
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Trop. Dis., 23 July 2024
Sec. Neglected Tropical Diseases
Volume 5 - 2024 | https://doi.org/10.3389/fitd.2024.1429266
This article is part of the Research TopicClinical Review of Neglected Tropical DiseasesView all 8 articles
Scabies is a global public health issue, with approximately 455 million new cases worldwide each year. Scabies is a parasitic skin disease caused by infestation with the mite Sarcoptes scabiei var. hominis that can lead to secondary skin infections such as impetigo. In 2017, scabies was added to the World Health Organization’s list of neglected tropical diseases renewing calls for effective management and control of the disease. Mass drug administration has emerged as an effective strategy to control scabies, especially in highly endemic settings. In this review, we detail scabies epidemiology and risk factors, clinical characteristics and diagnosis, as well as control options, and future areas for scabies research.
Scabies is a parasitic skin disease caused by infestation with the microscopic mite Sarcoptes scabiei var. hominis. It is an obligate parasite, completing its life cycle on humans over four stages: (i) egg, (ii) larva, (iii) nymph, and (iv) adult, taking place over a two-week period (1). The fertilised female mite, measuring approximately 0.4 mm in length and 0.3 mm in width, represents the largest stage of the mite and is found in the upper layer of the skin, the stratum corneum, where it lives and lays its eggs. Scabies mites do not usually survive more than 48-72 hours off a human host but can live 24-40 days on a person’s skin. Scabies infestation causes intense itching, and it presents as a papular skin rash that can spread to one or more areas of the body (2). Symptoms can appear within a few days in hosts with previous scabies exposure, while it takes longer (up to 4-8 weeks) in hosts being infested for the first time.
Scabies is a global health concern, with a significant burden on affected individuals and communities. While it is a very common skin condition, scabies management, particularly in low-and middle-income countries is challenging. In 2017, scabies was classified as a Neglected Tropical Disease (NTD) by the World Health Organization (WHO) and 2030 global targets for scabies were set to manage and control the disease (3). This article aims to provide an overview of the current knowledge on scabies, including its epidemiology, transmission, clinical presentation, and diagnosis. It discusses available treatment options and highlights the need for future research and interventions to effectively manage and control this NTD.
Globally, scabies is a highly prevalent disease and is estimated to affect more than 200 million people at any one time, with approximately 455 million new cases each year (4). In high income settings, scabies cases are documented but sporadic and scabies is generally not perceived as a public health problem. In the general population, the reported prevalence of scabies in countries of Europe and the Middle East is low (<2%) and it is unclear whether this is due to socioeconomic factors or climate conditions (5). However, recent reports have shown evidence of an increase in scabies incidence in some countries, especially in individuals with immunocompromised or deteriorated health and in institutions such as hospitals, nursing homes, schools, and prisons where outbreaks are more common. In Germany, there has been a notable increase in scabies cases in the past two decades, with an estimated 200% increase in treated outpatient cases recorded between 2014 and 2016 (6). In addition, scabies incidence increased 9-fold between 2012 and 2019 (7). Similarly, the incidence of reported scabies increased by 300% between 2011 and 2020 in the Netherlands (8). In Japan, a national report estimated the annual number of scabies cases to be between 80,000 and 150,000 (9). Although institutional outbreaks remain frequent, national prevalence is estimated to be less than 1% (10). In institutions such as prisons, the crowded living conditions and frequent transfers facilitate the spread of infections, including scabies infestations, while also hindering the processes of diagnosis, case management, contact tracing, and outbreak detection (11). Similar observations have been reported in European refugee camps and vulnerable populations such as asylum seekers and migrants, where scabies has been recorded as one of the most frequent dermatological presentations in Germany, the Netherlands and Greece (12–18).
Scabies is endemic in many low-and middle-income countries, particularly those with tropical climates where crowded living conditions and poverty are common, and access to affective treatment is limited (19). Countries in the Western Pacific region, Central America and Indigenous communities in Australia have reported some of the highest prevalence globally, with studies documenting prevalence rates of over 20% in the general population, and up to 60% in children (5). A nationwide study in Fiji, found a prevalence of 23.6% in the general population, with 43.6% of children aged 5-9 years diagnosed with scabies (20). Similarly, in Solomon Islands, the population prevalence of scabies was 19% and among school-aged children scabies prevalence was reported to be as high as 34% (21, 22). In Ethiopia, prevalence is estimated to range between 4% and 35%, with an average all age adjusted prevalence of 14% (23, 24).
Scabies is more prevalent in children and older people likely due to increased exposure, softer skin, and lack of immunity (children), and reduced mobility and residing in residential aged care facilities (the elderly) (25, 26). The prevalence and severity of scabies can vary between sexes. There is no consistent evidence indicating that scabies is significantly more common in either males or females. Some studies have shown slight variations, likely due to differences in context, exposure risks and behaviours. While in some settings, males might have higher prevalence rates due to increased exposure in communal living environments like military barracks, prisons, or certain types of work settings, in other contexts, females might be more at risk of contracting scabies. As they are often considered the household’s primary caregivers, closer and prolonged contact with children who are more likely to be scabies carriers makes women more at risk of infestation, in certain settings. The risk of infection increases in settings with high population density, such as aged care facilities, schools, prisons, refugee camps and communities with overcrowded housing. In these settings reinfestation due to contact with untreated individuals or household members is common (27). Immunosuppressed and immunodeficient people are at an increased risk of crusted scabies, the less common and more severe form of scabies (28, 29).
Scabies is transmitted from person to person through prolonged direct skin-to-skin contact but sometimes also through the sharing of clothing, towels or bedding (1). It takes approximately 20 minutes of close contact (e.g. holding hands, nursing a baby, sleeping the same bed or sexual contact) for successful transmission to take place, spreading easily throughout households, schools, and healthcare settings (30–32). Scabies mites cannot fly or jump, only crawl so being near an infected person does not lead to transmission (1). The more severe and contagious form of the disease, crusted scabies (where there is an extremely high mite burden), is more easily transmitted than classic scabies due to the large number of mites present in the shedding of old skin or scales (1). While hosts with classic scabies have on average 10-15 mites per person, two million mites may be present on crusted scabies making them likely sources of re-infestation even after treatment occurred (28). Crusted scabies is more common in elderly people (25, 33). Although seen in both sexes, some studies indicate that males may have higher rates of crusted scabies, possibly due to delays in seeking treatment (34).
Classic scabies presents with generalised and intense pruritus (itching), often becoming worse at night (35). Even after successful treatment, some individuals may experience persistent itching and skin inflammation for several weeks or months. The itching is caused by the host allergic response to the mite and can affect all body areas except the head and neck, where only infants have scabies lesions. The itching may be very mild or absent in infants, the elderly, and people with cognitive impairment or who are immunosuppressed (35). Scabies burrows or papules are typically found in the finger and toe web spaces, wrists, waistline, buttocks, genitals in men and breast in females (Figure 1). Young children, the elderly and immunocompromised people may also display atypical clinical presentations of scabies which includes distribution of lesions on the face, scalp, soles and palms (Figure 2) (35). The intense itching caused by scabies infestation can lead to scratching and breaking of the skin. The presence of bacterial skin infections associated with scabies is, therefore, a common occurrence. Numerous studies have found that up to 70% of individuals with scabies also present with bacterial skin infections, such as impetigo, particularly young children (20). Signs such as redness around the affected area, the formation of yellow or golden crusts, or the presence of pus may indicate a co-existing bacterial skin infection (Figure 3).
Crusted scabies is characterised by psoriasiform hyperkeratotic plaques and widespread scales, with fissuring in severe cases (36). Unlike common scabies, individuals with crusted scabies may not experience pruritus. Crusted scabies is usually seen in immunosuppressed and frail older people (36, 37).
Differential diagnoses should be considered both in clinical settings and during community evaluations. Conditions that might be misinterpreted for scabies may include insect bites and skin infections such as impetigo, folliculitis, eczema, and tinea. Immune-mediated dermatological conditions including bullous pemphigoid and popular urticaria should be considered. More careful evaluation by trained professionals may be necessary for crusted scabies. Differential diagnoses should include psoriasis, seborrheic dermatitis and widespread tinea.
Scabies is frequently observed in HIV-positive patients, who often present with various dermatological conditions such as psoriasis and seborrheic dermatitis (38). In HIV-positive patients, the immune system’s compromised ability to respond to infections can lead to atypical presentations of scabies, which may be misdiagnosed or overlooked (39). This delay can increase the risk of infestation spread within the community. It can also result in prolonged itching and an increased likelihood of secondary bacterial infections, such as impetigo or cellulitis, which can further complicate the clinical picture and require additional treatment. HIV-positive individuals are particularly susceptible to severe forms of scabies, including crusted scabies (37). This severe manifestation is not only harder to treat but also poses a higher risk of transmission to others. Timely and accurate diagnosis of scabies in HIV-positive patients is essential to prevent complications and to reduce the risk of transmission. Standard treatments, such as permethrin cream and oral ivermectin, can be used, but close monitoring is necessary to ensure effectiveness and to manage potential side effects (40).
Scabies infestation is often complicated by secondary skin infection with bacteria such as invasive Streptococcus pyogenes (Group A Streptococcus) and Staphylococcus aureus, which can in turn, lead to more severe bacterial infections (19). Bacterial infection may co-exist with scabies when the burrowing of mites and associated scratching breaches the skin barrier together with the scabies mites downregulating host immunity and creating an environment for bacterial growth (41–43). These organisms can cause subsequent invasive and potentially fatal skin and soft tissue infections such as impetigo, abscess, cellulitis or necrotising fasciitis (44). Repeat Streptococcus pyogenes infections can result in severe downstream disease such as acute post-streptococcal glomerulonephritis (APSGN) and acute rheumatic fever, which in turn increase the risk of systemic complications including septicemia, kidney disease, hypertension and possibly rheumatic heart disease (19, 45).
Beside the discomfort caused by intense itching and potential serious complications described above, scabies impacts quality of life in individuals and communities. By disrupting sleep, school or work performance, scabies impacts the economic productivity of communities and causes stigma, discrimination and social isolation (46–49).
The diagnosis of scabies is generally based on clinical skin examination, and high index of suspicion for those presenting with a widespread pruritic rash and symptoms reported by close contacts. In high-income settings and for more challenging cases and those not presenting with typical distribution of scabies lesions, other diagnostic methods can be applied to confirm parasitological diagnosis of scabies. A recent meta-analysis of diagnostic techniques, including dermoscopy, skin scraping, adhesive tape, and polymerase chain reaction (described below) showed no significant difference in sensitivity between techniques used to diagnose scabies (27, 28).
Dermoscopy (or dermatoscopy) aids in diagnosis through direct visualisation of a scabetic burrow, with a ‘delta sign’ characteristic of the mite, usually at the end of the burrow. The application of ink from a pen over the entrance of a burrow may confirm the diagnosis as the ink tracks along the burrow, which is a simple bedside test called the ‘burrow ink test’ (BIT) (29). Excess ink is removed using an alcohol wipe or equivalent and the remaining ink demarcates the burrow within the stratum corneum where the mite has tunnelled (29).
Simple light microscopy of skin scrapings may show direct visualisation of the mites, eggs or faeces in skin samples, but has reported detection rates varying from 10 to 70%, and a risk of false negative results, due to inadequate tissue or incorrect location of sampling, and operator error. Given the mobile nature of the mite incorrect sampling of site is a common error and the mite may be missed.
Adhesive tape applied to skin where a lesion is present, and removed rapidly can be transferred to a glass slide for microscopy, this facilitates direct visualisation of the mite, faeces or eggs that are adherent to tape media.
Newer techniques utilising probe-based real time polymerase chain reaction (PCR) assays to detect scabies DNA sequences present in samples from dry swabs of lesions has been developed (30).
In 2020, the International Alliance for the Control of Scabies (IACS), a global network of researchers, public health experts, and organizations dedicated to the control and elimination of scabies, undertook a Delphi process to develop consensus criteria to standardise and improve the accuracy of scabies diagnosis (31, 50, 51).
These criteria categorise scabies into three levels, based on level of certainty ranging from suspected, clinical and confirmed scabies for use in a variety of research, clinical or public health settings (see Table 1) (50, 51). Confirmed scabies is diagnosed by the presence of mites, eggs, or faeces on microscopy, dermoscopy, or a high-powered imaging device. Clinical scabies is diagnosed based on the presence of scabies burrows, typical lesions affecting male genitalia, or typical lesions in a typical distribution with two history features. Suspected scabies includes cases with typical lesions in a typical distribution with one history feature or atypical lesions with two history features. These criteria facilitate consistent diagnosis across different settings.
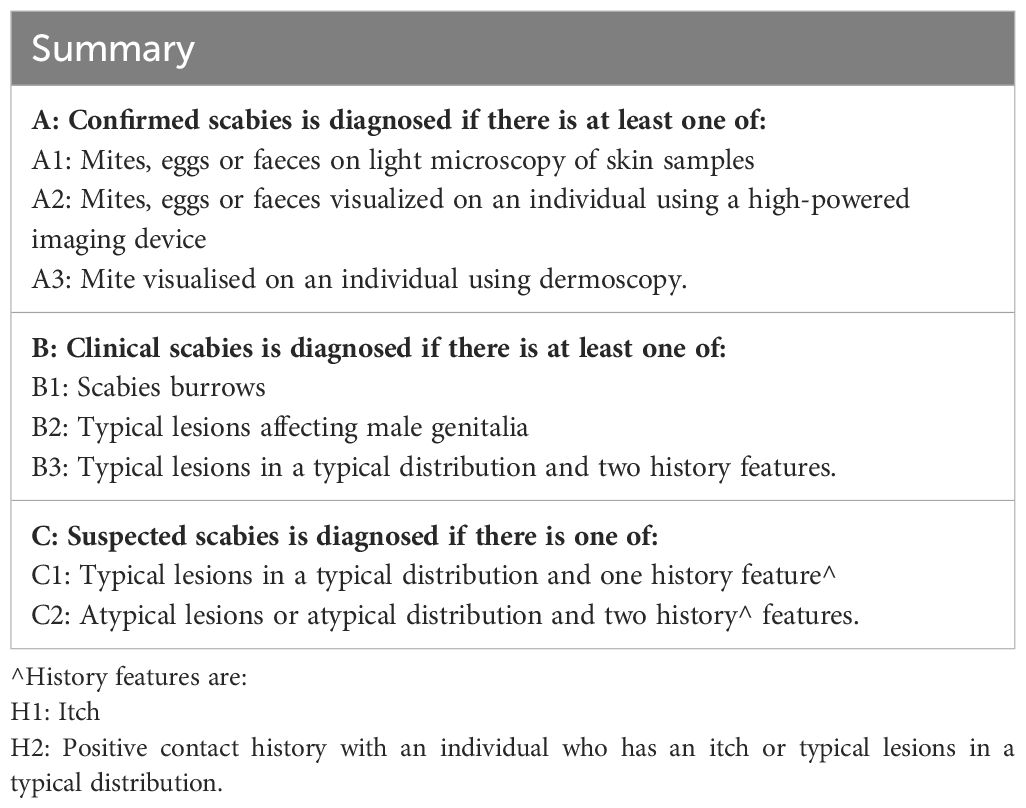
Table 1 Summary of The International Alliance for the Control of Scabies (IACS) updated consensus criteria for the diagnosis of scabies in 2020 (adapted from Engelman D et al.).
However, these criteria are complex so a simplified examination for mapping prevalence in endemic settings was developed, to determine high prevalence areas for scabies control in resource-poor settings. The simplified criteria for diagnosing scabies prioritise key diagnostic signs such as typical scabies lesions (e.g., burrows, papules, nodules) and intense nighttime itching, in common areas like wrists, elbows, finger webs, and genital areas. They are designed for use by non-specialist healthcare workers, requiring minimal equipment and training, and are suitable for application in community settings without advanced diagnostic tools. These criteria, validated in the Pacific region, demonstrated high levels of sensitivity and specificity in diagnosing scabies when compared to the 2020 IACS criteria. They are believed to be an accurate and efficient way to rapidly map scabies in countries with high levels of endemicity (52).
Effective treatment options for scabies are topical and oral agents (see Table 2). Permethrin 5% cream is a the most effective topical treatment, which must be applied to the whole body from neck to toe and washed off after 8-12 hours. Permethrin is active against all stages of the parasite’s life cycle so, if applied correctly, no repeat dose should be required (53). However, a second dose is often recommended 7-14 days after the first treatment. All household and close contacts (including sexual partners) should be treated at the same time. Benzyl benzoate 25% cream is also used for the treatment of scabies, once applied to the skin it must be left for 24 hours before being washed off. However, it has been found to cause discomfort and skin irritation (54). Despite being highly effective, compliance with topical treatments is often low (especially in asymptomatic contacts) due to lack of treatment priority, the time taken to apply the cream, difficulties to keep it on for the duration necessary, and discomfort experienced once the cream has been applied (27).
Ivermectin, an oral agent, is an antiparasitic drug belonging to the ivermectin family of medications (55). Due to its broad spectrum antiparasitic activity, it is used to treat NTDs such as lymphatic filariasis, onchocerciasis, and soil-transmitted helminths (55). Ivermectin is active against scabies mites, but not their eggs and has a short half-life (12-56 hours), so repeat dosing 7-14 days after the first dose is needed to kill newly hatched mites (53). Guidelines also recommend treatment for household contacts. Due to limited safety data, ivermectin is not recommended for use in pregnant women or children weighing less than 15kg (3). Some adverse side effects have been reported, including itching, headaches, abdominal and joint pain, and dizziness, but these are typically temporary and mild in nature (56). One benefit of ivermectin over topical permethrin is that it is consumed orally, possibly increasing the likelihood that household and close contacts will adhere to treatment, particularly in campaigns aiming to control scabies in communities with endemic disease. A 2018 Cochrane review showed no difference in efficacy between permethrin and ivermectin, suggesting instead that choice of treatment should be based on practicability, availability, drug licensing and cost (57).
While ivermectin is currently the only available oral treatment against scabies, new alternative oral treatments are under investigation. Moxidectin, a macrocyclic lactone, has shown promise as an alternative treatment for scabies. It works by binding to glutamate-gated chloride ion channels in the parasite’s nerve and muscle cells, leading to paralysis and death of the mites. Recent studies have demonstrated moxidectin’s efficacy in treating human scabies (58–60). It has several advantages over traditional treatments like ivermectin and permethrin: with a long half-life that spans the entire life cycle of the scabies mite, and a good safety profile shows promising preliminary results as an effective single-dose regimen, with clinical trials currently underway.
Treatment failure in scabies can occur for several reasons. Improper application of topical treatments, such incomplete body coverage, washing off the medication sooner than indicated, or inadequate dosing intervals with topical and oral treatments can contribute to treatment failure and persistent infestations. Although rare, there have been reports of resistance to medications and reduced efficacy in some regions, suggesting the emergence of resistance with permethrin and ivermectin. Scabies mites may develop resistance to treatments through various mechanisms. One common mechanism is genetic mutation that alters the target site of the drug, rendering it less effective. In the case of permethrin, resistance can occur due to mutations in the sodium channels of the mites, which the drug targets (61–64). For ivermectin, resistance may develop through mutations that affect the drug’s ability to bind to the mites’ nerve and muscle cells. Over time, the continuous and widespread use of these treatments can select for resistant mite populations, making standard therapies less effective. To mitigate this risk, it is essential to adhere to treatment protocols and consider alternative therapies or combination treatments when resistance is suspected. For example, combining topical permethrin with oral ivermectin has been shown to be effective in some cases of treatment-resistant scabies (56, 57, 65). Ongoing surveillance and research into new treatment options, such as moxidectin, are necessary to stay ahead of potential resistance developments.
In addition to antiparasitic treatments, accompanying dermatological therapy is crucial for managing the symptoms of scabies, particularly the intense itching and skin inflammation caused by the delayed hypersensitivity reaction. Topical corticosteroids and antihistamines can be prescribed to alleviate itching and reduce inflammation, improving patient comfort and adherence to the treatment regimen. Emollients may also be recommended to soothe the skin and prevent further damage and irritation.
Environmental measures such as washing clothes and linen in hot water and drying them at high temperatures to kill mites and eggs can complement medical therapies in communities with high prevalence. However, there is limited literature available on the efficacy of these environmental measures in controlling scabies. The effectiveness of environmental measures can vary depending on the severity of the infestation, climate, and other conditions. Therefore, while environmental management is a recommended component of scabies control, it should be part of a comprehensive strategy that includes direct treatment of affected individuals and broader public health interventions.
Control strategies endorsed by WHO to eliminate scabies as a public health problem in low and middle-income settings include preventative chemotherapy using mass drug administration (MDA). In settings where scabies is sporadic or with community prevalence below 10%, treatment is typically focused on an individual and household and close contacts. In endemic or outbreak settings, and in areas where prevalence is 10% or greater, community-wide treatment known as MDA, where the whole of the community is given medication regardless of infection status, is an effective and safe strategy (3, 26, 56). MDA using topical, oral or systemic medication can be delivered nationally or within confined settings such as nursing homes, schools, prisons and hospitals (5). MDA has been used worldwide to control a number of highly prevalent NTDs, including lymphatic filariasis, onchocerciasis, soil-transmitted helminths, and trachoma (66).
MDA for scabies control has been conducted in a range of settings using both topical and oral treatment regimes. These studies show MDA for scabies can substantially reduce both scabies and impetigo prevalence within target communities. MDA has been found to be particularly effective in communities with very high baseline prevalence (26). In Fiji, ivermectin-based MDA reduced scabies prevalence by 94% (from 32% to less than 2%) 12 months following the intervention (56, 67). Similar results were reported in other countries of the Pacific region (21, 44, 68–71). Studies showed that reduction in prevalence for both scabies and associated bacterial infections can be sustained up to three years (72, 73). A recent systematic review and meta-analysis showed a relative reduction of scabies (79%) and impetigo (66%) following MDA (67). WHO supports ivermectin-MDA for scabies and the medication is now included on the WHO essential medicines list for scabies (3). While ivermectin-based MDA is highly effective, the need for two doses 7-14 days apart is still considered the gold standard, posing a significant disadvantage to its use (26).
The WHO Informal Consultation on a Framework for Scabies Control meeting held in 2019 in Manila and the WHO’s first global meeting on skin NTDs held in 2023 in Geneva identified several key areas of importance to advance efforts to control scabies, globally. These included the need for increased visibility of skin NTDs, including scabies, within the broader context of NTDs, primary health care and universal health coverage. The importance of stronger country leadership, strategic advocacy, and increased funding at local and global levels to effectively control scabies are also highlighted (74, 75).
Several research gaps are identified by experts as priorities for effective scabies control in endemic communities. These include the need for standardised approaches, newer diagnostic tests, and digital technologies to aid in the field diagnosis of scabies, including the development of a simple, low-cost rapid assessment tool is also highlighted as key priority (19, 75). The simplified criteria for mapping scabies prevalence in endemic settings developed and validated in communities of the Pacific region and other countries, demonstrated its efficiency and accuracy in rapidly identifying high prevalence areas for scabies control (52).
The inclusion of implementation research that evaluates the integration of scabies control with other NTD programs to determine efficacy and cost-effectiveness is also needed. The significance of surveillance and mapping of co-endemicity to guide integrated interventions is often recognised and recommended by ministries of health of countries with high scabies prevalence, as well as the recent WHO Road Map for Neglected Tropical Diseases (76). However, integration of multiple health programs is only beneficial when there is strong collaboration and coordination among stakeholders, including healthcare providers, government agencies, community leaders, and non-governmental organizations. This highlights the importance of considering scabies control within a broader public health context.
One possible barrier for integration is that unlike other NTDs programs, ivermectin MDA for scabies requires two doses, 7-14 days apart. Further research to evaluate the definitive effectiveness of single dose ivermectin MDA versus the standard two-dose regimen would be beneficial to determine the feasibility and cost-effectiveness of integrating scabies control with other health programs. Moxidectin, another oral antiparasitic drug, has shown promising potential for the treatment of NTDs, including scabies. It has a longer half-life compared to ivermectin, making it a potential candidate for MDA programs, where a single dose can provide long-lasting protection (59, 60). In clinical trials, moxidectin has demonstrated high efficacy against scabies. A single oral dose of moxidectin has significantly reduced scabies infestation in treated individuals. The long-lasting effect of moxidectin provides an advantage over the standard two-dose regimen of ivermectin. Furthermore, moxidectin has also shown efficacy against other NTDs such as onchocerciasis and lymphatic filariasis and has been included as an alternative treatment option in the WHO guidelines for the control and elimination of these two NTDs. Further investigation into the safety of ivermectin and moxidectin in young children is needed, as well on the cost-effectiveness of both agents in community control programs for scabies.
Finally, limited social science research is available to inform NTD research and control efforts, globally (77). Rigorous qualitative research can provide much needed insights into the socio-structural factors that may enable or impede the success of NTD prevention, control and elimination efforts. Further research that utilises a qualitative social science lens to explore the social and structural determinants of scabies and scabies control, including MDA design and implementation would be highly beneficial (46, 75, 77). Operational research that identifies the most effective way to implement MDA in a range of settings, including peri-urban and urban settings and regions outside the Pacific is also a priority (75).
EM: Data curation, Project administration, Resources, Validation, Writing – original draft, Writing – review & editing. MWa: Writing – original draft, Writing – review & editing. JM: Conceptualization, Project administration, Resources, Writing – review & editing. MWh: Writing – review & editing. LR: Conceptualization, Data curation, Methodology, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. LR is supported by a National Health and Medical Research Council Investigator Grant RG220260.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Yotsu RR, Yoshizumi J, Izri A. Biology of Sarcoptes scabiei and its relevance to human scabies: clinical symptoms, treatment, and management. In: Scabies [Internet]. Springer Nature, Switzerland (2023). p. [19–34].
2. Mellanby K. The development of symptoms, parasitic infection and immunity in human scabies. Parasitology. (1944) 35:197–206. doi: 10.1017/S0031182000021612
3. WHO. Scabies Fact Sheet: World Health Organization. (2023). Available online at: https://www.who.int/news-room/fact-sheets/detail/scabies.
4. Vos T, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. (2017) 390:1211–59. doi: 10.1016/S0140-6736(17)32154-2
5. Romani L, Steer AC, Whitfeld MJ, Kaldor JM. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. (2015) 15:960–7. doi: 10.1016/S1473-3099(15)00132-2
6. Deutsche Dermatologie. Practice routine is significantly disrupted” Scabies cases have tripled in North Rhine. (2024). Available online at: https://www.springermedizin.de/praxisablauf-wird-erheblich-gestoert/12019044?fulltextView=true.
7. Sunderkotter C, Wohlrab J, Hamm H. Scabies: epidemiology, diagnosis, and treatment. Dtsch Arztebl Int. (2021) 118:695–704. doi: 10.3238/arztebl.m2021.0296
8. van Deursen B, Hooiveld M, Marks S, Snijdewind I, van den Kerkhof H, Wintermans B, et al. Increasing incidence of reported scabies infestations in the Netherlands, 2011-2021. PloS One. (2022) 17:e0268865. doi: 10.1371/journal.pone.0268865
9. Executive Committee of Guideline for the Diagnosis and Treatment of Scabies. Guideline for the diagnosis and treatment of scabies in Japan (third edition): Executive Committee of Guideline for the Diagnosis and Treatment of Scabies. J Dermatol. (2017) 44:991–1014. doi: 10.1111/1346-8138.13896
10. Yamaguchi Y, Murata F, Maeda M, Fukuda H. Investigating the epidemiology and outbreaks of scabies in Japanese households, residential care facilities, and hospitals using claims data: the Longevity Improvement & Fair Evidence (LIFE) study. IJID Reg. (2024) 11:100353. doi: 10.1016/j.ijregi.2024.03.008
11. Bedoya Del Campillo A, Lleopart N, Ch QG, Alvarez M, Montilla M, Martinez-Carpio PA. Intervention protocol to improve scabies control in enclosed communities: a case report. Rev Esp Sanid Penit. (2021) 23:37–42. doi: 10.18176/resp
12. Solbach P, Sdlacek L, Schmidt RE, Behrens G, Jablonka A. Mass therapy with Ivermectin for Scabies outbreak during the refugee crisis in Germany in 2015 [Systemischeantiinfektive Therapieeines Skabiesausbruchsmit Ivermectin in Einem Flüchtlingslager]. Anasthesiologie und Intensivmedizin. (2017) 58:534–41. doi: 10.19224/ai2017.534
13. Louka C, Logothetis E, Engelman D, Samiotaki-Logotheti E, Pournaras S, Stienstra Y. Scabies epidemiology in health care centers for refugees and asylum seekers in Greece. PloS Negl Trop Dis. (2022) 16:e0010153. doi: 10.1371/journal.pntd.0010153
14. Wollina U, Gaber B, Mansour R, Langner D, Hansel G, Koch A. Dermatologic challenges of health care for displaced people. Lessons from a German emergency refugee camp. Our Dermatol Online. (2016) 7:136–8. doi: 10.7241/ourd
15. Beeres DT, Ravensbergen SJ, Heidema A, Cornish D, Vonk M, Wijnholds LD, et al. Efficacy of ivermectin mass-drug administration to control scabies in asylum seekers in the Netherlands: A retrospective cohort study between January 2014 - March 2016. PloS Negl Trop Dis. (2018) 12:e0006401. doi: 10.1371/journal.pntd.0006401
16. Kuhne A, Gilsdorf A. [Infectious disease outbreaks in centralized homes for asylum seekers in Germany from 2004-2014]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. (2016) 59:570–7. doi: 10.1007/s00103-016-2332-9
17. Kwak R, Kamal K, Charrow A, Khalifian S. Mass migration and climate change: Dermatologic manifestations. Int J Womens Dermatol. (2021) 7:98–106. doi: 10.1016/j.ijwd.2020.07.014
18. Richardson NA, Cassell JA, Head MG, Lanza S, Schaefer C, Walker SL, et al. Scabies outbreak management in refugee/migrant camps in Europe 2014-2017: a retrospective qualitative interview study of healthcare staff experiences and perspectives. BMJ Open. (2023) 13:e075103. doi: 10.1136/bmjopen-2023-075103
19. Engelman D, Cantey PT, Marks M, Solomon AW, Chang AY, Chosidow O, et al. The public health control of scabies: priorities for research and action. Lancet. (2019) 394:81–92. doi: 10.1016/S0140-6736(19)31136-5
20. Romani L, Koroivueta J, Steer AC, Kama M, Kaldor JM, Wand H, et al. Scabies and impetigo prevalence and risk factors in Fiji: a national survey. PloS Negl Trop Dis. (2015) 9:e0003452. doi: 10.1371/journal.pntd.0003452
21. Romani L, Marks M, Sokana O, Nasi T, Kamoriki B, Cordell B, et al. Efficacy of mass drug administration with ivermectin for control of scabies and impetigo, with coadministration of azithromycin: a single-arm community intervention trial. Lancet Infect Dis. (2019) 19:510–8. doi: 10.1016/S1473-3099(18)30790-4
22. Lake SJ, Engelman D, Sokana O, Nasi T, Boara D, Marks M, et al. Health-related quality of life impact of scabies in the Solomon Islands. Trans R Soc Trop Med Hyg. (2022) 116:148–56. doi: 10.1093/trstmh/trab096
23. Walker SL, Lebas E, De Sario V, Deyasso Z, Doni SN, Marks M, et al. The prevalence and association with health-related quality of life of tungiasis and scabies in schoolchildren in southern Ethiopia. PloS Negl Trop Dis. (2017) 11:e0005808. doi: 10.1371/journal.pntd.0005808
24. Azene AG, Aragaw AM, Wassie GT. Prevalence and associated factors of scabies in Ethiopia: systematic review and Meta-analysis. BMC Infect Dis. (2020) 20:380. doi: 10.1186/s12879-020-05106-3
25. Raffi J, Suresh R, Butler DC. Review of scabies in the elderly. Dermatol Ther (Heidelb). (2019) 9:623–30. doi: 10.1007/s13555-019-00325-2
26. Engelman D, Steer AC. Control strategies for scabies. Trop Med Infect Dis. (2018) 3:98. doi: 10.3390/tropicalmed3030098
27. La Vincente S, Kearns T, Connors C, Cameron S, Carapetis J, Andrews R. Community management of endemic scabies in remote aboriginal communities of northern Australia: low treatment uptake and high ongoing acquisition. PloS Negl Trop Dis. (2009) 3:e444. doi: 10.1371/journal.pntd.0000444
28. Chosidow O. Clinical practices. Scabies N Engl J Med. (2006) 354:1718–27. doi: 10.1056/NEJMcp052784
29. Davis JS, McGloughlin S, Tong SY, Walton SF, Currie BJ. A novel clinical grading scale to guide the management of crusted scabies. PloS Negl Trop Dis. (2013) 7:e2387. doi: 10.1371/journal.pntd.0002387
30. Hay RJ, Steer AC, Engelman D, Walton S. Scabies in the developing world–its prevalence, complications, and management. Clin Microbiol Infect. (2012) 18:313–23. doi: 10.1111/j.1469-0691.2012.03798.x
31. Engelman D, Kiang K, Chosidow O, McCarthy J, Fuller C, Lammie P, et al. Toward the global control of human scabies: introducing the International Alliance for the Control of Scabies. PloS Negl Trop Dis. (2013) 7:e2167. doi: 10.1371/journal.pntd.0002167
32. Tjioe M, Vissers WH. Scabies outbreaks in nursing homes for the elderly: recognition, treatment options and control of reinfestation. Drugs Aging. (2008) 25:299–306. doi: 10.2165/00002512-200825040-00003
33. White LC, Lanza S, Middleton J, Hewitt K, Freire-Moran L, Edge C, et al. The management of scabies outbreaks in residential care facilities for the elderly in England: a review of current health protection guidelines. Epidemiol Infect. (2016) 144:3121–30. doi: 10.1017/S0950268816001746
34. Niode NJ, Adji A, Gazpers S, Kandou RT, Pandaleke H, Trisnowati DM, et al. Crusted scabies, a neglected tropical disease: case series and literature review. Infect Dis Rep. (2022) 14:479–91. doi: 10.3390/idr14030051
35. Coates SJ, Thomas C, Chang AY. Common scabies and special presentations. In: Scabies [Internet]. Springer Nature, Switzerland (2023). p. 207–19.
36. Slape D, Russell R, McMeniman E. Clinical manifestations of severe scabies. In: Fischer K, Chosidow O, editors. Scabies. Springer Nature, Switzerland (2023). p. 233–68.
37. Pakanati K, Jagota D, Ladogana M. Norwegian scabies in HIV/AIDS. Proc (Bayl Univ Med Cent). (2022) 35:346–7. doi: 10.1080/08998280.2022.2028705
38. Portu JJ, Santamaria JM, Zubero Z, Almeida-Llamas MV, Aldamiz-Etxebarria San Sebastian M, Gutierrez AR. Atypical scabies in HIV-positive patients. J Am Acad Dermatol. (1996) 34:915–7. doi: 10.1016/S0190-9622(96)90079-1
39. Thappa DM, Karthikeyan K. Exaggerated scabies: a marker of HIV infection. Indian Pediatr. (2002) 39:875–6.
40. Taplin D, Meinking TL. Treatment of HIV-related scabies with emphasis on the efficacy of ivermectin. Semin Cutan Med Surg. (1997) 16:235–40. doi: 10.1016/S1085-5629(97)80047-8
41. Steer AC, Jenney AW, Kado J, Batzloff MR, La Vincente S, Waqatakirewa L, et al. High burden of impetigo and scabies in a tropical country. PloS Negl Trop Dis. (2009) 3:e467. doi: 10.1371/journal.pntd.0000467
42. Swe PM, Zakrzewski M, Kelly A, Krause L, Fischer K. Scabies mites alter the skin microbiome and promote growth of opportunistic pathogens in a porcine model. PloS Negl Trop Dis. (2014) 8:e2897. doi: 10.1371/journal.pntd.0002897
43. Swe PM, Fischer K. A scabies mite serpin interferes with complement-mediated neutrophil functions and promotes staphylococcal growth. PloS Negl Trop Dis. (2014) 8:e2928. doi: 10.1371/journal.pntd.0002928
44. Thean LJ, Romani L, Engelman D, Wand H, Jenney A, Mani J, et al. Prevention of bacterial complications of scabies using mass drug administration: A population-based, before-after trial in Fiji, 2018-2020. Lancet Reg Health West Pac. (2022) 22:100433. doi: 10.1016/j.lanwpc.2022.100433
45. Parks T, Smeesters PR, Steer AC. Streptococcal skin infection and rheumatic heart disease. Curr Opin Infect Dis. (2012) 25(25):145–53. doi: 10.1097/QCO.0b013e3283511d27
46. Mitchell E, Bell S, Thean LJ, Sahukhan A, Kama M, Koroivueti A, et al. Community perspectives on scabies, impetigo and mass drug administration in Fiji: A qualitative study. PloS Negl Trop Dis. (2020) 14:e0008825. doi: 10.1371/journal.pntd.0008825
47. van der Linden N, van Gool K, Gardner K, Dickinson H, Agostino J, Regan DG, et al. A systematic review of scabies transmission models and data to evaluate the cost-effectiveness of scabies interventions. PloS Negl Trop Dis. (2019) 13:e0007182. doi: 10.1371/journal.pntd.0007182
48. Worth C, Heukelbach J, Fengler G, Walter B, Liesenfeld O, Feldmeier H. Impaired quality of life in adults and children with scabies from an impoverished community in Brazil. Int J Dermatol. (2012) 51:275–82. doi: 10.1111/j.1365-4632.2011.05017.x
49. Jackson A, Heukelbach J, Filho A, Campelo Júnior E, Feldmeier H. Clinical features and associated morbidity of scabies in a rural community in Alagoas, Brazil. Trop Med Int Health. (2007) 12:493–502. doi: 10.1111/j.1365-3156.2006.01809.x
50. Engelman D, Yoshizumi J, Hay R, Osti M, Micali G, Norton S, et al. The 2020 international alliance for the control of scabies consensus criteria for the diagnosis of scabies. Br J Dermatol. (2020) 183:808–20. doi: 10.1111/bjd.18943
51. Engelman D, Fuller LC, Steer AC, for the International Alliance for the Control of Scabies Delphi p. Consensus criteria for the diagnosis of scabies: A Delphi study of international experts. PloS Negl Trop Dis. (2018) 12:e0006549. doi: 10.1371/journal.pntd.0006549
52. Tsoi SK, Lake SJ, Thean LJ, Matthews A, Sokana O, Kama M, et al. Estimation of scabies prevalence using simplified criteria and mapping procedures in three Pacific and southeast Asian countries. BMC Public Health. (2021) 21:2060. doi: 10.1186/s12889-021-12039-2
54. Tilakaratne D, De Rosa N, Currie B. Management of common scabies. In: Fischer K, Chosidow O, editors. Scabies. Springer Nature, Switzerland (2023). p. 345–56.
55. Campbell WC. Lessons from the history of ivermectin and other antiparasitic agents. Annu Rev Anim Biosci. (2016) 4:1–14. doi: 10.1146/annurev-animal-021815-111209
56. Romani L, Whitfeld MJ, Koroivueta J, Kama M, Wand H, Tikoduadua L, et al. Mass drug administration for scabies control in a population with endemic disease. N. Engl J Med. (2015) 373:2305–13. doi: 10.1056/NEJMoa1500987
57. Rosumeck S, Nast A, Dressler C. Ivermectin and permethrin for treating scabies. Cochrane Database Syst Rev. (2018) 4:Cd012994. doi: 10.1002/14651858.CD012994
58. Sharaf M, Antonios S, Mina S, Eliwa K, Rayia DA. The scabicide effect of moxidectin in vitro and in experimental animals: Parasitological, histopathological and immunological evaluation. Exp Parasitol. (2020) 217:107961. doi: 10.1016/j.exppara.2020.107961
59. Bernigaud C, Fang F, Fischer K, Lespine A, Aho LS, Dreau D, et al. Preclinical study of single-dose moxidectin, a new oral treatment for scabies: efficacy, safety, and pharmacokinetics compared to two-dose ivermectin in a porcine model. PloS Negl Trop Dis. (2016) 10:e0005030. doi: 10.1371/journal.pntd.0005030
60. Mounsey KE, Bernigaud C, Chosidow O, McCarthy JS. Prospects for moxidectin as a new oral treatment for human scabies. PloS Negl Trop Dis. (2016) 10:e0004389. doi: 10.1371/journal.pntd.0004389
61. Walton SF, Myerscough MR, Currie BJ. Studies in vitro on the relative efficacy of current acaricides for Sarcoptes scabiei var. hominis. Trans R Soc Trop Med Hyg. (2000) 94:92–6. doi: 10.1016/S0035-9203(00)90454-1
62. Mounsey KE, Holt DC, McCarthy JS, Currie BJ, Walton SF. Longitudinal evidence of increasing in vitro tolerance of scabies mites to ivermectin in scabies-endemic communities. Arch Dermatol. (2009) 145:840–1. doi: 10.1001/archdermatol.2009.125
63. Currie BJ, Harumal P, McKinnon M, Walton SF. First documentation of in vivo and in vitro ivermectin resistance in Sarcoptes scabiei. Clin Infect Dis. (2004) 39:e8–12. doi: 10.1086/421776
64. De Sainte Marie B, Mallet S, Gaudy-Marqueste C, Baumstarck K, Bentaleb N, Loundou A, et al. [Therapeutic failure in scabies: An observational study]. Ann Dermatol Venereol. (2016) 143:9–15. doi: 10.1016/j.annder.2015.10.588
65. Rosumeck S, Nast A, Dressler C. Evaluation of ivermectin vs permethrin for treating scabies-summary of a cochrane review. JAMA Dermatol. (2019) 155:730–2. doi: 10.1001/jamadermatol.2019.0279
66. Hotez PJ. Mass drug administration and integrated control for the world's high-prevalence neglected tropical diseases. Clin Pharmacol Ther. (2009) 85:659–64. doi: 10.1038/clpt.2009.16
67. Lake SJ, Kaldor JM, Hardy M, Engelman D, Steer AC, Romani L. Mass drug administration for the control of scabies: A systematic review and meta-analysis. Clin Infect Dis. (2022) 75:959–67. doi: 10.1093/cid/ciac042
68. Hardy M, Samuela J, Kama M, Tuicakau M, Romani L, Whitfeld MJ, et al. Community control strategies for scabies: A cluster randomised noninferiority trial. PloS Med. (2021) 18:e1003849. doi: 10.1371/journal.pmed.1003849
69. Lake SJ, Engelman D, Sokana O, Nasi T, Boara D, Grobler AC, et al. Defining the need for public health control of scabies in Solomon Islands. PloS Negl Trop Dis. (2021) 15:e0009142. doi: 10.1371/journal.pntd.0009142
70. Willis GA, Kearns T, Mayfield HJ, Sheridan S, Thomsen R, Naseri T, et al. Scabies prevalence after ivermectin-based mass drug administration for lymphatic filariasis, Samoa 2018-2019. PloS Negl Trop Dis. (2023) 17:e0011549. doi: 10.1371/journal.pntd.0011549
71. Marks M, Toloka H, Baker C, Kositz C, Asugeni J, Puiahi E, et al. Randomized trial of community treatment with azithromycin and ivermectin mass drug administration for control of scabies and impetigo. Clin Infect Dis. (2019) 68:927–33. doi: 10.1093/cid/ciy574
72. Romani L, Whitfeld MJ, Koroivueta J, Kama M, Wand H, Tikoduadua L, et al. Mass drug administration for scabies - 2 years of follow-up. N Engl J Med. (2019) 381:186–7. doi: 10.1056/NEJMc1808439
73. Marks M, Romani L, Sokana O, Neko L, Harrington R, Nasi T, et al. Prevalence of scabies and impetigo 3 years after mass drug administration with ivermectin and azithromycin. Clin Infect Dis. (2020) 70:1591–5. doi: 10.1093/cid/ciz444
74. WHO. WHO’s first global meeting on skin NTDs calls for greater efforts to address their burden Geneva. World Health Organization (2023). Available at: https://www.who.int/news/item/31-03-2023-who-first-global-meeting-on-skin-ntds-calls-for-greater-efforts-to-address-their-burden.
75. WHO. WHO informal consultation on a framework for scabies control: meeting report. Manila: World Health Organization Regional Office for the Western Pacific (2020).
76. WHO. Ending the neglect to attain the Sustainable Development Goals: a road map for neglected tropical diseases 2021–2030. Geneva: World Health Organization (2020). Available at: https://www.who.int/publications/i/item/9789240010352.
77. Mitchell E, Kelly-Hanku A, Krentel A, Romani L, Robinson LJ, Vaz Nery S, et al. Community perceptions and acceptability of mass drug administration for the control of neglected tropical diseases in Asia-Pacific countries: A systematic scoping review of qualitative research. PloS Negl Trop Dis. (2022) 16:e0010215. doi: 10.1371/journal.pntd.0010215
Keywords: scabies, Sarcoptes scabiei, impetigo, neglected tropical diseases, mass drug administration, control strategies
Citation: Mitchell E, Wallace M, Marshall J, Whitfeld M and Romani L (2024) Scabies: current knowledge and future directions. Front. Trop. Dis 5:1429266. doi: 10.3389/fitd.2024.1429266
Received: 07 May 2024; Accepted: 10 July 2024;
Published: 23 July 2024.
Edited by:
Emanuele Nicastri, National Institute for Infectious Diseases Lazzaro Spallanzani (IRCCS), ItalyReviewed by:
Elham Kazemirad, Tehran University of Medical Sciences, IranCopyright © 2024 Mitchell, Wallace, Marshall, Whitfeld and Romani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lucia Romani, bHJvbWFuaUBraXJieS51bnN3LmVkdS5hdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.