- 1Nono District Health Office, Nono, Oromia Region, Ethiopia
- 2School of Medical Laboratory Sciences, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia
- 3Laboratory Bacteriology Research, Department of Diagnostic Sciences, Faculty of Medicine and Health Sciences, Ghent University, Ghent, Belgium
Background: Intestinal schistosomiasis caused by Schistosoma mansoni is a common helminthic infection recognized as an important public health problem in tropical and subtropical regions, particularly in sub-Saharan Africa, including Ethiopia. The disease is highly prevalent among schoolchildren with emerging data showing that these population groups are infected and carry heavy infection intensities. However, there was no prior data on this infection’s extent, intensity, and predisposing factors in the current study area. Therefore, this study aimed to assess the prevalence, intensity, and associated factors of intestinal schistosomiasis among primary school children in Nono District, Southwest Ethiopia.
Methods: A school-based cross-sectional study was conducted from January 20 to February 20, 2024 with 338 randomly selected primary school children. Data on socio-demographic and potential predisposing factors were collected using a structured questionnaire. A total of 5 g of stool samples was collected and processed using the Kato-Katz technique for parasitic investigation and infection intensity. Data were entered into Epi-data version 3.1 and then exported to Statistical Package for the Social Sciences version 26 for analysis. Binary logistic regression analyses were carried out to examine the associations between dependent and independent variables. A P-value <0.05 was considered statistically significant.
Results: In this study, only 21.5% of participants were infected with S. mansoni (95%CI: 11.77, 32.47). Of those infected participants, 49.3% had light infection intensity. Children who had no habit of wearing shoes (AOR = 3.27, 95%CI: 2.04, 8.47), wear shoes sometimes (AOR = 1.87, 95%CI: 1.22, 7.31), had open defecation practice at school (AOR = 1.21, 95%CI: 1.02, 3.58), from families who used river (AOR = 5.47, 95%CI: 2.53, 9.76) and spring water (AOR = 1.28, 95%CI: 1.01, 3.88) for drinking, from families who had no latrine at home (AOR = 8.14, 95%CI: 4.03,10.94), who had bathing habit in open water source once per day (AOR = 5.29, 95%CI: 3.01, 11.49), twice per week (AOR = 3.42, 95%CI:1.98, 7.92), and once per week (AOR = 2.56, 95%CI: 1.07, 5.96), and who did not know the possible modes of transmission (AOR = 1.15, 95%CI: 1.04, 3.27) were significantly associated with intestinal schistosomiasis.
Conclusion: The prevalence of S. mansoni infection in this study was medium according to the WHO (2022) classification. Factors like having no habit of wearing shoes, using river and spring water for drinking, having open defecation practice at school and home, bathing in open water sources, and not knowing the possible modes of transmission aggravated the occurrence of intestinal schistosomiasis. Therefore, health education should be given to children on the importance of wearing shoes, improving water sources, and environmental sanitation to alleviate the problem.
Introduction
Schistosomiasis is one of the most prevalent parasitic diseases and affects more than 236 million people globally despite it being considered as a neglected tropical disease (NTD) (1). This disease continues to pose a significant public health challenge, leading to notable socio-economic consequences, particularly in regions characterized by insufficient control measures and sanitation along with high poverty levels among the population (2). There are five species of Schistosoma with a tendency to occur in restricted geographical patterns. Schistosoma mansoni is the most prevalent species found in tropical and subtropical regions of sub-Saharan African, Middle East Asian, South American, and Caribbean countries (3). It is estimated that intestinal schistosomiasis infects around 200 million people globally, of whom 120 million are asymptomatic and 20 million have a severe disease. Moreover, about 600 million people are at risk of this infection (4).
Morbidity and mortality due to schistosomiasis are largely due to the consequences of a host T-cell-mediated immune response against parasite eggs trapped in the tissues. Antigens released from eggs stimulate a granulomatous reaction involving T cell, macrophages, and eosinophils, which results in clinical diseases. However, the magnitude of the resulting granulomatous and fibrosis inflammation varies greatly from individual to individual (5). Intestinal schistosomiasis causes abdominal pain, diarrhea, and bloody stool. In an advanced stage of the disease, there is an enlargement of the spleen and liver, which is associated with fluid accumulation in the peritoneal cavity and hypertension of abdominal vessels. In children, it causes anemia, stunted growth, and reduced ability to learn. In some chronic and severe cases, schistosomiasis can even lead to death (6). The World Health Organization Observatory data gives an estimate of 14,365 deaths due to schistosomiasis in 2020 (4).
According to WHO (2022), all school-aged children (SAC) and at-risk adults living in schistosomiasis-endemic areas with a high prevalence (≥50%) should get treatment annually. Thus, mass drug administration (MDA) campaign often uses the existing infrastructures of schools which ensure that an individual at a critical stage of physical and cognitive development can be reached and help to keep this intervention cost-effective. However, some groups of children who do not regularly attend school will not be reached through MDA measures (7).
Globally, over 250 million cases of schistosomiasis were reported in the sub-Saharan African region comprising about 90% of cases (8, 9). Next to Nigeria, Tanzania is the second country having the highest cases of schistosomiasis from the sub-Saharan African region. Approximately 51% of the Tanzanian population is either exposed or live in high-risk areas for this infection (10).
In Ethiopia, several epidemiological studies were conducted in different parts of the country. Those previous studies reported the presence of schistosomiasis, including S. mansoni infection, and also showed new transmission foci from time to time in the country. The reasons for the spreading of the disease to new localities seem to be due to an extensive population movement and water resource development (11, 12). Thus, about 3.4 million preschool children, 12.3 million SAC, and 21.6 million adults in Ethiopia live in schistosomiasis-endemic areas. These indicate that even though several control methods have been implemented in the country, still the magnitude and impact of schistosomiasis is high (13).
Instances of poor personal and environmental hygiene coupled with frequent contact habit with water bodies were reported to render individuals more vulnerable to schistosomiasis. A study conducted in the suburbs of Mekele City showed that SAC who had frequent contact with water bodies were 27 times more likely to acquire S. mansoni infection compared to their counterparts (14). Identifying potential predisposing factors for S. mansoni infection at different levels is crucial since it enables us to understand how transmission varies within small spatial scales and how it changes over time. In addition, identifying associated factors may facilitate disease control by targeting high-risk groups or by informing decision-makers to design possible intervention strategies (15). However, there was no previous study conducted on the extent, intensity, and associated factors of intestinal schistosomiasis among SAC in the current study area. Therefore, this study aimed to assess intestinal schistosomiasis’ prevalence, intensity, and associated factors among primary school children in Nono District, Southwest Ethiopia.
Materials and methods
Study area and period
The study was conducted in Nono District of West Shoa Zone, Oromia Regional State, from January 20 to February 20, 2024. Nono District is one of the districts in the West Shoa Zone, and it is found 101 km from Ambo Town and 316 km from Addis Ababa in the southwest direction. This district has a total population of 129,485. Regarding health facilities, there were four health centers and 33 health posts with 78 health extension workers and 201 health professionals with different fields of study.
In Nono District, there were water sources that schoolchildren used for domestic purposes. Three large rivers cross the district, and Gibe river has a long boundary with more parts of the villages of the district, and these could be potential risk factors for infection with S. mansoni. The district has 34 primary schools with a total number of students at 15,300 (7,191 girls and 8,109 boys) and 182 teachers (Nono District Administration Report, 2023). Four primary schools, namely, Nano Kondala, Nano Halo, Halo Dinki, and Biftu Jalala were randomly selected for this study. Schoolchildren in Nono District were treated with praziquantel once a year, and they were earlier treated with this drug 10 months prior to this study.
Study design and population
A school-based cross-sectional study was conducted. The source population for this study comprised all students attending those four selected primary schools in Nono District, West Shoa Zone. The study populations were all randomly selected children from those four primary schools during the study period. Primary schoolchildren who were severely ill and also those who had taken anti-helminthic drugs within the past 2 weeks prior to data collection were excluded from the study.
Sample size determination and sampling procedure
The required sample size for this study was determined using single population proportion formula by considering the prevalence of S. mansoni among primary school children at 27.6%, which was taken from a similar study done in Manna District, Jimma Zone (16), 95% confidence interval, 5% margin of error, and 10% non-response rate. Thus, the final sample size was 338.
Regarding the sampling procedure, Nono District has 34 primary schools, and from these, four primary schools were selected randomly using the lottery method. The number of students attending grades 1–8 in those selected schools were as follows: Nano Kondala—712, Nano Halo—690, Halo Dinki—547, and Biftu Jalala—740. To enroll students from each school in the study, a systematic random sampling technique was used by considering an alphabetically ordered list (name) of students on separate excel sheets as a sampling frame. To determine the interval of children in each school, the K-th value was used, where the K-th value was calculated by dividing the total number of students attending those selected primary schools by the calculated sample size (2,689/338 = 8). The calculated sample size was proportionally allocated to each school based on the number of students. Finally, the first participant from each school was selected randomly by lottery method from one to eight, and thereafter children at every eight intervals were included in the study. In case the selected child was absent, the next child was used.
Data collection methods
Questionnaire survey
Data were collected by two trained health extension workers and two senior public health officers and supervised by four senior BSc Laboratory professionals using a pretested structured questionnaire. To ensure consistency, the questionnaire was first developed in English, then translated into local language (Afan Oromo), and back-translated into English by different language experts. After written consent was obtained from the study participants/guardians, the trained health extension workers collected data through face-to-face interviews to assess socio-demographic, behavioral, and environmental factors.
Specimen collection and parasite identification
After the interview-related questionnaire was completed, a marked clean-labeled plastic stool cup and an applicator stick were given to all study participants, and they were requested to collect about 5 g of stool sample. Immediately after a fresh stool sample was received, a single Kato-Katz thick smear per stool sample was prepared by two medical laboratory professionals using a template delivering 41.7 mg of feces for both detection and quantification of S. mansoni eggs. The prevalence of S. mansoni was calculated by dividing the number of schoolchildren who were positive for S. mansoni infection during examination of Kato-Katz thick smear by the total number of schoolchildren involved in the study. Quantification of the egg load for evaluation of the intensity of infection was performed following standard operating procedures (SOPs), and a multiplication factor of 24 was used to convert the counted eggs into eggs per gram of feces (epg). The intensity of infection was estimated from the number of epg, and then cutoff values for the classification of infection intensity were used accordingly. Thus, for S. mansoni, the intensity of infection is classified as light (1–99 epg), moderate (100–399 epg), and heavy (≥400 epg) (17, 18).
Methods of data analysis
Data were entered into Epi-Data version 3.1 and analyzed using SPSS version 26. Descriptive statistical analysis was utilized to describe the study participants’ socio-demographic, behavioral, and environmental factors and the prevalence of S. mansoni. Both bi-variable and multivariable logistic regression analyses were done to assess the relationship between related variables and S. mansoni infection. In bi-variable logistic regression analysis, each associated factor was assessed with S. mansoni infection separately, and then all factors with a p-value less than or equal to 0.25 were considered for multivariable logistic regression analysis. In multivariable logistic regression analysis, a backward stepwise method was used, and all associated factors with a p-value less than 0.05 were considered in the model. Moreover, the variance inflation factor (VIF) was used to check the degree of multi-collinearity; if VIF values were greater than 10, there was significant multi-collinearity and then the correlated variables were considered to be removed from the model one at a time based on their entry order. The model goodness of fit was tested by using the Hosmer–Lemeshow statistic; the model was considered a good fit if it was found to be insignificant for the Hosmer–Lemeshow statistic (>0.05).
Data quality assurance
A total of 2 days of training was given to supervisors and data collectors. The questionnaire was pre-tested on 5% of the schoolchildren before the actual data collection, and required modifications and changes were made. The primary investigator also actively monitored the data collection every day, ensuring that surveys were fully completed and that the data being recorded made sense. Kato-Katz procedure was done by following SOPs in the detection and quantification of S. mansoni eggs. A 10% Kato-Katz thick smear (slide) was randomly selected and examined by a senior medical parasitologist who was blinded to the previous test results.
Ethical consideration
The study protocol was approved by Haramaya University, College of Health and Medical Sciences, Institutional Health Research Ethics Review Committee (IHRERC) (reference number IHRERC/016/2024). A letter of permission to conduct the study was obtained from Nono District Health and Education Office. A cooperation letter was submitted to those four primary schools. Informed voluntary written consent was obtained from each primary school director. Then, the objective and benefit of the study, the right to participate and/or withdraw at any time or not, and the procedures involved were briefly explained to the children, and informed assent was obtained accordingly. All collected data and laboratory results were kept confidential. Any positive test results for stool samples were reported to the nearby health facility for proper management of the infected children.
Results
Socio-demographic characteristics
A total of 338 study participants were included in this study with a response rate of 100%. More than half (57.1%) of the study participants were male students. The age range of the study participants was from 7 to 18 years, with a mean age ( ± SD) of 12.8 ( ± 0.5) years. Majority (78.3%) of the study participants were from a rural residence, and about 54% of the children were from grades 5–8. Of the children’s parents, 281 (83.1%) were farmers (Table 1).
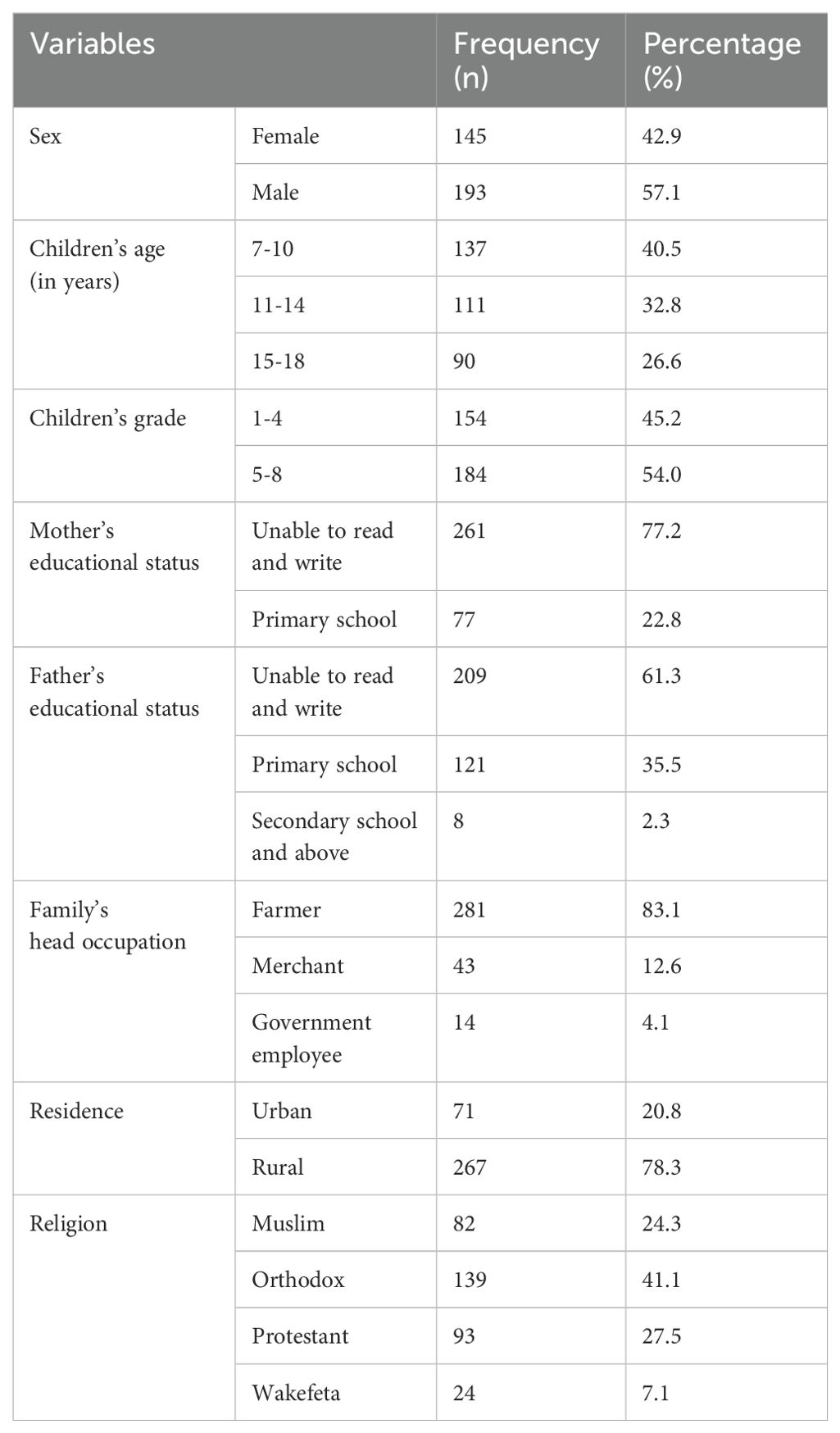
Table 1. Socio-demographic characteristics of study participants in Nono district, west Shoa zone, southwest Ethiopia, 2024 (n=338).
Behavioral and environmental factors
Out of the 338 students included in this study, a majority (42%) of them wore shoes sometimes, and an almost equal number (26% and 26.3%) of participants had bathing habits in open water sources once and twice per week, respectively. Moreover, most of the students (62.7%, 80.8%, and 73.1%) were from families who used pipe water for drinking, had latrines at home, and utilize the latrine at school, respectively (Table 2).
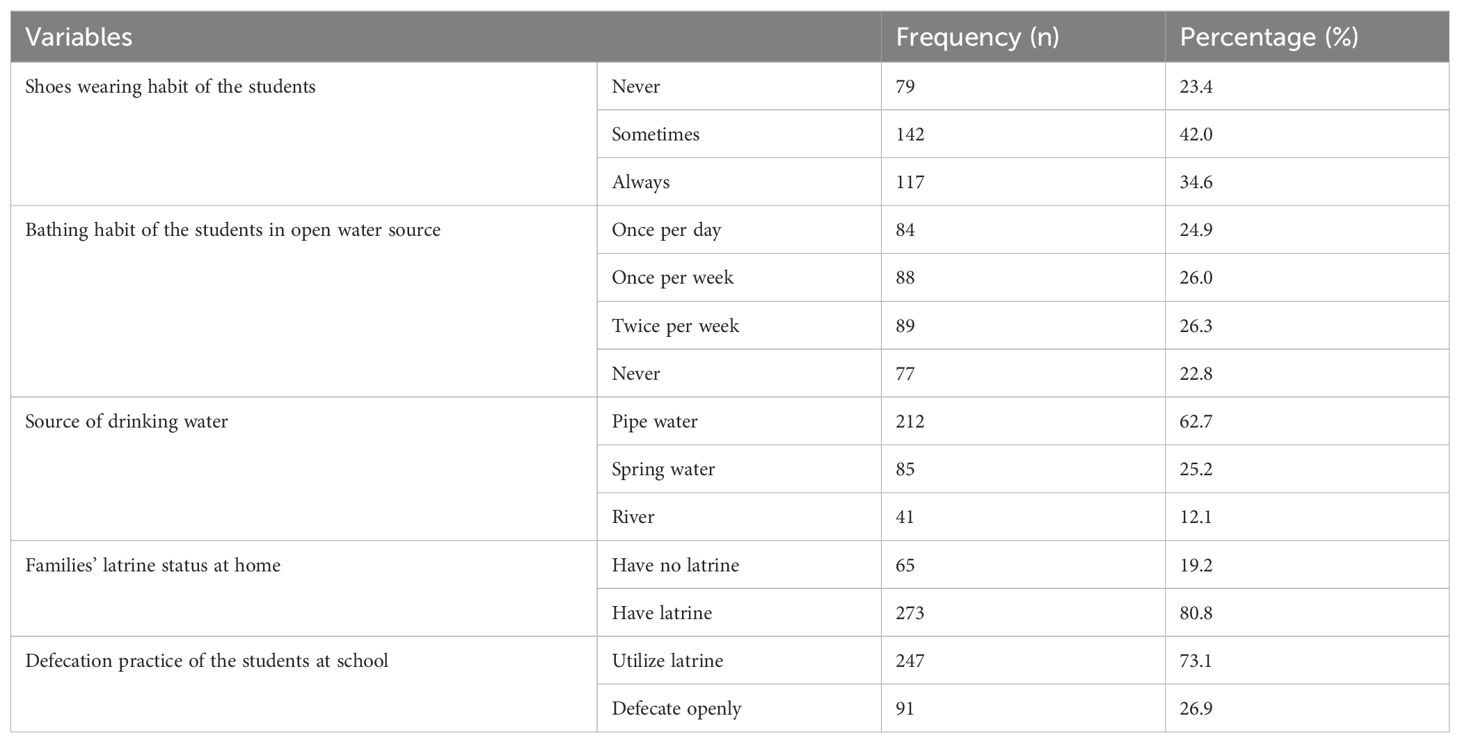
Table 2. Environmental and behavioral factors among primary school children in Nono district, west Shoa zone, southwest Ethiopia, 2024.
Awareness of the study participants about intestinal schistosomiasis
About 256 (75.7%), 309 (91.4%), 260 (76.9%), 266 (78.7%), 280 (82.8%), and 270 (99.9%) of the study participants did not ever hear about intestinal schistosomiasis, did not know the possible source of infection, did not know the mode of transmission, did not know the prevention, did not know the treatment, and did not know the sign/symptoms of intestinal schistosomiasis/bilharziasis, respectively (Table 3).
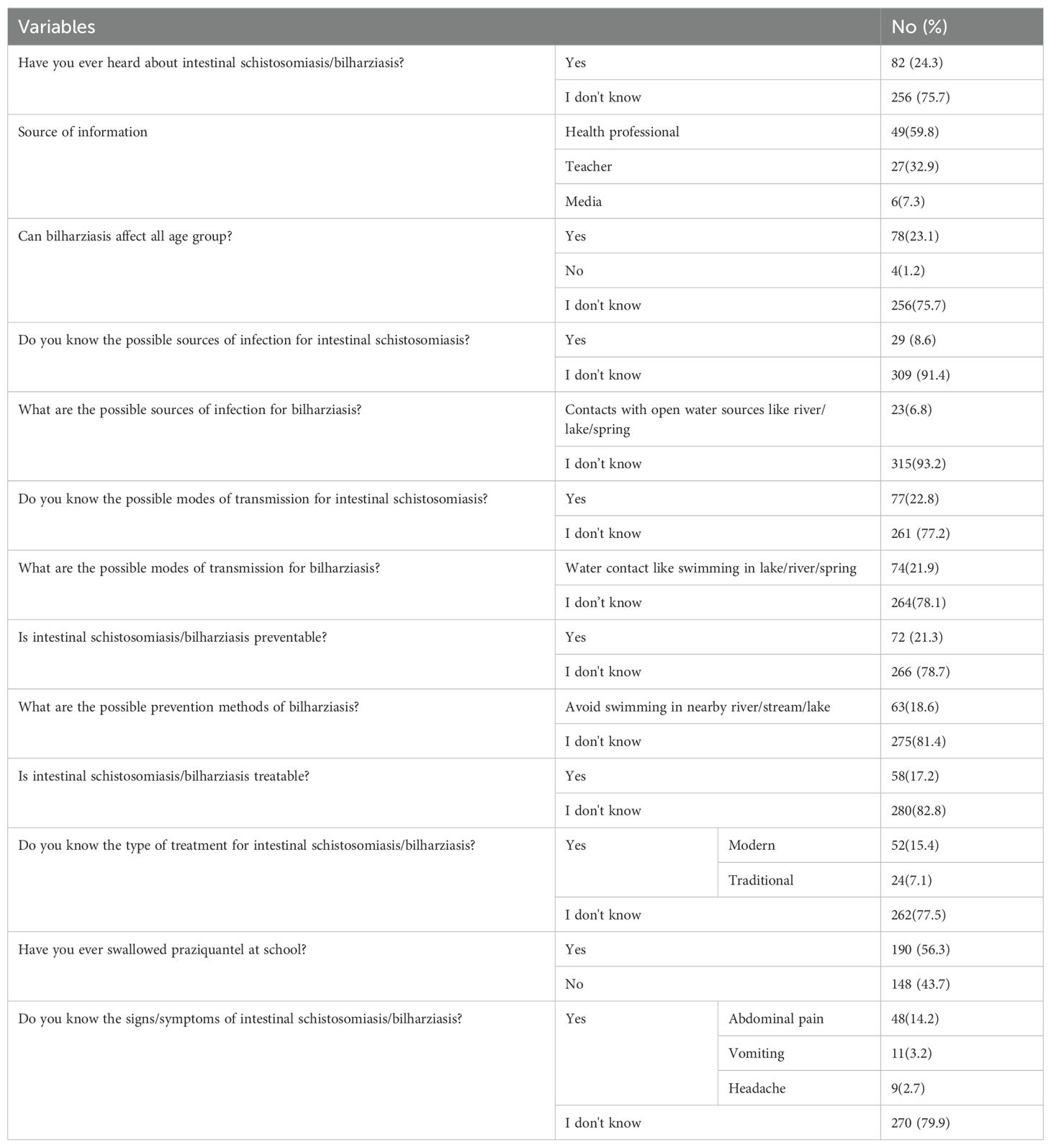
Table 3. Awareness of study participants about intestinal schistosomiasis in Nono district, west Shoa zone, southwest Ethiopia, 2024 (n=338).
Prevalence and intensity of intestinal schistosomiasis
From the total of 338 study participants enrolled in this study, 73 (21.5%; 95%CI: 11.77, 32.47) were positive for S. mansoni infection. Almost half (49.3% and 45.2%) of the students had light and moderate intensity of infection, respectively (Table 4). Relatively, the highest prevalence of S. mansoni was reported from Biftu Jalala primary school (22.6%), followed by Halo Dinki (21.7%), Nano Kondala (21.3%), and Nano Halo primary school (20.7%) (Figure 1). However, there was no statistically significant difference observed in the presence of S. mansoni infection between those primary schools (p = 0.89).
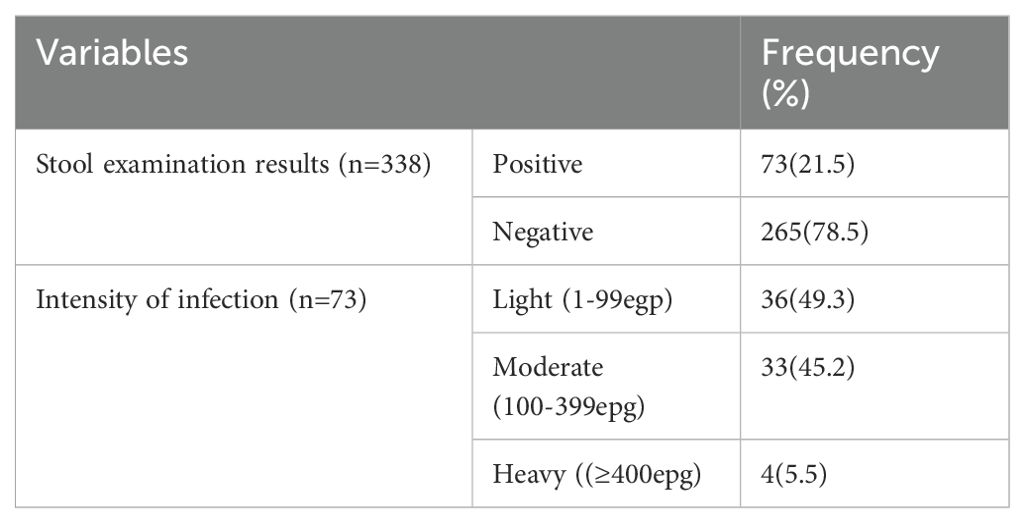
Table 4. Prevalence and intensity of intestinal schistosomiasis among primary school children in Nono district, west Shoa zone, southwest Ethiopia, 2024.
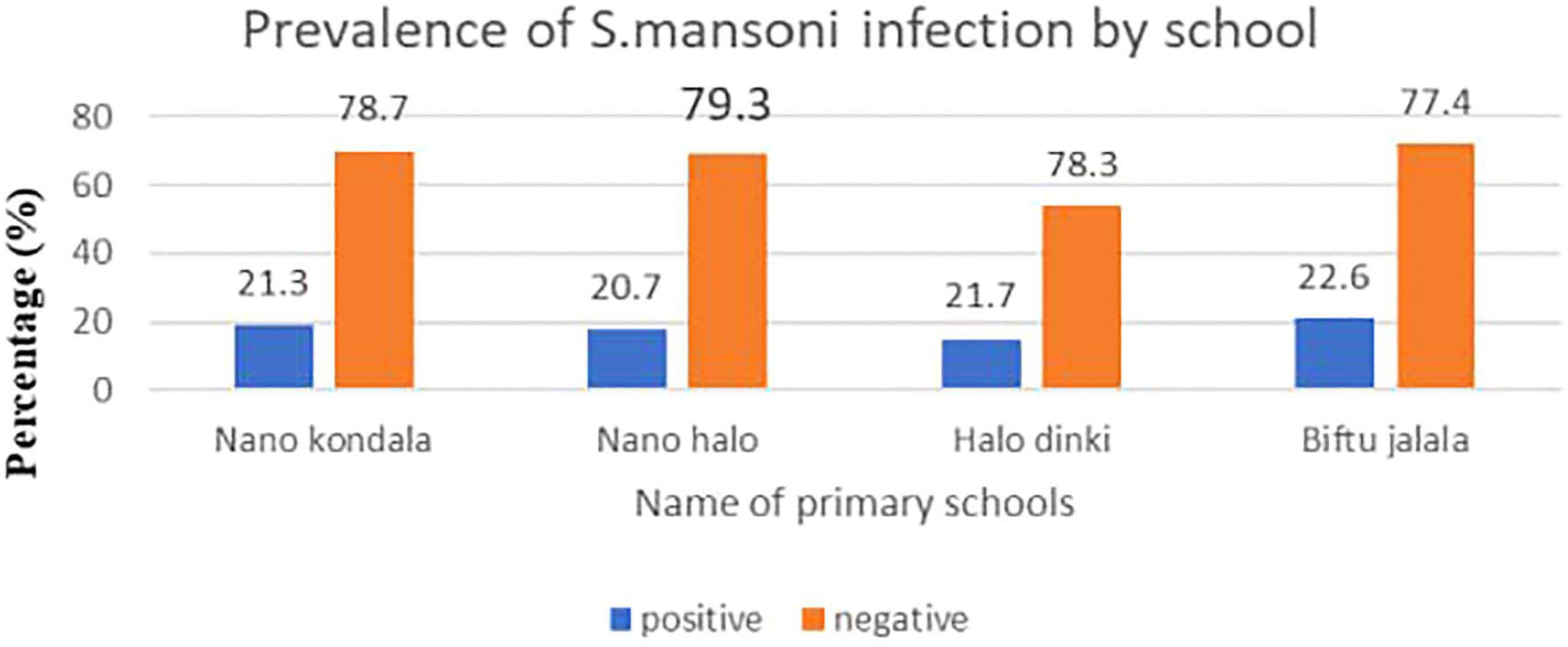
Figure 1. Prevalence of intestinal schistosomiasis among primary school children in Nono district, west Shoa zone, southwest Ethiopia, 2024.
Factors associated with intestinal schistosomiasis
In the bi-variable logistic regression analysis, variables like family latrine status at home, source of drinking water, mother’s educational status, shoes wearing habit of the students, bathing habit in open water source, defecation practice of the students at school, ever heard about intestinal schistosomiasis, and knowing the possible modes of transmission for intestinal schistosomiasis were found to be significant at a p-value less than 0.25 and considered as a candidate for the multivariable analysis. During the multivariable logistic regression analysis, variables including family latrine status, source of drinking water, shoes wearing habit, bathing habit in open water sources, defecation practice at school, and knowing the possible modes of transmission for intestinal schistosomiasis remained to have a statistically significant p-value less than 0.05 (Table 5).

Table 5. Bivariable and multivariable analysis of factors associated with intestinal schistosomiasis among primary school children in Nono district, west Shoa zone, southwest Ethiopia, 2024.
The odds of infection with intestinal schistosomiasis in schoolchildren who had no habit of shoes wearing and wore shoes sometimes were higher than in those who had a habit of wearing shoes always by 3.27 (AOR = 3.27; 95%CI: 2.04, 8.47) and 1.87 (AOR = 1.87; 95%CI: 1.22, 7.31), respectively. The odds of infection with intestinal schistosomiasis in schoolchildren who had a bathing habit in open water sources once per day, twice per week, and once per week were higher than in those who had no habit of bathing in open water sources by 5.29 (AOR = 5.29; 95%CI: 3.01, 11.49), 3.42 (AOR = 3.42; 95%CI: 1.98, 7.92), and 2.56 (AOR = 2.56; 95%CI: 1.07, 5.96), respectively (Table 5).
The odds of infection with intestinal schistosomiasis in schoolchildren from families who used river and spring water sources for drinking were higher than in those from families who used pipe water sources for drinking by 5.47 (AOR = 5.47; 95%CI: 2.53, 9.76) and 1.28 (AOR = 1.28; 95%CI: 1.01, 3.88), respectively. The risk of infection was higher in schoolchildren who had open defecation practice at school than those who utilized latrines (AOR = 1.21; 95%CI: 1.02, 3.58). The odds of infection with intestinal schistosomiasis in children who did not know the possible modes of transmission for this infection was higher than in their counterparts by 2.15 (AOR = 2.15; 95%CI; 1.04, 5.27). Moreover, the risk of infection with S. mansoni was higher in schoolchildren from families who had no latrine than those from families who had a latrine (AOR = 8.14; 95%CI: 4.03, 10.94) (Table 5).
Discussion
This study aimed to assess the prevalence, intensity, and associated factors of S. mansoni infection among primary school children. Thus, the prevalence of infection with S. mansoni was 21.5% (95%CI: 11.77, 32.47). This finding was in line with a study done in western Uganda (27.8%) (19). However, it was lower than the previous findings reported from different parts of Ethiopia: Gonder (33.7%) (20), Gomma District of Jimma Zone (73.8%) (21), and Sanja Area of Amhara Region (82.8%) (22). Moreover, our finding in this study was higher than the prevalence reported from Southwest Ethiopia (8.4%) (16), Jawe District (7%) (23), and Bahir Dar, Northwest Ethiopia (8.0%) (24). The observed differences might be due to the differences in water contact behavior of the communities (frequency of contact with infested water), ecological distribution of intermediate host (snail), local endemicity of the parasite, sample size, and also altitude and temperature, which are important for the development and survival of snails (16, 25).
In this study, schoolchildren who presented with S. mansoni heavy infection intensity accounted for 5.5%. This finding was almost similar to the study conducted in Lira District, Uganda (5.3%) (26). However, it was lower than the previous finding reported from Tanzania (17.8%) (10). The observed differences might be due to the differences in repeated exposure of schoolchildren to infested water bodies with the infective stage of the parasite (16).
In the current study, the habit of bathing in an open water source was significantly associated with intestinal schistosomiasis. This finding was consistent with the previous findings reported from Jimma Town (27) and Erer Health Center, Ethiopia (28). This might be due to the fact that when people swim, they may swallow contaminated water or have their skin exposed to the larvae which can penetrate through the skin and cause infection. The risk of infection is higher in areas where the prevalence of schistosomiasis is high and where people frequently come into contact with contaminated water (29). Many aspects of water contact, such as the frequency or total duration of contact and time of body exposure, may contribute to the likelihood of encountering infective cercariae (25, 29).
This study showed that using river or spring water as a source of drinking water was significantly associated with the occurrence of S. mansoni infection. Our finding in this case was supported by the study done in Mizan-Aman town, Ethiopia (30). The possible explanation for this could be that river or spring water is more likely contaminated with infection-causing cercariae than pipe water, so using river or spring water can increase the chance of acquiring intestinal schistosomiasis (31).
In the present study, having no habit of wearing shoes and wearing shoes sometimes were significantly associated with the occurrence of intestinal schistosomiasis. This finding was in line with the study finding reported from Jiga Town, Northwest Ethiopia (32). Schistosomiasis has been spread through contact with water that contains the infective larvae (cercariae), so students who have contact with a water body without shoes could have an increased probability of being infected with S. mansoni even though water contact is not in itself a means of exposure for schistosomiasis (23, 29).
The absence of toilet/open defecation practice at school and home was significantly associated with the prevalence of intestinal schistosomiasis in this study, revealing that children whose families had no latrine were 8.14 times more likely exposed to the infection than those whose families had a latrine. This finding was supported by a previous study conducted in Wondo District, West Arsi Zone, Ethiopia (33) and Tanzania (10). This might be due to open defecation practices that allow helminth eggs to contaminate the environment, including water sources, from the feces of infected persons. In addition, defecating and hygienic bathing in nearby water bodies may contaminate the water with feces containing schistosome eggs, which, in turn, results in the perpetuation of schistosome transmission. One of the possible explanations for this finding is that, in the transmission of S. mansoni infection, the infected intermediate host snails release cercariae into the water which may infect people who are exposed to such contaminated water (23, 34).
Children who did not know the possible modes of transmission for intestinal schistosomiasis were 2.15 times more likely to be infected compared to their counterparts. This finding was in line with the study conducted in Northwest Tanzania (10). This might be explained by the fact that having poor knowledge about the sources and modes of transmission among children must be a major concern, as in this group water contact generally increases intestinal schistosomiasis. In addition, the present study showed that the majority (75.7%, 77.2%, and 78.7%) of the respondents reported that they do not know about intestinal schistosomiasis, its modes of transmission, and prevention methods, respectively.
Overall, in this study, a relatively high prevalence of S. mansoni infection was observed in Biftu Jalala Primary School (22.6%) compared to other primary schools. The possible reason is that Biftu Jalala Primary School is located near the river and the school children had open defecation practice in this nearby river, and also most of them had frequent contact with this water source (daily) for bathing, swimming, and washing clothes.
The limitation of this study is that the cross-sectional nature of the study design does not confirm the definitive cause-and-effect relationship.
Conclusion
The infection rate of S. mansoni observed in this study was medium according to WHO (2022) classification. Almost half of the study participants had a light intensity of infection. Factors like having no habit of wearing shoes, using river and spring water sources for drinking, open defecation practice at school and home, bathing habits in open water sources, and not knowing the possible modes of transmission were significantly associated with intestinal schistosomiasis. Therefore, health education that focuses on the importance of wearing shoes, improving water sources, and environmental sanitation, especially relating to schistosomiasis, should be given to schoolchildren in particular and the community at large to alleviate the problem. Moreover, a further large-scale study involving the whole community should be done.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The study protocol was approved by Haramaya University, College of Health and Medical Sciences, Institutional Health Research Ethics Review Committee (IHRERC) (Ref. Number: IHRERC/016/2024). Letter of permission to conduct the study was obtained from Nono district health and education office. Cooperation letter was submitted to those four primary schools. Informed voluntary written consent was obtained from each primary school director. Then, the objective and benefit of the study, the right to participate, withdraw at any time or not and procedures involved were briefly explained for the children and informed assent was obtained accordingly. All collected data and laboratory results were kept confidential. Any positive test results for stool sample were reported to the nearby health facility for proper management of infected children.
Author contributions
BT: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. JM: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. UU: Supervision, Writing – original draft, Writing – review & editing. JA: Writing – original draft, Writing – review & editing. FT: Writing – original draft, Writing – review & editing. AS: Writing – original draft, Writing – review & editing. FW: Conceptualization, Validation, Writing – original draft, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and authorship of this article. Fund for data collection for this research was covered by Haramaya University postgraduate directorate. This organization or funder had no role in the study selection, data collection, analysis, interpretation, or conclusion.
Acknowledgments
We acknowledged Haramaya University, College of Health and Medical Sciences, Institutional Health Research Ethical Review Committee for giving the ethical clearance. We also thank the study participants and all individuals who have, in one way or another, contributed to the completion of this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Casulli A. New global targets for NTDs in the WHO roadmap 2021-2030 Vol. 15. San Francisco, CA USA: Public Library of Science (2021). p. e0009373.
2. Adenowo AF, Oyinloye BE, Ogunyinka BI, Kappo AP. Impact of human schistosomiasis in sub-Saharan Africa. Braz J Infect Dis. (2015) 19:196–205. doi: 10.1016/j.bjid.2014.11.004
3. Elbaz T, Esmat G. Hepatic and intestinal schistosomiasis. J Advanced Res. (2013) 4:445–52. doi: 10.1016/j.jare.2012.12.001
4. WHO. Prevention and control of schistosomiasis and soil-transmitted helminthiasis. WHO technical report series, vol. 912. (2020). Available at: http://www.who.int/intestinal_worms/resources/who_trs_912/en/ (Accessed July 1, 2024).
5. Rashad SB, Gamal ET, Tamer E-B. Human schistosomiasis: clinical perspective: review. J Advanced Res. (2013) 4:433–44. doi: 10.1016/j.jare.2013.01.005
6. Aron MS, Elizabeth M, Mwinzi PNM, Wiegand RE, Muchiri G, Ireri E. Schistosoma mansoni Morbidity among School-Aged Children: A Score Project in Kenya. Am J Trop Med Hygiene. (2012) 87:874–82. doi: 10.4269/ajtmh.2012.12-0397
7. Toor J, Rollinson D, Turner HC, Gouvras A, King CH, Medley GF, et al. Achieving elimination as a public health problem for Schistosoma mansoni and S. haematobium: when is community-wide treatment required? J Infect Dis. (2020) 221:S525–30. doi: 10.1093/infdis/jiz609
8. Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. (2014) 383:2253–64. doi: 10.1016/S0140-6736(13)61949-2
9. Muhsin MA, Wang X, Kabole FM, Zilabumba J, Yang K. The indispen- sability of snail control for accelerating schistosomiasis elimination: evidence from Zanzibar. Trop Med Infect Dis. (2022) 7:347. doi: 10.3390/tropicalmed7110347
10. Mazigo HD, Uisso C, Kazyoba P, Nshala A, Mwingira UJ. Prevalence, infection intensity and geographical distribution of schistosomiasis among preschool and school aged children in villages surrounding Lake Nyasa, Tanzania. Sci Rep. (2021) 11:295. doi: 10.1038/s41598-020-80317-x
11. Workineh L, Yimer M, Gelaye W, Muleta D. The magnitude of Schistosoma mansoni and its associated risk factors among Sebatamit primary school children, rural Bahir Dar, Northwest Ethiopia. BMC Res Notes. (2019) 12:447. doi: 10.1186/s13104-019-4498-3
12. Amsalu G, Mekonnen Z, Erko B. A new focus of schistosomiasis mansoni in Hayk town, northeastern Ethiopia. BMC Res Notes. (2015) 8:1–6. doi: 10.1186/s13104-014-0965-z
13. Federal Democratic Republic of Ethiopia Ministry of Health: Second Edition of Ethiopia National Master Plan for neglected tropical diseases. Ethiopia, Addis Ababa: Federal Ministry of Health (2016).
14. Assefa A, Dejenie T, Tomass Z. Infection prevalence of Schistosoma mansoni and associated risk factors among school children in suburbs of Mekelle city, Tigray, Northern Ethiopia. MEJS. (2013) 5:174–88. doi: 10.4314/mejs.v5i1.85339
15. Nagi S, Chadeka EA, Sunahara T, Mutungi F, Dan Justin YK, Kaneko S, et al. Risk Factors and Spatial Distribution of Schistosoma mansoni Infection among Primary School Children in Mbita District, Western Kenya. PloS Negl Trop Dis. (2014) 8:e3190. doi: 10.1371/journal.pntd.0002991
16. Bajiro M, Tesfaye S. Schistosoma mansoni infection prevalence and associated determinant factors among school children in Mana District, Jimma Zone, Oromia Region, South west Ethiopia. J Bacteriol Parasitol. (2017) 8:329. doi: 10.4172/2155-9597.1000329
17. World Health Organization. Prevention and control of schistosomiasis and soil-transmitted helminthiasis. WHO technical report series (2020). p. 912.
18. WHO. Guideline on control and elimination of human schistosomiasis. Geneva: World Health Organization (2022).
19. Stanton, Michelle C, Moses A, Alison H, Juliet D, Gillian Allison E, et al. Intestinal schistosomiasis in Uganda at high altitude (>1400 m): malacological and epidemiological surveys on Mount Elgon and in Fort Portal crater lakes reveal extra preventive chemotherapy needs. Infect Dis Poverty. (2017) 1)6:26–35. doi: 10.1186/s40249-017-0248-8
20. Mathewos B, Alemu A, Woldeyohannes D, Alemu A, Addis Z, Tiruneh M, et al. Current status of soil transmitted helminths and Schistosoma mansoni infection among children in two primary schools in North Gondar, Northwest Ethiopia: a cross sectional study. BMC Res Notes. (2014) 7:1–7. doi: 10.1186/1756-0500-7-88
21. Bekana T, Berhe N, Eguale T, Aemero M, Medhin G, Tulu B, et al. Prevalence and factors associated with intestinal schistosomiasis and human fascioliasis among school children in Amhara Regional State, Ethiopia. Trop Med Health. (2021) 49:1–11. doi: 10.1186/s41182-021-00326-y
22. Alebie G, Erko B, Aemero M, Petros B. Epidemiological study on Schistosoma mansoni infection in Sanja area, Amhara region, Ethiopia. Parasites Vectors. (2014) 7:1–10. doi: 10.1186/1756-3305-7-15
23. Hailu T, Mulu W, Abera B. Effects of Water Source, Sanitation and Hygiene on the Prevalence of Schistosoma mansoni among School Age Children in Jawe District, Northwest Ethiopia. Iran J Parasitol. (2020) 15:124–9. doi: 10.18502/ijpa.v15i1.2535
24. Hailu T, Alemu M, Abera B, Mulu W, Yizengaw E, Genanew A, et al. Multivariate analysis of factors associated with Schistosoma mansoni and hookworm infection among primary school children in rural Bahir Dar, Northwest Ethiopia. Trop Dis Travel Med Vaccines. (2018) 4:4. doi: 10.1186/s40794-018-0064-6
25. Mohammed J, Weldegebreal F, Teklemariam Z, Mitiku H. Clinico-epidemiology, malacology and community awareness of Schistosoma mansoni in Haradenaba and Dertoramis kebeles in Bedeno district, eastern Ethiopia. SAGE Open Med. (2018) 6:2050312118786748. doi: 10.1177/2050312118786748
26. Byagamy JP, Malinga GM, Angwech H, Opiro R, Echodu R, Odongo-Aginya E. Prevalence, infection intensity and associated risk factors of intestinal schistosomiasis among primary school children in Lira district, northern Uganda. (2019).
27. Mengistu M, Shimelis T, Torben W, Terefe A, Kassa T, Hailu A. Human intestinal schistosomiasis in communities living near three rivers of jimma town, South Western Ethiopia. Ethiop J Health Sci. (2011) 21:111–8. doi: 10.4314/ejhs.v21i2.69051
28. Kemal M, Tadesse G, Esmael A, Solomon MS, Kebede T. Schistosoma mansoni infection among preschool age children attending Erer Health Center, Ethiopia and the response rate to praziquantel. BMC Res Notes. (2019) 12:211. doi: 10.1186/s13104-019-4246-8
29. Reitzug F, Ledien J, Chami GF. Associations of water contact frequency, duration, and activities with schistosome infection risk: A systematic review and meta-analysis. PloS Negl Trop Dis. (2023) 17:e0011377. doi: 10.1371/journal.pntd.0011377
30. Jejaw A, Addisu A, Tegegne Y. Prevalence, intensity, and associated factors of Schistosoma mansoni among school children in Northwest Ethiopia. J Parasitol Res. (2020), 1–7. doi: 10.1155/2020/8820222
31. Ponce-Terashima R, Koskey AM, Reis MG, McLellan SL, Blanton RE. Sources and distribution of surface water fecal contamination and prevalence of schistosomiasis in a Brazilian village. PloS Negl Trop Dis. (2014) 8:e3186. doi: 10.1371/journal.pntd.0003186
32. Wubet K, Damtie D. Prevalence of Schistosoma mansoni Infection and Associated Risk Factors among School Children in Jiga Town, Northwest-Ethiopia: A Cross-Sectional Study. J Parasitol Res. (2020), 7. doi: 10.1155/2020/6903912
33. Ansha MG, Kuti KA, Girma E. Prevalence of intestinal schistosomiasis and associated factors among school children in Wondo District, Ethiopia. J Trop Med. (2020) 2020:1-8. doi: 10.1155/2020/9813743
Keywords: Schistosoma mansoni, primary school, children, Nono District, Ethiopia
Citation: Tolera B, Mohammed J, Umer U, Abamecha J, Tebeje F, Sime A and Weldegebreal F (2025) Prevalence, intensity, and associated factors of intestinal schistosomiasis among primary school children in Nono District, Southwest Ethiopia. Front. Trop. Dis. 5:1483164. doi: 10.3389/fitd.2024.1483164
Received: 19 August 2024; Accepted: 09 December 2024;
Published: 18 February 2025.
Edited by:
Nilanjan Lodh, Marquette University, United StatesReviewed by:
Hope Simpson, University of London, United KingdomHenry Kanyi, Kenya Medical Research Institute (KEMRI), Kenya
Copyright © 2025 Tolera, Mohammed, Umer, Abamecha, Tebeje, Sime and Weldegebreal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ukash Umer, dWthc2h1bWVyMTIzQGdtYWlsLmNvbQ==; Akewok Sime, YWtlc2ltZTc1QGdtYWlsLmNvbQ==
 Bayisa Tolera1
Bayisa Tolera1 Ukash Umer
Ukash Umer Akewok Sime
Akewok Sime Fitsum Weldegebreal
Fitsum Weldegebreal