- 1Department of Microbiology and Hygiene, Bangladesh Agricultural University, Mymensingh, Bangladesh
- 2Department of Physiology, Bangladesh Agricultural University, Mymensingh, Bangladesh
- 3Department of Agricultural Chemistry, Bangladesh Agricultural University, Mymensingh, Bangladesh
- 4Department of Cerebrovascular Diseases, The Second Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 5Department of Pediatrics, West China Second University Hospital, Sichuan University, Chengdu, China
- 6Department of Microbiology, Immunology and Infectious Diseases, University of Calgary, Calgary, AB, Canada
Antimicrobial resistance is a global threat. On the other hand, Bangladesh produces high-quality mangoes, yet the mango coat and seed kernel, which contain medicinal components, remain unused. Therefore, this study investigated the antibacterial potential and toxicity of ethanol extracts from mango (Mangifera indica) seed kernels, which specifically target the bacterial strains Staphylococcus aureus, Bacillus cereus, Escherichia coli, and Klebsiella sp. Amrapali variant mango seeds were collected, dried, ground into a fine powder, and extracted with ethanol at various ratios. The efficacy of the crude extract was tested via the disc diffusion method. The results demonstrated significant antibacterial activity against gram-positive bacteria (S. aureus and B. cereus), with clear zones of inhibition observed, especially in a dose-dependent manner. The pure crude extract inhibited the growth of S. aureus with a zone of 23 mm, identical to that produced by doxycycline. However, the extract exhibited limited activity against gram-negative bacteria (E. coli and Klebsiella sp.). Additionally, the extract was effective against multidrug-resistant S. aureus. The pure crude extract produced a 22.5 mm zone of inhibition against multidrug-resistant S. aureus, which was slightly smaller than that of gentamicin (23 mm) but larger than those of chloramphenicol (21 mm), vancomycin (20 mm), and tetracycline (16 mm). In vivo toxicity was assessed in mice, revealing no significant adverse effects on the hepatic structure or renal cortex at lower doses (100 μl of pure crude extract). However, higher doses caused mild histopathological changes in the liver and kidneys. These findings suggest that mango seed kernel extract holds promise as an alternative antibacterial agent, particularly against gram-positive and antibiotic-resistant bacteria, while being relatively safe at lower doses. Further research is needed to elucidate the active compounds, mechanisms of action, and broader applications of this extract in combating antibiotic-resistant bacterial infections.
Introduction
Humans and animals worldwide suffer from bacterial diseases, which pose significant health threats. Several bacterial pathogens, such as Escherichia coli, Staphylococcus aureus, Salmonella sp., Pseudomonas aeruginosa, and Bacillus subtilis, cause diseases in both humans and animals (1). These diseases can only be treated with antibiotics. However, many bacteria have developed resistance to a variety of commercially available synthetic antimicrobial agents. The World Health Organization (WHO) has identified antibiotic resistance as a public health problem of international concern (PHIC). Consequently, various investigations have shown that herbs offer convenient, safe, and affordable sources of alternative medications (2). Since the introduction of modern drugs, plant-derived products such as gums, oils, and extracts have been used for therapeutic purposes, and they continue to provide health coverage for more than eighty percent of the world’s population (3, 4). Serious attention is being given to medicinal plants, as evidenced by the recommendation given by the WHO in 1970 (5). The WHO emphasizes the need to include traditional remedies within national drug policies since these plants serve as the best sources of a variety of drugs.
Therefore, it is important to study plants to understand better their properties, safety, and efficacy for improved benefits. The first plant compound with antimicrobial activity was reported in the 1930s, and many plant compounds are readily available from herbal suppliers and natural food stores (6). In Africa, self-medication with these substances is common and growing in popularity (7).
Mango (Mangifera indica) is a broad evergreen canopy tree from the family Anacardiaceae and genus Mangifera, comprising sixty-nine different species (8, 9). It is presumed that mangoes originated in Asia approximately 4,000 years ago (10). It is indigenous to the Indian and Southeast Asian regions, especially Central America, the Andaman Islands, Burma, China, and Eastern India. Its global recognition has increased over time (11, 12). Mangoes are grown in tropical and subtropical regions with a broad range of latitudes and extremely diverse soil and climatic conditions. They may also be grown commercially in a variety of agroecological contexts with success (13).
Mango (M. indica) has complex pharmacological, ethnomedical, and phytochemical profiles. In traditional medicine, different parts of the M. indica tree have been utilized to treat various conditions (8). M. indica contains phytochemicals such as alkaloids, phenols, flavonoids, and saponins in its leaf and stem bark; steroids only in its stem bark; anthraquinones only in its leaves; and alkaloids, flavonoids, saponins, and tannins in its fruit pulp, mango kernel, and leaf extracts (14–16). The principal polyphenolic substances identified in M. indica include mangiferin, gallic acid, catechins, quercetin, kaempferol, protocatechuic acid, ellagic acids, propyl and methyl gallate, rhamnetin, and anthocyanins (17). Mangiferin, a well-known polyphenolic molecule, is known for its many biological effects and has been extensively studied (18). The portion and kind of mango determine the amounts of various polyphenols in the fruit (19). Mangiferin has long been used as an anti-inflammatory, antibacterial, analgesic, antipyretic, antioxidant, anticancer, antiviral, immunomodulatory, and anthelmintic agent for the treatment of obesity in various regions of the world (20). Despite its low protein content, the mango seed kernel contains a majority of its essential amino acids, such as valine (5.79), lysine (4.30), leucine (6.90), and isoleucine (5.41) (mg/100 g protein), on a dry weight basis. Mango seed kernels also contain xanthones, flavonoids, phenolic acids, stearic (24-57%), oleic (34–56%), and 52–56% unsaturated fatty acids, all of which are known to have medical importance (21–23). Thus, depending on the variety, mango seed kernels can serve as both a feed ingredient and an edible feed byproduct. They also contain balanced nutritional amounts, substances, and highly metabolizable energy comparable to that of maize (23–25).
Bangladesh produces high-quality mangoes, but some of them, such as the coat and mango kernel, still need to be used despite containing medicinal components. Although M. indica is an Asiatic plant that is easily accessible in our country, no investigations on the antimicrobial activities of M. indica seed kernels against common pathogenic bacteria in Bangladesh have been reported. Therefore, this study aimed to investigate the efficacy of M. indica seed kernel extract as an antimicrobial agent, compare it with that of commercial antibiotics and determine its toxicity in mice.
Results
Ethanol extraction of mango (M. indica) seed kernels
The dried and smashed pieces of the seed kernel were brown (Figure 1A). After evaporation, the solutions extracted with various concentrations of ethanol (10:1, 5:1, and 2.5:1) were sticky, semisolid, and liquid sticky, respectively. All three types of crude extracts were brown. However, further experiments were carried out using the 2.5:1 ratio extract, which, after evaporation, was liquid and sticky, making it easier to spread through the agar media (Figure 1B).

Figure 1. Sample preparation and types of extracts from mango seed kernels. (A) Different steps of seed kernel sample preparation. (B) Three types of extracts. After evaporation of the three solutions with various concentrations of ethanol and seed kernel (10:1, 5:1, 2.5:1), a sticky semisolid consistency (10:1, 5:1) and a sticky consistency (2.5:1) were obtained.
Efficacy of crude extracts against gram-positive bacteria
The antibacterial activities of the crude mango kernel extracts were investigated and compared with those of commercially available antibiotic discs via the disc diffusion test. The results demonstrated that the crude extract inhibited the growth of S. aureus, creating a clear zone of inhibition (Figure 2). The strength of the ability of the pure crude extract to inhibit the growth of S. aureus was 23 mm, which was the same as that of doxycycline (Figure 2 middle and bottom panels). Further investigation of the ability of the crude extract to inhibit the growth of S. aureus at different concentrations through serial fold dilution revealed that the mango kernel crude extract inhibited the growth of S. aureus up to a 125-fold dilution in a dose-dependent manner (Figure 3A). Additionally, the crude extract was tested against another gram-positive bacterium, B. cereus, and the results showed that both the pure and diluted extracts inhibited growth, creating clear zones of inhibition in a dose-dependent manner (Figure 3B). These results suggest that the crude extract of mango (M. indica) seed kernel has an antibacterial effect on gram-positive bacteria such as S. aureus and B. cereus.
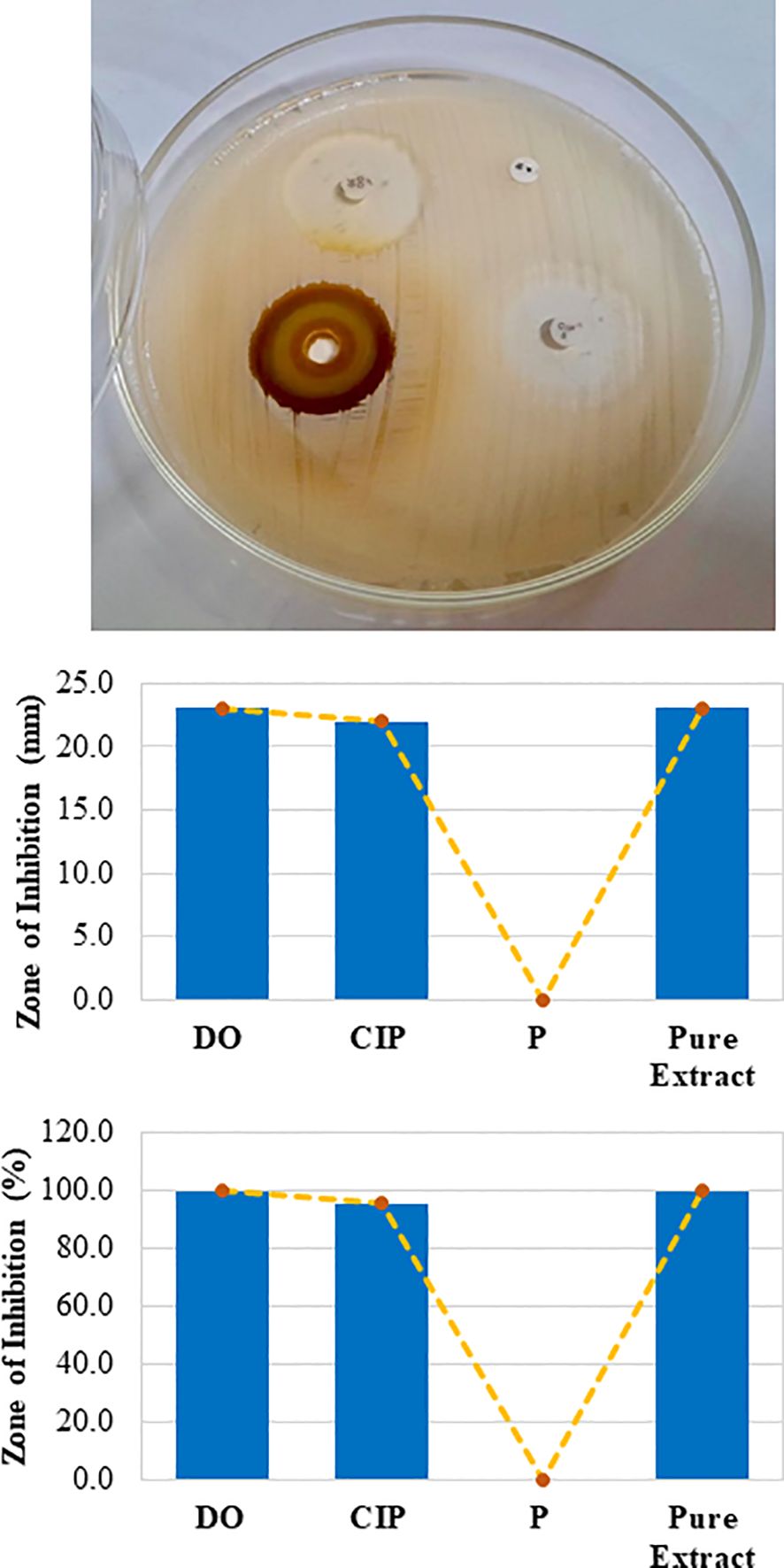
Figure 2. Zone of inhibition by the crude extract against gram-positive bacteria. A Mueller-Hinton agar plate was prepared and solidified. A small disc-shaped hole was made in the agar, and 15 µL of mango seed kernel extract was poured into it. Antibiotic discs of doxycycline (DO), ciprofloxacin (CIP), and penicillin (P) were used as controls. Following incubation at 37°C for 18 h, the plates were observed for zones of inhibition (upper panel). The zone of inhibition was measured with a millimetre (mm) (middle panel) and compared with the percentage of DO (bottom panel).
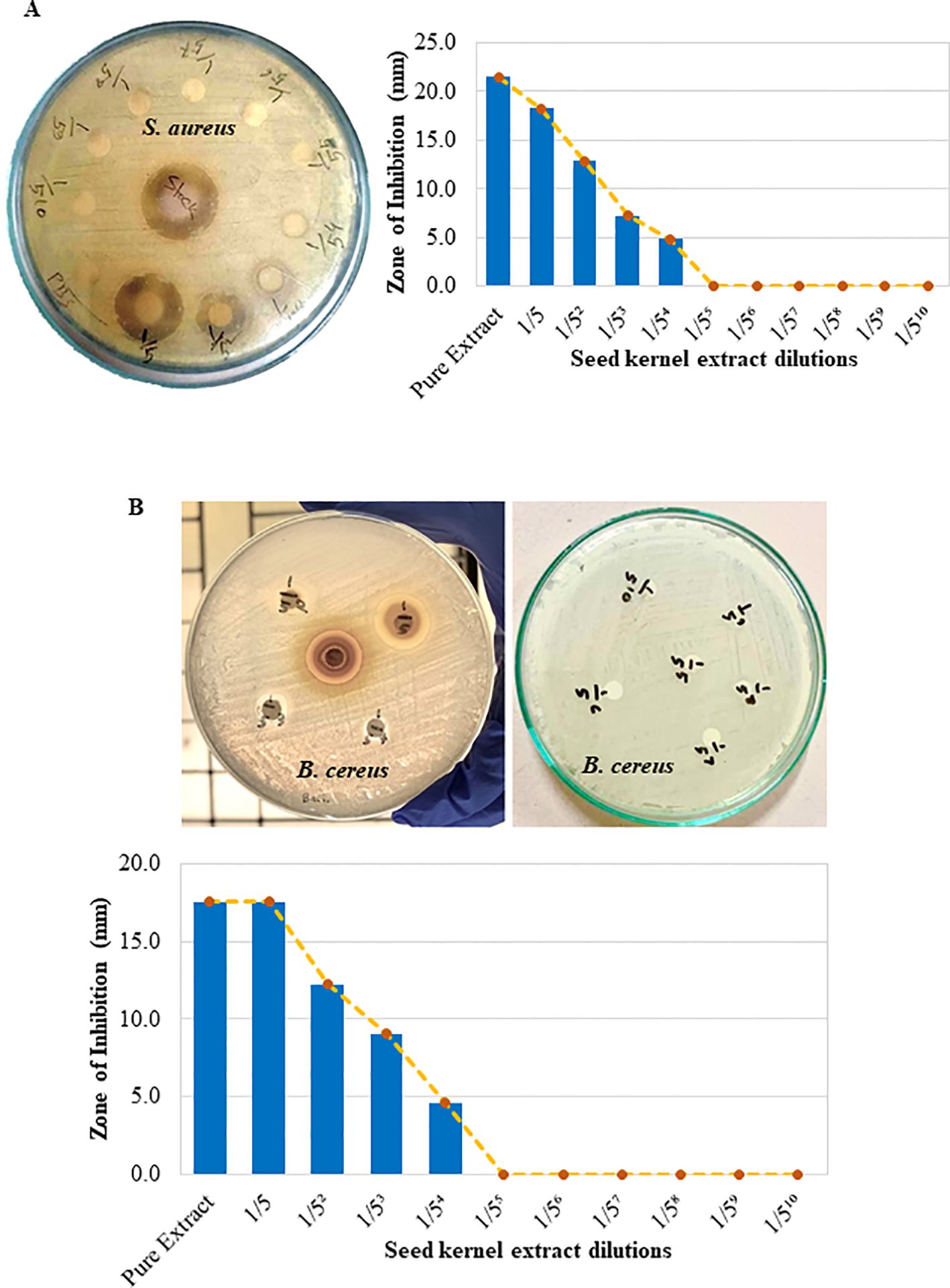
Figure 3. Mango seed kernel extract dilution and antibacterial effects against S. aureus and B. cereus. A fivefold serial dilution of the crude extract was prepared in PBS. Freshly cultured S. aureus and B. cereus were spread on separate Mueller–Hinton agar plates. Sterilized filter paper discs soaked in different concentrations of the extract and PBS were placed on agar, and zones of inhibition were observed after incubation at 37°C for 18 h. (A) Zone of inhibition on the agar plate and a bar graph showing zones of inhibition in millimeters (mm) compared with the pure crude extract (S. aureus). (B) The zone of inhibition on the agar plate and a bar graph showing zones of inhibition in millimeters (mm) compared with the pure crude extract (B. cereus).
Efficacy of crude extracts against gram-negative bacteria
The efficacy of the crude extract against gram-negative bacteria, such as E. coli and Klebsiella sp., was investigated similarly via the disc diffusion method in Mueller–Hinton agar. Surprisingly, the results demonstrated that the pure extract and its dilutions had a very limited capacity to inhibit the growth of both bacterial species, as shown by very small clear zones of inhibition (Figures 4A, B). Therefore, the results suggest that the crude extract of mango (M. indica) seed kernels has a very limited antibacterial effect against gram-negative bacteria such as E. coli and Klebsiella sp.
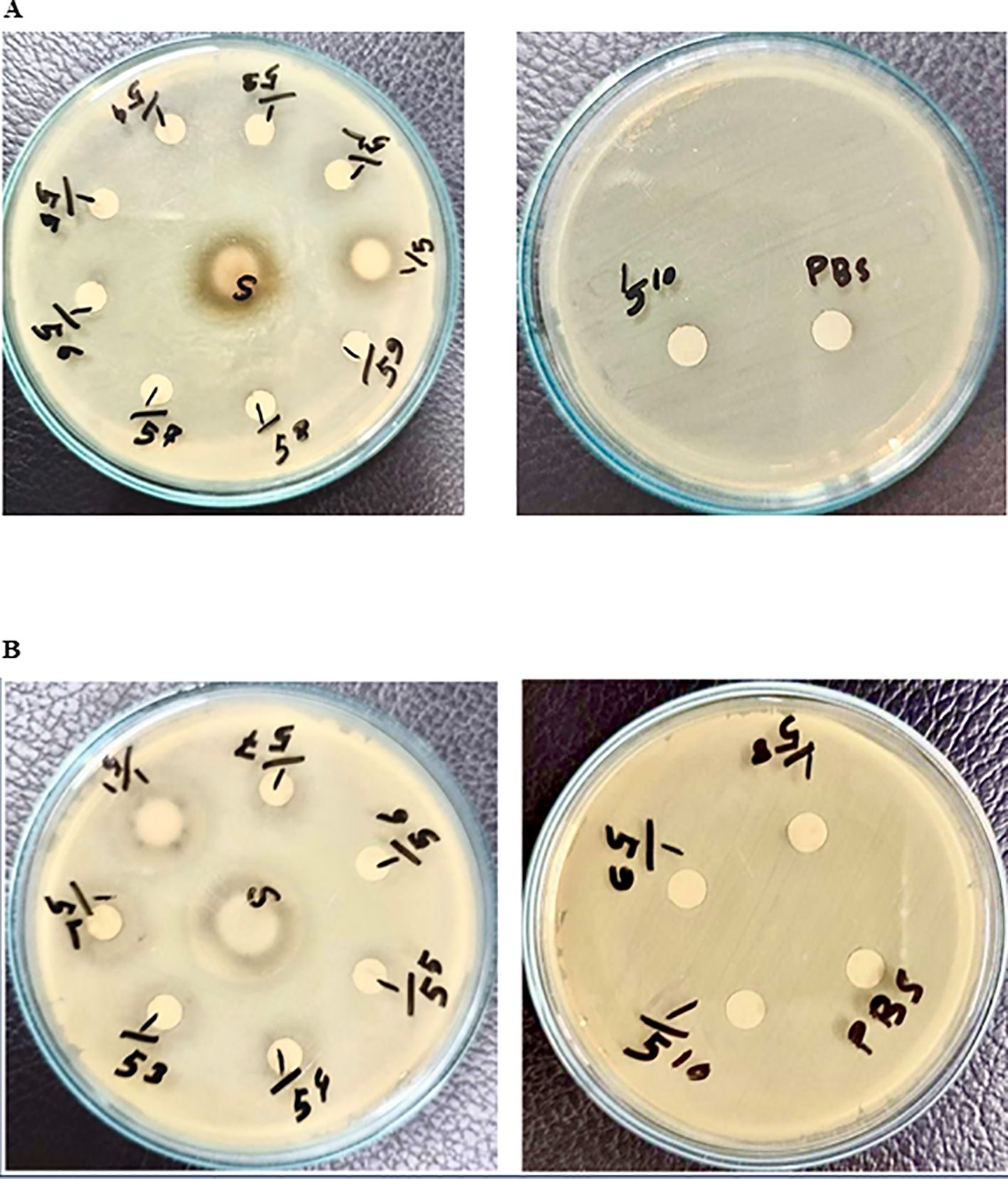
Figure 4. Investigation of mango seed kernel crude extract against gram-negative bacteria. The streak plate technique was used to disperse the bacteria across Mueller-Hinton agar plates. Filter paper discs soaked in varying concentrations (5-1 to 5-10) of extract, pure extract, or PBS were placed on agar. After incubation at 37°C for 18 h, zones of inhibition were observed. (A) coli. (B) Klebsiella sp.
Efficacy of the crude extract against antibiotic-resistant S. aureus
Additionally, the efficacy of the crude extract against antibiotic-resistant S. aureus was investigated. The results revealed that S. aureus was resistant to ampicillin-sulbactam (A/S), penicillin (P), and methicillin (MET) but susceptible to gentamicin (GEN), chloramphenicol (C), vancomycin (VA) and tetracycline (TET) to varying degrees. Quantitative measurements revealed that the pure crude extract created a zone of inhibition of 22.5 mm, which was slightly lower than that of GEN (23 mm) but greater than that of C (21 mm), VA (20 mm), and TET (16 mm) (lower panel of Figure 5). Surprisingly, the crude extract inhibited S. aureus in a dose-dependent manner (Figure 5). Hence, these findings suggest that the crude extract of mango (M. indica) seed kernels is capable of inhibiting the growth of multidrug-resistant S. aureus.
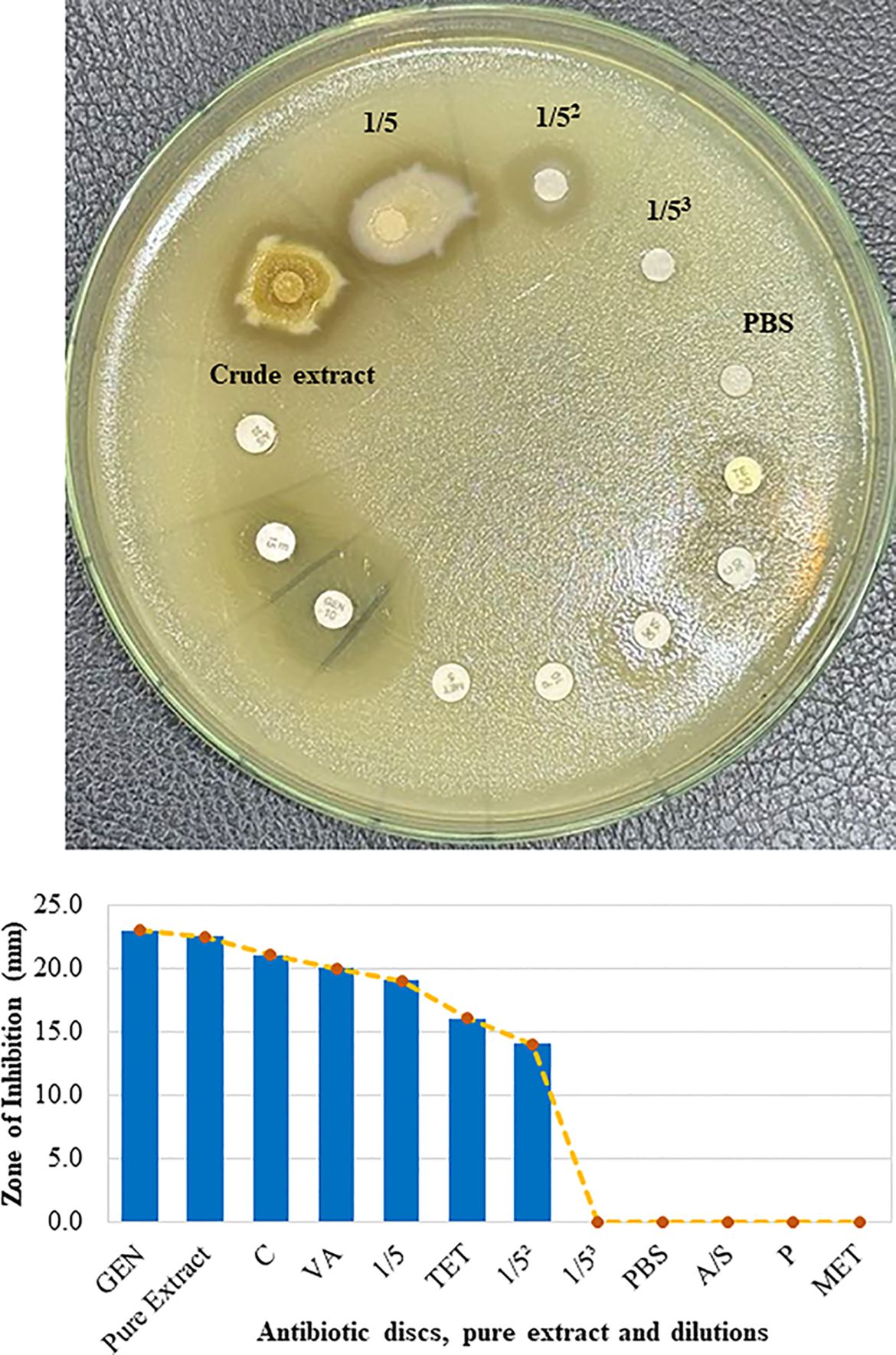
Figure 5. Efficacy of the crude extract against antibiotic-resistant S. aureus. Freshly cultured S. aureus was spread on Mueller-Hinton agar. Sterilized filter paper discs soaked in different concentrations of extract and PBS were placed on agar. Seven commercially available antibiotic discs, including those containing ampicillin-sulbactam (A/S), penicillin (P), methicillin (MET), gentamicin (GEN), vancomycin (VA), tetracycline (TET), and chloramphenicol (C), were used as controls. Zones of inhibition were observed after incubation at 37°C for 18 h (upper panel). The zone of inhibition in millimeters (mm) was measured and compared with that of GEN (bottom panel).
Toxicity analysis of the crude extract of mango (M. indica) seed kernels
The potential toxic effects of the crude extract of mango (M. indica) seed kernel on the host body were investigated in mice after oral administration of different doses for five d. The mice appeared healthy during the treatment period and up to 24 h posttreatment. Histopathological examination of the liver revealed that mice treated with 100 μl of pure crude extract had a normal hepatic structure, similar to those treated with PBS. In contrast, the mice treated with 500 μl or 1000 μl of pure crude extract presented mild or necrotic lymphocytic infiltration, respectively (Figure 6A). Histopathological examination of the kidneys revealed that mice treated with 100 μl of pure crude extract had a normal renal cortex, similar to those treated with PBS (Figure 6B). However, mice treated with 500 μl or 1000 μl of pure crude extract presented dilated renal tubules with hyaline deposition (Figure 6B). These results suggest that the crude extract might be nontoxic at lower doses in the host body.
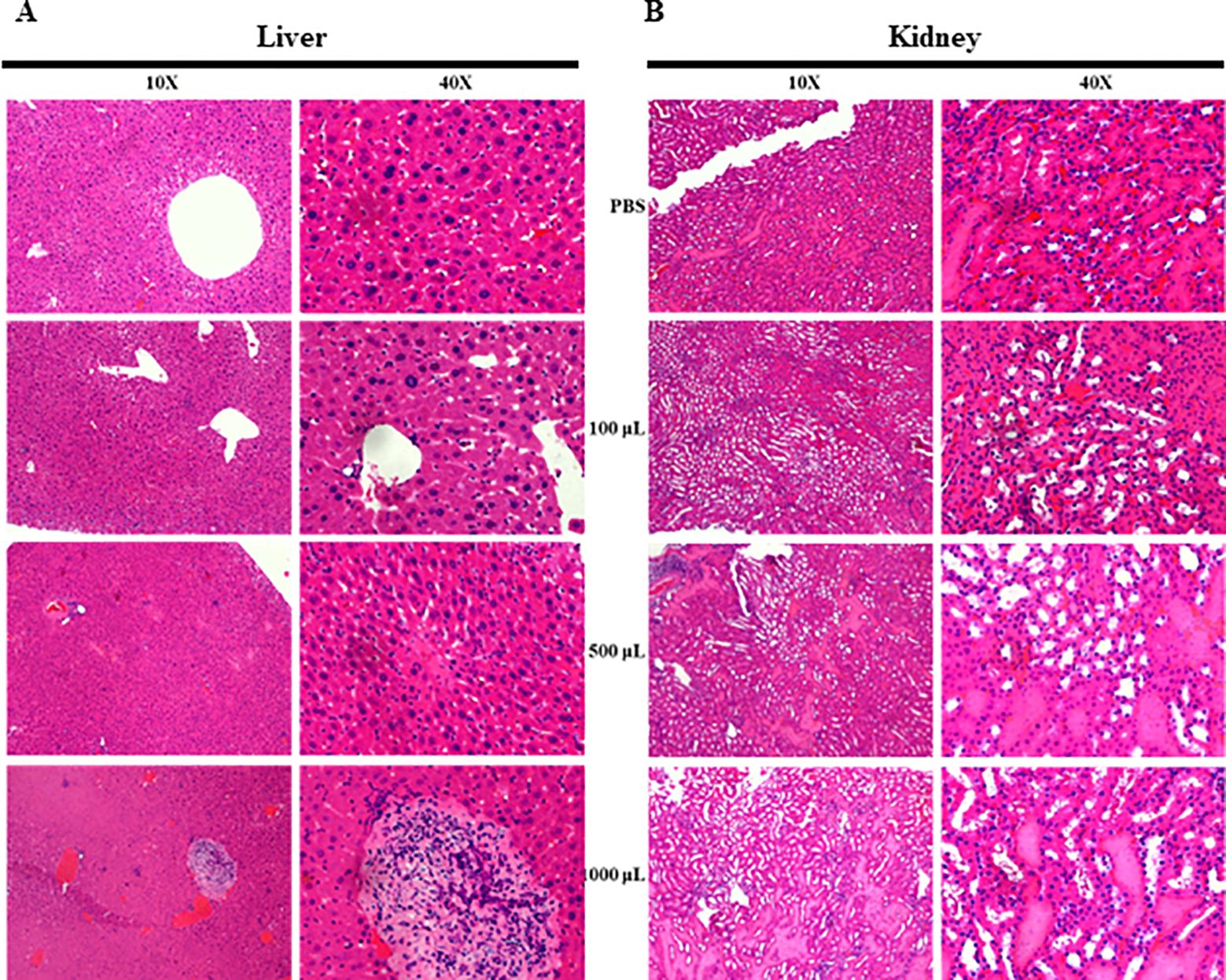
Figure 6. Histopathological examination of liver and kidney tissues from mice that were administered the crude extract. The mice were fed PBS or 100 μl, 500 μl, or 1000 μl of the crude extract daily for five d. Histopathological slides of the liver and kidney were prepared via H&E staining and examined under a microscope. (A) Liver sections showing hepatic architecture alterations due to crude extract and PBS administration. (B) Kidney sections showing renal tubule alterations due to crude extract and PBS administration.
Discussion
Microbial infections, particularly bacterial diseases, significantly impact global health and cause numerous illnesses. A systematic analysis revealed that in 2019, bacterial antimicrobial resistance (AMR) was linked to approximately 4.95 million deaths worldwide (26). Antibiotic resistance exacerbates this issue by making these infections more difficult to treat, leading to longer illnesses and higher mortality rates (27). Therefore, addressing antibiotic resistance is crucial for effective disease management and safeguarding public health. Alternative treatments, such as various plant- and herb-based medicines, are being investigated for their potential to treat antibiotic-resistant bacteria and viruses (28–30). Additionally, agri-food wastes contain a wide array of naturally occurring phytochemicals with significant bioactive potential for various animal and human applications (31). Therefore, this study investigated the efficacy of a crude extract of mango (M. indica) seed kernel from Bangladesh, which has demonstrated the expected antibacterial activity against gram-positive bacteria.
Various procedures exist for extracting bioactive compounds from medicinal herbs and plants via solvents such as ethanol, methanol, and phenol (32, 33). Ethanol is an effective method for obtaining bioactive plant compounds because of its ability to dissolve both polar and nonpolar substances. Additionally, ethanol is considered relatively safe and environmentally friendly compared with other solvents (33). Accordingly, the ethanolic extraction procedure used in this study yielded the expected results.
However, the findings revealed that the crude extract showed distinct antibacterial activities against gram-positive bacteria such as S. aureus and B. cereus rather than against gram-negative E. coli or Klebsiella sp. Gram-positive bacteria are usually more vulnerable to antibiotics because of their thick peptidoglycan cell wall, which is more accessible to drugs that target cell wall synthesis. This thick layer is simpler than the outer membrane of gram-negative bacteria, allowing antibiotics to penetrate more easily (34, 35). Qualitative phytochemical analysis revealed significant amounts of compounds, such as glycosides, saponins, flavonoids, tannins, and alkaloids, which exhibit antibacterial activity against various bacterial species (36, 37). These compounds may be more active and harmful to gram-positive bacteria, as revealed in the present study. However, our findings differ slightly from those of Seghosime et al., who reported that E. coli was sensitive to Mangifera indica seed kernel extract (38). These differences might be due to variations in the active compounds in different M. indica varieties, suggesting that further investigations into the active ingredients of M. indica varieties in Bangladesh are needed (37). Additionally, the crude extract of M. indica seed kernels was also effective against multidrug-resistant S. aureus, likely because diverse chemical compounds can disrupt bacterial cell walls, inhibit enzyme activity, or interfere with bacterial DNA/RNA synthesis (39).
Acute toxicity studies are crucial to avoid overdosing herbal drugs, as excessive doses could result in the removal of the drug from therapeutic use (40). Toxicity analysis in mice treated with different doses of the crude extract revealed minor changes in the liver and kidney, although no abnormal clinical symptoms were observed, especially at low doses (41).
Conclusion
The ethanol extract of mango (Mangifera indica) seed kernels has demonstrated significant antibacterial activity against gram-positive bacteria such as S. aureus and B. cereus and has potential as an alternative treatment for bacterial infections. However, it has shown limited effectiveness against gram-negative bacteria such as E. coli and Klebsiella sp. The extract also inhibited multidrug-resistant S. aureus. Toxicity analysis revealed that the extract is relatively safe at relatively low doses, with only mild effects observed at relatively high concentrations. These findings suggest that mango seed kernel extract could be a valuable resource for combating antibiotic-resistant bacteria. Further investigations are needed to identify its specific bioactive compounds, followed by an analysis of their mechanisms of action via electron microscopy and safety assessments in laboratory animals infected with various pathogenic bacterial species.
Methods
Material collection and preparation
M. indica of the Amrapali variant was collected from the local market of Mymensingh Sadar, which originated from Nowga, Rajshahi (Bangladesh). The fruits were 99% organic, each weighing an average of 200–250 g. The seed kernels were collected by manually removing the mango pulp and seed coat. The whole seed (seed coat with kernel) was kept at room temperature for 2 d to dry, making it easier to remove the seed coat. The coat was then removed with a knife. After the seed coat was removed, the seed kernels were dried at room temperature for one month. The seed kernels were subsequently chopped with a knife. The chopped kernels were ground via a grinder (Philips Mixer Grinder HL7505, 500 watts) and mixed homogeneously to form a fine powder. To remove excess water or moisture, the powdered kernels were dried again for 3 d at room temperature, and the fine powder was collected for further experimentation.
Extract preparation
The fine powder was placed into a Falcon tube with 99.9% alcohol at different ratios (1:10, 1:5, and 1:2.5; w/v) via a digital balance and a measuring cylinder. The tube was shaken with a shaker (200 rpm) for 72 h at 37°C. Afterward, the mixture of powder and ethanol was filtered through a syringe-driven 0.23 µM filter (Millipore® Filter). The ethanol was then evaporated from the filtrate via a rotary evaporator at 40°C and 150 rpm, and the extract was collected in Eppendorf tubes. The extract was stored at 4°C for further use. The extract was subjected to serial dilution using sterile PBS as a diluent.
Bacterial isolates
Four bacteria, Staphylococcus aureus, Bacillus cereus, Escherichia coli, and Klebsiella sp., were obtained from the repository of the Bacteriology Laboratory of the Department of Microbiology and Hygiene, Bangladesh Agricultural University. These bacteria were previously isolated from clinical samples, identified, characterized, and used for this study. The bacteria were revived and cultured in specific media according to standard protocols (42).
Antibacterial activity assay via the disc diffusion method
Mueller Hinton agar was prepared in Petri dishes following the standard protocol. The freshly cultured bacterial isolates were spread over solid agar media via a spreader to ensure uniform distribution throughout the plate surface and kept in the hood for 1 h to dry slightly to avoid creating hazy zones after incubation. Commercial antibiotic discs were placed onto agar plates. Additionally, small-diameter discs were prepared by blotting paper and sterilized. The dried prepared discs were soaked in the pure crude extract or diluted extract overnight at 4°C. The soaked discs were then placed onto agar plates. The plates were incubated in an upright position at 37°C for 18 h. The diameters of the inhibition zones were observed (43).
Administration of the crude extract to mice
Six- to eight-week-old laboratory-bred Swiss albino mice weighing 25-30 g were obtained from the Microbiology and Hygiene Laboratory Animal House, Bangladesh Agricultural University, and reared under normal laboratory conditions. The mice were divided into different groups and administered PBS or 100, 500, or 1000 μl of pure crude extract daily for 5 d. The mice were observed daily during the treatment period and up to 24 h posttreatment for their posture, gestures, and behavior. After the observation period, the mice were sacrificed, and their livers and kidneys were collected for histopathological examination. Histopathological examinations were conducted according to the standard protocol described previously (44).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Animal Welfare and Experimentation Ethics Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
RS: Methodology, Visualization, Writing – original draft. AM: Methodology, Visualization, Writing – review & editing. MM: Methodology, Writing – review & editing. SH: Data curation, Visualization, Writing – review & editing. CS: Methodology, Writing – review & editing. SA: Methodology, Writing – review & editing. BS: Validation, Writing – review & editing. SS: Writing – review & editing. MX: Writing – review & editing. HW: Writing – review & editing. CZ: Writing – review & editing. MH: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research is supported by the Science and Technology Department of Sichuan Province (2023JDKP0037), the National Key R&D Program of China (2023YFC2412900), and the Institutional Research Fund of Sichuan University (2023SCUH0018).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
°C, Degree Celsius; µM, Micrometre; A/S, Ampicillin-sulbactam; AMR, Antimicrobial resistance; B. cereus, Bacillus cereus; C, Chloramphenicol; DNA, Deoxyribonucleic acid; E. coli, Escherichia coli; GEN, Gentamicin; g, Gram; h, Hour; M. indica, Mangifera indica; MET, Methicillin; P, Penicillin; PBS, Phosphate-buffered saline; PHIC, Public health problem of international concern; RNA, Ribonucleic acid; rpm, Rotation per minute; S. aureus, Staphylococcus aureus; TET, Tetracycline; VA, Vancomycin; WHO, World Health Organization; μl, Microliter.
References
1. Stoilova I, Gargova S, Stoyanova A, Ho IJHp. Antimicrobial and antioxidant activity of the polyphenol mangiferin. Herba Polonica. (2005) 51:37–44.
2. Alaiya MA, Odeniyi MA. Utilisation of mangifera indica plant extracts and parts in antimicrobial formulations and as a pharmaceutical excipient: A review. Future J Pharm Sci. (2023) 9:29. doi: 10.1186/s43094-023-00479-z
3. Lima MEL, Cordeiro I, Young MCM, Sobra ME, Moreno P. Antimicrobial activity of the essential oil from two specimens of pimenta pseudocaryophyllus (Gomes) lr landrum (Myrtaceae) native from são paulo state-Brazil. Pharmacologyonline. (2006) 3:589–93.
4. Ndip R, Ajonglefac A, Wirna T, Luma H, Wirmum C, Efange S. In-vitro antimicrobial activity of ageratum conyzoides (Linn) on clinical isolates of helicobacter pylori. Afr J Pharm Pharmacol. (2009) 3:585–92.
5. Adjei B. Utilization of traditional herbal medicine and its role in health care delivery in Ghana: the case of wassa amenfi west district: college of art and social sciences. (2013).
6. Mushore J, Matuvhunye MJ. Antibacterial properties of mangifera indica on staphylococcus aureus. Afr J Clin Exp Microbiol. (2013) 14(2):62–74. doi: 10.4314/ajcem.v14i2.4
7. Okaiyeto K, Oguntibeju OO. African herbal medicines: adverse effects and cytotoxic potentials with different therapeutic applications. Int J Environ Res Public Health. (2021) 18(11):5988. doi: 10.3390/ijerph18115988
8. Ediriweera MK, Tennekoon KH, Samarakoon SR. A review on ethnopharmacological applications, pharmacological activities, and bioactive compounds of mangifera indica (Mango). Evidence-Based complementary Altern medicine: eCAM. (2017) 2017:6949835. doi: 10.1155/2017/6949835
9. Torres-León C, Rojas R, Contreras-Esquivel JC, Serna-Cock L, Belmares-Cerda RE, Aguilar CN. Mango seed: functional and nutritional properties. Trends Food Sci Technol. (2016) 55:109–17. doi: 10.1016/j.tifs.2016.06.009
10. Ubwa S, Ishu M, Offem J, Tyohemba R, Igbum GO. Proximate composition and some physical attributes of three mango (Mangifera indica L.) fruit varieties. Int J Agron Agric Res. (2014) 4:21–9.
11. Fowomola MJ. Some nutrients and antinutrients contents of mango (Magnifera indica) seed. Afr J Food Sci. (2010) 4:472–6.
12. Yatnatti S, Vijayalakshmi D, Chandru R. Processing and nutritive value of mango seed kernel flour. Curr Res Nutr Food Sci J. (2014) 2:170–5. doi: 10.12944/CRNFSJ
13. Anwar R, Ahmad S, Rajwana I, Khan A, Memon N-U-N, Nafees M. Phenological growth patterns and floral malformation of mango (Mangifera indica L.) tree under subtropical climate. Pakistan J Agric Sci. (2011) 48:107–13.
14. Sarker A, Amin N, Shimu I, Akhter B, Alam B, Rahman B, et al. Antimicrobial activity of methanolic extract of langra mango pulp. J Pharmacognosy Phytochem. (2017) 6:28–30.
15. Ogidi O, Okore C, Akpan U, Ayebabogha M, Onukwufo C. Evaluation of antimicrobial activity and bioactive phytochemical properties of mango (Mangifera indica) stem-bark extracts. Int J Pharmacognosy. (2021) 8:189–95. doi: 10.13040/IJPSR.0975-8232.IJP.8(5).189-95
16. Okareh OT, Alaiya M, Odeniyi M. Formulation of antiseptic ointments from mangifera indica kernel, leaf and psidium guajava leaf extracts. Trop J Natural Product Res. (2019) 3:307–13. doi: 10.26538/tjnpr/v3i10.2
17. Nayan V, Onteru S, Singh D. Mangifera indica flower extract mediated biogenic green gold nanoparticles: efficient nanocatalyst for reduction of 4-nitrophenol. Environ Prog Sustain Energy. (2017) 37:283–94. doi: 10.1002/ep.12669
18. Ichiki H, Miura T, Kubo M, Ishihara E, Komatsu Y, Tanigawa K, et al. New antidiabetic compounds, mangiferin and its glucoside. Biol Pharm Bull. (1998) 21:1389–90. doi: 10.1248/bpb.21.1389
19. Ma X, Wu H, Liqin Liu LL, Yao Q, Songbiao W, Zhan R, et al. Polyphenolic compounds and antioxidant properties in mango fruits. Scientia Hortic - Sci HORT-AMSTERDAM. (2011) 129:102–7. doi: 10.1016/j.scienta.2011.03.015
20. Biswas T, Sen A, Roy R, Maji S, Maji HS. Isolation of mangiferin from flowering buds of mangifera indica L and its evaluation of in vitro antibacterial activity. Res Reviews: J Pharm Anal. (2015) 4:49–56.
21. Abdelaziz SA. Physico chemical characteristics of mango kernel oil and meal. Middle East J Appl Sci. (2018) 8:01–6.
22. Ballesteros-Vivas D, Álvarez-Rivera G, Morantes SJ, del Pilar Sánchez-Camargo A, Ibáñez E, Parada-Alfonso F, et al. An integrated approach for the valorization of mango seed kernel: efficient extraction solvent selection, phytochemical profiling and antiproliferative activity assessment. Food Res Int. (2019) 126:108616. doi: 10.1016/j.foodres.2019.108616
23. Shehabeldin AM, El-Esawy GS, El-Sanafawy HA. Effect of dietary partial replacement of corn grains by mango seeds on productive and reproductive characterization of damascus goat bucks at prepubertal stage. J Anim Poultry Production. (2021) 12:61–9. doi: 10.21608/jappmu.2021.153289
24. Beyene G, Araya A. Review of mango (Mangifera indica) seed-kernel waste as a diet for poultry. J Biology Agric Healthcare. (2015) 5:156–9.
25. Diarra SS. Potential of mango (Mangifera indica L.) seed kernel as a feed ingredient for poultry: A review. World’s Poultry Sci J. (2014) 70:279–88. doi: 10.1017/S0043933914000294
26. Murray CJL, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet. (2022) 399:629–55. doi: 10.1016/S0140-6736(21)02724-0
27. Laxminarayan R, Duse A, Wattal C, Zaidi AKM, Wertheim HFL, Sumpradit N, et al. Antibiotic resistance—the need for global solutions. Lancet Infect Dis. (2013) 13:1057–98. doi: 10.1016/S1473-3099(13)70318-9
28. AlSheikh HM, Sultan I, Kumar V, Rather IA, Al-Sheikh H, Tasleem Jan A, et al. Plant-based phytochemicals as possible alternative to antibiotics in combating bacterial drug resistance. Antibiotics. (2020) 9(8):480. doi: 10.3390/antibiotics9080480
29. Sadiea RZ, Sultana S, Chaki BM, Islam T, Dash S, Akter S, et al. Phytomedicines to target hepatitis B virus DNA replication: current limitations and future approaches. Int J Mol Sci. (2022) 23(3):1617. doi: 10.3390/ijms23031617
30. Hossain KS, Hossain MG, Moni A, Rahman MM, Rahman UH, Alam M, et al. Prospects of honey in fighting against covid-19: pharmacological insights and therapeutic promises. Heliyon. (2020) 6(12):e05798. doi: 10.1016/j.heliyon.2020.e05798
31. Pereira JAM, Berenguer CV, Câmara JS. Delving into agri-food waste composition for antibacterial phytochemicals. Metabolites. (2023) 13(5):634. doi: 10.3390/metabo13050634
32. Fongang Fotsing Yannick S, Bankeu Kezetas Jean J, Gaber El-Saber B, Iftikhar A, Lenta Ndjakou B. Extraction of bioactive compounds from medicinal plants and herbs. In: Hany AE-S, editor. Natural medicinal plants. IntechOpen, Rijeka (2021).
33. Azmir J, Zaidul ISM, Rahman MM, Sharif KM, Mohamed A, Sahena F, et al. Techniques for extraction of bioactive compounds from plant materials: A review. J Food Eng. (2013) 117:426–36. doi: 10.1016/j.jfoodeng.2013.01.014
34. Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harbor Perspect Biol. (2010) 2:a000414. doi: 10.1101/cshperspect.a000414
35. Breijyeh Z, Jubeh B, Karaman R. Resistance of gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules (Basel Switzerland). (2020) 25(6):1340. doi: 10.3390/molecules25061340
36. Kaur J, Rathinam X, Kasi M, Leng KM, Ayyalu R, Kathiresan S, et al. Preliminary investigation on the antibacterial activity of mango (Mangifera indica L: anacardiaceae) seed kernel. Asian Pacific J Trop Med. (2010) 3:707–10. doi: 10.1016/S1995-7645(10)60170-8
37. Prastiyanto M, Darmawati SRI, Mukaromah A. Antibacterial activity of seed kernel extracts of seven mangoes (Mangifera indica) cultivars native to Indonesia against mdr-pseudomonas aeruginosa isolated from wounds. Biodiversitas J Biol Diversity. (2022) 23:5629–37. doi: 10.13057/biodiv/d231112
38. Seghosime A, Ebeigbe A, Awudza J. Potential use of mangifera indica seed kernel and citrus aurantiifolia seed in water disinfection. Nigerian J Technol. (2017) 36(4):1303–10. doi: 10.4314/njt.v36i4.41
39. Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. (1999) 12:564–82. doi: 10.1128/cmr.12.4.564
40. Bose S, Datta R, Kirlin WG. Toxicity studies related to medicinal plants. In: Mandal SC, Chakraborty R, Sen S, editors. Evidence based validation of traditional medicines: A comprehensive approach. Springer Singapore, Singapore (2021). p. 621–47.
41. Nwachukwu K, Ibe C. Hematological, biochemical and histopathological assessment of the toxicity potential of seed kernel extracts of mangifera indica linn. Varieties Mice. Internet Arch. (2022) 58(322):1117–25.
42. Younus MI, Sabuj AAM, Haque ZF, Sayem SM, Majumder S, Parvin MS, et al. Microbial risk assessment of ready-to-eat mixed vegetable salads from different restaurants of Bangladesh agricultural university campus. J advanced veterinary Anim Res. (2020) 7:34–41. doi: 10.5455/javar.2020.g390
43. Sabuj A, Haque Z, Barua N, Islam M, Saha S. Assessment of bacteriological quality of street vended fast foods and their antimicrobial resistance. Int J Curr Microbiol Appl Sci. (2018) 7:3059–. doi: 10.20546/ijcmas.2018.711.350
Keywords: mango (Mangifera indica), seed kernel, ethanol extraction, antimicrobial activities, AMR
Citation: Sadiea RZ, Mozumder A, Mou MJ, Hasan SMN, Sikder C, Akter S, Saha BK, Saha S, Xue M, Wang H, Zheng C and Hossain MG (2024) Evaluation of the antibacterial potential of mango (Mangifera indica) seed kernels in Bangladesh. Front. Trop. Dis 5:1473494. doi: 10.3389/fitd.2024.1473494
Received: 31 July 2024; Accepted: 14 October 2024;
Published: 30 October 2024.
Edited by:
Megha Raj Banjara, Tribhuvan University, NepalReviewed by:
Nishant Kumar, National Institute of Food Technology Entrepreneurship and Management, IndiaSupriya Kumari Sharma, Banasthali University, India
Copyright © 2024 Sadiea, Mozumder, Mou, Hasan, Sikder, Akter, Saha, Saha, Xue, Wang, Zheng and Hossain. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mengzhou Xue, eHVlbWVuZ3pob3VAenp1LmVkdS5jbg==; Huiqing Wang, d2FuZ2h1aXFpbmdAc2N1LmVkdS5jbg==; Chunfu Zheng, emhlbmcuYWxhbkBob3RtYWlsLmNvbQ==; Md. Golzar Hossain, bWdob3NzYWluQGJhdS5lZHUuYmQ=
 Rahila Zannat Sadiea1
Rahila Zannat Sadiea1 Anandha Mozumder
Anandha Mozumder Sharmin Akter
Sharmin Akter Biplob Kumar Saha
Biplob Kumar Saha Mengzhou Xue
Mengzhou Xue Chunfu Zheng
Chunfu Zheng Md. Golzar Hossain
Md. Golzar Hossain