- 1Department of Medical Parasitology and Entomology, School of Medicine, Catholic University of Health and Allied Sciences, Mwanza, Tanzania
- 2District Medical Department, Ukerewe District Council, Mwanza, Tanzania
- 3Deutsche Lepra- und Tuberkulosehilfe e.V (DAHW) - German Leprosy and Tuberculosis Relief Association, Würzburg, Germany
- 4Medical Mission Institute, Würzburg, Germany
- 5Medical Mission Hospital, Würzburg, Germany
- 6School of Public Health, Catholic University of Health and Allied Sciences, Mwanza, Tanzania
Background: The World Health Organization has called for the elimination of schistosomiasis as a public health problem by 2030 through expanding praziquantel delivery to all community members, specifically targeting the foci of the disease. The current project is responding to this call by implementing community-based mass preventive chemotherapy (PC) to an at-risk adult population on Ukerewe Island, northwestern Tanzania. To date, three rounds of mass preventive chemotherapy have been implemented and here we report the effect of these treatment rounds on the prevalence and intensity of S. mansoni infection. Secondarily, we report on the treatment coverage in all the treatment rounds.
Methods: A community-based cross-sectional study was conducted in 20 villages and included randomly selected adults aged ≥15 years. A single stool sample was obtained from each consenting participant and examined for S. mansoni infection using the Kato Katz technique. A questionnaire was used to collect the demographic information of the participants. The World Health Organization’s community evaluation survey (CES) methods were adapted to assess treatment coverage. Two weeks after each treatment round (In June 2021, January 2023, and June 2023), CESs were conducted among adults from randomly selected households.
Results: For parasitological surveys, a total of 2,041 participants (47.8% men and 52.2% women) were involved. After three rounds of treatment, the overall adjusted prevalence of S. mansoni infection was 9.5% (95%CI:8.3-10.8) and the geometrical mean egg intensity of infection was 79.9 eggs per gram (epg) of feces (95% CI:71.2-89.8). The prevalence declined by 68.8% (from 30.4% to 9.5%, P<0.0001) and intensity of infection declined by 24.1% (from 105.3 epg at baseline to 79.9 epg, P<0.0001). After three rounds of mass PC, the proportion of heavily infected adult individuals significantly declined by 81% (from 13.7% at baseline to 2.6%). For the coverage survey, a total of 12,531 adult individuals were interviewed after each round of treatment. Coverages of 80.8%, 78.5%, and 81.9% were recorded for treatment rounds one, two, and three, respectively.
Conclusion: Overall, the three rounds of mass preventive chemotherapy led to declines in the prevalence and intensity of S. mansoni infection in the targeted population. In all the targeted villages, the treatment coverage of praziquantel was above the recommended threshold of ≥ 75%, but improvement is needed to achieve higher coverage in the coming treatment rounds, which in turn will have a high impact on the disease prevalence. In addition, to achieve the elimination stage, the targeted villages still need additional treatment rounds which should be supported with other complementary interventions such as improved water supply, sanitation, and hygiene.
1 Introduction
The northwestern Tanzania region, which includes islands on Lake Victoria, remains endemic for intestinal schistosomiasis caused by the Schistosoma mansoni parasite (1–3). Intestinal schistosomiasis causes significant morbidities characterized by bloody stool, abdominal discomfort and pain, and diarrhea. At its chronic stage, the disease-related morbidities are characterized by hepatosplenic disease with hepatomegaly, splenomegaly, portal hypertension, esophageal varices, collateral veins, and hematemesis (4, 5). Evidence indicates that 52% of the Tanzanian population is either at risk of or infected by schistosome worms (6), with the Lake Victoria region carrying almost 70% of the disease burden due to the environmental conduciveness for transmission of the disease (1, 7).
The World Health Organization recommends the following public health strategies to control schistosomiasis: (i) large-scale mass treatment of at-risk population groups; (ii) improve access to safe water, improved sanitation, hygiene education, and behavior; and (iii) snail control and environmental management (8, 9). The current global goal described under the neglected tropical diseases road map 2021-2030 is to eliminate schistosomiasis as a public health problem in all endemic countries and to interrupt its transmission (absence of infection in human population) in selected countries (9). Of all the described intervention measures, mass preventive chemotherapy is widely implemented in endemic countries and it aims at reducing the disease burden through periodic treatment of the at-risk and affected populations (8, 9). The groups targeted for preventive chemotherapy are pre-school-aged children, school-aged children, adult community members considered at risk due to occupational exposure (such as those involved in fishing, paddy farming, farming, irrigation workers, and women whose domestic tasks bring them into contact with infested water sources), and entire communities living in highly endemic areas (8).
Tanzania follows the WHO recommendation on controlling schistosomiasis by implementing annual mass preventive chemotherapy, mainly targeting school-aged children within the school environment (8, 10). This approach is considered to be a highly cost-effective public health intervention (10, 11). The major drawback of this approach is leaving out other community members who are out of the school environment, such as pre-school children and adult community members. Adult community members not involved in mass preventive chemotherapy carry the highest burden of hepatosplenic disease, and at least 25% of those with hepatosplenic disease die from its complications (12). In addition, untreated community members maintain transmission of the disease in the environment and serve as a source of infection to treated school children (12). To reach the global goals of eliminating schistosomiasis as a public health problem in 2030 and responding positively to the global sustainable development goals (SDG) of not “leaving anyone behind” (9), public health interventions should not segregate anyone, meaning that these interventions should be inclusive and reach the entire endemic community. Understanding this concept and responding to the WHO’s new guidelines for controlling schistosomiasis (8), the current ongoing project on Ukerewe Island delivers different community-and-hospital-based interventions against intestinal schistosomiasis that target adult community members. The interventions include: (i) community-based mass drug administration that is led by community drug distributors and sub-village and village leaders; (ii) community-based public health education related to improving knowledge related to schistosomiasis epidemiology, prevention, and control; (iii) community test-and-treat campaigns; (iv) capacity building of healthcare facilities to diagnose and manage schistosomiasis and its related chronic morbidities; and (v) management of chronic morbidities (hepatosplenic morbidities, hematemesis and its sequelae, and esophageal varices) through the establishment of the endoscopy unit at the Nansio District Hospital, a district hospital in Ukerewe District Council in northwestern Tanzania. While the government of Tanzania, through the Ministry of Health, delivers annual school mass preventive chemotherapy targeting school-age children on Ukerewe Island, the activities of the current project cover the adult community members. To date, three rounds of mass preventive chemotherapy have been delivered to over 100,000 adult community members living in 20 villages. Therefore, it is important to assess the effects of these interventions, especially mass preventive chemotherapy, on S. mansoni prevalence and intensity of infection. This is important for refining the intervention strategies, adjusting the treatment plan based on new prevalence data, and sharing the success and challenges with the wider schistosomiasis community. In this context, this study conducted a mid-term review of this 5-year-long project to assess the effect of three rounds of mass preventive treatment (June 2021, January 2023, and June 2023) on the prevalence and intensity of S. mansoni infection among adult community members in 20 villages of Ukerewe District Council, northwestern Tanzania. Secondarily, we report the treatment coverage achieved during these rounds.
2 Methods
2.1 Study area
The project activities are implemented in 20 villages (selected as described previously) and hospital data, (which indicated that these were highly endemic for S.mansoni infection) of Ukerewe District Council, northwestern Tanzania. The project activities are implemented in the following villages: Bugorola, Bugula, Bukiko, Bukindo, Bukondo, Bukungu, Busumba, Bwisya, Chibasi, Chifule, Gallu, Kamea, Kome, Kweru, Muriti, Muritilima, Musozi, Nebuye, Nyamanga, and Nyang’ombe (Figure 1). The district is an island on Lake Victoria that experiences a tropical climate characterized by bimodal rainfall with annual rainfall of 1,090mm of rainfall and temperature ranges from 17°C to 27°C. According to the population census of 2022, the district had a total of 387,815 inhabitants (13). The common economic activities of the inhabitants are fishing and subsistence farming (mainly cultivating cassava, maize, banana, potatoes, and a variety of fruits). In terms of water supplies, none of the villages included in the current study had tap water supply systems. Lake Victoria is the main source of water for domestic, recreational, and agricultural use. Open defecation along the lake shoreline or within the lake is common (14).
The district is known to be endemic for intestinal schistosomiasis, and S. mansoni has been recorded in pre-school children (15), school-aged children (2), and adults (5). Hepatosplenic disease related to S. mansoni infection is common on the island (2, 16, 17). The routine control of schistosomiasis done by the National NTD program targets only school-aged children within the school environment and all those who are out of school are not included.
2.2 Pre-mass preventive chemotherapy intervention activities
2.2.1 Baseline assessment of prevalence and intensity of S. mansoni infection
Overall, the study utilized a quasi-experimental design (before and after intervention) characterized by baseline and post-intervention cross-sectional surveys. The baseline cross-sectional parasitological surveys were conducted prior to the implementation of mass preventive chemotherapy to determine the prevalence and infection intensity. From each village, a total of 200 randomly selected adult (≥15 years) community members were invited to participate and submitted a single stool sample that was processed using the Kato Katz technique. A sub-sample of study participants were requested to submit an additional single urine sample for detection of S. mansoni circulating cathodic antigen using a point-of-care Circulating Cathodic Antigen test following the manufacturer’s instructions (18).
2.2.1.1 Training of community health workers, community leaders, and community drug distributors
Before implementation of the project’s activities, community members through their leaders in each village and sub-villages were asked to identify community drug distributors (CDDs). The selected CDDs had the following qualities: (i) member of the community;(ii) completed at least primary or at least secondary school education, knows how to write Kiswahili, and keeps records; and (iii) knows how to speak and communicate using the local language (Kikerewe/Kijita) of the community to allow easy communication with other community members. In sub-villages or villages where community health workers (CHWs) existed, the CHWs were included in the training and participated in project activities. A total of 280 CDDs and 160 village and sub-village leaders (village chairperson=20 and sub-village chairperson=140) participated in 5 days of training. The training covered schistosomiasis epidemiology and common names of the disease and its related symptoms and signs, how the disease is transmitted, risk factors related to the disease transmission, and how to refer community members with severe signs such as vomiting blood to nearby health facilities for further assessment and preventive and control measures. The training also covered the treatment of schistosomiasis, specifically praziquantel (PZQ), its side effects, and how to manage mild side effects and refer those with severe side effects to nearby health facilities or call healthcare workers. The training also included how to use the praziquantel dose pole to estimate the number of tablets to be offered to community members. An additional topic was communication, specifically how CDDs and community leaders should communicate to the community members when educating the community about schistosomiasis and its related preventive measures and the importance of adhering to preventive measures and participating in mass preventive chemotherapy which was offered by CDD accompanied by their leaders.
The training integrated lectures with pictures, group discussion to allow community members to ask questions and learn about the presence of disease symptoms and signs among community members, and a practical session in which CDDs practiced how to measure height using the PZQ pole, offer drugs, and record information about the participants and number of tablets given. A CDD book was compiled in Kiswahili and was distributed to all community members for review and continued reading.
2.2.2 Population census, registration of community members, and community sensitization
A house-to-house population census was conducted in all the study villages and sub-villages among registered community members (≥15 years of age). This was important for planning the mass preventive chemotherapy and the number of PZQ tablets needed. This exercise was accompanied by offering public health education related to schistosomiasis to household members and informing them about the coming mass preventive chemotherapy exercise and the need and importance for them to participate fully. The exercise was led by trained CDDs and leaders in their respective jurisdictions. Because of the migratory nature of the fishing communities and accompanying populations, it was necessary to conduct a population census before each of the treatment rounds. Community leaders and CDDs/healthcare workers were tasked with and supported to conduct a population census of their sub-villages and provided data/census books to the district neglected tropical disease (NTD) coordinator for processing and planning of the next mass preventive chemotherapy exercise.
2.2.3 District team, community, and project microplanning for delivery of mass preventive chemotherapy
The Ukerewe District medical department, the project team, and the community teams conducted several meetings to understand the role of each team in the delivery of these interventions. The district medical department was responsible for ordering drugs from the Ministry of Health, receiving and storing drugs at the district center, coordinating all the dispensaries and health centers as distributing centers for drugs to CDDs and receiving back the drugs after the mass drug administration (MDA) was completed, continuing to distribute the drugs to community members who missed the community-based MDA in the next 30 days, and offering healthcare workers opportunities to participate in the exercise, especially for monitoring and managing side effects. The community team (comprising CDDs and village leaders) was responsible for conducting sensitization in their sub-villages. Furthermore, the community team was responsible for collecting the drugs from nearby health facilities and distributing them to community members using the agreed approach. Most teams opted to set a distribution point within a sub-village, where they usually met for different community-related activities or conducted house-to-house distribution, and recorded all participant information (age, sex, area of residence, height, and number of pills received). The project team was responsible for supporting the community awareness campaign by using loudspeakers to make public announcements on a moving vehicle from one village to another 3 days prior to offering MDA; ensuring the drugs were transported from the district center to all health facilities in the 20 villages; facilitating the transport of healthcare workers to reach all the villages on the days of MDA implementation; preparing and distributing training materials and sensitization materials; training of healthcare workers to manage minor and severe side effects; recording books and other stationaries; preparing and distributing sensitization materials; facilitating sensitization exercises; collecting and returning the remaining drugs to the district center; collecting all CDD recording books after MDA and data enumerations; conducting MDA coverage assessment and sharing data with the relevant stakeholders; and conducting feedback meetings with the community.
2.3 Implementation of mass preventive chemotherapy
After the baseline parasitological and ultrasonographical surveys were conducted (under publication consideration), the first round of mass preventive chemotherapy was implemented in June 2021 and two rounds were implemented in January and June 2023. There was no treatment in 2022 due to unavailability of PZQ in the country. Community village and sub-village leaders organized the treatment campaign, and trained CDDs delivered the drugs to the community members using a household-to-household (door-to-door) method or at an agreed site within the sub-village. Community drug distributors offered PZQ as a single dose of 40mg/kg body weight using the WHO PZQ dose pole (19, 20). Inclusion criteria for community treatment were age ≥15 years, both male and female community members, and willingly accepting the drug and taking it under the observation of CDD. Exclusion criteria were children below 15 years because children aged 6 to 14 years received treatment within the school environment. In addition, children aged between 2 and 5 years, adult individuals reported to be sick, and those reported to be pregnant at the time of treatment were not included (the national treatment guideline of Tanzania does not recommend the use of PZQ during pregnancy). To ensure school-aged children (SAC) were not involved in the community treatment, mass preventive chemotherapy was offered during the school days. Furthermore, to ensure that the school children continued to receive treatment at school, the project supported 36 schools located within the targeted 20 villages to receive annual mass preventive chemotherapy through the district council. This was important to reduce the rate of disease transmission. For the villages or sub-villages that opted for the household-to-household approach, consent to enter and include a household in the treatment was sought from the household head. Community members were free to decline treatment. During the mass treatment exercise, CDDs collected data on the participants’ age, sex, village, and sub-village of residence, measured the height of an individual using the PZQ dose pole, and estimated the number of PZQ tablets to be offered. The height and number of tablets were recorded in the CDD book. At the end of each treatment day (6 pm), CDDs returned the remaining drugs to the health facilities and collected them on the next day of treatment to continue with the exercise. The mass preventive chemotherapy exercise was conducted for 2 consecutive days.
From the community (house-to-house) population census data, the treatment coverage was estimated by comparing the number of adult community members who received PZQ as recorded by the CCDs with the eligible population in each of the villages involved in the study.
2.4 Data collection for the follow-up cross-sectional surveys
Coverage evaluation surveys: A total of 20,976 households were found in the 20 villages that took part in the project. Depending on the size of the village/community, the lowest number of households was 335 in Bukindo and the highest was 1,826 in Muritilima. The estimated sample size of households involved in the coverage surveys was based on the following: considering a margin error of 5% at a 95% confidence interval, a design effect of 1.6, and a non-response rate of 10%, , where n is the required sample size, r is the coverage indicator, deff is the design effect, p is the proportion, and ñ is the minimum household size (21–23). Thus, a minimum of 65 households were needed per village, giving a combined sample size of 1,300 (65 x 20) households. The study’s statistician used a simple random sampling method (a list of all households from each village was obtained prior to the exercises and a unique identification number for each house was used during randomization) to select the households involved in the CES. Except for round one of the CES (during which 1-2 participants were selected per household to be included in the study) (23), in the subsequent rounds, at least 4 to 6 adult participants (individuals aged ≥18 years) per household were involved in the CES. A household was defined as “a group of persons who normally live and eat together”. If there were more than six people in the household at the time of the interview, a simple random sampling technique was used to select six participants (23).
For the parasitological surveys, a technique described elsewhere (23, 24) was used to select participants to participate in the prevalence surveys to assess the effect of treatment on the prevalence and intensity of infection. In each village, to detect 5% changes in prevalence, with a power of 0.80 and a significance level of 5% (α=0.05), at least 100 adults were needed per village. Participants were randomly selected from randomly selected households (a list of all households from each village was obtained prior to the exercises and a unique identification number for each house was used during randomization). If a selected household had no inhabitants on the day of sample collection, a nearby household was selected. Similarly, if the selected individual(s) refused to participate, individuals from a nearby household were selected. A total of 2,041 adult participants were selected and consented to participate in the study.
2.5 Inclusion and exclusion criteria
2.5.1 Parasitological examination for S. mansoni infection and intensity
From each consenting participant, a single stool sample was obtained in a labeled container, from which duplicate Kato Katz thick smears were prepared using a template of 41.7 mg (25). Each of these slides was examined 24 hours after preparation for the presence of S. mansoni eggs and all quality standards described elsewhere were strictly followed (26).
2.6 Post-mass preventive chemotherapy coverage surveys
To evaluate the coverage reported by the CDDs, within 2 weeks after completion of the mass preventive chemotherapy, cross-sectional surveys were conducted to assess the community coverage of the mass preventive chemotherapy. The findings were compared with what was reported by the CDDs during the active mass preventive chemotherapy exercise. The surveys were conducted using the WHO-recommended methods (21, 22). The survey used a questionnaire that has been used in previous studies (23). Trained research assistants conducted the survey in the selected sub-villages using the questionnaire, which was designed using the Open Data Kit (ODK) tool.
2.7 Data management
Data were entered using Excel and transferred to Stata version 17 (StataCorp, statistical software, College Station, TX: StataCorp LP. Texas, USA). All continuous variables were summarized using mean, median, and their standard deviations or interquartile ranges depending on data distributions. A proportional test was used to compare the prevalence of S. mansoni infections by demographic characteristics and the participating villages. S. mansoni egg counts were obtained from two slides and the mean was multiplied by 24 to obtain eggs per gram of feces. S. mansoni geometrical mean eggs per gram of feces (GMepg) was calculated using the non-logarithmically transformed means. The comparison of mean S. mansoni egg counts was done using either Student’s t-test (for two groups) or ANOVA (for more than two groups). The intensity of infection was classified according to WHO criteria in which individuals with 1-99 epg, 100-399 epg, and ≥ 400 epg had a low, moderate, and heavy intensity of infection, respectively (8). To assess the changes or decline in prevalence and intensity of infection, data were compared between the baseline and after three rounds of treatment stratified by village, age, and sex. The overall comparison of prevalence and intensity was age-standardized. For all the analyses, the villages were used as a primary sampling unit.
The coverage was calculated as described previously (22, 23). Briefly, coverage was calculated as the proportion of adults who were reported to have received and swallowed PZQ among those who were interviewed (Coverage=Number of adults who swallowed PZQ/Total number of individuals surveyed]). This was compared with what was reported by the CDDs during the mass PC campaigns. These data were obtained from the registers used by the CDDs.
2.8 Ethical consideration
Ethical approval to implement the project’s activities including the current study was obtained from the National Ethical Review Committee board (NIMR/HQ/R.8a/Vol.IX/3590 and NIMR/HQ/R.8C/Vol.I/1973) and further implementation permissions were received from the Prime Minister’s Office for Local Governments, the Mwanza region’s administrative office, and the Ukerewe District Council, where the study is being implemented. Written informed consent to participate in the current study was obtained from all study participants. The informed consent form was written in Kiswahili and each participant received a copy of their written informed consent. For illiterate participants, the consent form was signed using a thumbprint after they had received a clear oral description of the study objectives and the treatment options. The consent form described the objectives, duration, risks, and benefits of participating in the study. Participation in the study was voluntary and the participants had the right to continue or to withdraw their consent from the study at any time.
2.9 Treatment
All participants received praziquantel (40mg/kgBWT) regardless of their infection status. The medication was administered under direct observation at the study site within the village.
3 Results
3.1 Parasitological surveys
3.1.1 Demographic information of the participants
A total of 2,041 adult participants from 20 villages were involved in the MDA follow-up study. Of these participants, 47.8% (n=975) and 52.2% (n=1,066) were men and women respectively. The mean age of the participants was 48.6 ± 16.7 years. Of all the participants, 82.7% (1688/2,041) reported having participated in a mass PC while 17.3% (353/2,041, women=184 and men=169) reported having never participated in MDAs implemented in their villages.
3.2 Post-treatment prevalence and intensity of S. mansoni infection
Briefly, at baseline, the overall prevalence of S. mansoni infection based on the Kato Katz technique was 30.4% (95%CI:29.0-31.9%), with male participants having a higher prevalence than female participants (32.2% versus 28%, χ2(1) = 11.2005, P = 0.001). The youngest age groups, 18 to 25 years and 26 to 35 years, had the highest prevalence compared to the age group ≥ 36 years. TheGMepg was 105.3 (95%CI:98.7-112.3 GMepg), with men having the highest GMepg value of 113.8 (95%CI:104.0–124.4 GMepg) while the GMepg value of women was 96.3 (87.8-105.6 GMepg) (t = 3.6076, P = 0.001). There was a significant difference in the intensity of infection between the age groups, with the youngest age groups, 18 to 25 years (126.6–95%CI:100.3-131.9 GMepg) and 25 to 35 years (115.04-95%CI:100.3-131.9 GMepg), having the highest intensity of infection (F = 9.61, P = 0.001). Regarding infection intensity categories, 53.9%, 32.4%, and 13.7% had light, moderate, and heavy intensity of infection, respectively.
In the post-treatment period, the overall prevalence of S. mansoni was 9.5% (95%CI:8.3-10.8) and the geometrical mean egg intensity of infection was 79.9 epg of feces (95%CI:71.2-89.8), with women and men having a geometrical mean egg per gram of feces of 78.2 (66.9-91.7) and 81.6 (95%CI:68.6-97.1), respectively (t=-0.3042, P=0.8). Figure 2 shows the prevalence of S. mansoni infection before and after the implementation of the MDA categorized by age and sex.
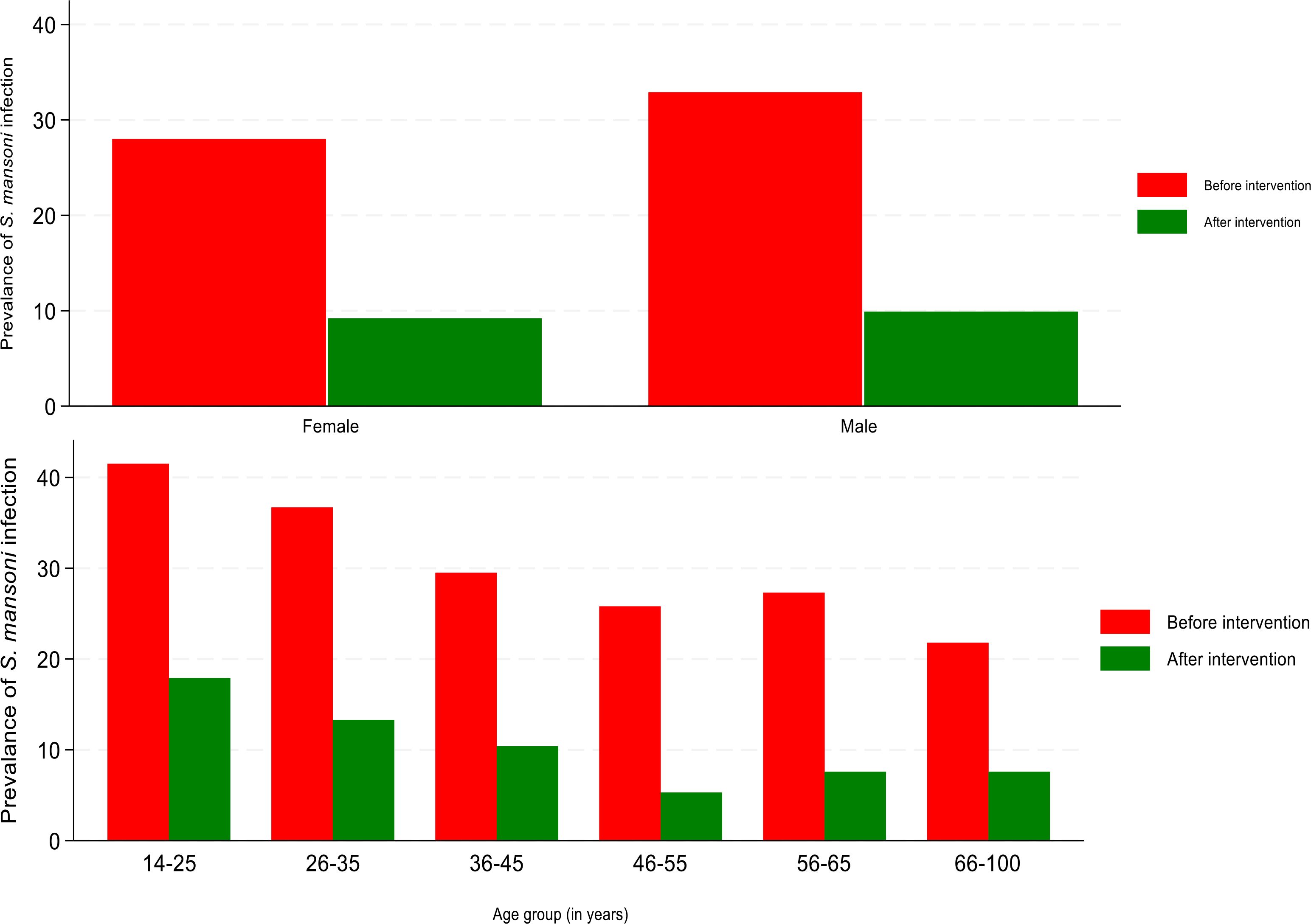
Figure 2. Changes in prevalence of S. mansoni infection at baseline and post-treatment categorized by age and sex.
When categorizing the prevalence by villages post-mass preventive chemotherapy, there was a significant variation in the prevalence of S. mansoni infection between villages (χ2 = 68.33771, P=0.001). Bugorola (11%), Bugula (17.5%), Bukungu (19.4%), Chifule (21.1%), Muritilima (10.8%), Musozi (16.2%), and Nyang’ombe (14.4%) all had a prevalence of S. mansoni of >10%. The remaining 13 villages recorded a prevalence of S. mansoni of <10%. Figure 3 shows the changes in the prevalence of S. mansoni infection at baseline and post-treatment in the targeted villages.
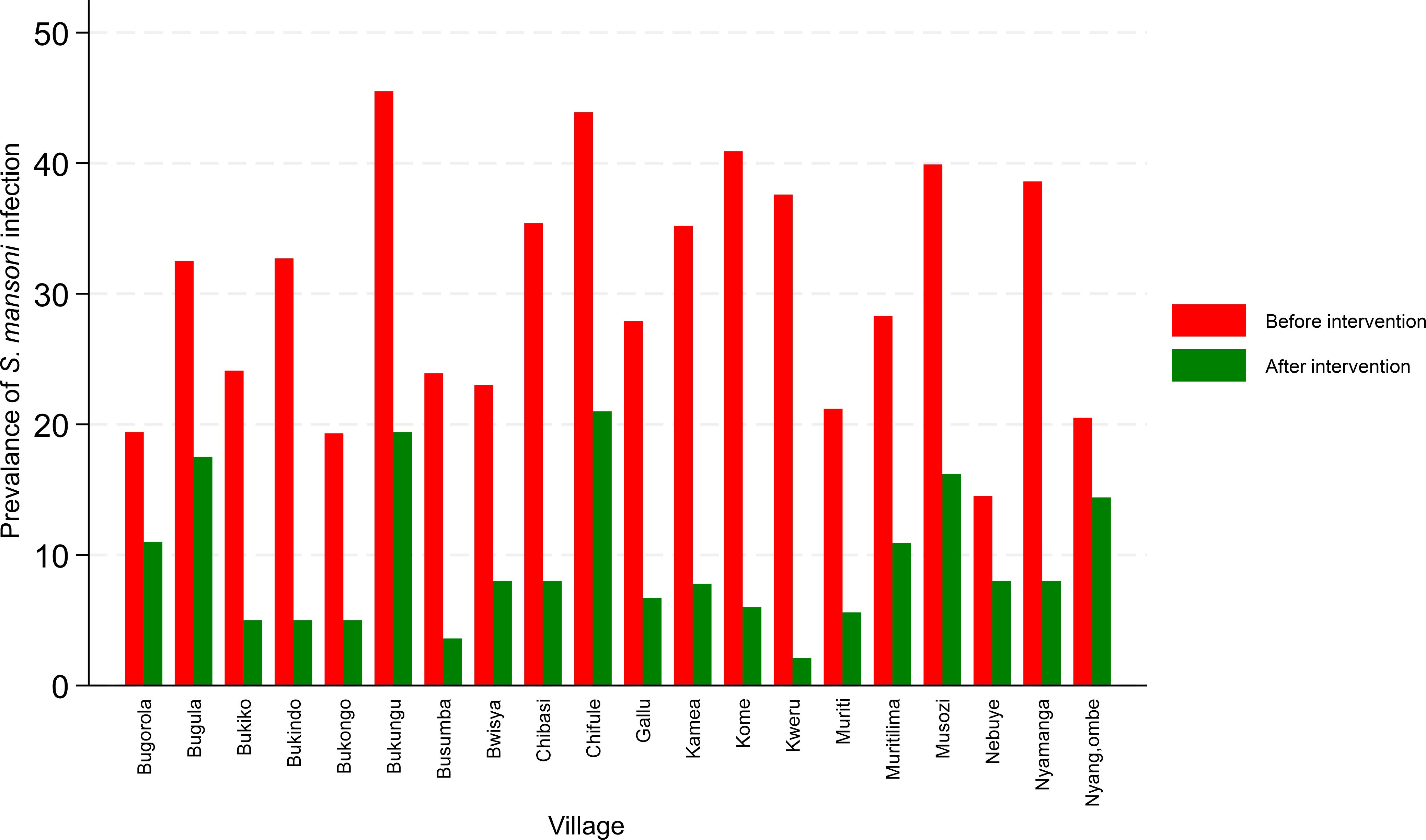
Figure 3. Changes in prevalence of S. mansoni infection at baseline and post-treatment in the targeted villages.
When categorizing the data based on reported participation in mass PC, the results indicate that those who reported having ever participated in any of the three treatment rounds had a significantly lower prevalence of S. mansoni infection than those who had not participated in any treatment round (8.1% versus 16.2%, χ2 = 21.8918, P<0.01).
Post-three mass PC rounds, the prevalence of S. mansoni infection declined from 30.4% (95%CI:29.0-31.9) to 9.5% (8.3% - 10.9%), representing a 68.7% reduction in the S. mansoni prevalence from baseline (P<0.0001). Across all 20 villages, Bugula (P=0.3), Bukungu (P=0.1), and Chifule (P=0.1) did not achieve significant changes in prevalence after three rounds of treatment. The remaining villages had a 46% to 94% reduction in S. mansoni prevalence. Conversely, a significant reduction in prevalence between the sex and age groups was observed. The reduction in S. mansoni prevalence after three rounds of mass preventive chemotherapy by villages, sex, and age groups is shown in Table 1.
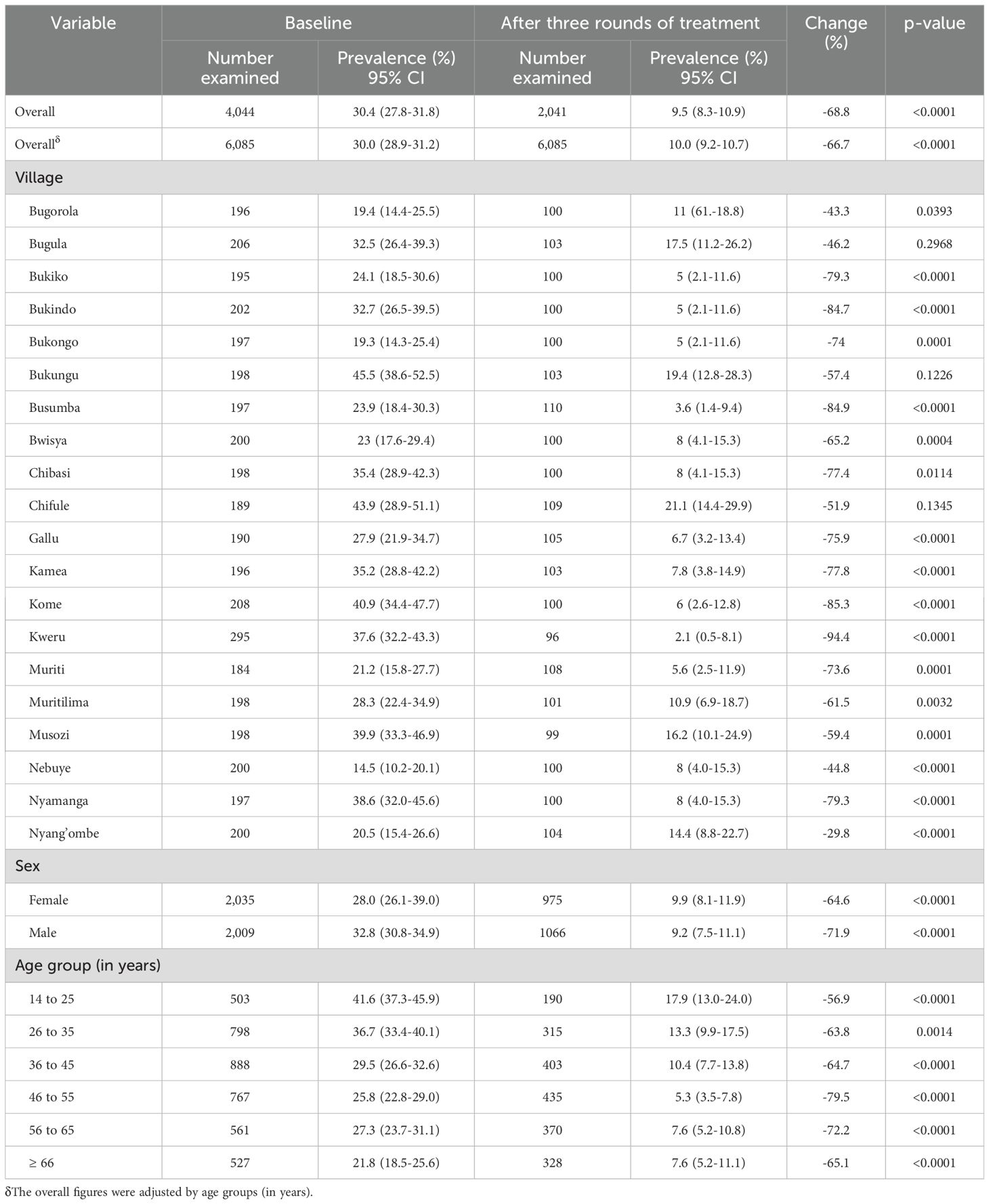
Table 1. Changes in prevalence of S. mansoni infection at baseline and post-three rounds of treatment in 20 villages in the Ukerewe District.
Table 2 summarizes the S. mansoni intensity of infection at baseline and post-treatment. The adjusted geometrical mean epg of feces from all the 20 villages post-treatment was 79.9 (95%CI:71.2-89.8). Compared with the baseline adjusted GMepg of 105.3 (95%CI:98.7-112.3), there was a significant decline in the intensity of infection from 105.3 GMepg to 79.9 GMepg, representing a reduction in the intensity of infection of 24.1% (P<0.0001). Across all 20 villages, a significant reduction in the intensity of S. mansoni infection was observed (Table 2). Significant reductions in the intensity of S. mansoni infection were observed between sex and age groups (Table 2).
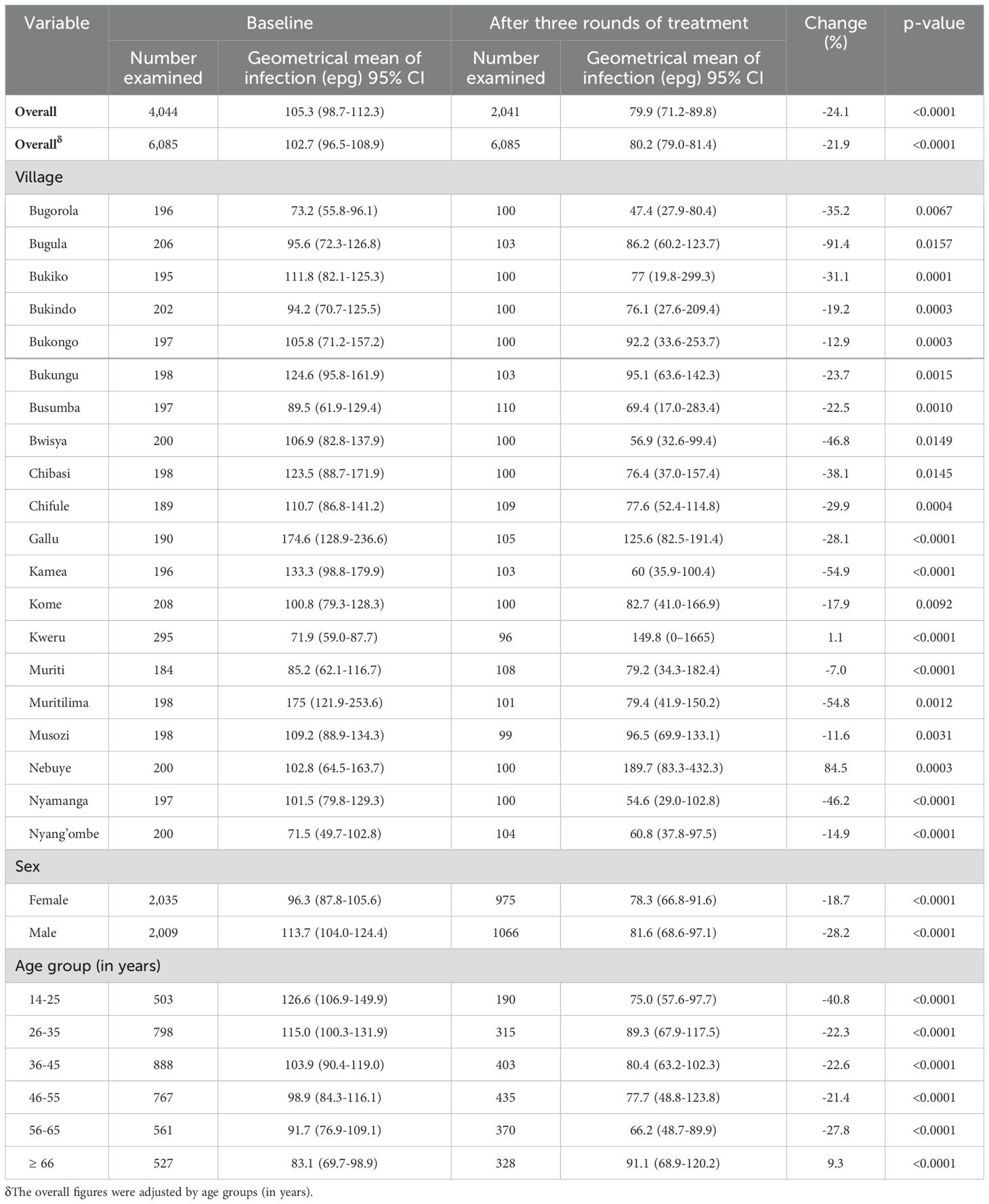
Table 2. Changes in the intensity of S. mansoni infection after three rounds of mass preventive chemotherapy in 20 villages in the Ukerewe District, northwestern Tanzania.
At baseline, 32.4% and 13.7% of the participants were either moderately or heavily infected with S. mansoni infection. After three rounds of mass preventive chemotherapy, 34.5% and 2.6% of the adults were moderately or heavily infected. After three rounds of mass preventive chemotherapy, the proportion of heavily infected adults declined significantly by 81%. Figure 4 shows the baseline and post-treatment changes in the proportions of individuals with low, moderate, and heavy intensity of infection.
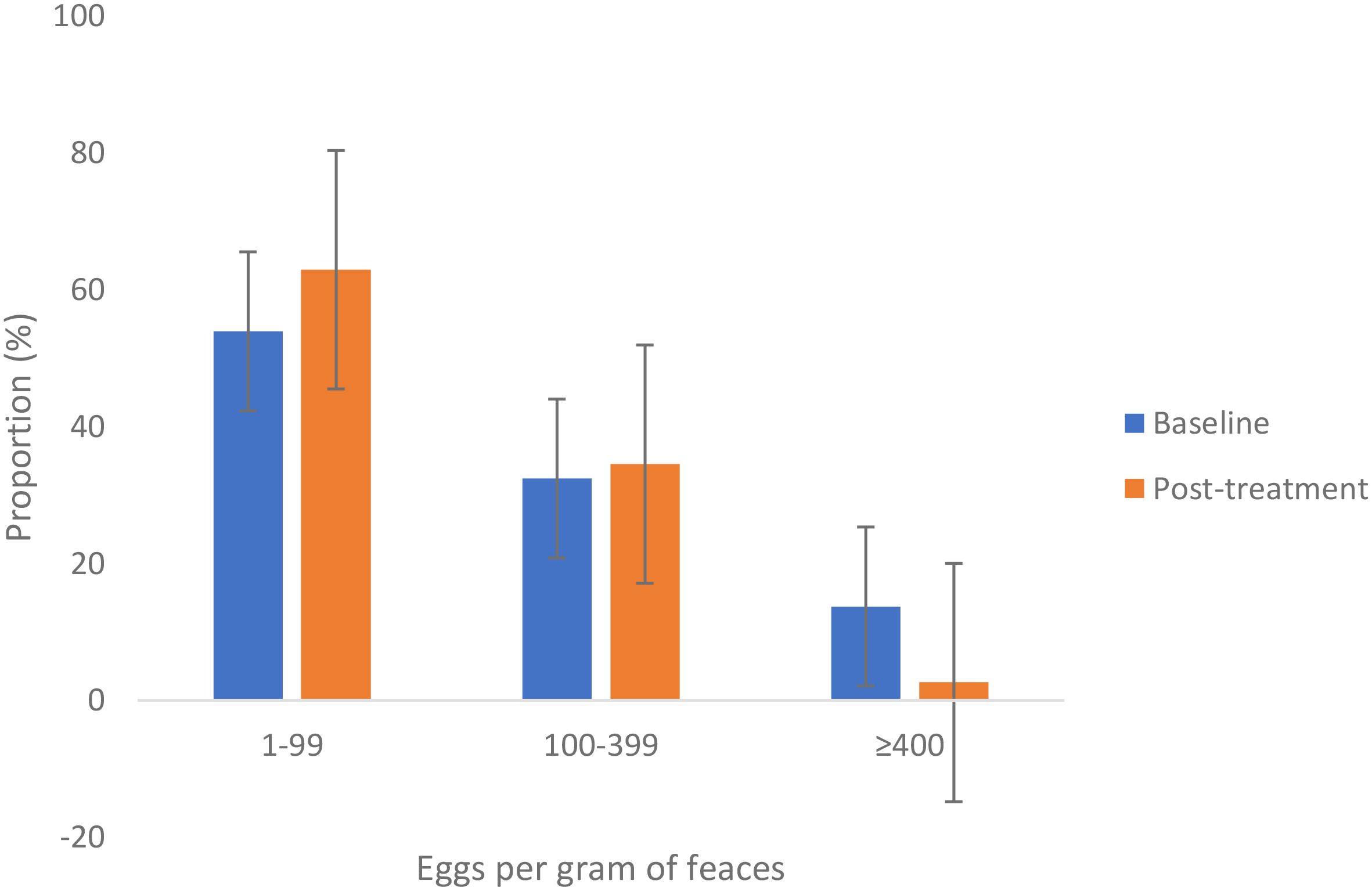
Figure 4. Proportion (%) of different degrees of intensity of S. mansoni infection among adult community members in Ukerewe District at baseline and after three rounds of treatment. Error bars represent the 95% confidence intervals.
3.3 Mass preventive chemotherapy coverage
The number targeted for treatment, the number of those treated, and the coverage as reported by the CCDs are shown in Table 3. The first round of mass preventive chemotherapy in July 2021 involved 15 villages located on the main island, Nansio Island. Later, in January and July (rounds two and three) 2023, mass preventive chemotherapy was expanded to an additional five villages on the neighboring Ukara Island.
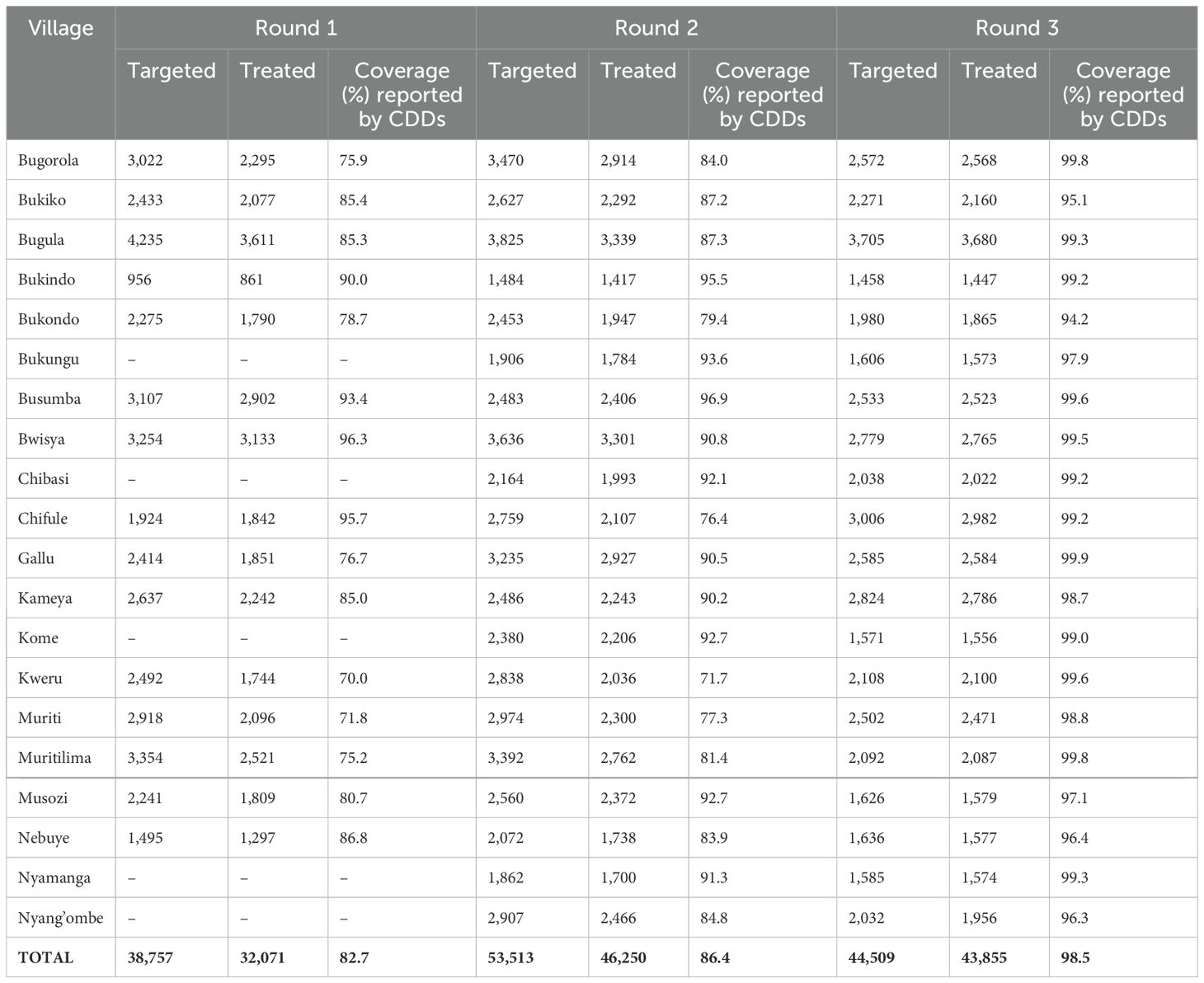
Table 3. The number of those treated and coverage for the three rounds of treatment for each village.
For the first 15 villages, a total of 38,757 adult individuals (≥15 years), were registered during the house-to-house census before the mass PC exercise. Of these, 32,071 participated and swallowed drugs during the mass PC, giving a coverage of 82.7%. The second round of treatment included 20 villages (140 sub-villages). A total of 53,513 adult individuals (aged ≥15 years) participated in the mass PC campaign. A total of 46,250 adult individuals swallowed the drugs, giving an overall coverage of 86.4%. The third round of MDA included a total of 44,509 adult individuals from 20 villages. A total of 43,855 adult individuals were reported to have swallowed drugs, giving an overall coverage of 98.5%. The migratory behavior of fishing communities affected the number targeted for treatment in round three as the mass PC was conducted during the time these communities, especially the men, moved away from their permanent villages for fishing.
Within 2 weeks after each treatment round, independent monitoring surveys were conducted in all the study villages. For round one, a total of 1,299 adult individuals from 15 villages were interviewed. In general, the mass PC coverage was 80.8%. For round two, a total of 5,615 adult individuals (≥ 15 years) from the 20 villages were randomly selected and involved in the coverage monitoring surveys. Of the interviewed adult participants, 78.5% confirmed they had taken part in the mass PC and swallowed the drugs. The mass PC coverage was 78.5%. For round three, a total of 5,617 adult individuals (≥15 years) were involved in the survey and a total of 81.9% reported to have swallowed the drug. The mass PC coverage was 81.9%. Table 4 summarizes the results of the monitoring surveys in all three rounds of treatment.
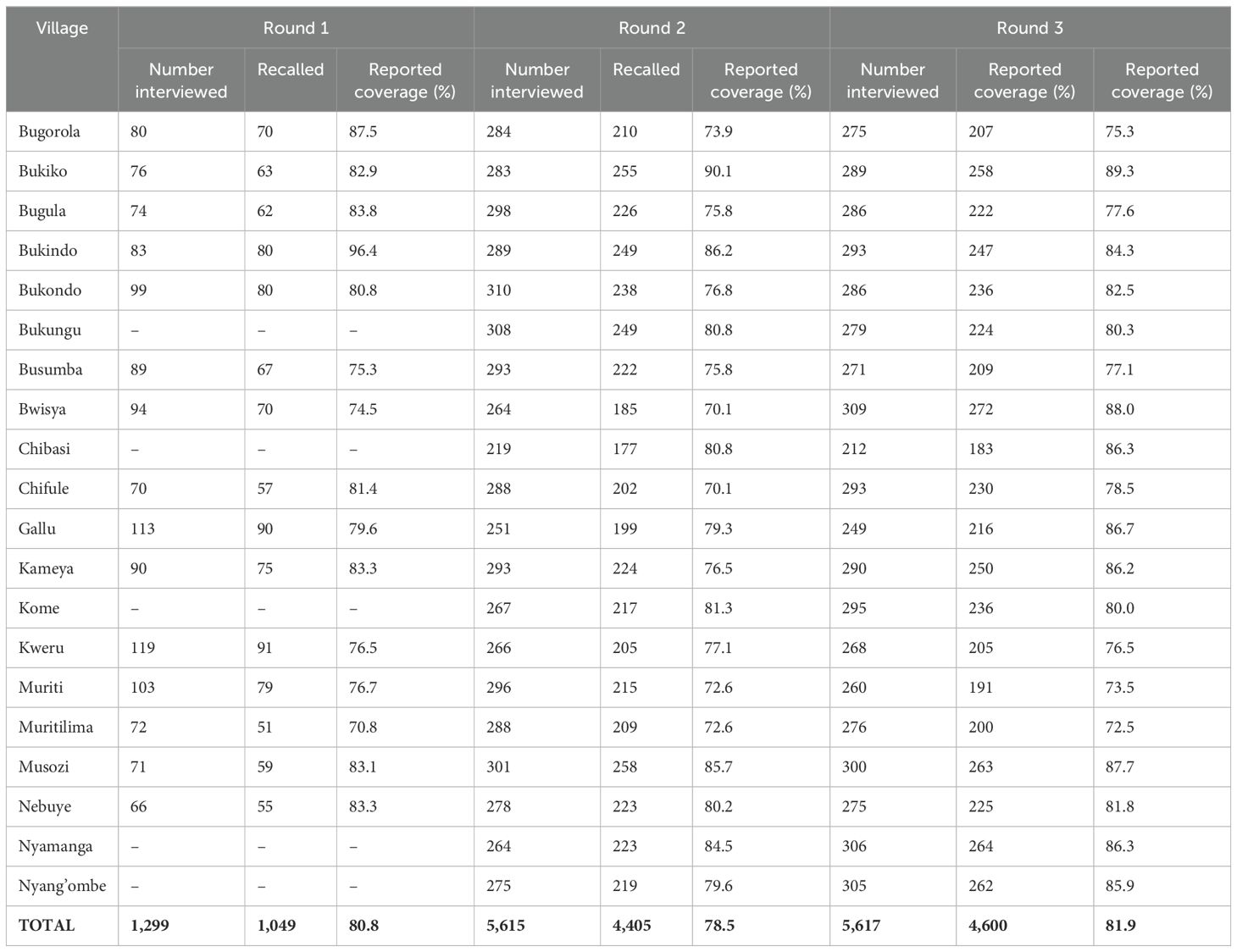
Table 4. Post-mass drug administration household coverage assessment during independent monitoring surveys in 20 villages.
4 Discussion
The parasitological assessment of the adult community members following three rounds of mass PC revealed that there was a decline in the prevalence and intensity of S. mansoni infection in the targeted villages. The prevalence of S. mansoni infection declined from an adjusted baseline prevalence of 30.4% to an adjusted prevalence of 9.5%, with significant variations between villages. Conversely, the intensity of S. mansoni infection declined from an adjusted mean of 105.3 GMepg at baseline to 79.9 GMepg after three rounds of treatment. A change in the proportion of adult individuals either heavily or moderately infected was also observed after three rounds of treatment. The average treatment coverage for all three treatment rounds reported by the CDDs and that of the monitoring team did not differ significantly and were above the WHO recommendation of ≥75%.
Mass PC using either the school or community-based approaches (24, 27–29) leads to rapid changes or decline in prevalence and intensity of infection. Furthermore, the treatment rounds lead to a reduction in the proportion of adults either heavily or moderately infected (27). This is because PZQ either kills or decreases the fecundity of the female worms (30), which leads to a decline in the number of eggs released from infected individuals. A similar study using community-based mass PC targeting an adult population in Western Kenya reported a rapid decline in the prevalence and intensity of S.mansoni infection in the targeted villages after two rounds of treatment (24). A significant reduction in the prevalence and intensity of infection using community-based mass PC targeting adults was reported in Uganda (28) and Burkina Faso (27). It is worthwhile to note that in the current study setting, school-aged children, a group at high risk and carrying high infection intensity, received treatment at school and for three consecutive years have received similar rounds of treatment. This can at least partly explain the rapid decline in the prevalence and intensity of infection observed in the current study villages, as it is similar to studies that combined school- and community-based treatment approaches (24, 27, 28). Statistical modeling studies have also pointed out that community-wide treatment that includes all at-risk populations out of the school environment can support endemic countries in eliminating schistosomiasis as a public health challenge (31).
The three rounds of treatment did not achieve a uniform decline in the prevalence of S. mansoni infection in all the targeted villages. This is a common observation in schistosomiasis-endemic countries (24, 28) and indicates that three rounds of treatment are not enough to halt the transmission. At present, the WHO recommends treatment to be offered based on the prevalence levels (8). Areas with a prevalence of schistosomiasis of ≥10% are recommended to receive annual mass PC (8) and those with a prevalence of <10%, depending on the availability of any mass PC program, should continue to receive the intervention (8). Observing the changes in prevalence after three rounds of treatment in these villages with seven villages having a prevalence of ≥ 10%, certainly, these villages still require more rounds of mass PC to reduce the rate of transmission. In addition, in order to achieve a high impact on prevalence, intensity of infection, and morbidities, it will be important to geographically expand the intervention to other villages that are equally at-risk and include other non-school-going groups, such as pre-school children.
In the current study, a decline in the proportion of adults with heavy intensity of infection was observed. This is a common observation in schistosomiasis-endemic areas following repeated rounds of treatment (27). The WHO estimates that after 2 or 3 years of effective mass PC, the proportion of heavily infected (>400 epg) should be <1% (32). In the current study, the proportion of heavily infected individuals declined significantly, from 13.7% to 2.6% after three rounds of treatment. This was also similar to the findings of a previous study in Sierra Leone after 3 years of effective mass PC (27). To continue reducing the force of transmission and the proportion of adult individuals who have heavy or moderate intensity of infection, continued mass PC is still needed and the integration of other measures such as improved water supply, sanitation, and hygiene is highly recommended. On Ukerewe Island, the availability of a clean water supply and improved sanitation are major public health challenges (33) and in order for this area to reach the elimination stage for schistosomiasis, water, sanitation, and hygiene (WASH) improvement should be integrated in future schistosomiasis control activities. The provision of proper sanitation facilities, a supply of clean piped water, and strategies to ensure proper personal/community hygiene will help reduce the transmission rate of the disease.
Effective mass PC and high coverage are key in the effort to control and eliminate schistosomiasis as a public health challenge. High mass PC coverage is highly recommended for endemic countries, with a coverage of ≥ 75% for schistosomiasis in each treatment round (8). In the current study, for the three rounds of treatment implemented in 20 villages, the CDD data and an independent survey revealed the average coverage was above the WHO-recommended threshold (8). The high coverage is important for schistosomiasis control and elimination programs, especially for programs that depend entirely on mass PC (8). The high mass PC coverage observed in the current study was partly related to high community involvement in the planning, mobilization, and implementation. Effective advocacy, social mobilization using all the community organization structures including beach management units and communal organizations such as fishing camps, involvement of all leaders (elected and governments), and coordination between the district council teams and the project staff lead to high coverage. The “in process” monitoring during the mass PC surveys that identified any challenges and gaps such as drug shortages or any issues led to high mass PC coverage. The project adapted the microplanning approach recommended by WHO, which emphasizes bottom-up planning to facilitate effective implementation and monitoring of public health program (34).
Despite the observed high coverage and the rapid decline in the prevalence and intensity of S. mansoni infection, there are still a number of challenges that need to be addressed in order to achieve 100% treatment coverage. A proportion of the population does not adhere to treatment campaigns. To achieve disease control and subsequent elimination of schistosomiasis as a public health challenge, individual and group compliance to treatment is needed (31). This not only will reduce the rate of disease transmission but also will lower the number of treatment rounds required to achieve the control objectives (31). A second challenge is that the current intervention does not target other non-school-going community members such as pre-school children, who are equally at-risk and infected by S. mansoni (15). Untreated pre-school children and adult community members who are systematically not complying with treatment serve as a source of infection for the treated groups and maintain transmission within the treated villages (8). The current WHO recommendation for schistosomiasis control recommends the inclusion of pre-school-aged children from the age of 2 years (8); however, the use of the currently available praziquantel in this age group is still controversial (35–37) and global efforts are ongoing to manufacture and distribute a more palatable pediatric formulation of the same drug in the near future (38). A final challenge is that although the current intervention includes public health education to raise community awareness, knowledge, and adherence to preventive measures, WASH interventions, which are important and complement the mass PC (8), are not covered. WASH interventions are very costly and beyond the budget of the current project but are very sustainable interventions to support the country moving towards control and elimination of schistosomiasis as a public health challenge (9).
5 Conclusion
The mid-term evaluation results from this 5-year ongoing schistosomiasis control project reveal that the three treatment rounds resulted in a significant reduction in the prevalence and intensity of S. mansoni infection among adult community members. The high treatment coverage levels above the WHO coverage recommendation observed in all targeted villages have contributed to the observed changes in the prevalence and intensity of infection. The decline in the prevalence and intensity of S. mansoni infection was not uniform for all the villages. Some continued to have a prevalence of S. mansoni infection >10%, meaning that these villages will require additional rounds of mass PC or more than one treatment round per year.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The ethical approval to implement the project activities including the current study was obtained from the National Ethical Review Committee board (NIMR/HQ/R.8a/Vol.IX/3590 and NIMR/HQ/R.8C/Vol.I/1973) and further implementation permission was received from the Prime Minister’s Office for Local Governments, the Mwanza region’s administrative office, and the Ukerewe District Council, where the study is being implemented. Written informed consent to participate in the current study was obtained from all study participants. The informed consent form was written in Kiswahili language, and each participant received a copy of their written informed consent. For illiterate participants, the consent form was signed using a thumbprint after they had received a clear oral description of the study objectives and the treatment options.
Author contributions
CC: Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. EM: Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. TB: Investigation, Methodology, Supervision, Writing – review & editing, Writing – original draft. CM: Investigation, Methodology, Supervision, Writing – review & editing, Writing – original draft. SK: Funding acquisition, Project administration, Writing – original draft, Writing – review & editing, Investigation. CK: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. AF: Formal Analysis, Funding acquisition, Investigation, Writing – review & editing, Writing – original draft. AM: Funding acquisition, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. PS: Formal Analysis, Writing – review & editing, Writing – original draft. HM: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This project is funded by the Else Kröner-Fresenius-Stiftung (EKFS) in Germany (Grant number:-2018_HA10SP), through the Else Kröner Center for advanced medical and medical humanitarian studies.
Acknowledgments
We would like to acknowledge the participants, community leaders, and community healthcare workers of the 20 villages involved in the study for their cooperation and support during the fieldwork. We are also grateful to the district authorities for supporting this study and ensuring that community members have access to the study. We acknowledge the technical team from the National Institute for Medical Research, Mwanza Centre, and the Bugando Medical Centre, Mwanza, for supporting the fieldwork in the 20 villages.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mazigo HD, Nuwaha F, Kinung’hi SM, Morona D, Pinot de Moira A, Wilson S, et al. Epidemiology and control of human schistosomiasis in Tanzania. Parasit Vect. (2012) 5:274. doi: 10.1186/1756-3305-5-274
2. Mugono M, Konje E, Kuhn S, Mpogoro FJ, Morona D, Mazigo HD. Intestinal schistosomiasis and geohelminths of Ukara Island, North-Western Tanzania: prevalence, intensity of infection and associated risk factors among school children. Parasit Vectors. (2014) 7:612. doi: 10.1186/s13071-014-0612-5
3. Mueller A, Fuss A, Ziegler U, Kaatano GM, Mazigo HD. Intestinal schistosomiasis of Ijinga Island, north-western Tanzania: prevalence, intensity of infection, hepatosplenic morbidities and their associated factors. BMC Infect Dis. (2019) 19:832. doi: 10.1186/s12879-019-4451-z
4. Mazigo HD, Dunne DW, Morona D, Lutufyo TE, Kinung’hi SM, Kaatano G, et al. Periportal fibrosis, liver and spleen sizes among S. mansoni mono or co-infected individuals with human immunodeficiency virus-1 in fishing villages along Lake Victoria shores, North-Western, Tanzania. Parasit Vect. (2015) 8:260. doi: 10.1186/s13071-015-0876-4
5. Malenganisho WL, Magnussen P, Friis H, Siza J, Kaatano G, Temu M, et al. Schistosoma mansoni morbidity among adults in two villages along Lake Victoria shores in Mwanza District, Tanzania. Trans R Soc Trop Med Hyg. (2008) 102:532–41. doi: 10.1016/j.trstmh.2008.03.006
6. Rollinson D, Knopp S, Levitz S, Stothard JR, Tchuem Tchuente LA, Garba A, et al. Time to set the agenda for schistosomiasis elimination. Acta Trop. (2013) 128:423–40. doi: 10.1016/j.actatropica.2012.04.013
7. Mbugi NO, Laizer H, Chacha M, Mbega E. Prevalence of human schistosomiasis in various regions of Tanzania Mainland and Zanzibar: A systematic review and meta-analysis of studies conducted for the past ten years (2013-2023). PloS Negl Trop Dis. (2024) 18:e0012462. doi: 10.1371/journal.pntd.0012462
8. World Health Organization. WHO guideline on control and elimination of human schistosomiasis. Geneva, Switzerland: World Health Organization (2021).
9. World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030. Geneva, Switzerland: WHO (2020).
10. World Health Organization. Guideline. Preventive chemotherapy to control-soil-transmitted helminth infections in at risk populations groups, 2017. Geneva: WOrld Health Organization Licence: CC BY-NC-SA 30 IGO (2017).
11. World Health Organization Expert Committee. Prevention and control of schistosomiasis and soil-transmitted helminthiasis. World Health Organization Technical Report Series,912. Geneva, Switzerland: World Health Organization. (2002). pp. 1–57.
12. Chofle AA, Jaka H, Koy M, Smart LR, Kabangila R, Ewings FM, et al. Oesophageal varices, schistosomiasis, and mortality among patients admitted with haematemesis in Mwanza, Tanzania: a prospective cohort study. BMC Infect Dis. (2014) 14:303. doi: 10.1186/1471-2334-14-303
13. Tanzania National Bureau of Statistics. Tanzania Populations census. Geneva, Switzerland: 2012Tanzania Government (2022).
14. Berling SM, Mgabo MR, Salu M. The persistence of open defecation in fishing communities of Lake Victoria; A reflection on inconsistent use of toilets in Ukerewe Island, Tanzania. Int J Res Soc Sci. (2013) 3(2):293–303.
15. Ruganuza DM, Mazigo HD, Waihenya R, Morona D, Mkoji GM. Schistosoma mansoni among pre-school children in Musozi village, Ukerewe Island, North-Western-Tanzania: prevalence and associated risk factors. Parasit Vectors. (2015) 8:377. doi: 10.1186/s13071-015-0997-9
16. El Scheich T, Hofer L, Kaatano G, Foya J, Odhiambo D, Igogote J, et al. Hepatosplenic morbidity due to Schistosoma mansoni in schoolchildren on Ukerewe Island, Tanzania. Parasitol Res. (2012) 110:2515–20. doi: 10.1007/s00436-011-2793-6
17. Kardorff R, Gabone RM, Mugashe C, Obiga D, Ramarokoto CE, Mahlert C, et al. Schistosoma mansoni-related morbidity on Ukerewe Island, Tanzania: clinical, ultrasonographical and biochemical parameters. Trop Med Intern Health. (1997) 2:230–9. doi: 10.1046/j.1365-3156.1997.d01-269.x
18. Mazigo HD, Chiombola CE, Mugassa S, Magambo M, Kaatano GM, Leeyio T, et al. Control and elimination of Schistosoma mansoni infection in adult individuals on Ukerewe island, northwestern Tanzania: baseline results before implementation of intervention measures. BMC Infect Dis. (2024) 24:1102. doi: 10.1186/s12879-024-10010-1
19. Hall A, Nokes C, Wen ST, Adjei S, Kihamia C, Mwanri L, et al. Alternatives to bodyweight for estimating the dose of praziquantel needed to treat schistosomiasis. Trans R Soc Trop Med Hyg. (1999) 93:653–8. doi: 10.1016/S0035-9203(99)90087-1
20. Montresor A, Engels D, Chitsulo L, Bundy DA, Brooker S, Savioli L. Development and validation of a ‘tablet pole’ for the administration of praziquantel in sub-Saharan Africa. Trans R Soc Trop Med Hyg. (2001) 95:542–4. doi: 10.1016/S0035-9203(01)90034-3
21. WHO. Preventive chemotherapy tools for improving the quality of reported data and information: a field manual for implementation. Geneva: World Health Organization (2019).
22. Liyew EF, Chernet M, Belay H, Maddren R, Landeryou T, Kalahasti S, et al. Coverage evaluation surveys following soil-transmitted helminthiasis and schistosomiasis mass drug administration in Wolaita Zone of Ethiopia-The Geshiyaro project. PloS One. (2021) 16:e0260722. doi: 10.1371/journal.pone.0260722
23. Knopp S, Person B, Ame SM, Ali SM, Muhsin J, Juma S, et al. Praziquantel coverage in schools and communities targeted for the elimination of urogenital schistosomiasis in Zanzibar: a cross-sectional survey. Parasites Vectors. (2016) 9. doi: 10.1186/s13071-015-1244-0
24. Onkanga IO, Mwinzi PN, Muchiri G, Andiego K, Omedo M, Karanja DM, et al. Impact of two rounds of praziquantel mass drug administration on Schistosoma mansoni infection prevalence and intensity: a comparison between community wide treatment and school based treatment in western Kenya. Int J Parasitol. (2016) 46:439–45. doi: 10.1016/j.ijpara.2016.01.006
25. Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. (1972) 14(6):397–400.
26. World Health Organization. Basic laboratory methods in medical parasitology. Geneva: World Health Organization 1991 (1991).
27. Sesay S, Paye J, Bah MS, McCarthy FM, Conteh A, Sonnie M, et al. Schistosoma mansoni infection after three years of mass drug administration in Sierra Leone. Parasit Vectors. (2014) 7:14. doi: 10.1186/1756-3305-7-14
28. Zhang Y, Koukounari A, Kabatereine N, Fleming F, Kazibwe F, Tukahebwa E, et al. Parasitological impact of 2-year preventive chemotherapy on schistosomiasis and soil-transmitted helminthiasis in Uganda. BMC Med. (2007) 5:27. doi: 10.1186/1741-7015-5-27
29. Touré S, Zhang Y, Bosqué-Oliva E, Ky C, Ouedraogo A, Koukounari A, et al. Two-year impact of single praziquantel treatment on infection in the national control programme on schistosomiasis in Burkina Faso. Bull World Health Organ. (2008) 86:780–7. doi: 10.2471/BLT.07.048694
30. Zdesenko G, Mutapi F. Drug metabolism and pharmacokinetics of praziquantel: A review of variable drug exposure during schistosomiasis treatment in human hosts and experimental models. PloS Negl Trop Dis. (2020) 14:e0008649. doi: 10.1371/journal.pntd.0008649
31. Kura K, Mutono N, Basáñez MG, Collyer BS, Coffeng LE, Thumbi SM, et al. How does treatment coverage and proportion never treated influence the success of schistosoma mansoni elimination as a public health problem by 2030? Clin Infect Dis. (2024) 78:S126–30. doi: 10.1093/cid/ciae074
32. WHO. Helminth control in school age children: a guide for managers of control programmes-2nd ed. Geneva: World Health Organization (2011).
33. Ngimbwa JP, Basinda N, Kapesa A, Ngallaba S. Evaluation of the school water, sanitation and hygiene national strategic implementation plan (2012 -2017) in ukerewe district, north-western Tanzania. Enliven: Int J Adv Civil Eng. (2020) 3:004. doi: 10.20944/preprints202008.0390.v1
34. World Health Organization. Microplanning manual to guide implementation of preventive chemotherapy to control and eliminate neglected tropical diseases Vol. 2022. Geneva: World Health Organization and Pan American Health Organization (2022).
35. Faust CL, Osakunor DNM, Downs JA, Kayuni S, Stothard JR, Lamberton PHL, et al. Schistosomiasis control: leave no age group behind. Trends Parasitol. (2020) 36:582–91. doi: 10.1016/j.pt.2020.04.012
36. Mduluza T, Mutapi F. Putting the treatment of paediatric schistosomiasis into context. Infect Dis Poverty. (2017) 6:85. doi: 10.1186/s40249-017-0300-8
37. Mutapi F, Rujeni N, Bourke C, Mitchell K, Appleby L, Nausch N, et al. Schistosoma haematobium treatment in 1-5 year old children: safety and efficacy of the antihelminthic drug praziquantel. PloS Negl Trop Dis. (2011) 5:e1143. doi: 10.1371/journal.pntd.0001143
38. N’Goran EK, Odiere MR, Assandé Aka R, Ouattara M, Aka NAD, Ogutu B, et al. Efficacy, safety, and palatability of arpraziquantel (L-praziquantel) orodispersible tablets in children aged 3 months to 6 years infected with Schistosoma in C&xf4;te d’Ivoire and Kenya: an open-label, partly randomised, phase 3 trial. Lancet Infect Dis. (2023) 23(7):867–76. doi: 10.1016/S1473-3099(23)00048-8
Keywords: schistosomiasis, Schistosoma mansoni, treatment coverage, mass preventive chemotherapy, adult, Ukerewe island, Tanzania
Citation: Chiombola CE, Mwangoka ES, Baumba T, Mkombe CG, Kreibich S, Kasang C, Fuss A, Mueller A, Sabuni PA and Mazigo HD (2025) Impact of three rounds of mass preventive chemotherapy on prevalence and intensities of Schistosoma mansoni infection among an adult population on Ukerewe Island, north-western Tanzania. Front. Trop. Dis. 6:1523177. doi: 10.3389/fitd.2025.1523177
Received: 05 November 2024; Accepted: 20 January 2025;
Published: 17 February 2025.
Edited by:
Roberta Iatta, University of Bari Aldo Moro, ItalyReviewed by:
Emmanuel Abraham Mpolya, Nelson Mandela African Institution of Science and Technology, TanzaniaCalvin Tonga, Ministry of Public Health, Cameroon
Copyright © 2025 Chiombola, Mwangoka, Baumba, Mkombe, Kreibich, Kasang, Fuss, Mueller, Sabuni and Mazigo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Humphrey D. Mazigo, aHVtcGhyZXltYXppZ29AZ21haWwuY29t
 Crecencia E. Chiombola
Crecencia E. Chiombola Erick Simon Mwangoka2
Erick Simon Mwangoka2 Andreas Mueller
Andreas Mueller Humphrey D. Mazigo
Humphrey D. Mazigo