- 1Bio-Manguinhos, Oswaldo Cruz Foundation, Rio de Janeiro, Brazil
- 2Aggeu Magalhães Institute, Oswaldo Cruz Foundation, Pernambuco, Brazil
- 3Department of Infectious Diseases and Microbiology, University of Pittsburgh, Pittsburgh, PA, United States
- 4School of Chemistry, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil
- 5Academy of Innovation and Intellectual Property, National Institute of Industrial Property, Rio de Janeiro, Brazil
Introduction: There has been a dramatic global surge in dengue fever (DF) in recent years, with a projected 100-400 million cases annually and large outbreaks expected every 3-4 years. This concerning scenario is in sharp contrast to the very low availability of dengue vaccines to meet this spiraling demand, particularly affecting developing countries in Latin America and Africa. In our study, we aim to identify current vaccine development constraints, from a technological perspective, based on clinical trials and patent landscape data.
Methods: This study was conducted in a two-step methodology. First, candidates and launched products and methods used in the development or as part of DF vaccines were identified from the Cortellis Drug Discovery Intelligence (CDDI) platform. Second, the Derwent Innovation database was used to retrieve patent documents related to dengue vaccines. Data were collected in the period 2014-2024 (to identify the candidate and or launched vaccines) and 2018-2022 (to identify patent documents) with claims involving applications for dengue vaccine development. We presented these descriptive data in the following format: quantitative and qualitative assessments describing the vaccine development scenario and the claimed vaccine technologies.
Results: Our study indicated that 65% of dengue vaccines are still in phase 1 of development. The few current dengue vaccines that have reached phase 3 (1) and launched (2) have shown limited levels of individual protection for one or more dengue serotypes and there is an anemic pipeline of the next generation of more effective tetravalent dengue vaccines. Although the intelligent use of multiple dengue vaccine formulations in immunization campaigns can result in effective protection against all serotypes at the population level, the implementation of this is complex and challenging. The results of our study thus provide evidence of a worrisome global situation regarding the development of new dengue vaccine candidates.
Conclusion: To reverse this scenario, there is an urgent need to implement new knowledge governance approaches and business models to stimulate an exponential increase in global vaccine development funding for dengue and several other vaccines.
1 Introduction
Dengue is a complex arboviral disease, possibly introduced in the Americas by Aedes mosquitoes on slave ships from Africa in the 15th through the 17th century. The dengue virus (DENV) has four different subtypes, DENV1-4, and belongs to the Flaviviridae family, genus Flavivirus. Severe cases can evolve into dengue hemorrhagic fever and dengue shock syndrome, which are often fatal and no effective therapy is thus far available.
A dramatic increase in the number of dengue fever cases has been globally reported in recent years, with an estimated 100-400 million cases every year and major outbreaks expected every 3-4 years (1). This concerning global scenario contrasts with a virtual lack of dengue vaccine availability (2) to respond to this spiraling demand for immunization. In our study, we examine the current challenges for vaccine development, discussing technological strategies and production scale-up, from a knowledge governance perspective, to overcome this impasse.
The recent dengue outbreak spirals in Latin American and Caribbean countries illustrate well this rapid increase in the number of cases and deaths. In spite of the previously successful eradication of Aedes aegypti mosquitoes in 18 countries in the Americas by 1962 due to a well-conceived continental plan (1947-1970), the reintroduction and reinfestation of mosquitoes from 1971 to 1999 completely changed the epidemiological scenario in the region. Brazil has experienced a severe outbreak, since the beginning of 2023, affecting most of the Brazilian states, with 6,3 million probable dengue fever (DF) cases and 4,400 deaths reported by the Minister of Health from January to June 2024, the highest historical record in decades (3). Nearly 80% of global cases have been reported in Brazil and Latin American countries as indicated in Figure 1 (3, 4).
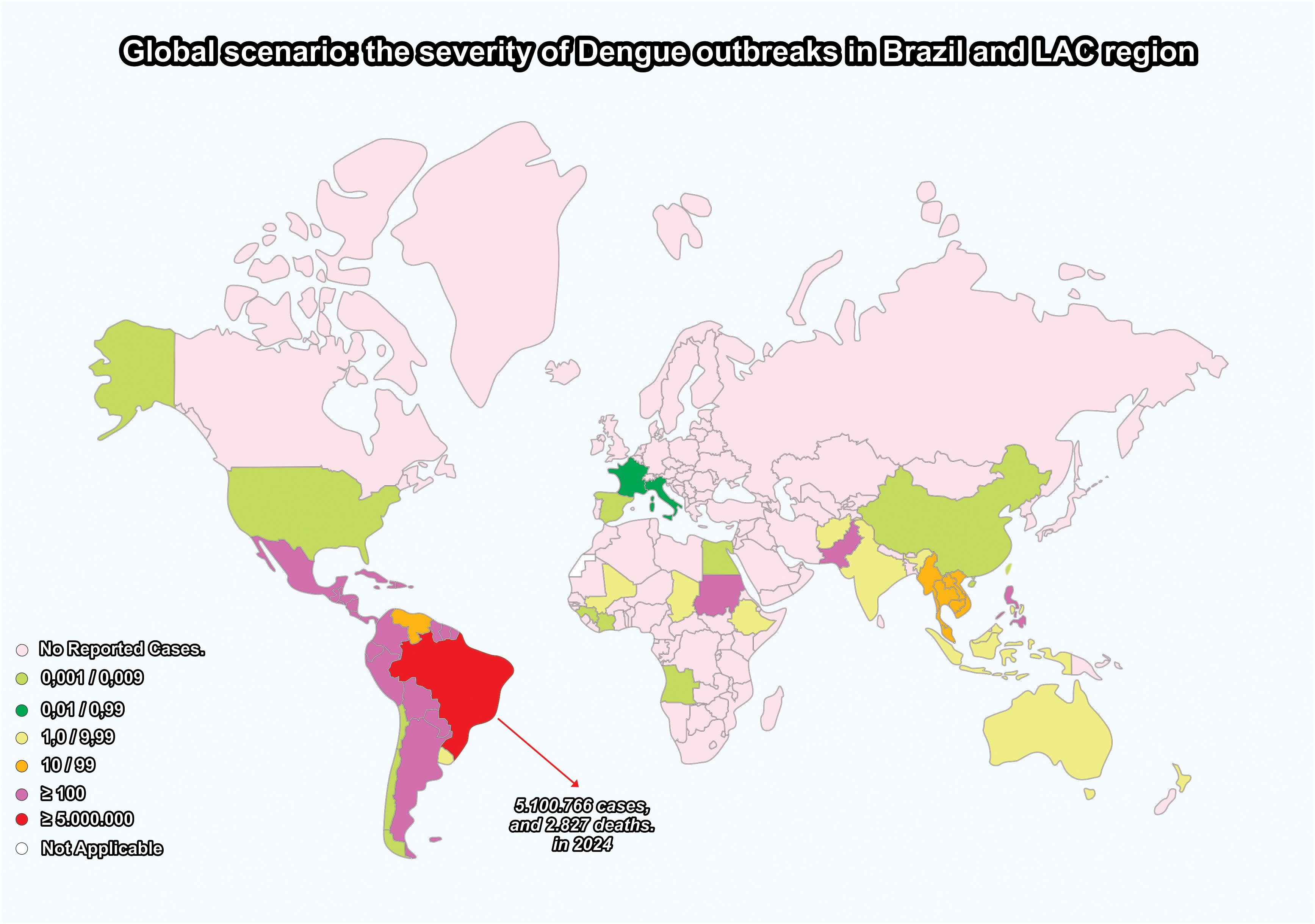
Figure 1. Brazil and the Latin America and the Caribbean (LAC) region: a dramatic increase in dengue outbreaks – 2024. created by the authors based on data from the WHO Health Emergencies Program (2023) (3) and the Brazilian Ministry of Health (2024) (4).
Nevertheless, it is important to underline that, in spite of this concentration of epidemics in tropical regions, dengue should not be considered exclusive to the tropics. Aedes reintroduction and DF outbreak spirals in the Americas and other continents have been attributed to complex interactions of herd immunity with climatic and eco-social determinants, i.e., global warming, El Niño, accelerated urbanization, travel, migration, poverty, lack of basic sanitation, deforestation, and low priority given to vector control activities (4). These multifaceted factors have contributed to the dislocation of vectors and infected populations from developing tropical regions to developed countries in the Northern Hemisphere. In Europe, imported dengue cases have been reported in 2024 in Germany, Italy, and France, although no autochthonous cases have been reported thus far (5).
Many diseases require the production of new vaccines in both low-income and high-income countries to reduce mortality and morbidity at a global level. For a vaccine to be developed and approved (including against DF) there are defined steps for use in humans, from research and discovery to monitoring safety after approval (Figure 2), that must be coupled with research and technological advances to develop safer and better vaccines (6, 7).
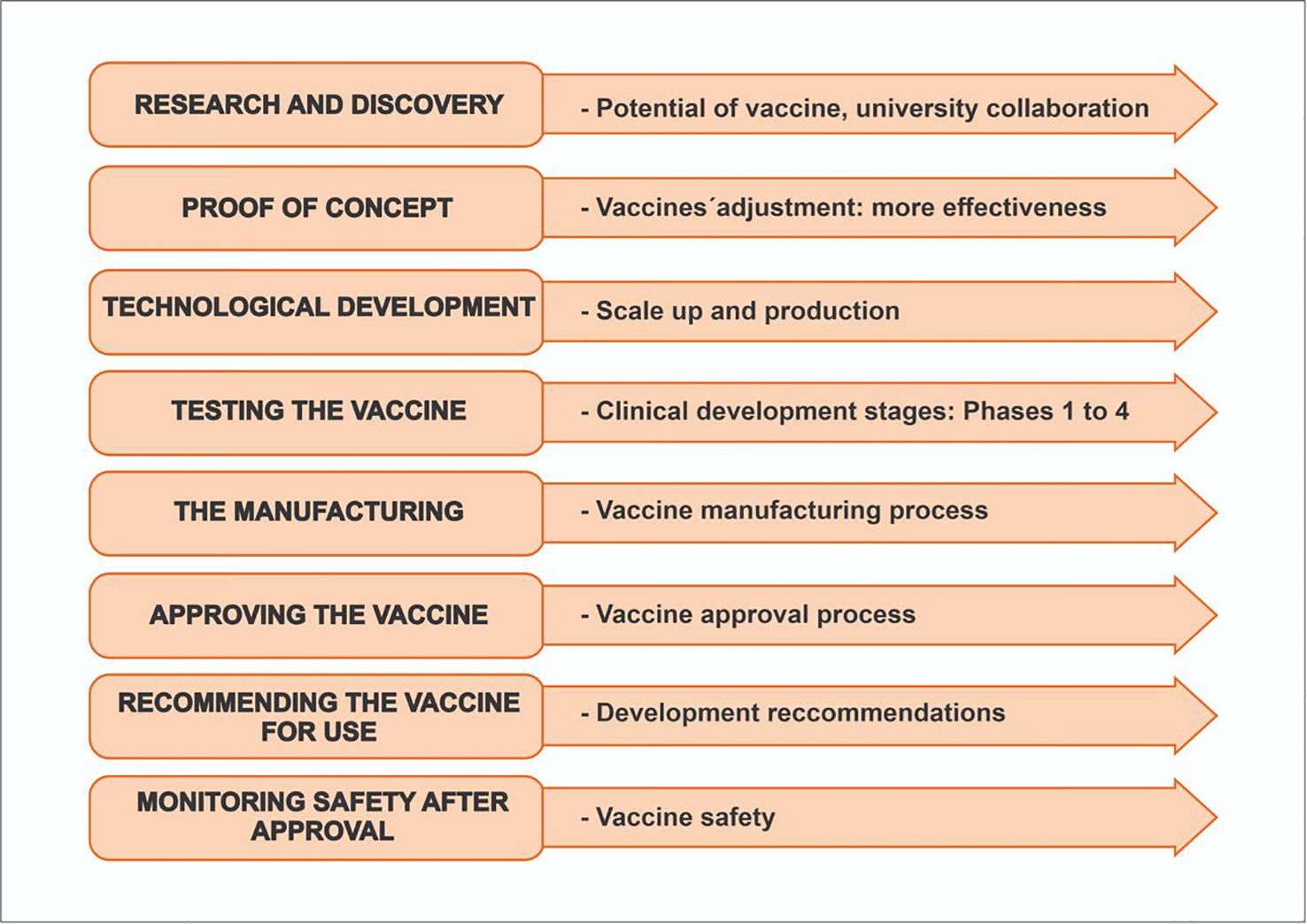
Figure 2. Steps for vaccine development and approval. created by the authors based on CDC 2023 (6).
Contrasting with this concerning scenario, there are few dengue vaccines available in the global market, and as we will indicate here, they have important limitations in protection against viral subtypes and significant bottlenecks in laboratory infrastructure and platforms, constraining large-scale production. Concerning protection, it is important to note that protective immunity against the DENV has not been complete in available vaccines due to a broad range of complex factors related to the host and to the diversity of viral subtypes (8). These factors and the issue of adverse events from dengue vaccines, especially related to antibody-dependent enhancement (ADE) evidenced in field trials, will be discussed.
Considering this complexity in dengue vaccine development, our study aims to provide an in-depth understanding of dengue vaccine research, development, and innovation (RD&I) from the patent landscape approach. Patents are the major indicator of innovation and can provide valuable information on the status and evolution of novel vaccine technologies and their potential to attract investments. In addition, some hybrid approaches were provided in our search for DF vaccines: research on the phases in clinical trials, on launched vaccines, and on the patent landscape for drugs and biologics for dengue.
2 Methods
To access the evolution of innovation in the research period, the study was conducted in a two-step methodology: first, for candidates and launched products, strategies used in development or as part of DF vaccines were identified from the Cortellis Drug Discovery Intelligence (CDDI) platform. Second, the Derwent Innovation database was used to retrieve patent documents related to dengue vaccines. Data were collected in the period 2014-2024 (to identify the candidate and or launched vaccines) and 2018-2022 (to identify patent documents), based on claims involving applications for dengue vaccine development. We present these descriptive data in the following format: quantitative and qualitative assessments describing the vaccine development scenario and the claimed vaccine technologies. The method attempts to provide an overview of new DF vaccine technologies following a two-step process (Figure 1).
2.1 Step 1: The first methodological strategy: clinical research/launched drugs
This practical and simple patent research strategy is an adequate methodology to find candidates or introduced products for vaccines/therapies against dengue infections. This strategy1 was developed to find candidates or introduced products to be used as vaccines against dengue infections. Retrieved from the selected platform (the CDDI2), vaccines in the most advanced phase of development were selected here, based on the date of last update to ensure the most recent information about the retrieved dengue vaccine products.
2.2 Step 2: The second methodological strategy: dengue vaccine processes and products
An important component of our research is patent data collection and analysis. To obtain filed patent documents related to dengue vaccines, a second search strategy was developed3 on the Derwent Innovation4 database (11), from 2018 to 20225. A patent search using general terms such as dengue or breakbone fever and vaccine to find patents which uses only specific keyword concepts (12). This search focused on technologies developed or related to the development of vaccines against DF, selecting the main priority countries, the development of these deposits over time per priority year and the main deposit companies. In addition, information on the main depositor companies around the world and their technological strategies is provided (11).
3 Results
The first methodological strategy provided the following results.
The results of our study evidence a worrisome global situation regarding the development of dengue vaccine candidates: most of these candidates are currently in the initial phases, 1 and 2, of clinical trials (23), with just one candidate in phase 3 and very few launched vaccines (2), as indicated in Figure 3.
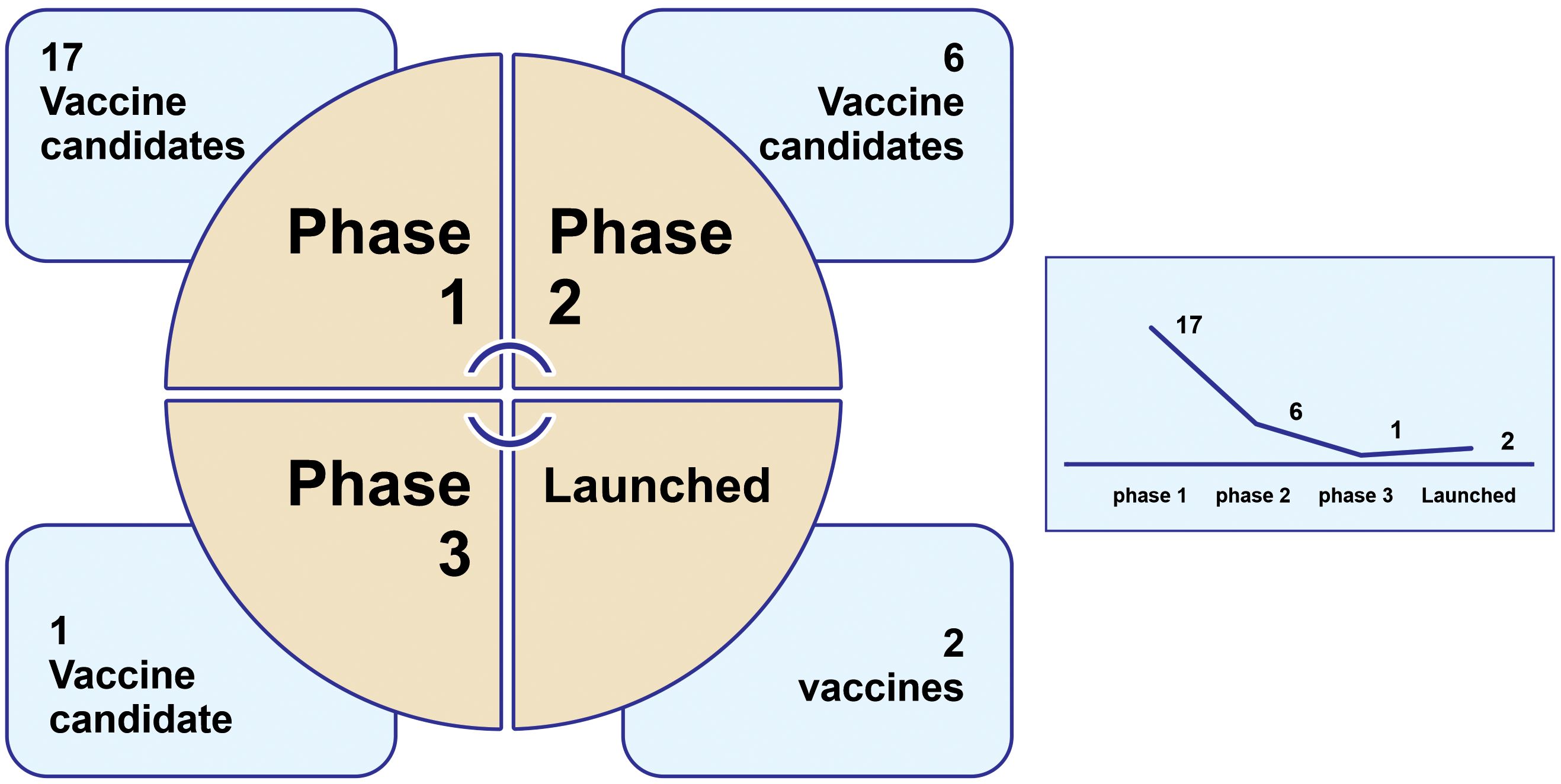
Figure 3. Development of vaccines against dengue infection in different phases of clinical trials last updated6 from 01/04/2014 to 01/04/2024. created by the authors. The search was carried out on 3 March 2024 (CDDI Platform). N=26.
3.1 Clinical research and launched drugs: vaccines against dengue infection
Based on the adopted strategy, we found very few clinical trials and novel launched drugs. In total, 26 clinical trials were found in their respective clinical phases: 17 were in Phase 1; 6 were in Phase 2, one was in Phase 3, and two had been launched, as shown in Figure 3. Only a few companies/institutes were found in the search strategy: the National Institute of Allergy and Infectious Diseases (NIAID/US) (five candidate vaccines), followed by Merck & Co (US), Sanofi (France), and the U.S. Army Medical Command (MEDCOM/US)7 with three results each.
Despite some progress in vaccine technology, these results confirm the general belief that very little investment is being made in the development of vaccines against neglected diseases. In addition, the correlates of protection against dengue are not clear. Some vaccines only focused on neutralizing antibodies, such as Dengvaxia, which induces very high levels of neutralizing antibodies and did not show clinically relevant protection 2 years after immunization in people that had never been infected with dengue, showing significantly increased hospitalization in this population. However, people that had been already infected with dengue and received the vaccine were protected. The other two leading vaccines, Qdenga and Butantan-DV, also generate strong antibodies but also generate strong T cell responses necessary to achieve a complete and lasting immune response. These vaccines have been shown to be effective in people who had never had dengue and had overall better protection levels.
The next generation of dengue vaccines must improve the production of humoral and memory B and T cell responses (without causing DF symptoms) against all four dengue serotypes (4, 15, 16). Furthermore, our research findings indicate that many companies and research institutions have focused the innovation on the engineering of the dengue antigens to minimize the risk of antibody enhancement but continue to utilize traditional vaccine platforms, such as attenuated or inactivated vaccines, to develop new processes or products for preventing or treating DENV.
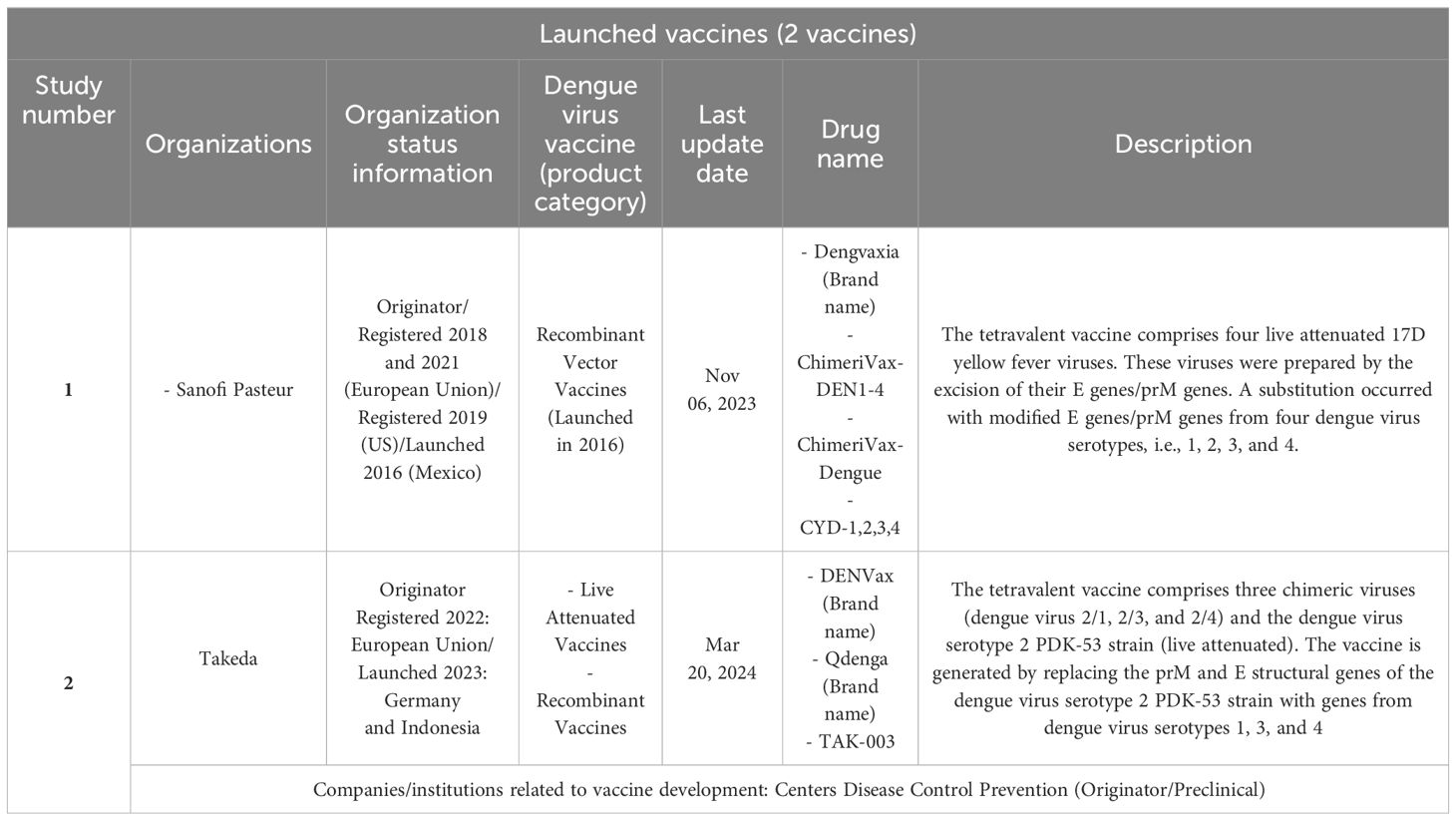
An overall summary of the results of the vaccine candidates (phase 3) and launched ones is provided in Tables 1–4. It is important to underline that several of these studies are for the same vaccine candidate. It should be noted that each component is tested individually and then combined and several combinations are tested. All are part of the process of developing the same vaccine, such as patented epitopes and nanoparticles tested for vaccine candidates or attenuated strains used in controlled human infectious models (CHIMs). Finally, an important observation is that several of these studies were discontinued or interrupted, indicating that the global vaccine scenario might be worse than our results indicate.
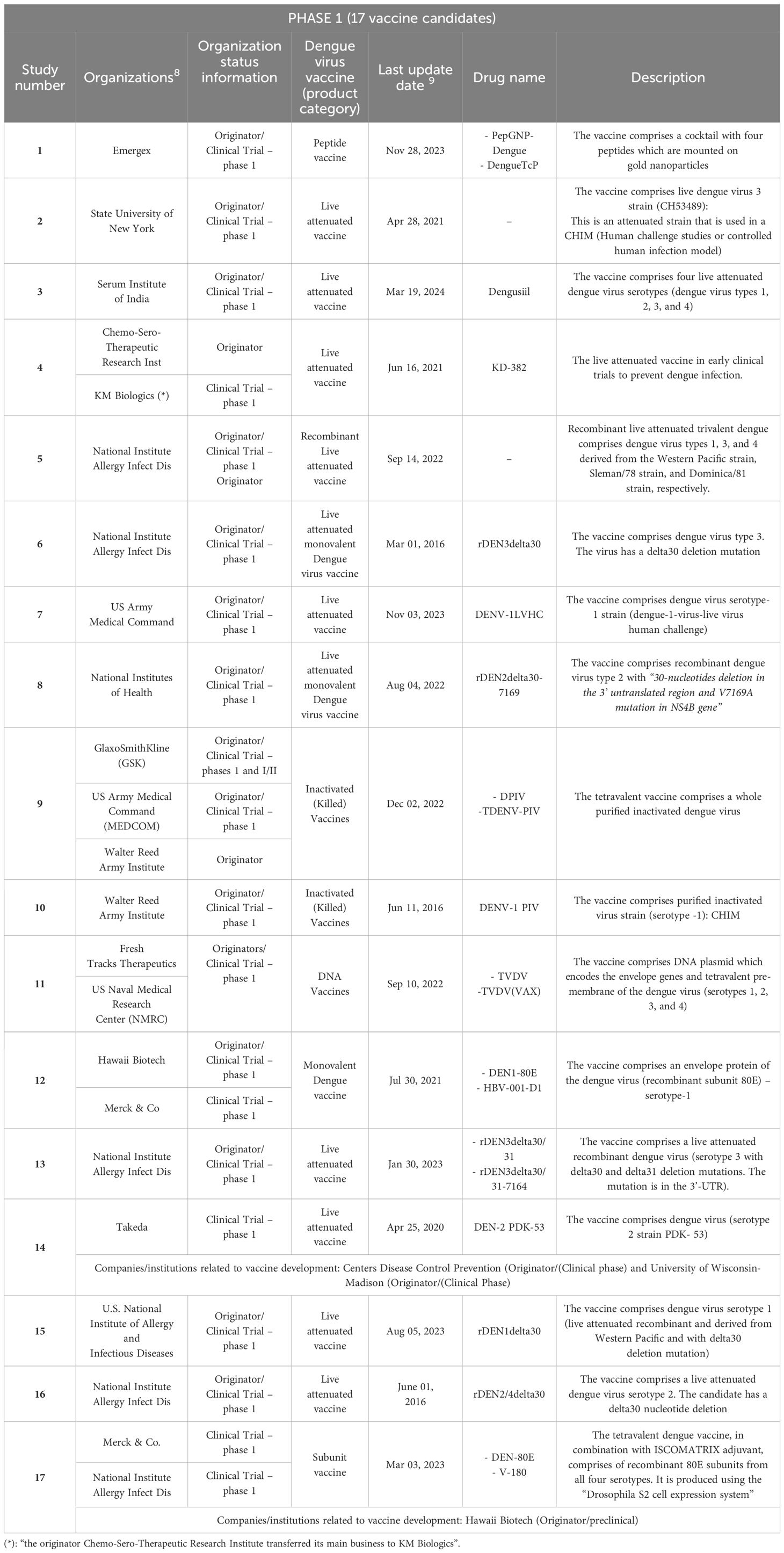
Table 1. Vaccine candidates against DF retrieved according to the search strategy: clinical trials in phase 1.
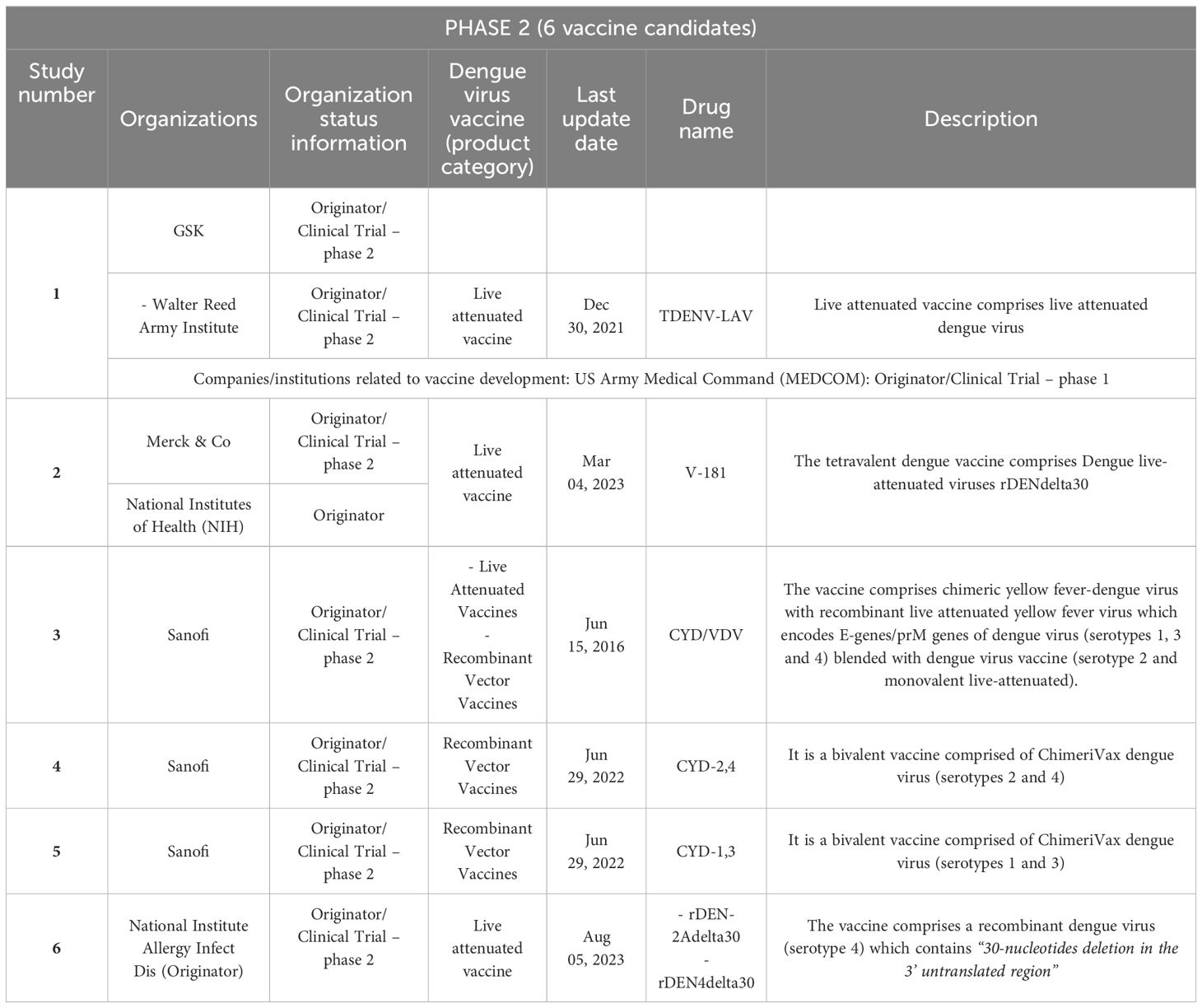
Table 2. Vaccine candidates against dengue fever according to the search strategy: clinical trials in phase 2.
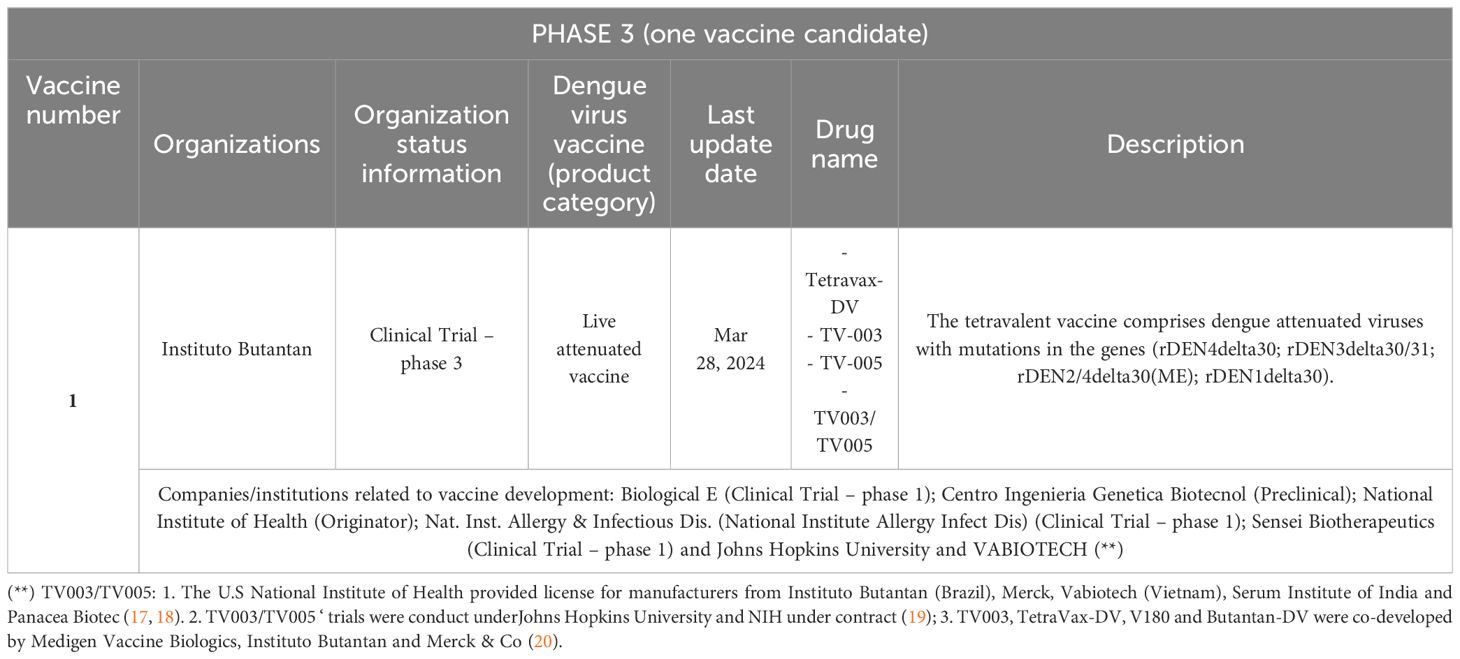
Table 3. Vaccine candidates against Dengue fever according to the search strategy: clinical trials in phase 3.
Finally, we present in Table 4 the two launched vaccines: Dengvaxia and Qdenga. In spite of significant breakthroughs, it is important to consider their current regulatory issues and restrictions by indicating in which countries the two launched vaccines presented have been approved. The approval of Dengvaxia was granted to Sanofi Pasteur. The vaccine has been approved in 19 countries including Brazil and in the European Union, but it is not approved by the US Food and Drug Administration (FDA) for use in individuals not previously infected by any dengue virus serotype or for whom this information is unknown. The approval of Qdenga has been approved for use in children and adults in the private market in countries in Europe, as well as in Indonesia, Thailand, and in private and some public programs in Argentina and Brazil. TAK-003 is not approved for use in India and the US. On 11 July 2023, Takeda announced withdrawal from the US FDA review. The WHO pre-qualified both the Dengvaxia and Qdenga vaccines.
We provide here a more detailed description of vaccines at a more advanced stage of development, i.e., candidate vaccines (clinical trial – phase 3) and vaccines that have already been introduced (launched vaccines: Dengvaxia and Qdenga9).
The candidate vaccine with the brand name Tetravax-DV (TV-003; TV-005, and TV-003/TV-005) has been licensed on a non-exclusive basis to manufacturers from developing countries. It was from the National Institute of Health (NIH/US). It includes the four DENV (live attenuated) serotypes and is used to prevent the disease in adults and children. It is in phase 3 clinical trials from Instituto Butantan10 (Brazil) (21). Similar to TV-003 formulated by the NIH, the Butantan–Dengue Vaccine (Butantan-DV) comprises the four DENV serotypes, so it is a live attenuated single-dose tetravalent vaccine. The vaccine was manufactured, formulated, and lyophilized at the institution and it comprises attenuated viruses with mutations in the genes rDEN4delta30, rDEN3delta30/31, rDEN2/4delta30(ME), and rDEN1delta30. In a clinical phase 3 trial (placebo-controlled, double-blind, multicenter, randomized, ID: NCT024067291112), the vaccine’s safety/efficacy will be evaluated. The trial will be community-based and will be carried out in several locations in Brazil (select urban areas with incidence of dengue transmission). According to Kalls and coworkers, the clinical trial demonstrated that the vaccine (Butantan-DV) is effective in preventing symptomatic disease during a 2-year follow-up in virologically confirmed dengue (against serotypes 1 and 2 and independent of prior exposure to the DENV) (21, 23).
Dengvaxia (immunizer from the French pharmaceutical company Sanofi-Pasteur) was the first live attenuated dengue vaccine approved for the prevention of dengue caused by the four serotypes (yellow fever chimeric DENV, serotypes 1, 2, 3 and 4). The approval happened in 2015 in three endemic countries: Brazil, the Philippines, and Mexico (the first approval in the world). In 2016, the vaccine was first introduced in the Philippines and later in Mexico (24–26). A study conducted by Salje and colleagues (ancillary study to a phase 3 vaccine trial) showed that the vaccine was effective in both the symptomatic and subclinical phases of the disease and achieved the greatest protective effect in the first 3 years after vaccination (27). It is approved for use in people between the ages of 9 and 45 who must have previously had dengue fever. According to several clinical studies, the vaccine, when administered in three doses (for people previously exposed to the DENV), can reduce the risk of dengue fever (almost 60%). For people who were not previously exposed, the reduction is 38%. It is important to highlight that Dengvaxia (in children under 9 years of age) was associated with a higher incidence of hospitalization (28).
Qdenga (from the Japanese Takeda Pharmaceutical Company) was first approved in Indonesia in 2022 for people aged 6 to 45 years against all types of DENV serotypes and has been approved by the European Commission for use in people aged 4 years and above, regardless of previous DENV exposure. In 2023, the vaccine was approved in Thailand, Argentina, the United Kingdom, and Brazil (29). The composition is based on live, weakened DENV (serotype 2), which can form the genetic backbone against four DENV serotypes (30). In Brazil (approved in March 2023), the vaccine was integrated into the Brazilian Public Health Network (SUS) and is manufactured in the country through Resolution 661/2313 (31).
The second methodological strategy provided the following results.
3.2 Dengue vaccines research and development: a patent landscape
This section provides a quantitative analysis of the results about most of the applicants. The resulting data is assessed in multiple stages. First, to examine the global dengue vaccine/therapeutic patent applications for the period 2018-2022 (earliest priority year), the adopted strategy retrieved 84 patent applications. Over 5 years (2018-2022), most of the applications were in 2018 (18 applications, 21.4%) and in 2021 (20 applications, 23.8%). Based on the available data, the average number of documents per year was just under 17 applications. The developed strategy also showed that the highest priority country in terms of number of applications was the United States with 38 documents, followed by China with 19, the Republic of Korea with 10, and India with 4 (Figure 4).
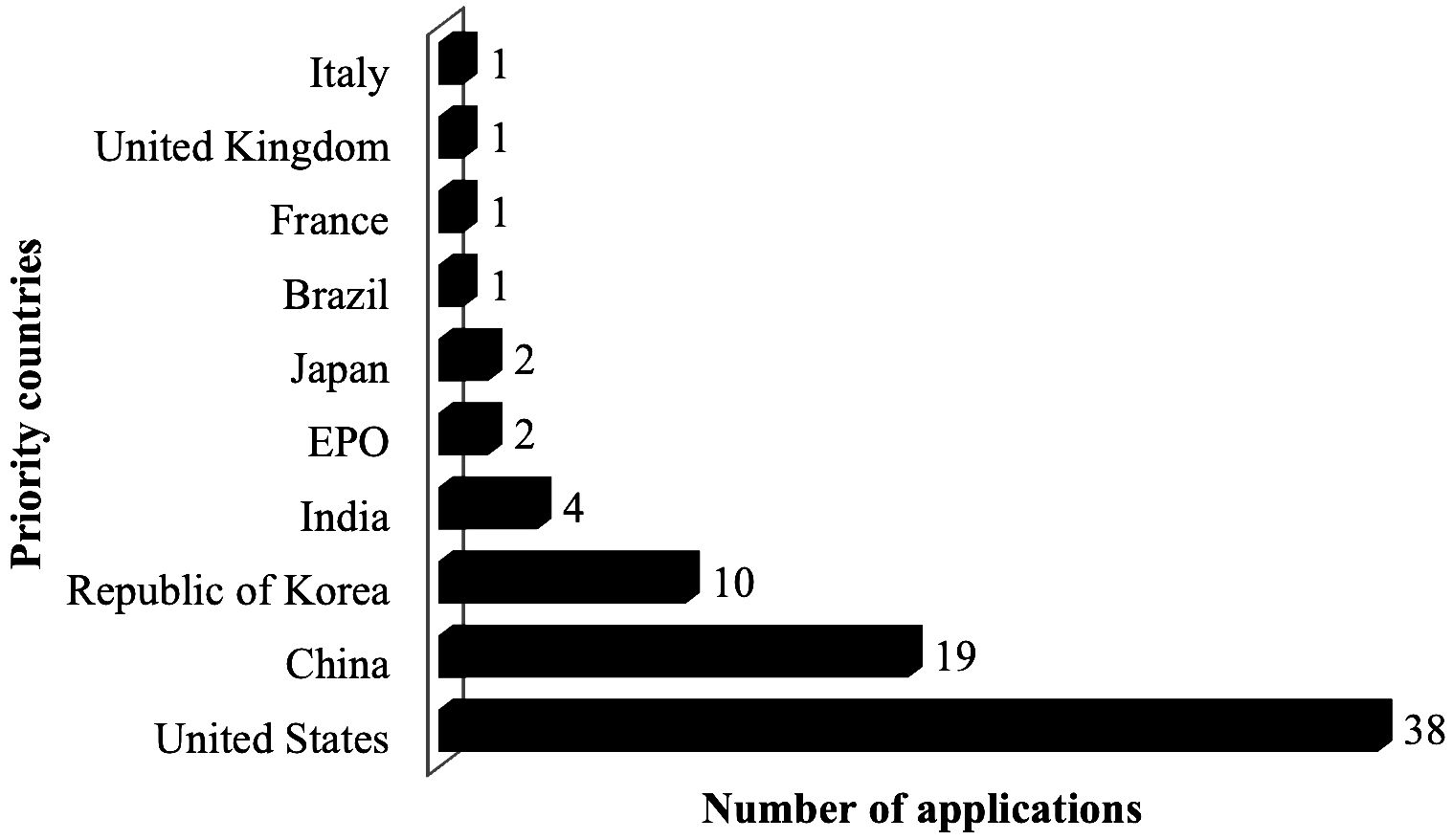
Figure 4. Top priority countries for vaccines against DF according to the adopted method from 2018-2022 (n=84). created by the authors using data from Derwent Innovation.
The leading company was Takeda Vaccines INC14 (with 4 applications), followed by the American universities The Regents of The University of California and The University of North Carolina at Chapel Hill, the company Cansino Biologics INC, and Chinese People’s Liberation Army - Army Medical University with 3 documents each as seen in Figure 5.

Figure 5. Top applicants for vaccines/therapeutics and related technologies against dengue fever according to the adopted method from 2018-2022 (n=84). created by the authors using data from Derwent Innovation.
The International Patent Classification (IPC) from the Strasbourg Agreement is useful for understanding document technology areas using a hierarchical language system. A further analysis was carried out to identify the IPCs most commonly used in corporate/institutional/university applications with the following results.
The main classifications were retrieved according to the search in the titles and claims, obtaining deposits with the prevalence of classifications, such as A61K 39/12 (“viral antigens” with 49 applications), A61K 39/00 (“Medicinal preparations containing antigens or antibodies” – 23 applications), and A61K 39/39 (“characterised by the immunostimulating additives, e.g. chemical adjuvants” – 14 applications).
Of all the applications retrieved, 84 documents were directly related to the development of dengue vaccines according to the adopted strategy. For a more detailed description of some of these documents (indicating the type of technology/technologies used in vaccine development), patents have been divided into three different groups to describe the subject matter of the claims, namely, 1) dengue vaccine, 2) vaccine against DF and other diseases, and 3) other approaches related to the development of dengue vaccines.
3.2.1 Application analysis: dengue vaccines
The resulting data (patent applications) is evaluated here step by step considering the most important technologies in dengue vaccine development (mainly its action on the immune system). Based on the applications related to dengue vaccines, we make the following observations. The unit dose of dengue vaccine developed by the company stimulates the immune response of a subject against the virus (in composition: viral strain of serotypes 1, 3, and 4 and non-chimeric serotype 2/live attenuated), thereby reducing the incidence of disease. In addition, the vaccine contains dengue serotype constructs at different concentrations (TDV-1, TDV-2, TDV-3, and TDV-4). It is important to consider that the effectiveness of the vaccine against serotype 2 was at least 60% in both vaccinated and unvaccinated individuals (PN EP4082568A1) (32). A tetravalent DNA vaccine against dengue fever has been claimed to induce neutralizing antibodies. It is important to note that this vaccine uses LAMP technology and its development stopped more than 10 years ago. It was considered redundant since other strategies were more advanced. The vaccine consists of DNA with nucleotide sequences and has antiviral-specific T cell and protective effects. It is reported to induce specific IgG antibodies against the four serotypes of DENV. It is a DNA vaccine (expression vector, preferably PVAX1) (PN: CN115990249A) (33). A drug-making liposome nanoparticle and a quadrivalent dengue m-RNA vaccine have m-RNA (containing 1976 nucleotides) to promote the expression of the DENV immunogen and produce neutralizing antibodies (PN: CN115779076A) (34). A claimed tetravalent inactivated dengue vaccine contains inactivated antigens (four DENV serotypes) with the following volume ratio for DENV serotype (I, II, III, and IV) inactivated antigens: 0.5-1.5:2-3:4-6:1-2. The vaccine has advantages such as no reproductive toxicity (mouse) and a long storage time at 4°C (PN: CN115518149A) (35). A vaccine has been developed that elicits a response against all DENV serotypes and reduces the induction of non-neutralizing antibodies. It generates immunity (neutralizing antibodies) against DENV and has a flavivirus virus-enveloping protein (PN: US10675343B2) (36). A subunit dengue vaccine to enhance the immune response against DENV comprises “a fusion protein of conjugating or connecting delta C nonstructural protein 1 (NS1ΔC or truncated NS1ΔC) to at least one polypeptides of NS3c (or truncated NS3c) and/or consensus envelope protein domain III (cEDIII)” (PN: US11786587B2) (37). A stable live attenuated recombinant tetravalent dengue vaccine has buffering agents (optional), bulking agent, stabilizer, and live attenuated recombinant dengue virus which is generated from mutated or 30 and/or 31 deleted DENV strains (PN: PH12018500675B1) (38). In addition, in spite of the technological limitations, our research highlights the growing use of novel and innovative mRNA platforms for DENV vaccines. A DENV mRNA vaccine induces the person to produce antibodies against the DENV. It has a carrier and mRNA capable of expressing the DENV immunogen (selected from the E80 protein, NS1, the prME protein, or a combination) (PN: CN113663063A) (39). A DENV vaccine which has an attenuated antibody-dependent enhancing effect was developed. The composition is able to elicit a stronger immune response, produce antibodies against DENV in target cells (four DENV serotypes), and overcome the shortcomings of the E protein D-III region monomer’s insufficient immunogenicity (PN: CN115850401A) (40). To treat/prevent the symptoms and infection caused by DENV, a new recombinant DNA vaccine was claimed. The pharmaceutical composition comprises a protein-coding region and an antigenic region of the envelope domain (PN: WO2023126882A1) (41). For the prevention/treatment of infection caused by the DENV (including the suppression of dengue hemorrhagic fever), a recombinant vaccinia virus (RVV) is used to prepare pharmaceutical compositions (e.g., vaccines) to induce cell-mediated immunity and humoral immunity. Moreover, the novelty of the RVV is that it comprises part of cDNA (or all). It is able to encode an expression promoter and a non-structural protein which is derived from the DENV (PN: EP3939607A4) (42).
3.2.2 Application analysis: vaccine for DF and other diseases
There is a group that is not exclusive to DF. The results reveal the development of vaccines against the DENV and other flavivirus/other diseases. In line with the findings of this study, another unit dose of the dengue vaccine was applied by Takeda to prevent pertussis, diphtheria, hepatitis A, yellow fever (these are another object of the invention), and dengue disease, which includes four live attenuated dengue virus serotypes (serotype 1, e.g., chimeric Dengue serotype 2/1 strain; serotype 2, e.g., a dengue serotype 2 strain; a dengue serotype 3, e.g., a chimeric dengue serotype 2/3 strain; and a dengue serotype 4, e.g., a chimeric dengue serotype 2/4 strain). The developed method induces cell-mediated immunity and cross-reactive multitypic antibodies (PN: US11590221B2) (43). The antigen containing the DENV or Zika virus’ Fusion Loop fusion region of the E protein has been used to develop Dengue/Zika vaccines. The vaccine consists of an adjuvant, diluent, and an acceptable vehicle. The vaccines include a viral particle vaccine or a subunit vaccine, an inactivated vaccine, a mRNA vaccine, a DNA vaccine, an adenovirus vaccine, an attenuated vaccine or other viral carrier vaccine, or an adenovirus vaccine (alternative) (PN: EP4056582A4) (44). Another dengue/Zika vaccine uses a new antigen. The antigen includes the DENV or Zika virus E protein fusion loop fusion region (with a combination of F108 site mutation and L107 site mutation or L107 site mutation). In addition, the antigen can be used to prepare a DENV or Zika virus detection kit or detection reagent (PN: CN114907456A) (45). To induce the antibodies’ neutralization, a vaccine against multiple mosquito-borne flavivirus (such as DENV) can be produced by the use of “highly-retained peptide sequence in E protein of mosquito-borne flavivirus”. It is important to highlight that the peptide immunogens consist of “one copy of amino acid sequence” with seven amino acids residues (PN: TWI756812B) (46).
3.2.3 Application analysis: other approaches related to dengue vaccine development
Regarding molecular development or dengue vaccine development, some application documents were very interesting. In one, a claimed quinoline-based compound (an adjuvant) is used to activate the immune system, elicit an immunological agent, elicit an adaptive immune response, and carry out vaccination. Composting is used to vaccinate against viral infections such as dengue fever (PN: WO2023249996A1) (47). New isolated polynucleotides to immunize, treat and/or prevent DENV are able to encode polypeptide and alpha-virus non-structural proteins (PN: US20230201333A1) (48). The immune effect of the dengue vaccine was evaluated using neonatal mice from adult Balb/c mice after immunization. It is a more economical and practical evaluation method for dengue vaccines (PN: CN109481699B) (49).
4 Discussion
From this industrial property perspective, innovative strategies by leading companies (Takeda Vaccines Inc, The Regents of The University of California, The University of North Carolina at Chapel Hill, the company Cansino Biologics INC, and Chinese People’s Liberation Army-Army Medical University) illustrate well the recent scientific and technological advances in novel vaccine platforms and related technologies. The promising potential of these innovative platforms to target a broad range of diseases has been also a goal of companies such as Takeda Vaccines Inc.
The two vaccines have been introduced against all four serotypes. Dengvaxia—the first live attenuated dengue vaccine—from the French pharmaceutical company Sanofi-Pasteur and Qdenga from the Japanese pharmaceutical company Takeda are essential tools to combat this neglected tropical disease and reduce the number of infections and deaths caused by the DENV. These results indicate important scientific, technological, and translational constraints from basic research to the final dengue vaccine product, evidencing a “valley of death” in vaccine development by research institutes and companies worldwide. Additional research will thus be necessary for in-depth analyses to clarify decision-making processes leading to patent interruption in each of these institutions, including economic issues involved in the decision to continue or not to pursue the final phases of product development.
In spite of these remarkable recent developments, this scenario of a reduced number of vaccines evidenced in our study, with detrimental impacts on the vulnerable poorest populations in the tropics, is in stark contrast to the recent Vaccinology 4.0 breakthroughs and developments. From this perspective, new knowledge of the immune system and innovative research based on RNA platforms are key to accelerating the technological processes, leading to faster development of more effective vaccines, consistent with recent vaccine advances in the post-COVID-19 era.
5 Conclusion
Contrasting with the concerning global epidemiological scenario, our research found that in spite of recent breakthroughs and novel platforms, there are very few dengue vaccines in development and available in the global market. Regarding patent deposits in different stages of development, this scenario is even worse and worrisome. Our study indicates that most of these patents are in fact related to Sanofi, Takeda, and Butantan vaccine developments. The remaining active patents related to vaccine developments from other research institutes and companies have been interrupted due to insufficient investments or other constraints.
In addition, this study evidences important limitations in protection against viral subtypes and significant bottlenecks in laboratory infrastructure and platforms, constraining technology transfer and large-scale production. Regarding protection, protective immunity against the DENV has been insufficient in available vaccines due to a broad range of complex factors related to the host and to the diversity of viral subtypes. There is a need to have more in-depth research on the correlates of protection in order to have a better understanding of the diverse viral interactions. It would be also important to develop a flavivirus combined vaccine, such as dengue/Zika/yellow fever, or combined with other arboviruses such as chikungunya/oropouche. The reasons for the low number of vaccine candidates reaching the final stages of development are therefore multifaceted. Several factors contribute to bottlenecks in dengue vaccine development such as the requirement of large amounts of capital investment and sustained public-private partnerships. Many years are required for observational studies and there is a need to enroll many participants. Furthermore, there is the requirement to prove efficacy for four dengue serotypes, a lack of reliable correlates of protection, the risk of ADE, and the interaction with populational exposure to arboviruses such as Zika, chikungunya, and yellow fever.
Brazil, the country most affected by dengue and other reemerging arboviral diseases in the current year, should revise its very low funding policy for science and vaccine development and reduce its high dependence on technology transfer and import of inputs for vaccine development. The Brazilian government can play a leading role among G20 countries in supporting the implementation of innovative strategies for these neglected vaccines through innovative knowledge governance approaches based on artificial intelligence and big data, and integrated surveillance for quality data (epidemiological, genomic, antigenic, epizootic, laboratory, and participatory). These strategies should be supported by new business models, such as biotechnology clusters for vaccine development, with strong participation of private companies attracted to a geographic location for the development of mRNA vaccines and new platforms.
A paradigm change in DENV vaccine development is thus imperative. An exponential increase in global long-term funding by G7 and G20 countries to support the World Health Organization; Gavi, the Vaccine Alliance; and the International Vaccine Institute Dengue Initiative, will be key to reverse this scenario and achieve UN Agenda 2030 sustainable development goals.
Data availability statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.
Author contributions
CP: Writing – original draft, Writing – review & editing. EM: Writing – original draft, Writing – review & editing. AO: Writing – original draft, Writing – review & editing. SS: Writing – original draft, Writing – review & editing. AA: Writing – original draft, Writing – review & editing. AH: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The authors thank the financial support of Bio-Manguinhos, Oswaldo Cruz Foundation Brazil, of the Chemical School of the Federal University of Rio de Janeiro, of the Brazilian National Institute of Industrial Property – INPI and of the University of Pittsburgh, US.
Acknowledgments
The authors thank Prof. Jose Viña for the technical support in the design of the figures.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
- ^ Search strategy: Condition (infection, dengue virus), product category (vaccines), and therapeutic group (antiviral drugs) last updated from 10/03/2014 to 10/03/2024. Phases: clinical trials: phases 1, 2, and 3; preclinical, pre-registered, or launched). The search was carried out on March 12th, 2024.
- ^ CDDI is a platform exclusively on pharmacological, chemical, and drug/pharma development (9).
- ^ Search strategy: TAB=(Dengue or "Breakbone Fever") AND (TAB=(vaccine*) or ICR=((A61K0039)) or MC=((B14-S11*))) AND DPRY>=(2018) AND DPRY<=(2022). The search was carried out on March 27th, 2024. Note: International Patent Classification: A61K0039: “Medicinal preparations containing antigens or antibodies” and Derwent Manual Code: B14-S11: “Vaccine (General)”. The words “dengue” and “vaccine” were used in the title or claims to retrieve dengue and related vaccines.
- ^ Derwent Innovation (from Clarivate): This is a platform of more than 40,000 innovators with data intelligence technology, proprietary search, and advanced content (10).
- ^ Most recent deposits (from 2023) are confidential for 18 months after deposit.
- ^ Last update: “Date when the record was last updated with new information”(11).
- ^ The U.S. Army Medical Research and Material Command (USAMRMC) is a subordinate command of MEDCOM/US that provides vaccines and other solutions (medical products) to the armed forces (14).
- ^ Organization (Originator): “The body that invented or created the drug” (13).
- ^ Last update date: “Date when the record was last updated with new information”13. Ibid..
- ^ Dengvaxia and Qdenga are the brand names selected to describe the launched vaccines in the text.
- ^ Instituto Butantan produces the major serums in Brazil against the rabies virus, bacterial toxins and poisons (originated from venomous animals). It is also the main producer of immunobiologicals in Brazil, and the largest of serums/vaccines in Latin America. Butantan Institute is expected to submit request to the Brazilian regulatory agency ANVISA registration of its new Dengue vaccine by July 2024.
- ^ “Phase III Trial to Evaluate Efficacy and Safety of a Tetravalent Dengue Vaccine” (Clinical trials gov ID: NCT02406729) (22).
- ^ Resolution 661/23: With this resolution, the National Health Surveillance Agency (ANVISA) approved the registration of a new vaccine from Takeda Pharma Ltda called Qdeng (consisting of four different serotypes of the DENV virus).
- ^ Takeda Pharmaceutical Company is a global Japanese biopharmaceutical company. TAKEDA VACCINES INC is headquartered in the US.
References
1. World Health Organization. Vaccines and immunization(2024). Available online at: https://www.who.int/health-topics/vaccines-and-immunization#tab=tab_1 (Accessed May 15, 2024).
2. World Health Organization. Dengue - Global situation: Disease Outbreak News(2023). Available online at: https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON498 (Accessed Feb 20, 2024).
3. Brazilian Ministry of Health. Painel de Monitoramento das Arboviroses. Atualização dos casos de arboviroses(2024). Available online at: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/a/aedes-aEgypti/monitoramento-das-arboviroses/painel (Accessed May 15, 2024).
4. Possas C, Antunes A, Oliveira A, Schumacher S, Marques ET, Homma A. Vaccine innovation for dengue, Chikungunya, Zika and yellow fever: accelerating global development agenda and partnerships in post-COVID era. Vaccine Res. (2022) 9:1–17.
5. European Centre for Disease Prevention and Control, ECDC. Dengue worldwide overview (2024). Stockholm, Sweden. Available online at: https://www.ecdc.europa.eu/en/dengue-monthly (Accessed July 11, 2024).
6. Centers for Disease Control and Prevention. How vaccines are developed and approved for use 2023(2023). Available online at: https://www.cdc.gov/vaccines/basics/test-approve.html (Accessed May 20, 2024).
7. Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol. (2021) 21:83–100.
8. Torresi J, Ebert G, Pellegrini M. Vaccines licensed and in clinical trials for the prevention of dengue. Hum Vaccines Immunotherapeutics. (2017) 13:1059–72.
9. Cortellis Drug Discovery Intelligence. Welcome to Cortellis Drug Discovery Intelligence, the next generation of integrity(2024). Available online at: https://access.clarivate.com/login?app=drugdiscovery (Accessed May 17, 2024).
10. Clarivate. Derwent innovation(2024). Available online at: https://clarivate.com/derwent/ru/solutions/derwent-innovation-2/ (Accessed Apr. 4, 2024).
11. Clarivate. Welcome to Cortellis Drug Discovery Intelligence, the next generation of Integrity(2024). Available online at: https://www.cortellis.com/marketing/drugdiscovery/ (Accessed May 17, 2024).
12. World Intellectual Property Organization. Generative artificial intellligence. In: Patent Landscape Report. WIPO, Geneva (2024). doi: 10.34667/tind.49740
13. Cortellis. Cortellis Drug Discovery Intelligence Glossary and Help File. Clarivate analytics. (2020).
14. Institute of medicine. Resources, responsibilities, and dynamics in the military's vaccine mission. In: Protecting Our Forces: Improving Vaccine Acquisition and Availability in the U.S. Military. The National Academies Press, Washington, DC (2002). p. 20–38. doi: 10.17226/10483
15. Vuitika L, Prates-Syed WA, Silva JDQ, Crema KP, Côrtes N, Lira A, et al. Vaccines against emerging and neglected infectious diseases: an overview. Vaccines. (2022) 10:1385.
16. Homma A, Freire MDS, Possas C. Vaccines for neglected and emerging diseases in Brazil by 2030: the “valley of death” and opportunities for RD&I in Vaccinology 4.0 Vol. 36. Cadernos de Saúde Pública (2020). doi: 10.1590/0102-311X00128819
17. Lim S-K, Lee YS, Namkung S, Lim JK, Yoon I-K. Prospects for dengue vaccines for travelers. Clin Exp Vaccine Res. (2016) 5:89.
18. Whitehead SS. Development of TV003/TV005, a single dose, highly immunogenic live attenuated dengue vaccine; what makes this vaccine different from the Sanofi-Pasteur CYD™ vaccine? Expert Rev Vaccines. (2016) 15:509–17.
19. Durbin AP. Historical discourse on the development of the live attenuated tetravalent dengue vaccine candidate TV003/TV005. Curr Opin Virol. (2020) 43:79–87.
20. Precision Vaccinations. TetraVax-DV (V180) dengue vaccine(2024). Available online at: https://www.precisionvaccinations.com/vaccines/tetravax-dv-v180-dengue-vaccine (Accessed May 17, 2024).
21. Cortellis. TV-003(2024). Available online at: https://access.clarivate.com/login?app=drugdiscovery&referrer=%2Fdrugdiscovery%2Fentity%2Fdrug%2F689351%2Fproduct%3Fent%3DdKFrY209 (Accessed Mar. 20, 2024).
22. ClinicalTrials.gov. Phase III trial to evaluate efficacy and safety of a tetravalent dengue vaccine(2024). Available online at: https://clinicaltrials.gov/study/NCT02406729 (Accessed Mar. 21, 2024).
23. Kallás EG, Cintra MAT, Moreira JA, Patiño EG, Braga PE, Tenório JCV, et al. Live, attenuated, tetravalent butantan–dengue vaccine in children and adults. New Engl J Med. (2024) 390:12.
24. Cortellis. CDDI - drugs & Biologics record - Dengvaxia(2024). Available online at: https://access.clarivate.com/login?app=drugdiscovery&referrer=%2Fdrugdiscovery%2Fentity%2Fdrug%2F292955%2Fproduct%3Fent%3D0qg6zvLW (Accessed Mar. 18, 2024).
25. European Medicines Agency. Dengvaxia(2024). Available online at: https://www.ema.europa.eu/en/medicines/human/EPAR/dengvaxia (Accessed Mar. 18, 2024).
26. Merieux Foundation. World's first dengue vaccine authorized fou use in three endemic countries(2016). Available online at: https://www.fondation-merieux.org/en/news/worlds-first-dengue-vaccine-authorized-for-use-in-3-endemic-countries/ (Accessed Mar. 18, 2024).
27. Salje H, Alera MT, Chua MN, Hunsawong T, Ellison D, Srikiatkhachorn A, et al. Evaluation of the extended efficacy of the Dengvaxia vaccine against symptomatic and subclinical dengue infection. Nat Med. (2021) 27:1395–400.
28. Zorzetto R. Vacina do Butantan contra a dengue tem eficácia de 80%(2023). Available online at: https://revistapesquisa.fapesp.br/vacina-do-butantan-contra-a-dengue-tem-eficacia-de-80/ (Accessed Mar. 19, 2024). Pesquisa Fapesp.
29. Cortellis. TAK-003(2024). Available online at: https://access.clarivate.com/login?app=drugdiscovery&referrer=%2Fdrugdiscovery%2Fentity%2Fdrug%2F464338%2Fproduct%3Fent%3DWCxrSPdU (Accessed Mar. 20, 2024).
30. Takeda. Takeda’s QDENGA® (Dengue tetravalent vaccine [Live, attenuated]) approved for use in European union(2022). Available online at: https://www.takeda.com/newsroom/newsreleases/2022/takedas-qdenga-dengue-tetravalent-vaccine-live-attenuated-approved-for-use-in-european-union/ (Accessed Mar. 19, 2024).
31. Agência Brasil. Takeda prioritizes dengue vaccine for Brazilian public health system(2024). Available online at: https://agenciabrasil.ebc.com.br/en/saude/noticia/2024-02/takeda-prioritizes-dengue-vaccine-Brazilian-public-health-system (Accessed Mar. 19, 2024).
32. Wallace DW, Lefevre I. Dengue vaccine unit dose and administration thereof. Eur Patent Office patent. (2022) EP4082568.
33. Li J, Zhou Y, Ding X, Quan W, He J, Hua D, et al. Dengue tetravalent DNA vaccine and application thereof. patent China Patent 115990249. (2023).
34. Li J, Ding X, Zhou Y, Quan W, He J, Hua D, et al. Dengue tetravalent mRNA (messenger ribonucleic acid) vaccine, liposome nanoparticle as well as preparation method and application of liposome nanoparticle. China patent CN115779076A. (2023).
35. Li J, Hua D, Liu M, Guo H. Tetravalent dengue inactivated vaccine. China patent CN115518149A. (2022).
36. Michael SF, Isern S. Vaccines and methods for creating a vaccine for inducing immunity to all dengue virus serotypes. United States patent US10675343B2. (2020).
37. Lin Y-S, Yeh T-M, Chuang Y-C, Yu C-Y, Chen H-W, Wan S-W. Composition of subunit dengue vaccine. United States patent US11786587B2. (2023).
38. Jain R, Singh S, Jambu L. Stable live attenuated recombinant dengue vaccine. Philippines patent PH12018500675. (2018).
39. Shanghai Public Health Clinical. Modified mRNA vaccine for dengue virus. China patent CN113663063A. (2021).
40. Zhang H, Chen Q. Dengue virus vaccine with weakened antibody-dependent enhancement effect. China patent CN115850401A. (2023).
41. Krishna S, Sankaradoss A. DENV EDIII-NS1 consensus sequence-based dengue DNA vaccine. World Intellectual Property Organisation patent WO2023126882A1. (2023).
42. Kohara M, Yasui F, Yamane D, Kohara K, Morita K, Yasutomi Y, et al. Nagasaki University, assignees. Dengue virus vaccine. Eur Patent Office patent EP3939607A4. (2023).
43. Wallace D. Dengue vaccine unit dose and administration thereof. United States patent US11590221B2. (2023).
44. Gao F, Dai L, Yan J, Xu K, Han Y, Wang Q, et al. Zika/dengue vaccine and application thereof. Eur Patent Office patent EP4056582A. (2022).
45. Gao F, Dai L, Yan J, Xu K, Han Y, Wang Q, et al. Zika/dengue vaccine and application thereof. China patent CN114907456A. (2022).
46. Liao C-L, Hsieh M-S, Lien S-P. Multiple mosquito-borne flavivirus vaccine and use thereof in inducing neutralizing antibodies. Taiwan patent TWI756812B. (2022).
47. David S. Vaccine adjuvants. World Intellectual Property Organisation patent WO2023249996A1. (2023).
48. Akahata W, Smith JF, Alexander JL, Sekida T. Efficient vaccine. United States patent US20230201333A1. (2023).
Keywords: dengue vaccine, immunotherapy, innovation, platforms, clinical trials, patents
Citation: Possas C, Marques ETA, Oliveira A, Schumacher S, Antunes A and Homma A (2024) Dengue vaccine endgame: why has the global response to exponential demand in the tropics been so slow? Front. Trop. Dis 5:1441973. doi: 10.3389/fitd.2024.1441973
Received: 31 May 2024; Accepted: 09 August 2024;
Published: 09 September 2024.
Edited by:
Darrick Carter, PAI Life Sciences, United StatesReviewed by:
Andréa Nazaré Monteiro Rangel Da Silva, Federal University of Pará, BrazilJaime Torres, Central University of Venezuela, Venezuela
Copyright © 2024 Possas, Marques, Oliveira, Schumacher, Antunes and Homma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cristina Possas, Y3Jpc3RpbmEucG9zc2FzQGJpby5maW9jcnV6LmJy; Ernesto T. A. Marques, bWFycXVlc0BwaXR0LmVkdQ==
†These authors have contributed equally to this work
 Cristina Possas
Cristina Possas Ernesto T. A. Marques
Ernesto T. A. Marques Alessandra Oliveira
Alessandra Oliveira Suzanne Schumacher
Suzanne Schumacher Adelaide Antunes
Adelaide Antunes Akira Homma
Akira Homma