10.1371/journal.pone.0218052
40. Shabil M, Bushi G, Beig MA, Rais MA, Ahmed M, Padhi BK. Cardiovascular manifestation in tuberculosis cases: a systematic review and meta-analysis. Curr Probl Cardiol. (2023) 48:101666. doi: 10.1016/j.cpcardiol.2023.101666
41. Workneh MH, Bjune GA, Yimer SA. Prevalence and associated factors of diabetes mellitus among tuberculosis patients in South- Eastern Amhara Region, Ethiopia: a cross-sectional study. PLoS ONE. (2016) 11:e0147621. doi: 10.1371/journal.pone.0147621
42. Wang Q, Ma A, Han X, Zhao S, Cai J, Ma Y, et al. Prevalence of type 2 diabetes among newly detected pulmonary tuberculosis patients in China: a community based COHORT study. PLoS ONE. (2013) 8:e82660. doi: 10.1371/journal.pone.0082660
43. Adegbite BR, Edoa JR, Agbo Achimi Abdul J, Epola M, Mevyann C, Dejon-Agobé JC, et al. Non-communicable disease co-morbidity and associated factors in tuberculosis patients: a cross-sectional study in Gabon. EClinicalMedicine. (2022) 45:101316. doi: 10.1016/j.eclinm.2022.101316
44. Sarvamangala K, Banerjee A. Comparative study of type II diabetes mellitus and HIV comorbidity among tuberculosis patients attending tertiary care hospital in Davangere. Indian J Public Health Res Dev. (2014) 5:193–7. doi: 10.5958/j.0976-5506.5.2.102
45. Bates M, Marais BJ, Zumla A. Tuberculosis comorbidity with communicable and noncommunicable diseases. Cold Spring Harb Perspect Med. (2015) 5:a017889. doi: 10.1101/cshperspect.a017889
46. Thapa B, Paudel R, Thapa P, Shrestha A, Poudyal AK. Prevalence of diabetes among tuberculosis patients and associated risk factors in Kathmandu valley. SAARC J Tuber Lung Dis HIV/AIDS. (2015) 12:20–7. doi: 10.3126/saarctb.v12i2.15951
47. Cai J, Ma A, Wang Q, Han X, Zhao S, Wang Y, et al. Association between body mass index and diabetes mellitus in tuberculosis patients in China: a community based cross-sectional study. BMC Public Health. (2017) 17:228. doi: 10.1186/s12889-017-4101-6
48. Alavi SM, Khoshkhoy MM. Pulmonary tuberculosis and diabetes mellitus: co-existence of both diseases in patients admitted in a teaching hospital in South West of Iran. Caspian J Intern Med. (2012) 3:421–4.
49. Tulu B, Amsalu E, Zenebe Y, Abebe M, Fetene Y, Agegn M, et al. Diabetes mellitus and HIV infection among active tuberculosis patients in Northwest Ethiopia: health facility-based cross-sectional study. Trop Med Health. (2021) 49:68. doi: 10.1186/s41182-021-00358-4
50. Raghuraman S, Vasudevan KP, Govindarajan S, Chinnakali P, Panigrahi KC. Prevalence of diabetes mellitus among tuberculosis patients in urban Puducherry. N Am J Med Sci. (2014) 6:30–4. doi: 10.4103/1947-2714.125863
51. Lin CH, Lin CJ, Kuo YW, Wang JY, Hsu CL, Chen JM, et al. Tuberculosis mortality: patient characteristics and causes. BMC Infect Dis. (2014) 14:5. doi: 10.1186/1471-2334-14-5
52. Costa-Veiga A, Briz T, Nunes C. Unsuccessful treatment in pulmonary tuberculosis: factors and a consequent predictive model. Eur J Public Health. (2018) 28:352–8. doi: 10.1093/eurpub/ckx136
53. Liu Q, Li W, Xue M, Chen Y, Du X, Wang C, et al. Diabetes mellitus and the risk of multidrug resistant tuberculosis: a meta-analysis. Sci Rep. (2017) 7:1090. 1090. doi: 10.1038/s41598-017-01213-5
54. Khattak M, Rehman AU, Muqaddas T, Hussain R, Rasool MF, Saleem Z, et al. Tuberculosis (TB) treatment challenges in TB-diabetes comorbid patients: a systematic review and meta-analysis. Ann Med. (2024) 56:2313683. doi: 10.1080/07853890.2024.2313683
55. Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis. (2009) 9:737–46. doi: 10.1016/S1473-3099(09)70282-8
56. World Health Organization. Collaborative framework for care and control of tuberculosis and diabetes (No. WHO/HTM/TB/2011.15). Geneva: World Health Organization (2011).
57. Saito N, Yoshii Y, Kaneko Y, Nakashima A, Horikiri T, Saito Z, et al. Impact of renal function-based anti-tuberculosis drug dosage adjustment on efficacy and safety outcomes in pulmonary tuberculosis complicated with chronic kidney disease. BMC Infect Dis. (2019) 19:374. doi: 10.1186/s12879-019-4010-7
58. Seegert AB, Patsche CB, Sifna A, Gomes VF, Wejse C, Storgaard M, et al. Hypertension is associated with increased mortality in patients with tuberculosis in Guinea-Bissau. Int J Infect Dis. (2021) 109:123–8. doi: 10.1016/j.ijid.2021.06.062
Keywords: Mycobacterium tuberculosis, comorbidities, non-communicable diseases, tuberculosis, diabetes, cardiovascular disease, kidney disease
Citation: Magha C, Nchang LC, Weldeslassie M, Nkimbeng DA, Tchatat NM, Meriki HD, Deribe K, Nietcho FN, Foyet JV, Fombad FF, Katcho TD, Cho JF, Gebremeskel EI, Waddell SJ, Bobosha K, Newport MJ, Hoerauf A, Ritter M and Wanji S (2024) Comorbidity profiles among sputum-positive tuberculosis patients in Cameroon. Front. Tuberc. 2:1433856. doi: 10.3389/ftubr.2024.1433856
Received: 17 May 2024; Accepted: 02 September 2024;
Published: 02 October 2024.
Edited by:
Fraser Wares, KNCV TB Foundation, NetherlandsReviewed by:
Rebeca Briceno Robaugh, United States Agency for International Development, United StatesVineet Bhatia, World Health Organization, India
Copyright © 2024 Magha, Nchang, Weldeslassie, Nkimbeng, Tchatat, Meriki, Deribe, Nietcho, Foyet, Fombad, Katcho, Cho, Gebremeskel, Waddell, Bobosha, Newport, Hoerauf, Ritter and Wanji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samuel Wanji, c3dhbmppQHlhaG9vLmZy; Manuel Ritter, bWFudWVsLnJpdHRlckB1a2Jvbm4uZGU=
†These authors share first authorship
 Chefor Magha
Chefor Magha Lucy Cho Nchang
Lucy Cho Nchang Michael Weldeslassie
Michael Weldeslassie Desmond Akumtoh Nkimbeng1,2
Desmond Akumtoh Nkimbeng1,2 Kebede Deribe
Kebede Deribe Frank Noel Nietcho
Frank Noel Nietcho Juluis Visnel Foyet
Juluis Visnel Foyet Simon J. Waddell
Simon J. Waddell Melanie J. Newport
Melanie J. Newport Manuel Ritter
Manuel Ritter Samuel Wanji
Samuel Wanji