- 1Harvard Medical School, Boston, MA, United States
- 2African Center for Community Investment in Health, Nginyang, Baringo County, Kenya
- 3University of Florida College of Medicine, Gainesville, FL, United States
- 4College of Science, Northeastern University, Boston, MA, United States
- 5Ministry of Health, Lusaka, Zambia
- 6Chreso University, Lusaka, Zambia
- 7Department of Research and Innovation, Terrelle University, Lusaka, Zambia
- 8Department of Behavioral Neuroscience, College of Science, Northeastern University, Boston, MA, United States
- 9Department of Biology, Northeastern University, College of Science, Boston, MA, United States
- 10Department of Research & Instruction, Northeastern University Library, Northeastern University, Boston, MA, United States
- 11Department of Cultures, Societies and Global Studies, College of Social Sciences and Humanities, Integrated Initiative for Global Health, Northeastern University, Boston, MA, United States
- 12Department of Global and Public Health, University of Nairobi, Nairobi, Kenya
- 13Nigerian Institute of Medical Research, Federal Ministry of Health, Lagos, Nigeria
Lymphatic filariasis (LF) is a neglected tropical disease caused by microfilariae of the Wuchereria and Brugia genus and spread by mosquitoes. Chronic infection and associated morbidity leads to poor psychosocial and economic outcomes. LF elimination programs have been underway in many countries, including Zambia, a country in Sub-Saharan Africa where LF has long been endemic. Zambia has made great progress in moving towards elimination through a multipronged strategy involving mass drug administration, regular surveillance, and morbidity management and disease prevention. This scoping review aims to capture the breadth of literature published on LF in Zambia to support further research into the disease that may support ongoing elimination efforts, research gaps, and funding opportunities. PRISMA-ScR and JBI scoping review guidelines were used in the design and conduct of this scoping review, leading to 475 full-text articles screened and included in data analysis, with analyzed information including publication year, journal, study theme, study type, citation number, and funding sources. The resulting screen found many articles focused on disease epidemiology in Zambia, but less research on vectors, treatment and prevention. Therefore, we conclude there may be opportunities to better understand this disease in the Zambian context through filling in these research gaps.
Systematic review registration: https://doi.org/10.17605/OSF.IO/7W62G
Introduction
In 1997, lymphatic filariasis (LF) became one of a handful of neglected tropical diseases (NTDs) targeted for elimination (1, 2). The Global Programme to End LF (GPELF) was initiated in 2000 with the goal of eliminating LF by 2020. While significant progress has been made over the subsequent two decades, in 2019 over 50 million people were infected with LF among the 793 million people still at risk within 72 endemic countries (3–5). Elimination is achieved and validated through a series of steps, including mapping surveys, mass drug administration (MDA), and transmission assessment surveys (TAS) (5). Bangladesh and Lao People’s Democratic Republic most recently achieved elimination in 2023 following completion of multiple rounds of MDA and confirmation that the disease prevalence is below transmission thresholds (6, 7). Globally, LF causes the loss of an estimated 1.6 million disability adjusted life years (DALYs) (8).
LF is spread when Anopheles and Culex mosquitoes infected with Wuchereria bancrofti, Brugia malayi, or B. timori filariae bite a human (9). During this blood meal, the infected mosquitoes introduce third-stage filarial larvae into the human host’s skin via the bite site. These larvae ultimately develop into adults that reside in the lymphatic system, and themselves produce sheathed microfilariae that enter the peripheral circulation. A mosquito biting an infected human host then ingests the microfilariae during a blood meal, that then matures to become an L3 larvae in the mosquito and is transmitted to another human during that mosquito’s next blood meal (10). The migration of filariae to the host’s lymphatic vessels results in dysfunction that can present clinically with lymphedema and hydrocele (11). Acute attacks, also referred to as secondary bacterial infections, may also occur from insufficient daily hygiene practices related to limb washing and drying and ultimately drive disease progression (12, 13). The clinical consequences of infection can be stigmatizing, resulting in social, psychological, and economic consequences beyond the health effects (14). Treatment consists of a package of morbidity management and disability prevention, which consists of antimicrobial agents for the primary filarial infection and secondary acute adenolymphangitis, topical treatments and hygiene for lymphedema, and surgical care for hydrocele (15). Antifilarial agents are selected based on co-endemicity of onchocerciasis and loiasis. In locales where onchocerciasis and loiasis are not endemic, triple therapy consisting of ivermectin, diethylcarbamazine (DEC), and albendazole is recommended (16). This triple therapy has been shown to be effective in clearing microfilariae from the blood stream (17). Where onchocerciasis is present, DEC is contraindicated due to a severe inflammatory effect, called the Mazzotti reaction (18). Ivermectin is associated with severe encephalopathy in patients with high serum Loa loa burden as is therefore avoided in regions known to be endemic with loiasis (19).
LF imparts a significant burden of disease in Africa, where the disease remains endemic in 34 countries placing an estimated 288 million people at risk of infection (5). A study estimated the prevalence of LF in Africa to be 10 million in 2018, down from 74 million in 2000 (3) While likely underestimates, over 160 thousand lymphedema patients and over 147 thousand hydrocele patients were reported to the World Health Organization (WHO) by endemic countries in Africa in 2022 (5). While almost all endemic countries have made progress towards reducing the burden of LF, Malawi and Togo were the first in Sub-Saharan Africa (SSA) to be validated as having eliminated the disease (5). Several other countries, including Zambia, have completed the necessary number of rounds of MDA, and have transitioned into an impact survey stage to assess for any evidence of ongoing disease transmission.
Zambia, a landlocked country in SSA, has made significant progress towards reducing the burden of LF (20). The country is made up of 10 provinces and 116 districts and is home to over 19 million people (21, 22). LF is endemic in 96 of those 116 districts (23). LF in Zambia was first noted in the literature by Buckley in 1946 (24), while the first definitive locally transmitted cases were described by Hira in the 1970s (25, 26). In 2003, following the establishment of the GPELF, the government of Zambia launched a program to eliminate LF. Epidemiological mapping of the disease was completed in 2011, which found an average prevalence of 7.4% across the country (27). Five rounds of MDA have since been conducted in Zambia, and despite funding challenges, the country now awaits the results of TAS to determine if MDAs are no longer needed (5). Both the challenges and successes along the road to elimination may be useful to inform similar efforts in neighboring countries (20). As Zambia steadily moves from active MDA towards disease surveillance and, eventually, elimination, we conducted a scoping review of the literature on LF in Zambia to identify general research gaps as to guide further research and elimination efforts.
Methods
Article screening
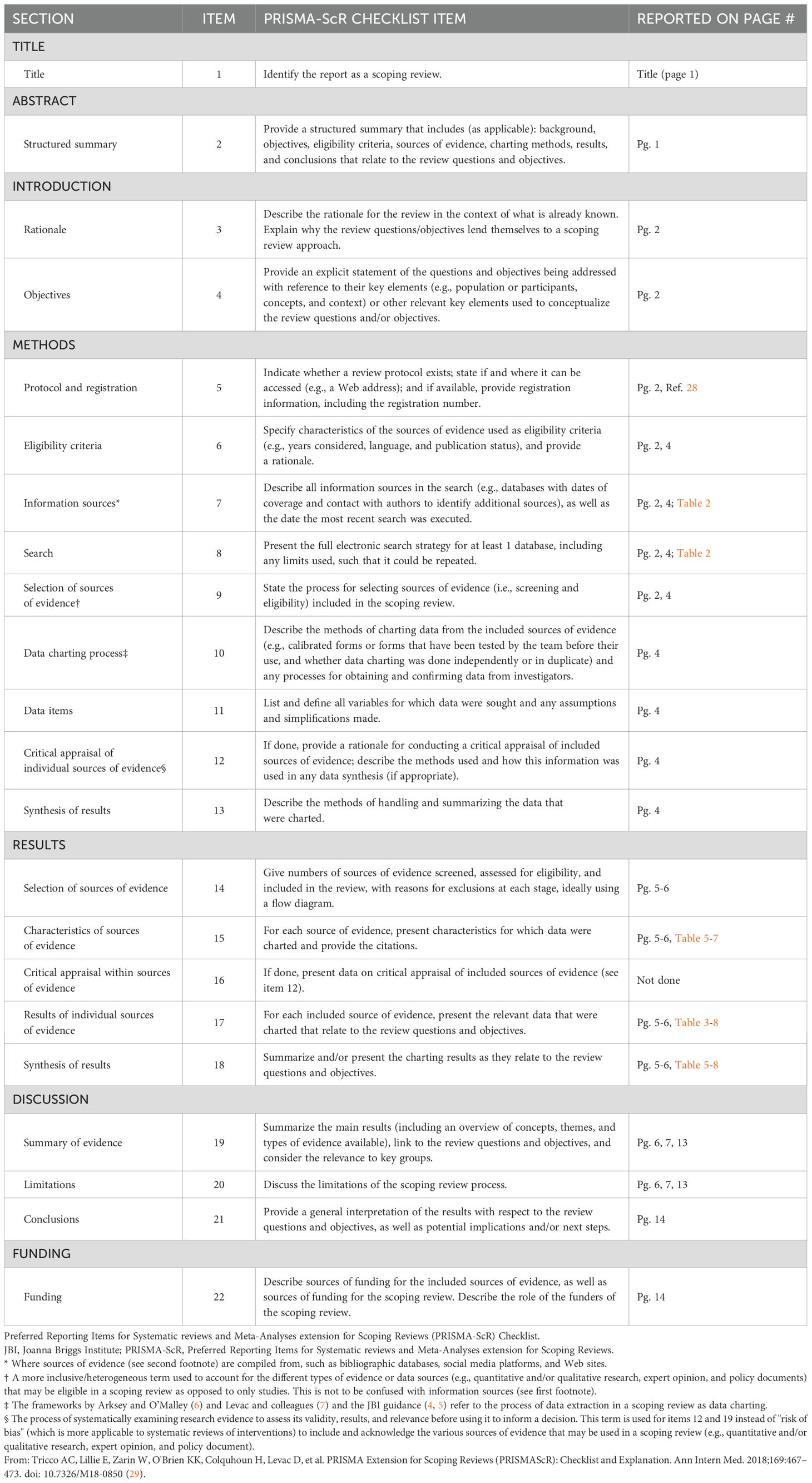
We conducted a scoping review of the existing literature on LF in Zambia. The full protocol is published here (28). PRISMA-Scoping Review (ScR) and Joanna Briggs Institute (JBI) scoping review guidelines were used in the design and conduct of this scoping review (29–31) The PRISMA Scoping Review checklist can be found in Table 1. In brief, a search was conducted with the goal of collecting articles that may include a discussion of LF in Zambia. The search strings used for each database are detailed in Table 2. Following de-duplication, 6363 primary records were uploaded into Rayyan (32). Primary and secondary inclusion criteria and exclusion criteria were formulated and are described below. The overall screening strategy across title-abstract and full-text screens is visualized in Figure 1.
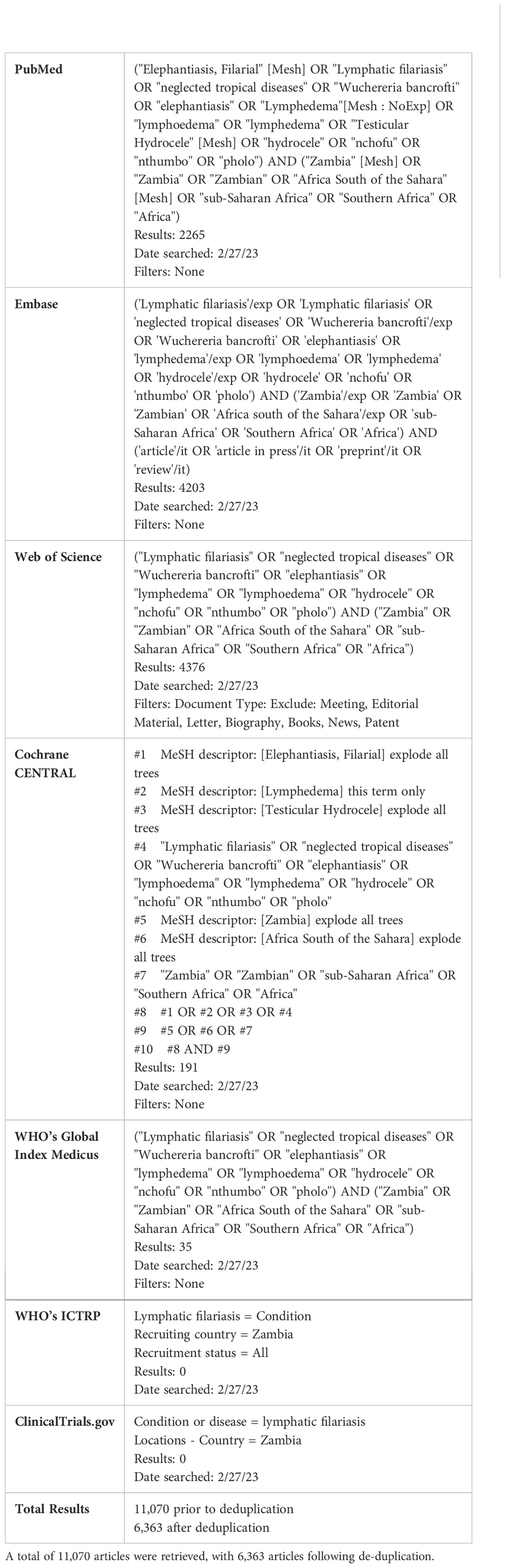
Table 2. Search strings, results, date of search and filters used for 7 databases for article retrieval.
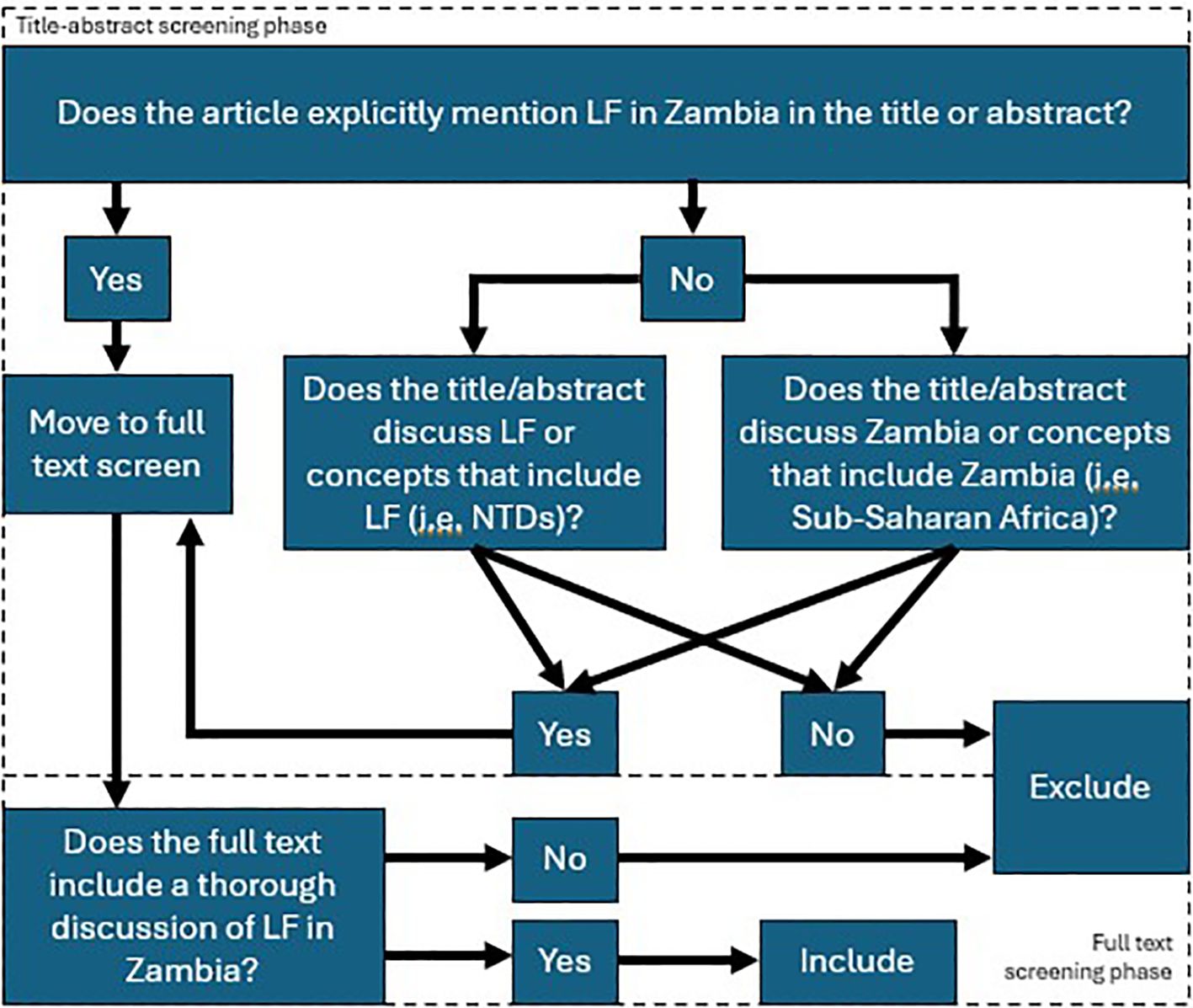
Figure 1. Screening strategy employed following article retrieval. The top half shows the title-abstract screening strategy, whereby two independent reviewers determined if the article explicitly mentioned lymphatic filariasis and Zambia together, or separately. Articles that emphasized categories that include lymphatic filariasis, such as the neglected tropical diseases, or Zambia, such as Sub-Saharan Africa, where screened in at this step. Otherwise, articles were excluded. In the full text screen, articles were screened in if they included a thorough discussion of lymphatic filariasis in the Zambian context, where thorough was operationalized with respect to LF to mean “containing LF-related discussion or data, including incidence, prevalence, GIS data, etc.” and with respect to Zambia to mean “containing discussion or data that is specific to Zambia or includes Zambia among a set of data or discussions of multiple countries/contexts”.
Prior to initiation of the screening phase, a test batch of 20 articles was used to train all screeners. Screening was not initiated until reviewers achieved >90% concordance with the results of a senior author (HS) on the same set of 20 articles. The title-abstract screen was conducted by 8 reviewers (HS, AO, DH, KC, AB, KS, JL, ST). Each article was analyzed by two independent reviewers, and a third senior author (HS, AO) resolved any disagreements. Articles were screened-in if they explicitly mention lymphatic filariasis in the Zambian context in their title or abstract or if they mentioned categories of which LF or Zambia were a part (i.e. NTDs, Sub-Saharan Africa, etc.) and there was a reasonable possibility that the full text would include a discussion of LF in Zambia, specifically. Articles were excluded if not in English.
A total of 513 articles were screened in from the title-abstract phase while 5850 articles were excluded. Of those 513, the full text of 38 articles was unretrievable. The remaining 475 full text articles were then screened by 6 reviewers (HS, AO, DH, AB, JL, ST). At this stage, articles were included in the next stage of analysis if their full text included a thorough discussion of LF in Zambia. Thorough was operationalized for LF to mean “containing LF-related discussion or data, including incidence, prevalence, GIS data, etc.” Thorough was operationalized for Zambia to mean “containing discussion or data that is specific to Zambia or includes Zambia among a set of data or discussions of multiple countries/contexts”. Additionally, included articles must discuss LF in Zambia specifically; separate thorough discussions of each subject were not sufficient. Exclusion criteria included articles that were unretrievable, were not published in peer-reviewed journals or by government and international regulatory bodies, were published as part of conference proceedings or poster abstracts or were published as part of a book. Reference lists from screened-in articles were reviewed and cross-referenced with the list of articles acquired from the screening process when the title was suggestive of possible relevance. Articles that were absent from the list of screened articles but were deemed possibly relevant were screened using the same criteria for possible inclusion in the final set of articles. Expert opinion was sought on the inclusion of official government literature, as many of these articles are not indexed in searchable databases.
For relevant articles that have been published as part of a series of regular updates, for example the annual Global Programme to Eliminate LF (GPELF) updates published in the Bulletin of the WHO, only the most recent was included to avoid diluting results with similar or equivalent articles.
Data extraction
Article information, including publication year, journal, study theme, study type, citation number, and funding sources, was extracted into Microsoft Excel for further analysis. Study theme describes the article’s core focus as it relates to LF, for example, LF epidemiology or LF health policy. Study type describes the methodology used to carry out the study, such as secondary or clinical research. The study themes included general epidemiology, diagnostics, treatment, health systems/policy, vectors, and co-infections, as well as a general topics category aimed at articles that touch on multiple themes equally or that focused on themes that were not well captured by the existing framework. Study types included clinical research, epidemiological research, secondary research, and institutional or government reports. A codebook was adapted from Grifferty et al. to capture the expected study themes and types relevant to LF research (33). General epidemiology was defined as “studies that aimed to describe the prevalence, distribution, or other epidemiological indicators of disease.” The study type, epidemiological research, was defined as “studies that collected and/or independently analyzed epidemiological data.” Two authors (HS, AO) independently assigned labels to each article then collaboratively reviewed and resolved any disagreements. These data were analyzed by two authors (HS, GG) to generate summary statistics and data visualizations. Citation numbers were drawn from Google Scholar citation estimates.
Results
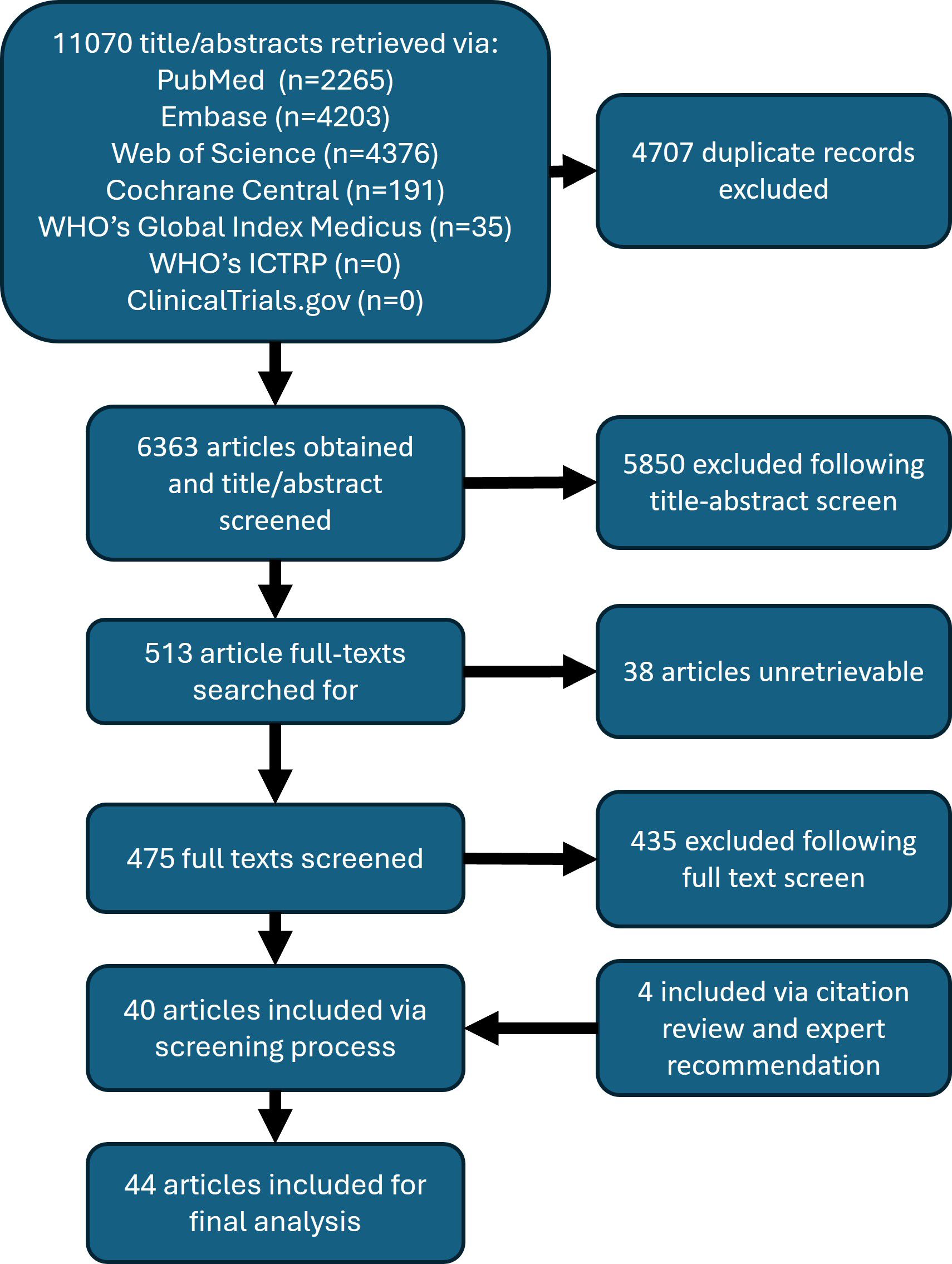
From an initial search that obtained over 6,000 unique articles, the yield following the screening process was approximately 0.6%, or 40 articles. An additional 4 articles were included for analysis, 2 government documents and 2 screened-in article references, for a total of 44 articles. Figure 2 shows the PRISMA flow diagram of this scoping review, which describes the number of articles at each step of the review process (34). The final article list is included in Table 3.
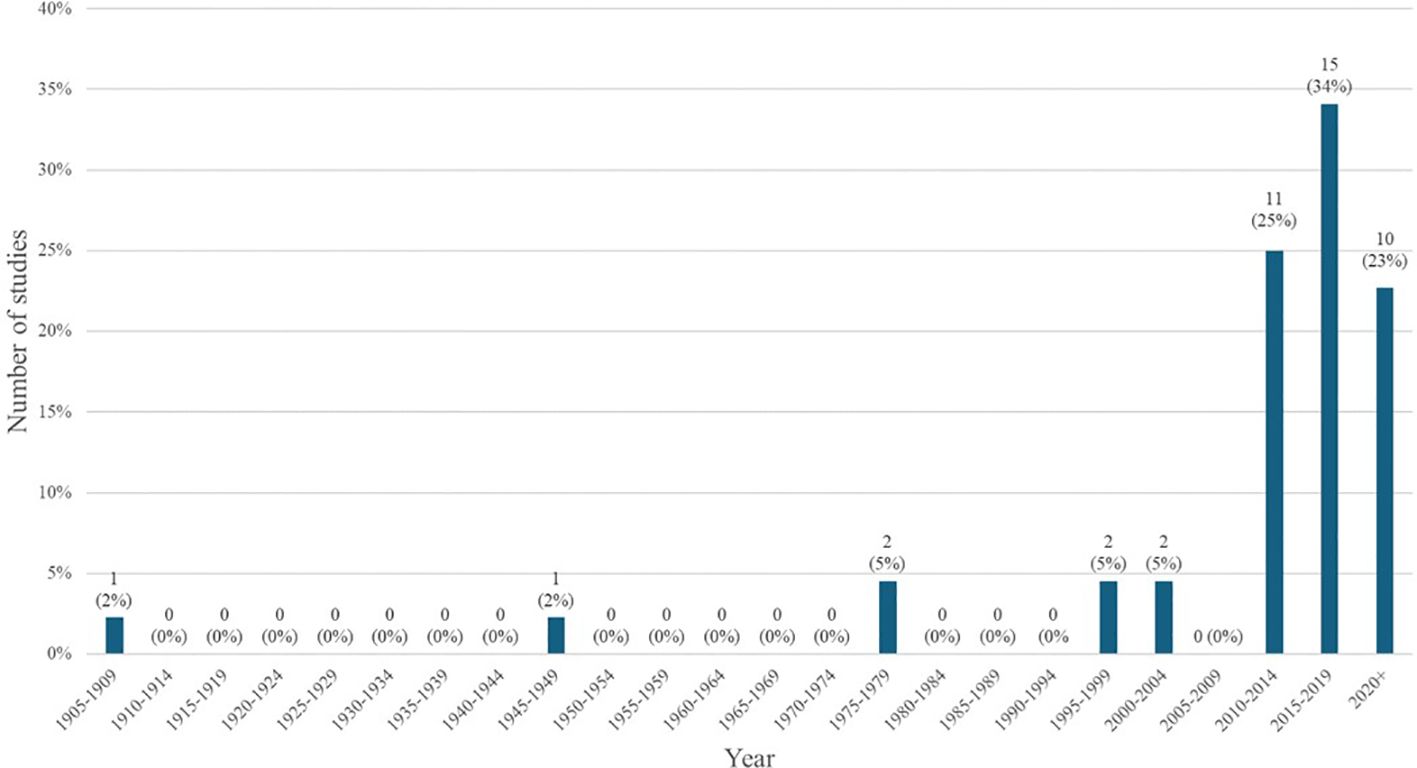
Generally, there has been a slight increase in the number of articles published about LF in Zambia over time; with most articles being published after 2010 and relatively few before that point. Figure 3 depicts the number and proportion of articles published per half decade, with the greatest share being published in the period between 2015-2019.
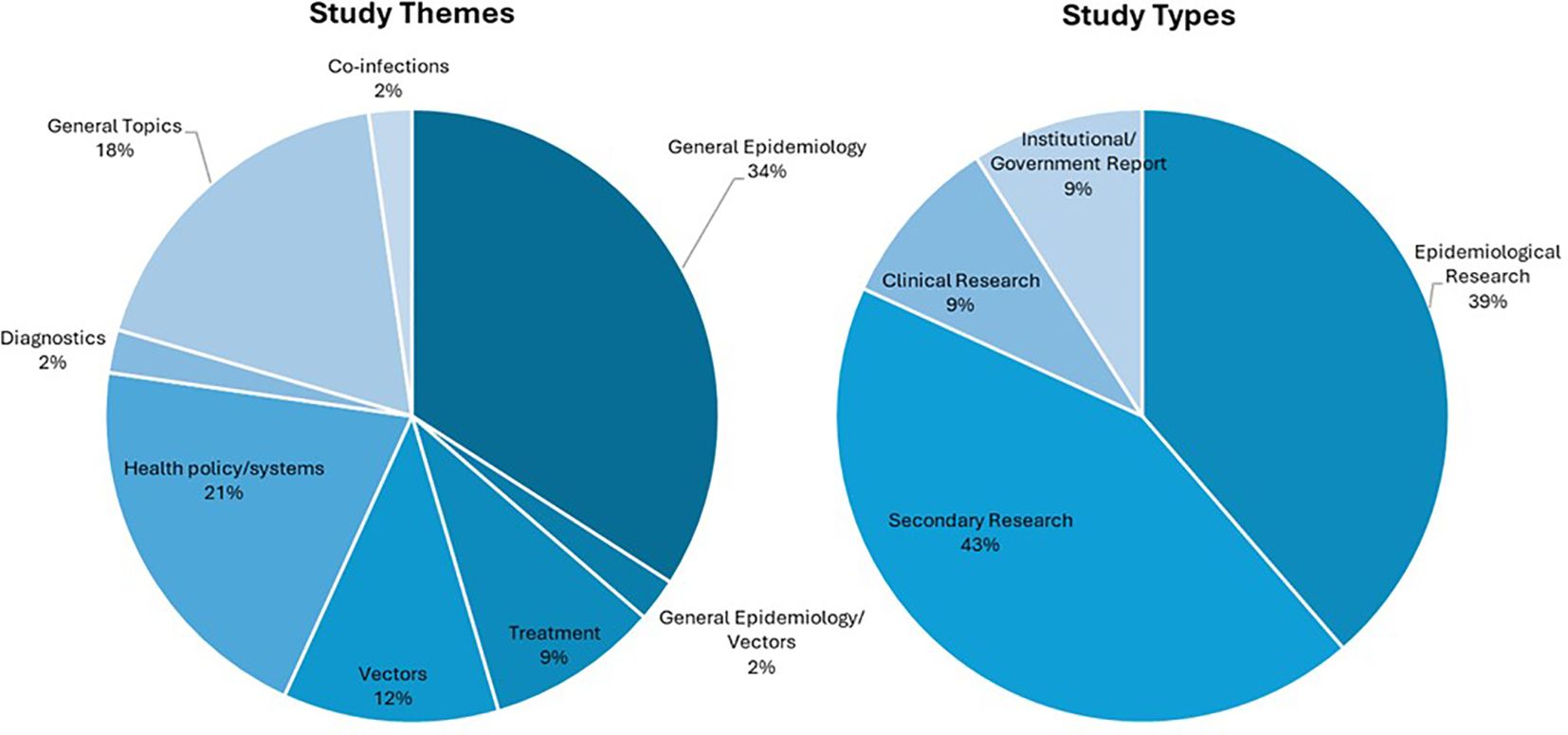
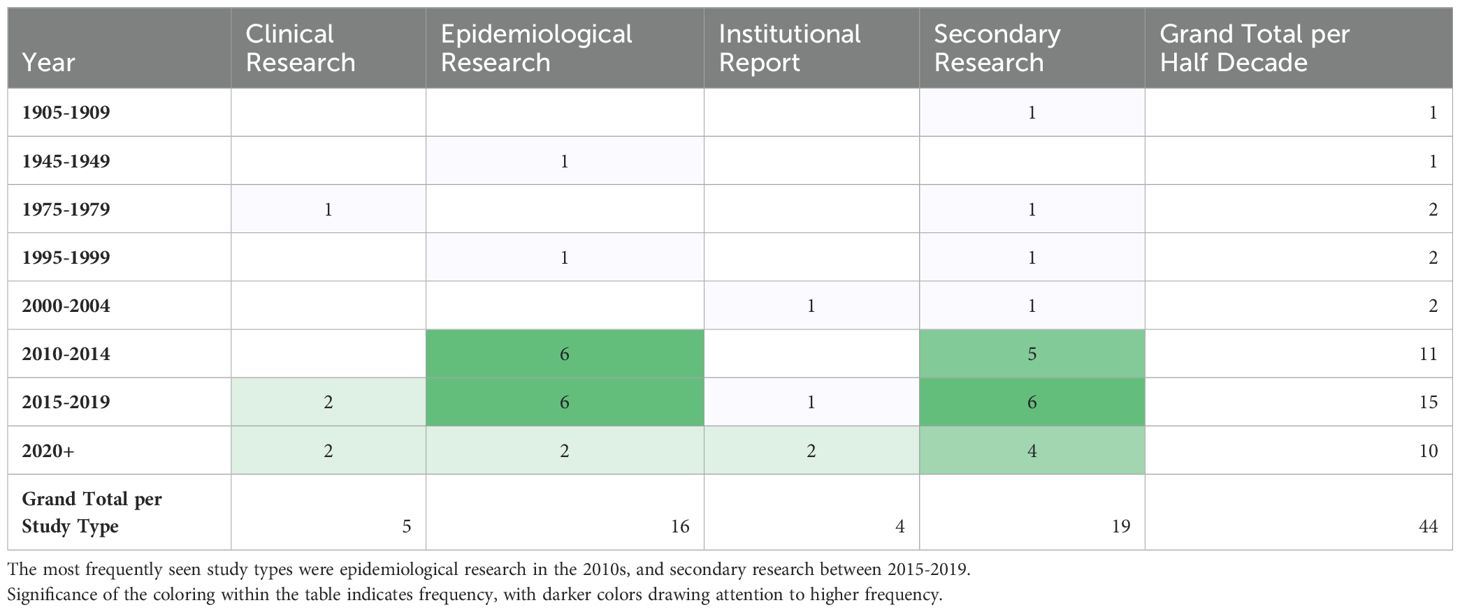
The most frequently noted study theme was general epidemiology (n = 16; 37%) followed by health policy/systems (n = 9; 21%) (Figure 4). The next most common study themes were general topics (n =8; 18%), vectors (n = 5;11%), and diagnostics (n = 4; 9%), respectively. There was limited research coverage of co-infections with LF and diagnostics in Zambia, each with only one article, 2% of the total number of articles. There were no articles focused on disease prevention or pathophysiology. The most frequent study type was secondary research (n = 19; 43%) followed closely by epidemiological research (n = 17; 39%). There were relatively few clinical research articles published on LF in Zambia (n=4; 9%). There were no basic science articles found during this review. One article by Shawa et al., 2013 was found to fit into two thematic categories, general epidemiology, and vectors. Researchers took community serum samples to assess anti-filarial antibody and filarial antigen seropositivity and conduct vector assessments with light traps. On reviewer discussion, we determined that this paper would be given its own mixed category of general epidemiology/vectors, rather than be double counted in each category. Table 4 depicts the study theme per half decade, highlighting trends in article focus over time. Study theme by study type and study type by decade can be found in Tables 5, 6, respectively, and in addition to Figure 4.
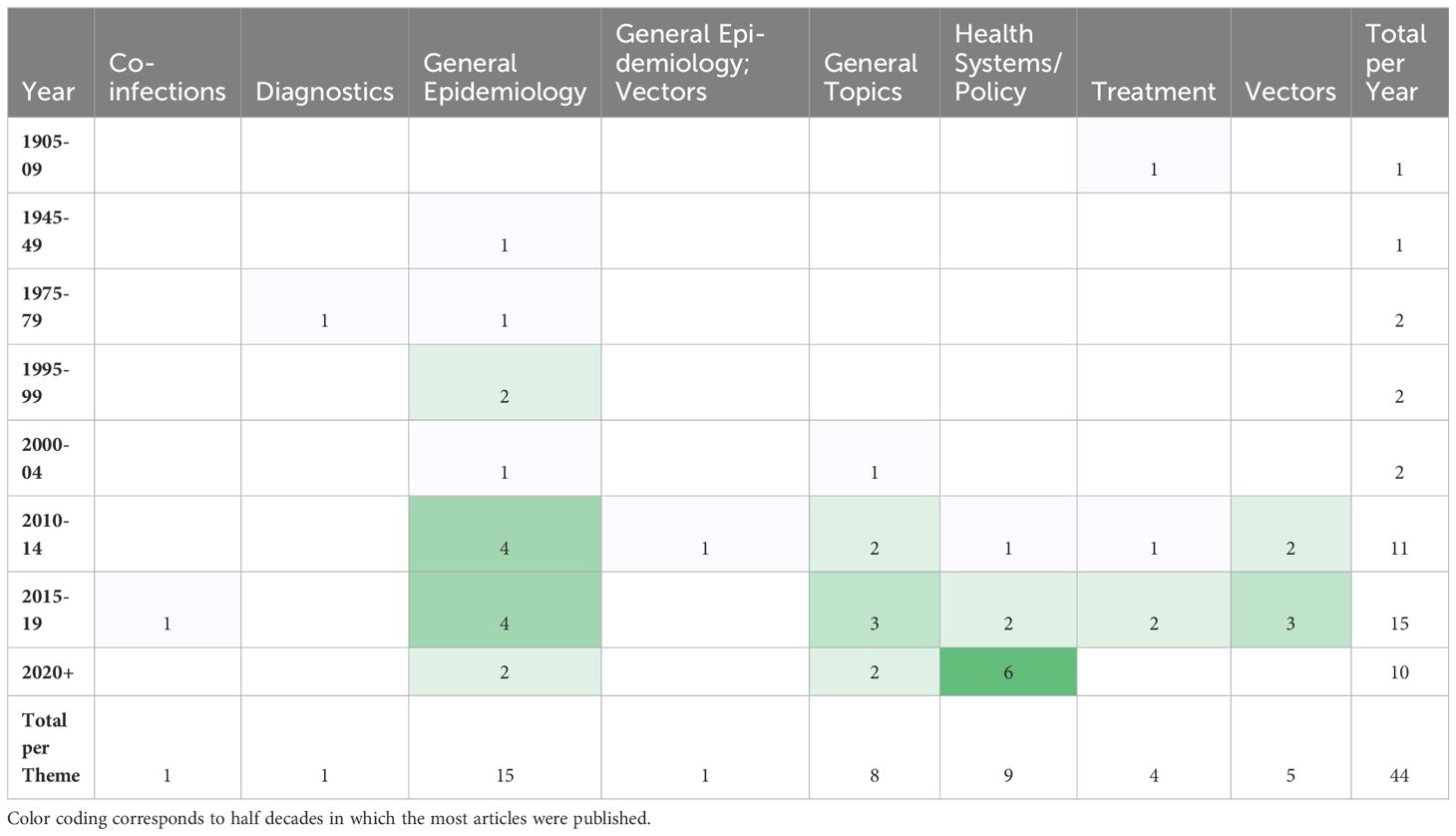
Table 4. Number of articles in each study theme by half decade, excluding half decades in which no articles were published.
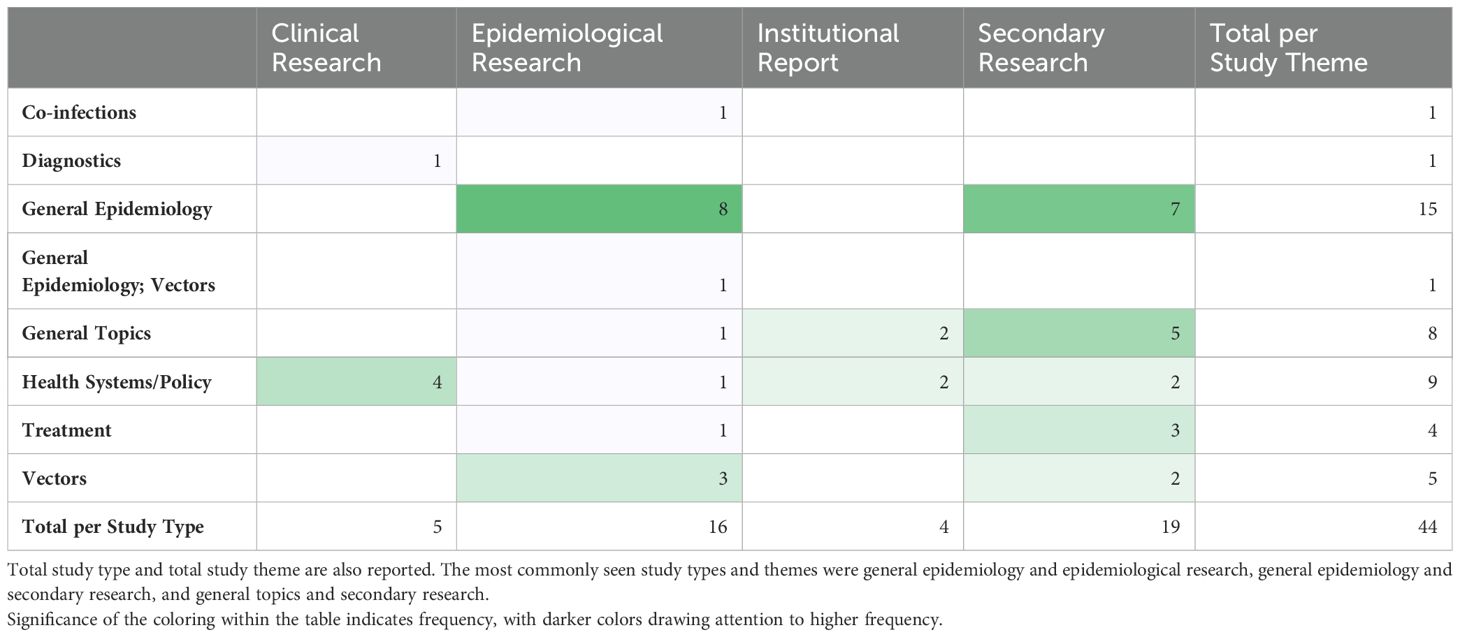
Table 6. Study theme by study type, color gradient indicates most common intersection between theme and type.
The full list of funding organizations acknowledged by articles are detailed in Table 7. The Bill and Melinda Gates Foundation provided funding to the greatest number of articles (n = 9; 20%). The Department of International Development, United Kingdom, and the Wellcome Trust contributed to the second greatest number of articles, at 4 each. No funders were reported in 16 articles, which is the largest collection of articles included for final analysis. In total, 24 unique funding organizations were identified across 44 articles.
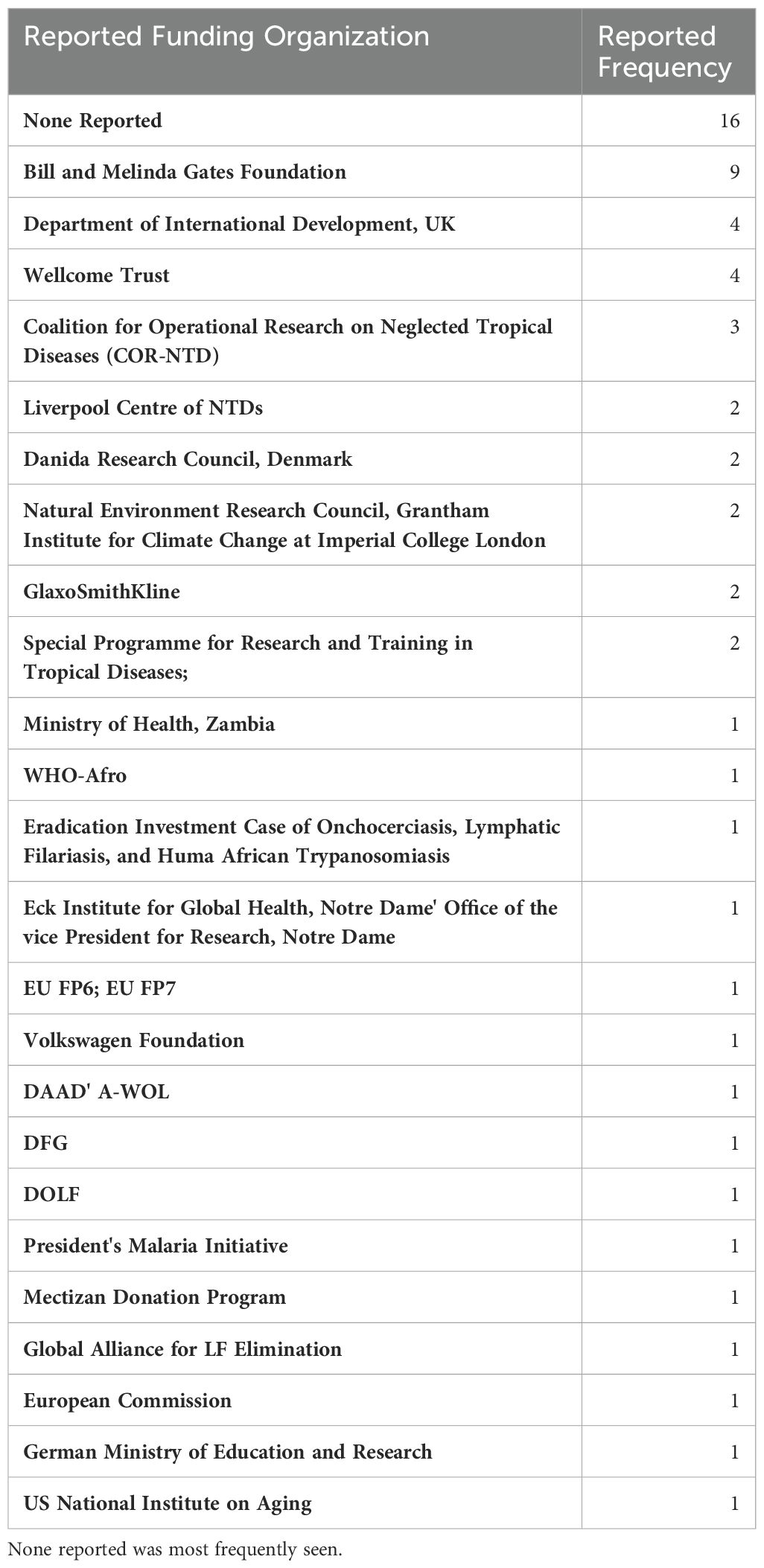
Table 7. List of organizations and donors acknowledged by articles as providing support in order from most frequently reported.
In reviewing the most cited articles that include a thorough discussion of LF in Zambia, which are detailed in Table 8, the 2015 Global Burden of Disease Study has the greatest number of citing articles at 5221. This is followed by “Re-assessing the global prevalence and distribution of lymphatic filariasis,” at 637. The most cited article that focuses exclusively on LF in Zambia was “Mapping the geographical distribution of lymphatic filariasis in Zambia,” at 29 citing articles, and was the 26th most cited article included in the analysis. Articles were published in 26 unique peer-reviewed journals. Only 6 journals published more than one article on LF in Zambia. The journals in which articles were most frequently published were PLoS NTD (n = 7; 16%), Parasites & Vectors (n = 5; 11%), and Trends in Parasitology (n = 3; 6.8%).
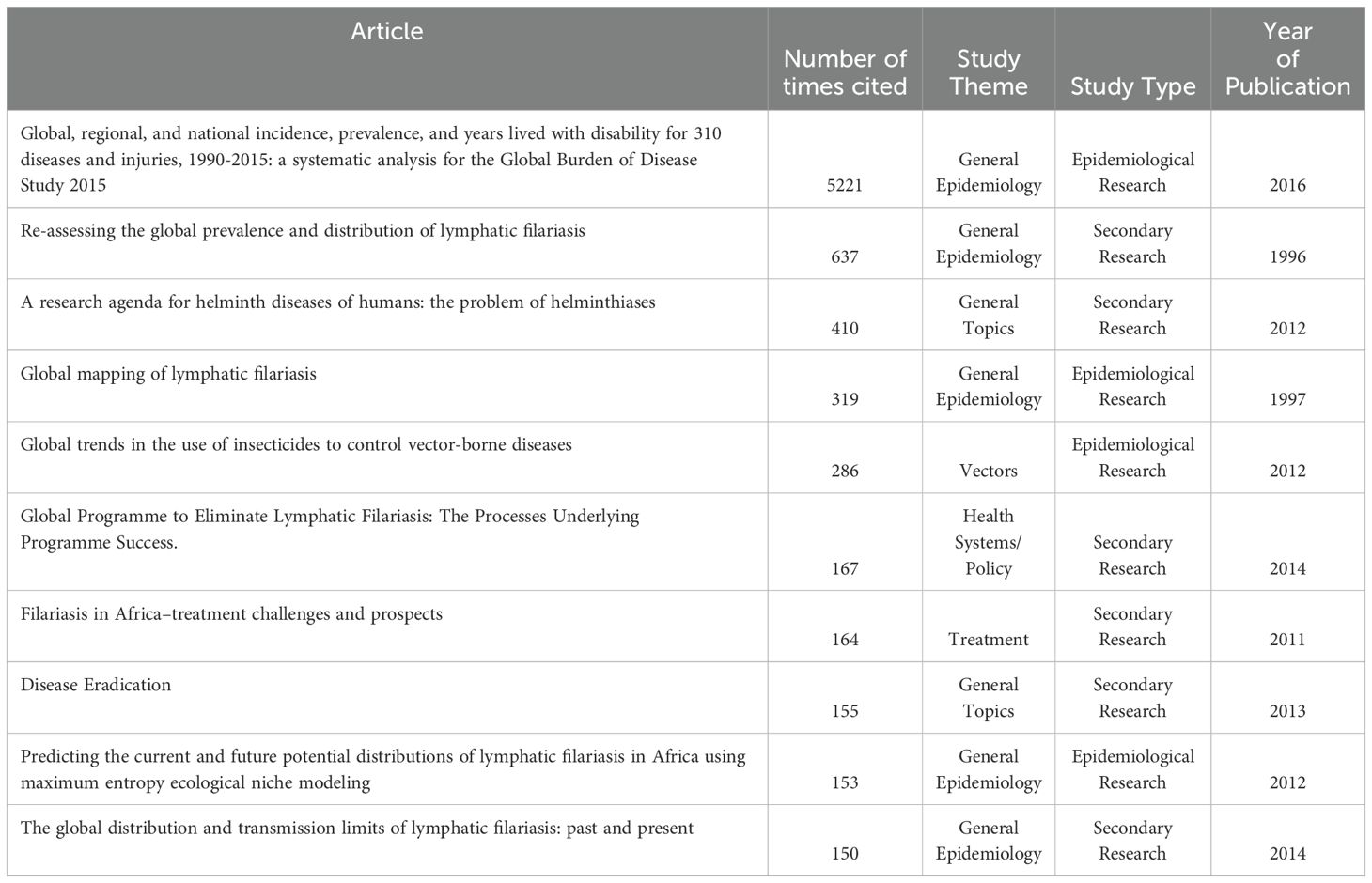
Table 8. Article title, number of citations as indexed by Google Scholar, study theme, type and year of publication for the 10 most highly cited articles found in this scoping review.
Discussion
This article is, to our knowledge, the first scoping review of LF in Zambia, or of LF research generally. Our goal was to capture and describe the body of literature at this intersection as completely as possible. We found that most studies focused on the epidemiology of LF as a theme and used secondary research methodologies, such as systematic reviews. There were no articles that fit the study themes of prevention or pathophysiology or the study type of basic science research. The absence of prevention focused articles was especially surprising, and potentially a reflection of limitations inherent in this study. Prevention as a research focus may be captured by those articles that were labeled as vector studies, given research into host-vector interactions may be targeted at preventing future infections.
A significant number of studies were published with maps of the distribution and prevalence of LF globally or in Africa, which included information on Zambia, but beyond these maps, did not further conversation around LF in Zambia specifically. These studies were included in our analysis as they met our inclusion criteria and definition for a ‘thorough’ discussion of LF in Zambia. The greatest number of studies were published in the 2010s, which corresponds to the completion of the initial LF mapping studies conducted prior to the initiation of MDAs (27). Prior to this, there was relatively little knowledge about the disease in Zambia published in the literature. Many of the subsequent epidemiological studies draw from the paper published by Mwase et al. which outlines the results of those initial surveys (27). Those papers that referenced epidemiological information about LF in Zambia but did not perform a primary analysis of previously collected epidemiological data, were considered secondary research for the purposes of this scoping review. There were 8 articles fitting this description. There is another subset of 9 articles that gathered primary epidemiological data or independently analyzed existing data, for example (35, 36). Information about the epidemiology of LF in Zambia is helpful in determining where MDAs are necessary and continued monitoring needs to occur.
There were relatively few studies that focused on the vectors of LF in Zambia. However, the Anopheles mosquito that spreads the disease similarly spreads malaria. There were a number of studies screened out that focused on the vectors of malaria, but made no explicit mention of lymphatic filariasis, while a much smaller number did reference their co-endemicity in the region (37). Malaria researchers may consider the impact of their work on Anopheles mosquitos on LF, thereby allowing the scientific community to better understand how to control both diseases, perhaps even synergistically.
There was only one study that focused on co-infections with LF (38). Given LF co-endemicity has important implications for selecting therapeutics and that infrastructure needed to deploy LF programming could be coopted for other disease target, further research into co-endemic infections could be useful in Zambia. Additionally, there were no basic science studies or articles that focused on the pathophysiology of lymphatic filariasis in Zambia, however there’s no clear reason that research into LF pathophysiology conducted in Zambia specifically would yield novel results. Articles that specifically focused on Zambia had relatively few citations, suggesting the limited impact of the field, while those studies that had a broader focus than specifically LF or Zambia on their own, such as the 2015 Global Burden of Disease study, had significantly more citations, the vast majority likely from papers that are unrelated to this study’s focus. Given our inclusive approach, we retained these broadly encompassing articles. Most articles were concentrated around a select few journals that had scopes that focused on either NTDs or parasitology.
There are several limitations in the design and conduct of this study. There were several older studies, for example those by Hira (25, 26), that were not included in the analysis despite their significant contribution to the understanding of LF in Zambia, as the full text for these articles could not be located and thus could not be carefully analyzed. It is possible that articles that should have been included in this scoping review were missed. However, we attempted to mitigate this by being as inclusive as possible during the title-abstract screening process to ensure that any article that may have reasonably contained a thorough discussion of LF in Zambia was included in the full-text screen. Additionally, efforts were made to ensure that screeners were properly trained and had achieved a high level of inter-reviewer concordance prior to the initiation of the title-abstract screening phase. While we performed a review of the relevant government and institutional literature that discusses LF in Zambia, these articles are often less readily accessible than those published in peer-reviewed journals, thus we may have missed some of these articles during our search.
Interestingly, the most prevalent funding status for published articles was ‘None reported’. Some articles explicitly stated that no funding was obtained for the purposes of that research, while others made no mention of funding in any explicit way. These were described the same way in this study. This suggests that much of the work that has been done on LF in Zambia has been unfunded and serves to highlight the neglected status of this neglected tropical disease.
Conclusions
Global efforts to eliminate LF are making progress worldwide. In Zambia, following 5 rounds of MDA, the country is awaiting verification that no further rounds are necessary. On this backdrop, we conducted a scoping review of the literature on LF in Zambia to describe and contextualize the existing body of knowledge. Through an analysis of 44 records that discuss LF in Zambia, we found that the most frequent articles focus was on the epidemiology of LF, while the most frequently used methodology was secondary research. Consolidating the body of knowledge on LF in Zambia may be helpful in several ways, including to support the efforts other countries that aim to follow the same path Zambia has taken toward elimination and to guide future research efforts in Zambia through the identification of potential gaps. Those gaps include further research into the vectors of LF and co-infections, given the Anopheles mosquito that spreads the disease is also a principal vector for malaria.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
HS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. AO: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. DH: Data curation, Writing – original draft, Writing – review & editing. KC: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing. AB: Data curation, Writing – review & editing. KS: Data curation, Writing – review & editing. JL: Data curation, Writing – review & editing. ST: Data curation, Writing – review & editing. GG: Formal analysis, Methodology, Visualization, Writing – review & editing. PC: Data curation, Methodology, Project administration, Software, Supervision, Writing – review & editing. RW: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to acknowledge the institutional support of the College of Social Sciences and Humanities at Northeastern University and the support of the Northeastern University Library.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ending the neglect to attain the Sustainable Development Goals: a road map for neglected tropical diseases 2021–2030. Geneva: World Health Organization (2020). Licence: CC BY-NC-SA 3.0 IGO.
2. Elimination of lymphatic filariasis as a public health problem. World Health Organization (1997). Elimination of lymphatic filariasis as a public health problem. World Health Organization. Available online at: https://iris.who.int/handle/10665/179773
3. Cromwell EA, Schmidt CA, Kwong KT, Pigott DM, Mupfasoni D, Biswas G, et al. The global distribution of lymphatic filariasis, 2000–18: a geospatial analysis. Lancet Global Health. (2020) 8:e1186–e94. doi: 10.1016/S2214-109X(20)30286-2
4. Progress report 2000-2009 and strategic plan 2010-2020 of the global programme to eliminate lymphatic filariasis: halfway towards eliminating lymphatic filariasis. Geneva: World Health Organization (2010). https://iris.who.int/handle/10665/44473
5. Global programme to eliminate lymphatic filariasis: progress report, 2022 Vol. 98. Weekly Epidemiological Record (2023) p. 489–502. WHO.
6. The Lao People’s Democratic Republic eliminates lymphatic filariasis [press release]. Manila: World Health Organization (2023).
7. Bangladesh eliminates lymphatic filariasis [press release]. New Delhi: World Health Organization (2023).
8. Lymphatic filariasis-Level 3 cause. Seattle: Institute for Health Metrics and Evaluation (2023). Available at: https://www.healthdata.org/research-analysis/diseases-injuries/factsheets?title=lymphatic+filariasis.
9. Deribe K, Bakajika DK, Zoure HM-G, Gyapong JO, Molyneux DH, Rebollo MP. African regional progress and status of the programme to eliminate lymphatic filariasis: 2000–2020. Int Health. (2020) 13:S22–S7. doi: 10.1093/inthealth/ihaa058
10. Lymphatic Filariasis. Atlanta: CDC (2019). Available at: https://www.cdc.gov/dpdx/lymphaticfilariasis/index.html.
11. Partono F, Evered D, Clark S. The spectrum of disease in lymphatic filariasis. In Ciba Foundation Symposium 127 - Filariasis. (2007), 15–31 p. doi: 10.1002/9780470513446.ch3
12. Douglass J, Hailekiros F, Martindale S, Mableson H, Seife F, Bishaw T, et al. Addition of lymphatic stimulating self-care practices reduces acute attacks among people affected by moderate and severe lower-limb lymphedema in Ethiopia, a cluster randomized controlled trial. J Clin Med. (2020) 9:4077. doi: 10.3390/jcm9124077
13. Debrah LB, Mohammed A, Osei-Mensah J, Mubarik Y, Agbenyega O, Ayisi-Boateng NK, et al. Morbidity management and surveillance of lymphatic filariasis disease and acute dermatolymphangioadenitis attacks using a mobile phone-based tool by community health volunteers in Ghana. PloS Negl Trop Dis. (2020) 14:e0008839. doi: 10.1371/journal.pntd.0008839
14. Zeldenryk LM, Gray M, Speare R, Gordon S, Melrose W. The emerging story of disability associated with lymphatic filariasis: A critical review. PloS Negl Trop Dis. (2011) 5:e1366. doi: 10.1371/journal.pntd.0001366
15. Lymphatic filariasis: managing morbidity and prevention disability: an aide-mémoire for national programme managers. 2nd edition. Geneva: World Health Organization (2021). Licence: CC BY-NC-SA 3.0 IGO.
16. Guideline: Alternative mass drug administration regimens to eliminate lymphatic filariasis. Geneva: World Health Organization (2017). Licence: CC BY-NC-SA 3.0 IGO.
17. Jambulingam P, Kuttiatt VS, Krishnamoorthy K, Subramanian S, Srividya A, Raju HKK, et al. An open label, block randomized, community study of the safety and efficacy of co-administered ivermectin, diethylcarbamazine plus albendazole vs. diethylcarbamazine plus albendazole for lymphatic filariasis in India. PloS Negl Trop Dis. (2021) 15:e0009069. doi: 10.1371/journal.pntd.0009069
18. Francis H, Awadzi K, Ottesen EA. The Mazzotti reaction following treatment of onchocerciasis with diethylcarbamazine: clinical severity as a function of infection intensity. Am J Trop Med Hyg. (1985) 34:529–36. doi: 10.4269/ajtmh.1985.34.529
19. Gardon J, Gardon-Wendel N, Demanga N, Kamgno J, Chippaux JP, Boussinesq M. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet. (1997) 350:18–22. doi: 10.1016/S0140-6736(96)11094-1
20. Chimfwembe K, Shirley H, Baker N, Wamai R. Zambia: A narrative review of success and challenges in lymphatic filariasis elimination. Trop Med Infect Dis. (2024) 9. doi: 10.3390/tropicalmed9010021
24. Buckley JJC. A helminthological survey in Northern Rhodesia. J Helminthol. (1946) 21:111–74. doi: 10.1017/S0022149X00032041
25. Hira PR. Bancroftian Filariasis in Zambia. Ann Trop Med Parasitol. (1975) 69:521–2. doi: 10.1080/00034983.1975.11687044
26. Hira PR. Bancroftian filariasis. An autochthonous case in Zambia. Med J Zambia. (1976) 10:160–4.
27. Mwase ET, Stensgaard A-S, Nsakashalo-Senkwe M, Mubila L, Mwansa J, Songolo P, et al. Mapping the geographical distribution of lymphatic filariasis in Zambia. PloS Negl Trop Dis. (2014) 8:e2714. doi: 10.1371/journal.pntd.0002714
28. Shirley H, Orriols A, Hogan D, Chimfwembe K, Balya A, Sibbuku K, et al. Lymphatic filariasis in Zambia: A scoping review protocol. PloS One. (2023) 18:e0292237. doi: 10.1371/journal.pone.0292237
29. Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Internal Med. (2018) 169:467–73. doi: 10.7326/M18-0850
30. Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. (2015) 13:141–6. doi: 10.1097/XEB.0000000000000050
31. Peters MDJ, Marnie C, Tricco AC, Pollock D, Munn Z, Alexander L, et al. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth. (2020) 18:2119–26. doi: 10.11124/JBIES-20-00167
32. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. (2016) 5:210. doi: 10.1186/s13643-016-0384-4
33. Grifferty G, Shirley H, O’Brien K, Hirsch JL, Orriols AM, Amechi KL, et al. The leishmaniases in Kenya: A scoping review. PloS Negl Trop Dis. (2023) 17:e0011358. doi: 10.1371/journal.pntd.0011358
34. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
35. Slater H, Michael E. Predicting the current and future potential distributions of lymphatic filariasis in Africa using maximum entropy ecological niche modelling. PloS One. (2012) 7:e32202. doi: 10.1371/journal.pone.0032202
36. Michael E, Singh BK, Mayala BK, Smith ME, Hampton S, Nabrzyski J. Continental-scale, data-driven predictive assessment of eliminating the vector-borne disease, lymphatic filariasis, in sub-Saharan Africa by 2020. BMC Med. (2017) 15. doi: 10.1186/s12916-017-0933-2
37. Kelly-Hope LA, Molyneux DH, Bockarie MJ. Can malaria vector control accelerate the interruption of lymphatic filariasis transmission in Africa; capturing a window of opportunity? Parasites Vectors. (2013) 6:39. doi: 10.1186/1756-3305-6-39
38. Cano J, Basáñez M-G, O’Hanlon SJ, Tekle AH, Wanji S, Zouré HG, et al. Identifying co-endemic areas for major filarial infections in sub-Saharan Africa: seeking synergies and preventing severe adverse events during mass drug administration campaigns. Parasites Vectors. (2018) 11. doi: 10.1186/s13071-018-2655-5
Keywords: lymphatic filariasis, neglected tropical diseases (NTDs), sub-Saharan Africa, Wuchereria bancrofti-parasite, vector-borne disease (VBD), mosquito borne disease
Citation: Shirley H, Orriols AM, Hogan D, Chimfwembe K, Bwalya A, Sibbuku K, Lardizabal J, Tillotson S, Grifferty G, Coombs PE and Wamai R (2024) A scoping review of lymphatic filariasis research in Zambia. Front. Trop. Dis 5:1449719. doi: 10.3389/fitd.2024.1449719
Received: 16 June 2024; Accepted: 29 July 2024;
Published: 23 August 2024.
Edited by:
Norbert Brattig, Bernhard Nocht Institute for Tropical Medicine (BNITM), GermanyReviewed by:
Manuel Ritter, University Hospital Bonn, GermanyThomas Unnasch, University of South Florida, United States
Chester Kalinda, University of Global Health Equity, Rwanda
Copyright © 2024 Shirley, Orriols, Hogan, Chimfwembe, Bwalya, Sibbuku, Lardizabal, Tillotson, Grifferty, Coombs and Wamai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hugh Shirley, aHVnaHNoaXJsZXk5N0BnbWFpbC5jb20=
 Hugh Shirley
Hugh Shirley Adrienne M. Orriols
Adrienne M. Orriols Dylan Hogan4
Dylan Hogan4 Kingford Chimfwembe
Kingford Chimfwembe Alinaswe Bwalya
Alinaswe Bwalya