- 1School of Medical Laboratory Sciences, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia
- 2Laboratory Bacteriology Research, Faculty of Medicine and Health Sciences, Ghent University, Ghent, Belgium
- 3Department of Medical Laboratory Sciences, Ibro Salama Health Center, Kersa Town, Ethiopia
- 4Department of Pediatrics and Child Health Nursing, School of Nursing, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia
- 5Department of Obstetrics and Gynecology, Leiden University Medical Centre, Leiden University, Leiden, Netherlands
- 6School of Public Health, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia
Background: Trachoma is a neglected tropical disease that mainly affects impoverished and marginalized communities with inadequate shelter and sanitation. Nevertheless, the prevalence of active trachoma and the specific factors contributing to it among communities residing in former leprosy settlements in eastern Ethiopia are not well explored. Therefore, the objective of this study was to compare the prevalence of active trachoma and its associated factors among children aged 1-9 years in the previous leprosarium and non-leprosarium areas, as well as urban and rural areas in eastern Ethiopia.
Methods: A community-based comparative cross-sectional study was conducted among 580 systematically selected households from January 1 to 30, 2024. Data were collected by interviewing the children’s caregivers, observing the child and environment, and conducting a clinical examination of their eyes. Data were entered in EpiData version 4.6 and exported to Statistical Package of Social Science (SPSS) version 26 software for analysis. A chi-square test was also done. The association was presented as an adjusted odds ratio with a 95% confidence interval, and variables with a p-value less than 0.05 were regarded as statistically significant.
Results: The overall prevalence of active trachoma was 12.9% (95% CI: 10.5%-15.3%). The prevalence of active trachoma was 15.6%, 9.8%, 18.3%, and 7.5% among children in previous leprosy and non-leprosy settlements and rural and urban areas, respectively (x²=5.65, p-value = 0.017). Having eye discharge (AOR = 10.7’; 95% CI: 4.32, 26.51), latrine distance from home of less than 10 m(AOR=3.12; 95% CI: 1.16–8.34), being a rural resident (AOR=4.1; 95% CI: 1.69-10.18), presence of solid waste around their home (AOR=6.5; 95% CI: 2.14-19.72), and household monthly income less than 5000 Ethiopian birrs (AOR=2.9; 95%CI: 1.04-8.30) were statistically associated with active trachoma in the previous leprosy settlements. In the non-leprosy settlements, children who had eye discharge (AOR = 7.6; 95% CI: 5.37, 58.05), latrine distance from home of less than 10 m (AOR=3.12; 95%CI: 1.11, 8.77), habit of playing with soil (AOR=9.0; 95% CI: 2.92, 28.24), and presence of animal dung (AOR=6.98; 95% CI: 3.44, 48.47) were statistically associated with active trachoma.
Conclusion: In this study, the prevalence of active trachoma among children aged from 1-9 years old was higher than the WHO target for the elimination of active trachoma (<5%) in every district. Therefore, targeted treatments and raising awareness on proper hygiene and sanitation are required to alleviate the problem.
Introduction
Trachoma is caused by the intracellular bacterium Chlamydia trachomatis serovars A, B, Ba, and C, and is the leading cause of blindness due to infectious diseases worldwide (1, 2). Active trachoma is recognized by recurrent tarsal conjunctivitis (3). The main route of transmission is the passage of ocular and nasal secretions from person to person on fingers, fomites (such as clothing), and eye-seeking flies (particularly Musca sorbents) (4). Children under 9 years of age who reside in situations where there is a high risk of infection may experience the blinding consequences of trachoma (5). Additionally, it causes conjunctival scarring and, sometimes, trichiasis with or without entropion of the inner surface of the eyelids, resulting in blindness (6, 7).
Globally, there are 1,224 areas with > 5% prevalence of follicular trachoma (TF) in children aged between 1 and 9 and a total of 145.6 million people live in these areas and nearly 86% (124.7 million) of these were in Africa (8). Prevalence rates for active trachoma in preschool-aged children in endemic areas can range from 60% to 90% and as individuals age illnesses become less frequent and persist for shorter periods (8). This infection is most prevalent in impoverished rural regions of Africa, Central and South America, Asia, Australia, and the Middle East (9–11). It is endemic in 44 nations across the globe, 33 of which are in Africa (3) and 136 million people are at risk, with the majority being in east and west sub-Saharan Africa, North Africa, and a few endemic coastal states in central Africa (3). Furthermore, it has caused visual impairment in 1.9 million people and contributes to 1.4% of overall blindness cases (8).
The country with the most incidence is Ethiopia, with 10.2 million, followed by Sudan (3.6 million), Tanzania, Kenya, and Niger (2.0–2.1 million each) (12, 13). Ethiopia launched its first trachoma action plan in 2012 and its second master plan in 2016 (14). However, a recent study showed that the prevalence of active trachoma among children in Ethiopia was 26.9 (15), of which the Southern Nation, Nationality and Peoples (SNNP) region had the highest prevalence (35.8%), followed by the Amhara region (30.2%) and other regions (21.4%). A study conducted among school children in the Harari region in eastern Ethiopia in 2016 showed that the prevalence of active trachoma was 1.3%, of which 0.8% was follicular trachoma and 0.5% was follicular and intense (16).
Each year, approximately 1.3 million disability-adjusted life years are lost due to trachoma (17). Trachoma also has a negative impact on affected individuals and communities. In terms of lost productivity, blindness and visual impairment are expected to have an economic cost of between US$2.9 and US$5.3 billion (8). Several poverty-related factors can influence the development of active trachoma. Because trachoma is one of the neglected tropical diseases (NTDs) that affect impoverished communities, there may be a bidirectional causal association between poverty and trachoma (16, 18).
To eliminate blinding trachoma as a public health problem, all countries must reduce the number of active trachoma in children aged 1 to 9 years to less than 5% in all districts (17). In cases where the prevalence of follicular trachoma exceeds 10% among children aged 1 to 9 years, it is recommended that districts implement mass distribution campaigns of either tetracycline eye ointment or oral azithromycin antibiotics (14, 19). In any district where the prevalence of follicular trachoma is between 5% and 10% in children aged 1–9 years, targeted treatments may be used instead of mass treatments (20). To achieve success in the integrated control of NTDs, integrated mapping, the rapid scale-up of interventions, and operational research on the co-implementation of intervention packages are crucial. Therefore, this study was designed to compare the prevalence of active trachoma and its associated factors among unique communities, identify risk groups, and can be used to guide public health interventions to target areas with high trachoma prevalence and implement effective control measures.
Materials and methods
Study setting
This study was conducted in the Harari Regional State, in 1 and 2 Kebeles, Amir Nur District, and Oromia Regional State, in Bisidimo and Ifadin Kebeles, Babile District, from January 1 to 30, 2024. The Harari Regional State is 523 km away from Addis Ababa to the east. This region has nine districts with 19 urban and 17 rural kebeles.
Amir Nur District is an urban district with 5,380 households and a total population of 24215. There were 4,027 children aged 1–9 years in the district. Gandaa Ferroo village is one of the villages in 1 Kebele, and it was a leprosy settlement area (21).
The other study sites were Babile District, Bisidimo, and Ifadin Kebeles, which are 540 km from Addis Ababa and 23 km from Harar to the east. Bisidimo Kebele is an area around Bisidimo Hospital, and it includes 1,237 households, a total population of 7229, and 2,228 children aged 1–9 years. Bisidimo Kebele is a historical leprosy settlement area (21, 22). Patients with leprosy visited Bisidimo Hospital for treatment and made their residences around Bisidimo Hospital. Ifadin Kebele is a non-leprosy settlement area around Bisidimo Kebele, and it includes 1,381 households with a total population of 6627, and 2,178 children aged 1–9 years.
Study design and populations
A community-based comparative cross-sectional study was conducted. The source population for this study included all households with children aged from 1 to 9 years old who were living at 1 and 2 Kebeles, Amir Nur District, and Bisidimo and Ifadin Kebeles, Babile District. The study population included children aged 1-9 years and their caregivers from the selected households who gave full consent to participate. Children with acute ocular infection, recent ocular treatment, recent ocular trauma, or surgery, whose families or caregivers were not present at home during the data collection period, and those children whose caregivers did not sign consent, were excluded.
Sample size determination and sampling technique
The sample size was determined using a double population proportion formula by considering the prevalence of active trachoma (11.8%) among children aged 1 to 9 years taken from a previous study conducted in Metema district, Ethiopia (23) and the prevalence among 50% at a confidence interval of 95% and 5% margin of error. Furthermore, 10% of the sample size was added to reduce errors resulting from the likelihood of non-compliance, resulting in a final sample size of 598. 1 Kebele, Amir Nur District, and Bisidimo Kebele, Babile District were purposively selected as previous leprosy settlements and the other two kebeles were selected randomly as non-leprosy settlements. The study sites were stratified into leprosy and non-leprosy settlements. Households were selected using a systemic random sampling technique from each selected kebeles by using the family registration book found at the health post as a sample frame [k=10, where k was calculated by dividing the total number of households by the calculated sample size (6200/598 = 10)] and every 10th value was used. In cases where the selected household had no children aged from 1-9 years, the next household was used. All eligible children who were at home during the data collection were included in the study. The number of households was proportionally allocated. The total number of households was almost the same, i.e., 3,105 and 3,095 households in the leprosy and non-leprosy settlement areas, respectively. Finally, the same number of households (299) from both areas were considered using the systematic sampling technique.
Data collection methods and measurement
Data were collected by interviewing children’s caregivers using a pretested structured questionnaire, which was adopted from the literature (19, 23–26), observing the child’s face, and conducting clinical eye examinations.
During data collection, public health officers and ophthalmic nurses looked for flies on the child’s face and if there was at least one fly on the child’s face they recorded it as the presence of flies on the child’s face. The examiner then looked for discharge from the eyes and nose of children during data collection. If there was eye or nose discharge, they recorded it as discharge on the child’s face. If there was no discharge from either the child’s eye or nose, the examiner/data collectors were recorded it as a clean face (27). The same procedures were used to examine each eye and all children were examined by two different examiners to correlate the results. Clinical signs of trachoma, such as trachoma follicular (TF), trachomatous inflammation-intense (TI), trachomatous conjunctival scar (TS), trachomatous trichiasis (TT), and corneal opacity were identified by the trained data collectors using a pen torch and a lens with a 2.5x magnifying power. The upper eyelid was slightly pushed upward to expose the lid margins to look for embedded eyelashes. They then looked for inflammation (TF and TI) and scarring on the upper eyelid (TS) (28). An ophthalmologist was ready to check if there were discordant results among data collectors during the data collection. To prevent cross-infection between successive participants, the necessary hygienic measures were taken by cleaning hands with alcohol-based hand gel after each examination.
Methods of data analysis
Data were double-entered, edited, and cleaned using Epidata version 4.6, and exported to the SPSS version 26 program for analysis. Descriptive statistics were computed and summarized in the tables, figures, and text as frequencies, means, or standard deviations where appropriate.
Potential co-linearity was checked using the multicollinearity model considering tolerance and variance inflation factor (VIF). Bi-variable and multivariable logistic regression analyses were computed to identify the association between the independent variables and the outcome variable. In the bivariate analysis, variables with a p-value ≤ 0.25 were included in the multivariable analysis to control for all confounding variables. The model goodness of fit was tested by the Hosmer–Lemeshow statistic. The model was considered a good fit if it was found to be p >0.05 for the Hosmer–Lemeshow statistic. The association between active trachoma and the independent variables was reported as an odds ratio with a 95% CI and a p-value less than 0.05 was considered statistically significant.
Data quality assurance
The structured questionnaires were prepared in English and translated into local languages (i.e., Afan Oromo and Amharic) by a language expert, and back-translated into English by another language expert to maintain its consistency. Questionnaires were pre-tested 7 days before the actual data collection on 5% of the caregivers of children aged from 1-9 years in Kebele 7, Amir Nur District, who were not included in the actual data collection. The required modifications and changes were implemented based on the results of the pretest. Both supervisors and data collectors received 2 days of training. Supervisors monitored data collectors at each site throughout the data collection process. Data collectors used a pen torch to obtain adequate light for the eye examination and a 2.5x power magnifying lens to see conjunctival follicles that were not seen by the naked eye. The primary investigator also actively monitored the fieldwork every day, ensuring that surveys were fully completed and that the data being recorded made sense.
Ethical consideration
The Institutional Health Research Ethics Review Committee (IHRERC) at Haramaya University College of Health and Medical Science granted ethical approval (Reference number: IHRERC/185/2023). Permission letters were obtained from the Amir Nur District and Babile District administrative offices. The interviews and clinical eye examinations of the chosen children took place in the presence of the children’s caregivers after the signed informed voluntary consent was given. Their names and other personal identifiers were not registered to maintain confidentiality. The study’s goals, as well as the rights of and advantages for the study participants, were explained. Individuals who were positive for signs of active trachoma (204) were referred to nearby health facilities.
Results
Sociodemographic characteristics
A total of 580 households were included in this study, with a response rate of 96.9%. Among the included households, 290 were from leprosy settlements, and 290 were from non-leprosy settlements. The age range of the caregivers was 15 and 63 years and the mean age (± SD) was 30.68 (± 7.23).
Regarding the educational status of the caregivers, 178 (61.2%) of those who were living in the leprosy settlement and 110 (38.1%) who were living in the non-leprosy settlement area had no formal education. The mean age (± SD) of the children living in leprosy and non-leprosy settlements was 5.23 (± 2.51) and 5.53 (± 2.49) years, respectively. Regarding the children’s educational status, 146 (36.8%) from the leprosy settlements and 184 (52.9%) from the non-leprosy settlements attended elementary school (Table 1).
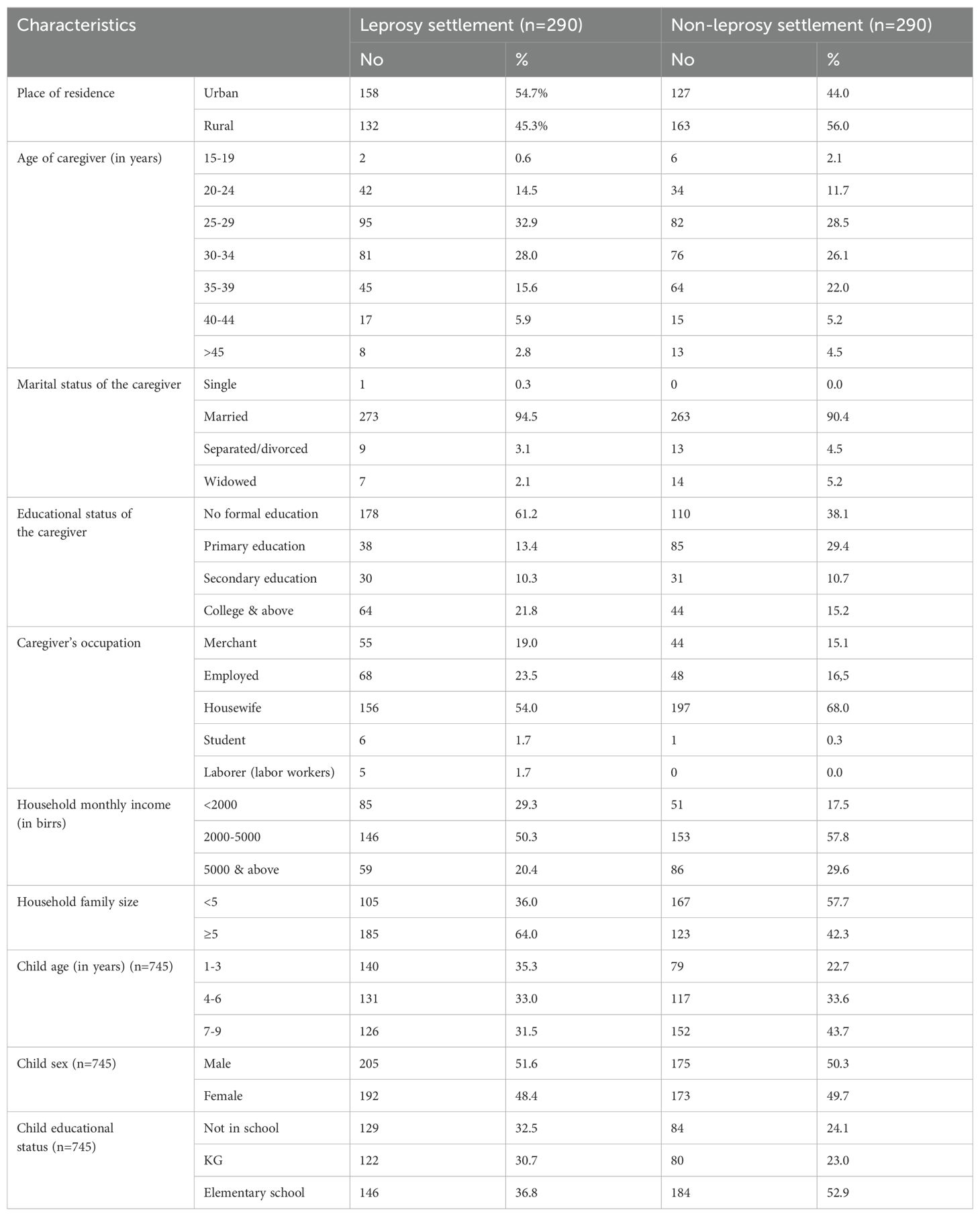
Table 1. Sociodemographic characteristics of caregivers and children from leprosy and non-leprosy settlements in eastern Ethiopia in 2024 (n=580).
Environmental and water, sanitation, and hygiene (WASH) conditions of the households
In total, 222 (76.8%) and 234 (80.8%) households had latrine coverage in the leprosy and non-leprosy settlements, respectively. Regarding solid waste, 76.1% (222) of the households in the leprosy settlements and 45.0% (223) of the households in the non-leprosy settlements had solid waste around their homes. In the leprosy settlements, 61.1% of households had animal waste in their living compound, while in the non-leprosy settlements, the prevalence was 49.3% (Table 2).
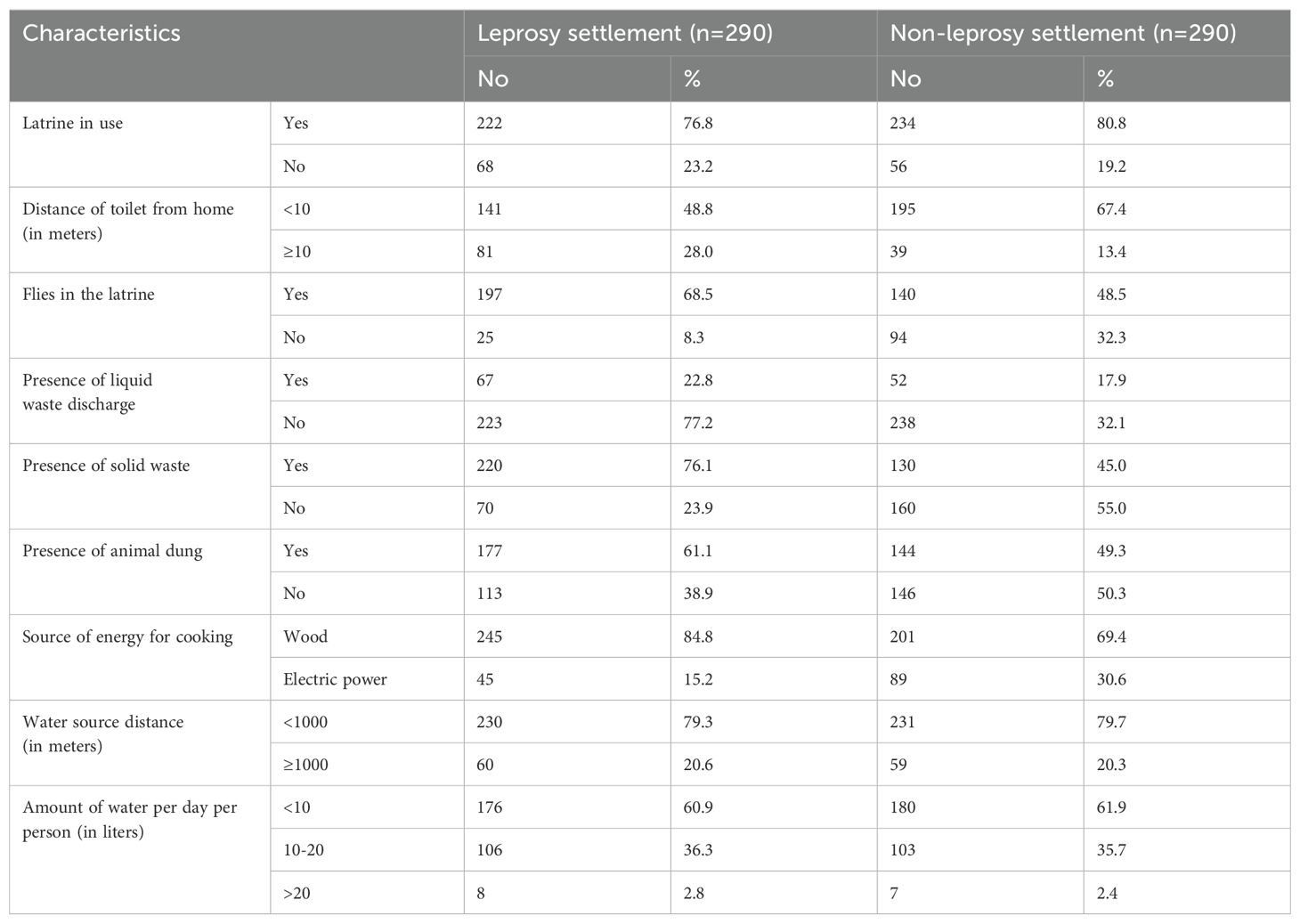
Table 2. Households’ environmental and WASH conditions in the leprosy and non-leprosy settlements in eastern Ethiopia in 2024 (n=580).
Child-related factors
The prevalence of the habit of sharing clothes was 73.8%, and 60.6% among children living in the leprosy and non-leprosy settlements, respectively. In the leprosy settlements, 78.3% of children played with soil, whereas only 55.7% of children in the non-leprosy settlements did the same. Approximately 63.2% and 65.5% of children in the leprosy and non-leprosy settlements did not use soap for face washing, respectively (Table 3).
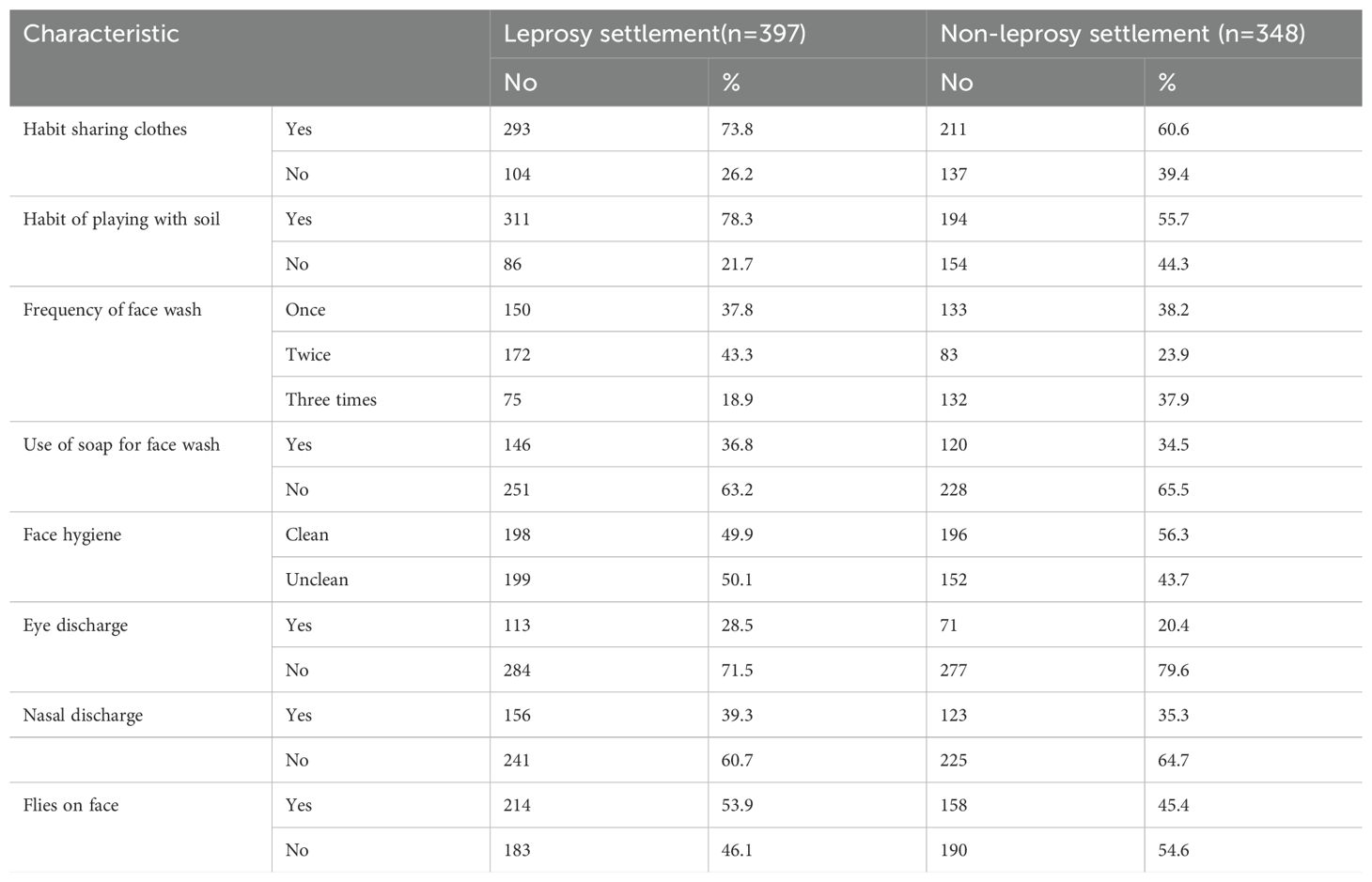
Table 3. Childhood-related behavioral factors in the leprosy and non-leprosy settlement areas in eastern Ethiopia in 2024 (n=745).
Prevalence of active trachoma
The overall prevalence of active trachoma among children who were enrolled in this study was 12.9% (95% CI: 10.5%, 15.3%). The prevalence was found to be 15.6% (95% CI: 12.0%, 19.2%) and 9.8% (95% CI: 6.6%, 12.9%) among children who were living in the leprosy settlements and non-leprosy settlements, respectively. This difference was statistically significant (x²=5.65, p-value = 0.017). Among the children living in the leprosy settlements, the prevalence of trachomatous follicular and trachomatous intense was 7.1% (28) and 8.6% (34), respectively. In the non-leprosy settlements, the prevalence of TF and TI cases was 4.3% (15) and 5.5% (19), respectively. Among the urban and rural children screened for active trachoma, 7.5% (95% CI: 3.8%–9.7%) and 18.3% (95% CI: 14.4%–22.2%) were positive, respectively (Figure 1).
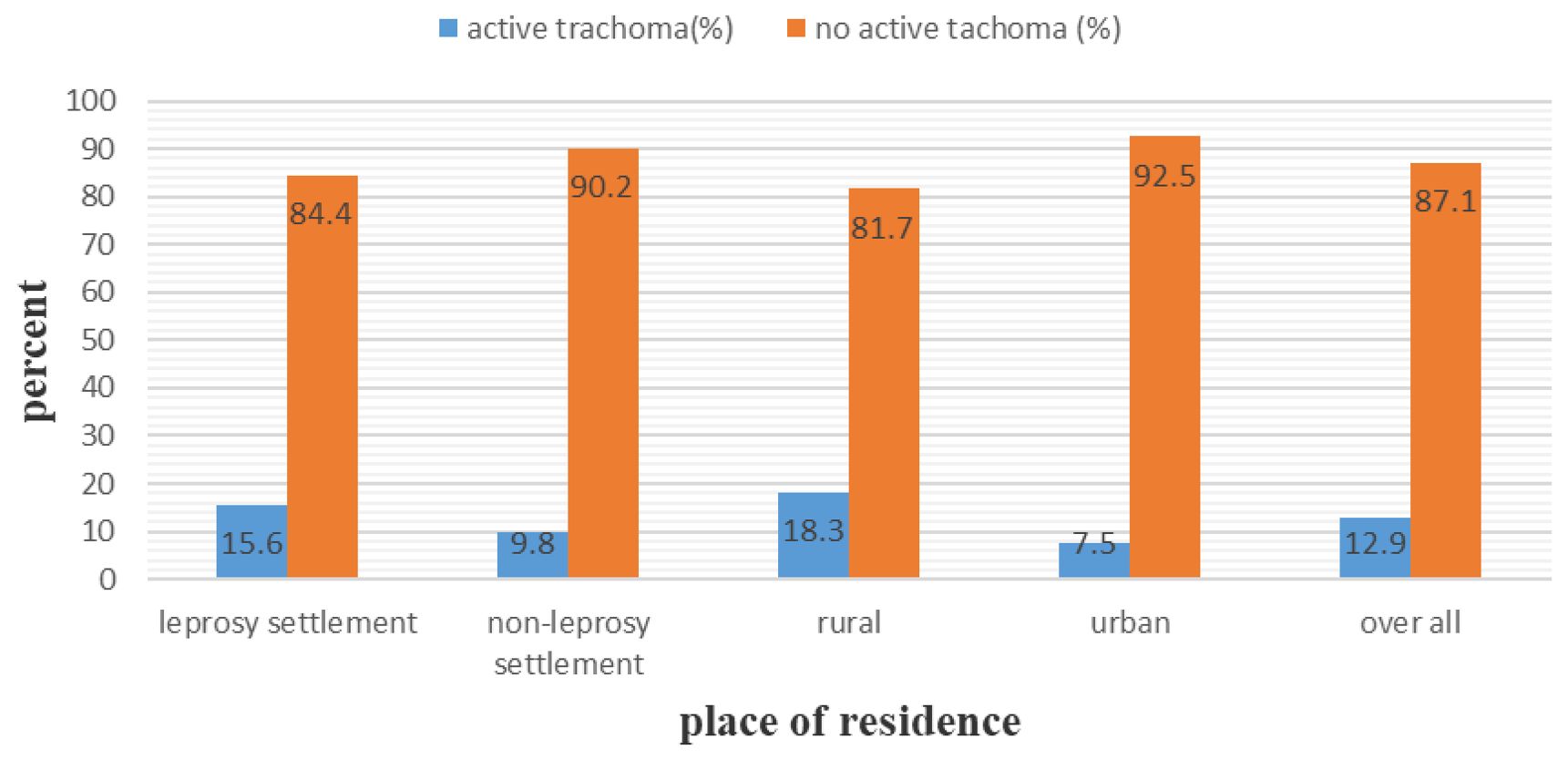
Figure 1. Prevalence of active trachoma among children aged 1 years in the leprosy and non leprosy settlements in eastern Ethiopia in 2024.
There was a statistically significant difference between the urban and rural children (x²=18.47, P-value = 0.000). Among the children from the leprosy settlements, 23.3% of the rural children and 6.2% of the urban children were positive for active trachoma. In contrast, 10.9% of the rural and 8.5% of the urban children from the non-leprosy settlements were positive for active trachoma. Of the total number of girls who tested positive for active trachoma, 16.7% were from the leprosy settlements (Table 4) and 10.9% were from the non-leprosy settlements (Table 5). Of the children living in the rural areas, 32 (8.4%) tested positive for TF and 37 (9.7%) for TI. Among the urban children screened for active trachoma, 17 (4.7%) had TI and 10 (2.8%) had TF (Figure 2).
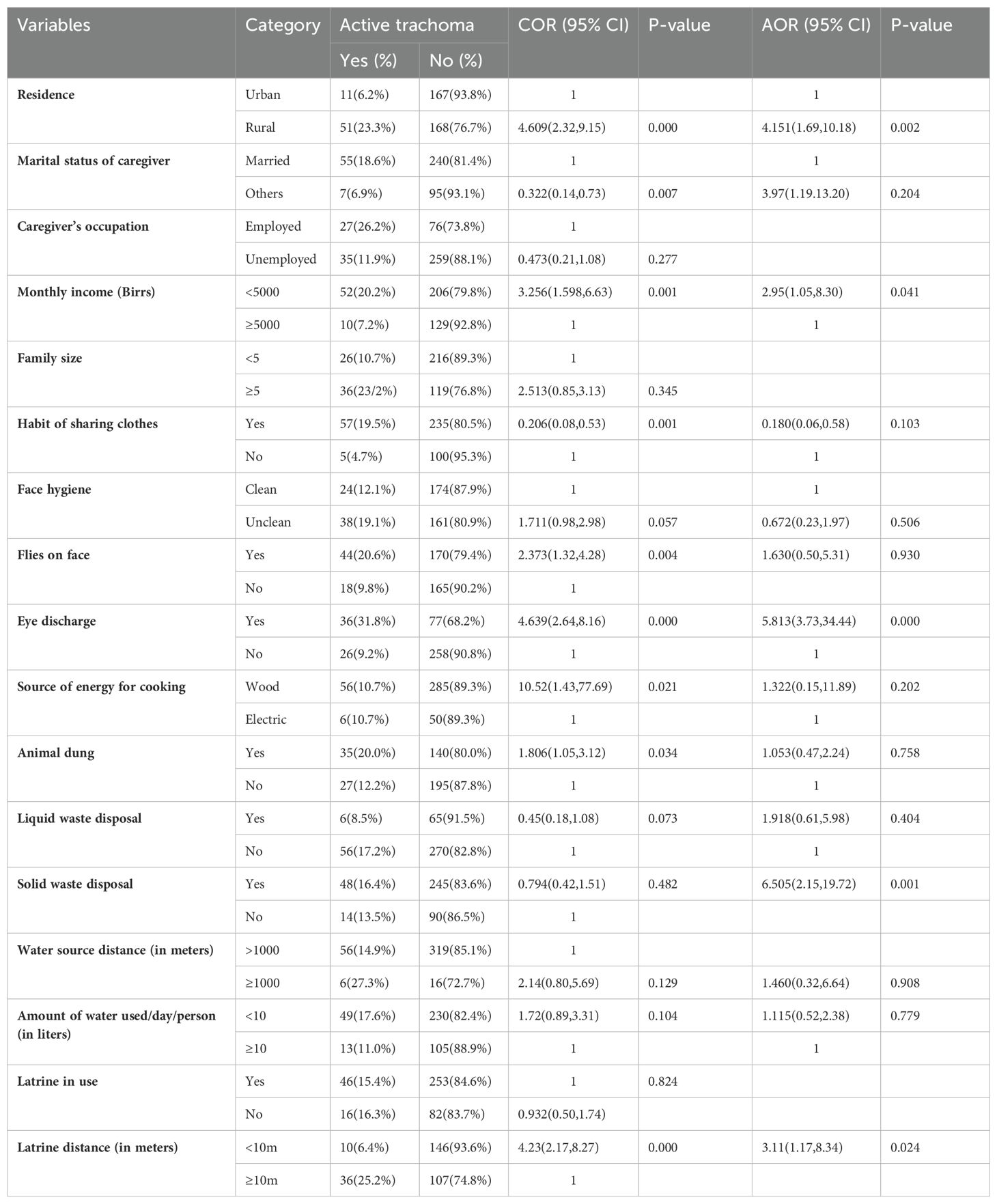
Table 4. Bivariate and multivariable analyses of factors associated with active trachoma among children in the leprosy settlements in eastern Ethiopia in 2024.
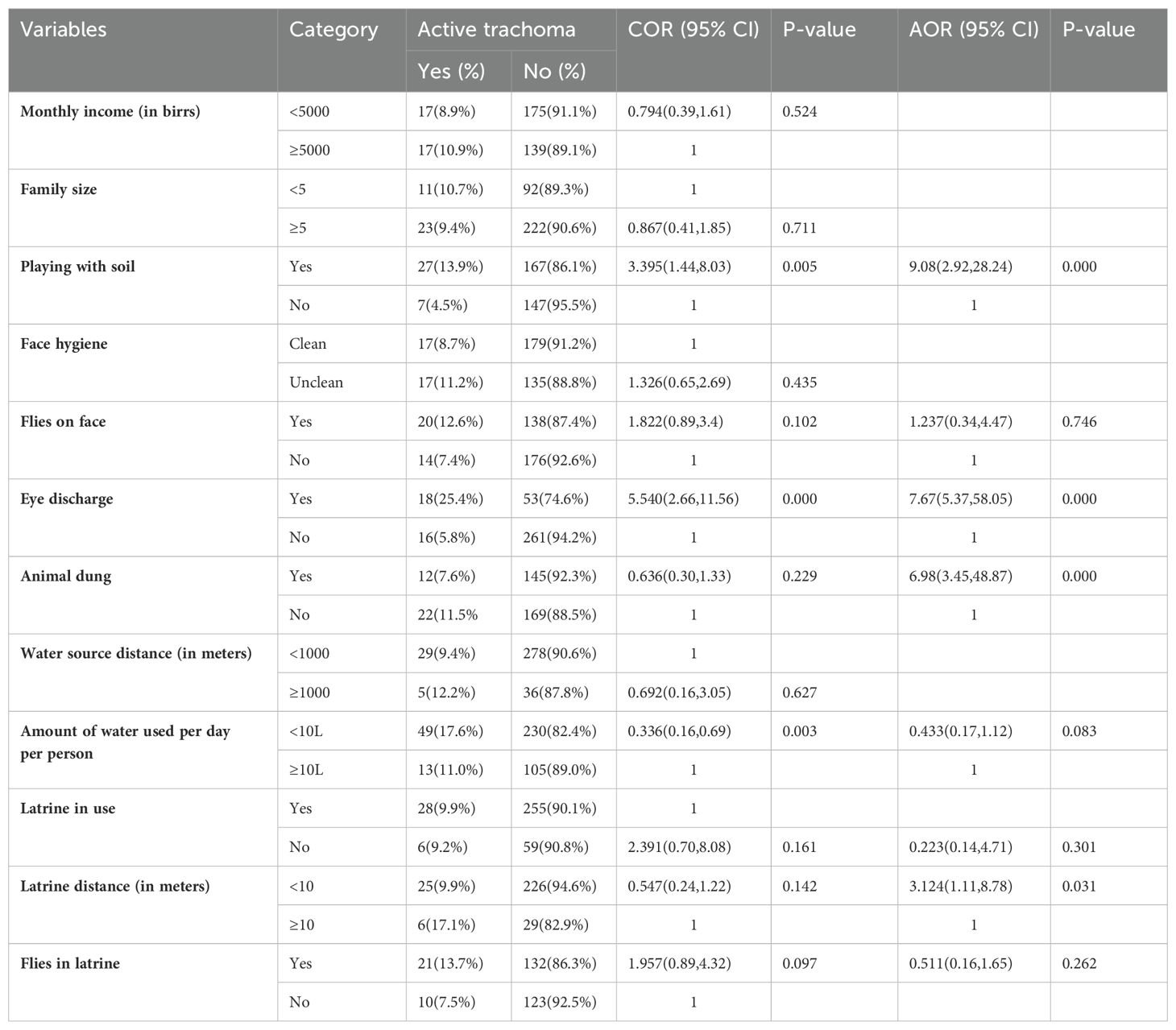
Table 5. Bivariate and multivariable analyses of factors associated with active trachoma among children in the non-leprosy settlements in eastern Ethiopia in 2024.
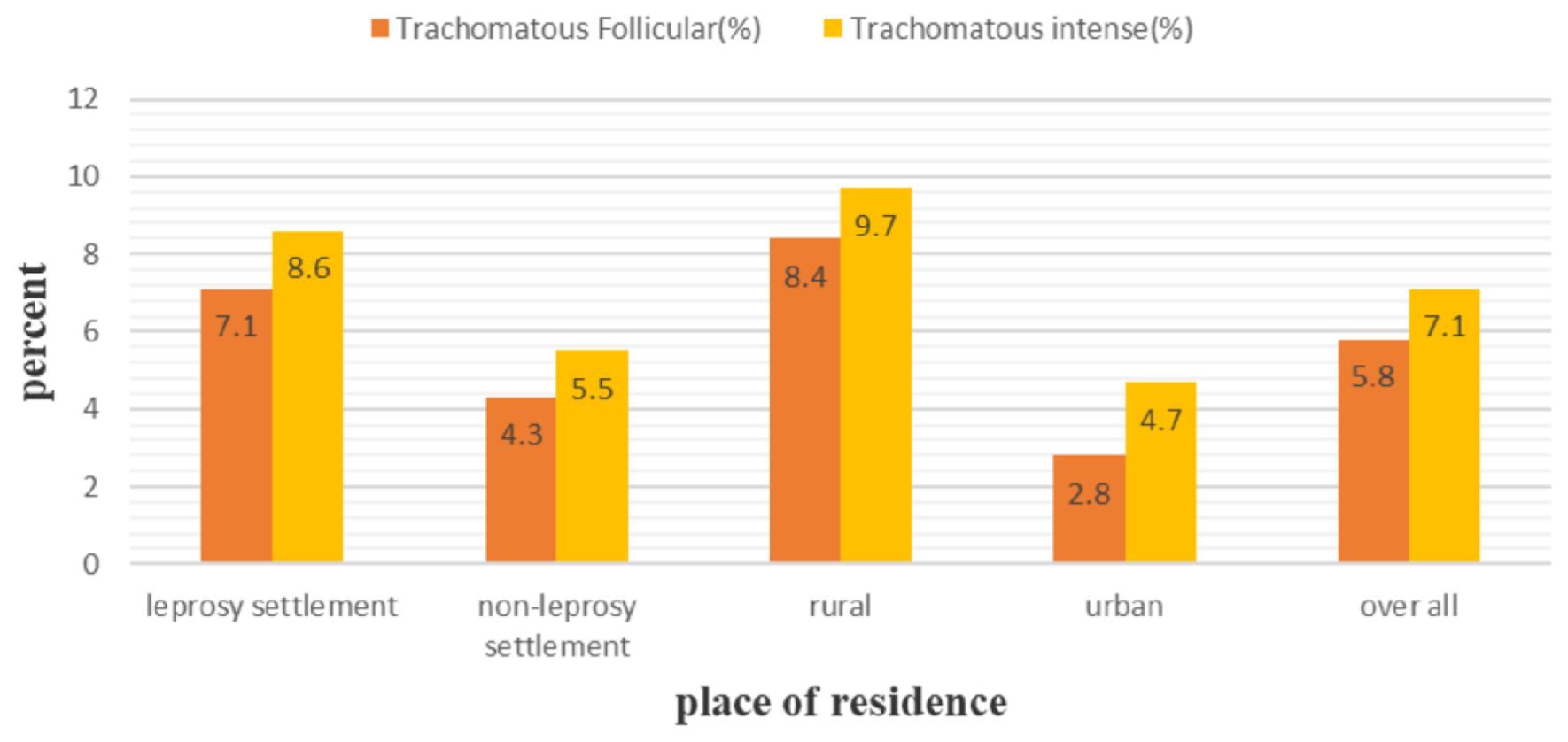
Figure 2. Prevalence of trachomatous follicular and trachomatous intense among children aged 1-9 years in the leprosy and non-leprosy settlements in eastern Ethiopia in 2024.
Among the children in the leprosy settlement areas
In the bivariate logistic regression, place of residence, caregiver’s marital status, household monthly income, habit of sharing clothes, use of soap for washing face, face hygiene, flies on child’s face, eye discharge, source of energy for cooking, presence of animal dung, presence of liquid waste, amount of water per day per person, latrine distance, and water source distance were found to have a p-value less than or equal to 0.25 and were selected as candidates for the final multivariable analysis of the leprosy settlements. In the multivariable logistic regression, place of residence, household monthly income, eye discharge, latrine distance, and presence of solid waste were significantly associated with the prevalence of active trachoma at a p-value of less than 0.05 (Table 4). The likelihood of active trachoma among the rural children was 4.15 times (AOR = 4.15, 95% 268 CI: 1.69–10.18) higher than that of the urban children. The children who had discharge in their eyes were 10.7 times (AOR = 10.7, 95% CI: 4.32–26.51) more likely to have active trachoma than their counterparts. The likelihood of active trachoma among the children who were from households that had a latrine less than 10m from home was 3.12 times (AOR = 3.12, 95% CI: 1.17–8.34) higher compared to their counterparts. Children from households that had solid waste around the home were 6.5 times (AOR = 6.51, 95% CI: 2.15–19.72) more likely to have active trachoma compared to those children from households that had no solid waste around the home. The odds of active trachoma among children from households with a monthly income of less than 5,000 birr was 2.9 times (AOR = 2.95, 95%CI: 1.05–8.30) higher compared to their counterparts (Table 4).
Among the children in the non-leprosy settlement areas
In the bivariate logistic regression, a habit of playing with soil, flies on child’s face, eye discharge, presence of animal dung, amount of water per day per person, latrine distance, latrine in use, and flies in latrine were found to be a p-value less than or equal to 0.25 and were selected as candidates for the final multivariable analysis for the non-leprosy settlements. Thus, a habit of playing with soil, eye discharge, presence of animal dung, and latrine distance were found to be significantly associated with the prevalence of active trachoma at a p-value less than 0.05 (Table 5). Children who had discharge in their eye were 7.6 times (AOR = 7.66, 95% CI: 5.37–58.06) more likely to have active trachoma than their counterparts. Children who had a habit of playing with soil were 9.1 times (AOR = 9.09, 95% CI: 2.92–28.24) more likely to acquire active trachoma than children who had no habit of playing with soil. The likelihood of active trachoma among children who were from households that possessed latrines less than 10m from home was 3.12 times (AOR = 3.12, 95% CI: 1.11–8.78) higher as compared to their counterparts. Children who were living in households that had animal dung around the home were 6.98 times (AOR = 6.98, 95% CI: 3.45–48.87) higher than children from households that had no animal dung around the home (Table 5).
Among the rural children
In the bivariate logistic regression, caregiver’s age, family size, child age, female sex, child educational status, habit of sharing clothes, habit of playing with soil, frequency of face wash, use of soap for washing face, face hygiene, eye discharge, nasal discharge, presence of liquid waste, amount of water per day per person, latrine distance, and flies in latrine were found have a p-value less than or equal to 0.25 and were selected as candidates for the final multivariable analysis for the non-leprosy settlements. Factors such as sex of the child, a habit of sharing clothes, face hygiene, eye discharge, presence of liquid waste, and flies in the latrine were significantly associated with the prevalence of active trachoma at a p-value less than 0.05 (Table 6). The likelihood of active trachoma among girls was 2.8 times (AOR=2.88, 95% CI: 1.38-6.03) higher than boys. Children who had discharge in their eyes were 9.26 times (AOR = 9.26, 95% CI: 4.05–21.15) more likely to have active trachoma as compared to those who had no discharge in their eyes. The likelihood of active trachoma among children with an unclean face was 3.3 times (AOR = 3.3, 95% CI: 1.47–7.49) higher than their counterparts. Children who shared clothes were 3.4 times (AOR = 3.44, 95% CI: 1.22–9.77) more likely to have active trachoma than their counterparts. Children who were living in households that had liquid waste around the home were 4.5 times (AOR = 4.46, 95% CI: 1.20–16.61) more likely to have active trachoma compared to children from households who did not. Active trachoma among children who had flies in the latrine was 7.8 times higher than their counterparts (AOR=7.85, 95% CI: 2.40-25.69) (Table 6).
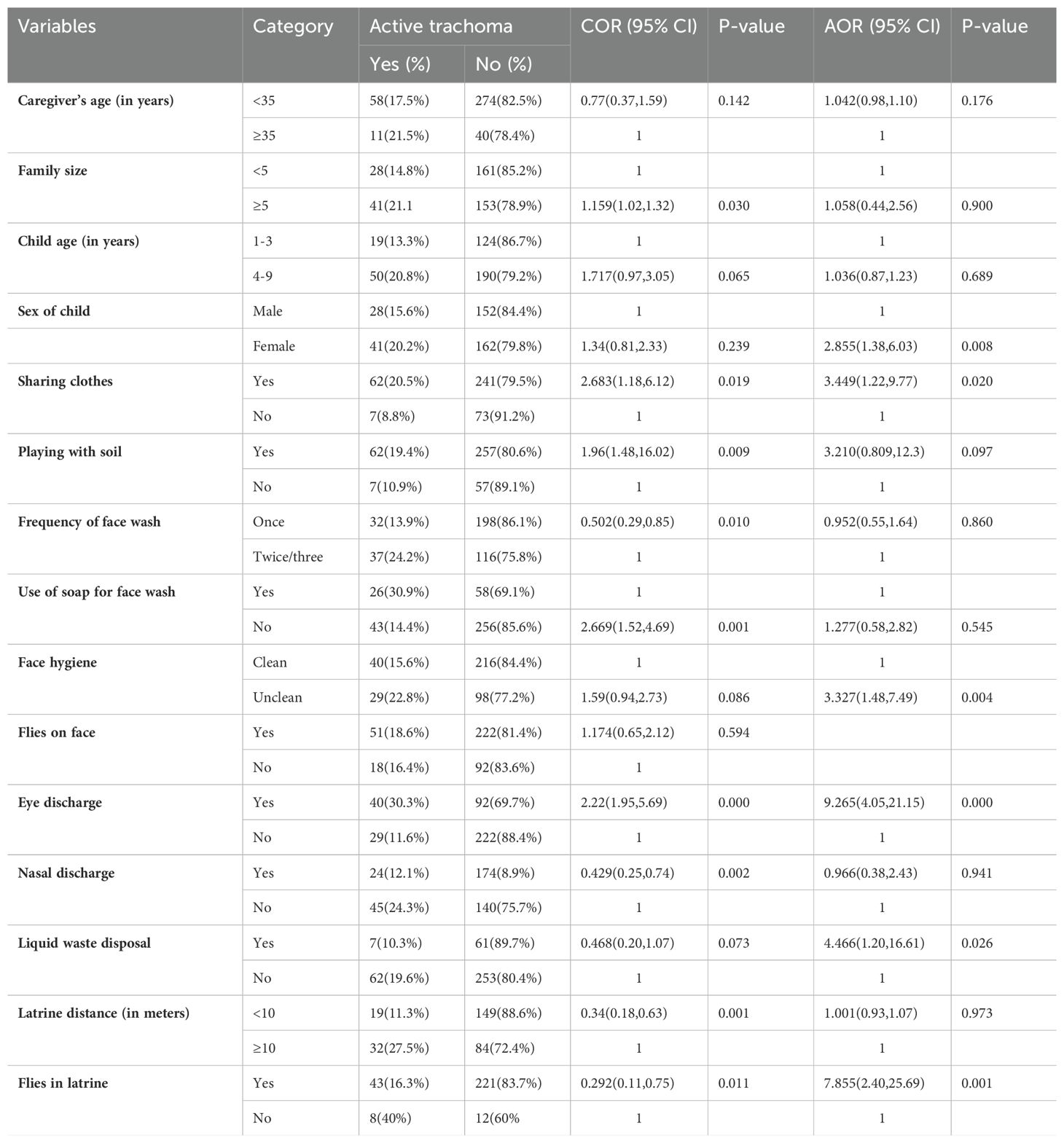
Table 6. Bivariate and multivariable analyses of factors associated with active trachoma among rural children in eastern Ethiopia in 2024.
Among the urban children
In the bivariate logistic regression, child age, caregiver’s educational status, family size, a habit of sharing clothes, frequency of washing face, face hygiene, flies on child’s face, eye discharge, source of energy for cooking, presence of liquid waste, and amount of water per day per person were found to have a p-value less than or equal to 0.25 and were selected as candidates for the final multivariable analysis for the non-leprosy settlements. Thus, a habit of sharing clothes, eye discharge, and the presence of liquid waste were significantly associated with the prevalence of active trachoma at a p-value less than 0.05 (Table 7). Children who shared clothes were 4.6 times (AOR = 4.60, 95% CI: 1.60–13.17) more likely to have active trachoma than children who did not share clothes. The likelihood of active trachoma among children with eye discharge was 8.6 times (AOR = 8.56, 95% CI: 4.62– 333 74.57) higher than that of children with no discharge from their eyes. Children who were living in households that had liquid waste around the home were 2.9 (AOR = 2.98, 95% CI: 1.53–11.09) times more likely to have active trachoma compared to children from households that had no liquid waste around the homes (Table 7).
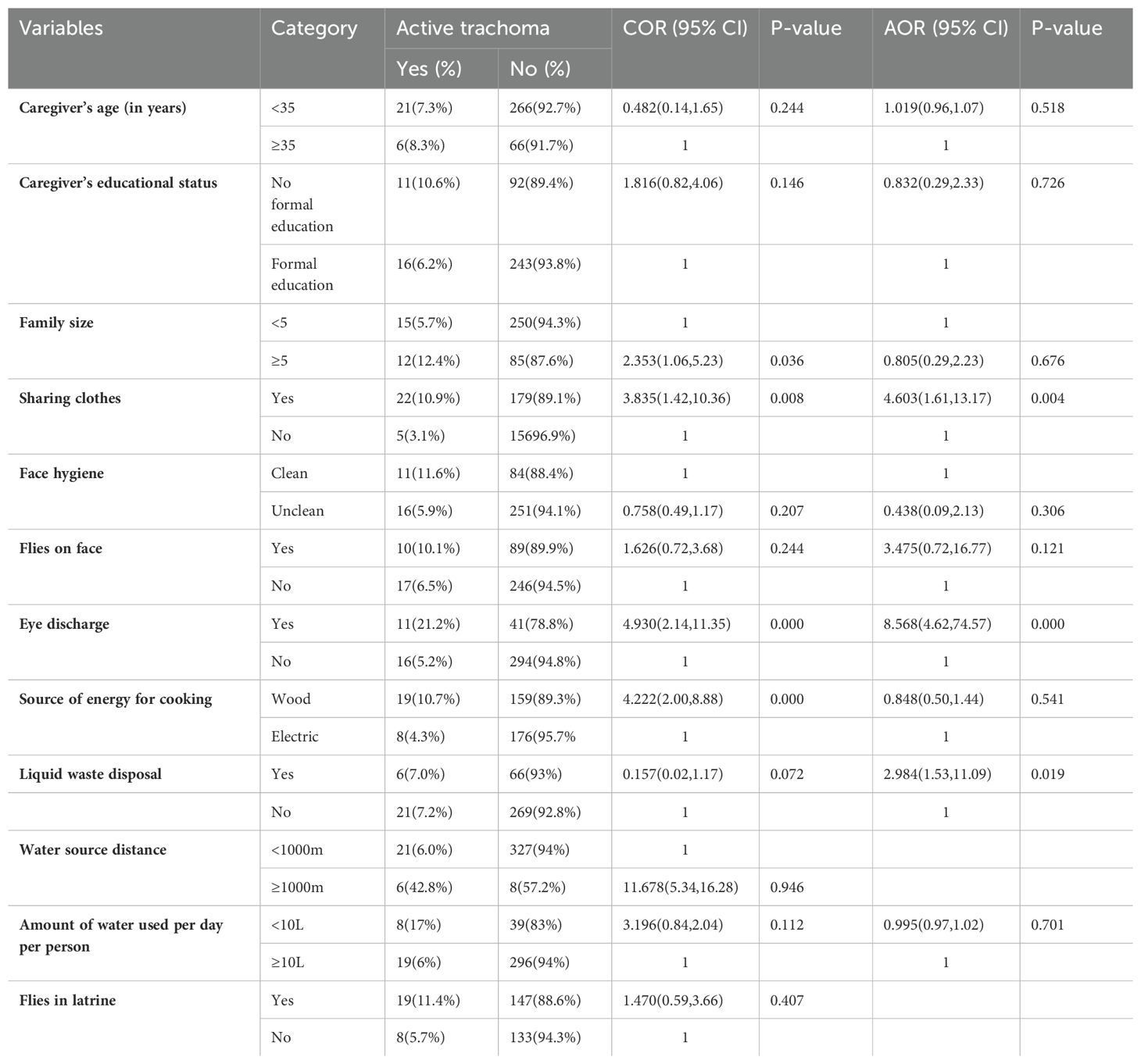
Table 7. Bivariate and multivariable analyses of factors associated with active trachoma among urban children in eastern Ethiopia in 2024.
Discussion
Trachoma is an NTD caused by infection with conjunctival strains of C. trachomatis and is the leading cause of preventable blindness worldwide. This study aimed to assess the prevalence and associated factors of active trachoma among children aged from 1-9 years in previous leprosy and non-leprosy settlement areas. In this study, the overall prevalence of active trachoma was 12.9% (95% CI: 10.5%, 15.3%), with 15.6% in the leprosy settlements and 9.8% in the non-leprosy settlements. In addition, the prevalence of active trachoma among rural and urban children was 18.3% and 7.5%, respectively. Factors such as being a rural resident; household with low monthly income; being female; the presence of solid waste, liquid waste, and animal dung around their home; toilet distance from home; the presence of flies in the toilet; a habit of playing with soil; a habit of sharing clothes; facial hygiene; and eye discharge were statistically associated with active trachoma.
In this study, the overall prevalence of active trachoma was 12.9%. This is in line with the finding in the Metema District (11.8%) (23). However, this study’s finding is lower than the previous findings reported in the Lare District, Southwest Zone (21.6%) (29), Deguatemben, Tigray (21.5%) (30), Tigray Region (26.1%) (3), Ebinat District, South Gondar Zone (36.1%) (31), Ethiopia (24%) (7), South Sudan (63.3%) (32), and the Sagar District, Madhya Pradesh, India (16.36%) (33). This difference might be due to better accessibility to health facilities and religious-based personal hygiene practices in this study area (34). However, the findings of this study were higher than the WHO threshold for the elimination of active trachoma and also the findings of studies conducted in the Dangila District (6.1%) (35), Dire Dawa (4.3%) (34), the Brazilian state of Roraima (4.5%) (36), Brazil (3.4%) (37), Dholpur state in India (2.1%) (38), India (6.8%) (39), China (5.2%) (40), Nepal (4.3%) (41), Kwara State in Nigeria (1.3%) (42), and Sana, Yemen (9.1%) (43). This could be attributed to the difference in the implementation of the Surgery for trichiasis, antibiotics for active disease, facial hygiene, and environmental improvement (SAFE) strategy, study setting, study period, and age of study population (44, 45). Although the WHO recommends a reduction of TF cases to less than 5% among children aged 1–9 years to eliminate blinding trachoma, the prevalence of active trachoma in the leprosy and non-leprosy settlements in this study was 15.6% and 9.8%, respectively. This difference was statistically significant (x²=5.65, p-value = 0.017). This could be explained by poorer quality of life in the leprosarium group than the general population (46). The results of this study show that living in a leprosy settlement as a rural resident was strongly linked to having active trachoma. This may be due to the NTDs (trachoma and leprosy in this case) being associated with poverty, which can attributed to the inequity in access to preventive chemotherapy and morbidity management, stigma and discrimination, poor housing conditions, and low socioeconomic status of the community residing in the leprosy settlement (44, 47–49).
The current study found that prevalence of active trachoma was 18.3% and 7.5% among children in rural and urban areas, respectively. There were statistically significant differences in the prevalence of active trachoma between the rural and urban children (x²=18.47, p-value = 0.000). This could be explained in the rural area by poor access to clean water sources (leading to reliance on unsafe water from rivers, ponds, or unprotected wells), inadequate water supply (individuals may prioritize water for drinking and cooking over personal hygiene activities such as face washing), lack of access to latrines or inadequate sanitation facilities, and poorer housing conditions in rural households (50, 51).
In both the leprosy and non-leprosy settlements, a latrine distance of less than 10 m from the home was significantly associated with the risk of trachoma. This finding is similar to other studies conducted in the Zala District, Gamo Gofa Zone (19) and the Ebinat District, South Gonder Zone (31), in Ethiopia. This might be due to the proximity of latrines to homes influencing community sanitation practices and overall cleanliness. If latrines are close but not well maintained or utilized correctly, this could contribute to a higher prevalence of active trachoma due to the persistence of unsanitary conditions in the surrounding area (52). In this study, eye discharge was significantly associated with active trachoma in both settings, indicating that the infected discharge from the nose and eye transmitted infection via fingers, flies, or fomites (44) and through direct contact with nasal and ocular secretions. This finding is in line with studies conducted in the Dera District (53) and Dangila District (35).
In this study, having a family monthly income of less than 5,000 Ethiopian birr was significantly associated with the occurrence of active trachoma among children in the leprosy settlements. This finding is congruent with other similar studies conducted in the Dire Dawa (34), Gondar (54), and Harari regions (16). This could be due to the fact that economic constraints among the leprosarium community may impact a family’s ability to afford basic hygiene and sanitation resources, such as clean water, soap, and sanitation facilities (34).
In this study, having solid waste around the home was significantly associated with the prevalence of active trachoma among children in the leprosy settlements. This is explained by the fact that disposing of solid waste in an open field attracts a high number of eye-seeking flies, which leads to a high chance of transmission of active trachoma among children (55).
This finding is in line with the findings of studies conducted in Arba Minch (56), Lemo District, southern Ethiopia (29) and Gondar Zuria District, North Gondar (54).
In this study, having animal dung in their living compound was significantly associated with active trachoma among children in the non-leprosy settlements. This result is in line with a study conducted in Arba Minch (56). It is possible that this association results from children being exposed to more flies, which breed in exposed animal and human feces, and can spread trachoma (57). In this study, having a habit of playing in soil was significantly associated with the occurrence of active trachoma among children in the non-leprosy settlements. This is explained by the fact that when children play in soil and touch their faces, particularly their eyes or nose, they can introduce the pathogens into their mucous membranes, increasing the risk of infection.
This study showed that having an unclean face was significantly associated with active trachoma among rural children. This is in line with the findings of a study done in Arba Minch (56). A possible explanation is that unclean faces attract eye-seeking flies (Musca sorbens), which are potential mechanical vectors of C.trachomatis infections (57). In this study, having a habit of sharing clothes was significantly associated with active trachoma. The primary ways that trachoma is transmitted is through direct contact with secretions from the eyes, nose, and throat; however, sharing towels, handkerchiefs, or clothing can also result in direct contact which could be the explanation for the above finding (44).
In this study, liquid waste around the home was significantly associated with the prevalence of active trachoma among the rural and urban children. This result is the same as a study in the Dambia District, Northwest Ethiopia (24). A potential reason for this is that improper garbage disposal practices create an environment conducive to fly reproduction. This study revealed that the presence of flies in the toilet was significantly associated with the occurrence of active trachoma among rural children. This finding in line with the findings of studies in the Wadla District, northern Ethiopia (58) and Dire Dawa (34). This could be because flies are attracted to decaying organic matter, including feces, and their presence in and around toilets can facilitate trachoma transmission (59). In this study, being female was significantly associated with the prevalence of active trachoma among rural children. It is clear that regardless of age, women have a higher likelihood of having trachomatous trichiasis by adulthood than men (60, 61). The differences in risk of infection between girls and boys are similar to those between adult women and men. This could be explained by the proximity of women and girls to children, which exposes them to more repeated infections than men (61).
The strength of this study is that it was conducted in leprosy and non-leprosy settlement areas. Furthermore, it considered both urban and rural children. However, the study might have some limitations; the cross-sectional nature of study design does not confirm a definitive cause-and-effect relationship. In addition, a bacteriological laboratory test was not used for confirmation of clinical diagnoses. There are also some limitations related to the diagnostic tools such as the magnifying lens. While magnifying lenses can be helpful for basic eye examinations, they are limited in magnification, illumination, field of view, and specialized features. Even though the data collectors were trained prior to data collection, there is observation or interpretation bias.
Conclusions
This study investigated the prevalence of active trachoma and its associated factors among children between 1 and 9 years old who were living in previously identified leprosy settlements and non-leprosy settlements. The findings revealed that the prevalence of active trachoma was 12.9%. This finding was higher than the WHO threshold (<5%) for the elimination of active trachoma in every district. According to this study, children living in the leprosy settlements had a higher prevalence of active trachoma than children living in the non-leprosy settlements. In addition to this, the children in the rural areas had a higher prevalence of active trachoma than the children in the urban areas. The study further revealed factors such as place of residence, presence of liquid waste, toilet distance less than 10m from the home, and eye discharge were factors that increased the occurrence of active trachoma in both settlements. Community-based behavioral change campaigns to promote hygienic practices; discourage the sharing of clothes; raise awareness of the risks of playing with soil, exposure to animal dung, and inappropriate latrine utilization should be enacted. This study provides an insight into the districts that require targeted drug distribution campaigns with appropriate eye ointment or antibiotics.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Institutional Health Research Ethics Review Committee at Haramaya University College of Health and Medical Science were granted ethical approval (IHRERC) (Reference number: IHRERC/185/2023). Permission letter was obtained from Amir Nur District and Babble District administrative offices. The interview and clinical eye examination of the chosen children were taking place in the presence of the children’s caregivers after the signed informed voluntary assent was found. Their names and other personal identifiers were not registered to maintain confidentiality. The study’s goals, as well as the rights and advantages of study participants, were explained. Individuals with positive for sign of active trachoma was linked to nearby health facilities. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
FW: Data curation, Formal Analysis, Methodology, Software, Supervision, Validation, Visualization, Writing – review & editing, Conceptualization, Investigation. MG: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – review & editing, Funding acquisition, Project administration, Resources, Writing – original draft. GK: Conceptualization, Data curation, Formal Analysis, Methodology, Software, Supervision, Validation, Visualization, Writing – review & editing. AD: Conceptualization, Data curation, Formal Analysis, Methodology, Software, Supervision, Validation, Visualization, Writing – review & editing. SM: Data curation, Formal Analysis, Methodology, Software, Supervision, Validation, Visualization, Writing – review & editing. TR: Conceptualization, Formal Analysis, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – review & editing. UU: Conceptualization, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Visualization, Writing – review & editing. KU: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was not received for the research, authorship, and/or publication of this article.
Acknowledgments
We were grateful for the ethical approval provided by the Institutional Health Research Ethics Review Committee of the Haramaya University Colleges of Health and Medical Sciences. We also extend our gratitude to the study participants, healthcare workers, data collectors, supervisors, and everyone else who helped us finish this research in any way. Human and material resource was covered by Haramaya University’s College of Health and Medical Science.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hotez PJ, Aksoy S, Brindley PJ, Kamhawi S. What constitutes a neglected tropical disease. CA USA: Public Library of Science San Francisco (2020).
2. Potroz MG, Cho N-J. Natural products for the treatment of trachoma and Chlamydia trachomatis. Molecules. (2015) 20:4180–203. doi: 10.3390/molecules20034180
3. Sherief ST, Macleod C, Gigar G, Godefay H, Abraha A, Dejene M, et al. The prevalence of trachoma in Tigray Region, Northern Ethiopia: results of 11 population-based prevalence surveys completed as part of the global trachoma mapping project. Ophthalmic Epidemiol. (2016) 23:94–9. doi: 10.1080/09286586.2016.1250917
4. Alliance WHO, Elimination G. Weekly epidemiological record Relevé épidémiologique hebdomadaire Geneva: WHO, Vol. 30. (2020). pp. 349–60.
5. Burton MJ, Mabey DC. The global burden of trachoma: a review. PloS Negl Trop Dis. (2009) 3:e460. doi: 10.1371/journal.pntd.0000460
6. WHO. Report of the 4th global scientific meeting on trachoma Geneva: World Health Organization, Vol. 27–29. (2018).
7. Asgedom YS, Melaku T, Gebrekidan AY, Meskele M, Asnake G, Alemu A, et al. Prevalence of active trachoma among 1–9 years of age children in Ethiopia: a systematic review and meta-analysis. BMJ Open. (2024) 14:e079623. doi: 10.1136/bmjopen-2023-079623
8. WHO. Report of the 21st meeting of the WHO alliance for the global elimination of trachoma by 2020. Geneva, Switzerland: World Health Organization (2019).
9. Miller HA, López de Mesa CB, Talero SL, Meza Cárdenas M, Ramírez SP, Moreno-Montoya J, et al. Prevalence of trachoma and associated factors in the rural area of the department of Vaupés, Colombia. PloS One. (2020) 15:e0229297. doi: 10.1371/journal.pone.0229297
10. WHO. Towards zero leprosy: global Leprosy (Hansen’s disease) Strategy 2021–2030. Regional Office for South-East Asia: World Health Organization (2021).
11. Polack S, Brooker S, Kuper H, Mariotti S, Mabey D, Foster A. Mapping the global distribution of trachoma. Bull World Health Organ. (2005) 83:913–9.
12. Gebre T. Trachoma control & elimination in Africa. Int J Infect Dis. (2014) 21:42. doi: 10.1016/j.ijid.2014.03.504
13. Tanywe AC, Matchawe C, Fernandez R, Lapkin . Perceptions and practices of community members relating to trachoma in Africa: a qualitative systematic review protocol. JBI Evidence Synthesis. (2019) 17:2350–6. doi: 10.11124/JBISRIR-2017-003820
14. WHO. Second Edition of National Neglected Tropical Diseases Master Plan, Federal Ministry of Health, Addis Ababa Ethiopia Addis Ababa. (2016) Ethiopia: Federal Ministry of Health.
15. Gebrie A, Alebel A, Zegeye A, Tesfaye B, Wagnew F. Prevalence and associated factors of active trachoma among children in Ethiopia: A systematic review and meta-analysis. BMC Infect Dis. (2019) 19:1–12. doi: 10.1186/s12879-019-4686-8
16. Assefa N, Aklilu AR, Tekabe A, Jelalu K, Eskindir D. Prevalence and factors associated with trachoma among primary school children in Harari region, eastern Ethiopia. Ophthalmol Research: Int J. (2017) 7:37212. doi: 10.9734/OR/2017/37212
17. Hu VH, Harding-Esch EM, Burton MJ, Bailey RL, Kadimpeul J, Mabey DC. Epidemiology and control of trachoma: systematic review. Trop Med Int Health. (2010) 15:673–91. doi: 10.1111/j.1365-3156.2010.02521.x
18. Habtamu E, Wondie T, Aweke S, Tadesse Z, Zerihun M, Zewdie Z, et al. Trachoma and relative poverty: a case-control study. PloS Negl Trop Dis. (2015) 9:e0004228. doi: 10.1371/journal.pntd.0004228
19. Mengistu K, hegaze M, Woldemichael K, Gesesew H, Markos Y. Prevalence and factors associated with trachoma among children aged 1–9 years in Zala district, Gamo Gofa Zone, Southern Ethiopia. Clin Ophthalmol. (2016) 10:1663–70. doi: 10.2147/OPTH.S107619
20. Kassim K, Kassim J, Aman R, Abduku M, Tegegne M, Sahiledengle B. Prevalence of active trachoma and associated risk factors among children of the pastoralist population in Madda Walabu rural district, Southeast Ethiopia: a community-based cross-sectional study. BMC Infect Dis. (2019) 19:1–7. doi: 10.1186/s12879-019-3992-5
21. Center., T.C. Trachoma control program summary of 2019 activities. (2019) 2019:. The Carter center's. Available online at: https://www.cartercenter.org/resources/pdfs/news/health_publications/trachoma/trachomareview-final-eng-2020-protected.pdf
22. Kebede MT. Leprosy, leprosaria, and society in Ethiopia: a historical study of selected sites, 1901-2001. Armauer Hansen Res Inst. (2005).
23. Ayelgn K, Guadu T, Getachew A. Low prevalence of active trachoma and associated factors among children aged 1–9 years in rural communities of Metema District, Northwest Ethiopia: a community based cross-sectional study. Ital J Pediatr. (2021) 47:114. doi: 10.1186/s13052-021-01064-x
24. Ferede AT, Dadi AF, Tariku A, Adane AA. Prevalence and determinants of active trachoma among preschool-aged children in Dembia District, Northwest Ethiopia. Infect Dis Poverty. (2017) 6:1–7. doi: 10.1186/s40249-017-0345-8
25. Nash SD, Chernet A, Moncada J, Stewart AEP, Astale T, Sata E, et al. Ocular chlamydia trachomatis infection and infectious load among pre-school aged children within trachoma hyperendemic districts receiving the safe strategy, Amhara Region, Ethiopia. PloS Negl Trop Dis. (2020) 14:1–16. doi: 10.1371/journal.pntd.0008226
26. Shiferaw D, Moges HG. Risk factors for active trachoma among children aged 1-9 years in Maksegnit town, Gondar Zuria District, Northwest Ethiopia. Saudi J Health Sci. (2022) 2:1–5. doi: 10.4103/2278-0521.127069
27. Mpyet C, Lass BD, Yahaya HB, Solomon AW. Prevalence of and risk factors for trachoma in kano state, Nigeria. PLoS ONE. (2012) 7:3–8. doi: 10.1371/journal.pone.0040421
28. Hylefors B, Dawson CR, Jones BR, West SK, Taylor H. A simple system for the assessment of trachoma and its complications. Bull World Health Organ. (1987) 65:477–83.
29. Belsti Y, Fekadu SA, Assem AS. Active trachoma prevalence and its associated factors among children aged 1-9 years in rural residents of Lare District, Southwest Ethiopia. Int J Ophthalmol. (2021) 14:1756–64. doi: 10.18240/ijo.2021.11.16
30. Reda G, Yemane D, Gebreyesus A. Prevalence and associated factors of active trachoma among 1-9 years old children in Deguatemben, Tigray, Ethiopia, 2018: Community cross-sectional study. BMC Ophthalmol. (2020) 20:1–9. doi: 10.1186/s12886-020-01394-0
31. Getalem A, Nigist A, Gedefaw A. Factors Associated with Active Trachoma among Children in Ebinat District, South Gondar Zone, North West Ethiopia: A community-based cross-sectional study. medRxiv. (2022), 1–26. doi: 10.1101/2022.02.07.22270570
32. Ngondi J, Ole-Sempele F, Onsarigo A, Matende I, Baba S, Reacher M, et al. Blinding trachoma in postconflict southern Sudan. PloS Med. (2006) 3:e478. doi: 10.1371/journal.pmed.0030478
33. Agarwal AK, Rakesh M, Nandeswar S, Prasad P. A clinico-epidemiological study of trachoma in urban and rural population of Sagar District Madhya Pradesh, India: Study of active trachoma in Sagar District (MP). Community Acquired Infection. (2016) 3:10–15. doi: 10.4103/2225-6482.179227
34. Mohamed H, Weldegebreal F, Mohammed J, Gemechu A. Prevalence of Trachoma and its Associted Factors Among Children Aged 4-9 Years in Dire Dawa Administration,Eastern Ethiopia: School-based Cross-sectional study. East Afri Health Biomed Sci. (2019) 3(2):45–54.
35. Genet A, Dagnew Z, Melkie G, Keleb A, Motbainor A, Mebrat A, et al. Prevalence of active trachoma and its associated factors among 1-9 years of age children from model and non-model kebeles in Dangila district, northwest Ethiopia. PloS One. (2022) 17:1–15. doi: 10.1371/journal.pone.0268441
36. De Brito CMG, Barbosa CC, Andrade SMC, Oliveira ALS, Montarroyos UR, Ferraz C, et al. Household survey of trachoma among children living in pernambuco, Brazil. Pathogens. (2019) 8:1–13. doi: 10.3390/pathogens8040263
37. Favacho J, Alves da Cunha AJL, Gomes STM, Freitas FB, Queiroz MAF, Vallinoto ACR, et al. Prevalence of trachoma in school children in the Marajó Archipelago, Brazilian Amazon, and the impact of the introduction of educational and preventive measures on the disease over eight years. PloS Negl Trop Dis. (2018) 12:e0006282. doi: 10.1371/journal.pntd.0006282
38. Gupta N, Vashist P, Senjam SS, Gupta V, Wadhwani M, Manna S, et al. Current status of trachoma in India: Results from the National Trachoma Prevalence Survey. Indian J Ophthalmol. (2022) 70:3260–5. doi: 10.4103/ijo.IJO_503_22
39. Malhotra S, Vashist P, Gupta N, Kalaivani M, Satpathy G, Shah A, et al. Prevalence of trachoma in Car-Nicobar Island, India after three annual rounds of mass drug administration with azithromycin. PloS One. (2016) 11:e0158625. doi: 10.1371/journal.pone.0158625
40. Xue W, Lu L, Zhu J, He X, He J, Zhao R, et al. A cross-sectional population-based survey of trachoma among migrant school aged children in Shanghai, China. BioMed Res Int. (2016) 2016:8692685. doi: 10.1155/2016/8692685
41. Sharmaa S, Ngondi JM, Mishra S, Prasad RD, Crowley K, Bonuedi D, et al. Completing baseline mapping of trachoma in Nepal: results of 27 population-based prevalence surveys conducted in 2013 and 2014. Ophthal Epidemiol. (2018) 25:115–20. doi: 10.1080/09286586.2018.1489972
42. Alada JJ, Mpyet C, Florea VV, Boisson S, Willis R, Muhammad N, et al. Prevalence of and risk factors for trachoma in Kwara state, Nigeria: Results of eight population-based surveys from the Global Trachoma Mapping Project. Ophthalmic Epidemiol. (2018) 25:53–61. doi: 10.1080/09286586.2018.1437188
43. Al-Eryani SA, Alshamahi EYA, Al-Shamahy HA, Al-Moyed KAA, Shawkany Al AM, AA, et al. Prevalence and risk factors for trachoma among primary school children in Sana’a City, Yemen. Univers J Pharm Res. (2021) 6(4):636. doi: 10.22270/ujpr.v6i4.636
44. Ageed A, Khan M. Eliminating trachoma in Africa: the importance of environmental interventions. Cureus. (2024) 16:1–17. doi: 10.7759/cureus.52358
45. Ramesh A KS, Haslam D, Schmidt E, Gilbert CE. The impact of climatic risk factors on the prevalence, distribution, and severity of acute and chronic trachoma. PloS Negl Trop Dis. (2013) 7:e2513. doi: 10.1371/journal.pntd.0002513
46. Howard G, Bartram J, Williams A, Overbo A, Fuente D, Geere J-A. Domestic Water Quantity, Service Level and Health, second edition. Geneva: World Health Organization (2020). 76 p. Available at: http://www.who.int/water_sanitation_health/diseases/wsh0302/en/.
47. Houweling T, Karim-Kos HE, Kulik MC, Stolk WA, Haagsma JA, Lenk EJ, et al. Socioeconomic inequalities in neglected tropical diseases: A systematic review. PloS Negl Trop Dis. (2016) 10:1–28. doi: 10.1371/journal.pntd.0004546
48. Sun N, Amon J. Addressing inequity: neglected tropical diseases and human rights. Heal Hum Rights Journa. (2018) 20:11–26.
49. Van‘t Noordende AT AM, Schippers A. The impact of leprosy, podoconiosis and lymphatic filariasis on family quality of life: A qualitative study in Northwest Ethiopia. PloS Negl Trop Dis. (2020) 14:1–17. doi: 10.1371/journal.pntd.0008173
50. Girma M, Samuel A, Pradeilles R, Genye T, van Zy C. Progress in water, sanitation and hygiene service coverage in Ethiopia: what more do we need to do and why? Natl Inf Platforms Nutr. (2021), 2–7.
52. WHO. Water, Sanitation & Hygiene for accelerating and sustaining progress on Neglected Tropical Diseases(2017). Available online at: https://apps.who.int/iris/bitstream/handle/10665/182735/WHO_FWC_WSH_15.12_eng.pdf?sequence=1%0Ahttp://www.unwater.org/statistics_san.html. (accessed October 15, 2023).
53. Alemayehu M, Koye DN, Tariku A, Yimam K. Prevalence of active trachoma and its associated factors among rural and urban children in dera woreda, northwest Ethiopia: A comparative cross-sectional study. BioMed Res Int. (2015) 2015:8. doi: 10.1155/2015/570898
54. Asres M, Endeshaw M, Yeshambaw M. Prevalence and risk factors of active trachoma among children in gondar zuria district North Gondar, Ethiopia. Prevent. Med. (2016) 1:5. doi: 10.21767/2572-5483.100005
55. Karimurio J, Gichangi M, Ilako DR, Adala HS, Kilima P. Prevalence of trachoma in six districts of Kenya. East Afr Med J. (2006) 83:63–8. doi: 10.4314/eamj.v83i4.9417
56. Abdilwohab MG, Abebo ZH. High prevalence of clinically active trachoma and its associated risk factors among preschool-aged children in arba minch health and demographic surveillance site, southern Ethiopia. Clin Ophthalmol. (2020) 14:3709–18. doi: 10.2147/OPTH.S282567
57. CDC. Center for Global Health Division of Parasitic Diseases and Malaria The Targeted Neglected Tropical Diseases (NTDs) (2022). Available online at: https://www.cdc.gov/globalhealth/ntd/resources/targeted_ntds.pdf. (accessed December 5, 2023).
58. Kassaw MW, Abebe AM, Tegegne KD, Getu MA, Bihonegn WT. Prevalence and associations of active trachoma among rural preschool children in Wadla district, northern Ethiopia. BMC Ophthalmol. (2020) 20:1–10. doi: 10.1186/s12886-020-01585-9
59. Emerson PM, Lindsay SW, Alexander N, Bah M, Dibba SM, Faal HB, et al. Role of flies and provision of latrines in trachoma control: cluster-randomised controlled trial. Lancet. (2004) 363:1093–8. doi: 10.1016/S0140-6736(04)15891-1
Keywords: active trachoma, children, leprosy, non-leprosy, settlement, Ethiopia
Citation: Weldegebreal F, Getachew M, Mekonnen GK, Desalew A, Mekonnen S, Raru TB, Umer U and Urgesa K (2024) Prevalence of active trachoma and its associated factors among children aged 1–9 years in previous leprosarium and non-leprosarium areas in eastern Ethiopia: a community-based comparative study. Front. Trop. Dis 5:1476778. doi: 10.3389/fitd.2024.1476778
Received: 06 August 2024; Accepted: 11 October 2024;
Published: 13 November 2024.
Edited by:
Emanuele Nicastri, National Institute for Infectious Diseases Lazzaro Spallanzani (IRCCS), ItalyReviewed by:
Bernard Naafs, Stichting Global Dermatology, NetherlandsFrancesco Travaglino, Campus Bio-Medico University Hospital, Italy
Copyright © 2024 Weldegebreal, Getachew, Mekonnen, Desalew, Mekonnen, Raru, Umer and Urgesa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shambel Mekonnen, bWVrb25uZW5zaGFtYmVsOTE2QGdtYWlsLmNvbQ==
 Fitsum Weldegebreal
Fitsum Weldegebreal Mitiku Getachew
Mitiku Getachew Getachew Kabew Mekonnen
Getachew Kabew Mekonnen Assefa Desalew
Assefa Desalew Shambel Mekonnen
Shambel Mekonnen Temam Beshir Raru
Temam Beshir Raru Ukash Umer
Ukash Umer Kedir Urgesa
Kedir Urgesa