
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Trop. Dis., 25 March 2025
Sec. Antimicrobial Resistance
Volume 6 - 2025 | https://doi.org/10.3389/fitd.2025.1499098
This article is part of the Research TopicAntimicrobial Resistance Response Perspectives in AfricaView all 6 articles
 Eyosias Teklemariam1
Eyosias Teklemariam1 Mekonnen Damessa2*
Mekonnen Damessa2* Mamo Nigatu3
Mamo Nigatu3 Bikila Alemu4
Bikila Alemu4 Kumale Tolesa5
Kumale Tolesa5 Daba Abdissa6
Daba Abdissa6 Korinan Fanta2
Korinan Fanta2Background: Bacterial conjunctivitis is a significant cause of ocular morbidity globally, with increasing antimicrobial resistance posing a challenge to effective treatment. In Ethiopia, data on bacterial profiles and antimicrobial susceptibility patterns in conjunctivitis are limited. This study aimed to assess the bacterial profile and antimicrobial susceptibility patterns among patients clinically suspected of bacterial conjunctivitis at Jimma Medical Center, Ethiopia.
Methods: A facility-based cross-sectional study was conducted from January to June 2022. Conjunctival swabs were collected, and bacterial identification and antimicrobial susceptibility testing were performed using standard microbiological methods.
Results: Among 190 patients, 160 (84.2%) had culture-confirmed bacterial conjunctivitis. Gram-positive bacteria, particularly Coagulase-negative staphylococci (35.6%) and Staphylococcus aureus (21.9%), were predominant. High resistance rates were observed for penicillin, ampicillin, and tetracycline, while meropenem and piperacillin/tazobactam showed better efficacy. Multidrug resistance was detected in 77.5% of isolates.
Conclusions: Coagulase-negative staphylococcus and Staphylococcus aureus were the two most predominant bacterial isolates with high resistance to frequently used antibiotics such as penicillin, ampicillin, and tetracycline. Therefore, empirical treatment of bacterial conjunctivitis should be supported by antimicrobial susceptibility tests in the study area.
Conjunctivitis, an inflammation of the conjunctiva, is a common ocular condition that can be caused by infectious (bacterial or viral) or non-infectious factors (e.g., allergies, irritants). While viral conjunctivitis is more prevalent, bacterial conjunctivitis remains a significant public health concern, particularly in resource-limited settings like Ethiopia. Although bacterial conjunctivitis is often self-limiting, it can lead to substantial morbidity, economic burden, and, in some cases, complications if not properly managed. The economic impact of bacterial conjunctivitis is considerable, with the United States alone reporting over 6 million cases annually, resulting in an estimated direct cost of $800 million (1). In Ethiopia, conjunctivitis accounts for up to 60.4% of all external ocular infections and is a leading cause of ocular morbidity, particularly among rural children (2, 3).
The management of bacterial conjunctivitis typically involves the use of topical antibiotics, which can shorten the duration of symptoms and reduce transmission. However, the rapid emergence of antimicrobial resistance (AMR) has complicated treatment strategies and outcomes. In Ethiopia, the prevalence of antibiotic-resistant pathogens, particularly Staphylococcus aureus and Pseudomonas aeruginosa, is alarmingly high. Studies have shown that S. aureus, a leading cause of ocular infections, is highly resistant to commonly used antibiotics, including those prescribed in ophthalmology (4, 5) Additionally, the increasing prevalence of extended beta-lactamase-producing Enterobacteriaceae (e.g., E. coli and K. pneumoniae) and multidrug-resistant P. aeruginosa poses a significant challenge to effective treatment (6). These resistant pathogens not only compromise patient outcomes but also increase healthcare costs and the risk of treatment failure.
Despite the growing threat of AMR, data on bacterial conjunctivitis in Ethiopia remain limited. Previous studies have primarily focused on general external ocular infections, with few specifically addressing bacterial conjunctivitis. Moreover, the dynamic nature of AMR necessitates continuous surveillance to monitor resistance patterns and inform treatment guidelines. The World Health Organization (WHO) and the Ethiopian Food and Drug Authority (EFDA) have emphasized the importance of AMR surveillance to guide antibiotic stewardship and improve patient care (7) However, there is a critical gap in understanding the bacterial profile and antimicrobial susceptibility patterns specific to bacterial conjunctivitis in Ethiopia.
This study was conducted to address this gap by assessing the bacterial profile and antimicrobial susceptibility patterns among patients with clinically suspected bacterial conjunctivitis at Jimma Medical Center. By identifying the predominant bacterial pathogens and their resistance patterns, this study aims to provide evidence-based recommendations for empirical treatment and contribute to antimicrobial stewardship efforts in the region. The findings will help healthcare providers tailor treatment strategies, reduce the spread of resistant infections, and improve patient outcomes in the face of rising AMR.
A prospective cross-sectional study was performed from January to June 2022 at the Ophthalmologic Clinic of Jimma Medical Center, Southwest Ethiopia. All patients with signs and symptoms of bacterial conjunctivitis fulfilling the inclusion criteria were enrolled by ophthalmology nurses and confirmed through clinical examination by an ophthalmologist. Patients who were clinically diagnosed with bacterial conjunctivitis regardless of their age, Patients who were willing to participate in the study and Children whose guardian agreed to participate and give assent were the inclusion criteria; while, Patients with clinical signs of viral or allergic conjunctivitis (e.g., watery discharge, itching, and seasonal recurrence) and who had used antibiotics within the last 7 days prior to sample collection were also excluded.
Every patient was examined on a slit-lamp biomicroscope and diagnosed by ophthalmologists. Visual acuity (VA) was measured under conditions of high contrast using printed or projected charts with optotypes. Sociodemographic characteristics such as age, sex, residence, educational status, source of light, and source of power for cooking were recorded by interviewing patients. Histories of comorbid medical conditions and past medication history were abstracted from patient medical records using structured questionnaires.
Specimens from the conjunctiva were collected by an ophthalmologist using a sterile saline moistened cotton swab, applied by passing the swab gently over the lower tarsal and fornix conjunctiva twice (8). Two swabs were collected from each patient: one for Gram staining and the other for culture. The swabs were immersed in 3 ml of Amiens transport media with charcoal (Himedia®, India), placed in a cold box, and transported to the JMC Microbiology laboratory for bacterial isolation and identification. Standard operating procedures were followed in specimen collection and handling (9).
Each ocular specimen was inoculated onto MacConkey agar (MAC), mannitol salt agar (MAS), and blood agar plate (BAP), and chocolate agar plate (CAP) culture media. The media were incubated at 37°C for 24 and 48 hours and aerobic conditions were maintained. For fastidious organisms, chocolate agar (heated 5% sheep’s blood agar) was incubated at 37°C for 24 to 48 hours in a 5-10% CO2 atmosphere (10). All plates were examined for bacterial growth after 24 hours and plates with no bacterial growth were reincubated for another 24 hours. After obtaining pure colonies, specific bacterial pathogens were identified by Gram stain, colony morphology, and a series of biochemical tests. Catalase, coagulase, optochin disk sensitivity, novobiocin, and bacitracin tests were conducted to identify Gram-positive bacteria. Biochemical tests such as lysine decarboxylase, citrate utilization, lactose fermentation, indole, urease, oxidase and satellitism tests were used to identify Gram-negative bacteria.
Antimicrobial susceptibility tests were carried out on Muller Hinton agar (MHA) (Oxoid Ltd. Basingstoke, Hampshire, UK) using the modified Kirby-Bauer disk diffusion method on each isolated bacterium. For fastidious bacterial isolates, MHA medium containing 5% defibrinated sterile sheep blood was used. Three-five bacterial colonies were emulsified in 5 ml of sterile nutrient broth and mixed gently. A 0.5 McFarland standard solution was used to stabilize the density of the inoculums for the susceptibility test. Then the plates were inoculated by streaking the swab over the entire agar surface and antibiotic-impregnated disks were placed on the agar surface and incubated at 37°C for 24 hours. After that, the zone of inhibition was measured using a caliper and categorized as resistant, intermediate, and sensitive according to CLSI guidelines (11).
The suspension was diluted and incubated at 37°C until the turbidity of the suspension was adjusted to 0.5 McFarland standards. The suspension was swabbed uniformly onto MHA agar entirely by rotating the plate 60 degrees between the streak for nonfastidious organisms and MHA with defibrinated sterile sheep blood (5%) for fastidious organisms. The antimicrobial impregnated disks were placed using sterile forceps on the MHA plate surface and the plates were incubated at 37°C for 18-24 hours. The zone of inhibition around the disk was measured to the nearest millimeter using a graduated caliper in millimeters, and the isolates were classified as sensitive, intermediate and resistant according to CLSI (11). The antibiotic disks used in this study were sourced from company name (Oxoid Ltd.), city (Basingstoke), country (UK). The following twelve antibiotic disks were used; Ampicillin (AMP) 10μg, Ceftriaxone (CRO) 30μg, Chloramphenicol (C) 30μg, Ciprofloxacin (CIP) 5μg, Clindamycin (DA) 2μg, Erythromycin (E) 15μg, Gentamicin (CN) 10μg, Penicillin-G (P) 10IU, Tetracycline (TE) 30μg, Trimethoprim-sulphamethoxazole (SXT) 1.25/23.75μg, piperacillin/tazobactam (PIP) 100/10μg and meropenem (MER) 10 μg. Multidrug resistance was considered if bacterial isolates were resistant to three or more antibiotics from different classes (12).
Standard operating procedures were followed for specimen collection, handling, and transport. The sterility of culture media was ensured by incubating 5% of each batch of prepared media at 37°C for 24 hours. Standard strains (S. aureus ATCC 25923, E. coli ATCC 25922, and P. aeruginosa ATCC 27853) were used for quality control of culture media and biochemical tests.
The collected data were checked for completeness and entered into EPI data version 4.2. Then, the data were imported and analyzed using IBM SPSS software (Version 23:0; IBM SPSS Inc., New York, USA). Descriptive statistics were calculated and data were presented using tables. Sociodemographic variables, bacterial isolates, and antimicrobial susceptibility patterns were summarized using frequency and percentage. A chi-square test value <0.05 was considered statistically significant for categorical variables.
Among the 190 study participants included in this study, the proportions of males 97 (51.1%) and females 93 (48.9%) were similar. More than half 107 (56.3%) of the patients were younger than 18 years. One hundred thirteen (59.5%) of the total participants were from urban areas and used electricity as a source of light. All 185 (97.4%) of the study participants/their families use wood as a source of power for cooking (Table 1).
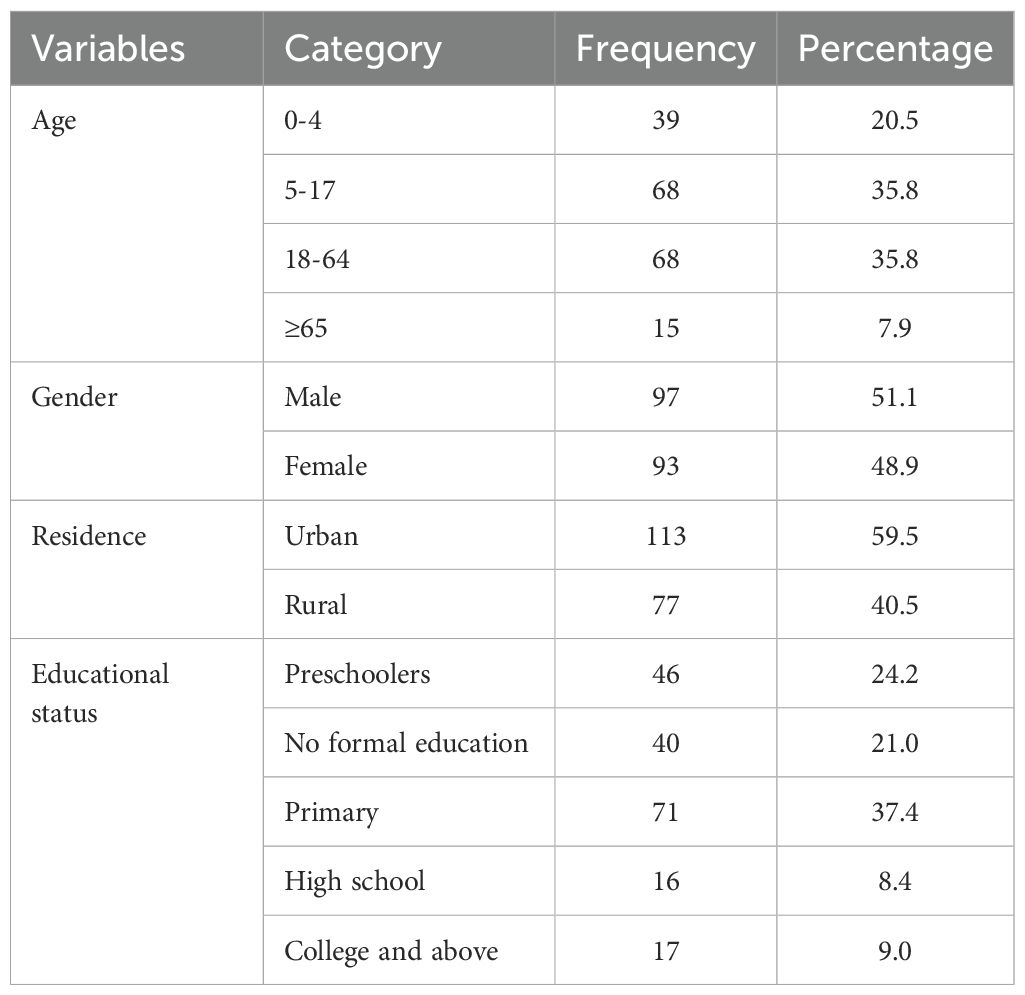
Table 1. Sociodemographic characteristics of patients clinically diagnosed with bacterial conjunctivitis presented at the Ophthalmic Clinic of JMC, 2022.
Out of 190 study participants, 65 (34.2%) of them had a history of chronic medical conditions with diabetes mellitus being the most common 28 (14.7%) followed by HIV/AIDS 19 (10%). History of systemic steroid use was recorded in 54 (28.4%) of study participants (Table 2).
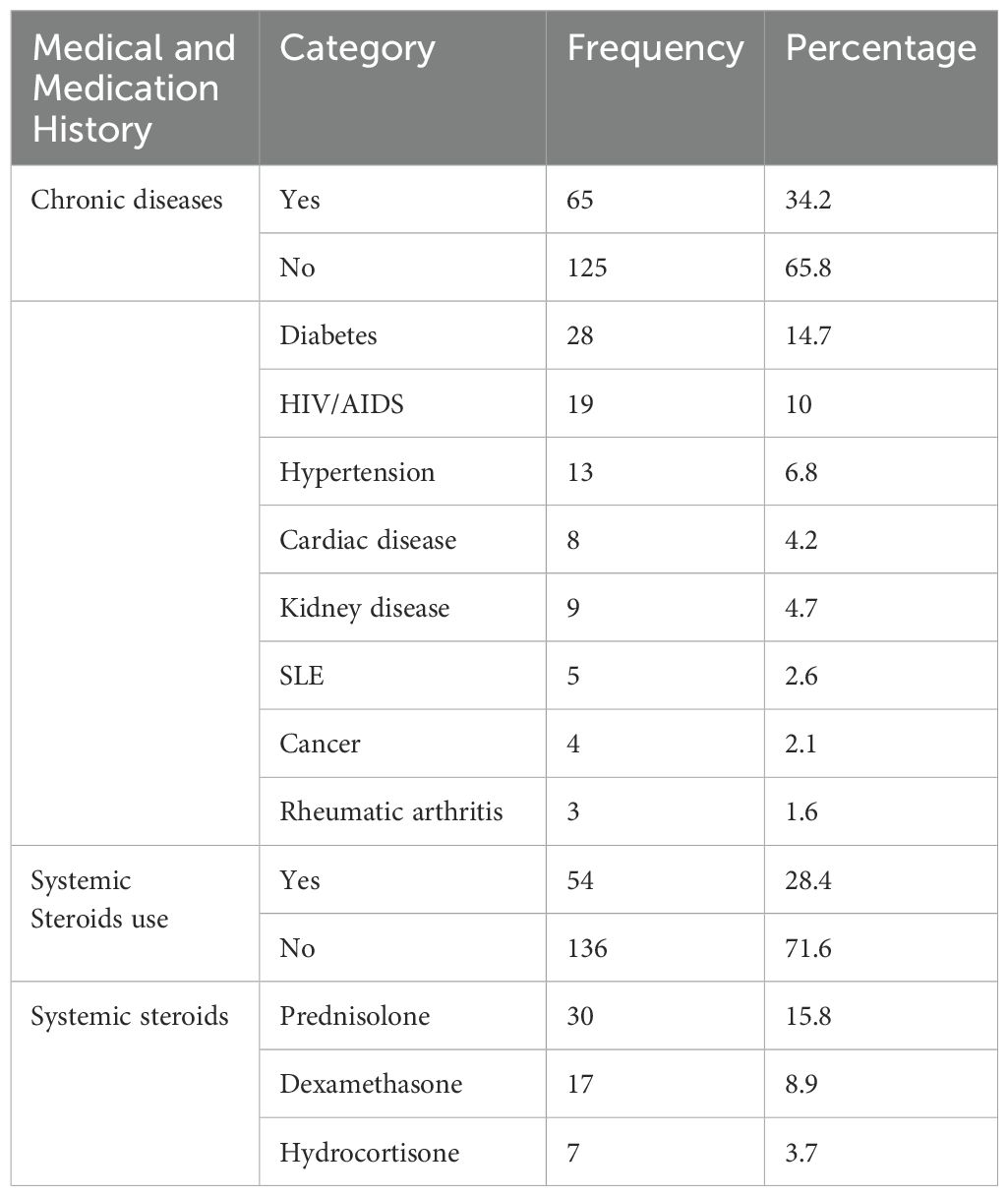
Table 2. Medical and medication history of patients diagnosed with bacterial conjunctivitis at Ophthalmologic Clinic of JMC, January-June 2022.
Among the 190 conjunctival swabs cultured, 160 (84.2%) had bacterial growth. Approximately three-quarters of bacterial isolates were Gram-positive (124, 77.5%). Coagulase-negative staphylococcus was the most predominant 57 (35.6%) bacterial isolate followed by S. aureus 35(21.9%). The two common Gram-negative bacterial isolates were P. aeruginosa 13 (8.1%) and K. pneumoniae 7 (4.4%) (Table 3).
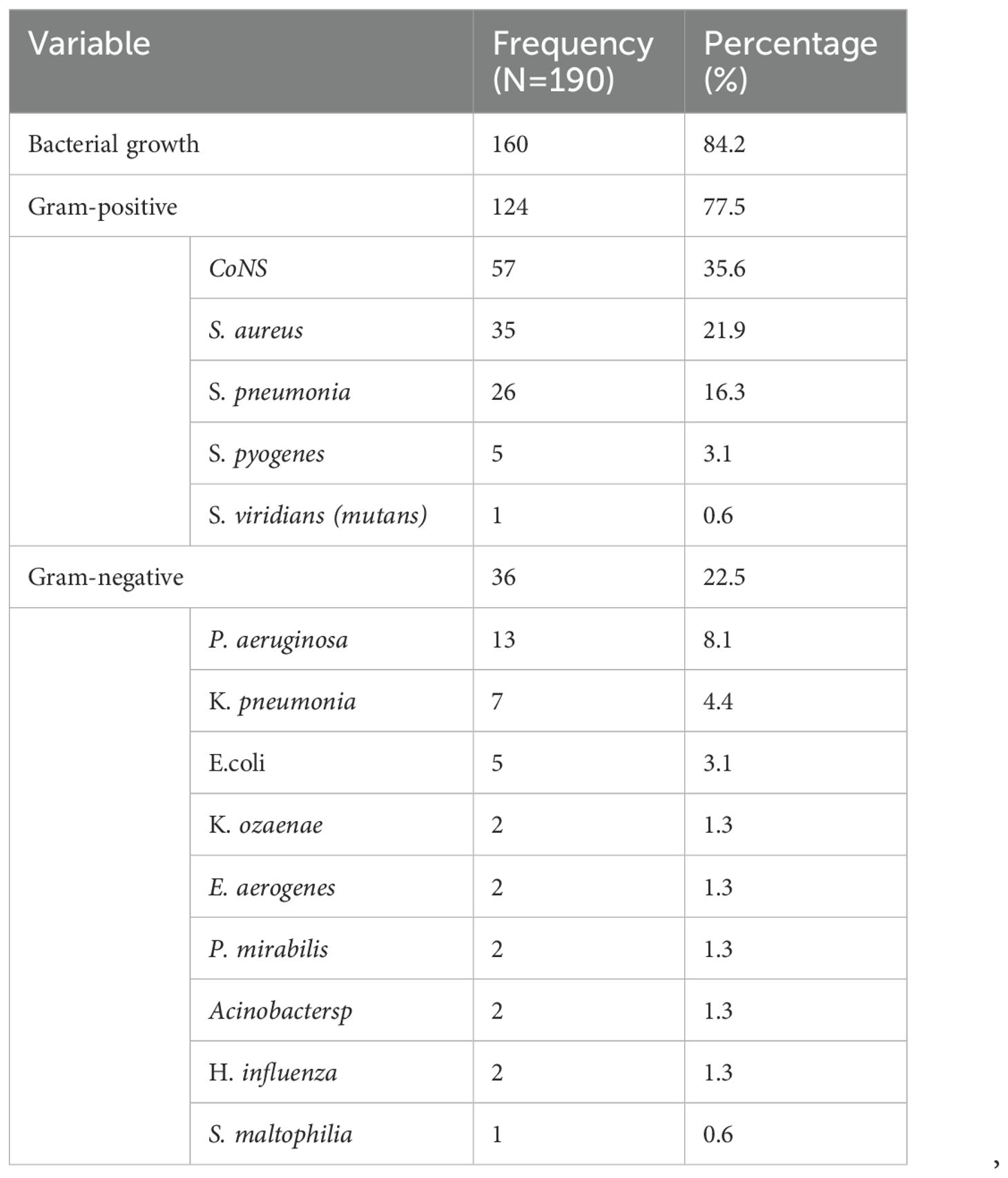
Table 3. The prevalence of bacterial pathogens isolated from patients clinically diagnosed with bacterial conjunctivitis at the Ophthalmologic Clinic of JMC, 2022.
Antimicrobial susceptibility patterns were determined for twelve antibiotics belonging to ten drug classes. Among Gram-positive isolates, coagulase-negative staphylococcus acquired a higher resistance rate to penicillin 55 (96.5%), ampicillin 54 (94.7%), and tetracycline 47 (82.5%). Likewise, S. aureus showed a high resistance rate to penicillin 34 (97.1%), ampicillin 33 (94.3%), and tetracycline 32 (91.4%). Both coagulase-negative staphylococcus and S. aureus maintained susceptibility to meropenem (71.9% and 74.3%) and piperacillin/tazobactam (68.4% and 71.4%) respectively (Table 4).
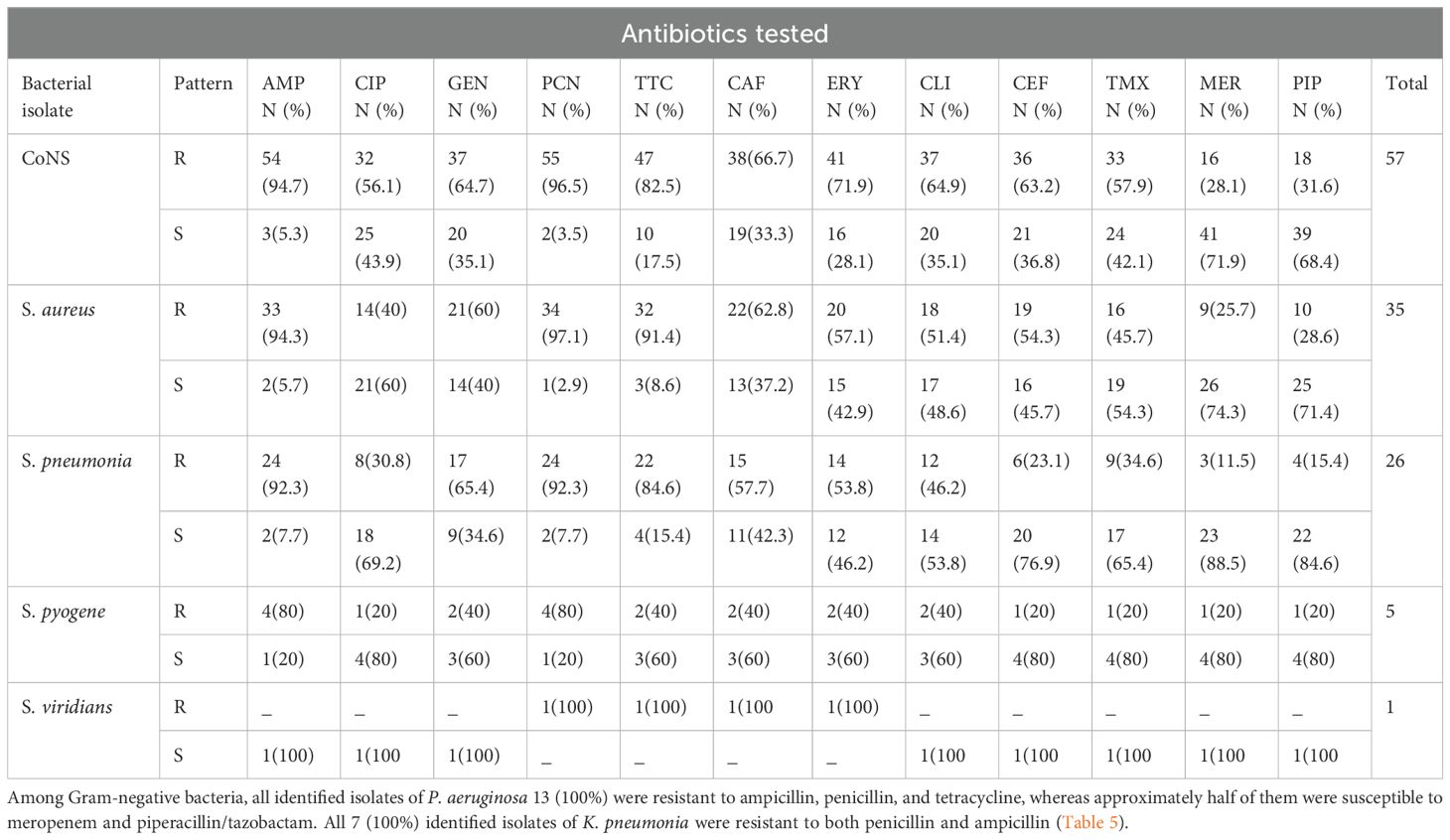
Table 4. Antimicrobial susceptibility patterns of Gram positive isolates from patients diagnosed with bacterial conjunctivitis presented at Ophthalmologic Clinic of JMC, January-June 2022Antimicrobial susceptibility pattern among Gram-negative bacterial isolates.
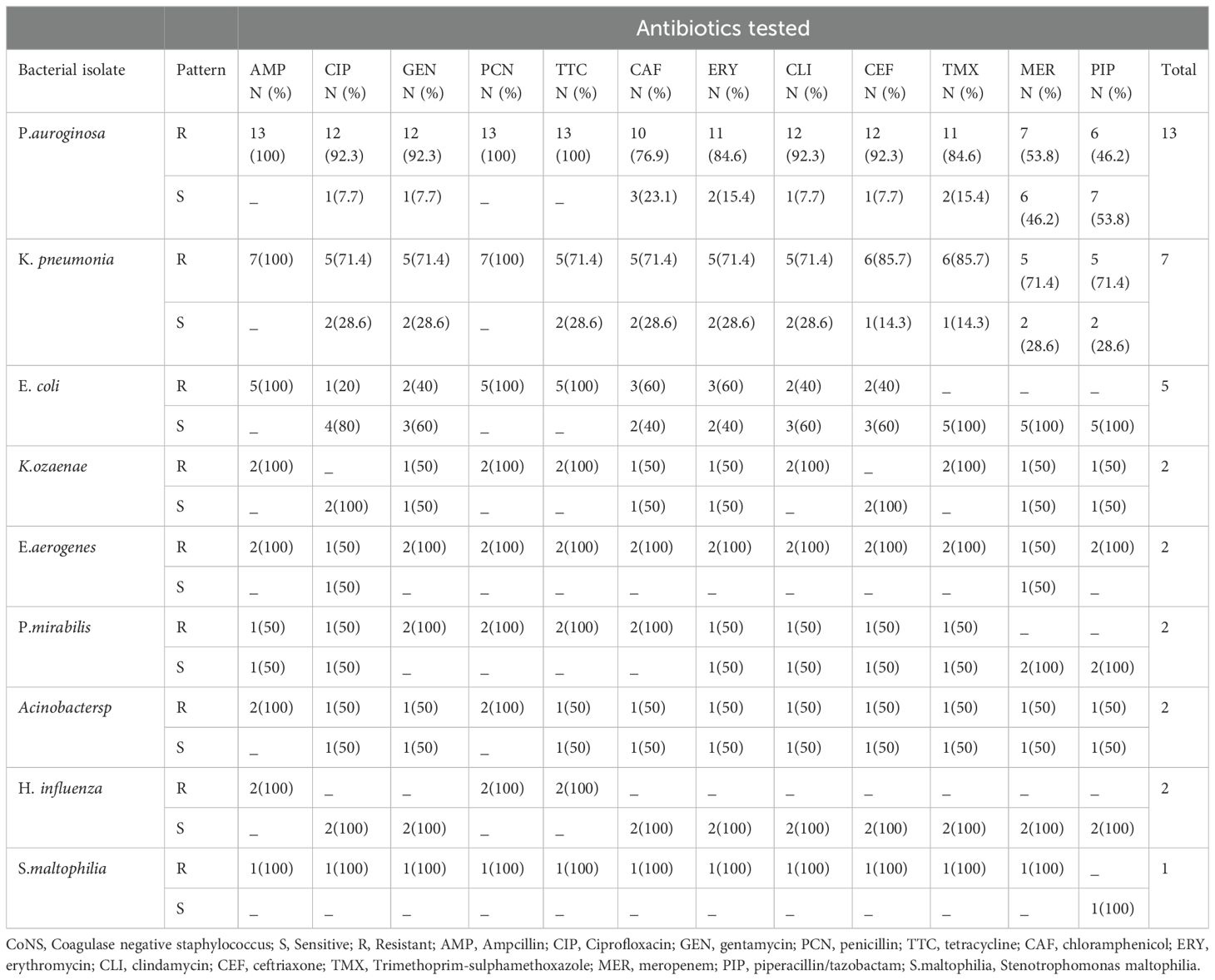
Table 5. Antimicrobial susceptibility patterns of Gram negative isolates identified from patients diagnosed with bacterial conjunctivitis presented at Ophthalmologic Clinic of JMC, January-June 2022.
Of the 160 bacterial isolates, 124 (77.5%) were found to be multidrug resistant. Coagulase-negative staphylococcus 48 (30%) and S. aureus 26 (16.3%) exhibited a higher rate of multidrug resistance among Gram-positive pathogens. Regarding Gram-negative isolates, almost all identified P. aeruginosa 12 (7.5%) and K. pneumoniae 6 (3.8%) isolates showed multidrug resistance (Table 6).
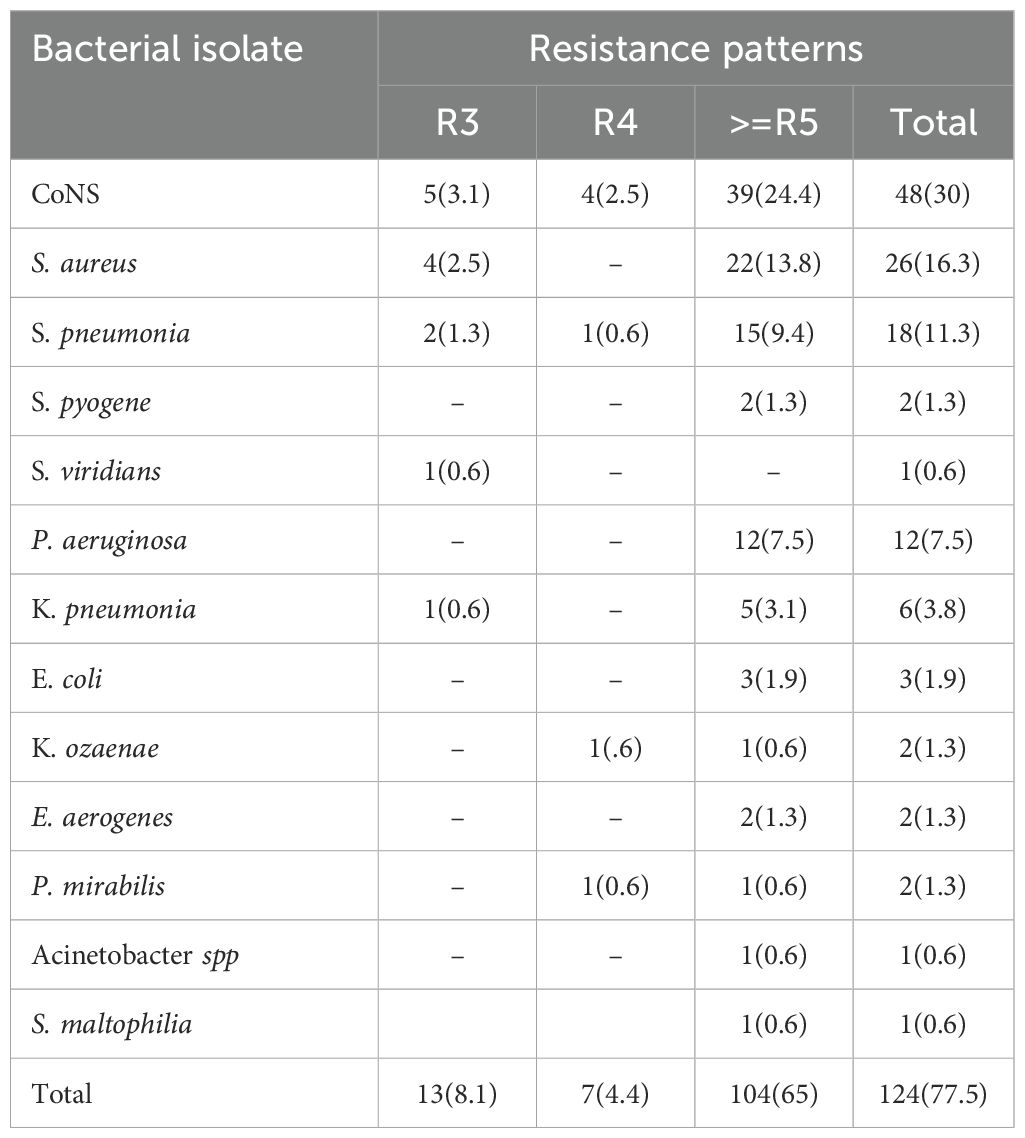
Table 6. Multidrug resistance pattern among bacterial isolates from patients diagnosed with bacterial conjunctivitis at the Ophthalmologic Clinic of JMC, 2022.
This study reveals important insights into bacterial conjunctivitis at the Ophthalmologic Clinic of Jimma Medical Center, Ethiopia, where 56.3% of patients are under 18 years old. The high prevalence in children is linked to their immature immune systems and exposure to unsanitary conditions. In developing countries like Ethiopia, poor hygiene and limited access to clean water increase the risk of ocular infections. Additionally, parents are more likely to seek treatment for symptomatic children than for adults, further contributing to the higher rates of bacterial conjunctivitis in pediatric patients.
The overall prevalence of bacterial growth in the present study was 84.2%. This finding is higher than previous studies conducted in Ethiopia, which reported bacterial growth rates of 46% to 75% [12]–[15], [22], [23].
This difference might be because our study participants were patients with presumed bacterial conjunctivitis rather than general patients with ocular infections. Another reason for the differences might be differences in the study population, climate, specimen collection, handling, and microbiological tests. On the other hand, comparable results were reported from Saudi Arabia, which reported a bacterial growth rate of 78.7% among patients with red eye (13).
Gram-positive bacteria accounted for three-fourths of bacterial isolates in the present study. Previous studies from Ethiopia (14, 15) Nigeria (16) and Japan (17) also reported that the majority of bacterial isolates from external ocular infections, including conjunctivitis, were Gram-positive. Overall, CoNS was the most predominant pathogen, with a prevalence of 35.6% in the present study. This finding is in line with studies conducted in Addis Ababa (41.3%) (15) Rwanda (51.4%) (17) and Uganda (65.9%) (18) Staphylococcus aureus (21.9%) was the second most common bacterial isolate in the present study, although other studies (8, 19, 20) had reported it as a predominant bacterial isolate from external ocular infection. This difference might be due to differences in the study population (conjunctivitis vs. general external ocular infections), environmental factors, age, and site of infections.
Gram-negative bacteria account for 22.5% of overall bacterial isolates, which is lower compared to previous studies conducted in different parts of Ethiopia, which reported that Gram-negative bacteria account for 31.8% to 48.0%[6], [12]–[14], [30].
The relatively low prevalence of Gram-negative bacteria in the present study might be due to improved personal hygiene, lack of contact lens use, and the exclusion of other external ocular infections such as keratitis. The common Gram-negative isolates were P. aeruginosa 8.1% and K. pneumonia 4.4%, which is in line with other studies (2, 3, 21, 22).
As members of the ESKAPE pathogens group—comprising Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species—these organisms are globally recognized for their multidrug resistance and clinical significance. In this study, we identified S. aureus, P. aeruginosa, and K. pneumoniae as key contributors to antimicrobial resistance, underscoring their role as major pathogens in the context of bacterial conjunctivitis. These findings align with global trends, highlighting the urgent need for targeted antimicrobial stewardship and infection control measures to address the growing challenge of multidrug-resistant infections (23–26).
A significant number (50-70%) of both Gram-positive and Gram-negative bacteria were susceptible to meropenem and piperacillin/tazobactam. In contrast, two previous studies performed on external ocular infections from Jimma [12], [33], reported that most Gram-positive and Gram-negative isolates were sensitive (69-100%) to gentamycin, ciprofloxacin, and amoxicillin-clavulanic acid. The coverage of gentamycin and ciprofloxacin in the present study was less than 50% in most of the bacterial isolates. This finding alerts the prudent use of these antibiotics as empiric treatment of bacterial conjunctivitis in the study area.
Regarding the antimicrobial resistance pattern, Gram-positive isolates were highly resistant (90%) to commonly used antibiotics such as penicillin, ampicillin, and tetracycline. This finding was supported by other studies conducted in Ethiopia [15, 22, 29, 34, 35]. Likewise, almost all (≈100%) of the Gram-negative isolates were resistant to ampicillin, penicillin, and tetracycline. High resistance to these antibiotics could be due to the overutilization of these medications with or without prescription. Furthermore, it is known that Gram-positive cocci such as Staphylococcus produce beta-lactamase and modify penicillin-binding proteins that enable the bacteria to resist most beta-lactam antibiotics (36).
The study showed the growing concern of Klebsiella pneumoniae with reduced susceptibility to third-generation cephalosporins like ceftriaxone (85.7%) and carbapenems (71.4%), particularly in developing countries like Ethiopia. Additionally, the resistance of Pseudomonas aeruginosa to meropenem (53.8% in our study). Beside high price and limited availability of carbapenems in Ethiopia, patients are forced to bought and used these antibiotics in the study area to obtain the desired treatment outcomes. These resistant pathogen poses a significant threat to public health, and our findings align with previous study which stated the growing trend of antimicrobial resistance in the region (27).
The prevalence of MDR (resistance to ≥3 antimicrobials) in the present study was 77.5%. This is higher than the previous study performed in the same setting where the rate of MDR was reported to be 68.7% (2). However, the rate of MDR observed in this study is lower compared to studies conducted at Gondar (35) and Alert Hospital which reported MDR rates of 87.1% and 93.0%, respectively. This difference might be due to the difference in the operationalization of the term multidrug resistance because those studies defined it as resistance to two or more antibiotics. In general, the emergence of MDR bacteria is increasing steadily, which indicates the urgent need for antimicrobial stewardship and infection prevention and control practices in hospitals.
The present study provided the recent trend of antimicrobial resistance in bacterial conjunctivitis that can be used by ophthalmologists and healthcare providers to tailor patient treatment and clinical guidelines accordingly. However, this study also has limitations. First, the study was conducted at a single tertiary care center, which may limit the generalizability of the findings to other settings. Second, the exclusion of patients with viral or allergic conjunctivitis may have introduced selection bias. Third, the small sample size and limited number of antibiotics tested may affect the generalizability of the findings. Future studies with larger sample sizes and extended incubation periods for fastidious organisms are needed to confirm these findings.
The prevalence of bacterial isolates from conjunctival specimens among patients with bacterial conjunctivitis was high in the present study area and the isolates were predominantly Gram-positive cocci (CoNS and S. aureus). The two common Gram-negative bacteria identified were P. aeruginosa and K. pneumoniae. Over three-quarters of bacterial isolates were multidrug-resistant, and a high resistance rate was observed to frequently use antibiotics such as penicillin, ampicillin, and tetracycline. Meropenem and piperacillin/tazobactam were the two effective antimicrobials against most identified bacteria in the present study area.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Jimma university ethical review board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
ET: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. MD: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MN: Conceptualization, Data curation, Formal analysis, Methodology, Software, Validation, Writing – original draft. BA: Conceptualization, Investigation, Supervision, Writing – original draft. KT: Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – review & editing. DA: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing. KF: Conceptualization, Data curation, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Institute of Health, Jimma University. The funding body had no involvement in study design, data collection, analysis, interpretation, manuscript writing, or the decision to submit the article for publication.
We would like to thank the Jimma University Institute of Health for financially supporting this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AMR, Antimicrobial Resistance; ATCC, American Type Culture Collection; CLSI, Clinical and Laboratory Standard Institute; CoNS, Coagulase-negative Staphylococcus; JMC, Jimma Medical Center; MDR, Multidrug Resistance; MHA, Muller Hinton Agar; MRSA, Methicillin-resistant Staphylococcus.
1. Pepose JS, Sarda SP, Cheng WY, McCormick N, Cheung HC, Bobbili P, et al. Direct and indirect costs of infectious conjunctivitis in a commercially insured population in the United States. Clin Ophthalmol. (2020) 14:377–87. doi: 10.2147/OPTH.S233486
2. Tesfaye T, Beyene G, Gelaw Y, Bekele S, Saravanan M. Bacterial profile and antimicrobial susceptibility pattern of external ocular infections in Jimma University specialized hospital, Southwest Ethiopia. Am J Infect Dis Microbiol. (2013) 1:13–20. doi: 10.12691/ajidm-1-1-3
3. Teweldemedhin M, Saravanan M, Gebreyesus A, Gebreegziabiher D. Ocular bacterial infections at Quiha Ophthalmic Hospital, Northern Ethiopia: an evaluation according to the risk factors and the antimicrobial susceptibility of bacterial isolates. BMC Infect Dis. (2017) 17:1–11. doi: 10.1186/s12879-017-2304-1
4. Reta A, Bitew Kifilie A, Mengist A. Bacterial infections and their antibiotic resistance pattern in Ethiopia: a systematic review. Adv Prev Med. (2019) 2019:4380309. doi: 10.1155/2019/4380309
5. Getahun E, Gelaw B, Assefa A, Assefa Y, Amsalu A. Bacterial pathogens associated with external ocular infections alongside eminent proportion of multidrug resistant isolates at the University of Gondar Hospital, northwest Ethiopia. BMC Ophthalmol. (2017) 17:1–10. doi: 10.1186/s12886-017-0548-6
6. Subedi D, Vijay AK, Willcox M. Overview of mechanisms of antibiotic resistance in Pseudomonas aeruginosa: an ocular perspective. Clin Exp Optom. (2018) 101:162–71. doi: 10.1111/cxo.12621
7. Ibrahim RA, Teshal AM, Dinku SF, Abera NA, Negeri AA, Desta FG, et al. Antimicrobial resistance surveillance in Ethiopia: Implementation experiences and lessons learned. Afr J Lab Med. (2018) 7:1–4. doi: 10.4102/ajlm.v7i2.770
8. Fenta F, Alemu D, Alelign D. Magnitude of drug-resistant gram-positive bacterial pathogens, and its associated factors from external ocular infected patients attending at jinka general hospital ophthalmic clinic, southern Ethiopia. Infect Drug Resist. (2022), 947–59. doi: 10.2147/IDR.S356974
9. Ren Z, Liu Q, Li W, Wu X, Dong Y, Huang Y. Profiling of diagnostic information of and latent susceptibility to bacterial keratitis from the perspective of ocular bacterial microbiota. Front Cell Infect Microbiol. (2021) 11:645907. doi: 10.3389/fcimb.2021.645907
10. Ma L, Jakobiec FA, Dryja TP. A review of next-generation sequencing (NGS): applications to the diagnosis of ocular infectious diseases. Basingstoke, Hampshire, UK: Taylor & Francis (2019) p. 223–31.
11. Humphries R, Bobenchik AM, Hindler JA, Schuetz AN. Overview of changes to the clinical and laboratory standards institute performance standards for antimicrobial susceptibility testing, M100. J Clin Microbiol. (2021) 59:10–1128. doi: 10.1128/JCM.00213-21
12. Ayehubizu Z, Mulu W, Biadglegne F. Common bacterial causes of external ocular infections, associated risk factors and antibiotic resistance among patients at ophthalmology unit of Felege Hiwot Referral Hospital, Northwest Ethiopia: a cross-sectional study. J Ophthalmic Inflammation Infect. (2021) 11:1–10. doi: 10.1186/s12348-021-00238-2
13. Shahaby A, Alharthi A, El Tarras A. Potential bacterial pathogens of red eye infections and their antibiotic susceptibility patterns in Taif, KSA. Int J Curr Microbiol App Sci. (2015) 4:383–93. doi: 10.1186/s12348-021-00238-2
14. Shiferaw B, Gelaw B, Assefa A, Assefa Y, Addis Z. Bacterial isolates and their antimicrobial susceptibility pattern among patients with external ocular infections at Borumeda hospital, Northeast Ethiopia. BMC Ophthalmol. (2015) 15:1–8. doi: 10.1186/s12886-015-0078-z
15. Woreta AN, Kebede HB, Tilahun Y, Teklegiorgis SG, Abegaz WE. Antibiotic susceptibility pattern and bacterial spectrum among patients with external eye infections at Menelik II Referral Hospital in Addis Ababa, Ethiopia. Infect Drug Resist. (2022), 765–79. doi: 10.2147/IDR.S352098
16. Ubani UA. Bacteriology of external ocular infections in Aba, South Eastern Nigeria. Clin Exp Optom. (2009) 92:482–9. doi: 10.1111/j.1444-0938.2009.00425.x
17. Semanyenzi S, Abahuje E. Normal conjunctival flora as seen in adult patients at Kigali university Teaching Hospital. RMJ. (2013) 70:22–4. doi: 10.1186/1471-2415-13-7
18. Mshangila B, Paddy M, Kajumbula H, Ateenyi-Agaba C, Kahwa B, Seni J. External ocular surface bacterial isolates and their antimicrobial susceptibility patterns among pre-operative cataract patients at Mulago National Hospital in Kampala, Uganda. BMC Ophthalmol. (2013) 13:1–6. doi: 10.1186/1471-2415-13-71
19. Sarmah P, Shenoy P. Profile of microbial isolates in ophthalmic infections and antibiotic susceptibility of the bacterial isolates: a study in an eye care hospital, Bangalore. J Clin Diagn Res JCDR. (2014) 8:23. doi: 10.1371/journal.pone.0205814
20. Asbell PA, DeCory HH. Antibiotic resistance among bacterial conjunctival pathogens collected in the Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) surveillance study. PloS One. (2018) 13:e0205814. doi: 10.1371/journal.pone.0205814
21. Belyhun Y, Moges F, Endris M, Asmare B, Amare B, Bekele D, et al. Ocular bacterial infections and antibiotic resistance patterns in patients attending Gondar Teaching Hospital, Northwest Ethiopia. BMC Res Notes. (2018) 11:1–7. doi: 10.1186/s13104-018-3705-y
22. Aklilu A, Bitew A, Dessie W, Hailu E, Asamene N, Mamuye Y. Prevalence and drug susceptibility pattern of bacterial pathogens from ocular infection in St. Paul’s Hospital Millennium Medical College, Ethiopia. J Bacteriol Mycol. (2018) 5:1085. doi: 10.3390/antibiotics10020142
23. Mulani MS, Kamble EE, Kumkar SN, Tawre MS, Pardesi KR. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: a review. Front Microbiol. (2019) 10:539. doi: 10.3389/fmicb.2019.00539
24. Diriba K, Kassa T, Alemu Y, Bekele S. In vitro biofilm formation and antibiotic susceptibility patterns of bacteria from suspected external eye infected patients attending ophthalmology clinic, Southwest Ethiopia. Int J Microbiol. (2020) 2020:1–12. doi: 10.1186/s13104-018-3705-y
25. Muluye D, Wondimeneh Y, Moges F, Nega T, Ferede G. Types and drug susceptibility patterns of bacterial isolates from eye discharge samples at Gondar University Hospital, Northwest Ethiopia. BMC Res Notes. (2014) 7:1–5. doi: 10.1186/1756-0500-7-292
26. Christaki E, Marcou M, Tofarides A. Antimicrobial resistance in bacteria: mechanisms, evolution, and persistence. J Mol Evol. (2020) 88:26–40. doi: 10.1007/s00239-019-09914-3
27. Akinyemi KO, Abegunrin RO, Iwalokun BA, Fakorede CO, Makarewicz O, Neubauer H, et al. The Emergence of Klebsiella pneumoniae with reduced susceptibility against third generation cephalosporins and carbapenems in Lagos Hospitals, Nigeria. Antibiotics. (2021) 10:142. doi: 10.3390/antibiotics10020142
Keywords: antimicrobial resistance, antibiotic susceptibility pattern, conjunctivitis, ocular infections, red eye
Citation: Teklemariam E, Damessa M, Nigatu M, Alemu B, Tolesa K, Abdissa D and Fanta K (2025) Bacterial profile and antimicrobial susceptibility patterns among patients clinically suspected of bacterial conjunctivitis at the ophthalmologic clinic of Jimma Medical Center, Southwest Ethiopia. Front. Trop. Dis. 6:1499098. doi: 10.3389/fitd.2025.1499098
Received: 20 September 2024; Accepted: 28 February 2025;
Published: 25 March 2025.
Edited by:
Sylvia Opanga, University of Nairobi, KenyaReviewed by:
Gamal Wareth, Friedrich Loeffler Institut, GermanyCopyright © 2025 Teklemariam, Damessa, Nigatu, Alemu, Tolesa, Abdissa and Fanta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mekonnen Damessa, bWVrb25uZW5tb2tlNEBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.