
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Trop. Dis., 05 March 2025
Sec. Antimicrobial Resistance
Volume 6 - 2025 | https://doi.org/10.3389/fitd.2025.1497199
This article is part of the Research TopicAntimicrobial Resistance Response Perspectives in AfricaView all 6 articles
 Joseph Mbuthia1
Joseph Mbuthia1 Nkatha Gitonga2
Nkatha Gitonga2 Winnie Akoth1*
Winnie Akoth1* Susan Mutua1
Susan Mutua1 Ndinda Kusu2
Ndinda Kusu2 Tamara Hafner3
Tamara Hafner3 Mohan P. Joshi3
Mohan P. Joshi3 Martha Embrey4
Martha Embrey4Introduction: A baseline point prevalence survey (PPS) was conducted at Gertrude’s Children’s Hospital (GCH), a private pediatric hospital in Kenya, to assess antimicrobial use patterns, adherence to antimicrobial stewardship (AMS) protocols, and compliance with treatment guidelines. The survey aimed to provide insights into antimicrobial prescribing practices in a pediatric setting and address a critical gap in data from private healthcare facilities in Kenya.
Methods: The PPS included all inpatients as of 13 May 2022, excluding those discharged on that date, day cases, and those prescribed topical antimicrobials. Data were collected using a tool adapted from the World Health Organization (WHO) PPS standardized methodology, focusing on antimicrobial prescribing trends, compliance with GCH treatment guidelines, and adherence to WHO’s Access, Watch, and Reserve categorization.
Results: The results showed that 61% of inpatients were on antimicrobials, with systemic antibacterials (J01) being the most prescribed, particularly third-generation cephalosporins, penicillin combinations, and imidazole derivatives. Ceftriaxone was the most commonly used antimicrobial, and the average number of antimicrobials prescribed per patient was 1.2. Prescribing practices showed a high use of Watch category antibiotics (51%) and a predominant use of the intravenous (IV) route (75%).
Discussion: Only 50% of prescriptions complied with guidelines and microbiological findings; we identified significant areas for improvement, including the need for structured reviews of antimicrobial prescriptions, better alignment with AMS objectives, and enhanced training on treatment guidelines, diagnostic stewardship, and infection prevention and control.
Conclusion: The PPS provided valuable data to inform AMS interventions at GCH, the development of policies for IV-to-oral switch criteria, enhancement of the health management system, establishment of antimicrobial ward rounds, and improved education on laboratory result interpretation and appropriate sample collection.
Antimicrobial resistance (AMR) threatens global health and sustainable development with immense adverse health and economic effects. An estimated 1.27 million deaths globally from bacterial AMR occurred in 2019 (1); furthermore, a recent World Health Organization (WHO) study across 47 African countries estimated 1.05 million deaths associated with bacterial AMR and 250,000 deaths directly attributed to bacterial AMR in 2019, with Sub-Saharan Africa bearing the highest burden (2). The prevalence of AMR in Kenyan hospitals is concerning. For example, Escherichia coli and Klebsiella spp. isolates have been highlighted as having high resistance to second- and third-generation cephalosporins (>70%) (3). Furthermore, a retrospective study of data collected between 2016 and 2018 from 16 laboratories within the Kenyan AMR surveillance network revealed alarmingly high resistance levels to third-generation cephalosporins among Enterobacterales, carbapenem in Pseudomonas aeruginosa and methicillin in Staphylococcus aureus (4). The overuse and misuse of antimicrobials are major contributing factors to AMR, and evidence-based efforts are needed for containment (5).
Antimicrobial stewardship (AMS) is an important strategic objective of the Kenyan National Action Plan (NAP) on the prevention and containment of AMR, which is in line with the WHO Global Action Plan on AMR (6, 7). Stewardship initiatives aim to significantly reduce AMR’s emergence and spread, thereby improving the safety and quality of patient care (8). However, limited data exists on the implementation of AMS programs in Africa. A WHO Africa region assessment of 31 countries revealed that only one achieved advanced implementation. Key challenges included insufficient funding, weak policies, limited use of the WHO Access, Watch and Reserve (AWaRe) classification, and scarce surveillance of antimicrobial consumption and antimicrobial use (AMU) to inform AMS efforts (9). One of the Kenyan NAP-AMR’s primary objectives is to ensure the continuous monitoring of AMR and AMU in healthcare facilities and to understand better the trends related to AMR spread; however, Kenya currently lacks comprehensive and structured AMU data and surveillance processes (6, 10).
WHO developed a standardized point prevalence survey (PPS) tool to collect AMU data at healthcare facilities (11). Although several point prevalence surveys on AMU have recently been conducted in Kenya, most have been in public hospitals; therefore, we have scant data from private hospitals, especially those specializing in pediatric care (12, 13). A PPS is part of an ongoing continuous quality improvement approach for healthcare facilities and supports efforts to build capacity for monitoring AMU. As per the NAP-AMR and National Antimicrobial Stewardship Guidelines for Health Care Settings in Kenya (14), the overall goal is for the PPS results to link hospitals’ AMR containment programs to national efforts. The 2020 national AMS guidelines recommend conducting a PPS either bi-annually or annually in all inpatient settings to evaluate adherence to national and WHO treatment guidelines (15–17) and to support the country’s surveillance efforts. However, the current recommendations in the national treatment guidelines are outdated (15), and as of February 2025, the revised guidelines had not yet been endorsed or disseminated.
Gertrude’s Children’s Hospital (GCH), a private pediatric hospital in Kenya, plans to conduct a PPS annually to evaluate the frequency and patterns of antimicrobial prescribing and adherence to protocols, guidelines, and policies for AMR in the inpatient setting. The hospital will use the results from this first PPS to assess adherence to best practices in antimicrobial prescribing, evaluate changes in prescribing patterns, track AMU trends, monitor the success of the facility’s AMS program interventions, and promote the appropriate use of antimicrobials.
This survey is strategic and significant as it addresses a critical gap in data on AMU in private healthcare settings, which are often underrepresented in the current national surveillance efforts. GCH’s unique position as a private tertiary pediatric hospital, allows for an in-depth understanding of AMU specific to pediatric care and the private sector. The findings from this survey will directly inform the national strategy for AMR containment by providing a data-driven basis to refine the country’s NAP-AMR and AMS guidelines. Additionally, the findings will contribute to more accurate national AMU estimates and patterns by providing insights into the utilization of antimicrobials in the private sector. These insights will support the country in tailoring targeted interventions for private healthcare settings, influencing policy decisions to contain AMR, and promoting sustainable, evidence-based antimicrobial practices.
The survey was conducted at GCH, a 100-bed private tertiary referral and teaching children’s hospital in Nairobi, Kenya. The hospital is the largest pediatric hospital in the East African region and admits approximately 9,000 patients annually. GCH is one of 21 hospitals supported by the United States Agency for International Development (USAID) Medicines, Technologies, and Pharmaceutical Services (MTaPS) Program.
This survey adopted a point prevalence cross-sectional design to determine antimicrobial use in GCH, examining variation and characteristics of antimicrobial use across all the hospital’s open inpatient departments. Data collection was based on the WHO PPS methodology, with forms tailored to the facility, and included all admitted patients on the day of the survey.
The survey population typically consists of patients aged 0 to 21 years from a tertiary referral and teaching hospital serving a diverse demographic.
All admitted patients in the wards during the survey were included, following the WHO PPS methodology for hospitals with < 500 total inpatient beds.
The PPS was conducted over a single day on 13 May 2022, using the “WHO Methodology for Point Prevalence Survey on Antibiotic Use in Hospitals” (11), a standardized global methodology for assessing antimicrobial use in hospital settings. The methodology guides information collection on antibiotic prescribing practices and other data relevant to treating and managing infectious diseases in hospitalized patients (6). We adapted the PPS to collect data on ward occupancy, patient demographics, and antimicrobial prescriptions (Supplementary File 1). The inclusion criteria encompassed all inpatients at 6 am on 13 May 2022 receiving antimicrobial therapy via oral, parenteral, or inhalation routes. Primary exclusion criteria included patients discharged on 13 May 2022, day-case patients, and those receiving topical antimicrobial agents.
We obtained data from the electronic prescription system and the hospital’s multidisciplinary treatment plan notes, which contained medical notes on all patients prescribed antimicrobial agents. Additional information was extracted from laboratory results, including antimicrobial susceptibility testing data. The survey included the 2023 Anatomical Therapeutic Chemical (ATC) medicine categories that WHO recommends to include in antimicrobial consumption surveillance: J01 (antibacterials for systemic use); J02 (antimycotics for systemic use); J04 (antimycobacterials); J05 (antivirals for systemic use); P01AB (nitroimidazole derivatives); and P01B (antimalarials) (18).
We used basic descriptive statistics to analyze the data using Microsoft Excel™ 2021. Using the 2023 WHO ATC codes, we entered the antimicrobials’ International Non-proprietary Names (INNs) into Microsoft Excel™ 2021 and then analyzed the data to characterize the macro (above-molecule) AMU trends. AMU by percentage share of each antibiotic molecule was labeled as either Access, Watch, or Reserve (AWaRe) in accordance with the 2021 WHO AWaRe list (19), and we then determined the total percentage share of prescribing by WHO AWaRe category. This analysis omits antibiotics not categorized within the 2021 WHO AWaRe list. Other analysis parameters were compliance with prescribing standards, where each prescription was assessed for its completeness in recording generic prescribing by INN, dose, frequency, duration, indication, and route of administration information; the prevalence of antimicrobial use; microbiology utilization; and adherence to GCH’s standard treatment guidelines (20), GCH antimicrobial use policies (21, 22) and microbiology findings.
The survey analysis employed descriptive methods to examine patterns of antimicrobial use. Key outcome measures included patient characteristics; percentage utilization of antimicrobials by pharmacological subgroups and individual antimicrobial molecules within the defined scope; the proportion of prescriptions meeting documentation standards, including antimicrobial name (as INN), treatment duration, dosing frequency, dose, route of administration and clinical indication; number and percentage of antimicrobials prescribed for specific indications; and prevalence of antimicrobial prescribing aligned with GCH guidelines, policies, and microbiology findings.
At the time of the survey, the hospital had 71 open inpatient beds across five open wards (i.e., medical, surgical, and intensive care units) with 56 inpatients (79% occupancy rate, 63% male). The inpatients’ age range was 2 months to 18 years and distributed as 20% < 1-year old (n=11), 55% 1- to 5-year-old (n=31), and 25% > 5-year-old (n=14).
Of the 56 inpatients, 34 (61%) were on antimicrobials, with more males than females (i.e., 68% [n=23] and 32% [n=11], respectively). The age range of patients prescribed antimicrobials was 24% < 1-year-old (n=8), 50% 1-5-year-old (n=17), and 26% > 5-year-old (n=9).
Systemic antibacterials (J01) were the most prescribed antimicrobials, representing 80% (n=42) of all active prescriptions (Table 1). The most prescribed pharmacological subgroup of antimicrobials was third-generation cephalosporins at 21%, followed by a combination of penicillins, including beta-lactamase inhibitors (19%), then imidazole derivatives (12%) (Figure 1). These three antimicrobial pharmacological subgroups accounted for >50% of all antimicrobial prescriptions.
Ceftriaxone was the most prescribed antimicrobial at 18%, followed by amoxicillin/clavulanic acid (16%) and metronidazole (12%) (Figure 2). Five antimicrobials (ceftriaxone, amoxicillin/clavulanic acid, metronidazole, cefuroxime, and fluconazole) accounted for 58% of all prescriptions. The average number of antimicrobials prescribed per patient was 1.2 (range 1-5). Of the 41 antibiotics analyzed, the average proportion prescribed by WHO AWaRe classification was 49% Access, 51% Watch, and 0% Reserve. Supplementary File 2 includes prescribing by ward.
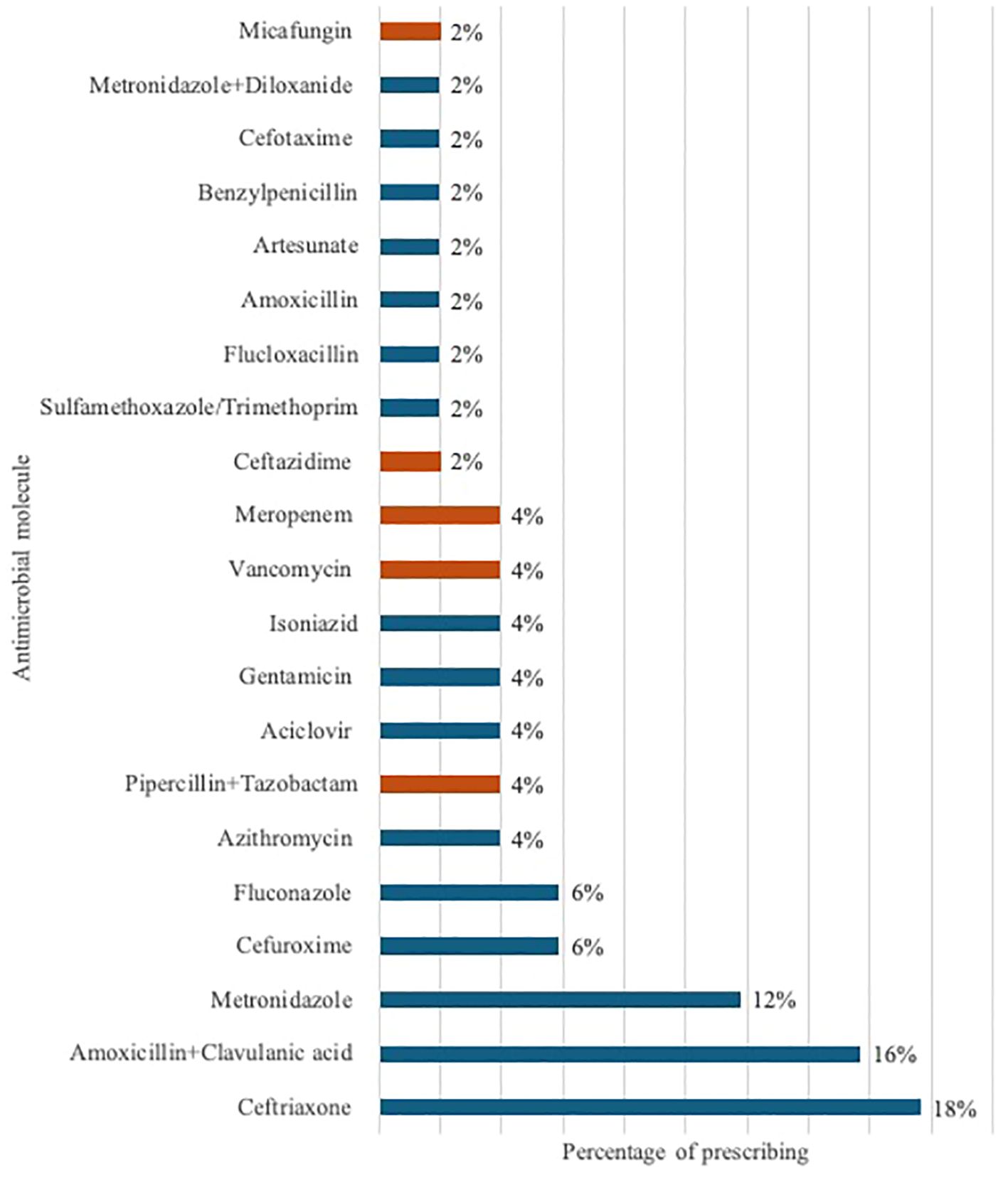
Figure 2. Percentage of antimicrobial prescribing by molecule, with orange markers indicating antimicrobials from the hospital’s restricted list.
GCH guidelines restrict selected antimicrobials by controlling how and who prescribes them. We found that 16% of all antimicrobials prescribed were from the hospital’s restricted list of antimicrobials: vancomycin (4%), meropenem (4%), piperacillin/tazobactam (4%), ceftazidime (2%), and micafungin (2%) (highlighted in Figure 2).
The PPS revealed that all prescribers used the generic INN and recorded the indication for prescribing in the multidisciplinary notes for 94% of prescriptions and on the treatment chart for no prescriptions. However, all 52 prescriptions had duration of treatment, frequency, dose, and route of administration recorded in the treatment chart.
Of the 52 indications recorded, 60% were community-acquired infections (Table 2). Upper respiratory tract infection (17%) was the most common indication of community-acquired infections, followed by gastrointestinal infection (15%).
The average treatment duration was 5.3 days (excluding the medical prophylaxis prescription outliers). Twenty-three prescriptions (44%) were prescribed for more than seven days across five patients. Six of these (11%) indicated an underlying long-term condition, which may have required medical prophylaxis (e.g., acute myeloid leukemia, Bartter’s Syndrome, suspected cystic fibrosis, systemic lupus erythematosus). Thirty percent (n=7) of the prescriptions had a clear rationale documented in the notes for continuing treatment beyond 7 days; 16 prescriptions (70%) had no such documentation.
Analysis revealed that 75% of antimicrobial prescriptions were administered via the intravenous (IV) route, 23% were prescribed for oral administration, and 2% were delivered through nebulization.
Fifty percent of prescriptions complied with the GCH standard treatment guidelines, GCH restricted antimicrobial policy, and, where applicable, microbial testing results. The audit also examined why prescribers did not comply with these information sources. Two cases of the 17 samples processed were inappropriately treated based on their microbial testing results, which showed bacterial contamination or colonization rather than an actual infection. Six of the eight prescriptions for medical prophylaxis were inappropriate, as they were not in keeping with the hospital guidelines.
Our survey showed that the prevalence of antimicrobial prescribing on 13 May 2022 in GCH was 61% (n=34), which is comparable to the other PPS in Kenya that had rates of 62% (12) and 68% (13). It is, however, essential to note GCH’s unique patient demographics as a pediatric hospital; the other two studies were conducted in public hospitals with a wide range of patient ages. Also, the small sample size of 34 patients on antimicrobials is attributed to data from a single private facility specializing in pediatric care.
The complete documentation of antimicrobial prescribing parameters—such as prescribing by generic (INN) name, dose, frequency, route of administration, and duration of use—was achieved for all prescriptions, attributable to the hospital’s electronic health management information system (HMIS), which mandates the entry of these core elements by prescribers. However, while indications for AMU were documented in 94% of prescriptions within the multidisciplinary care notes, the HMIS lacked a designated field for recording this critical information.
The spectrum of antimicrobials used in the facility revealed that over half came from only five antimicrobials. This AMU pattern may be suboptimal as evolutionary pressure driving resistance would be focused only among this narrow band (23).
Evaluation of antibiotic prescriptions by WHO AWaRe categories showed that 49% of antibiotics were in the Access category (narrow-spectrum antibiotics); this finding was lower than a county referral hospital's in Kenya (57% Access antibiotics) (12), but higher than a hospital in Bangladesh whose use of Access antibiotics was 36% (24). While there is no globally defined facility-level recommendation for Access use rate, WHO recommends a 60% country-level AMU rate for Access antibiotics (19). In addition, broader-spectrum Watch category antibiotics accounted for more than half of the total antibiotic use recorded, surpassing the recommended narrow-spectrum first- and second-line treatments for common infections. This finding highlights an urgent need to regulate the use of Watch category antibiotics, which contain broad-spectrum antibiotics with a higher potential of developing resistance (25).
The survey also revealed that an overwhelming number of antimicrobials (75%) were prescribed for parenteral administration, which is high compared to 54% at a county referral hospital (12); however, the GCH PPS only included children, who may be less able to tolerate oral medications than adults. Most patients presenting with a severe infection requiring parenteral therapy can be switched to oral treatment after 24-72 hours if they are improving and can tolerate an oral formulation. An early switch from parenteral treatment reduces the likelihood of hospital-acquired bacteremia and infected or phlebitic IV lines (26). In addition, it improves patients’ mobility and potential for earlier discharge, thereby saving both money and time for healthcare workers.
From the two cases that were inappropriately treated based on their microbial testing results (contamination vs. colonization), we can draw lessons on interpreting laboratory findings, which are key to diagnostic stewardship. Not all culture growths require antimicrobial sensitivity testing (27).
Hospital-acquired infections (HAIs), also known as nosocomial infections, occur in patients during hospitalization—up to 48 hours after hospital admission, up to 3 days after discharge, or up to 30 days after surgery. The nine prescriptions to treat HAIs were for three patients: one an oncology patient, one a referral, and one with an extended hospital stay. GCH has a well-structured infection prevention and control (IPC) program, but additional training is needed on IPC practices for oncology patients who are more susceptible to infections due to their immunosuppressed state; in addition, referral cases may need isolation to prevent the spread of HAIs, especially in open-ward settings and critical units. The GCH IPC committee monitors IPC practices regularly and implements targeted interventions to lower the incidence of HAIs, as per WHO guidance (28).
The PPS showed only 50% compliance with guidelines, policy, and microbiological evidence, including surgical and medical prophylaxis. For example, surgical prophylaxis with pre or postoperative antibiotics is an accepted practice governed by various guidelines to reduce the risk of surgical site infections and optimize postoperative recovery. All four prescriptions for surgical antimicrobial prophylaxis cases were noncompliant. Three indications were prescribed for procedures that did not require postoperative antibiotics per the GCH guidelines (i.e., lipoma removal, cleft lip and palate repair, and microfracture repair). The fourth surgical case was a urethroplasty, where literature indicates that postoperative use of antimicrobials does not reduce the incidence of surgical site infections (29); however, the GCH guidelines do not explicitly include this indication. Although patients undergoing urethroplasty with bacteriuria or urinary tract infections should be treated postoperatively to reduce infection risk, this patient did not fall into that category.
Medical prophylaxis is the routine use of an antimicrobial to lower infection risk due to a chronic health condition such as cystic fibrosis, chronic obstructive lung diseases, cancers, etc. In our survey, a patient with acute aspiration pneumonia secondary to poisoning and gastroesophageal reflux disease was prescribed five prophylactic antimicrobials—neither indication required antimicrobial prophylaxis. A second patient treated for recurrent lung infections had a sputum culture that grew Pseudomonas aeruginosa sensitive to gentamicin and piperacillin/tazobactam, which were prescribed. However, the patient was also on unnecessary prophylactic macrolide therapy. While long-term use of low-dose macrolides in chronic airway disease is acceptable due to the macrolides’ antibacterial and immunomodulatory effects, the current infection should have been cleared using the appropriate antimicrobial first.
While an electronic HMIS may be a good structural intervention to strengthen AMS programs, its design should be customized to include essential factors. The findings revealed that 94% of the prescriptions had an indication documented on the multidisciplinary notes, which is commendable. However, implementing structural improvements to the HMIS to include indications for each antimicrobial in the treatment chart will ensure that this crucial information is captured.
While clinicians documented their daily reviews of patients, there was no structured review specifically for antimicrobial use. Implementing a structured mandatory review of antimicrobial prescriptions would be necessary at key points in treatment (e.g., 24-72 hours post-therapy initiation). This structured review of antimicrobial prescriptions would trigger the prescriber to examine the efficacy of and need for the antimicrobial and, therefore, the implementation of critical AMS actions, such as discontinuing therapy, switching to the oral route, escalating or de-escalating based on microbiology results, or confirming the continuation of the same treatment until the next review period. Based on this, we recommend that the GCH AMS team establish a multidisciplinary team to carry out ward rounds to support AMU in the facility.
As only five antimicrobials accounted for over 50% of prescriptions, we recommend that the facility’s AMS program explore ways to expand the variety of antimicrobials consumed and increase healthcare workers’ awareness and use of GCH standard treatment guidelines, which align with appropriate use principles.
Additionally, to enhance oversight of Watch and Reserve antibiotic prescribing, we recommend that the GCH classify all antibiotics used at the hospital according to AWaRe. This approach would expand the current GCH-restricted list of antimicrobials, enabling the committee to establish clear parameters for regulating Watch and Reserve antibiotics and other restricted antimicrobials.
The AMS team at GCH should also develop guidance for switching from IV to oral to ease the clinician’s decision-making regarding de-escalation from parenteral to oral antimicrobials.
To support appropriate diagnostic stewardship in informing treatment recommendations, the GCH AMS team must prioritize strengthening the laboratory’s capacity to provide their users with accurate information on whether isolated organisms are colonizers, contaminants, or pathogenic. Furthermore, the laboratory should ensure that sensitivity testing is only conducted for pathogenic organisms and selectively report results (suppressing results of second/third-line antimicrobials, where first-line treatment is available) to guide clinician prescribing. Proper sample collection techniques will help limit the contamination of specimens collected by normal flora found on the skin and mucosal membranes or by microorganisms in the environment. We recommend that healthcare providers receive refresher training on the appropriate techniques.
GCH has implemented a surgical antimicrobial prophylaxis policy to regulate AMU for various surgical procedures performed in its theaters, complemented by regular audit feedback to surgeons for continuous improvement. We recommend supplementing this policy with guidance that defines specific postoperative scenarios that warrant antimicrobial use, ensuring a more precise and evidence-based approach.
Additionally, a guideline for antimicrobial use in medical prophylaxis, along with targeted training of prescribers on the rationale for its use, would provide clear guidance and support optimal antimicrobial use for medical prophylaxis.
Implementing the PPS at GCH aligned with the country’s NAP-AMR and the national AMS guidelines. The findings from this survey contribute valuable data on antimicrobial use, particularly within the private sector, and provide a reproducible methodology for conducting PPS, which can be utilized across the country and region. These insights will strengthen the local evidence base and support the revision of the NAP-AMR, AMS guidelines, and AMR policies to incorporate targeted interventions to address the gaps identified.
Moreover, the results provide the first step in generating data on antimicrobial use in the private sector, which can be compared with existing data from the public sector. This comparison will help inform the development of tailored AMS interventions for private sector health institutions, considering their unique operational contexts, such as the availability of electronic HMIS. By advancing HMIS to include AMS-related actions, these interventions can be standardized and recommended for integration into public health facilities, ultimately strengthening AMS efforts across the sectors.
The sample size analyzed was relatively small, encompassing only 56 pediatric inpatients, with only 34 on antimicrobials. Additionally, the data represents findings from a single facility in Kenya, which has over 10,000 healthcare facilities. Therefore, the generalization of the findings to the broader pediatric population or all private healthcare facilities in Kenya may be limited. However, these findings provide a critical first look into AMU patterns and associated gaps in antimicrobial prescribing within pediatric care and the private healthcare sector in Kenya.
This PPS produced AMU data that provide unique insights for the GCH stewardship program and for formulating policies, guidelines, and protocols to stem the emergence of AMR. Our major recommendations include developing a policy to define IV-to-oral switch criteria; enhancing the HMIS to include key AMS actions; establishing antimicrobial ward rounds to review therapy appropriateness; sensitizing various cadres on proper sample collection and rationale for requesting laboratory tests; training healthcare providers on the appropriate interpretation of laboratory findings; and communicating with any healthcare facility that is referring patients to GCH on any potential HAI cases.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The requirement of ethical approval was waived by Gertrude’s Children’s Hospital Research Committee for the studies involving humans because it serves as part of the institution’s continuous quality improvement program. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board also waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the research held no identifiable data elements.
JM: Supervision, Writing – review & editing, Formal analysis. NG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. WA: Data curation, Formal analysis, Investigation, Project administration, Writing – original draft, Writing – review & editing. SM: Conceptualization, Project administration, Resources, Supervision, Writing – review & editing. NK: Project administration, Resources, Writing – review & editing. TH: Writing – review & editing. MJ: Writing – review & editing. ME: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the USAID under contract number (7200AA18C00074). The contents are the responsibility of the authors and do not necessarily reflect the views of USAID or the US Government. The funder had no role in survey design, data collection, data analysis, data interpretation, or writing of the manuscript.
We would like to appreciate Charles Matheka, Veronica Mwikali, and Uri Karanja who took part in the data collection process.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fitd.2025.1497199/full#supplementary-material
1. Murray CJL, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. (2022) 399:629–55. doi: 10.1016/S0140-6736(21)02724-0
2. Sartorius B, Gray AP, Davis Weaver N, Robles Aguilar G, Swetschinski LR, Ikuta KS, et al. The burden of bacterial antimicrobial resistance in the WHO African region in 2019: a cross-country systematic analysis. Lancet Glob Health. (2024) 12:e201–16. doi: 10.1016/S2214-109X(23)00539-9
3. National Antimicrobial Stewardship Interagency Committee. Kenya Antimicrobial Resistance Surveillance Report. Nairobi: Ministry of Health (2022).
4. Mapping Antimicrobial Resistance and Antimicrobial Use Partnership. National Situation of Antimicrobial Resistance and Consumption Analysis from 2016-2018 (2022). Available online at: https://africacdc.org/download/mapping-antimicrobial-resistance-and-antimicrobial-use-partnership-maap-country-reports/ (Accessed September 9, 2024).
5. Ahmed SK, Hussein S, Qurbani K, Ibrahim RH, Fareeq A, Mahmood KA, et al. Antimicrobial resistance: Impacts, challenges, and future prospects. J Med Surg Public Health. (2024) 2:100081. doi: 10.1016/j.glmedi.2024.100081
6. Ministry of Health. National Action Plan on Prevention and Containment of Antimicrobial Resistance (2017). Available online at: https://www.afro.who.int/publications/national-action-plan-prevention-and-containment-antimicrobial-resistance-2017-2022 (Accessed September 9, 2024).
7. World Health Organization. Global Action Plan on Antimicrobial Resistance. Geneva, Switzerland: World Health Organization (2015). Available at: https://www.who.int/publications/i/item/9789241509763 (Accessed September 9, 2024).
8. World Health Organization. Antimicrobial stewardship programmes in health-care facilities in low- and middle-income countries. A Pract toolkit. Geneva: World Health Organization (2019). Available online at: https://iris.who.int/bitstream/handle/10665/329404/9789241515481-eng.pdf?sequence=1 (Accessed September 9, 2024).
9. WHO African Region. Status on national core elements for antimicrobial stewardship programmes in the WHO African Region. Brazzaville: WHO African Region (2024). (Accessed December 28, 2024).
10. World Health Organization. Kenya national action plan on antimicrobial resistance: review of progress in the human health sector. Geneva, Switzerland: Antimicrobial resistance policy information and action brief series. World Health Organization (2022).
11. World Health Organization. WHO Methodology for Point Prevalence Survey on Antibiotic Use in Hospitals (2019). Available online at: https://apps.who.int/iris/rest/bitstreams/1175969/retrieve (Accessed May 9, 2024).
12. Kamita M, Maina M, Kimani R, Mwangi R, Mureithi D, Nduta C, et al. Point prevalence survey to assess antibiotic prescribing pattern among hospitalized patients in a county referral hospital in Kenya. Front Antibiot. (2022) 1:993271. doi: 10.3389/frabi.2022.993271
13. Okoth C, Opanga S, Okalebo F, Oluka M, Baker Kurdi A, Godman B. Point prevalence survey of antibiotic use and resistance at a referral hospital in Kenya: findings and implications. Hosp Pract 1995. (2018) 46:128–36. doi: 10.1080/21548331.2018.1464872
14. Ministry of Health. National Antimicrobial Stewardship: Guidelines for Health Care settings. Nairobi, Kenya: Ministry of Health (2020). Available online at: https://www.health.go.ke/wp-content/uploads/2021/09/National-Antimicrobial-Stewardship-Guidelines-for-health-care-settings-In-Kenya-2020.pdf (Accessed September 9, 2024).
15. Ministry of Health. Clinical Management and Referral Guidelines Volume 3. In: Clinical Guidelines for Management and Referral of Common Conditions at Levels 4-6: Primary Care. Nairobi, Kenya: Ministry of Health (2009).
16. Ministry of Health. BASIC PAEDIATRIC PROTOCOLS: for ages up to 5 years (2022). Available online at: http://guidelines.health.go.ke//category/55/478/meta (Accessed September 9, 2024).
17. World Health Organization. Pocket book of hospital care for children: guidelines for the management of common childhood illnesses (2016). Available online at: https://iris.who.int/bitstream/handle/10665/81170/9789241548373_eng.pdf?sequence=1 (Accessed September 9, 2024).
18. World Health Organization. GLASS guide for national surveillance systems for monitoring antimicrobial consumption in hospitals. Geneva, Switzerland: World Health Organization (2020). Available at: https://www.who.int/publications/i/item/9789240000421 (Accessed May 9, 2024).
19. World Health Organization. WHO Access, Watch, Reserve (AWaRe) classification of antibiotics for evaluation and monitoring of use. Geneva, Switzerland: World Health Organization (2021). Available online at: https://www.who.int/news/item/01-10-2019-who-releases-the-2019-aware-classification-antibiotics (Accessed September 9, 2024).
21. Gertrude’s Children’s Hospital. GCH Policies and Procedures: Restricted Antimicrobials Policy - GCH/MMU/1.1/2. Nairobi, Kenya: Gertrude’s Children’s Hospital (2019).
23. Laxminarayan R, Matsoso P, Pant S, Brower C, Røttingen JA, Klugman K, et al. Access to effective antimicrobials: a worldwide challenge. Lancet. (2016) 387:168–75. doi: 10.1016/S0140-6736(15)00474-2
24. Rashid MM, Akhtar Z, Chowdhury S, Islam MA, Parveen S, Ghosh PK, et al. Pattern of Antibiotic Use among Hospitalized Patients according to WHO Access, Watch, Reserve (AWaRe) Classification: Findings from a Point Prevalence Survey in Bangladesh. Antibiot Basel Switz. (2022) 11. doi: 10.3390/antibiotics11060810
25. Mudenda S, Daka V, Matafwali SK. World Health Organization AWaRe framework for antibiotic stewardship: Where are we now and where do we need to go? An expert viewpoint. Antimicrob Steward Healthc Epidemiol ASHE. (2023) 3:e84. doi: 10.1017/ash.2023.164
26. McCarthy K, Minyon A. Oral or intravenous antibiotics? Aust Prescr. (2020) 43:45–8. doi: 10.18773/austprescr.2020.008.
27. CDC. Selective Reporting of Antimicrobial Susceptibilty Testing Results: A Primer of Antimicrobial Stewardship Programs (2020). Available online at: https://www.cdc.gov/antibiotic-use/pdfs/Selective-Reporting-508.pdf (Accessed September 11, 2024).
28. World Health Organization. Prevention of Hospital Acquired Infections; A practical guide. 2nd edition. Geneva, Switzerland: World Health Organization (2002). Available online at: https://apps.who.int/iris/bitstream/handle/10665/67350/WHO_CDS_CSR_EPH_2002.12.pdf (Accessed September 11, 2024).
Keywords: antimicrobial use (AMU), point prevalence survey (PPS), antimicrobial stewardship (AMS), antimicrobial resistance (AMR), pediatric hospital, private healthcare, antibiotic
Citation: Mbuthia J, Gitonga N, Akoth W, Mutua S, Kusu N, Hafner T, Joshi MP and Embrey M (2025) Antimicrobial use in a pediatric hospital in Kenya: a point prevalence survey to inform antimicrobial stewardship. Front. Trop. Dis. 6:1497199. doi: 10.3389/fitd.2025.1497199
Received: 16 September 2024; Accepted: 30 January 2025;
Published: 05 March 2025.
Edited by:
Annick Lenglet, University of KwaZulu-Natal, South AfricaReviewed by:
Joshua Mbanga, National University of Science and Technology, ZimbabweCopyright © 2025 Mbuthia, Gitonga, Akoth, Mutua, Kusu, Hafner, Joshi and Embrey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Winnie Akoth, d2Frb3RoQGdlcnRpZXMub3Jn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.